Abstract
Objective:
To describe the safety, feasibility and outcome of redo buccal mucosal graft urethroplasty in patients presenting with recurrent anterior urethral stricture following previous failed BMG urethroplasty.
Materials and Methods:
This was a retrospective chart review of 21 patients with recurrent anterior urethral stricture after buccal mucosal graft urethroplasty, who underwent redo urethroplasty at our institute between January 2008 to January 2014. All patients underwent preoperative evaluation in the form of uroflowmetry, RGU, sonourethrogram and urethroscopy. Among patients with isolated bulbar urethral stricture, who had previously undergone ventral onlay, redo dorsal onlay BMG urethroplasty was done and vice versa (9+8 patients). Three patients, who had previously undergone Kulkarni-Barbagli urethroplasty, underwent dorsal free graft urethroplasty by ventral sagittal urethrotomy approach. One patient who had previously undergone urethroplasty by ASOPA technique underwent 2-stage Bracka repair. Catheter removal was done on 21st postoperative day. Follow-up consisted of uroflow, PVR and AUA-SS. Failure was defined as requirement of any post operative procedure.
Results:
Idiopathic urethral strictures constituted the predominant etiology. Eleven patients presented with stricture recurrence involving the entire grafted area, while the remaining 10 patients had fibrotic ring like strictures at the proximal/distal graft-urethral anastomotic sites. The success rate of redo surgery was 85.7% at a mean follow-up of 41.8 months (range: 1 yr-6 yrs). Among the 18 patients who required no intervention during the follow-up period, the graft survival was longer compared to their initial time to failure.
Conclusion:
Redo buccal mucosal graft urethroplasty is safe and feasible with good intermediate term outcomes.
Keywords: Buccal mucosal graft-urethroplasty, recurrent anterior urethral strictures, redo surgery
INTRODUCTION
Urethral reconstruction using a buccal mucosal graft (BMG) to substitute the urethral mucosa has become a well-established modality in the management of bulbar and penile urethral strictures, not amenable for excision and anastomosis.[1,2] The various configurations of BMG urethroplasty have included ventral onlay,[3] dorsal onlaly,[4] dorsal inlay via a ventral sagittal urethrotomy approach,[5] dorsolateral onlay with one sided mobilization of the urethra,[6] combined dorsal plus ventral double mucosal grafts,[7,8] two stage repairs,[9] and augmented anastomotic urethroplasty.[10]
Regardless of the surgical technique, all buccal graft-urethroplasty have the potential to fail or deteriorate with time. Stricture recurrence after substitution urethroplasty may take the form of either short segment fibrous ring strictures at the proximal or distal anastomotic sites or extensive fibrosis involving the entire grafted area.[11] Patients with anastomotic ring strictures may be managed with minimally invasive procedures, such as dilatation or optical internal urethrotomy (OIU). However, management of patients who fail these minimally invasive options or those with extensive fibrosis involving the entire grafted area is challenging. Furthermore, there is sparse literature regarding the optimal management of such patients. The objective of this study was to describe the safety, feasibility, and medium term outcome of redo BMG urethroplasty in patients presenting with recurrent anterior urethral stricture following previously failed substitution (BMG) urethroplasty.
MATERIALS AND METHODS
This was a retrospective analysis of our urethroplasty database. Between January 2008 and January 2014, we treated 25 patients who presented to our institute with recurrent anterior urethral stricture, having previously undergone BMG urethroplasty. Out of the total of 25 patients, two patients were excluded upfront from the analysis because of lack of adequate operative records/documentation and two further patients were excluded because of the loss to follow-up. Hence, 21 patients were included in the final analysis. Previous operative notes and discharge summary were thoroughly evaluated and following points were noted:
Type of urethroplasty previously undergone, with specific reference to placement of the buccal graft (dorsal or ventral onlay or dorsal inlay)
Location and length of the urethral stricture at the time of the first operation
Etiology
Time to failure after the first BMG urethroplasty
Failure was defined as a requirement of any postoperative procedure like dilatation, OIU, or redo urethroplasty. Time to failure was defined as the interval between catheter removal and first intervention (dilatation/OIU/urethroplasty). A similar definition was used after redo BMG urethroplasty. Patients with no previous operative and follow-up records were excluded from analysis upfront.
