Abstract
In this study the development of a model tracheal mucus with chemical composition and physical properties (bulk viscoelasticity and surface tension) matched to that of native tracheal mucus is described. The mucus mimetics were formulated using components that are abundant in tracheal mucus (glycoproteins, proteins, lipids, ions and water) at concentrations similar to those found natively. Pure solutions were unable to achieve the gel behavior observed with native mucus. The addition of a bi-functional crosslinking agent enabled control over the viscoelastic properties of the mucus mimetics by tailoring the concentration of the crosslinking agent and the duration of crosslinking. Three mucus mimetic formulations with different bulk viscoelastic properties, all within the normal range for non-diseased tracheal mucus, were chosen for investigation of surfactant spreading at the air-mimetic interface. Surfactant spread quickly and completely on the least viscoelastic mimetic surface, enabling the surface tension of the mimetic to be lowered to match native tracheal mucus. However, surfactant spreading on the more viscoelastic mimetics was hindered, suggesting that the bulk properties of the mimetics dictate the range of surface properties that can be achieved.
Keywords: Biomimetic material, Lung, Mechanical properties, Surface modification, Viscoelasticity
INTRODUCTION
The mucosal epithelium of the large conducting airways is coated with a fluid layer known as airway lining fluid. The primary functions of this fluid layer are to protect the underlying lung tissues by trapping and clearing inhaled foreign material and to hydrate the epithelial cells on the mucosal surface. The airway lining fluid is able to carry out these various functions due to its unique structural properties. It is composed of two primary layers: a periciliary layer and a mucus gel layer which lies atop the periciliary layer. The periciliary fluid allows unhindered movement of the cilia that extend from the cell surface to the mucus gel. The mucus gel layer exhibits viscoelastic behavior which assists in the entrapment of foreign material in the gel and provides energy storage for use in bulk transport during mucociliary and cough clearance.1,2 Recently, a gel-on-brush model for the airway lining fluid has been proposed by Button et al. in which the periciliary fluid layer is occupied by densely tethered macromolecules of membrane-spanning mucins and mucopolysaccharides that form an extracellular brush.3 This periciliary brush establishes a mesh of a pore size of ~20 to 40 nm that prevents the penetration of the mucus gel into the periciliary space thus stabilizing the two distinct layers.
The mucus gel is composed of 93–97% w/w water and 3–7% w/w solids, which includes proteins, glycoproteins (mucins), lipids, minerals and DNA.4–6 It forms a highly crosslinked network due to a number of covalent and non-covalent bonds, which leads to its viscoelastic nature.7 Physical entanglement between the glycoproteins, proteins and DNA contribute to the gel network. Intra-molecular disulfide bridges covalently link glycoprotein subunits to form an extended mucin polymer. In addition, non-covalent linkages such as hydrogen bonds, ionic interactions, and Van der Waals forces aid the formation of a crosslinked network.
Many of the proteins and lipids found natively in the airway lining fluid are surface active. These molecules collect at the air-fluid interface, leading to the relatively low surface tension reported for the large conducting airways, ~ 30–34 mN/m, in literature.8–10 This surfactant film is crucial for normal function of the tracheobronchial tree in that it aids the penetration of foreign material (such as pathogens, environmental contaminants and therapeutics) into the sticky mucus gel to enhance clearance of these materials,10,11 participates in innate lung defense,12 and reduces evaporation of the subphase.13 In the large conducting airways of mammalian lungs, the air-fluid interface experiences a variety of stresses, including shearing by air during inhalation-exhalation cycles and bulk fluid movement due to mucociliary and cough clearance. Knowledge of the interfacial properties of the airway lining fluid under stress could provide new insight into the function of these fluids in the large conducting airways. However, the interfacial properties of the fluids in the large conducting airways remain largely unstudied, despite their importance in a variety of physiological processes. Therefore, a better understanding of the properties of surfactants at the air-mucus interface would provide critical information about the function of the tracheobronchial tree.14–16 It has been shown that the interfacial behavior of an air-fluid surface is influenced by the surface composition as well as the physical properties of the underlying fluid.14,17 Given the viscoelastic nature of the mucus underlying the surfactant film at the interface,5,18–21 it is expected that the surface behavior of this fluid will be distinct from the commonly studied Newtonian case, where the underlying subphase is water.
Current models of the airway lining fluid include synthetic gels, native secretions, and in vitro secretions derived from cell cultures. While these models have been used successfully to better understand some of the functional properties of lung fluids, each has its own limitations. Synthetic viscoelastic gels made of polymeric materials (such as locust bean gum,22 gum tragacanth,23 guar gum,24 scleroglucan,24 Polyox water-soluble resin,25 carrageenan,22 and pig gastric mucins26 are formulated to match the bulk viscoelastic behavior of mucus. However, they do not match the chemical composition or surface properties, which are critical to our understanding of mucus function and to enable investigation of the interactions of mucus with foreign entities such as bacteria, drugs, and inhalable particles. Native secretions, on the other hand, are ideal for their chemical and physical properties, but suffer from difficulties in collection, inaccessibility, and large inter-subject and intra-subject variability. Patients with certain chronic airway diseases such as asthma, cystic fibrosis and chronic obstructive pulmonary diseases produce large quantities of sputum, enabling direct removal by hypertonic saline induction or by expectoration.27,28 The collection of mucus from patients without any known lung disease, however, is burdensome and results in extremely small quantities of collected fluid. Primary cultures of human bronchial epithelial (HBE) cells grown under an air-liquid interface are able to produce mucus whose composition is similar to that of native secretions.29–32 Mucus harvested from these cell cultures can serve as a model system in studies where small quantities of mucus are required. However, extensive experimental analysis where large quantities are required also limit the use of this model.
We set out to develop a model of the airway lining fluid that would match both the bulk and interfacial properties of native, non-diseased mucus to enable large-scale studies of mucus properties. We formulated a series of airway mucus mimetics where the chemical composition and concentration, bulk viscoelastic properties, and surface tension have been matched to that of native, non-diseased tracheal mucus. The mucus mimetics were developed utilizing components that are abundant in tracheal mucus (glycoproteins, proteins, lipids, ions and water), at concentrations similar to those found natively. The in vitro model mucus mimetic was crosslinked using a bi-functional crosslinking agent to control the viscoelastic properties of the mucus mimetic. Finally, the ability to lower the surface tension of the mucus mimetic to that of native tracheal mucus via surfactant spreading at the air-mimetic interface was studied.
MATERIALS and METHODS
Materials
Pig gastric mucin (PGM)-type III, n-hexane (99.0% purity) and glutaraldehyde (G7651, 50% w/w solution) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO); bovine serum albumin (fraction V, lyophilized powder) from Spectrum (Gardena, CA); 1, 2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) from Genzyme Pharmaceuticals (Cambridge, MA); methanol (99.9% purity) from Fisher Scientific (Fair Lawn, NJ); and Texas Red-DHPE (1, 2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt) from Invitrogen. Infasurf® was a gift from Ony, Inc. (Amherst, NY). Purified water (18 MΩ·cm) used in all experiments was obtained from a NANOpure Infinity Ultrapure Water System (Barnstead International, Northbrook, IL). All other chemical reagents were of analytical grade and used without further purification.
Preparation of Uncrosslinked Mucin Solutions
A series of mucin solutions was formulated to contain 2%, 3%, 4% or 6% w/v of PGM-type III, 1% w/v protein (albumin), and 1% w/v DPPC in buffer (154 mM NaCl, 3 mM CaCl2, 15 mM NaH2PO4/Na2HPO4 in water; pH 7.4). To prepare 30 ml of the mucin solution, 0.3 g DPPC was dispersed in 5 ml of buffer solution in an amber glass bottle for 2 hours using a magnetic stirrer. Then, 0.3 g albumin, PGM-type III and additional buffer solution were added to the amber glass bottle. Ingredients were mixed at 4°C on a tube rotator (Glas-Col, IN) for at least 6 days to homogenize the sample.
Preparation of Crosslinked Mucus Mimetics
To prepare the mucus mimetic, 0.3 g albumin and 1.2 g (4%) PGM-type III were added to 28.2 ml buffer solution (154 mM NaCl, 3 mM CaCl2, 15 mM NaH2PO4/Na2HPO4; pH 7.4) in an amber glass bottle and mixed at 4°C on a tube rotator (Glas-Col, IN) for at least 6 days. The bi-functional crosslinking agent, glutaraldehyde (GA), was used to induce crosslinking to mucus mimetic. The bulk viscoelastic properties of the mucus mimetic were controlled by altering the concentration of GA added to mucus mimetic formulations (1.4%, 2.7%, 4.0%, 5.3%, 6.5% and 21.7% w/w of total solids) and/or the crosslinking time (1 day, 2 days and 3 days). Crosslinking of the mucus mimetic was achieved by adding the GA solution to the glass bottle and mixing using a tube rotator (Glas-Col, IN) at 4°C. To lower the surface tension at the mimetic interface, the mimetic was placed into a Langmuir trough and a surfactant solution containing either DPPC or Infasurf® spread onto the mimetic surface using a Hamilton micro-syringe (0–100 μl of a 1 mg/ml solution of DPPC in 95:5 v/v n-hexane:methanol or 28 μL of Infasurf® suspension at a concentration of 1 mg of total phospholipids in 1.0 ml of 90:10 v/v chloroform:methanol). Prior to any experiments with the surfactant-laden mucus mimetic, the solvent was allowed to evaporate from the surface for 10 min.
