ABSTRACT
All live attenuated respiratory syncytial virus (RSV) vaccines that have advanced to clinical trials have been produced in Vero cells. The attachment (G) glycoprotein in virions produced in these cells is smaller than that produced in other immortalized cells due to cleavage. These virions are 5-fold less infectious for primary well-differentiated human airway epithelial (HAE) cell cultures. Because HAE cells are isolated directly from human airways, Vero cell-grown vaccine virus would very likely be similarly inefficient at initiating infection of the nasal epithelium following vaccination, and therefore, a larger inoculum would be required for effective vaccination. We hypothesized that Vero cell-derived virus containing an intact G protein would be more infectious for HAE cell cultures. Using protease inhibitors with increasing specificity, we identified cathepsin L to be the protease responsible for cleavage. Our evidence suggests that cleavage occurs in the late endosome or lysosome during endocytic recycling. Cathepsin L activity was 100-fold greater in Vero cells than in HeLa cells. In addition, cathepsin L was able to cleave the G protein in Vero cell-grown virions but not in HeLa cell-grown virions, suggesting a difference in G-protein posttranslational modification in the two cell lines. We identified by mutagenesis amino acids important for cleavage, and these amino acids included a likely cathepsin L cleavage site. Virus containing a modified, noncleavable G protein produced in Vero cells was 5-fold more infectious for HAE cells in culture, confirming our hypothesis and indicating the value of including such a mutation in future live attenuated RSV vaccines.
IMPORTANCE Worldwide, RSV is the second leading infectious cause of infant death, but no vaccine is available. Experimental live attenuated RSV vaccines are grown in Vero cells, but during production the virion attachment (G) glycoprotein is cleaved. Virions containing a cleaved G protein are less infectious for primary airway epithelial cells, the natural RSV target. In the study described here we identified the protease responsible, located the cleavage site, and demonstrated that cleavage likely occurs during endocytic recycling. Moreover, we showed that the infectivity of Vero cell-derived virus for primary airway epithelial cells is increased 5-fold if the virus contains a mutation in the G protein that prevents cleavage. The blocking of cleavage should improve RSV vaccine yield, consequently reducing production costs. Posttranslational cleavage of the fusion glycoprotein of many viruses plays an essential role in activation; however, cleavage of the RSV G protein is a novel example of a detrimental effect of cleavage on virus infectivity.
INTRODUCTION
In adults and teenagers, most respiratory syncytial virus (RSV) infections produce upper respiratory tract infection and symptoms. However, in infants, the immunocompromised, and the elderly, RSV has the potential to cause life-threatening lower respiratory tract infection (1–4). Worldwide, in 2010 alone, over 230,000 children under 5 years of age died from RSV illness, with the majority of these being infants under the age of 1 year (5). Currently, only supportive care is available to treat individuals with lower respiratory tract disease. A neutralizing monoclonal antibody (MAb), palivizumab, is used prophylactically, but only for infants considered at the greatest risk for severe disease. There is a clear need for vaccines to combat this virus.
The attachment (G) glycoprotein and the fusion (F) glycoprotein are important for the initial steps in virus infection and attachment and in membrane fusion, respectively. The G protein is a 33-kDa type II membrane protein with a large number of posttranslational modifications, including N- and O-glycans, that increase its apparent molecular mass to 90 kDa when produced in most immortalized cell lines. Historically, it was difficult to determine if the G protein was a viral or host protein (6–8) because differences in the cell line, virus strain, and method of protein detection all had an effect on the apparent size and presence of the G protein (6–8). While HEp-2 cells produced a 90-kDa G protein, BS-C-1 cells, an African green monkey kidney cell line, produced a 55-kDa G protein, and the size discrepancy was assumed to be due to differences in glycosylation (9).
More recently, in the G protein produced by another African green monkey kidney cell line, the Vero cell line, this size difference was found to be caused by cleavage, a different posttranslational modification. Although the G protein is not absolutely essential for infection of immortalized cells (10), virus lacking the G protein is 10-fold less infectious for these cells (11). Virus lacking the G protein is a further 10-fold less infectious for primary well-differentiated human airway epithelial (HAE) cells in culture (12). HAE cell cultures are an excellent in vitro model for the natural target cells in the human respiratory tract (13, 14). Similarly, virions produced by Vero cells, which primarily contain the cleaved G protein, are 5-fold less infectious for HAE cells in culture, suggesting that cleavage severely compromises the attachment function of the G protein (12).
This finding is of particular importance for live attenuated vaccine development because the viruses used in the initial formalin-inactivated RSV vaccine tested in the 1960s (15, 16) and in live attenuated vaccine candidates tested in clinical trials since the 1990s have been grown in African green monkey kidney or Vero cells (10, 17–23).
Currently, only 3 cell lines are approved for use by the Food and Drug Administration for live attenuated vaccine production, MRC-5, WI-38, and Vero cells. MRC-5 and WI-38 cells are much less proliferative than Vero cells, and the G protein is also cleaved in MRC-5 cells (12) and WI-38 cells (unpublished data). In addition, Vero cells do not produce interferon (IFN) (24), which is especially appealing if a vaccine candidate is deficient in inhibiting the IFN response.
We hypothesized that virus with an intact G protein produced in Vero cells would more efficiently infect HAE cells in culture and in vivo would more efficiently infect the cells that line the human airway. To test our hypothesis, we used protease inhibitors to identify cathepsin L to be the protease responsible for cleaving the G protein in Vero cells and demonstrated that cleavage likely occurs during endocytic recycling. Others have shown that the Nipah virus fusion protein is activated by cathepsin L during recycling (25, 26). In addition, endocytic recycling is important in the envelopment of RSV (27, 28). However, our work demonstrates for the first time that endocytic recycling and cathepsin L can be detrimental to the production of infectious viral progeny in one cell type but not another.
In addition, we demonstrate that growing the virus in Vero cells in the presence of a cathepsin L inhibitor resulted in RSV with an intact G protein that was 5-fold more infectious for HAE cells in culture. We also identified the amino acids that are important for G-protein cleavage in Vero cells, built into the viral genomic cDNA the mutation that most efficiently inhibited cleavage, rescued the modified virus, and demonstrated that this Vero cell-derived virus has an intact G protein and is 5-fold more infectious for HAE cells in culture without the addition of a protease inhibitor.
MATERIALS AND METHODS
Cell culture.
HeLa and Vero cells (ATCC, Manassas, VA) were cultured in Dulbecco modified Eagle medium (DMEM; Corning Incorporated, Corning, NY) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), referred to throughout as “medium.” Cells were incubated at 37°C in a 5% CO2 atmosphere. Primary, well-differentiated HAE cell cultures were generated from human airway tissue as previously described (29). HAE cell cultures were grown on collagen-coated Transwell inserts (Corning Incorporated). Once the cells reached confluence and formed tight junctions, the apical medium was removed and the cells were maintained at the air-liquid interface for 6 to 8 weeks to form well-differentiated, polarized cells before use. The basal medium was changed three times weekly, and the apical surface was washed for 2 h once weekly with Dulbecco's phosphate-buffered saline (D-PBS).
Virus infection and drug treatment.
