ABSTRACT
The porcine sapovirus (SaV) (PoSaV) Cowden strain is one of only a few culturable enteric caliciviruses. Compared to the wild-type (WT) PoSaV Cowden strain, tissue culture-adapted (TC) PoSaV has two conserved amino acid substitutions in the RNA-dependent RNA polymerase (RdRp) and six in the capsid protein (VP1). By using the reverse-genetics system, we identified that 4 amino acid substitutions in VP1 (residues 178, 289, 324, and 328), but not the substitutions in the RdRp region, were critical for the cell culture adaptation of the PoSaV Cowden strain. The other two substitutions in VP1 (residues 291 and 295) reduced virus replication in vitro. Three-dimensional (3D) structural analysis of VP1 showed that residue 178 was located near the dimer-dimer interface, which may affect VP1 assembly and oligomerization; residues 289, 291, 324, and 328 were located at protruding subdomain 2 (P2) of VP1, which may influence virus binding to cellular receptors; and residue 295 was located at the interface of two monomeric VP1 proteins, which may influence VP1 dimerization. Although reversion of the mutation at residue 291 or 295 from that of the TC strain to that of the WT reduced virus replication in vitro, it enhanced virus replication in vivo, and the revertants induced higher-level serum and mucosal antibody responses than those induced by the TC PoSaV Cowden strain. Our findings reveal the molecular basis for PoSaV adaptation to cell culture. These findings may provide new, critical information for the cell culture adaptation of other PoSaV strains and human SaVs or noroviruses.
IMPORTANCE The tissue culture-adapted porcine sapovirus Cowden strain is one of only a few culturable enteric caliciviruses. We discovered that 4 amino acid substitutions in VP1 (residues 178, 289, 324, and 328) were critical for its adaptation to LLC-PK cells. Two substitutions in VP1 (residues 291 and 295) reduced virus replication in vitro but enhanced virus replication and induced higher-level serum and mucosal antibody responses in gnotobiotic pigs than those induced by the tissue culture-adapted strain. Structural modeling analysis of VP1 suggested that residue 178 may affect VP1 assembly and oligomerization; residues 289, 291, 324, and 328 may influence virus binding to cellular receptors; and residue 295 may influence VP1 dimerization. Our findings will provide new information for the cell culture adaptation of other sapoviruses and possibly noroviruses.
INTRODUCTION
Caliciviruses, in the family Caliciviridae, are small, icosahedral, and nonenveloped viruses with a diameter of 27 to 35 nm, which have a positive-sense, single-stranded RNA genome of 6.5 to 8.3 kb (1, 2). Caliciviruses have been classified into five genera (Norovirus, Sapovirus, Vesivirus, Lagovirus, and Nebovirus) and several proposed genera (3, 4). Among them, noroviruses (NoVs) and sapoviruses (SaVs) are the leading causes of gastroenteritis in humans of all ages. SaVs are often associated with sporadic, self-limiting gastroenteritis, the severity of which is reportedly milder than that of NoVs (5, 6). However, SaVs also cause outbreaks worldwide (7–10), and deaths associated with SaV infection have been reported for long-term-care facilities (11).
Because most enteric caliciviruses are unculturable, research on pathogenesis and immunity, as well as the development of antivirals, has been hampered. The porcine SaV (PoSaV) Cowden strain, previously known as porcine enteric calicivirus (PEC), belongs to genogroup III (GIII) of the SaVs and is one of only a few culturable enteric caliciviruses (2, 12, 13). The PoSaV Cowden strain was adapted to a porcine kidney cell line (LLC-PK) after passage of the virus in gnotobiotic (Gn) pigs, followed by 20 passages in primary porcine kidney cells in the presence of an intestinal content preparation from uninfected Gn pigs (12, 14).
Gn pigs have been established as a relevant animal model because of the similarity of anatomy, genetics, physiology, and immunity with humans (15–17). PoSaV naturally infects pigs and causes mild-to-moderate gastroenteritis in Gn pigs (14, 18, 19), thus mimicking SaV diarrhea in humans and providing an animal model suitable for studies of the replication and pathogenesis of enteric caliciviruses.
The genome of PoSaV is composed of two open reading frames (ORFs). ORF1 encodes a polyprotein that is processed into several nonstructural proteins (NSs) and the major structural protein VP1 by a viral protease. ORF2 encodes a small structural protein, VP2 (20). VP1 is divided into two domains: a shell (S) domain (amino acid positions 3 to 216) and a protruding (P) domain (amino acid positions 217 to 544) (21). The P domain is further divided into the P1 (amino acid positions 217 to 272 and 425 to 544) and P2 (amino acid positions 273 to 424) subdomains (21, 22).
Reverse-genetics systems are important tools to rescue unculturable viruses and to study virus replication mechanisms. A reverse-genetics system, pCV4A, that was constructed for the PoSaV Cowden strain contained the full-length genomic cDNA of the tissue culture-adapted (TC) PoSaV Cowden strain, directly downstream from the T7 RNA polymerase promoter (20). Infectious TC PoSaV particles were rescued after transfection of LLC-PK cells with the in vitro-transcribed and -capped PoSaV genomic RNA (20).
In this study, we investigated the genetic basis of cell culture adaptation of the PoSaV Cowden strain by comparative sequence analyses of the genomes of different passages of wild-type (WT) and TC PoSaVs and the generation of a series of PoSaV mutants using the reverse-genetics system. We further investigated their differences in replication both in vivo and in vitro as well as their putative structural differences. To our knowledge, our studies are the first to identify which amino acid residues are critical for the cell culture adaptation of a SaV. This study provides new information on cell culture adaptation of SaVs that may be applicable to other human SaVs or to NoVs.
MATERIALS AND METHODS
Cells and viruses.
The LLC-PK cell line (ATCC CL-101) and a human embryonic kidney cell line, HEK 293T/17 (ATCC CRL-11268), were obtained from the American Type Culture Collection (ATCC). LLC-PK cells were passaged and maintained as previously described (20, 23). HEK 293T/17 cells and a baby hamster kidney cell line (BHK-T7) stably expressing T7 RNA polymerase were cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, NY, USA) with 10% fetal bovine serum (FBS; Thermo Scientific, MA, USA), 1% nonessential amino acids (NEAA; Invitrogen, NY, USA), and 1% antibiotic-antimycotic (Invitrogen, NY, USA).
Two passage levels of the WT PoSaV Cowden strain (Gn pig passage level 5 [I-1113] and level 13 [R418]) from the small intestinal contents (SICs) or large intestinal contents (LICs) of Gn pigs were used for sequencing. TC PoSaV was propagated in LLC-PK cells (TC PoSaV-2010; cell culture passage level 30) with 50 μM glycochenodeoxycholic acid (GCDCA; Sigma-Aldrich, MO, USA) as previously described (24).
Sequence analyses.
The genomes of TC PoSaV-2010 (passage level 30) and WT PoSaV I-1113 (Gn pig passage level 5) and the VP1 region of WT PoSaV R418 (Gn pig passage level 13) were sequenced by the primer walking method based on the TC PoSaV genome (GenBank accession no. AF182760), as previously described (25). The 5′ and 3′ ends were determined by using 5′-rapid amplification of cDNA ends (RACE) and 3′-RACE methods. Sequence editing and assembly were performed by using the Lasergene software package (v10; DNASTAR Inc., WI, USA). Multiple-sequence alignment was done with ClustalW using the DNA Data Bank of Japan (DDBJ) (http://www.ddbj.nig.ac.jp).
Generation of full-length cDNA clones of PoSaV WT and TC chimeric genomes, mutants, and revertant mutant strains.
Plasmid pCV4A containing the full-length cDNA of TC PoSaV (TC PoSaV-2005; cell culture passage level 27) was provided by Kyeong-Ok Chang (20). The primers for the generation of these chimeric clones are listed in Table 1. The genomic organization and mapping of the mutations are illustrated (Fig. 1). TC-WTVP1 was generated by replacing a partial VP1 fragment (nucleotide [nt] positions 5227 to 6060 and amino acid positions 30 to 308) of pCV4A with the corresponding sequence fragment of the WT PoSaV Cowden strain. Briefly, the WT PoSaV Cowden VP1 fragment containing two ApaI restriction enzyme sites (nucleotide positions 5227 to 5232 and 6055 to 6060) was reverse transcribed by using SuperScript III reverse transcriptase (Life Technologies, NY, USA) and amplified by PCR with primers ApaI-F and ApaI-R using PrimeStar HS high-fidelity DNA polymerase (Clontech Laboratories Inc., CA, USA). The amplicons were digested by the ApaI restriction enzyme and cloned into the ApaI-digested pCV4A plasmid.
TABLE 1.