Dynamic (Oscillatory) Bulk Rheological Analysis
The bulk viscoelastic properties of crosslinked mucus mimetics were determined using a controlled-stress cone and plate rheometer (RheoStress 1, Haake®; 60 mm diameter, 4° cone angle) maintained at 23°C via a temperature control system (Haake® F3-CH refrigerated circulator). A solvent trap was used to cover the sample during analysis to minimize sample dehydration. For each experiment, 5 ml of mimetic was loaded onto the lower plate, spread evenly over the entire surface area of the plate and allowed to relax and equilibrate at 23°C for 60 seconds. The upper cone was lowered until the gap between the cone and plate was 0.138 mm and then the cone oscillated under the defined frequency conditions. The strain-dependence of the mimetic over a strain range of 0.01 to 1.1 at a constant frequency (ω) of 2 rad/sec was measured to determine the linear viscoelastic region (LVR). Within the LVR, the shear moduli remain constant and are independent of strain. Thus, the material response is independent of the magnitude of the deformation, ensuring that the observed viscoelastic behavior of the material is only related to its molecular structure. The point at which the viscoelastic moduli deviate by more than 10% from a constant (plateau) value indicated departure from LVR. On the basis of this test, an applied strain of 0.1, within the LVR, was chosen for subsequent frequency-dependent experiments. The frequency-dependence of the mimetics were determined over a frequency range of 0.5 to 100 rad/sec. The response of each mimetic to the imposed oscillatory shear was characterized using the bulk shear storage (or elastic) modulus G′ and the bulk shear loss (or viscous) modulus G″. Both quantities were determined using RheoWin® Data Pro Manager (v.2.96). The degree of solid-like behavior in a material is reflected by the storage or elastic modulus (G′) and the liquid-like behavior is reflected by the loss or viscous modulus (G″). The response of these two moduli to shear frequency provides a measure of how the mimetic responds to shear forces generated by fluid flow. Bulk viscoelastic properties were determined for at least three independent samples.
Surface Tension Analysis and Modification
The surface tension of each mucus mimetic was determined using a Wilhelmy plate balance (KSV NIMA Instruments, Finland) or the du Noüy ring method (Sigma 70, KSV NIMA Instruments, Finland). The Wilhelmy plate method was used in this study to determine the surface tension of mucus mimetic of more liquid-like behavior, where a meniscus is formed on the plate perimeter and the surface tension can be measured over time. For more solid-like materials, the Wilhelmy plate method does not provide accurate measurements and other techniques, such as the du Noüy ring method or contact angle measurements, must be used. In the Wilhelmy plate method, a platinum plate (20 mm × 10 mm) was attached to a force transducer and dipped into the surface of the mucus mimetic sample, oriented perpendicular to the interface. The force exerted by the fluid on the plate was measured and used to calculate the surface tension using the following equation: γ = F/ (2Lcosθ), where γ is the surface tension of the interface, F is the force exerted on the plate, L is the perimeter of the plate, and θ is the contact angle made between the interface and the plate, which is assumed to be zero. The surface tension was calculated using the KSV software. In the du Noüy ring method, a platinum iridium du Noüy ring (circumference 5.910 cm, R/r 52.94) was attached to the balance and put into contact with the mucus sample to a wetting depth of 6.0 mm. The ring was then raised at a speed up of 20 mm/min and the force required to separate the ring from the sample directly measured by a force transducer. The surface tension was calculated using the equation: γ = F/ (4πR), where F is the detachment force for the meniscus and 4πR is the periphery of the surface in contact with the ring. At least three surface tension measurements were conducted for each sample.
RESULTS
Bulk Rheology of Uncrosslinked Mucin Solutions
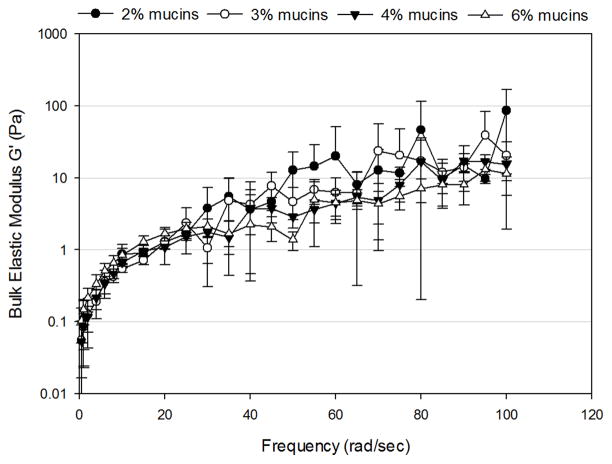
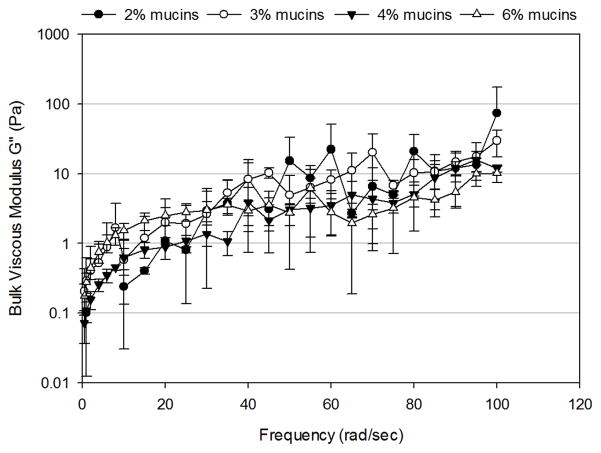
For solutions composed of 2–6% mucins, the bulk storage modulus (G′) and bulk loss modulus (G″) increased as a function of frequency (Figure 1). At low frequencies (< 20 rad/sec), both G′ and G″ exhibited an exponential increase with frequency. At higher frequencies (> 20 rad/sec), G′ and G″ increased linearly with frequency. No significant differences were observed in G′ or G″ as a function of mucin concentration. Therefore, control over the bulk rheological properties were not possible based on mucin concentration alone.
Figure 1.
Frequency-dependence of (A) bulk shear storage modulus, G′, and (B) bulk shear loss modulus, G″ for uncrosslinked mimetic solutions containing 2% (n=3), 3% (n=3), 4% (n=4) and 6% (n=5) mucins. Data are represented as the mean ± SD.
Controlling the Bulk Viscoelastic Properties via Crosslinking
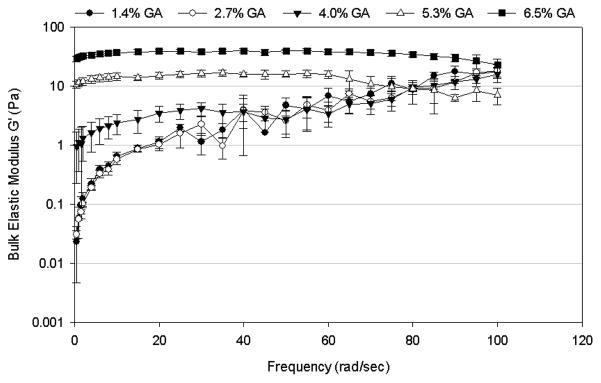
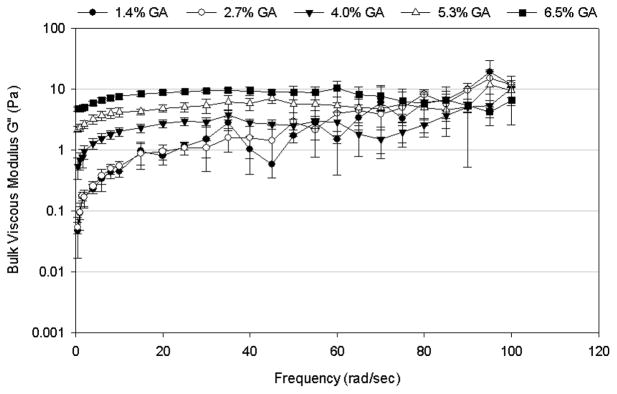
Using the crosslinking agent glutaraldehyde (GA) to induce crosslinking to the 4% mucin containing mimetic significantly increased the bulk viscoelastic properties of the mimetic. The crosslinked mucus mimetics exhibited more solid-like (elastic) behavior at all GA concentrations (Figure 2) and crosslinking times (Figure 3) tested, with G′ larger than G″ at all frequencies. Both G′ and G″ increased with increasing GA concentration and/or the duration of crosslinking process. The effect of increasing GA concentration from 1.4 to 6.5% w/w of total solids on the bulk rheological properties of the mucus mimetics crosslinked for 3 days is illustrated in Figure 2. The addition of GA at low concentration (1.4 – 2.7% w/w of total solids) did not alter the viscoelastic properties of the mimetic. However, at GA concentrations above 2.7% w/w of total solids, both G′ and G″ increased with increasing GA concentration. For mimetics crosslinked using 1.4, 2.7 and 4% w/w of total solids of GA, both G′ and G″ increased with increasing frequency. However, this trend disappears at higher GA concentrations (5.3 and 6.5% w/w of total solids), where G′ exhibits a plateau region that extends from frequencies of about 5 rad/sec to higher frequencies of 60 – 80 rad/sec and G″ exhibits a plateau from about 10 rad/sec to the highest frequency tested (100 rad/sec). At very high frequencies (> 60 rad/sec for the 5.3% w/w of total solids of GA mimetic or > 80 rad/sec for the 6.5% w/w of total solids of GA mimetic), a decrease in G′ was observed with increasing frequency.
