Abstract
Using a HeLa cell line stably transfected with the tat gene from human immunodeficiency virus type 1, we have found that the expression of the regulatory Tat protein suppresses the expression of cellular Mn-containing superoxide dismutase (Mn-SOD). This enzyme is one of the cell's primary defenses against oxygen-derived free radicals and is vital for maintaining a healthy balance between oxidants and antioxidants. The parental HeLa cells expressed nearly equivalent amounts of Cu,Zn- and Mn-SOD isozymes. Those cells expressing the Tat protein, however, contained 52% less Mn-SOD activity than parental cells, whereas that of the Cu,Zn enzyme was essentially unchanged. The steady-state levels of Mn-SOD-specific RNAs were also lower in the HeLa-tat cell line than in the parental line. No difference was seen in the steady-state levels of Cu,Zn-SOD-specific RNAs. In addition to the decreased Mn-SOD-activity, HeLa-tat cell showed evidence of increased oxidative stress. Carbonyl proteins were markedly higher, and total cellular sulfhydryl content decreased in cell extracts at a faster rate, probably reflecting ongoing lipid peroxidation. HeLa and HeLa-tat extracts were incubated with radiolabeled Mn-SOD transcripts, and the reaction products were subjected to UV crosslinking, digestion with ribonuclease A, and electrophoretic analysis. The results suggest a direct interaction between Tat protein and Mn-SOD gene transcripts.
Full text
PDF
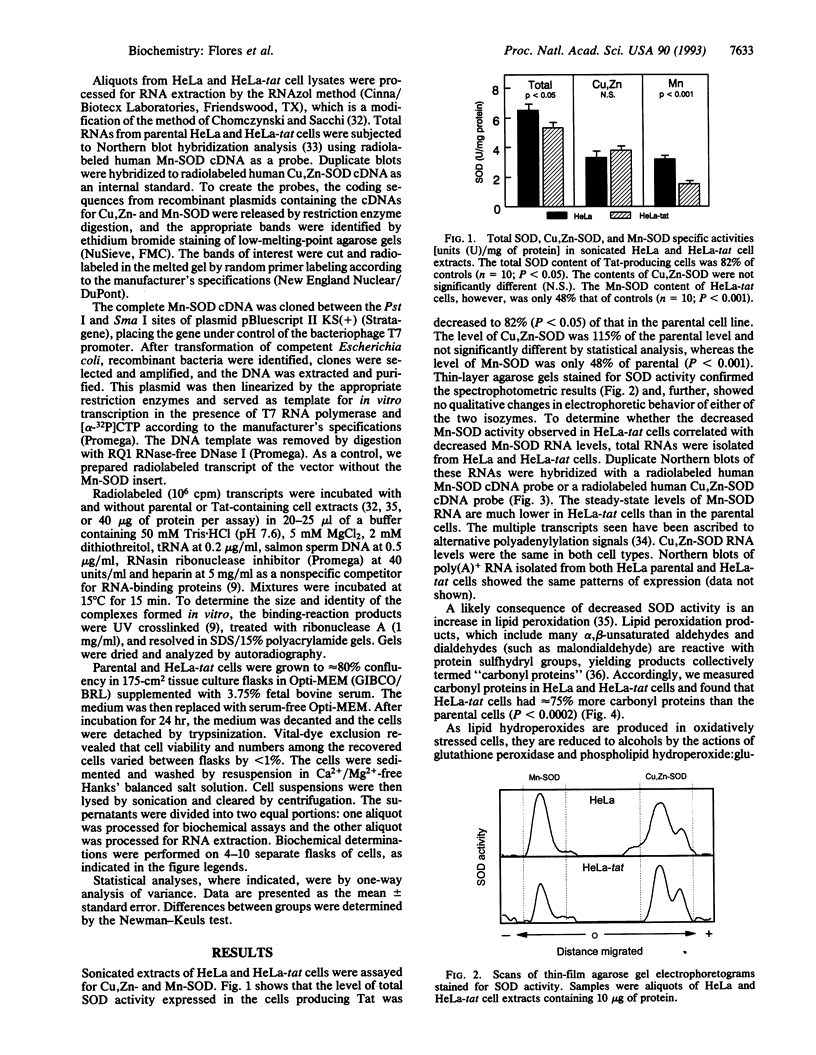
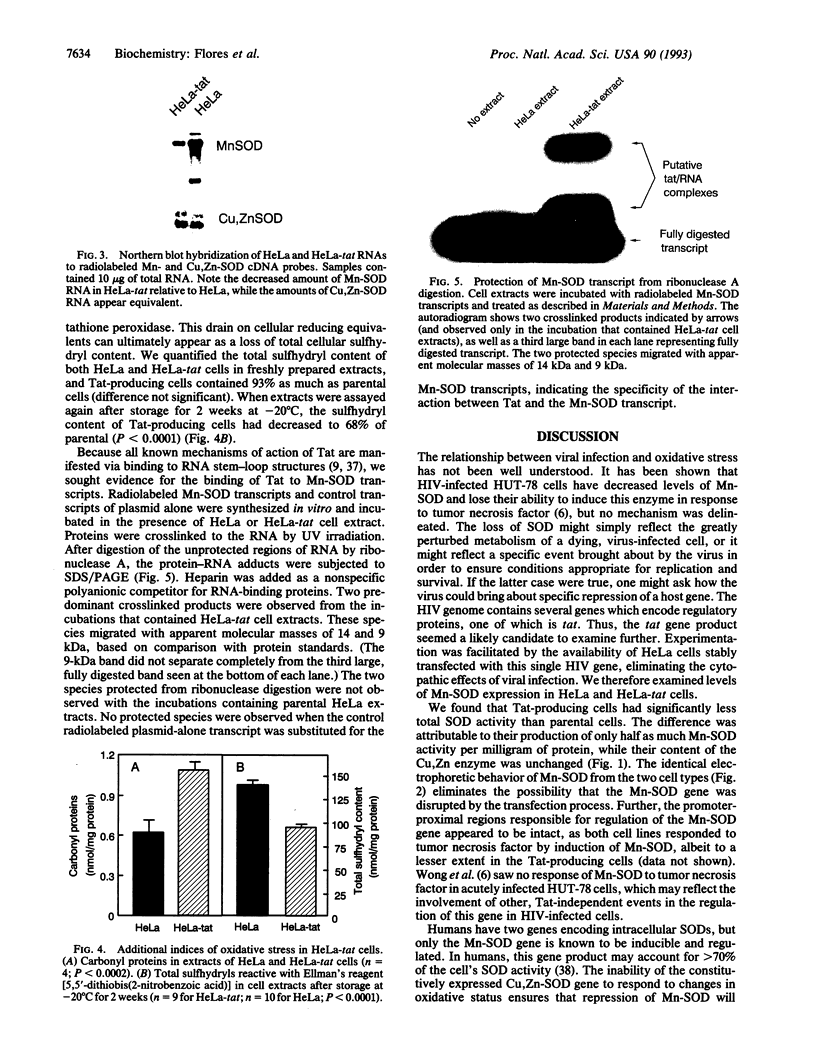


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike T., Ando M., Oda T., Doi T., Ijiri S., Araki S., Maeda H. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Invest. 1990 Mar;85(3):739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman B. S., Balin A. K., Allen R. G. Superoxide dismutase induces differentiation of Friend erythroleukemia cells. J Cell Physiol. 1989 May;139(2):370–376. doi: 10.1002/jcp.1041390220. [DOI] [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrakasan G., Bhatnagar R. S. Stimulation of collagen synthesis in fibroblast cultures by superoxide. Cell Mol Biol. 1991;37(7):751–755. [PubMed] [Google Scholar]
- Chojkier M., Houglum K., Solis-Herruzo J., Brenner D. A. Stimulation of collagen gene expression by ascorbic acid in cultured human fibroblasts. A role for lipid peroxidation? J Biol Chem. 1989 Oct 5;264(28):16957–16962. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chowdhury M., Taylor J. P., Chang C. F., Rappaport J., Khalili K. Evidence that a sequence similar to TAR is important for induction of the JC virus late promoter by human immunodeficiency virus type 1 Tat. J Virol. 1992 Dec;66(12):7355–7361. doi: 10.1128/jvi.66.12.7355-7361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church S. L., Farmer D. R., Nelson D. M. Induction of manganese superoxide dismutase in cultured human trophoblast during in vitro differentiation. Dev Biol. 1992 Jan;149(1):177–184. doi: 10.1016/0012-1606(92)90274-k. [DOI] [PubMed] [Google Scholar]