Primers for generation of chimeric PoSaV clones
| Primer | Sequencea | Location in PEC genome (positions) | Orientation |
|---|---|---|---|
| ApaI-F | 5′-GAGTCCAGACCAGTCCAGCCAGC | 5203–5225 | Forward |
| ApaI-R | 5′-TGGGTAGTGGTTGATGATGTTG | 6090–6069 | Reverse |
| TC-3763-CTF | 5′-CGTGAATGACCCAAGGTACCCCTTCTCACAACA | 3747–3779 | Forward |
| TC-3763-CTR | 5′-TGTTGTGAGAAGGGGTACCTTGGGTCATTCACG | 3779–3747 | Reverse |
| TC-4145-AGF | 5′-AGAAAAGAATGACCAAGGCAAAAGACGCCTGCTGTG | 4122–4157 | Forward |
| TC-4145-AGR | 5′-CACAGCAGGCGTCTTTTGCCTTGGTCATTCTTTTCT | 4157–4122 | Reverse |
| TC-5671-ATF | 5′-TTGGTGGGGCTATAGCATGTTTGGCACTTTACGTG | 5654–5688 | Forward |
| TC-5671-ATR | 5′-CACGTAAAGTGCCAAACATGCTATAGCCCCACCAA | 5688–5654 | Reverse |
| TC-6004-CTF | 5′-CCCGTGTCAATGGAAAGTACACTGACAACACAGGT | 5987–6021 | Forward |
| TC-6004-CTR | 5′-ACCTGTGTTGTCAGTGTACTTTCCATTGACACGGG | 6021–5987 | Reverse |
| TC-6010-GAF | 5′-CCCGTGTCAATGGAAAGCACACTAACAACACAGGTA | 5987–6022 | Forward |
| TC-6010-GAR | 5′-TACCTGTGTTGTTAGTGTGCTTTCCATTGACACGGG | 6022–5987 | Reverse |
| TC-6023-GAF | 5′-AGCACACTGACAACACAGGTAAGGCAGTGTTTCA | 6002–6035 | Forward |
| TC-6023-GAR | 5′-TGAAACACTGCCTTACCTGTGTTGTCAGTGTGCT | 6035–6002 | Reverse |
| WT-5671-TAF | 5′-TTGGTGGGGCTATAGCAAGTTTGGCACTTTACGTG | 5654–5688 | Forward |
| WT-5671-TAR | 5′-CACGTAAAGTGCCAAACTTGCTATAGCCCCACCAA | 5688–5654 | Reverse |
| WT-6004-TCF | 5′-CCCGTGTCAATGGAAAGCACACTAACAACACAGGT | 5987–6021 | Forward |
| WT-6004-TCR | 5′-ACCTGTGTTGTTAGTGTGCTTTCCATTGACACGGG | 6021–5987 | Reverse |
| EcoRI-F | 5′-GAGGCCTACGAGGAATTCAAG | 4570–4590 | Forward |
| EcoRI-R | 5′-GAGCCTGATTAAAAGAATTCATAATA | 6891–6866 | Reverse |
| 6111-F | 5′-CAACAATGTTCAACACAGGAAC | 6104–6125 | Forward |
| 6111-R | 5′-GTTGAACATTGTTGATGCAGC | 6117–6097 | Reverse |
| 6122-F | 5′-CAACACAGAAACTGCCGTAAATG | 6114–6138 | Forward |
| 6122-R | 5′-GGCAGTTTCTGTGTTGAATATTG | 6129–6107 | Reverse |
| 6111-6122-F | 5′-CAATGTTCAACACAGAAACTGCC | 6107–6129 | Forward |
| 6111-6122-R | 5′-CAGTTTCTGTGTTGAACATTGTTG | 6127–6104 | Reverse |
| QCback-F | 5′-GGCTGCATCAACAATATTCAACACAGGAACTGCC | 6096–6129 | Forward |
| QCback-R | 5′-GGCAGTTCCTGTGTTGAATATTGTTGATGCAGCC | 6129–6096 | Reverse |
Boldface and underlined nucleotides are the mutation sites; underlined sequences are EcoRI digestion sites.
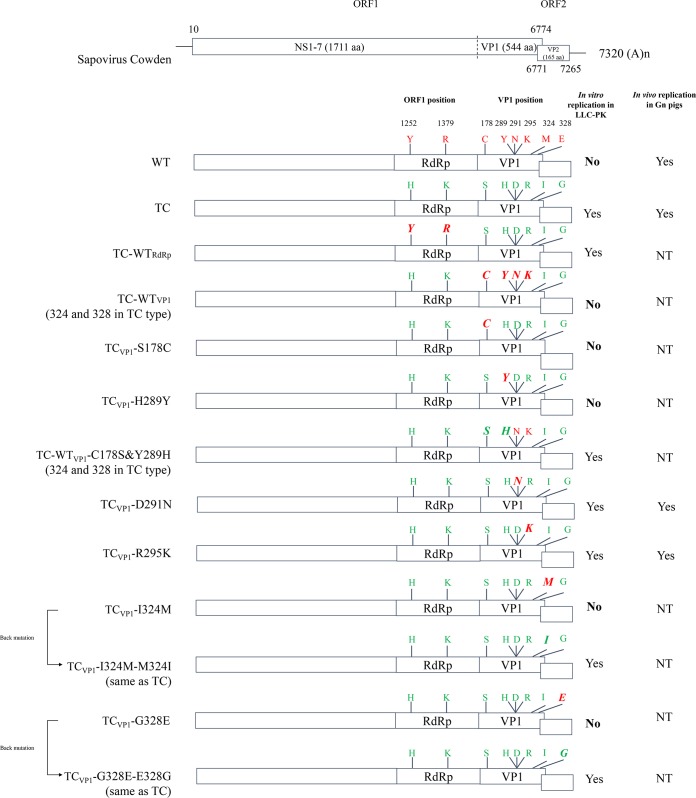
FIG 1.
Diagrams of constructions of TC and WT PoSaVs and the mutants derived from the pCV4A backbone. The 8 amino acid (aa) residues, at positions 1252 and 1379 in the ORF1 polyprotein and at positions 178, 289, 291, 295, 324, and 328 in VP1, that differed between the WT (red) and TC (green) PoSaVs are indicated in boldface and italic type for each mutant. The TCVP1-D291N and TCVP1-R295K mutants were tested in Gn pigs with WT and TC strains as controls. The TC-WTVP1-C178S&Y289H mutant was not tested (NT) due to the 2-log10-lower virus infectivity titer that could not be equalized by further concentration. Replication of the mutants in cell culture or in Gn pigs is noted.
Full-length cDNA clones of TC-WTRdRp, VP1 of the TC strain with an S-to-C mutation at position 178 (TCVP1-S178C), TCVP1-H289Y, TCVP1-D291N, TCVP1-R295K, TCVP1-I324M→M324I, and TCVP1-G328E→E328G were generated based on the pCV4A backbone by using a QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, TX, USA) according to the manufacturer's instructions (Fig. 1). For example, TC-WTRdRp was generated when the two RNA-dependent RNA polymerase (RdRp) amino acid residues at positions 1252 and 1379 of pCV4A were mutated from the TC to the WT residues (H1252Y and K1379R). Three full-length cDNA clones of chimeric genomes (TC-WTVP1-C178S, TC-WTVP1-Y289H, and TC-WTVP1-C178S&Y289H) were generated based on the TC-WTVP1 backbone, whose residues at amino acid positions 324 and 328 in VP1 were of the TC type. Full-length cDNA clones of TCVP1-I324M and TCVP1-G328E were generated by digestion of pCV4A with the EcoRI restriction enzyme (nucleotide positions 4582 to 6877) and replacement with I324M or G328E engineered PCR products. Using TCVP1-I324M as an example, two fragments were PCR amplified with primers EcoRI-F and 6111-R and with primers 6111-F and EcoRI-R, respectively, using pCV4A as the template. The PCR product containing the I324M mutation was assembled by overlap PCR with primers EcoRI-F and EcoRI-R, using the two fragments as the templates. The overlap PCR products containing the I324M mutation were digested with the EcoRI restriction enzyme and inserted into EcoRI restriction enzyme-digested pCV4A. The recombinant plasmid was transformed and amplified in competent Escherichia coli 10-beta cells (New England BioLabs Inc., MA, USA).
In vitro transcription and capping of viral genomic RNA.
In vitro transcription and capping of viral genomic RNA were performed according to the manufacturer's instructions. Briefly, the reverse-genetics plasmid DNA was extracted from E. coli, linearized by NotI restriction enzyme digestion, and purified by phenol-chloroform extraction twice. Subsequently, genomic RNA was transcribed in vitro from the linearized plasmid by using a MEGAscript T7 transcription kit (Life Technologies, NY, USA). The reaction mixture was treated with DNase, and the RNA was purified with an RNeasy minikit (Qiagen, CA, USA) and analyzed by agarose gel (1%) electrophoresis under denaturing conditions with formaldehyde. The transcribed RNA was capped by using the ScriptCap m7G capping system (Cellscript, WI, USA), followed by RNA purification using the RNeasy minikit. The RNA transcripts were suspended in RNase-free water to a final concentration of 500 ng/μl for transfection.
Transfection of BHK-T7 cells or HEK 293T/17 cells to rescue infectious viruses.
The purified RNA transcripts were transfected into 1-day-old HEK 293T/17 or BHK-T7 cells (∼50 to 70% confluent) in 24-well cell culture plates with Lipofectamine 2000 (Invitrogen, NY, USA). Briefly, 1-day-old HEK 293T/17 or BHK-T7 cells were washed with Opti-MEM I (Invitrogen, NY, USA). Approximately 1.5 μg capped RNA and 4 μl Lipofectamine 2000 were diluted in 50 μl Opti-MEM I separately and incubated at room temperature for 5 min. The RNA and the Lipofectamine 2000 solution were then mixed (total volume of 100 μl) and incubated at room temperature for 20 min before being added to HEK 293T/17 or BHK-T7 cell monolayers. After 6 h of incubation at 37°C, the supernatant was replaced with DMEM (10% FBS, 1% NEAA, and 1% antibiotic-antimycotic). After 1 day of incubation at 37°C, HEK 293T/17 or BHK-T7 cell lysates were harvested by freezing and thawing once, followed by centrifugation at 2,095 × g for 5 min to remove cell debris. The cell lysates were used to inoculate LLC-PK cells to generate virus pools.
Recovery of progeny virus in LLC-PK cells.
The LLC-PK cell monolayers in 6-well plates were washed with minimum essential medium (MEM) and inoculated with HEK 293T/17 or BHK-T7 cell lysates in the presence of 50 μM GCDCA. Cytopathic effects (CPEs) were monitored daily. Infected cells were incubated for up to 6 days postinoculation, before harvesting of the first passage of each mutant. Each mutation was confirmed by reverse transcription-PCR (RT-PCR) with primer sets covering the mutated region, followed by sequence analysis.
Plaque assays.
Tenfold serially diluted samples were inoculated into wells of 6-well cell culture plates. After incubation at 37°C for 1.5 h with rocking, the inoculum was removed, and the cell monolayer was overlaid with 0.85% low-melting-temperature agarose (Sigma-Aldrich, MO, USA) in MEM supplemented with 50 μM GCDCA (20). After plaques formed (5 days postinoculation), cell monolayers were stained with 1 ml of a 0.03% neutral red–phosphate-buffered saline (PBS) solution for 30 min at 37°C. The solution was removed, and the plaques were counted and observed under a microscope. The plaque sizes were quantified by using the Icy bioimage program (26).
Growth kinetics test for progeny mutants in LLC-PK cells.
A growth kinetics curve for each mutant was determined by collecting cell lysates at different postinoculation time points. LLC-PK cells in 6-well plates were incubated with each mutant virus at a multiplicity of infection (MOI) of 0.01 for 1 h. The inoculum was removed, and the plate was washed once before the addition of maintenance MEM in the presence of 50 μM GCDCA. Supernatants and cell lysates were collected at 24, 48, 72, and 96 h postinoculation (hpi) after three cycles of freezing and thawing. Virus infectivity titers were determined in LLC-PK cells as the 50% tissue culture infectious dose (TCID50) by immunohistochemistry (IHC) staining using 96-well plates, as described below (23).
IF and IHC staining for detection of VP1 proteins in cell culture.