Figure 2.
Frequency-dependence of (A) bulk shear storage modulus, G′, and (B) bulk shear loss modulus, G″, for mucus mimetic crosslinked with varying concentrations of GA solution (1.4% w/w of total solids, n=3; 2.7% w/w of total solids, n=4; 4% w/w of total solids, n=5; 5.3% w/w of total solids, n=3; and 6.5% w/w of total solids, n=5) for 3 days. Data are represented as the mean ± SD.
Figure 3.
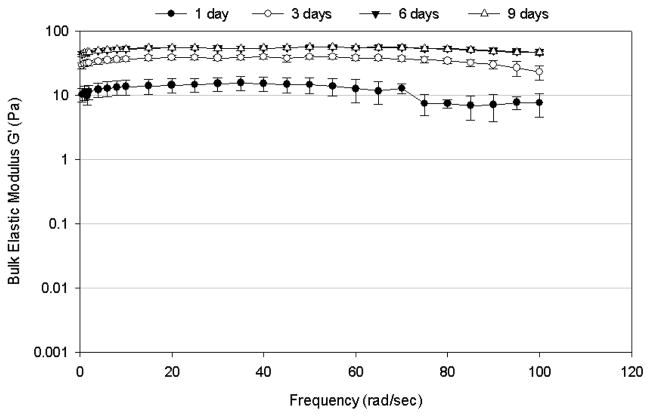
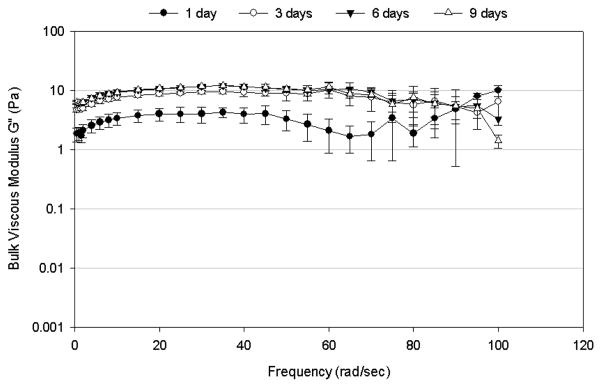
Frequency-dependence of (A) bulk shear storage modulus, G′, and (B) bulk shear loss modulus, G″, for mucus mimetic crosslinked using 6.5% w/w of total solids of GA for varying crosslinking times (1 day, n=5; 3 days, n=5; 6 days, n=4; and 9 days, n=3). Data are represented as the mean ± SD.
The bulk viscoelastic properties of the mucus mimetics varied with the duration of the crosslinking process (Figures 3). After 1 day of crosslinking, the mucus mimetic exhibited a G′ of about 10 Pa over a frequency range of 0.5 to 70 rad/sec, then a reduction in G′ at higher frequencies (Figure 3A). Upon crosslinking for 3 days, the storage modulus increased to about 40 Pa and the plateau region extended to about 85 rad/sec. After 6 days, the mucus mimetic exhibited a near constant G′ of about 55 Pa over the entire frequency range tested (0.5–100 rad/sec). Additional crosslinking time above 6 hours did not increase the storage modulus. Similar trends were observed for G″ (Figure 3B), where G″ increased with increasing the duration of crosslinking up to 3 days, with no significant change in G″ after 3 days.
Classification of Crosslinked Mucus Mimetics
The mucus mimetic formulations examined within this study displayed rheological properties that were controlled by altering the crosslinker concentration and/or duration of crosslinking process. To represent the wide range of viscoelastic properties exhibited in native, non-diseased mucus samples, three mucus mimetic formulations with different bulk rheological properties were chosen for further study and labeled mucus mimetic 1 (MM1; crosslinked using 4% w/w of total solids of GA and mixed for 24 hours), mucus mimetic 2 (MM2; crosslinked using 6.5% w/w of total solids of GA and mixed for 3 days) and mucus mimetic 3 (MM3; crosslinked using 21.7% w/w/ of total solids of GA and mixed for 3 days).
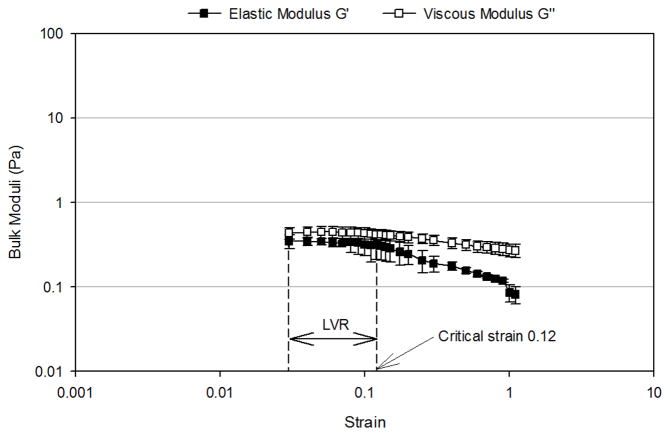
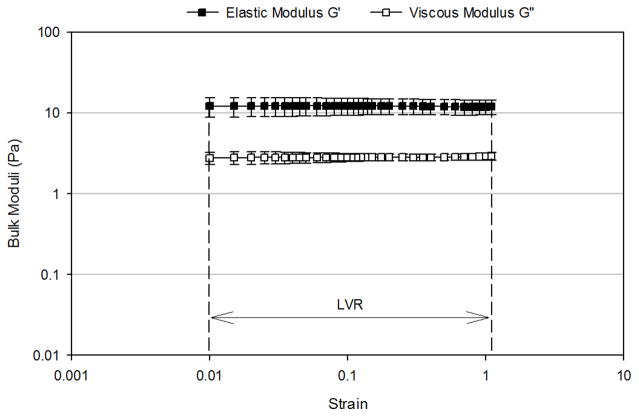
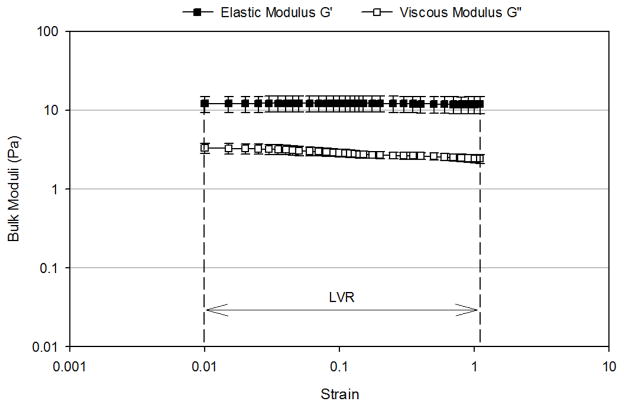
Figure 4 presents the oscillation strain sweep study of the three mimetics conducted at a fixed frequency of 2 rad/sec. The linear viscoelastic region (LVR), where the observed viscoelastic properties were independent of the applied strain, for the mucus mimetic 1 occurred over a strain range of 0.03 to 0.12 (Figure 4A). Beyond a critical strain of 0.12, G′ and G″ strongly decreased with deformation and the network was disrupted. The LVR for the mucus mimetic 2 occurred over a strain range of 0.01 to 1.1 (Figure 4B). The LVR for the mucus mimetic 3 occurred over a strain range of 0.025 to 0.4. The LVR for the mucus mimetics 2 and 3 extended over a longer range and exhibited a higher critical strain value compared to that of mucus mimetic 1. This observation is consistent with mucus mimetics 2 and 3 forming a significantly stronger crosslinked network due to the higher GA concentration. Similar observations have been made for other hydrated crosslinked systems, such as with cellulose hydrogels.33 Based on these tests, a value of an applied strain of 0.1 was chosen and used in subsequent bulk rheological analysis.
Figure 4.
Strain-dependence of bulk shear storage modulus, G′, and bulk shear loss modulus, G″, at a fixed frequency of 2 rad/sec for (A) MM1 over a strain range of 0.03 to 1.1 (n=4), (B) MM 2 over a strain range of 0.01 to 1.1 (n=4), (C) MM3 over a strain range of 0.01 to 1.1 (n=3). The linear viscoelastic region (LVR) and the critical strain are indicated by arrows. Data are represented as the mean ± SD.