- Collison M. W., Thomas J. A. S-thiolation of cytoplasmic cardiac creatine kinase in heart cells treated with diamide. Biochim Biophys Acta. 1987 Apr 22;928(2):121–129. doi: 10.1016/0167-4889(87)90112-1. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Regulation of HIV-1 gene expression. FASEB J. 1991 Jul;5(10):2361–2368. doi: 10.1096/fasebj.5.10.1712325. [DOI] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Lau L. F., Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991 May;11(5):2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale C. M., Pavlakis G. N. Rapid activation and subsequent down-regulation of the human immunodeficiency virus type 1 promoter in the presence of Tat: possible mechanisms contributing to latency. J Virol. 1991 Jun;65(6):3044–3051. doi: 10.1128/jvi.65.6.3044-3051.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G., Lysko H. A precise method for the determination of whole blood and plasma sulfhydryl groups. Anal Biochem. 1979 Feb;93(1):98–102. [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Zollner H. Methods for determination of aldehydic lipid peroxidation products. Free Radic Biol Med. 1989;7(2):197–203. doi: 10.1016/0891-5849(89)90015-4. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988 Dec 23;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J Biol Chem. 1992 May 5;267(13):8757–8763. [PubMed] [Google Scholar]
- Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. AIDS. 1992 Apr;6(4):347–363. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- Harrison G. S., Maxwell F., Long C. J., Rosen C. A., Glode L. M., Maxwell I. H. Activation of a diphtheria toxin A gene by expression of human immunodeficiency virus-1 Tat and Rev proteins in transfected cells. Hum Gene Ther. 1991 Spring;2(1):53–60. doi: 10.1089/hum.1991.2.1-53. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Schutt A. D., Holly M., Slice L. W., Sherman M. I., Richman D. D., Potash M. J., Volsky D. J. Discovery and characterization of an HIV-1 Tat antagonist. Biochem Soc Trans. 1992 May;20(2):525–531. doi: 10.1042/bst0200525. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Schutt A. D., Holly M., Slice L. W., Sherman M. I., Richman D. D., Potash M. J., Volsky D. J. Inhibition of HIV replication in acute and chronic infections in vitro by a Tat antagonist. Science. 1991 Dec 20;254(5039):1799–1802. doi: 10.1126/science.1763331. [DOI] [PubMed] [Google Scholar]
- Huber B. E., Richards C. A., Martin J. L., Wirth P. J. Alterations in tumor angiogenesis associated with stable expression of the HIV tat gene. Mol Carcinog. 1992;5(4):293–300. doi: 10.1002/mc.2940050410. [DOI] [PubMed] [Google Scholar]
- Hurt J., Hsu J. L., Dougall W. C., Visner G. A., Burr I. M., Nick H. S. Multiple mRNA species generated by alternate polyadenylation from the rat manganese superoxide dismutase gene. Nucleic Acids Res. 1992 Jun 25;20(12):2985–2990. doi: 10.1093/nar/20.12.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J., Whitney P. Use of cyanide and diethyldithiocarbamate in the assay of superoxide dismutases. Free Radic Biol Med. 1991;10(1):69–77. doi: 10.1016/0891-5849(91)90023-v. [DOI] [PubMed] [Google Scholar]
- Kalebic T., Kinter A., Poli G., Anderson M. E., Meister A., Fauci A. S. Suppression of human immunodeficiency virus expression in chronically infected monocytic cells by glutathione, glutathione ester, and N-acetylcysteine. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):986–990. doi: 10.1073/pnas.88.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. M., Vogel J., Jay G., Rhim J. S. The HIV tat gene transforms human keratinocytes. Oncogene. 1992 Aug;7(8):1525–1529. [PubMed] [Google Scholar]