HeLa or Vero cells were inoculated with recombinant green fluorescent protein (GFP)-expressing RSV (rgRSV), the D53 variant of the A2 strain (30, 31) or a mutant virus, diluted in the medium. Cells were tipped at 37°C for 2 h, and the inoculum was replaced with fresh medium. At 48 h postinoculation (hpi), fresh medium was added, and at 72 hpi, the cells were scraped and the medium containing the cells was collected and pulse vortexed. Cells were pelleted by centrifugation at 1,200 × g for 5 min in a Megafuge centrifuge (Baxter Scientific Products), and the supernatant was divided into equal aliquots, snap-frozen on dry ice, and stored at −80°C. All viruses were titrated on HeLa cells. HeLa or Vero cells were inoculated with rgRSV or rgRSV with the L208A mutation (rgRSV-L208A) as described above. For protease inhibitor experiments, at 7 hpi medium containing 0.5 μM cathepsin L inhibitor III (Calbiochem, San Diego, CA) or an equal volume of vehicle (dimethyl sulfoxide [DMSO]) was added to the cells. At 72 hpi, virus was harvested as described above and partially purified by layering over 35% sucrose in 1× Hanks balanced salt solution with calcium and magnesium and centrifuged overnight at 4°C and 26,000 × g in an F14-14x50cy rotor for the Sorvall Lynx 6000 centrifuge (Thermo Fisher Scientific). A portion of these virus preparations was further purified through a sucrose gradient by centrifugation in a TH-641 rotor (Thermo Fisher Scientific) and Beckman ultracentrifuge at 287,000 × g for 20 h. Gradient fractions were examined by immunoblotting and staining with an MAb to the N protein (32). Fractions containing virus were separated by SDS-PAGE, and G protein was detected by immunoblotting using an MAb, L9 (33).
The full-length RSV cDNA construct RW30 (34), representing the D53 variant of the A2 strain, was used as the backbone for modification of the G protein at amino acid 208 from leucine to alanine. Synthetic double-stranded DNA (gBlock; Integrated DNA Technologies, Coralville, IA) containing the G gene with the L208A mutation was inserted into plasmid RW30 using restriction sites that flank the G-protein gene and a Gibson assembly kit (New England BioLabs, Ipswich, MA). The G-protein mutant virus, rgRSV-L208A, was rescued from this plasmid as previously described (31).
HeLa and Vero cells were inoculated with rgRSV (multiplicity of infection, 1) or mock inoculated, at 2 hpi the inoculum was replaced with fresh medium, and at 4 hpi the medium was again replaced with fresh medium containing 2-fold dilutions of the protease inhibitor (Sigma-Aldrich, St. Louis, MO) aprotinin (3.125 to 50 μg/ml), leupeptin (6.25 to 100 μg/ml), or E-64 (6.25 to 100 μg/ml) dissolved in water or equal volumes of water in medium. In other experiments, cells were treated at 6 hpi with drugs that block calpains and cathepsins, consisting of 10-fold dilutions (0.1 to 100 μM) of cathepsin inhibitor I (Calbiochem), N-acetyl-leucine-leucine-methionine (ALLM; Santa Cruz, Dallas, TX), chloroquine diphosphate salt (Sigma-Aldrich), or equivalent volumes of vehicle (water) or of CA-074 (Calbiochem), cathepsin L inhibitor III (Calbiochem), or an equal volume of vehicle (DMSO) in medium. ALLM is a calpain and cathepsin inhibitor, and CA-074 is a cathepsin B inhibitor.
Biotinylation and immunoblot analysis.
At 24 hpi, the cells were biotinylated with EZ-link sulfo-NHS-LC-biotin (Thermo Fisher Scientific, Waltham, MA). The cells were lysed with lysis buffer containing 150 mM NaCl, 1% Triton X-100, 50 mM Tris, 0.1% SDS, and 1× Halt protease cocktail inhibitor (Thermo Fisher Scientific). Proteins were quantified using a bicinchoninic acid (BCA) protein assay kit (Pierce, Waltham, MA), and equal amounts of protein were added to high-capacity streptavidin agarose beads (Thermo Fisher Scientific). The mixtures were rotated for 1 h at 4°C, the beads were pelleted and washed with lysis buffer (without protease cocktail inhibitor), the proteins were separated on NuPAGE Novex 4 to 12% bis-Tris protein gels (Life Technologies, Carlsbad, CA), and immunoblots were probed with mouse MAb L9 to the RSV G protein or D14 to the RSV N protein (Ed Walsh, University of Rochester) or a polyclonal rabbit anti-cathepsin L antibody that recognizes cathepsin L (Sigma), followed by the appropriate human serum-adsorbed and peroxidase-labeled secondary antibody: anti-mouse IgG (H+L) antibody or anti-rabbit IgG (H+L) antibody (KPL, Inc. Gaithersburg, MD).
Cathepsin L treatment.
Viruses grown in the presence of vehicle or a cathepsin L inhibitor were pelleted through a sucrose cushion and resuspended in citric acid-sodium phosphate buffer at pH 5.5. Active cathepsin L enzyme (Sigma) or vehicle was added to a final concentration of 50 ng/μl. Samples were incubated for 2 h at 37°C, and the G protein was assayed by immunoblotting with the L9 MAb.
PCR.
Primers against cathepsin L were designed to amplify a 294-bp product and to cross exon-exon boundaries to decrease the chance of amplifying genomic DNA (forward primer, GAGGCAACAGAAGAATCC; reverse primer, CCCAGCTGTTCTTCACC). Total mRNA was isolated from uninfected cells at 24 hpi, reverse transcribed, and amplified by PCR. The PCR products were separated on 2% agarose gels and visualized with ethidium bromide.
Cathepsin activity assays.
At 24 hpi, inoculated or mock-infected cells were treated with lysis buffer without protease inhibitors and maintained on ice. Protein concentrations were determined with a BCA protein assay kit (Pierce) and assayed by use of a fluorogenic InnoZyme cathepsin L activity kit (Calbiochem). The results were normalized for the amount of protein added and are displayed as the log10 amount of amido-4-methylcoumarin (AMC) released per gram of protein. Protein from HeLa or Vero cells was similarly assayed using the fluorogenic InnoZyme cathepsin B activity kit (Calbiochem).
Mutagenesis.
A soluble version of the A2 strain G protein was constructed by replacing its cytoplasmic tail and transmembrane domain with the cytoplasmic tail, the transmembrane domain, and a portion of the stalk of the Schwarz measles virus H protein and inserting a furin cleavage site, a 6-His tag, and a factor Xa site between the measles virus stalk and the G protein.
For all other G-protein mutants, a codon-optimized strain A2 G-protein gene was mutagenized using synthetic gBlock DNA (Integrated DNA Technologies) inserted into pcDNA3.1.
frG-protein transfection.
Wild-type G protein or a furin-releasable (fr) version of the G protein (frG protein) was expressed in HeLa or Vero cells following plasmid transfection with the FuGene HD (Promega) or Lipofectamine LTX (Life Technologies) reagent, respectively, in medium containing 2% FBS. In frG experiments, the medium was collected and concentrated using Ultracel 10K centrifugal filters (EMD Millipore, Billerica, MA). For other transfection experiments, Vero cells were transfected using Lipofectamine 3000 (Life Technologies) in DMEM containing 10% FBS. For all transfection experiments, cells were lysed and protein was quantified by the BCA assay. Equivalent amounts of HeLa or Vero cell lysate protein and equivalent volumes of proteins concentrated from the medium were analyzed by immunoblotting.
HAE cell viral infections.
Partially purified virions were titrated on HeLa cells. The apical surface of well-differentiated HAE cells in Transwells was washed with D-PBS for 2 h, and the basal medium was changed before equivalent numbers of PFU (between 2,000 and 10,000 PFU, referred to as HeLa cell-infectious units) of the appropriate RSV stock were diluted in HAE cell medium and added to the apical chamber of the Transwell. In parallel, HeLa cells were inoculated with 200 PFU. At 2 hpi, the inoculum was removed from HeLa cells and replaced with fresh medium only. Fluorescent (35) cells were visualized with an EVOS fl inverted fluorescence microscope (Life Technologies), and the numbers of cells in HeLa cell cultures were counted at 24 hpi and the numbers of cells in HAE cell cultures were counted at 48 hpi.