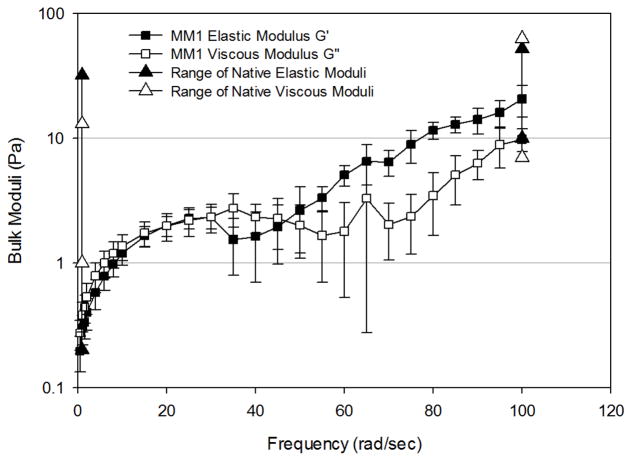
Figure 5 presents the frequency-dependence of G′ and G″ of the three mimetics, conducted in the linear viscoelastic region (LVR) for the mimetic under study. Mucus mimetic 1 (MM1) exhibited rheological characteristics of an entangled polymer solution with few crosslinks. Both G′ and G″ were frequency-dependent and two distinct regions in bulk rheological behavior were observed (Figure 5A). In region I, G′ and G″ were statistically equivalent at all frequencies (0.5–55 rad/sec) and both moduli increased rapidly with frequency. In region II, (frequencies of 55–100 rad/sec) the mimetic exhibited more elastic behavior where G′ dominated G″. Both moduli increased more slowly with frequency throughout region II. MM1 remained intact throughout the frequency range tested, with no apparent breakdown in the network structure.
Figure 5.
Frequency-dependence of bulk shear storage modulus, G′, and bulk shear loss modulus, G″, for (A) MM1 crosslinked using 4% w/w/ of total solids of GA for 24 hours (n=11), (B) MM2 crosslinked using 6.5% w/w of total solids of GA for 3 days (n=6), and (C) MM3 crosslinked using 21.7% w/w of total solids of GA for 3 days (n=5). Data are represented as the mean ± SD. The range of bulk elastic (solid triangles) and viscous (open triangles) moduli for non-diseased mucus at 1 and 100 rad/sec are provided for comparison.
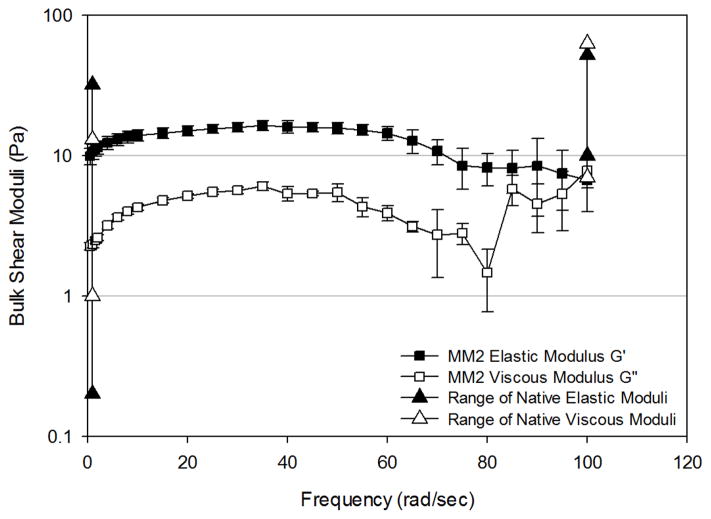
Mucus mimetic 2 (MM2) exhibited higher G′ and G″ values at frequencies from 0–70 rad/sec compared to MM1, but lower G′ and G″ values compare to MM1 at higher frequencies due to breaking of the crosslinked network (Figure 5B). G′ exhibited a broad plateau region across a frequency range of 0 to 70 rad/sec, similar to that observed for other crosslinked polymer gels. Beyond a frequency of 70 rad/sec, G′ decreased with increasing frequency. G″ shows a slight increase with frequency up to about 10 rad/sec, then exhibited a plateau from 10–55 rad/sec. Beyond 55 rad/sec, G″ exhibited inconsistent behavior, likely due to breaking of crosslinks in the samples.
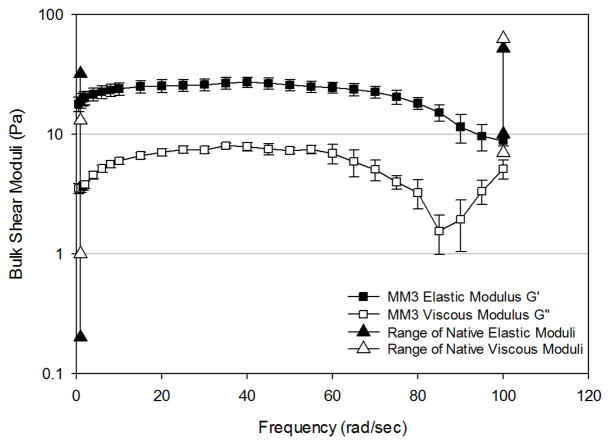
Mucus mimetic 3 (MM3) exhibited similar behavior to MM2, indicating again the crosslinking of the polymeric mucins to create a gel. Both G′ and G″ were larger for MM3 than MM2 at frequencies from 0–80 rad/sec (Figure 5C). The mimetic exhibited a broader plateau region which extended over a frequency range of 0–80 rad/sec for G′. Beyond a frequency of 80 rad/sec, G′ decreased as a function of frequency, indicating network breakdown at high frequency.
Altering the Surface Tension of Crosslinked Mucus Mimetics
The surface tension of MM1, measured using the Wilhelmy plate balance, was 53.3 ± 1.22 mN/m (n=9) (Table 1). The surface tensions of MM2 and MM3 were difficult to measure using the Wilhelmy plate balance due to their high elasticity. Therefore, the du Noüy ring method was employed. The surface tensions of MM2 and MM3 were 76.83 ± 5.83 mN/m (n=3) and 95.04 ± 7.78 mN/m (n=3), respectively (Table 1).
Table 1.
Surface tensions of native, non-diseased tracheal mucusand mucus mimetics 1, 2, and 3 measured using the Wilhelmy plate balance, du Noüy ring, or droplet spreading method.
| Type of Mucus | Surface Tension (mN/m) | Method Used |
|---|---|---|
| Native tracheal mucus | 31.9 ± 0.5 (n=21, horses)4 | Droplet spreading method |
| 32.5 ± 2.5 (n=10, horses)6 | ||
| 33.3 ± 0.7 (n=19, rats)5 | ||
| 32.2 ± 0.7 (n=39, guinea pigs)5 | ||
|
| ||
| Mucus mimetic 1 | 53.3 ± 1.2 (n= 9) | Wilhelmy plate |
| 56.4 ± 0.4 (n=3) | Du Noüy ring | |
|
| ||
| Mucus mimetic 2 | 78.6 ± 5.8 (n=3) | du Noüy ring |
|
| ||
| Mucus mimetic 3 | 95.0 ± 7.8 (n=3) | du Noüy ring |
Data are represented as the mean ± SD.
Fluorescence microscopy was used to monitor the spreading of DPPC on each of the mimetic surfaces. The surfactant solution was doped with Texas Red-DHPE to enable visualization of the fluid front during spreading. DPPC quickly spread on the MM1 surface and complete coverage of the surface was achieved (data not shown). As the amount of DPPC solution added to the surface was increased from 30 to 90 μl, the surface tension of the interface decreased from 47.4 to 36.4 mN/m (Table 2). The addition of 100 μl of a 1.02 mg/ml DPPC solution to the surface lowered the surface tension of MM1 to 30.2 ± 2.4 (n=4). This was 70.0% more than the amount of DPPC required to achieve the same surface tension on a water subphase. On MM2 and MM3, the surfactant drops did not spread upon contact with the mimetic surface, leading to a non-uniform surfactant concentration at the interface.
Table 2.
Surface tension and calculated surface area per surfactant molecule (if an equivalent amount of DPPC was added to a water subphase) after the spreading of different volumes of surfactant solution onto a MM1 or water subphase, measured using the Wilhelmy plate method.
| Subphase/Surfactant | Volume of surfactant solution added (μL) | Surface tension (mN/m) | Equivalent surface area/DPPC molecule (Å2) |
|---|---|---|---|
| MM1 | 0 | 53.3 ± 1.2 (n=9) | NA |
| MM1/DPPC | 30 | 47.4 ± 2.1 (n=5) | 40 |
| MM1/DPPC | 60 | 43.3 ± 2.1 (n=6) | 20 |
| MM1/DPPC | 90 | 36.4 ± 4.1 (n=6) | 13 |
| MM1/DPPC | 100 | 30.2 ± 2.4 (n=4) | 12 |
| Water/DPPC | 36 | 33.2 ± 1.7 (n=12) | 30 |
| MM1/Infasurf® | 28 | 31.4±3.8 (n=5) | NA |
| Water/Infasurf® | 28 | 29.2±1.0 (n=6) | NA |
Data are represented as the mean ± SD.