Immunofluorescence (IF) staining was performed for the detection of VP1 protein in mutant-infected cells (20), while IHC staining was performed for virus infectivity titration (23). Briefly, cell monolayers were fixed with 10% neutral formalin buffer at room temperature for 30 min, and the fixed cells were then permeabilized with 1% Triton X-100 in PBS at room temperature for 10 min. Gn pig hyperimmune serum to the WT PoSaV Cowden strain was used as a primary antibody (18). Fluorescein isothiocyanate (FITC)-conjugated goat anti-swine IgG(H+L) serum (KPL, MD, USA) or horseradish peroxidase (HRP)-conjugated goat anti-swine IgG(H+L) serum (KPL, MD, USA) was used as a secondary antibody. The IF signal was observed by using an IX70 fluorescence microscope (Olympus, PA, USA). For IHC, cells were stained with the substrate 3-amino-9-ethylcarbazole (AEC) (Sigma-Aldrich, MO, USA) at room temperature for at least 2 h and observed by using light microscopy.
Three-dimensional structural analyses of the VP1 proteins of WT and TC PoSaVs.
The three-dimensional (3D) structure of a SaV VP1 protein is not available in the database. The VP1 protein of WT PoSaV shares higher sequence identity (38%) with that of feline calicivirus (FCV) (PDB accession no. 3M8L) than with those of San Miguel sea lion virus (SMSV) (34%) (PDB accession no. 2GH8) and recombinant Norwalk virus (rNV) (29%) (PDB accession no. 1IHM). When the sequence identity is 30 to 50%, the obtained model tends to have ∼90% of the main chain modeled with a 1.5-Å root mean square error (27). Therefore, the VP1 dimer structural models of WT and TC PoSaVs were constructed based on the crystal structure of the FCV VP1 protein at a resolution of 3.40 Å by the homology modeling method using “MOE-Align” and “MOE-Homology” in the Molecular Operating Environment (MOE) (version 2014-09; Chemical Computing Group Inc., Quebec, Canada). Twenty-five intermediate models were obtained from one homology modeling with the MOE, among which the intermediate models with the best scores were selected according to the generalized Born (GB)/volume integral (VI) scoring function. The final 3D models were thermodynamically simulated by energy minimization using the AMBER10 extended Huckel theory (EHT) force field combined with the GB model of aqueous solvation implemented in the MOE (28–30). Physically unacceptable local structures of the optimized 3D models were further refined on the basis of evaluation using the Ramachandran plot in the MOE. The structures of WT PoSaV VP1 dimers were generated from the monomeric structures by the MOE on the basis of the assembly information for the FCV VP1 crystal structures. The quality of the models was assessed by using the 3D structure evaluation program Verify3D (31).
Prediction of effects of point mutations on stability of PoSaV.
The change in the stability of the WT PoSaV VP1 protein by each mutation was analyzed by using the Protein Design application in the MOE (version 2014-09). The structure of WT PoSaV VP1 was constructed as described above. The single point mutations in VP1 were generated individually, and ensembles of protein conformations were generated by using the LowMode molecular dynamics (MD) module with Boltzmann distribution in the MOE to calculate average stability. The stability scores in the structures refined by energy minimization were obtained by using the stability scoring function of the Protein Design application in the MOE.
Gn pigs and experimental design.
Gn pigs were derived and maintained as previously described (18, 32). A total of 36 Gn pigs were assigned to five groups (Table 2) and inoculated orally with (i) TC PoSaV (cell culture passage level 30) (n = 9), (ii) TCVP1-R295K (n = 7), (iii) TCVP1-D291N (n = 9), (iv) WT PoSaV (PS799) (Gn pig passage level 13) (n = 7), or (v) MEM (n = 4). TC PoSaV and the TCVP1-D291N and TCVP1-R295K mutants were harvested from cell culture and concentrated to ∼7.0 log10 TCID50/ml (equivalent to a real-time quantitative RT-PCR [RT-qPCR] titer of ∼11.5 log10 genome equivalents [GE]/ml) by ultracentrifugation at 126,000 × g for 1.5 h at 4°C. The WT PoSaV Cowden strain inoculated into Gn pigs was filtered through 0.22-μm-pore-size filters prior to inoculation. Each pig was inoculated orally with a 5-ml inoculum containing TC PoSaV or mutant PoSaVs (∼7.0 log10 TCID50/ml) or containing WT PoSaV with a RT-qPCR titer of ∼11.5 log10 GE/ml.
TABLE 2.
Experimental design for inoculation of Gn pigs with WT or TC PoSaV Cowden or mutants
| Groupa | Oral inoculum | No. of Gn pigs | Titer (log10 GE/ml in MEM [5 ml/pig])b | No. of Gn pigs euthanized at acute infection phase (PIDs 5–7) |
|---|---|---|---|---|
| 1 | TC Cowden strain | 9 | 11.5 | 3 |
| 2 | TCVP1-R295K mutant | 7 | 11.5 | 2 |
| 3 | TCVP1-D291N mutant | 9 | 11.5 | 3 |
| 4 | WT Cowden strain PS799 | 7 | 11.5 | 3 |
| 5 | MEM | 4 | 2 |
All pigs were 4 to 7 days of age at inoculation.
A RT-qPCR titer of 11.5 log10 GE/ml is equivalent to ∼7.0 log10 TCID50/ml by an infectivity assay.
All inoculated Gn pigs were observed daily for clinical signs. Their feces were scored as 0 for normal, 1 for pasty, 2 for semiliquid, and 3 for liquid, with fecal scores of ≥2 indicating diarrhea (18). Rectal swabs (RSs) were collected daily for titration of virus shedding. Blood was collected for serum from each Gn pig before inoculation; at postinoculation days (PIDs) 1, 3, 6, 9, 16, 23, and 27; and at euthanasia. Two to three Gn pigs of each group were euthanized at the acute phase of infection: on the next day after an increase of the fecal viral RNA titer or on the day following the onset of clinical signs. The remaining pigs were euthanized after fecal virus shedding was no longer detected at the termination of the experiment. Animal care and use for these studies were approved by the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University. Mucosal antibody samples were collected at necropsy by scraping the mucosa from the ileum and centrifuging the sample at 2,095 × g for 20 min at 4°C.
Histopathological examination.
At necropsy, blood, SICs, and LICs were collected from each Gn pig. Fresh duodenum; proximal, mid-, and distal jejunum; ileum; colon; cecum; liver; spleen; lung; and kidney specimens were collected and immersed immediately in 10% neutral buffered formalin (NBF). Tissues fixed in 10% NBF were trimmed, embedded in paraffin, sectioned at 4 μm, stained with Harris' hematoxylin and alcoholic eosin Y solution (H&E; Sigma-Aldrich, MO, USA), and examined for histopathology microscopically.
Duodenum; proximal, mid-, and distal jejunum; ileum; colon; and cecum specimens were collected in duplicate, immersed in a sucrose solution (130 mM Na2HPO4, 30 mM KH2PO4, 10% [wt/vol] sucrose, and 0.01% sodium azide [pH 7.2]) on ice, embedded in a optimum-cutting-temperature (OCT) compound (Sakura, PA, USA), stored at −20°C overnight, and then sectioned at 4 to 7 μm in a cryostat microtome. To detect the PoSaV antigen in tissues, frozen sections were fixed with acetone for 20 min at −20°C, followed by washing with PBS twice for 5 min each. Tissue sections were then blocked with 5% normal goat serum in 0.01 M PBS–0.05% Tween 20 (PBST) (pH 7.2) for 20 min. After blocking, the tissue sections were incubated with hyperimmune guinea pig serum to PoSaV virus-like particles (VLPs) (33) at 4°C overnight, followed by incubation with Alexa Fluor 488-conjugated goat anti-guinea pig IgG(H+L) serum (Life Technologies, NY, USA) at room temperature for 1 h. Thereafter, tissue sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for nuclei and examined by using fluorescence microscopy.
Detection of PoSaV RNA in rectal swabs.
RSs were collected, suspended in 4 ml of MEM, and centrifuged at 2,095 × g for 30 min. The supernatant was collected as a 10% fecal suspension and stored at −20°C until RNA extraction. Total RNA was extracted from 50-μl RS suspensions by using a MagMax RNA extraction kit (Life Technologies, NY, USA) according to the manufacturer's instructions. The virus titer was determined by using One-Step TaqMan SaV-specific RT-qPCR as described previously (23).
Detection of PoSaV-specific antibodies in serum and mucosal samples by an enzyme-linked immunosorbent assay (ELISA).
A recombinant baculovirus expressing PoSaV VP1 was generated as previously described and used to infect Sf9 cells for VLP production (33). Briefly, purified PoSaV VLPs were used as antigens to coat Nunc 96-well plates (MaxSorp surface; Thermo Scientific, MA, USA) at 4°C overnight at a final concentration of 2 μg/ml (100 ng/well) in 0.05 M carbonate buffer (pH 9.6). The plates were blocked with 2% nonfat dry milk (NFDM) in PBST at 37°C for 1 h. After being washed three times with PBST, serum samples were 4-fold serially diluted in PBST containing 2% NFDM and added to the wells. The plates were incubated at 37°C for 1 h and washed with PBST three times. HRP-conjugated goat anti-swine IgG(H+L) serum (KPL, MD, USA) diluted 1:3,000 or HRP-conjugated goat anti-swine IgA serum (AbD Serotec, NC, USA) diluted 1:5,000 in PBST containing 2% NFDM was added to wells, followed by incubation at 37°C for 1 h. After the plates were washed three times with PBST, the substrate 3,3′,5,5′-tetramethylbenzidine (TMB; KPL, MD, USA) was added to each well for color development at room temperature. An equal volume of 1 M phosphoric acid was added to terminate the reactions after 5 min of incubation at room temperature. The absorbance at 450 nm was measured by using a spectrometer (SpectraMax 430 PC; Molecular Devices LLC, CA, USA). The antibody titer was determined as the reciprocal of the highest serum dilution with an absorbance value greater than or equal to the mean absorbance of a series of negative-control serum samples plus 3 times the standard deviation (SD) of the negative controls (33).
Virus neutralization test.
Serum samples were tested for virus neutralization (VN) antibodies to PoSaV by a 50% cell culture infectivity reduction test. A total of 100 TCID50/well of TC PoSaV was incubated with an equivalent volume of 4-fold serially diluted serum samples at 37°C for 1 h before application to cell monolayers. Quadruplicate wells were used for each serum dilution. Nonneutralized PoSaV was detected on LLC-PK cells by IHC staining as described above. The VN antibody titers were determined by using the Reed-Muench method (34) and expressed as the reciprocal of the highest serum dilution that inhibited PoSaV infection in 50% of wells.
Statistical analysis.
One-way analysis of variance (ANOVA) followed by Duncan's multiple-range test was used to assess differences in plaque sizes, mean durations of virus shedding, and log-transformed titers (including antibody titer, VN antibody titer, and viral RNA titer) among groups. One-way ANOVA was used to assess ratios of villus height to crypt depth (VH/CD ratios) and the mean numbers of antigen-positive cells per villus. A significance level of a P value of <0.05 was used for all comparisons.