- Kirby T., Blum J., Kahane I., Fridovich I. Distinguishing between Mn-containing and Fe-containing superoxide dismutases in crude extracts of cells. Arch Biochem Biophys. 1980 May;201(2):551–555. doi: 10.1016/0003-9861(80)90544-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laspia M. F., Rice A. P., Mathews M. B. HIV-1 Tat protein increases transcriptional initiation and stabilizes elongation. Cell. 1989 Oct 20;59(2):283–292. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
- Maeda H., Akaike T. Oxygen free radicals as pathogenic molecules in viral diseases. Proc Soc Exp Biol Med. 1991 Nov;198(2):721–727. doi: 10.3181/00379727-198-43309c. [DOI] [PubMed] [Google Scholar]
- Marciniak R. A., Garcia-Blanco M. A., Sharp P. A. Identification and characterization of a HeLa nuclear protein that specifically binds to the trans-activation-response (TAR) element of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 May;87(9):3624–3628. doi: 10.1073/pnas.87.9.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcuzzi A., Weinberger J., Weinberger O. K. Transcellular activation of the human immunodeficiency virus type 1 long terminal repeat in cocultured lymphocytes. J Virol. 1992 Jul;66(7):4228–4232. doi: 10.1128/jvi.66.7.4228-4232.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Munday R., Winterbourn C. C. Reduced glutathione in combination with superoxide dismutase as an important biological antioxidant defence mechanism. Biochem Pharmacol. 1989 Dec 15;38(24):4349–4352. doi: 10.1016/0006-2952(89)90641-2. [DOI] [PubMed] [Google Scholar]
- Nakashima J. M., Levin J. R., Hyde D. M., Giri S. N. Repeated exposures to enzyme-generated oxidants cause alveolitis, epithelial hyperplasia, and fibrosis in hamsters. Am J Pathol. 1991 Dec;139(6):1485–1499. [PMC free article] [PubMed] [Google Scholar]
- Nose K., Shibanuma M., Kikuchi K., Kageyama H., Sakiyama S., Kuroki T. Transcriptional activation of early-response genes by hydrogen peroxide in a mouse osteoblastic cell line. Eur J Biochem. 1991 Oct 1;201(1):99–106. doi: 10.1111/j.1432-1033.1991.tb16261.x. [DOI] [PubMed] [Google Scholar]
- Oliver C. N., Starke-Reed P. E., Stadtman E. R., Liu G. J., Carney J. M., Floyd R. A. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis S. F., Georges D. L., Williams T. M., Lederman M. M. Suppression of interleukin-2 and interleukin-2 receptor expression in Jurkat cells stably expressing the human immunodeficiency virus Tat protein. Cell Immunol. 1992 Oct 1;144(1):32–42. doi: 10.1016/0008-8749(92)90223-c. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Berk B. C. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992 Mar;70(3):593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- Roederer M., Staal F. J., Raju P. A., Ela S. W., Herzenberg L. A., Herzenberg L. A. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-L-cysteine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4884–4888. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Campbell K., Haseltine W. A. Construction of recombinant murine retroviruses that express the human T-cell leukemia virus type II and human T-cell lymphotropic virus type III trans activator genes. J Virol. 