A complex surfactant, Infasurf®, was also used to alter the surface tension of MM1. Spreading 28 μL of Infasurf® solution (1 mg/ml of total phospholipid) at the air-mimetic interface led to a reduction in surface tension to 31.4 ± 3.8 mN/m (n=5) (Table 2). The amount of Infasurf® needed to lower the surface tension of MM1 was equivalent to that needed for a water subphase.
DISCUSSION
To investigate the properties of mucus, researchers have generally relied on secretions from individuals who overproduce mucus, i.e. those with chronic airway disease, secretions from canines, mucus harvested from primary cultures of human bronchial epithelial cells, or synthetic viscoelastic gels. While samples from canines and individuals with diseased lungs match the native properties of airway fluids, the small quantity of fluid obtained with each sample limits the number and scope of studies that can be conducted with the samples. Synthetic gels offer larger quantities of fluids and have been formulated to match the bulk rheological properties of native tracheal mucus, however they lack chemical similarity to normal mucus and surface properties that match native mucus. To overcome these limitations, we developed a series of mucus mimetics formulated using compounds found in native mucus and then altered the surface tension of the mimetic to match that of native, non-diseased tracheal mucus.
Tracheal mucus contains approximately 93–97% w/w water and 3–7% w/w solids, of which the solids content is about 1–3% w/w glycoproteins, 1% w/w proteins, 0.5–1% w/w lipids and 0.70–1.4% w/w minerals.4,5,34 Mucins are high molecular weight (2–20 × 105 Da) glycoproteins which are essential for maintaining the rheological properties of mucus.4 In the present study, we used commercially available pig gastric mucins (PGM, type III) with 0.5–1.5% sialic acid as the glycoprotein source, due to their easy availability and similarity to sialic acid content in native respiratory mucus.35,36 PGM type III is partially purified from crude mucin via the method of Glenister et al.37 Chemical analysis of the purified mucin performed by Glenister et al. showed that it was comprised of 20% w/w protein (which makes up the mucin core), 37% hexosamine, 27% total hexose (of which 10% is fucose), and 6% sialic acid. The latter three components are contained in the carbohydrate side-chain content of the mucins and account for 70% of the mucin content. In comparison, respiratory glycoproteins have been observed to contain about 10–25% protein and 75–85% carbohydrate.38,39 Therefore, the total protein and carbohydrate content of mucins from these two sources are similar. The gastric mucosa of healthy individuals primarily contains two secreted mucin proteins, MUC5AC and MUC6, which are characterized by the composition of their protein core.40 In comparison, three secreted mucin proteins, MUC2, MUC5AC and MUC5B, have been identified in the respiratory tract mucus of healthy individuals.41,42 However, MUC2 mucin is detected only at very low levels in airway samples. There is a commonality between gastric and respiratory mucins in that the primary mucin proteins from both sources are cysteine-rich and that both express MUC5AC. However, the differences in the expression profiles between the gastric and respiratory mucosa (MUC6 in gastric mucosa versus MUC5B in respiratory mucosa) may present a limitation of this model. In particular, it is not currently known if the different mucins form gels with different functional properties.43
The mucin concentration in pulmonary secretions has been evaluated in a single paper from 1963 by Matthews et al.44 In this paper, measurements were conducted on “normal” secretions from nine patients, ages 55–65, that had been laryngectomized 6 months to 5 years prior to the analysis. The mucin content from this study was estimated to be about 1–3%. Given the wide range of bulk rheological properties that have been reported in the literature for healthy airway mucus, it is conceivable that the solids concentration in mucus across the general population may vary to a larger extent than was observed in this one study. In our work, we chose to work at a slightly higher concentration of mucins, i.e. a fixed concentration of 4%. We observed in our early studies that mucin concentration did not have a large effect on the bulk rheological properties when not crosslinked, however would be expected to increase the number of available sites for crosslinking. This would have a two-fold effect on the formulation of the mimetics by decreasing the crosslinking time required and increasing the possible range of bulk rheological properties of the mimetics. The protein concentration in native pulmonary secretions was reported by Matthews et al. to be 1% of the overall composition.45 Many types of serum proteins have been identified in the tracheobronchial secretions, including immunoglobulins, albumin, fibrinogen, lysozyme, and lactoferrin.46 Albumin is the major serum protein component in the secretions, making up 14% of the total protein content.47 Thus, bovine serum albumin (BSA) was used at a concentration of 1% in the mimetic. While this study focused on a single protein and mucin concentration, future studies will extend the range of concentrations investigated to cover a wider range of properties observed in healthy mucus.
Human airway secretions contain an ion content of 165 ± 42 mmol/1000 g sodium, 13.2 ± 5.4 mmol/1000 g potassium, 162 ± 60 mmol/1000 g chloride, 3.1 ± 1.0 mmol/1000 g calcium, and 27 ± 16 mmol/1000 g phosphorous, leading to an osmolarity of 359 ± 56 mOsmol/L.20,46 The pH of human airway mucus has been reported between 5.4–8.2.4,34 In the current study, an aqueous buffer (pH 7.4; osmolarity 392 mOsmol/L) containing the minerals sodium, chloride, calcium and phosphorus was used. Overall, the synthetic mucus mimetics developed in this study were composed of 4% PGM-type III, 1% BSA, 154 mM NaCl, 3 mM CaCl2, 15 mM NaH2PO4/Na2HPO4 and 94% water.
Native mucus is a sheer-thinning fluid that exhibits viscoelastic behavior due primarily to entanglement of large macromolecules and to crosslinking by covalent and non-covalent bonds between the macromolecules. The frequency-dependent bulk rheological properties of non-diseased tracheal mucus from canines and humans has revealed average elastic moduli (G′) of 0.2–32 Pa at 1 rad/sec, which increase with frequency to 10–52 Pa at 100 rad/sec, and viscous moduli (G″) of 1–13 Pa at 1 rad/sec, which increase to 7–63 Pa at 100 rad/sec.18,21,48,49 While these values show high variability between subjects, the trend of increasing the moduli with frequency existed for all samples tested. The values of G′ and G″ at 1 and 100 rad/sec served as the target values for the development of the mucus mimetics in this study. As a first attempt to match the bulk rheological properties, the ability of solutions of mucins (concentrations ranging from 2–6%) to form a crosslinked polymer network naturally (i.e. no chemical crosslinking) was investigated. While the mucin solutions formed loosely entangled networks, as shown by the bulk rheological characterization, we could not modulate the viscoelastic properties of the mimetics. This is line with a study by Madsen et al.50 who observed that solutions containing 15% w/w pig gastric mucins could not match the rheological properties of native secretions.
Since crosslinking was not induced after 6 days of mixing the mucin solutions, the bi-functional crosslinking agent glutaraldehyde (GA) was used to induce crosslinking to the mucin solution. This crosslinking strategy enabled precise control over the viscoelastic properties of the mimetic by tailoring either the GA concentration or the duration of the crosslinking process. Increasing the concentration of the GA to ≥ 4% w/w of total solids and prolonging the duration of the crosslinking process to 3 days or longer significantly increased both G′ and G″ of the mimetics. In all cases, the mimetic exhibited more elastic behavior, as expected for a crosslinked polymer network. The extended plateau regions observed for highly crosslinked mimetics, i.e. those crosslinked with 5.3 to 6.5% w/w of total solids of GA, indicate the formation of stable covalent bonds and a gel network. This behavior has been observed both with non-diseased tracheal mucus51 and with mucus obtained from individuals with CF.52,53 The effect of GA on the rheological properties of bovine cervical mucus has been reported by Gelman and Meyer 54 who found that GA increases the elasticity of bovine cervical mucus. The researchers indicated that this increase in mucus elasticity was due to the formation of additional intermolecular links by the formation of Schiff bases between the epsilon-amino groups of the lysines in the mucin chains and the aldehyde groups of GA. Therefore, it is possible that the lysine content of mucus from different sources might produce different gels properties. Human cervical mucus contains about 14 lysine residues per 1000 amino acids, or 1.4% of the protein content of its mucins; whereas the protein content of pig gastric mucins contain about 2.2% lysine.55,56 This difference in lysine content can be attributed to the different MUC content of cervical and pig gastric mucus. It would be expected that cervical mucus, given its lower lysine content, would either be less crosslinked using the same quantity of GA or would require a longer time for crosslinking to complete.