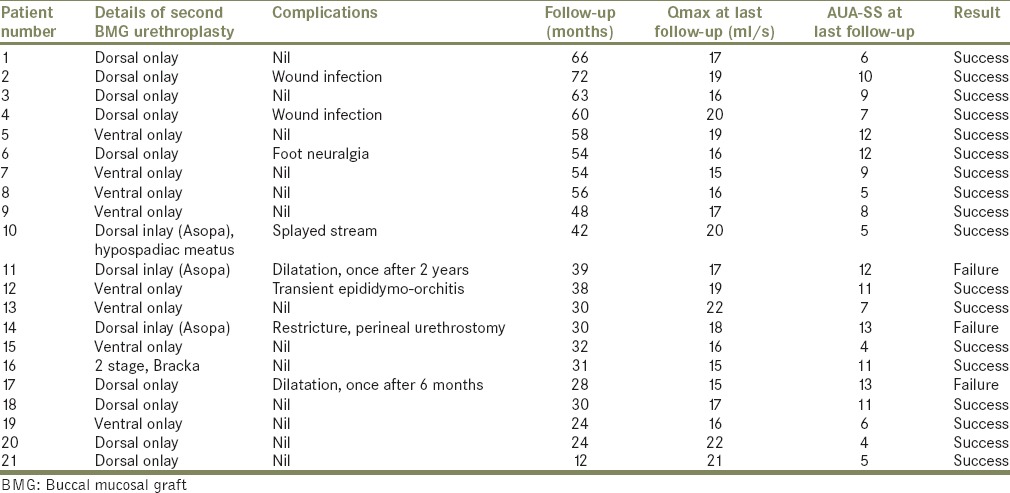
Further preoperative evaluation consisted of a detailed clinical history and physical examination, urine culture, uroflowmetry, post void residual urine, serum creatinine, ultrasound of kidney-ureter-bladder, retrograde urethrography, sonourethrogram, and urethroscopy with a 6/7.5 Fr semi-rigid ureteroscope. Present stricture length and location were noted. Institutional review board approval was obtained. All surgeries were performed by either one of two surgeons: HKN/TDJ. Table 1 gives the details of first BMG urethroplasty. Table 2 analyses failure after first BMG urethroplasty. Table 3 gives results after second BMG urethroplasty.
Table 1.
Details of first BMG urethroplasty surgery
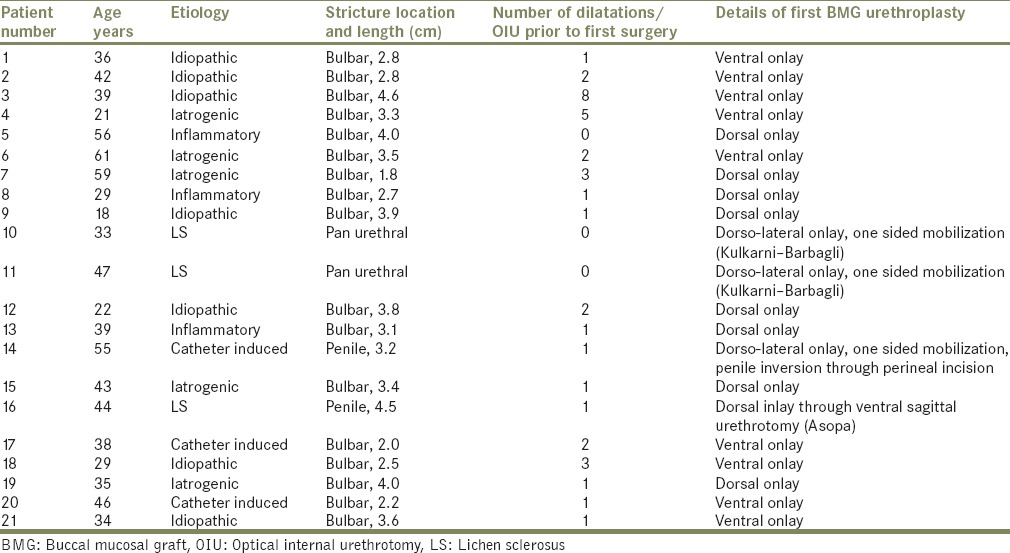
Table 2.