Statistical analysis.
All data are representative of those from one of three experiments unless otherwise noted. Data from three to six replicates of each experimental condition are expressed as the mean ± standard deviation. All experiments were repeated ≥3 times. A 2-tailed Student's t test was employed to determine the significance of the differences between experimental conditions. A P value of <0.05 was considered statistically significant.
RESULTS
Protease identification with protease inhibitors.
We hypothesized that Vero cell-derived virus containing an intact G protein would be more infectious for HAE cells in culture. To test this, we set out to identify the protease responsible for cleavage. Vero cells were inoculated with recombinant green fluorescent protein expressing RSV (rgRSV) and treated with the following protease inhibitors: aprotinin (a serine protease inhibitor), leupeptin (a serine/cysteine protease inhibitor), or E-64 (a cysteine protease inhibitor). Since glycoproteins on the cell surface are the most likely to be incorporated into the virion, at 24 h postinoculation (hpi) the cell surface proteins were labeled with biotin. Biotinylated proteins were pulled down with streptavidin beads, and the G protein was detected by immunoblotting with an antibody to the G protein (Fig. 1A). As expected, in the untreated and vehicle-treated samples, most of the cell surface G protein was cleaved (∼55 kDa), leaving only a small portion of full-length (∼90 kDa) G protein. Aprotinin had no effect on cleavage. However, both leupeptin and E-64 prevented G-protein cleavage, suggesting that a cysteine protease is responsible for cleavage of the G protein in Vero cells.
FIG 1.
Protease inhibition of G-protein cleavage in Vero cells. Immunoblotting was used to detect biotinylated cell surface RSV G protein (55 or 90 kDa) produced in rgRSV-inoculated Vero cells treated with the following protease inhibitors: 2-fold dilutions of aprotinin (3.125 to 50 μg/ml) or leupeptin or E-64 (6.25 to 100 μg/ml) (A), 10-fold dilutions of the inhibitor ALLM (0.1 to 100 μM) (B), or 10-fold dilutions of CA-074 (a cathepsin B inhibitor) or cathepsin L inhibitor III (0.1 to 100 μM) (C). The immunoblots were probed with MAb L9. Data are representative of those from three independent experiments.
To narrow the field of cysteine proteases, rgRSV-infected Vero cells were treated with ALLM, a protease inhibitor that blocks the activity of cathepsin B, cathepsin L, calpain I, and calpain II. While nearly all of the cell surface G protein in Vero cells was cleaved in the absence of the inhibitor, ALLM inhibited G-protein cleavage in a concentration-dependent manner (Fig. 1B).
The G protein is a type II membrane protein, whose N-terminal signal sequence is not cleaved, serving also as its anchor in cell and virion membranes. During transit to the cell surface, the C-terminal ectodomain of the protein would be inside vesicles and only the N-terminal cytoplasmic tail would be exposed to the cytoplasm. Since calpains are exclusively cytoplasmic proteases, it is unlikely that they would be responsible for cleavage. Cathepsins B and L, however, reside inside vesicles and organelles or are secreted from cells and, therefore, would be more likely to have access to the G protein.
To test the possibility of cathepsin cleavage of the G protein, RSV-infected Vero cells were treated with a cathepsin B inhibitor (CA-074) or a cathepsin L inhibitor (cathepsin L inhibitor III). Vehicle-treated RSV-infected cells displayed a mixture of cleaved and uncleaved G protein, and the cathepsin B inhibitor did not change this pattern (Fig. 2B). However, the cathepsin L inhibitor strongly prevented G-protein cleavage even at the lowest concentration tested, suggesting that cathepsin L is the protease that cleaves the G protein.
FIG 2.
Cathepsin L and B expression and activity in HeLa and Vero cells. (A) Cathepsin L mRNA from mock-treated or rgRSV-infected HeLa and Vero cells was reverse transcribed and amplified by reverse transcription-PCR (RT-PCR), and the specific 294-bp product was displayed by agarose gel electrophoresis. (B) Immunoblot detection of cathepsin L protein species in mock- or rgRSV-infected cell lysates. (C and D) Enzymatic activity of cathepsin L (C) and cathepsin B (D) in lysates. (A and B) Data are representative of those from three independent experiments. (C and D) Data from four independent experiments were combined. *, P < 0.05 (unpaired, 2-tailed t test, unequal variance); **, P < 10−7 (unpaired, 2-tailed t test, unequal variance).
Cathepsin L expression and activity.
Both Vero and HeLa cells expressed cathepsin L mRNA in rgRSV-infected and uninfected cells (Fig. 2A). However, only Vero cells contained detectable cathepsin L protein, and expression was independent of infection (Fig. 2B). Cathepsin L mRNA is present in HeLa cells, but the protein is undetectable, suggesting that there could be a block in protein synthesis, but determination of the mechanism of this inhibition was not pursued.
Though cathepsin L is present in Vero cells, the protein may not be active. Cathepsin L has multiple splice variants (34, 36, 37) and at least two isoforms (38, 39). Like other acid hydrolases, cathepsin L is a zymogen that needs to be cleaved three times for full protease activity (35, 40–43). Mock- or rgRSV-infected cells were harvested at 24 hpi in the absence of protease inhibitors and assayed for cathepsin L activity. Vero cell cathepsin L was 100-fold more active than HeLa cell cathepsin L (Fig. 2C). However, a comparable cathepsin B assay found a much smaller difference in cathepsin B activity between infected HeLa and Vero cells (Fig. 2D). These data support the inhibitor data suggesting that cathepsin L is the protease that cleaves the G protein in Vero cells.
To directly determine if cathepsin L is sufficient to cleave the G protein, we incubated G protein produced in Vero cells with pure, active cathepsin L protein. To generate the intact G-protein substrate, HeLa and Vero cells were infected with rgRSV and treated with vehicle or the cathepsin L inhibitor during virus production. Progeny virions were purified to remove the cathepsin L inhibitor and incubated with cathepsin L or vehicle. As expected, virus grown in Vero cells without the protease inhibitor contained a mix of cleaved and intact G protein, while virus grown in the presence of the protease inhibitor primarily contained intact G protein (Fig. 3A, first and second lanes). Exogenously added cathepsin L cleaved most of the G protein that had remained uncleaved in virions grown in vehicle-treated Vero cells. It also cleaved the intact G protein produced in Vero cells that were treated with a cathepsin L inhibitor (Fig. 3A, third and fourth lanes). In both cases, the size of the cathepsin L-cleaved G protein was 55 kDa, the same as that of the G protein cleaved in Vero cells. Interestingly, the G protein in virus grown in HeLa cells was not cleaved when the cells were incubated with exogenous cathepsin L (Fig. 3B), suggesting a posttranslational modification of the G protein in HeLa cells that prevents cleavage by cathepsin L; this posttranslational modification does not occur in Vero cells.
FIG 3.
Cathepsin L treatment of purified virions that had been grown in the presence of a cathepsin L inhibitor (inh). Vero cells (A) or HeLa cells (B) were inoculated with rgRSV and treated with medium containing vehicle (first and third lanes) or 0.5 μM cathepsin L inhibitor III (second and fourth lanes). Virus produced from these cells was partially purified and incubated with vehicle (first and second lanes) or 50 ng/μl cathepsin L (third and fourth lanes). G protein was detected by immunoblotting. Data are representative of those from three independent experiments.
Cellular location of cleavage.