GA has been widely used for the crosslinking of proteins,57,58 polyaminosaccharides,59,60 and glycoproteins, including pig gastric mucins,61 inducing both covalent intra- and intermolecular crosslinks.62 Natively, macromolecules in the tracheal mucus form an entangled network, which is further crosslinked by covalent and non-covalent bonds.7 Covalent linkages between macromolecules occur natively due to disulfide bonds and cleavage of these bonds leads to breakdown of the mucus gel structure. In our study, we have used GA as a substitute for the disulfide bridges. Covalent crosslinking of the mucus mimetics with GA restricts the local fluctuations in mucin conformation, particularly when using high concentration of the crosslinker. The irreversibility of this bond lends the mimetics less susceptible to network cleavage. This is useful for the stability of the system, but does limit the use of the mimetic in studies where disulfide bond cleavage is important (such as in the study of mucolytics that disrupt disulfide bonds). Secondary bonds, including ionic, hydrogen and hydrophobic bonds, also play an important role in the overall structure of the mucus network. These linkages could occur spontaneously in our mimetics, although we did not attempt to control their formation in this study.
In a previous study by Celli et al., purified pig gastric mucins were crosslinked by altering the pH of the mimetic.63 The rheological properties of the mimetic were matched to that of native gastric mucus using this approach, where low pH values (2 to 4) induced gelation (G′>G″), but higher pH values (~ 6) led to a liquid-like response. In instances where a low pH is advantageous, such as in the gastrointestinal tract, this model serves well. In the respiratory tract, where pH values have been reported to range from about 5–8, the lack of gelation at higher pHs would not allow the use of this model for respiratory mucus.4
In the present study, three mucus mimetic formulations were chosen for further investigation to determine the dependence of the each mimetic on shear frequency, which provides a measure of how the mimetic responds to shear forces generated by fluid flow. This property is of obvious relevance to the ability of respiratory mucus to withstand the shear forces generated in the respiratory tract due to fluid movement. MM1 exhibited bulk viscoelastic properties of an entangled polymer network, with G′ and G″ values increasing with increasing frequency. At high frequencies, G′ was slightly greater than G″, suggesting that more energy is stored by the mimetic than is dissipated at higher frequencies. Both MM2 and MM3 exhibited a crosslinked gel structure, with G′ and G″ not changing considerably with frequency. In both cases, G′ was greater than G″ at all frequencies, until network breakdown occurred at very high frequencies, again showing that these mimetics can store energy. The ability of native mucus to store energy has been linked to proper function of the mucociliary escalator.2
To enable comparison to bulk rheological properties of native mucus, reported moduli values for non-diseased tracheal mucus obtained from canines and humans in four different studies18,21,48,49 were compiled together and are presented in Figures 5–7. Moduli across the 3 mimetics show the greatest variation at 1 rad/sec, which is in accordance with the variation observed in native mucus samples. At this frequency, MM1 exhibited elastic behavior at the lower end of the reported native mucus values, whereas MM2 and MM3 exhibited elastic moduli at the upper end of the range. The viscous moduli of MM1 are slightly below native values, whereas MM2 and MM2 fall mid-range compared to native values. At 100 rad/sec, native samples exhibit significantly smaller variation in elastic and viscous moduli. All three mimetics fall within the native range at this high frequency.
The surface tension of the tracheal mucosal surface has been measured directly in horses by the droplet spreading method to be ~ 30–34 mN/m.8–10,64 The mucus mimetics developed in this study exhibited an increase in surface tension with crosslinking. MM1 exhibited very low elastic and bulk moduli under low shear conditions and a relatively low surface tension (53–57 mN/m). The surface tension of MM1 was in good agreement with reported surface tension values for aqueous solutions containing bovine mucins, proteins, or polysaccharides, which all have surface tensions between 45 and 60 mN/m.8,65 The surface tensions of MM2 and MM3 (79 and 95 mN/m, respectively) as measured via the Du Nuoy ring method were higher than that of water. These high surface tension values may be due to a high force of separation required to remove the ring from the mimetic surface. The du Noüy ring method requires an empirical correction, which accounts for the complex shape of the meniscus during the detachment of the ring. However, the correction factors have been developed only for Newtonian liquids. Therefore, the surface tension becomes less reliable as the flow characteristics of the sample become less Newtonian due to film slippage on the surface of the ring prior to detachment or cohesion of the sample. This can lead to a higher force required to detach the ring and thus an artificially high calculated surface tension. Therefore, the surface tension values are not accurate for these mimetic systems, but can be used to compare against published data of the surface tension of native sputum or mucus measured using the same technique. The surface tension values obtained for MM2 and MM3 are in line with a study by Albers et al. on sputum, who observed that more solid-like sputum surfaces were characterized by higher surface tension compared to more liquid-like sputum surfaces.66 None of the mimetics exhibited surface tension properties of native mucus due to the lack of surfactants in the mimetic. Therefore, we investigated the ability to surfactant addition to decrease surface tension of the mucus mimetics.
Native mucus contains a complex array of surface-active molecules. Phospholipids represent the second major lipid component in tracheal secretions and are the primary surface active component.6,67 Phosphatidylcholine is the most abundant phospholipid, with DPPC accounting for ~50% of the phosphatidylcholine molecular species in tracheal secretions.68–70 Therefore, the ability of DPPC alone to lower the surface tension of the mimetics was investigated. In addition, a complex surfactant, Infasurf®, which is used as a surfactant replacement therapy in premature infants, was investigated. Infasurf® contains 90–94% phospholipids, of which 79% is phosphatidylcholine and the remainder is 6% phosphatidylglycerol, 6% neutral lipids and about 1% protein, and is a fair representation of the known composition of surface active molecules in secretions of the conducting airways.
DPPC added directly into the mimetic formulation did not lower the surface tension of the mimetic (data not shown), which might be attributed to the ability of mucins to bind non-covalently to lipids.71 Therefore, the ability of surfactant that has been spread directly onto the mimetic surface to lower surface tension was measured. Spreading DPPC onto the surface of MM1 lowered the surface tension to ~ 30 mN/m, but required significant quantities of surfactant (100 μl of a 1.0 mg/ml solution). If all the DPPC molecules remained at the surface as a monolayer, the equivalent molecular area per molecule would be 12Å2. This is physically impossible given the molecular size of the DPPC polar headgroups (~ 30 Å2), thereby suggesting the formation of multi-layers at the interface.72 In comparison, the surface tension of MM1 was decreased to ~30 mN/m with only 28 μl of Infasurf® (compared to 100 μl required for DPPC). This is equivalent to the amount of surfactant required to lower the surface tension of water to 30 mN/m and indicates the relative fluidity of the Infasurf® solution.
We attempted to spread DPPC onto the surface of MM2 and MM3, but were unsuccessful given the more solid nature of the mimetics. Spreading of surfactant droplets onto viscoelastic surfaces is a dynamic process that is driven by the Marangoni effect, which requires a convective flow to push surfactant from areas of high surfactant concentration (low surface tension) to area of low surfactant concentration (high surface tension).73 The inability of solutions to spread on viscoelastic subphases has been reported by Kaneko et al.,74 who found that the spreading exponent (α) of a liquids decreases linearly with an increase in the elasticity (G′) of the underlying viscoelastic polymeric subphase. Therefore, the liquid forms a drop on the solid-like gel surface and does not spread to wet the surface.66 Further studies are required to evaluate the spreading of Infasurf® onto the solid-like surfaces of MM2 and MM3 and to understand the surface behavior of these highly crosslinked mimetics.
CONCLUSIONS
A more physiologically relevant in vitro mimetic of tracheal mucus was developed by matching the chemical composition and key physical properties (bulk viscoelasticity and surface tension) of the mimetic to that of native tracheal mucus. A series of mucus mimetic formulations with different bulk viscoelastic properties that were within the normal range for non-diseased tracheal mucus were developed by tailoring of the crosslinker concentration and the duration of the crosslinking process. The range of bulk viscoelastic properties represented by the three mimetics will enable further study of the variability in mucus behavior across healthy individuals. By matching the surface tension of the mucus mimetic to that of native secretions, new insight into the surface properties of mucus, which remain relatively unstudied, can be obtained.
Contributor Information
R. Hamed, Department of Pharmaceutical Sciences and Experimental Therapeutics, The University of Iowa, Iowa City, IA, 52242, USA.
J. Fiegel, Department of Pharmaceutical Sciences and Experimental Therapeutics & Department of Chemical and Biochemical Engineering, The University of Iowa, Iowa City, IA, 52242, USA.