Stricture recurrence after first BMG urethroplasty
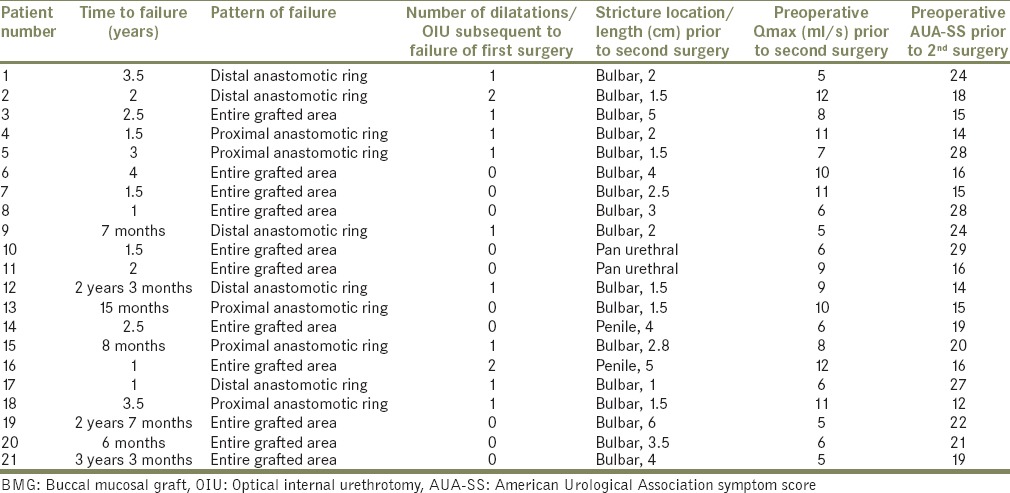
Table 3.
Results after second BMG urethroplasty
The surgical technique of redo BMG urethroplasty was selected based on the knowledge of previous operative procedure, the site and length of the present stricture, current intraoperative findings (tissue planes and ease of dissection) as well as surgeon preference.
The buccal mucosal graft was harvested from the cheek using a two-team approach. The donor site was closed with 3–0 polyglactin. The graft was preferentially harvested from the cheek opposite to the one used in the previous surgery. However, when longer grafts were necessary, both cheeks were used. Lingual grafts were not used in any patients.
The urethroplasty was done in the lithotomy position using either a midline or λ incision. The bulbocavernosus muscle was divided and the bulbar urethra was exposed. Among patients with isolated bulbar urethral stricture, who had previously undergone ventral onlay (nine patients), dorsal onlay redo BMG urethroplasty was done; and in those with a previous dorsal onlay (eight patients), a ventral onlay BMG urethroplasty was performed by the standard surgical techniques.[3,4] In both situations, the urethrotomy (dorsal/ventral) through the strictured tract was extended 1 cm into the normal urethra, to calibrate the distal and proximal lumina to at least 24 Fr. This procedure remained the same, whether the pattern of recurrence was an anastomotic ring stricture or stricture of the entire grafted area.
Three patients (pt. nos. 10, 11, and 14) who had previously undergone BMG urethroplasty by the Kulkarni-Barbagli technique,[6] (two of whom had pan-anterior urethral stricture recurrence due to lichen sclerosus [LS] and the other who had a catheter induced isolated penile stricture) underwent dorsal free graft-urethroplasty by ventral sagittal urethrotomy approach.[5] In all these three patients midline, perineal incision and penile inversion technique were used to access the penile urethra.[12]
Patient number 16, with LS induced penile stricture, who had previously undergone urethroplasty by the Asopa technique, underwent two-stage Bracka repair.[9]
In all these 21 patients with redo-BMG urethroplasty, perioperative antibiotics consisted of intravenous second generation cephalosporin and aminoglycoside at induction of anesthesia and two subsequent doses, followed by oral cephalosporin till catheter removal.
In all patients, the catheter was removed at 21 days and voiding cystourethrography was performed to rule out extravasation. Follow-up protocol consisted of uroflowmetry and postvoid residual assessment, as well as urine culture and American Urological Association symptom score (AUA-SS), every 4 months for the first 2 years and then 6 monthly thereafter. Retrograde urethrography and urethroscopy was performed if a stricture was suspected based on obstructive symptoms, deterioration of flow rate or AUA-SS scores or increase in postvoid residual volumes. Failure was defined as requirement of any postoperative procedure like dilatation or OIU.
RESULTS
A total of 21 patients were included in the final analysis. Of these 21 patients, 4 were previously operated at our own institute. Mean patient age was 39.33 years. Idiopathic urethral strictures constituted the predominant etiology (7 cases), followed by iatrogenic (5 cases), inflammatory, catheter-induced and LS (3 cases each)., followed by iatrogenic (5 cases), inflammatory, catheter-induced and LS (3 cases each).