Cathepsin L is found in the nucleus and in the late endosomes/lysosomes and can be secreted. While there is little likelihood and no evidence that the membrane-anchored G glycoprotein would transit through the nucleus, the G protein is transported through the cell in vesicles, though no evidence for transit through the late endosomes/lysosomes has been presented. The G protein might also contact secreted cathepsin L at the cell surface. A soluble G protein would allow us to test if the G protein is cleaved during transit to the cell surface or after it is secreted into the medium.
The second methionine in the G protein gene can also be used as a start codon, producing a secreted version of the G protein in some cell lines (44–46). However, this G protein is released from the cell as a monomer, whereas cell-associated G protein appears to be in a tetramer form (47). In the hope of producing a soluble tetramer, we constructed a gene that expresses a furin-releasable version of the G protein (the frG protein). The construct (Fig. 4A) contains the G-protein ectodomain attached to the measles virus hemagglutinin (H) protein stalk, transmembrane, and cytoplasmic domains. A furin cleavage site was inserted between the measles virus H stalk and the RSV G-protein ectodomain. Our hope was that the measles virus stalk would stabilize the G protein in its tetramer form until it reached the trans-Golgi network, where it would be released as the frG protein by furin cleavage and secreted into the medium. The H stalk was utilized as a spacer to separate the furin cleavage site from the plasma membrane so that furin could more easily access this cleavage site, resulting in efficient release of the frG protein from the H stalk. This frG protein is in a tetramer form (C. Capella, S. M. Johnson, M. Mejias, O. Ramilo, and M. E. Peeples, unpublished data).
FIG 4.
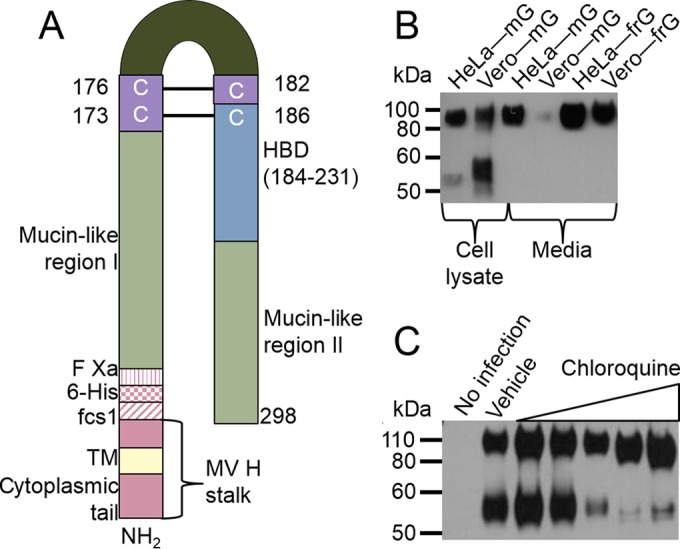
Furin-released and membrane-bound G-protein processing. (A) Chimeric protein that contains the N terminus of the measles virus hemagglutinin, including its cytoplasmic tail, its transmembrane domain, and a portion of its stalk, followed by the RSV G-protein ectodomain. The ectodomain contains two hypervariable mucin-like regions flanking a central conserved domain, a cysteine noose, and a heparin binding domain (HBD) (72). Amino acid numbering is based on the complete G protein from the A2 lab strain without the MV stalk. (B) Membrane-bound G protein (mG protein) or frG protein from HeLa and Vero cells detected by immunoblotting. (C) Immunoblot of biotinylated cell surface RSV G protein (55 or 90 kDa) on Vero cells inoculated with rgRSV and treated with vehicle or 10-fold dilutions of chloroquine (0.1 to 100 μM). Data are representative of those from three independent experiments.
HeLa and Vero cells were transfected with plasmids expressing either full-length, membrane-bound G protein (mG protein) or frG protein. At 48 h posttransfection, the cell culture medium was collected and concentrated. While cleaved mG protein was detected in Vero cell lysates, medium from both cell types contained only the intact, 90-kDa frG protein (Fig. 4B). This result indicates that the released frG protein is not cleaved during transit to the cell surface or while exposed to the medium, strongly suggesting that the full-length G protein is unlikely to be cleaved on its way to or at the cell surface. The 90-kDa size of the released frG protein also indicates that the protein is fully glycosylated.
If the G protein is not cleaved during transit to the cell surface, the next most likely possibility would be for it to be cleaved during endocytic recycling. As cathepsin L is present primarily in the interior of the late endosome/lysosome and is optimally active at acidic pH, we treated rgRSV-infected Vero cells with chloroquine to raise the pH of acidic organelles. Chloroquine-treated Vero cells primarily produced a full-length G protein, unlike the control treated samples (Fig. 4C). Taken together, these data suggest that the G protein encounters cathepsin L during endocytic recycling.
Infectivity for HAE cells of virus grown in the presence of a cathepsin L inhibitor.
We used the virus produced and assayed in the experiment whose results are presented in Fig. 3 to test if virus grown in the presence of a protease inhibitor is more infectious for HAE cells in culture than the control treated virus. First, we determined the infectious titer of these viruses on HeLa cells, and on the basis of those titers, HAE cells in culture were inoculated with equivalent numbers of HeLa cell-infectious units of all viruses. The same dilution series from each virus stock that was used to inoculate the HAE cells was used to inoculate HeLa cells. The numbers of infected cells in both cell cultures were counted, and the data are displayed as the ratio of HAE cell-infectious units to HeLa cell-infectious units (Fig. 5). The cathepsin L inhibitor provided during viral growth increased the infectivity of Vero cell-derived virus for HAE cells by 6-fold, such that there was no significant difference in the infectivity of virus derived from cathepsin L-treated Vero cells and virus derived from cathepsin L-treated HeLa cells. Virus produced in HeLa cells treated with the cathepsin L inhibitor displayed 30% lower infectivity for HAE cells than virus grown under the same conditions but without the inhibitor. This relatively minor inhibitory effect on virus production may reflect the loss of cathepsin L activity on one or more of its many cellular functions.
FIG 5.
Ability of rgRSV grown in the presence of a cathepsin L inhibitor to infect HAE cells. The infectivity for HAE cells of partially purified rgRSV virions that were produced in HeLa or Vero cells in the presence of a cathepsin L inhibitor or DMSO (Fig. 3, the left two lanes of each panel) was tested. HAE cells were inoculated with equal numbers of HeLa cell-infectious units of these 4 viruses, as were HeLa cells, on the same day and from the same dilution series for comparison. The ratio of the number of infected HAE cells to the number of infected HeLa cells is plotted. Data from three experiments were combined. *, P < 0.01 (unpaired, 2-tailed t test); **, P < 10−4 (unpaired, 2-tailed t test).
Identification of the G-protein cleavage site.
Another, more permanent way to reduce G-protein cleavage during virus growth in Vero cells would be to identify the site of cleavage using mutagenesis and insert the mutation that most efficiently prevents cleavage into the viral cDNA and rescue virus. Such a virus produced in Vero cells should display full infectivity for HAE cells.
To estimate the position of cleavage, we took into account the positions of the 4 N-linked glycans in the strain A2 G protein and the predicted positions of the many O-linked glycan sites using NetOGlyc software (48). We predicted that the G protein is cleaved in Vero cells at about amino acid 210. To test this estimate, we mutated codon 211 to a stop codon. The resultant protein was 50 to 60 kDa (Fig. 6A), similar to the size of the cleaved G protein in Vero cells.
FIG 6.