References
- 1.Cone RA. Barrier properties of mucus. Adv Drug Del Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 2.King M, Rubin BK. Pharmacological approaches to discovery and development of new mucolytic agents. Adv Drug Del Rev. 2002;54:1475–1490. doi: 10.1016/s0169-409x(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 3.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337(6097):937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boat T, Cheng P, Leigh M. Biochemistry of mucus. In: Lenfant C, editor. Lung Biology in Health and Disease. New York: Marcel Dekker; 1994. pp. 217–282. [Google Scholar]
- 5.Matthews L, Spector S, Lemm J, Potter J. Studies on pulmonary secretions. I. The over-all chemical composition of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am Rev Respir Dis. 1963;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- 6.Slomiany A, Murty V, Aono M, Snyder C, Herp A, Slomiany B. Lipid composition of tracheobronchial secretions from normal individuals and patients with cystic fibrosis. Biochim Biophys Acta. 1982;710(1):106–111. doi: 10.1016/0005-2760(82)90196-5. [DOI] [PubMed] [Google Scholar]
- 7.Verdugo P, Tam PY, Butler J. Conformational structure of respiratory mucus studied by laser correlation spectroscopy. Biorheology. 1983;20(2):223–230. doi: 10.3233/bir-1983-20212. [DOI] [PubMed] [Google Scholar]
- 8.Im Hof V, Gehr P, Gerber V, Lee M, Schurch S. In vivo determination of surface tension in the horse trachea and in vitro model studies. Respir Physiol. 1997;109(1):81–93. doi: 10.1016/s0034-5687(97)84032-7. [DOI] [PubMed] [Google Scholar]
- 9.Lee M, Schurch S, Roth S, Jiang X, Cheng S, Bjarnason S, Green F. Effects of acid aerosol exposure on the surface properties of airway mucus. Exp Lung Res. 1995;21(6):835–851. doi: 10.3109/01902149509031766. [DOI] [PubMed] [Google Scholar]
- 10.Schurch S, Gehr P, Im Hof V, Geiser M, Green F. Surfactant displaces particles toward the epithelium in airways and alveoli. Respir Physiol. 1990;80(1):17–32. doi: 10.1016/0034-5687(90)90003-h. [DOI] [PubMed] [Google Scholar]
- 11.Im Hof V, Patrick G. Particle retention and clearance. J Aerosol Med. 1994;7(1):39–47. [Google Scholar]
- 12.Khubchandani K, Snyder J. Surfactant protein A (SP-A): The alveolus and beyond. FASEB J. 2001;15(1):59–69. doi: 10.1096/fj.00-0318rev. [DOI] [PubMed] [Google Scholar]
- 13.Hohlfeld J. The role of surfactant in asthma. Respir Res. 2002;3:4. doi: 10.1186/rr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneko D, Gong J, Zrínyi M, Osada Y. Kinetics of fluid spreading on viscoelastic substrates. J Polym Sci. 2005;43(5):562–572. [Google Scholar]
- 15.Nishimura S, Magana G, Ketelson H, Fuller G. Effect of lysozyme adsorption on the interfacial rheology of DPPC and cholesteryl myristate films. Langmuir. 2008;24(20):11728–11733. doi: 10.1021/la8016485. [DOI] [PubMed] [Google Scholar]
- 16.Roberts S, Kellaway I, Taylor K, Warburton B, Peters K. Combined surface pressure-interfacial shear rheology studies of the interaction of proteins with spread phospholipid monolayers at the air-water interface. Int J Pharm. 2005;300(1–2):48–55. doi: 10.1016/j.ijpharm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Daniels K, Mukhopadhyay S, Behringer R. Starbursts and wispy drops: Surfactants spreading on gels. Chaos. 2005;15(4):041107. doi: 10.1063/1.2139968. [DOI] [PubMed] [Google Scholar]
- 18.Jeanneret-Grosjean A, King M, Michoud M, Liote H, Amyot R. Sampling technique and rheology of human tracheobronchial mucus. Am Rev Respir Dis. 1988;137(3):707–710. doi: 10.1164/ajrccm/137.3.707. [DOI] [PubMed] [Google Scholar]
- 19.King M, Macklem P. Rheological properties of microliter quantities of normal mucus. J Appl Physiol. 1977;42(6):797–802. doi: 10.1152/jappl.1977.42.6.797. [DOI] [PubMed] [Google Scholar]
- 20.Potter J, Matthews L, Spector S, Lemm J. Studies on pulmonary secretions. II. osmolality and the ionic environment of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am Rev Respir Dis. 1967;96(1):83–87. doi: 10.1164/arrd.1967.96.1.83. [DOI] [PubMed] [Google Scholar]
- 21.Rubin B, Ramirez O, Zayas J, Finegan B, King M. Collection and analysis of respiratory mucus from subjects without lung disease. Am Rev Respir Dis. 1990;141(4 Pt 1):1040–1043. doi: 10.1164/ajrccm/141.4_Pt_1.1040. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe W, Thomas M, Clarke R, Klibanov A, Langer R, Katstra J, Fuller G, Griel L, Fiegel J, Edwards D. Why inhaling salt water changes what we exhale. J Colloid Interface Sci. 2007;307(1):71–78. doi: 10.1016/j.jcis.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee R, Puniyani R. Effects of clove oil-phospholipid mixtures on rheology of gum tragacanth - possible application for surfactant action on mucus gel simulants. Biomed Mater Eng. 2000;10(3–4):189–197. [PubMed] [Google Scholar]
- 24.Zahm J, King M, Duvivier C, Pierrot D, Girod S, Puchelle E. Role of simulated repetitive coughing in mucus clearance. Eur Respir J. 1991;4(3):311–315. [PubMed] [Google Scholar]
- 25.Mujica-Lopez K, Pearce M, Narron K, Perez J, Rubin B. In vitro evaluation of endotracheal tubes with intrinsic suction. Chest. 2010;138(4):863–869. doi: 10.1378/chest.09-3117. [DOI] [PubMed] [Google Scholar]
- 26.Kocevar-Nared J, Kristl J, Smid-Korbar J. Comparative rheological investigation of crude gastric mucin and natural gastric mucus. Biomaterials. 1997;18(9):677–681. doi: 10.1016/s0142-9612(96)00180-9. [DOI] [PubMed] [Google Scholar]
- 27.King M, Dasgupta B, Tomkiewicz RP, Brown NE. Rheology of cystic fibrosis sputum after in vitro treatment with hypertonic saline alone and in combination with recombinant human deoxyribonuclease I. Am J Respir Crit Care Med. 1997;156(1):173–177. doi: 10.1164/ajrccm.156.1.9512074. [DOI] [PubMed] [Google Scholar]
- 28.Riedler J, Reade T, Button B, Robertson CF. Inhaled hypertonic saline increases sputum expectoration in cystic fibrosis. J Paediatr Child Health. 1996;32(1):48–50. doi: 10.1111/j.1440-1754.1996.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 29.Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, Knight D, Thornton DJ, Sheehan JK. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol. 2009;296:L92–L100. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill DB, Button B. Establishment of respiratory air-liquid interface cultures and their use in studying mucin production, secretion, and function. In: McGuckin MA, Thornton DJ, editors. Mucins: Methods and Protocols. New York, N.Y: Humana Press; 2012. pp. 245–258. [DOI] [PubMed] [Google Scholar]
- 31.Holmén JM, Karlsson NG, Abdullah LH, Randell SH, Sheehan JK, Hansson GC, Davis CW. Mucins and their O-Glycans from human bronchial epithelial cell cultures. Am J Physiol Lung Cell Mol Physiol. 2004;287:L824–L834. doi: 10.1152/ajplung.00108.2004. [DOI] [PubMed] [Google Scholar]
- 32.Kim KC. Biochemistry and pharmacology of mucin-like glycoproteins produced by cultured airway epithelial cells. Exp Lung Res. 1991;17(3):533–545. doi: 10.3109/01902149109062863. [DOI] [PubMed] [Google Scholar]
- 33.Rudraraju VS, Wyandt CM. Rheology of microcrystalline cellulose and sodiumcarboxymethyl cellulose hydrogels using a controlled stress rheometer: Part II. Int J Pharm. 2005;292(1–2):63–73. doi: 10.1016/j.ijpharm.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Kwart H, Moseley WW, Jr, Katz M. The chemical characterization of human tracheobronchial secretion: A possible clue to the origin of fibrocystic mucus. Ann NY Acad Sci. 1963;106:709–721. doi: 10.1111/j.1749-6632.1963.tb16679.x. [DOI] [PubMed] [Google Scholar]
- 35.Kaliner M, Shelhamer J, Borson B, Nadel J, Patow C, Marom Z. Human respiratory mucus. Am Rev Respir Dis. 1986;134(3):612–621. doi: 10.1164/arrd.1986.134.3.612. [DOI] [PubMed] [Google Scholar]
- 36.Sachdev G, Myers F, Horton F, Fox O, Wen G, Rogers R, Carubelli R. Isolation, chemical composition, and properties of the major mucin component of normal human tracheobronchial secretions. Biochem Med. 1980;24(1):82–94. doi: 10.1016/0006-2944(80)90090-3. [DOI] [PubMed] [Google Scholar]
- 37.Glenister DA, Salamon K, Smith K, Beighton D, Keevil CW. Enhanced growth of complex communities of dental plaque bacteria in mucin-limited continuous culture. Microbial Ecol Health Dis. 1988;1:31–38. [Google Scholar]