Nucleotide sequence accession numbers.
The genomes of WT PoSaV I-1113 (Gn pig passage level 5) and TC PoSaV-2010 (passage level 30) were deposited in GenBank under accession numbers KT922087 and KT922088, respectively. The VP1 region of WT PoSaV R418 (Gn pig passage level 13) was deposited in GenBank under accession number KT945132.
RESULTS
Consistent mutations occur in the RdRp and VP1 regions at different passages of TC PoSaV compared to WT PoSaV.
To investigate which genes were critical for PoSaV adaptation to cell culture, the genome of TC PoSaV at passage level 30 in LLC-PK cells was sequenced in this study and compared with those of WT PoSaV at pig passage levels 5 and 13, TC PoSaV at cell culture passage level 20 (25), and the infectious clone pCV4A carrying the cDNA of TC PoSaV-2005 (cell culture passage level 27) (20). Two and six conserved amino acid mutations were observed in the RdRp (residues 1252 and 1379) and VP1 of (residues 178, 289, 291, 295, 324, and 328) regions TC PoSaV (Fig. 1 and Table 3), respectively.
TABLE 3.
Summary of amino acid substitutions in the genomes from different passages of the WT or TC PoSaV Cowden strain
| Gene | Amino acid positionc | Nucleotide position | WT-PoSaV sequence in Gn pigs at passage: |
TC-PoSaV sequence in LLC-PK cells at passage: |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 5b | 5 (I-1113a) | 13 (R418a) | 13 (PS499a) | 20b | 27 | 30 | |||
| NS1 | 17 | 59 | TTT | TTT | TTT | TTT | TTT | TCT (S17F) | TTT |
| 18 | 62 | GAC | GGC (G18D) | GGC | GGC | GAC | GGC | GGC | |
| 24 | 80 | CCA | CCA | CCA | CCA | CCA | CCA | CTA (L24P) | |
| 29 | 94 | GCG | GCG | GCG | GCG | GCG | GCG | ACG (T29A) | |
| NS3 | 356 | 1075 | ATT | ATT | ATT | ATT | ATT | GTT (V356I) | ATT |
| 367 | 1109 | AAG | AAG | AAG | AAG | AAG | AGG (R367K) | AAG | |
| NS4 | 733 | 2207 | GGC | GAC/GGC | GGC | GGC | GGC (D733G) | GGC | GGC |
| NS7 | 1252 | 3763 | TAC | TAC | TAC | TAC | CAC (Y1252H) | CAC | CAC |
| 1379 | 4145 | AGA | AGA | AGA | AGA | AAA (R1379K) | AAA | AAA | |
| 1392 | 4185 | ATA | ATA | ATG/ATA | ATA | ATA(M1392I) | ATA | ATA | |
| VP1 | 75 | 5362 | ACA | ACA | ACA | ACA | ACA | ACA | GCA (A1785T) |
| 178 | 5671 | TGT | TGT | TGT | TGT | AGT (C178S) | AGT | AGT | |
| 289 | 6004 | TAC | TAC | TAC | TAC | CAC (Y289H) | CAC | CAC | |
| 291 | 6010 | AAC | AAC | AAC | AAC | GAC (N291D) | GAC | GAC | |
| 295 | 6023 | AAG | AAG | AAG | AAG | AGG (K295R) | AGG | AGG | |
| 324 | 6111 | ATA | ATG | ATG | ATG | ATA (M324I) | ATA | ATA | |
| 328 | 6122 | GGA | GAA | GAA | GAA | GGA (E328G) | GGA | GGA | |
| ORF2 | 27 | 6851 | CAT | CAT | CAT | CAT | CAT | CAT | CAA (E27H) |
| 35 | 6873 | AAT | AAT | AAT | AAT | AAT | AAT | GAT (D35N) | |
Sample identification based on the labeling system in the laboratory.
See reference 25.
Boldface numbers refer to consensus amino acid substitutions among different passages of TC compared with WT PoSaV.
The VP1 region is critical for cell culture adaptation of PoSaV.
To examine whether the RdRp or the VP1 region was critical for PoSaV adaptation to cells, we engineered two chimeric genomes (Fig. 1) based on the previously established PoSaV reverse-genetics system, pCV4A: (i) TC-WTRdRp, whose amino acid residues 1252 and 1379 of the polyprotein (RdRp region) were mutated from the TC to the WT phenotype (H1252Y and K1379R), and (ii) TC-WTVP1, whose partial VP1 region (nt 5227 to 6060 and amino acids 30 to 308, excluding amino acid residues 324 and 328) was replaced with the corresponding WT fragment. The capped genomic RNA transcripts were transfected into BHK-T7 cells. After infection of LLC-PK cells with BHK-T7 cell lysates, the PoSaV VP1 proteins were detected exclusively by an immunofluorescence assay (IFA) in LLC-PK cells inoculated with TC-WTRdRp- but not TC-WTVP1-transfected products. We further compared the growth kinetics and plaque sizes of the TC-WTRdRp virus to those of the TC-pCV4A virus in LLC-PK cells (Fig. 2). TC-WTRdRp had plaque sizes (0.168 ± 0.052 mm2) similar to those of TC-pCV4A (0.146 ± 0.066 mm2) and had growth kinetics similar to those of TC-pCV4A in LLC-PK cells, showing increasing titers between 0 and 72 hpi with similar peak titers (7.1 ± 0.2 log10 TCID50/ml for TC-pCV4A and 7.0 ± 0.0 log10 TCID50/ml for TC-WTRdRp). These results suggested that the VP1 region, but not the RdRp region, was critical for cell culture adaptation of PoSaV.
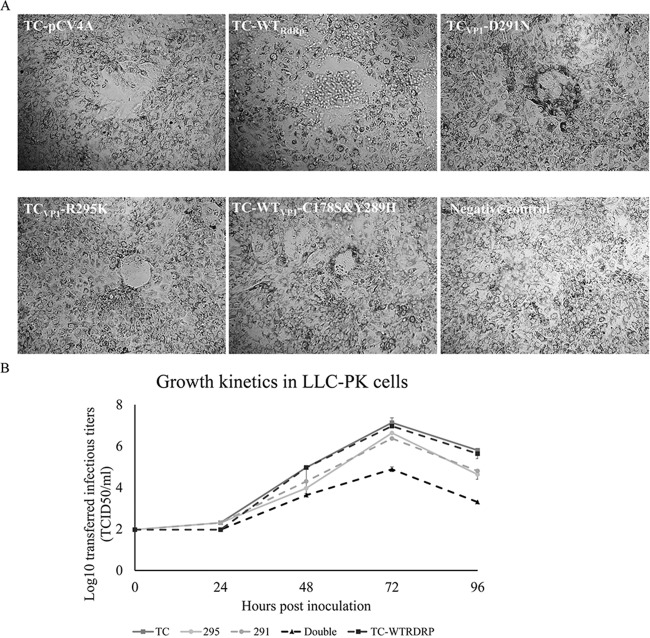
FIG 2.
Growth kinetics and representative plaques of TC-pCV4A (TC) and the culturable mutants TC-WTRdRp, TCVP1-D291N (291), TCVP1-R295K (295), and TC-WTVP1-C178S&Y289H (Double) in LLC-PK cells. (A) The plaque sizes of the TCVP1-D291N, TCVP1-R295K, and TC-WTVP1-C178S&Y289H mutants are smaller than those of TC-pCV4Aand TC-WTRdRp in LLC-PK cells. (B) LLC-PK cells were inoculated with each virus at a MOI of 0.01. Cell lysates were collected at 24, 48, 72, and 96 hpi for titration of infectious virus. TC-pCV4A and TC-WTRdRp replicated in LLC-PK cells to significantly higher titers (72 hpi) than did the culturable mutants (P < 0.05 by one-way ANOVA followed by Duncan's multiple-range test on log10-transformed titers).
Four (residues 178, 289, 324, and 328) of the six amino acid residues in the VP1 region were essential for PoSaV adaptation to LLC-PK cells. To address which of the individual mutations in the VP1 region was essential for PoSaV adaptation, each of the sites was mutated to the WT sequence individually (Fig. 1): TCVP1-S178C, TCVP1-H289Y, TCVP1-D291N, TCVP1-R295K, TCVP1-I324M, and TCVP1-G328E. The TCVP1-D291N and TCVP1-R295K mutants replicated in LLC-PK cells. No infectious virus was rescued from LLC-PK cells infected with the transfection lysates of TCVP1-S178C, TCVP1-H289Y, TCVP1-I324M, or TCVP1-G328E infectious clones. Furthermore, when the point mutations of TC-WTVP1 were mutated back to TC sequences, the double mutant (TC-WTVP1-C178S&Y289H, carrying TC amino acids at residues 324 and 328), instead of the individual point mutants (TC-WTVP1-C178S and TC-WTVP1-Y289H), was rescued in LLC-PK cells. Infectious virus was rescued from infectious clones of the TCVP1-I324M→M324I and TCVP1-G328E→E328G back mutations. These results indicate that the 4 amino acids at residues 178, 289, 324, and 328 in the VP1 region are essential for PoSaV adaptation to LLC-PK cells.
Single amino acid substitutions in the VP1 region alter PoSaV growth kinetics in vitro.
To examine whether the amino acid substitutions D291N, R295K, and TC-WTVP1-C178S&Y289H can alter viral replication in LLC-PK cell cultures, the growth kinetics and plaque sizes of TCVP1-D291N, TCVP1-R295K, and TC-WTVP1-C178S&Y289H were compared to those of TC-pCV4A in LLC-PK cells (Fig. 2). The TCVP1-D291N (0.041 ± 0.011 mm2) and TCVP1-R295K (0.047 ± 0.016 mm2) viruses and especially the TC-WTVP1-C178S&Y289H (0.018 ± 0.003 mm2) virus formed significantly smaller (P < 0.05) plaques than those formed by TC-pCV4A (0.146 ± 0.066 mm2) and TC-WTRdRp (0.168 ± 0.052 mm2) (Fig. 2A). The infectious titers of TCVP1-D291N, TCVP1-R295K, and TC-WTVP1-C178S&Y289H increased postinoculation. However, the peak titers of TC-pCV4A (7.1 ± 0.2 log10 TCID50/ml) and TC-WTRdRp (7.0 ± 0.0 log10 TCID50/ml) were the highest, followed by TCVP1-R295K (6.6 ± 0.0 log10 TCID50/ml), TCVP1-D291N (6.4 ± 0.0 log10 TCID50/ml), and TC-WTVP1-C178S&Y289H (4.9 ± 0.1 log10 TCID50/ml) (Fig. 2B). These data led us to conclude that the D291N and R295K amino acid substitutions in the VP1 region reduced PoSaV replication in LLC-PK cells.