Mutagenesis of the G protein to identify the cleavage site. The results of immunoblot analysis of transiently expressed RSV G protein with a premature stop codon at amino acid 211 in HeLa cells (A), with overlapping deletion mutations in this region in Vero cells (B), and with alanine mutations in this region in Vero cells (C) are shown. Data are representative of those from three independent experiments. wt, wild type.
To identify amino acids important for G-protein cleavage in Vero cells, we deleted overlapping regions around amino acid 210 (amino acids 200 to 211, 204 to 213, 206 to 215, 208 to 217, or 209 to 213), with the expectation that deletion of the cleavage site would prevent G-protein cleavage in Vero cells. All of these deletion mutants reduced cleavage compared to that for the wild-type G protein (Fig. 6B), confirming that this region contains the protease site. In addition, all deletion mutants were missing amino acids 209 to 211, suggesting that cleavage requires these amino acids but not ruling out the importance of other amino acids in this region. Surprisingly, constructs with the deletion of amino acids 206 to 210 and 206 to 215, both of which contain deletions of all or the majority of amino acids 209 to 211, only partially inhibited cleavage. However, deletions have the potential of bringing together amino acids that might serve as a proteolytic site, and this is likely the case here.
To further delineate the amino acids that are important for cleavage, we individually mutated amino acids around amino acid 210 to alanine. Alanine substitutions at L208, K209, K212, K213, and D214 were resistant to cleavage, consistent with our hypothesis that amino acids 209 to 211 are important for cleavage. Amino acid substitutions at positions 197,199, 200, 201, 204, 206, 210, and 211 had little or no effect on cleavage.
Cathepsin L is able to cleave substrates with a variety of sequences but has preferential cleavage sites. Importantly, the L208/K209 motif is consistent with a known cathepsin L cleavage preference consisting of a hydrophobic amino acid in the P-2 position and a basic residue in P-1 (49), with cleavage occurring between P-1 and P-1′. In this case, cleavage would be between amino acids 209 and 210.
Infection of HAE cells with virus containing an uncleavable G protein.
We built the L208A mutation into the rgRSV genomic cDNA (rgRSV-L208A) and rescued the virus. Both rgRSV and rgRSV-L208A were grown in HeLa or Vero cells and sucrose gradient purified (Fig. 7A). As expected, HeLa cell-derived rgRSV contained an intact G protein and Vero cell-derived rgRSV contained a mix of cleaved and uncleaved G proteins. However, consistent with the transiently expressed modified G protein, Vero cell-grown rgRSV-L208A virions almost exclusively contained uncleaved G protein. Partially purified viruses were titrated on HeLa cells, and equivalent numbers of HeLa cell-infectious units were used to inoculate HAE cell or HeLa cell cultures. Infected cells were counted, and data are displayed as the ratio of the number of HAE cell-infectious units to the number of HeLa cell-infectious units (Fig. 7B). Consistent with our hypothesis, rgRSV-L208A was 5-fold more infectious for HAE cells than rgRSV when they were grown in Vero cells.
FIG 7.
Comparison of rgRSV and rgRSV-L208A infection of HAE cells. HeLa cell-derived (H-D) and Vero cell-derived (V-D) rgRSV and rgRSV-L208A were produced in HeLa or Vero cells. (A) Virus was purified by use of a sucrose gradient, and G protein was detected by immunoblotting. (B) Viruses were partially purified by centrifugation through a sucrose cushion and titrated on HeLa cells. Equal numbers of HeLa cell-infectious units were used to inoculate HAE and HeLa cells on the same day, as described in the legend to Fig. 5. Data from 4 experiments were combined. *, P < 10−8 (unpaired, 2-tailed t test, unequal variance).
DISCUSSION
Cells use endocytic recycling to transport proteins from the trans-Golgi network, turn over their membranes, bring in nutrients from the extracellular space (50), and process proteins for loading into major histocompatibility complex class I complexes (51). All enveloped viruses have hijacked this system to deliver their glycoproteins to the correct membranes to envelop their capsid structures (52–63). In at least one instance, the Nipah virus F protein requires endocytic recycling to activate its F protein in the late endosome/lysosome by either cathepsin B or cathepsin L (26, 64, 65).
Unlike these examples where recycling is required for the production of infectious virus, we have found that RSV G-protein recycling in Vero cells is detrimental to the infectivity of progeny virus for its in vivo target, the ciliated airway epithelial cell. In this context, the cathepsin L encountered during endocytic recycling serves as a viral restriction factor. Similar restriction factors could reduce the production of other enveloped viruses in particular cell lines, animals, or organs.
Cathepsins are critically important for lysosomal degradation and a number of other cellular processes, and, as such, many are ubiquitously expressed (66). However, the G protein is cleaved in only a few cell lines (12). One reason for this difference is that cathepsin L activity is much lower in HeLa cells than in Vero cells (Fig. 2). Another is that the mature HeLa cell-derived G protein is not susceptible to cathepsin L cleavage, while the Vero cell-derived G protein is (Fig. 3). This result suggests that there is something fundamentally different about the G protein when it is produced in HeLa cells than when it is produced in Vero cells, and this is most likely a difference in posttranslational modification. Each G protein is modified by the addition of an estimated 35 O-glycans, but their specific locations on the G protein have not been determined for any cell line. In any case, these results demonstrate that not all immortalized cell lines are equivalent for viral production.
The immortalized cell receptor for the G protein is heparan sulfate (HS) (67–72), a long-chain glycan modification found on a specific subset of cell surface glycoproteins on many cell types (73). The first indication that Vero cell-derived RSV was different from HEp-2 cell-derived RSV came when we found that HEp-2 cell-derived virus was 20-fold more infectious than Vero cell-derived virus for CHO cells that express HS than for CHO cells that are deficient in HS production (12). This finding prompted us to examine the G protein in virions, and we found that most of it was 55 kDa rather than 90 kDa. We went on to demonstrate that its smaller size was due to cleavage. We concluded that the loss of the G-protein C terminus reduced the ability of the Vero cell-derived virus to infect cells expressing HS, assuming that the HS binding domain had been lost when the C terminus was removed by cleavage.
Originally, a highly basic peptide from the G protein representing amino acids 184 to 198 was shown to bind to cells and to inhibit infection (74). This region of the G protein was dubbed the heparin binding domain (HBD). In the current study, we identified the site of cleavage in Vero cells to be between amino acids 209 and 210. This cleavage site is C terminal to amino acids 184 to 198, the originally described HBD, so cleavage at this site does not remove these amino acids from the 55-kDa G protein. However, in another study, Shields et al. found that the basic amino acids between amino acids 198 and 231 are also important for G-protein binding to immortalized cells (72). Taken together, these results support an extension of the HBD to encompass amino acids 184 to 231. Furthermore, from our current study the most important region for HS binding would seem to be the portion of the region from amino acids 183 to 231 that is lost by cleavage in Vero cells: amino acids 210 to 231.
Though it is important for entry into immortalized cells, HS is detectable only on the basal surface of HAE cells (75). RSV infects only via the apical surface and, more specifically, targets the ciliated epithelial cells (88), strongly suggesting that there is a different virus receptor on HAE cells. Consistent with this hypothesis, we have found that soluble HS readily inhibits RSV infection of HeLa cells but not HAE cells (89). In addition, we and others have recently shown that a molecule known to interact with the G protein, CX3CR1 (76), is present on the cilia of HAE cells (77, 89) and colocalizes with bound RSV virions in these cultures (77). Further, we have shown that the RSV G-protein CX3C motif (amino acids 182 to 186) is important for infection of HAE cells (89).