Out of the 21 patients, 9 patients had undergone ventral onlay BMG urethroplasty and 8 patients had undergone dorsal onlay BMG urethroplasty for isolated bulbar urethral stricture. The mean stricture length in these 17 patients with bulbar stricture was 3.18 cm (range: 1.8–4.6 cm). Two patients with pan-anterior urethral stricture due to LS had undergone single stage dorsal onlay BMG urethroplasty by Kulkarni-Barbagli technique. The remaining two patients had isolated penile urethral stricture, one of which was catheter induced and the other due to LS The mean number of dilatations prior to the first BMG urethroplasty was 1.76 (range: 0–8). The mean time to failure in patients with isolated bulbar urethral stricture who had undergone either a dorsal/ventral BMG urethroplasty was 24.4 months. The three patients with LS had a comparatively shorter time to failure (1.5, 2, and 1-year). Eleven patients presented with stricture recurrence involving the entire grafted area while the remaining 10 patients had fibrotic ring like strictures at the proximal/distal graft-urethral anastomotic sites.
Some of the patients with recurrent strictures were again temporarily managed with dilatation/OIU by either their primary surgeon/elsewhere, before presenting to our institute with intractable symptoms.
On evaluation at our institute, the mean Qmax among the 21 patients prior to the redo urethroplasty was 8 ml/s and mean preoperative AUA-SS score was 19.62. Details of the second BMG urethroplasty are given in Table 3 and have already been discussed in the materials and methods section above.
Postoperative complications included wound infection in two patients, foot neuralgia in one patient and transient epididymo-orchitis in one patient. One patient (no. 10) who had undergone dorsal inlay urethroplasty had splayed stream because of the surgically created hypospadiac meatus.
The success rate was 85.7% at a mean follow-up of 42.4 months (range: 1–6 years). One patient developed pan-anterior urethral stricture and underwent permanent perineal urethrostomy. Two patients (pt. no. 11 and 17) required a single session of dilatation, 6 months and 2 years after the redo surgery respectively, and then remained symptom-free till the end of their follow-up period. Among the 18 patients who required no intervention during the follow-up period, the graft survival was longer compared to their initial time to failure.
DISCUSSION
There is a dearth of literature regarding the management of patients with recurrent anterior urethral strictures following previous BMG urethroplasty as well as the outcomes of these patients who undergo redo BMG urethroplasty. Blaschko et al. analyzed the outcomes of 130 patients who underwent repeat urethroplasty after initial failed urethral reconstruction.[13] However, in this study, in 73% of patients the details of previous urethral reconstruction were not known. Furthermore, the technique employed during redo surgery was end-to-end anastomosis in 42% of patients, fasciocutaneous flap in 23% of patients and combined flap plus graft in 12% of patients. They also included urethral stricture due to hypospadias, which is a totally different entity altogether. Only 31 out of the 130 patients underwent onlay graft-urethropalsty, but the nature of the graft (skin/buccal mucosa/lingual mucosa) is not mentioned in the paper, as is the type of previous urethral reconstruction undergone by this cohort of patients. Contrary to the Blashcko paper, our study, specifically deals with redo BMG urethroplasty in patients with previous failed urethral reconstruction using BMG. To the best of our knowledge, our study is the largest series of redo BMG urethroplasty with intermediate-term outcomes. The first point we would like to highlight is the safety and feasibility of redo BMG urethroplasty. There are no uniform rules regarding the management of failed BMG urethroplasty cases. However, in our experience, employing ventral onlay technique for those who had previously undergone dorsal onlay and vice versa, ensures easier dissection. The history of urethral reconstructive surgery has witnessed wide-ranging debates regarding the best technique for placement of the buccal graft, with each group highlighting the superiority of one over the other. Barbagli et al. showed that the placement of BMGs into the vental, dorsal or lateral surface of the urethra showed the same success rate and the outcome were not affected by the surgical technique.[14] Surgeons involved in the urethral reconstructive surgery should be well versed in the various techniques of BMG urethroplasty so that they can employ the technique best suited for the particular situation in redo cases.
Anastomotic ring strictures cannot be indefinitely managed by minimally invasive techniques like dilatation/OIU; though this may be the only viable option in some patients. Ultimately a proportion of these patients may present with intractable strictures that are best treated with redo BMG urethroplasty. Recurrences in the form of strictures involving the entire grafted area, either a result of a technical error or a progression of the native urethral disease are also amenable for redo urethroplasty. This is borne out the mean stricture free period (45.06 months) among the successful 16 cases of isolated bulbar stricture redo cases in our series, which was longer than the mean time to failure after the first BMG urethroplasty in these cases (25.18 months). However, technical aspect could have contributed to failure after the first surgery in these cases.