Comparative structural analyses of the VP1 proteins of TC and WT PoSaVs predicted the location and potential function of amino acid residues.
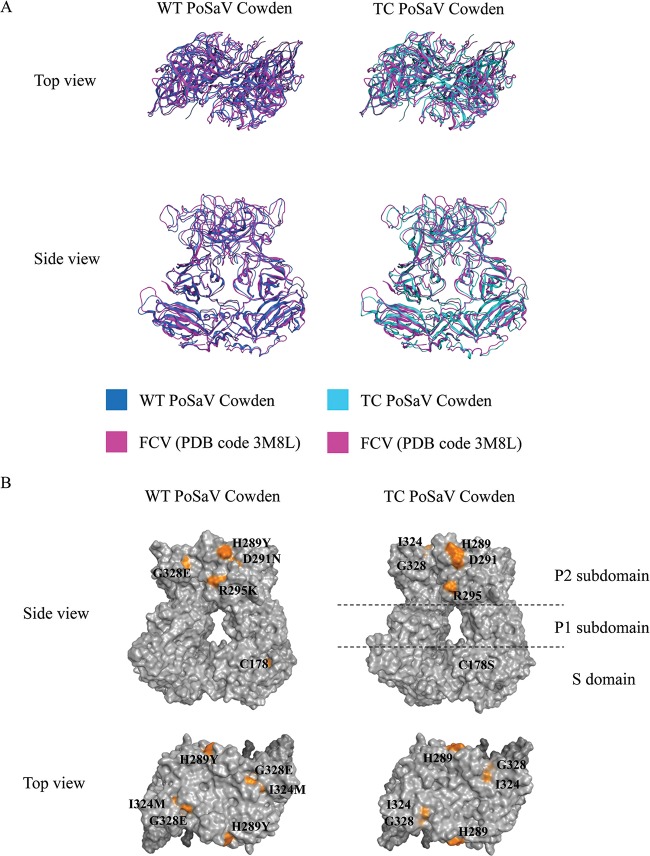
The 3D structural models of both the TC and WT PoSaV Cowden strains matched the template structure (FCV VP1 [PDB accession no. 3M8L]) (Fig. 3A). Subsequently, 3D structural analysis was performed to examine whether the residue changes in VP1 between the WT and TC PoSaVs resulted in structural changes. Amino acid residue 178 was located in the S domain near the dimer-dimer interface (Fig. 3B). C178 of the WT was exposed, but S178 of the TC strain was hidden. Amino acid residues 289, 291, 324, and 328 in PoSaV were located at the P2 region, while amino acid 295 was located at the interface of two monomeric VP1 proteins forming a dimer at P2 (Fig. 3B).
FIG 3.
Superimposition of the modeled structures of PoSaV Cowden and the template FCV structure and the 3D models of WT and TC PoSaV VP1s. (A) Superimposition of the modeled structures (WT PoSaV Cowden on the left and TC PoSaV Cowden on the right) and the template structure (FCV VP1 [PDB accession no. 3M8L]) by homology modeling. The blue ribbon structure denotes WT PoSaV Cowden VP1, the cyan ribbon structure denotes TC PoSaV Cowden VP1, and the magenta ribbon structure denotes the template structure of FCV VP1. (B) Amino acid residue 178 was located at the dimer-dimer interface in the S domain; residues 289, 291, 324, and 328 were located in the P2 region; and residue 295 was located on the interface of two monomeric VP1 proteins in the P2 region.
To investigate whether the mutations at residues 178, 289, 291, 295, 324, and 328 influenced the stability of WT PoSaV, we analyzed the changes in thermodynamic stability by the mutations using the Protein Design application in the MOE. A positive number indicated decreased stability, while a negative number indicated increased stability. The changes in ΔG caused by the C178S, Y289H, N291D, K295R, M324I, and E328G point mutations were 0.54 ± 0.04, 4.51 ± 0.02, 1.80 ± 0.24, −1.43 ± 0.31, −0.42 ± 0.15, and 1.86 ± 0.37 kcal/mol, respectively (Fig. 4). The C178S, Y289H, N291D, and E328G mutations decreased the stability, whereas K295R and M324I, especially K295R, compensated for the changes.
FIG 4.
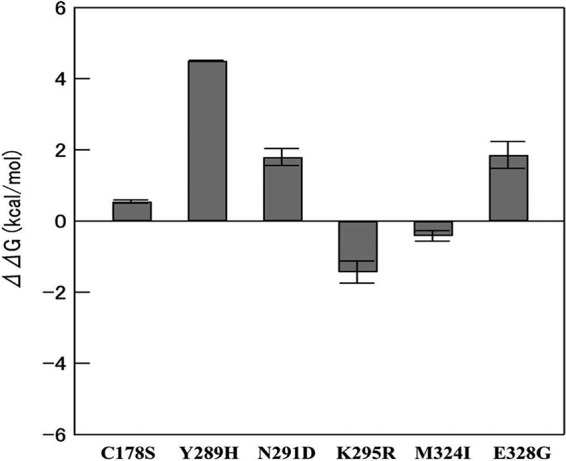
Changes in ΔG for the C178S, Y289H, N291D, K295R, M324I, and E328G point mutations. Mutations C178S, Y289H, N291D, and E328G decreased thermodynamic stability, whereas K295R and M324I compensated for the changes.
Single amino acid substitutions in the VP1 region alter PoSaV replication in pigs.
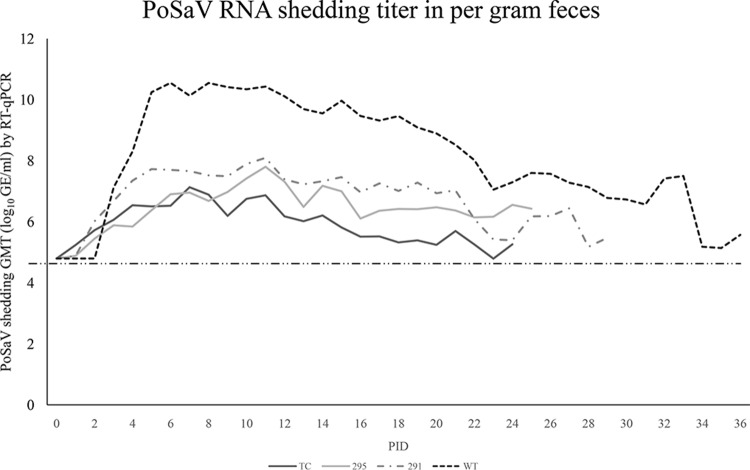
To investigate whether the amino acid substitutions from the TC to the WT sequence in VP1 could restore the virulence of WT PoSaV in vivo, we performed pathogenesis studies in Gn pigs. Among the experimental groups, the WT PoSaV-inoculated Gn pigs had a significantly longer duration of viral RNA shedding (30.3 ± 3.8 days) and higher peak viral RNA titers (10.8 ± 0.4 log10 GE/g) than those of the other three inoculated Gn pig groups. Relative to the mutants, the TCVP1-D291N-inoculated Gn pigs had a longer duration of viral RNA shedding (23.2 ± 3.4 days) and a higher peak RNA titer (8.6 ± 0.8 log10 GE/g) than those of the TCVP1-R295K- or TC PoSaV Cowden strain-inoculated Gn pigs (Fig. 5 and Table 4).
FIG 5.
Geometric mean titers (GMT) of viral RNA shedding of TCVP1-D291N and TCVP1-R295K compared with those of TC-pCV4A and the WT PoSaV Cowden strain in Gn pigs on various PIDs. Gn pigs were inoculated with the corresponding virus inoculum. Rectal swabs were collected daily for titration of viral RNA shedding. The WT PoSaV Cowden strain had the highest peak titer and longest duration of viral RNA shedding among the experimental groups. The TC PoSaV Cowden strain had the lowest peak titer and shortest duration of shedding among the experimental groups. Peak viral RNA shedding titers and durations of shedding in TCVP1-D291N- and TCVP1-R295K-inoculated Gn pigs were intermediate between the WT and TC groups.
TABLE 4.
Comparative viral RNA shedding parameters for TC, WT, and mutant PoSaV Cowden strains
| Groupa | No. of Gn pigs | Time of onset (PIDs)b | Duration (days) (SD)c | Peak titer (log10 GE/ml) (SD)c | Time of peak titer (PIDs)d |
|---|---|---|---|---|---|
| TC | 6 | 1–4 | 19.8 (2.6)a | 7.7 (0.4)a | 3–14 |
| 295 | 5 | 1–4 | 20.8 (3.4)a | 7.9 (1.0)a | 5–15 |
| 291 | 6 | 1–6 | 23.2 (3.4)b | 8.6 (0.8)b | 3–19 |
| WT | 4 | 1–3 | 30.3 (3.8)c | 10.8 (0.4)c | 6–10 |
| NC | 2 | NAe | NA | NA | NA |
TC, 295, 291, WT, and NC refer to the TC PoSaV Cowden strain, TCVP1-R295K, TCVP1-D291N, the WT PoSaV Cowden strain, and the negative control, respectively.
The onset of RNA shedding refers to the PIDs when fecal viral RNA was first detected by RT-qPCR.
Superscript letters denote significant differences among the groups (determined by one-way ANOVA followed by Duncan's multiple-range test).
Time of peak titer refers to PIDs that have the highest viral RNA titers by RT-qPCR.
NA, not applicable.
Clinical signs and histopathological lesions are observed exclusively in WT PoSaV-infected Gn pigs.
Moderate diarrhea (fecal score of 2) was observed in three of seven (43%) WT PoSaV-inoculated Gn pigs. Diarrhea developed by PIDs 3 to 12 and persisted for 2 to 16 days. No diarrhea or other clinical signs were observed in TC PoSaV Cowden-, TCVP1-D291N-, TCVP1-R295K-, or mock-inoculated Gn pigs.