If amino acids 182 to 186 are important for attachment to the HAE cellular receptor CX3CR1 and these amino acids are retained in the Vero cell-cleaved G protein, as they would be with cleavage taking place between amino acids 209 and 210, it is not clear why HAE cell infection would be impaired (Fig. 6 and 7). This result suggests that the CX3C motif is not the only portion of the G protein that is important for infection of HAE cells. The C-terminal domain of the G protein, which is lost due to cleavage in Vero cells, might also be involved in binding to the CX3CR1 receptor, in binding to a second receptor, or in some other aspect of infection initiation. However, it is also possible that protein cleavage changes the structure of the G protein such that its CX3C motif is no longer able to bind CX3CR1.
An important role for the C-terminal portion of the G protein is consistent with the appearance, in 2003, of an RSV B strain virus in Buenos Aires, Argentina, containing a 60-nucleotide duplication in the C-terminal third of the G protein (78). Since then, these BA strain viruses have become the prominent circulating B strains (79–85). More recently, an A strain with a 72-nucleotide duplication in the C-terminal third of its G protein has become a predominant circulating A strain (79–81, 83, 85–87). The duplicated regions in these A and B strains do not overlap, but the fact that these viruses have arisen independently of one another and have become the predominant circulating strains also suggests that this C-terminal region of the G protein may play an important functional role.
We identified key residues in the C-terminal third of the G protein that are important for cathepsin L cleavage and chose the most efficient cleavage-inhibiting modification, L208A, for inclusion in the virus. rgRSV-L208A was equally infectious regardless of the cell type that produced the virus. rgRSV-L208A grown in Vero cells and rgRSV grown in HeLa cells were also equally infectious, confirming the importance of the full-length G protein for RSV infection of HAE cells. Importantly, the comparable infectivity of Vero cell-derived rgRSV-L208A and HeLa cell-derived rgRSV indicates that the L208A mutation does not affect G-protein function during virus entry into HeLa or HAE cells.
If a mutation that prevents G-protein cleavage in Vero cells is incorporated into a live attenuated RSV vaccine candidate, the virus produced would have an intact G protein and be 5-fold more infectious for the nasal epithelium, the site of vaccine inoculation. As a result, 5 times less inoculum could be used for each vaccination, resulting in a more economically feasible vaccine candidate. Because cleavage is a posttranslational modification, airway epithelial cells infected in vivo should spread within the airway epithelium similarly to the wild-type virus because both viruses would produce an intact, functional G protein. While the work presented here appears to solve the problem of G-protein cleavage in Vero cells for live attenuated vaccine production, efforts to reach the right balance between attenuation and immunogenicity continue.
ACKNOWLEDGMENTS
We thank Sara Mertz, Erin Gorrell, and Leah Rogers for technical assistance, Edward Walsh and Peter Collins for providing reagents, and Joan Durbin for establishing the Lung Cell Facility at The Research Institute at Nationwide Children's Hospital.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Obata K, Mukai K, Tsujimura Y, Ishiwata K, Kawano Y, Minegishi Y, Watanabe N, Karasuyama H. 2007. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110:913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Falsey AR, Walsh EE. 2005. Respiratory syncytial virus infection in elderly adults. Drugs Aging 22:577–587. doi: 10.2165/00002512-200522070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falsey AR, Cunningham CK, Barker WH, Kouides RW, Yuen JB, Menegus M, Weiner LB, Bonville CA, Betts RF. 1995. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis 172:389–394. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine S. 1977. Polypeptides of respiratory syncytial virus. J Virol 21:427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kingsbury DW. 1991. The paramyxoviruses. Plenum Press, New York, NY. [Google Scholar]
- 8.Pringle CR, Shirodaria PV, Gimenez HB, Levine S. 1981. Antigen and polypeptide synthesis by temperature-sensitive mutants of respiratory syncytial virus. J Gen Virol 54:173–183. doi: 10.1099/0022-1317-54-1-173. [DOI] [PubMed] [Google Scholar]
- 9.Elango N, Prince GA, Murphy BR, Venkatesan S, Chanock RM, Moss B. 1986. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci U S A 83:1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karron RA, Buonagurio DA, Georgiu AF, Whitehead SS, Adamus JE, Clements-Mann ML, Harris DO, Randolph VB, Udem SA, Murphy BR, Sidhu MS. 1997. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci U S A 94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Techaarpornkul S, Barretto N, Peeples ME. 2001. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J Virol 75:6825–6834. doi: 10.1128/JVI.75.15.6825-6834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwilas S, Liesman RM, Zhang L, Walsh E, Pickles RJ, Peeples ME. 2009. Respiratory syncytial virus grown in Vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J Virol 83:10710–10718. doi: 10.1128/JVI.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright PF, Ikizler MR, Gonzales RA, Carroll KN, Johnson JE, Werkhaven JA. 2005. Growth of respiratory syncytial virus in primary epithelial cells from the human respiratory tract. J Virol 79:8651–8654. doi: 10.1128/JVI.79.13.8651-8654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaap-Nutt A, Scull MA, Schmidt AC, Murphy BR, Pickles RJ. 2010. Growth restriction of an experimental live attenuated human parainfluenza virus type 2 vaccine in human ciliated airway epithelium in vitro parallels attenuation in African green monkeys. Vaccine 28:2788–2798. doi: 10.1016/j.vaccine.2010.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. [DOI] [PubMed] [Google Scholar]
- 16.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89:405–421. [DOI] [PubMed] [Google Scholar]
- 17.Hall CB, Walsh EE, Long CE, Schnabel KC. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 18.Karron RA, Wright PF, Crowe JE Jr, Clements-Mann ML, Thompson J, Makhene M, Casey R, Murphy BR. 1997. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. J Infect Dis 176:1428–1436. doi: 10.1086/514138. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead SS, Firestone CY, Karron RA, Crowe JE, Elkins WR, Collins PL, Murphy BR. 1999. Addition of a missense mutation present in the L gene of respiratory syncytial virus (RSV) cpts530/1030 to RSV vaccine candidate cpts248/404 increases its attenuation and temperature sensitivity. J Virol 73:871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE Jr, Boyce TG, Halburnt LL, Reed GW, Whitehead SS, Anderson EL, Wittek AE, Casey R, Eichelberger M, Thumar B, Randolph VB, Udem SA, Chanock RM, Murphy BR. 2000. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis 182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 21.Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, Polack FP, Randolph VB, Deatly A, Hackell J, Gruber W, Murphy BR, Collins PL. 2005. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis 191:1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 22.Wright PF, Karron RA, Madhi SA, Treanor JJ, King JC, O'Shea A, Ikizler MR, Zhu Y, Collins PL, Cutland C, Randolph VB, Deatly AM, Hackell JG, Gruber WC, Murphy BR. 2006. The interferon antagonist NS2 protein of respiratory syncytial virus is an important virulence determinant for humans. J Infect Dis 193:573–581. doi: 10.1086/499600. [DOI] [PubMed] [Google Scholar]