We acknowledge certain limitations in our study. This was a retrospective study with a limited sample size. Furthermore, it was a heterogeneous cohort of patients consisting of pan-anterior urethral as well as isolated bulbar and penile urethral strictures of varied etiology and operated at various centers. There were no strict predetermined criteria regarding the management of patients with recurrent anterior urethral strictures with repeated dilatations versus subjecting them to redo surgery. This was left to the discretion of the treating surgeon taking various factors into account like length and location of stricture based on retrograde urethrogram, degree of spongiofibrosis based on sonourethrogram, number and frequency of minimally invasive procedure already undergone by the patient after the previous failed surgery and patient preference regarding long-term cure with a redo surgery versus continuing to remain on frequent dilatation.
CONCLUSIONS
Redo BMG urethroplasty is safe and feasible with good intermediate-term outcomes. Surgeons need to be well versed in various techniques of BMG urethroplasty to manage patients with recurrent anterior urethral strictures.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Andrich DE, Mundy AR. What is the best technique for urethroplasty? Eur Urol. 2008;54:1031–41. doi: 10.1016/j.eururo.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 2.Barbagli G, Lazzeri M. Surgical treatment of anterior urethral stricture diseases: Brief overview. Int Braz J Urol. 2007;33:461–9. doi: 10.1590/s1677-55382007000400002. [DOI] [PubMed] [Google Scholar]
- 3.Morey AF, McAninch JW. When and how to use buccal mucosal grafts in adult bulbar urethroplasty. Urology. 1996;48:194–8. doi: 10.1016/S0090-4295(96)00154-9. [DOI] [PubMed] [Google Scholar]
- 4.Barbagli G, Palminteri E, Rizzo M. Dorsal onlay graft urethroplasty using penile skin or buccal mucosa in adult bulbourethral strictures. J Urol. 1998;160:1307–9. [PubMed] [Google Scholar]
- 5.Asopa HS, Garg M, Singhal GG, Singh L, Asopa J, Nischal A. Dorsal free graft urethroplasty for urethral stricture by ventral sagittal urethrotomy approach. Urology. 2001;58:657–9. doi: 10.1016/s0090-4295(01)01377-2. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni S, Barbagli G, Sansalone S, Lazzeri M. One-sided anterior urethroplasty: A new dorsal onlay graft technique. BJU Int. 2009;104:1150–5. doi: 10.1111/j.1464-410X.2009.08590.x. [DOI] [PubMed] [Google Scholar]
- 7.Palminteri E, Manzoni G, Berdondini E, Di Fiore F, Testa G, Poluzzi M, et al. Combined dorsal plus ventral double buccal mucosa graft in bulbar urethral reconstruction. Eur Urol. 2008;53:81–9. doi: 10.1016/j.eururo.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 8.Gelman J, Siegel JA. Ventral and dorsal buccal grafting for 1-stage repair of complex anterior urethral strictures. Urology. 2014;83:1418–22. doi: 10.1016/j.urology.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Bracka A. A versatile two-stage hypospadias repair. Br J Plast Surg. 1995;48:345–52. doi: 10.1016/s0007-1226(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 10.Abouassaly R, Angermeier KW. Augmented anastomotic urethroplasty. J Urol. 2007;177:2211–5. doi: 10.1016/j.juro.2007.01.140. [DOI] [PubMed] [Google Scholar]
- 11.Barbagli G, Palminteri E, Lazzeri M, Turini D. Interim outcomes of dorsal skin graft bulbar urethroplasty. J Urol. 2004;172:1365–7. doi: 10.1097/01.ju.0000139727.70523.30. [DOI] [PubMed] [Google Scholar]
- 12.Gupta NP, Ansari MS, Dogra PN, Tandon S. Dorsal buccal mucosal graft urethroplasty by a ventral sagittal urethrotomy and minimal-access perineal approach for anterior urethral stricture. BJU Int. 2004;93:1287–90. doi: 10.1111/j.1464-410X.2004.04822.x. [DOI] [PubMed] [Google Scholar]
- 13.Blaschko SD, McAninch JW, Myers JB, Schlomer BJ, Breyer BN. Repeat urethroplasty after failed urethral reconstruction: Outcome analysis of 130 patients. J Urol. 2012;188:2260–4. doi: 10.1016/j.juro.2012.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbagli G, Palminteri E, Guazzoni G, Montorsi F, Turini D, Lazzeri M. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: Are results affected by the surgical technique? J Urol. 2005;174:955–7. doi: 10.1097/01.ju.0000169422.46721.d7. [DOI] [PubMed] [Google Scholar]