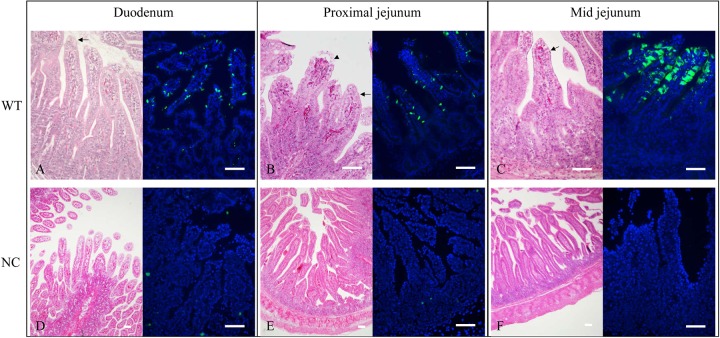
Microscopically, histopathological lesions were not observed in organs from the TC PoSaV-, TCVP1-D291N-, or TCVP1-R295K-inoculated or control Gn pigs. WT PoSaV-inoculated Gn pigs euthanized at the acute phase of infection exhibited mild-to-moderate, diffuse, and atrophic enteritis, demonstrating shortened and blunt villi from the duodenum to the midjejunum of the small intestine (Fig. 6A to C). Duodenal, proximal, and midjejunal tissues showed moderate, diffuse villous atrophy (Fig. 6A to C). The VH/CD ratios for duodenal and midjejunal tissues were 3.54 ± 1.06 and 3.98 ± 1.84, respectively, significantly lower than those for control pigs (5.42 ± 2.26 and 5.95 ± 2.25, respectively) (Table 5). Interestingly, the VH/CD ratio for jejunal tissues from the TCVP1-D291N-inoculated Gn pigs was statistically lower than those for the TCVP1-R295K- and TC PoSaV Cowden-inoculated and control Gn pigs (Table 5), indicating more severe villous atrophy in the jejuna of TCVP1-D291N-inoculated Gn pigs than in TCVP1-R295K- and TC PoSaV-inoculated and control Gn pigs.
FIG 6.
Histopathological examination of small intestinal samples from WT PoSaV Cowden- or mock (negative-control [NC])-infected Gn pigs. Shown are H&E staining (left) and IF staining (right) of samples collected from different regions of the small intestine. (A to C) Samples from WT PoSaV Cowden-inoculated Gn pigs at PID 5, showing fusion, shortening (arrows), or blunting (arrowhead) of villi in the duodenum (A), proximal jejunum (B), and midjejunum (C). (D to F) Samples from mock-inoculated Gn pigs at PID 5, showing normal villi in the duodenum (D), proximal jejunum (E), and midjejunum (F). Intestinal samples were collected in duplicate for IF staining and for H&E staining. Bar, 50 μm.
TABLE 5.
Ratios of villus length to crypt depth in different regions of small intestines of Gn pigs
| Groupa | Avg VL/CD ratio (SD)b |
||
|---|---|---|---|
| Duodenum | Jejunum | Ileum | |
| TC | 5.21 (0.86)a | 5.93 (1.60)a | 4.68 (0.52) |
| 295 | 5.97 (1.15)a | 6.44 (0.78)a | 5.26 (0.66) |
| 291 | 5.32 (0.94)a | 5.09 (1.47)b | 5.20 (0.76) |
| WT | 3.54 (1.06)b | 3.98 (1.84)c | 5.23 (1.20) |
| NC | 5.42 (2.26)a | 5.95 (2.25)a | 6.57 (0.33) |
TC, 295, 291, WT, and NC refer to the TC PoSaV Cowden strain, TCVP1-R295K, TCVP1-D291N, the WT PoSaV Cowden strain, and the negative control, respectively.
Superscript letters denote significant differences among the groups not sharing the same letter (determined by one-way ANOVA).
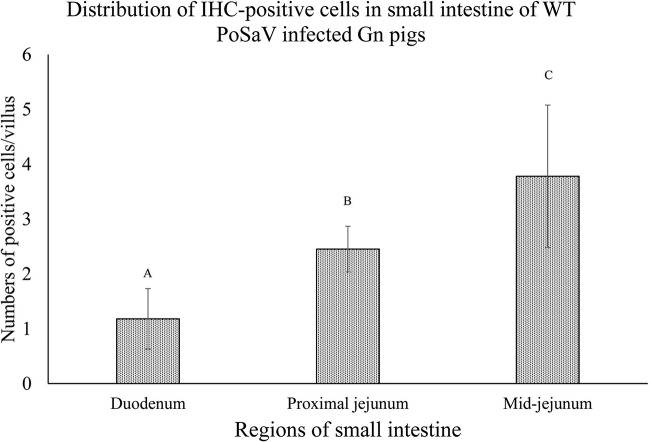
Virus VP1 antigens were detected in the frozen tissues of WT PoSaV-inoculated Gn pigs by IF staining. Most antigen-positive cells were distributed in the mature enterocytes lining the intestinal villi of the midjejunum and, to a lesser extent, in the duodenum and proximal jejunum (Fig. 6). Antigen-positive cells were rarely detected in the distal jejunum and ileum. The mean numbers of antigen-positive cells per villus differed significantly among different regions of the small intestine, with most of the positive epithelial cells being observed in the duodenum to midjejunum, with an increasing trend from duodenum (1.18 ± 0.55 cells) to proximal jejunum (2.45 ± 0.42 cells) and to midjejunum (3.78 ± 1.30 cells) (Fig. 7). Antigen-positive cells were not observed in any tissue sections from TC PoSaV-, TCVP1-D291N-, or TCVP1-R295K-inoculated Gn pigs.
FIG 7.
Distribution of antigen-positive cells per villus in different regions of the small intestine of WT PoSaV-inoculated pigs. Mean numbers of antigen-positive cells per villus were significantly different among the duodenum, proximal jejunum, and midjejunum of the small intestine (P < 0.05 by one-way ANOVA).
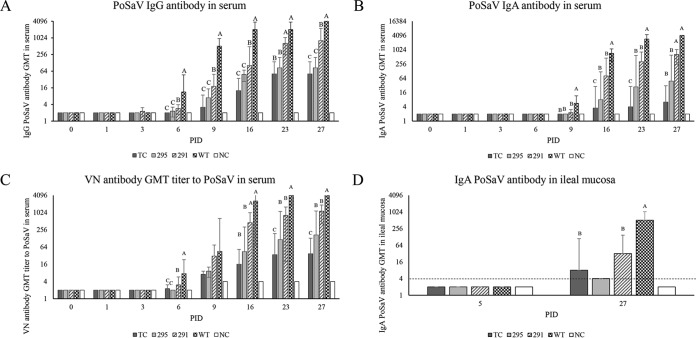
IgG, IgA, and VN antibody titers in pig serum samples were titrated (Fig. 8A to C). From PIDs 6 to 27, Gn pigs inoculated with the WT had the highest IgG, IgA, and VN serum antibody titers. Gn pigs inoculated with TCVP1-D291N had higher serum IgG, IgA, and VN antibody titers than did those inoculated with TC or TCVP1-R295K mutant PoSaV at PIDs 6 to 27. Gn pigs inoculated with the WT also had the highest IgA mucosal antibody titers at PID 27 (Fig. 8D). Gn pigs inoculated with TCVP1-D291N and TC PoSaV had significantly higher IgA mucosal antibody titers than did those inoculated with TCVP1-R295K (P < 0.05 by one-way ANOVA followed by Duncan's multiple-range test on log4-transformed titers).
FIG 8.
Serum and mucosal antibody responses in Gn pigs. (A) Geometric mean titers (GMT) of IgG antibodies to the corresponding PoSaV strains or mock (NC) in serum samples from Gn pigs. (B) Geometric mean titers of IgA antibodies to the corresponding PoSaV strains or mock infection in serum samples of Gn pigs. (C) Geometric mean titers of virus-neutralizing antibodies to the corresponding PoSaV strains or mock in serum samples of Gn pigs. (D) Geometric mean titers of IgA antibodies to the corresponding PoSaV strains in ileal mucosal samples from Gn pigs. Serum samples were collected from each Gn pig before inoculation and at PIDs 1, 3, 6, 9, 16, 23, and 27. Ileal mucosal samples were collected at PID 5 and PID 27. Data points marked with different letters at each day differed significantly (P < 0.05 by one-way ANOVA followed by Duncan's multiple-range test on log10-transformed titers).
DISCUSSION
The genomes of the WT (Gn pig passage level 5) and TC PoSaV Cowden strains (cell culture passage level 20) were reported in 1999 (25). It was also reported that the WT PoSaV Cowden strain grew in porcine primary kidney cell culture after 13 passages in Gn pigs but only with mock Gn pig intestinal contents in medium (14). Therefore, we selected one Gn pig intestinal sample from both the 5th and the 13th passages of WT PoSaV for genomic sequence analysis. Besides the reported 6 amino acid substitutions between WT and TC PoSaVs (25), we identified two additional substitutions at amino acid residues 324 and 328 of VP1. We compared seven genomes from different passages of the WT and TC PoSaV Cowden strains (Table 3). Compared to the WT PoSaV Cowden strain, 8 of 19 amino acid mutations were conserved among all TC PoSaV genomes. The differences between the reported WT PoSaV Cowden genome and our results are likely due to the intestinal content samples from different pigs and the different passage levels tested. Because the two newly identified sites (residues 324 and 328 in the VP1 region) were conserved in the newly sequenced genomes from the 5th and 13th Gn pig passages of WT PoSaV, both sites were also included in this study. The establishment of the reverse-genetics system pCV4A for the PoSaV Cowden strain provided an important tool to study the molecular mechanisms of PoSaV cell culture adaptation (20). In this study, we generated a series of PoSaV mutants to test which of the 8 amino acids were critical for cell culture adaptation. In previous reports, a one-step in vitro transcription-and-capping procedure was used to generate infectious PoSaV genomic RNA for transfection (20). Infectious PoSaV Cowden virions were successfully rescued from LLC-PK cells by using the one-step method (20, 35). However, for unknown reasons, we failed to rescue infectious virus. Because infectious murine norovirus (MNV) was rescued from RAW 264.7 cells by using two-step in vitro transcription followed by capping (36), we tried the two-step method and rescued infectious PoSaV from LLC-PK cells. The genome-linked virus protein (VPg) is encoded by the NS5 gene and is covalently linked to the 5′ end of the SaV genomic RNA (20, 37, 38). In our system, the cap structure analog m7G(5′)ppp(5′)G was added to the 5′ end of the SaV genomic RNA transcripts to simulate SaV VPg (36, 39).