- 23.Malkin E, Yogev R, Abughali N, Sliman J, Wang CK, Zuo F, Yang CF, Eickhoff M, Esser MT, Tang RS, Dubovsky F. 2013. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLoS One 8:e77104. doi: 10.1371/journal.pone.0077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desmyter J, Melnick JL, Rawls WE. 1968. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J Virol 2:955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diederich S, Dietzel E, Maisner A. 2009. Nipah virus fusion protein: influence of cleavage site mutations on the cleavability by cathepsin L, trypsin and furin. Virus Res 145:300–306. doi: 10.1016/j.virusres.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diederich S, Thiel L, Maisner A. 2008. Role of endocytosis and cathepsin-mediated activation in Nipah virus entry. Virology 375:391–400. doi: 10.1016/j.virol.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Utley TJ, Ducharme NA, Varthakavi V, Shepherd BE, Santangelo PJ, Lindquist ME, Goldenring JR, Crowe JE Jr. 2008. Respiratory syncytial virus uses a Vps4-independent budding mechanism controlled by Rab11-FIP2. Proc Natl Acad Sci U S A 105:10209–10214. doi: 10.1073/pnas.0712144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brock SC, Goldenring JR, Crowe JE Jr. 2003. Apical recycling systems regulate directional budding of respiratory syncytial virus from polarized epithelial cells. Proc Natl Acad Sci U S A 100:15143–15148. doi: 10.1073/pnas.2434327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. 2005. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107:183–206. [DOI] [PubMed] [Google Scholar]
- 30.Juhasz K, Whitehead SS, Bui PT, Biggs JM, Crowe JE, Boulanger CA, Collins PL, Murphy BR. 1997. The temperature-sensitive (ts) phenotype of a cold-passaged (cp) live attenuated respiratory syncytial virus vaccine candidate, designated cpts530, results from a single amino acid substitution in the L protein. J Virol 71:5814–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci U S A 92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh EE, Hall CB, Schlesinger JJ, Brandriss MW, Hildreth S, Paradiso P. 1989. Comparison of antigenic sites of subtype-specific respiratory syncytial virus attachment proteins. J Gen Virol 70(Pt 11):2953–2961. doi: 10.1099/0022-1317-70-11-2953. [DOI] [PubMed] [Google Scholar]
- 33.Walsh EE, Falsey AR, Sullender WM. 1998. Monoclonal antibody neutralization escape mutants of respiratory syncytial virus with unique alterations in the attachment (G) protein. J Gen Virol 79(Pt 3):479–487. doi: 10.1099/0022-1317-79-3-479. [DOI] [PubMed] [Google Scholar]
- 34.Gal S, Gottesman MM. 1988. Isolation and sequence of a cDNA for human pro-(cathepsin L). Biochem J 253:303–306. doi: 10.1042/bj2530303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason RW, Green GD, Barrett AJ. 1985. Human liver cathepsin L. Biochem J 226:233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chauhan SS, Popescu NC, Ray D, Fleischmann R, Gottesman MM, Troen BR. 1993. Cloning, genomic organization, and chromosomal localization of human cathepsin L. J Biol Chem 268:1039–1045. [PubMed] [Google Scholar]
- 37.Joseph LJ, Chang LC, Stamenkovich D, Sukhatme VP. 1988. Complete nucleotide and deduced amino acid sequences of human and murine preprocathepsin L. An abundant transcript induced by transformation of fibroblasts. J Clin Invest 81:1621–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goulet B, Baruch A, Moon NS, Poirier M, Sansregret LL, Erickson A, Bogyo M, Nepveu A. 2004. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol Cell 14:207–219. doi: 10.1016/S1097-2765(04)00209-6. [DOI] [PubMed] [Google Scholar]
- 39.Ritonja A, Popovic T, Kotnik M, Machleidt W, Turk V. 1988. Amino acid sequences of the human kidney cathepsins H and L. FEBS Lett 228:341–345. doi: 10.1016/0014-5793(88)80028-0. [DOI] [PubMed] [Google Scholar]
- 40.Ishidoh K, Kominami E. 1995. Procathepsin L degrades extracellular matrix proteins in the presence of glycosaminoglycans in vitro. Biochem Biophys Res Commun 217:624–631. doi: 10.1006/bbrc.1995.2820. [DOI] [PubMed] [Google Scholar]
- 41.Nomura T, Fujisawa Y. 1997. Processing properties of recombinant human procathepsin L. Biochem Biophys Res Commun 230:143–146. doi: 10.1006/bbrc.1996.5905. [DOI] [PubMed] [Google Scholar]
- 42.Salminen A, Gottesman MM. 1990. Inhibitor studies indicate that active cathepsin L is probably essential to its own processing in cultured fibroblasts. Biochem J 272:39–44. doi: 10.1042/bj2720039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura Y, Kawabata T, Furuno K, Kato K. 1989. Evidence that aspartic proteinase is involved in the proteolytic processing event of procathepsin L in lysosomes. Arch Biochem Biophys 271:400–406. doi: 10.1016/0003-9861(89)90289-0. [DOI] [PubMed] [Google Scholar]
- 44.Hendricks DA, Baradaran K, McIntosh K, Patterson JL. 1987. Appearance of a soluble form of the G protein of respiratory syncytial virus in fluids of infected cells. J Gen Virol 68(Pt 6):1705–1714. doi: 10.1099/0022-1317-68-6-1705. [DOI] [PubMed] [Google Scholar]
- 45.Hendricks DA, McIntosh K, Patterson JL. 1988. Further characterization of the soluble form of the G glycoprotein of respiratory syncytial virus. J Virol 62:2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts SR, Lichtenstein D, Ball LA, Wertz GW. 1994. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J Virol 68:4538–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escribano-Romero E, Rawling J, Garcia-Barreno B, Melero JA. 2004. The soluble form of human respiratory syncytial virus attachment protein differs from the membrane-bound form in its oligomeric state but is still capable of binding to cell surface proteoglycans. J Virol 78:3524–3532. doi: 10.1128/JVI.78.7.3524-3532.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Julenius K, Molgaard A, Gupta R, Brunak S. 2005. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 15:153–164. [DOI] [PubMed] [Google Scholar]
- 49.Puzer L, Cotrin SS, Alves MF, Egborge T, Araujo MS, Juliano MA, Juliano L, Bromme D, Carmona AK. 2004. Comparative substrate specificity analysis of recombinant human cathepsin V and cathepsin L. Arch Biochem Biophys 430:274–283. doi: 10.1016/j.abb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Olson JK, Grose C. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol 71:4042–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdel Motal UM, Sentman CL, Zhou X, Robinson PJ, Dahmen J, Jondal M. 1995. Glycosylphosphatidylinositol-linked Db does not induce an influenza-specific cytotoxic T lymphocyte response or recycle membrane-bound peptides. Eur J Immunol 25:1121–1124. doi: 10.1002/eji.1830250441. [DOI] [PubMed] [Google Scholar]
- 52.Hedman K, Goldenthal KL, Rutherford AV, Pastan I, Willingham MC. 1987. Comparison of the intracellular pathways of transferrin recycling and vesicular stomatitis virus membrane glycoprotein exocytosis by ultrastructural double-label cytochemistry. J Histochem Cytochem 35:233–243. doi: 10.1177/35.2.3025294. [DOI] [PubMed] [Google Scholar]