1986 Jan;57(1):379–384. doi: 10.1128/jvi.57.1.379-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Terwilliger E., Dayton A., Sodroski J. G., Haseltine W. A. Intragenic cis-acting art gene-responsive sequences of the human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2071–2075. doi: 10.1073/pnas.85.7.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O., Virelizier J. L., Montagnier L., Hazan U. A microtransfection method using the luciferase-encoding reporter gene for the assay of human immunodeficiency virus LTR promoter activity. Gene. 1990 Apr 16;88(2):197–205. doi: 10.1016/0378-1119(90)90032-m. [DOI] [PubMed] [Google Scholar]
- Shahabuddin M., Volsky B., Hsu M. C., Volsky D. J. Restoration of cell surface CD4 expression in human immunodeficiency virus type 1-infected cells by treatment with a Tat antagonist. J Virol. 1992 Nov;66(11):6802–6805. doi: 10.1128/jvi.66.11.6802-6805.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal F. J., Ela S. W., Roederer M., Anderson M. T., Herzenberg L. A., Herzenberg L. A. Glutathione deficiency and human immunodeficiency virus infection. Lancet. 1992 Apr 11;339(8798):909–912. doi: 10.1016/0140-6736(92)90939-z. [DOI] [PubMed] [Google Scholar]
- Stanley S. K., Folks T. M., Fauci A. S. Induction of expression of human immunodeficiency virus in a chronically infected promonocytic cell line by ultraviolet irradiation. AIDS Res Hum Retroviruses. 1989 Aug;5(4):375–384. doi: 10.1089/aid.1989.5.375. [DOI] [PubMed] [Google Scholar]
- Taylor J. P., Cupp C., Diaz A., Chowdhury M., Khalili K., Jimenez S. A., Amini S. Activation of expression of genes coding for extracellular matrix proteins in Tat-producing glioblastoma cells. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9617–9621. doi: 10.1073/pnas.89.20.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. E., Morehouse L. A., Aust S. D. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985 Mar 25;260(6):3275–3280. [PubMed] [Google Scholar]
- Togashi H., Shinzawa H., Wakabayashi H., Nakamura T., Yong H., Yamada N., Ukai K., Okuyama Y., Takahashi T., Ishikawa M. Superoxide is involved in the pathogenesis of paraquat-induced injury in cultured rat liver slices. Hepatology. 1991 Oct;14(4 Pt 1):707–714. doi: 10.1016/0270-9139(91)90062-z. [DOI] [PubMed] [Google Scholar]
- Toledano M. B., Leonard W. J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F., Maiorino M., Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta. 1985 Mar 29;839(1):62–70. doi: 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Vaishnav Y. N., Wong-Staal F. The biochemistry of AIDS. Annu Rev Biochem. 1991;60:577–630. doi: 10.1146/annurev.bi.60.070191.003045. [DOI] [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Reynolds R. K., Luciw P. A., Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- Wong G. H., McHugh T., Weber R., Goeddel D. V. Tumor necrosis factor alpha selectively sensitizes human immunodeficiency virus-infected cells to heat and radiation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4372–4376. doi: 10.1073/pnas.88.10.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]