Among the mutants, TCVP1-S178C, TCVP1-H289Y, TCVP1-I324M, and TCVP1-G328E could not be recovered in 4 to 5 repeats of the experiment. Whether virus particles were formed was unknown. However, HEK 293T/17 cells transfected with in vitro-transcribed and -capped RNAs of TCVP1-S178C, TCVP1-H289Y, TCVP1-I324M, and TCVP1-G328E showed several VP1-positive cells by IHC staining using hyperimmune serum against the VLPs of the PoSaV Cowden strain (data not shown). This suggests that the defect may occur at any step post-VP1 expression, such as virion assembly and binding to the receptors on LLC-PK cells or entry into LLC-PK cells. TCVP1-D291N, TCVP1-R295K, and TC-WTVP1-C178S&Y289H were culturable in the LLC-PK cell line, although the titer of the TC-WTVP1-C178S&Y289H strain was 2 log10 units lower than those of the other culturable strains. In the in vitro study, both the TCVP1-D291N and TCVP1-R295K mutants showed reduced replication compared with that of TC PoSaV. However, in our Gn pig study, both the TCVP1-D291N and TCVP1-R295K mutants had relatively higher peak viral RNA titers, a longer duration of fecal viral RNA shedding, and higher-level serum and mucosal antibody responses than those of TC PoSaV but lower than those of WT PoSaV. This indicates that the replication efficiencies of PoSaV mutants were discordant in vitro and in vivo. In vitro, peak virus infectivity titers (highest to lowest) were TC PoSaV > TCVP1-R295K > TCVP1-D291N > TC-WTVP1-C178S&Y289H; in vivo, peak viral RNA titers (highest to lowest) were WT PoSaV > TCVP1-D291N > TCVP1-R295K > TC PoSaV. Because the critical mutation sites are all located in VP1, different receptors were probably used by the PoSaV Cowden strain in vivo to infect small intestinal epithelial cells in pigs and in vitro to infect porcine kidney LLC-PK cells.
Three passages of MNV1 in the macrophage cell line RAW 264.7 resulted in a total of 3 amino acid substitutions, which included V716I and H845R in the 3A-like protease (NS4) region and E296K in the P2 region of VP1. Only V716I and E296K were suspected to be related to decreased virulence in mice and increased titers in RAW 264.7 cells (40, 41). Using reverse genetics, the K296E but not the I716V back mutation restored the virulence of the MNV1 revertant in mice (41). Therefore, a single amino acid substitution in the P2 region of VP1 of a norovirus may affect its virulence in the host and replication efficacy in cell culture. A previous study concluded that the TC PoSaV Cowden strain was attenuated in vivo compared with the WT PoSaV Cowden strain after oral inoculation of Gn pigs with a TC PoSaV Cowden strain cell culture supernatant (cell culture passage 20) or a filtrate of the WT PoSaV Cowden strain (18). In our study, we concentrated the TC PoSaV Cowden strain, TCVP1-D291N, and TCVP1-R295K, which were 1-log10-higher doses than those used in the previous study. However, clinical signs were observed exclusively in the WT PoSaV-infected pigs but not in the TC PoSaV Cowden strain-, TCVP1-D291N-, or TCVP1-R295K-infected Gn pigs. These results indicated that even at a 1-log10-higher inoculation dose, the TC PoSaV Cowden strain did not cause diarrhea in Gn pigs. Although the TCVP1-D291N and TCVP1-R295K revertants did not cause diarrhea in Gn pigs, they replicated more efficiently and induced relatively higher-level immune responses than TC PoSaV, suggesting that the two amino acid substitutions in the P2 subdomain of VP1 affected PoSaV virulence in the host and could be better candidates than the TC PoSaV Cowden strain for PoSaV vaccine development.
The first calicivirus structure was reported in 1994 for a primate calicivirus (42). Currently, structures of VLPs or virion particles of caliciviruses have been determined for GI.1 and GII.10 human NoVs (43, 44), GV MNV (45, 46), San Miguel sea lion virus (47), Tulane virus (48), rabbit hemorrhagic disease virus (49), and FCV (50). Therefore, structures are available for all classified genera of the family Caliciviridae, except for the genera Sapovirus and Nebovirus. Chen et al. reported that sapovirus showed more structural similarity to vesivirus (22). The FCV VP1 protein has the closest phylogenetic relatedness (37% amino acid identity) to that of the PoSaV Cowden strain among the existing calicivirus VP1 structures. To understand the location of the mutation sites based on structural predictions, we performed 3D structure modeling and analysis of the VP1 protein of the PoSaV Cowden strain using FCV VP1 as the template. In a previous study, the complete removal of the P domain of recombinant Norwalk virus-like particles resulted in the formation of smooth particles, which demonstrated that the S domain is sufficient for assembly of the capsid (51). Since the P2 region of caliciviruses is the most protruding part of VP1 and is highly variable, it has been considered responsible for binding to host receptors (52). A recent study indicated that the α2,3- and α2,6-linked sialic acids on O-linked glycoproteins are receptors on LLC-PK cells for the PoSaV Cowden strain (53). In our study, based on their locations in the structural model, amino acid position 178 was located in the S domain, which may influence VP1 oligomerization, virion assembly, and stability. Amino acid positions 289, 291, 324, and 328 were located in the P2 region, which affects binding to the receptors on LLC-PK cells and may impair virus replication. Amino acid position 295 was located in the P2 region at the interface of two monomeric VP1 proteins, which may influence VP1 dimerization.
The changes in thermodynamic stability caused by the mutations indicate whether or not the protein structure can be maintained. The overall change in thermodynamic stability is the sum of each estimated value. Decreasing the stability is a disadvantage to maintain the structure, whereas increasing the stability is an advantage. Therefore, mutations that decrease stability are critical for function, e.g., cell culture adaptation, rather than structure. The mutations that increase stability then play a role in the compensation for decreasing stability. In this study, thermodynamic stability analysis indicated that C178S, Y289H, N291D, and E328G decrease stability, while K295R and M324I increase stability. These results suggest that C178S, Y289H, N291D, and E328G would provide essential functions for cell culture adaptation, whereas K295R and M324I would compensate for the decreased stability of C178S, Y289H, N291D, and E328G.
We compared 12 WT GIII PoSaV VP1 protein sequences available in GenBank (http://www.ncbi.nlm.nih.gov/nucleotide/). We found that C178 in the S domain is conserved among all 12 WT GIII PoSaVs (WT PoSaV Cowden [GenBank accession no. KT922087], SaV1-CHN [accession no. ACP43737], HW20-KOR [accession no. ADN84680], ID3-HUN [accession no. ABD38714], LL14-US [accession no. AAR37376], MM280-US [accession no. AAX32888], JJ259-US [accession no. AAX37311], QW270-US [accession no. AAX37314], PES-VENEZ [accession no. AAY88248], PoS6-HUN [accession no. ACS68238], PoS9-HUN [accession no. ACS68240], and s20-JAP [accession no. BAE94661]), suggesting that C178S may be the most critical mutation during PoSaV Cowden strain tissue culture adaptation.
In this study, WT PoSaV antigen was observed in epithelial cells of the Gn pig small intestine from the duodenum to midjejunum but rarely in the distal jejunum or ileum. We confirmed the region of PoSaV infection in Gn pigs, as reported previously (18). Also, as previously reported, morphological alterations in duodenal and jejunal villi were observed in WT PoSaV Cowden-infected Gn pigs (18, 54). IF staining of small and large intestinal impression smears as well as villus length measurement also confirmed that the small intestine was the major infection site (18, 54). Our data support results of previous studies of the pathogenesis of the WT PoSaV Cowden strain in Gn pigs.
This study demonstrated that cell culture adaptation of the PoSaV Cowden strain is due to amino acid substitutions in the VP1 region. The single-revertant mutation (from the TC to the WT phenotype) at certain positions, positions 291 and 295, in the VP1 region reduced virus replication in vitro but partially regained PoSaV replication efficiency in vivo. The genetic basis delineated for cell culture adaptation of PoSaV may provide new critical information for the rescue of other uncultivable PoSaVs and human SaVs. In future studies, we plan to construct reverse-genetics systems for selected PoSaV and human SaV strains by introducing site-directed mutations at structurally corresponding positions in the VP1 protein to rescue such unculturable SaVs.
ACKNOWLEDGMENTS
We acknowledge Juliette Hanson, Jeff Ogg, Andrew Wright, Ronna Wood, and Megan Strother for assistance with animal care and Chun-Ming Lin, Xiaohong Wang, and Susan Sommer-Wagner for technical assistance.