- 53.Tirabassi RS, Enquist LW. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J Virol 72:4571–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olson JK, Grose C. 1998. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J Virol 72:1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, Feng Y, Chen D, Wandinger-Ness A. 1998. Rab11 is required for trans-Golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell 9:3241–3257. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogt C, Eickmann M, Diederich S, Moll M, Maisner A. 2005. Endocytosis of the Nipah virus glycoproteins. J Virol 79:3865–3872. doi: 10.1128/JVI.79.6.3865-3872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moll M, Diederich S, Klenk HD, Czub M, Maisner A. 2004. Ubiquitous activation of the Nipah virus fusion protein does not require a basic amino acid at the cleavage site. J Virol 78:9705–9712. doi: 10.1128/JVI.78.18.9705-9712.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rowe RK, Suszko JW, Pekosz A. 2008. Roles for the recycling endosome, Rab8, and Rab11 in hantavirus release from epithelial cells. Virology 382:239–249. doi: 10.1016/j.virol.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beitia Ortiz de Zarate I, Cantero-Aguilar L, Longo M, Berlioz-Torrent C, Rozenberg F. 2007. Contribution of endocytic motifs in the cytoplasmic tail of herpes simplex virus type 1 glycoprotein B to virus replication and cell-cell fusion. J Virol 81:13889–13903. doi: 10.1128/JVI.01231-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krzyzaniak MA, Mach M, Britt WJ. 2009. HCMV-encoded glycoprotein M (UL100) interacts with Rab11 effector protein FIP4. Traffic 10:1439–1457. doi: 10.1111/j.1600-0854.2009.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruce EA, Digard P, Stuart AD. 2010. The Rab11 pathway is required for influenza A virus budding and filament formation. J Virol 84:5848–5859. doi: 10.1128/JVI.00307-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zenner HL, Yoshimura S, Barr FA, Crump CM. 2011. Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for herpes simplex virus 1 secondary envelopment. J Virol 85:8012–8021. doi: 10.1128/JVI.00500-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakatsu Y, Ma X, Seki F, Suzuki T, Iwasaki M, Yanagi Y, Komase K, Takeda M. 2013. Intracellular transport of the measles virus ribonucleoprotein complex is mediated by Rab11A-positive recycling endosomes and drives virus release from the apical membrane of polarized epithelial cells. J Virol 87:4683–4693. doi: 10.1128/JVI.02189-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diederich S, Sauerhering L, Weis M, Altmeppen H, Schaschke N, Reinheckel T, Erbar S, Maisner A. 2012. Activation of the Nipah virus fusion protein in MDCK cells is mediated by cathepsin B within the endosome-recycling compartment. J Virol 86:3736–3745. doi: 10.1128/JVI.06628-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diederich S, Moll M, Klenk HD, Maisner A. 2005. The Nipah virus fusion protein is cleaved within the endosomal compartment. J Biol Chem 280:29899–29903. doi: 10.1074/jbc.M504598200. [DOI] [PubMed] [Google Scholar]
- 66.Guha S, Padh H. 2008. Cathepsins: fundamental effectors of endolysosomal proteolysis. Indian J Biochem Biophys 45:75–90. [PubMed] [Google Scholar]
- 67.Bourgeois C, Bour JB, Lidholt K, Gauthray C, Pothier P. 1998. Heparin-like structures on respiratory syncytial virus are involved in its infectivity in vitro. J Virol 72:7221–7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hallak LK, Spillmann D, Collins PL, Peeples ME. 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 74:10508–10513. doi: 10.1128/JVI.74.22.10508-10513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feldman SA, Audet S, Beeler JA. 2000. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J Virol 74:6442–6447. doi: 10.1128/JVI.74.14.6442-6447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hallak LK, Collins PL, Knudson W, Peeples ME. 2000. Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection. Virology 271:264–275. doi: 10.1006/viro.2000.0293. [DOI] [PubMed] [Google Scholar]
- 71.Feldman SA, Hendry RM, Beeler JA. 1999. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J Virol 73:6610–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shields B, Mills J, Ghildyal R, Gooley P, Meanger J. 2003. Multiple heparin binding domains of respiratory syncytial virus G mediate binding to mammalian cells. Arch Virol 148:1987–2003. doi: 10.1007/s00705-003-0139-0. [DOI] [PubMed] [Google Scholar]
- 73.Walsh EE, Schlesinger JJ, Brandriss MW. 1984. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun 43:756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Collins PL, Wertz GW. 1985. Nucleotide sequences of the 1B and 1C nonstructural protein mRNAs of human respiratory syncytial virus. Virology 143:442–451. doi: 10.1016/0042-6822(85)90384-8. [DOI] [PubMed] [Google Scholar]
- 75.Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL, Pickles RJ. 2005. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol 79:1113–1124. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2:732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 77.Jeong KI, Piepenhagen PA, Kishko M, DiNapoli JM, Groppo RP, Zhang L, Almond J, Kleanthous H, Delagrave S, Parrington M. 2015. CX3CR1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on cilia in a G protein-dependent manner. PLoS One 10:e0130517. doi: 10.1371/journal.pone.0130517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trento A, Galiano M, Videla C, Carballal G, Garcia-Barreno B, Melero JA, Palomo C. 2003. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J Gen Virol 84:3115–3120. doi: 10.1099/vir.0.19357-0. [DOI] [PubMed] [Google Scholar]
- 79.de-Paris F, Beck C, de Souza Nunes L, Machado AB, Paiva RM, da Silva Menezes D, Pires MR, dos Santos RP, de Souza Kuchenbecker R, Barth AL. 2014. Evaluation of respiratory syncytial virus group A and B genotypes among nosocomial and community-acquired pediatric infections in southern Brazil. Virol J 11:36. doi: 10.1186/1743-422X-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choudhary ML, Anand SP, Wadhwa BS, Chadha MS. 2013. Genetic variability of human respiratory syncytial virus in Pune, western India. Infect Genet Evol 20:369–377. doi: 10.1016/j.meegid.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 81.Visser A, Delport S, Venter M. 2008. Molecular epidemiological analysis of a nosocomial outbreak of respiratory syncytial virus associated pneumonia in a kangaroo mother care unit in South Africa. J Med Virol 80:724–732. doi: 10.1002/jmv.21128. [DOI] [PubMed] [Google Scholar]
- 82.Zhang ZY, Du LN, Chen X, Zhao Y, Liu EM, Yang XQ, Zhao XD. 2010. Genetic variability of respiratory syncytial viruses (RSV) prevalent in southwestern China from 2006 to 2009: emergence of subgroup B and A RSV as dominant strains. J Clin Microbiol 48:1201–1207. doi: 10.1128/JCM.02258-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pretorius MA, van Niekerk S, Tempia S, Moyes J, Cohen C, Madhi SA, Venter M, SARI Surveillance Group . 2013. Replacement and positive evolution of subtype A and B respiratory syncytial virus G-protein genotypes from 1997-2012 in South Africa. J Infect Dis 208(Suppl 3):S227–S237. doi: 10.1093/infdis/jit477. [DOI] [PubMed] [Google Scholar]
- 84.Tran DN, Pham TM, Ha MT, Tran TT, Dang TK, Yoshida LM, Okitsu S, Hayakawa S, Mizuguchi M, Ushijima H. 2013. Molecular epidemiology and disease severity of human respiratory syncytial virus in Vietnam. PLoS One 8:e45436. doi: 10.1371/journal.pone.0045436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Auksornkitti V, Kamprasert N, Thongkomplew S, Suwannakarn K, Theamboonlers A, Samransamruajkij R, Poovorawan Y. 2014. Molecular characterization of human respiratory syncytial virus, 2010-2011: identification of genotype ON1 and a new subgroup B genotype in Thailand. Arch Virol 159:499–507. doi: 10.1007/s00705-013-1773-9. [DOI] [PubMed] [Google Scholar]
- 86.Tabatabai J, Prifert C, Pfeil J, Grulich-Henn J, Schnitzler P. 2014. Novel respiratory syncytial virus (RSV) genotype ON1 predominates in Germany during winter season 2012-13. PLoS One 9:e109191. doi: 10.1371/journal.pone.0109191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pierangeli A, Trotta D, Scagnolari C, Ferreri ML, Nicolai A, Midulla F, Marinelli K, Antonelli G, Bagnarelli P. 2014. Rapid spread of the novel respiratory syncytial virus A ON1 genotype, central Italy, 2011 to 2013. Euro Surveill 19(26):pii=20843 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20843. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol 76:5654–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson SM, McNally BA, Ioannidis I, Flano E, Teng MN, Oomens AG, Walsh EE, Peeples ME. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathogens, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]