- 38.Sachdev GP, Myers FJ, Horton FO, Fox OF, Wen G, Rogers RM, Carubelli R. Isolation, chemical composition, and properties of the major mucin components of normal human tracheobronchial secretions. Biochem Med. 1980;24:82–94. doi: 10.1016/0006-2944(80)90090-3. [DOI] [PubMed] [Google Scholar]
- 39.Boat TF, Cheng P, Leigh MW. Biochemistry of mucus. In: Takishima T, Shimura S, editors. Airway Secretion: Physiological Bases for the Control of Mucous Hypersecretion. New York: Marcel Dekker; 1994. [Google Scholar]
- 40.Cornfield A, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589–594. doi: 10.1136/gut.47.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Vilkar J, Mabolo R. Gel forming mucins. Notions from in vitro studies. Histol Histopathol. 2007;22:455–464. doi: 10.14670/HH-22.455. [DOI] [PubMed] [Google Scholar]
- 42.Thorton DJ, Gray T, Nettesheim P, Howard M, Koo JS, Sheehan JK. Characterization of mucins from cultured normal human tracheobronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1118–L1128. doi: 10.1152/ajplung.2000.278.6.L1118. [DOI] [PubMed] [Google Scholar]
- 43.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:5.1–5.28. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 44.Matthews LW, Spector S, Lemm J, Potter JL. Studies on pulmonary secretions I. The over-all chemical composition of pulmonary secretions from patients with cystic fibrosis, bronchiestasis, and laryngectomy. Am Rev Respir Dis. 1963;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- 45.Matthews LW, Spector S, Lemm J, Potter JL. Studies on pulmonary secretions. I. The over-all chemical composition of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am Rev Respir Dis. 1963;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- 46.Boat T, Cheng P. Biochemistry of airway mucus secretions. Fed Proc. 1980;39(13):3067–3074. [PubMed] [Google Scholar]
- 47.Boucher R, Stutts M, Gatzy J. Regional differences in bioelectric properties and ion flow in excised canine airways. J Appl Physiol. 1981;51:706–714. doi: 10.1152/jappl.1981.51.3.706. [DOI] [PubMed] [Google Scholar]
- 48.Zayas J, Man G, King M. Tracheal mucus rheology in patients undergoing diagnostic bronchoscopy. Interrelations with smoking and cancer. Am Rev Respir Dis. 1990;141(5 Pt 1):1107–1113. doi: 10.1164/ajrccm/141.5_Pt_1.1107. [DOI] [PubMed] [Google Scholar]
- 49.Litt M, Khan M, Chakrin L, Wardell J, Christian P. The viscoelasticity of fractionated canine tracheal mucus. Biorheology. 1974;11(2):111–117. doi: 10.3233/bir-1974-11202. [DOI] [PubMed] [Google Scholar]
- 50.Madsen F, Eberth K, Smart J. A rheological evaluation of various mucus gels for use in in-vitro mucoadhesion testing. J Pharm Sci. 1996;2:563–566. [Google Scholar]
- 51.Litt M, Khan M, Wolf D. Mucus rheology: Relation to structure and function. Biorheology. 1976;13(1):37–48. doi: 10.3233/bir-1976-13106. [DOI] [PubMed] [Google Scholar]
- 52.Dasgupta B, King M. Reduction in viscoelasticity in cystic fibrosis sputum in vitro using combined treatment with Nacystelyn and rhDNase. Pediatr Pulmonol. 1995;22:161–166. doi: 10.1002/(SICI)1099-0496(199609)22:3<161::AID-PPUL4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 53.Puchelle E, Bajolet O, Abely M. Airway mucus in cystic fibrosis. Pediatr Respri Rev. 2002;3:115–119. doi: 10.1016/s1526-0550(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 54.Gelman R, Meyer F. Mucociliary transference rate and mucus viscoelasticity dependence on dynamic storage and loss modulus. Am Rev Respir Dis. 1979;120(3):553–557. doi: 10.1164/arrd.1979.120.3.553. [DOI] [PubMed] [Google Scholar]
- 55.Yurewicz EC, Moghissi KS. Purification of human midcycle cervical mucin and characterization of its oligosaccharides with respect to size, composition, and microheterogeneity. J Biol Chem. 1981;256(22):11895–11904. [PubMed] [Google Scholar]
- 56.Mantle M, Allen A. Isolation and characterization of the native glycoprotein from pig small-intestinal mucus. Biochem J. 1981;195:267–275. doi: 10.1042/bj1950267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin C, Xu J, Zhang Y. Spectroscopic investigation of the function of aqueous 2-hydroxyethylmethacrylate/glutaraldehyde solution as a dentin desensitizer. Eur J Oral Sci. 2006;114(5):354–359. doi: 10.1111/j.1600-0722.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 58.Farris S, Song J, Huang Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J Agric Food Chem. 2010;58(2):998–1003. doi: 10.1021/jf9031603. [DOI] [PubMed] [Google Scholar]
- 59.Tomihata K, Ikada Y. Crosslinking of hyaluronic acid with glutaraldehyde. J Polym Sci A. 1997;35(16):3553–3559. [Google Scholar]
- 60.Gupta KC, Kumar MNVR. Semi-interpenetrating polymer network beads of crosslinked chitosan-glycine for controlled release of chlorphenramine maleate. J Appl Polym Sci. 2000;76(5):672–683. [Google Scholar]
- 61.Capra RH, Baruzzi AM, Quinzani LM, Strumia MC. Rheological, dielectric and diffusion analysis of mucin/carbopol matrices used in amperometric biosensors. Sensors Actuators B: Chem. 2007;124(2):466–476. [Google Scholar]
- 62.Molin SO, Nygren H, Dolonius L. A new method for the study of glutaraldehyde-induced crosslinking properties in proteins with special reference to the reaction with amino groups. J Histochem Cytochem. 1978;26(5):412–414. doi: 10.1177/26.5.96177. [DOI] [PubMed] [Google Scholar]
- 63.Celli JP, Turner BS, Afdhal NH, Ewoldt RH, McKinley GH, Bansil R, Erramilli S. Rheology of gastric mucin exhibits a pH-dependent sol-gel transition. Biomolecules. 2007;8(5):1580–1586. doi: 10.1021/bm0609691. [DOI] [PubMed] [Google Scholar]
- 64.Rubin B. Mucus, phlegm, and sputum in cystic fibrosis. Respir Care. 2009;54(6):726–732. doi: 10.4187/002013209790983269. [DOI] [PubMed] [Google Scholar]
- 65.Baszkin A, Proust J, Monsenego P, Boissonnade M. Wettability of polymers by mucin aqueous solutions. Biorheology. 1990;27(3–4):503–514. doi: 10.3233/bir-1990-273-429. [DOI] [PubMed] [Google Scholar]
- 66.Albers GM, Tomkiewicz RP, May MK, Ramirez OE, Rubin BK. Ring distraction technique for measuring surface tension of sputum: Relationship to sputum clearability. J Appl Physiol. 1996;81(6):2690–2695. doi: 10.1152/jappl.1996.81.6.2690. [DOI] [PubMed] [Google Scholar]
- 67.Bhaskar K, O’Sullivan D, Opaskar-Hincman H, Reid L, Coles S. Density gradient analysis of secretions produced in vitro by human and canine airway mucosa: Identification of lipids and proteoglycans in such secretions. Exp Lung Res. 1986;10(4):401–422. doi: 10.3109/01902148609058290. [DOI] [PubMed] [Google Scholar]
- 68.Bernhard W, Haagsman H, Tschernig T, Poets C, Postle A, van Eijk M, von der Hardt H. Conductive airway surfactant: Surface-tension function, biochemical composition, and possible alveolar origin. Am J Respir Cell Mol Biol. 1997;17(1):41–50. doi: 10.1165/ajrcmb.17.1.2594. [DOI] [PubMed] [Google Scholar]
- 69.Griese M, Duroux A, Schams A, Lenz A, Kleinsasser N. Tracheobronchial surface active material in cystic fibrosis. Eur J Med Res. 1997;2(3):114–120. [PubMed] [Google Scholar]
- 70.Wright S, Hockey P, Enhorning G, Strong P, KBMR, Holgate S, Djukanovie R, Postle A. Altered airway surfactant phospholipid composition and reduced lung function in asthma. J Appl Physiol. 2000;89(4):1283–1292. doi: 10.1152/jappl.2000.89.4.1283. [DOI] [PubMed] [Google Scholar]
- 71.Gong DH, Turner B, Bhaskar KR, Lamont JT. Lipid binding to gastric mucins: protective effect against oxygen radicals. Am J Physiol Gastrointest Liver Physiol. 1990;259:G681–G686. doi: 10.1152/ajpgi.1990.259.4.G681. [DOI] [PubMed] [Google Scholar]
- 72.Duncan S, Larson R. Comparing experimental and simulated pressure-area isotherms for DPPC. Biophys J. 2008;94:2965–2986. doi: 10.1529/biophysj.107.114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marcinkowski A, Garoff S, Tilton R, Pilewski J, Corcoran T. Postdeposition dispersion of aerosol medications using surfactant carriers. J Aerosol Med Pulm Drug Deliv. 2008;21(5):361–370. doi: 10.1089/jamp.2008.0699. [DOI] [PubMed] [Google Scholar]
- 74.Kaneko D, Gong JP, Zrínyi M, Osada Y. Kinetics of fluid spreading on viscoelastic substrates. J Polym Sci. 2005;43:562–572. [Google Scholar]