Zhongyan Lu was supported by a scholarship provided by the China Scholarship Council. Salaries and research support were provided by state and federal funds provided to the Ohio Agricultural Research and Development Center (OARDC), The Ohio State University.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Clarke IN, Lambden PR. 1997. The molecular biology of caliciviruses. J Gen Virol 78(Part 2):291–301. [DOI] [PubMed] [Google Scholar]
- 2.Farkas T, Sestak K, Wei C, Jiang X. 2008. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J Virol 82:5408–5416. doi: 10.1128/JVI.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. 2012. Virus taxonomy. Classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA. [Google Scholar]
- 4.Oka T, Wang Q, Katayama K, Saif LJ. 2015. Comprehensive review of human sapoviruses. Clin Microbiol Rev 28:32–53. doi: 10.1128/CMR.00011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang XL, Honma S, Nakata S, Vesikari T. 2000. Human caliciviruses in acute gastroenteritis of young children in the community. J Infect Dis 181(Suppl 2):S288–S294. doi: 10.1086/315590. [DOI] [PubMed] [Google Scholar]
- 6.Sakai Y, Nakata S, Honma S, Tatsumi M, Numata-Kinoshita K, Chiba S. 2001. Clinical severity of Norwalk virus and Sapporo virus gastroenteritis in children in Hokkaido, Japan. Pediatr Infect Dis J 20:849–853. doi: 10.1097/00006454-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Hansman GS, Saito H, Shibata C, Ishizuka S, Oseto M, Oka T, Takeda N. 2007. Outbreak of gastroenteritis due to sapovirus. J Clin Microbiol 45:1347–1349. doi: 10.1128/JCM.01854-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cubitt WD, Pead PJ, Saeed AA. 1981. A new serotype of calicivirus associated with an outbreak of gastroenteritis in a residential home for the elderly. J Clin Pathol 34:924–926. doi: 10.1136/jcp.34.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svraka S, Vennema H, van der Veer B, Hedlund KO, Thorhagen M, Siebenga J, Duizer E, Koopmans M. 2010. Epidemiology and genotype analysis of emerging sapovirus-associated infections across Europe. J Clin Microbiol 48:2191–2198. doi: 10.1128/JCM.02427-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi S, Fujiwara N, Yasui Y, Yamashita T, Hiramatsu R, Minagawa H. 2012. A foodborne outbreak of sapovirus linked to catered box lunches in Japan. Arch Virol 157:1995–1997. doi: 10.1007/s00705-012-1394-8. [DOI] [PubMed] [Google Scholar]
- 11.Lee LE, Cebelinski EA, Fuller C, Keene WE, Smith K, Vinje J, Besser JM. 2012. Sapovirus outbreaks in long-term care facilities, Oregon and Minnesota, USA, 2002-2009. Emerg Infect Dis 18:873–876. doi: 10.3201/eid1805.111843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parwani AV, Flynn WT, Gadfield KL, Saif LJ. 1991. Serial propagation of porcine enteric calicivirus in a continuous cell line. Effect of medium supplementation with intestinal contents or enzymes. Arch Virol 120:115–122. [DOI] [PubMed] [Google Scholar]
- 13.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 14.Flynn WT, Saif LJ. 1988. Serial propagation of porcine enteric calicivirus-like virus in primary porcine kidney cell cultures. J Clin Microbiol 26:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saif LJ, Ward LA, Yuan L, Rosen BI, To TL. 1996. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol Suppl 12:153–161. [DOI] [PubMed] [Google Scholar]
- 16.Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. 2012. The pig: a model for human infectious diseases. Trends Microbiol 20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart EA, Caccamo M, Harrow JL, Humphray SJ, Gilbert JG, Trevanion S, Hubbard T, Rogers J, Rothschild MF. 2007. Lessons learned from the initial sequencing of the pig genome: comparative analysis of an 8 Mb region of pig chromosome 17. Genome Biol 8:R168. doi: 10.1186/gb-2007-8-8-r168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo M, Hayes J, Cho KO, Parwani AV, Lucas LM, Saif LJ. 2001. Comparative pathogenesis of tissue culture-adapted and wild-type Cowden porcine enteric calicivirus (PEC) in gnotobiotic pigs and induction of diarrhea by intravenous inoculation of wild-type PEC. J Virol 75:9239–9251. doi: 10.1128/JVI.75.19.9239-9251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saif LJ, Bohl EH, Theil KW, Cross RF, House JA. 1980. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J Clin Microbiol 12:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang KO, Sosnovtsev SV, Belliot G, Wang Q, Saif LJ, Green KY. 2005. Reverse genetics system for porcine enteric calicivirus, a prototype sapovirus in the Caliciviridae. J Virol 79:1409–1416. doi: 10.1128/JVI.79.3.1409-1416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu MS, Jung EH, Cho KO, Kang SY. 2012. Expression of porcine sapovirus VP1 gene and VP1 specific monoclonal antibody production. Hybridoma (Larchmt) 31:155–162. doi: 10.1089/hyb.2011.0112. [DOI] [PubMed] [Google Scholar]
- 22.Chen R, Neill JD, Noel JS, Hutson AM, Glass RI, Estes MK, Prasad BV. 2004. Inter- and intragenus structural variations in caliciviruses and their functional implications. J Virol 78:6469–6479. doi: 10.1128/JVI.78.12.6469-6479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Zhang Z, Saif LJ. 2012. Stability of and attachment to lettuce by a culturable porcine sapovirus surrogate for human caliciviruses. Appl Environ Microbiol 78:3932–3940. doi: 10.1128/AEM.06600-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang KO, Sosnovtsev SV, Belliot G, Kim Y, Saif LJ, Green KY. 2004. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc Natl Acad Sci U S A 101:8733–8738. doi: 10.1073/pnas.0401126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo M, Chang KO, Hardy ME, Zhang Q, Parwani AV, Saif LJ. 1999. Molecular characterization of a porcine enteric calicivirus genetically related to Sapporo-like human caliciviruses. J Virol 73:9625–9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Chaumont F, Dallongeville S, Chenouard N, Herve N, Pop S, Provoost T, Meas-Yedid V, Pankajakshan P, Lecomte T, Le Montagner Y, Lagache T, Dufour A, Olivo-Marin JC. 2012. Icy: an open bioimage informatics platform for extended reproducible research. Nat Methods 9:690–696. doi: 10.1038/nmeth.2075. [DOI] [PubMed] [Google Scholar]
- 27.Baker D, Sali A. 2001. Protein structure prediction and structural genomics. Science 294:93–96. doi: 10.1126/science.1065659. [DOI] [PubMed] [Google Scholar]
- 28.Case DA, Darden TA, Cheatham TEI, Simmerling CL, Wang J, Duke RE, Luo R, Crowley M, Walker RC, Zhang W, Merz KM, Wang B, Hayik S, Roitberg A, Seabra G, Kolossváry I, Wong KF, Paesani F, Vanicek J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, Yang L, Tan C, Mongan J, Hornak V, Cui G, Mathews DH, Seetin MG, Sagui C, Babin V, Kollman PA. 2008. AMBER 10. University of California, San Francisco, CA. [Google Scholar]
- 29.Gerber PR, Muller K. 1995. MAB, a generally applicable molecular force field for structure modelling in medicinal chemistry. J Comput Aided Mol Des 9:251–268. doi: 10.1007/BF00124456. [DOI] [PubMed] [Google Scholar]
- 30.Summa CM, Levitt M. 2007. Near-native structure refinement using in vacuo energy minimization. Proc Natl Acad Sci U S A 104:3177–3182. doi: 10.1073/pnas.0611593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberg D, Luthy R, Bowie JU. 1997. VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol 277:396–404. doi: 10.1016/S0076-6879(97)77022-8. [DOI] [PubMed] [Google Scholar]
- 32.Meyer RC, Bohl EH, Kohler EM. 1964. Procurement and maintenance of germ-free Seine for microbiological investigations. Appl Microbiol 12:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo M, Qian Y, Chang KO, Saif LJ. 2001. Expression and self-assembly in baculovirus of porcine enteric calicivirus capsids into virus-like particles and their use in an enzyme-linked immunosorbent assay for antibody detection in swine. J Clin Microbiol 39:1487–1493. doi: 10.1128/JCM.39.4.1487-1493.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am J Epidemiol 27:493–497. [Google Scholar]
- 35.Shivanna V, Kim Y, Chang KO. 2014. The crucial role of bile acids in the entry of porcine enteric calicivirus. Virology 456–457:268–278. doi: 10.1016/j.virol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yunus MA, Chung LM, Chaudhry Y, Bailey D, Goodfellow I. 2010. Development of an optimized RNA-based murine norovirus reverse genetics system. J Virol Methods 169:112–118. doi: 10.1016/j.jviromet.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhry Y, Nayak A, Bordeleau ME, Tanaka J, Pelletier J, Belsham GJ, Roberts LO, Goodfellow IG. 2006. Caliciviruses differ in their functional requirements for eIF4F components. J Biol Chem 281:25315–25325. doi: 10.1074/jbc.M602230200. [DOI] [PubMed] [Google Scholar]
- 38.Hosmillo M, Chaudhry Y, Kim DS, Goodfellow I, Cho KO. 2014. Sapovirus translation requires an interaction between VPg and the cap binding protein eIF4E. J Virol 88:12213–12221. doi: 10.1128/JVI.01650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinnebusch AG. 2014. The scanning mechanism of eukaryotic translation initiation. Annu Rev Biochem 83:779–812. doi: 10.1146/annurev-biochem-060713-035802. [DOI] [PubMed] [Google Scholar]
- 40.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol 2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey D, Thackray LB, Goodfellow IG. 2008. A single amino acid substitution in the murine norovirus capsid protein is sufficient for attenuation in vivo. J Virol 82:7725–7728. doi: 10.1128/JVI.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasad BV, Matson DO, Smith AW. 1994. Three-dimensional structure of calicivirus. J Mol Biol 240:256–264. doi: 10.1006/jmbi.1994.1439. [DOI] [PubMed] [Google Scholar]
- 43.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 44.Hansman GS, Taylor DW, McLellan JS, Smith TJ, Georgiev I, Tame JR, Park SY, Yamazaki M, Gondaira F, Miki M, Katayama K, Murata K, Kwong PD. 2012. Structural basis for broad detection of genogroup II noroviruses by a monoclonal antibody that binds to a site occluded in the viral particle. J Virol 86:3635–3646. doi: 10.1128/JVI.06868-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katpally U, Voss NR, Cavazza T, Taube S, Rubin JR, Young VL, Stuckey J, Ward VK, Virgin HW IV, Wobus CE, Smith TJ. 2010. High-resolution cryo-electron microscopy structures of murine norovirus 1 and rabbit hemorrhagic disease virus reveal marked flexibility in the receptor binding domains. J Virol 84:5836–5841. doi: 10.1128/JVI.00314-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katpally U, Wobus CE, Dryden K, Virgin HW IV, Smith TJ. 2008. Structure of antibody-neutralized murine norovirus and unexpected differences from viruslike particles. J Virol 82:2079–2088. doi: 10.1128/JVI.02200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen R, Neill JD, Estes MK, Prasad BV. 2006. X-ray structure of a native calicivirus: structural insights into antigenic diversity and host specificity. Proc Natl Acad Sci U S A 103:8048–8053. doi: 10.1073/pnas.0600421103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu G, Zhang D, Guo F, Tan M, Jiang X, Jiang W. 2013. Cryo-EM structure of a novel calicivirus, Tulane virus. PLoS One 8:e59817. doi: 10.1371/journal.pone.0059817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Z, Tian X, Zhai Y, Xu W, Zheng D, Sun F. 2010. Cryo-electron microscopy reconstructions of two types of wild rabbit hemorrhagic disease viruses characterized the structural features of Lagovirus. Protein Cell 1:48–58. doi: 10.1007/s13238-010-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ossiboff RJ, Zhou Y, Lightfoot PJ, Prasad BV, Parker JS. 2010. Conformational changes in the capsid of a calicivirus upon interaction with its functional receptor. J Virol 84:5550–5564. doi: 10.1128/JVI.02371-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertolotti-Ciarlet A, White LJ, Chen R, Prasad BV, Estes MK. 2002. Structural requirements for the assembly of Norwalk virus-like particles. J Virol 76:4044–4055. doi: 10.1128/JVI.76.8.4044-4055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bu W, Mamedova A, Tan M, Xia M, Jiang X, Hegde RS. 2008. Structural basis for the receptor binding specificity of Norwalk virus. J Virol 82:5340–5347. doi: 10.1128/JVI.00135-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim DS, Hosmillo M, Alfajaro MM, Kim JY, Park JG, Son KY, Ryu EH, Sorgeloos F, Kwon HJ, Park SJ, Lee WS, Cho D, Kwon J, Choi JS, Kang MI, Goodfellow I, Cho KO. 2014. Both alpha2,3- and alpha2,6-linked sialic acids on O-linked glycoproteins act as functional receptors for porcine sapovirus. PLoS Pathog 10:e1004172. doi: 10.1371/journal.ppat.1004172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flynn WT, Saif LJ, Moorhead PD. 1988. Pathogenesis of porcine enteric calicivirus-like virus in four-day-old gnotobiotic pigs. Am J Vet Res 49:819–825. [PubMed] [Google Scholar]