Abstract
Newcastle disease virus (NDV) expressing HIV-1 BaL gp160 was evaluated either alone or with monomeric BaL gp120 and BaL SOSIP gp140 protein in a prime-boost combination in guinea pigs to enhance envelope (Env)-specific humoral and mucosal immune responses. We showed that a regimen consisting of an NDV prime followed by a protein boost elicited stronger serum and mucosal Th-1-biased IgG responses and neutralizing antibody responses than NDV-only immunizations. Additionally, these responses were higher after the gp120 than after the SOSIP gp140 protein boost.
TEXT
It is believed that an effective vaccine against HIV-1 should induce potent systemic and mucosal immune responses. Therefore, the use of live-virus vectored vaccines capable of replicating at mucosal surface has become very attractive. To induce robust antibody (Ab) responses, several of these vectored vaccines are being evaluated either alone or in several prime-boost combinations using different forms of HIV envelope (Env) protein (1–4). It has been reported that a viral vector prime and protein boost regiment could elicit protective immunity to HIV-1 in nonhuman primates (4–7) and, recently, in humans in an RV-144 vaccine trial (8). Among the different viral vector systems under evaluation for HIV, Newcastle disease virus (NDV), an avian paramyxovirus, has the characteristics desired for an HIV-1 vaccine. There is no preexisting immunity to NDV in humans. NDV infects via the intranasal and oral routes and induces both mucosal and systemic immune responses (9–17). Previously, we demonstrated the potential of NDV as a vaccine vector for HIV-1 (14–17). However, the concept of an NDV vector prime followed by Env protein boost to increase immune responses to HIV has never been evaluated previously.
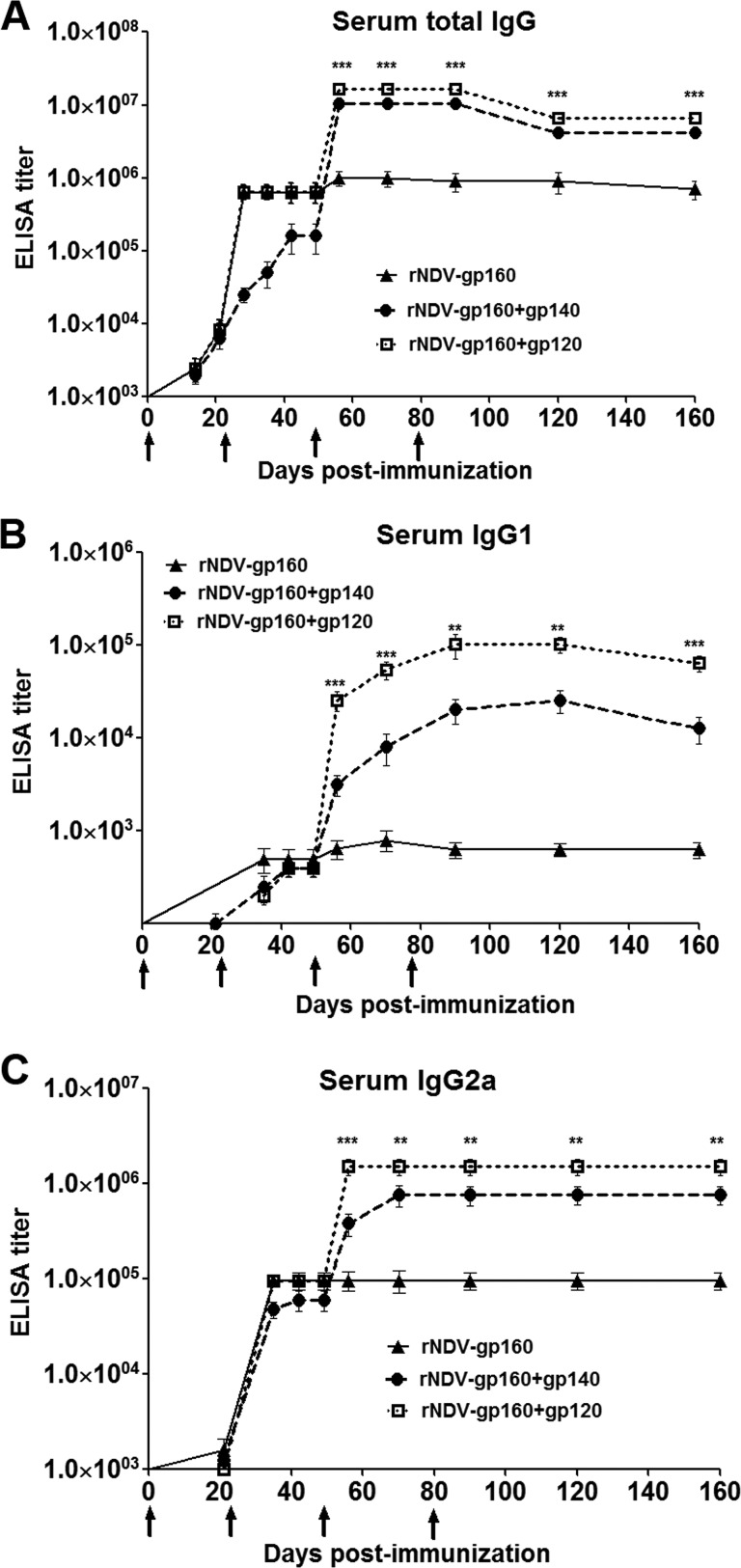
In order to identify an improved vaccination regimen that elicits a higher level of anti-HIV humoral as well as mucosal immune responses, an avirulent recombinant NDV (rNDV) strain, LaSota, expressing gp160 of HIV-1 strain BaL.1 was used as a prime followed by a boost with purified monomeric gp120 and trimeric SOSIP gp140 proteins in this study. The construction and characterization of rNDV expressing gp160 (rNDV-gp160) were described before (16). Briefly, the gp160 protein expressed by rNDV was detected on infected cell surfaces and was also incorporated into the NDV virion. Further, gp160 present in infected cells and in the virion formed oligomers which were recognized by conformationally dependent monoclonal Abs (MAbs). The BaL gp120 and SOSIP gp140 proteins (18) were produced as described previously (19). The BaL SOSIP gp140 protein has been characterized by Dey et al. (19), and they demonstrated that BaL SOSIP gp140, expressed in HEK 293 cells, was a mixture of monomers, dimers, and trimers. Guinea pigs were used to evaluate the humoral and mucosal immune responses induced by this vaccine regimen. Female Hartley guinea pigs obtained from Charles River Laboratories were assigned to four groups (n = 3/group) as shown in Fig. 1. Each animal in all the groups received a dose of 200 μl (100 μl in each nostril) of allantoic fluid containing 106 PFU/ml of rNDV. The animals in the parental rNDV (control) group were primed with parental rNDV on day 0 and boosted with parental rNDV on days 21, 49, and 79 via the intranasal (i.n.) route. The animals in the rNDV-gp160, rNDV-gp160 plus gp120 (rNDV-gp160+gp120), and rNDV-gp160+gp140 groups were primed and boosted with rNDV-gp160 on days 0 and 21 via the i.n. route. The animals in rNDV-gp160 group were further boosted with rNDV-gp160 on days 49 and 79 via the i.n. route, whereas each animal in the rNDV-gp160+gp120 and rNDV-gp160+gp140 groups was boosted via the intramuscular route with 50 μg of gp120 protein and SOSIP gp140 protein formulated in Montanide ISA 50 V2 adjuvant (Seppic Inc., NJ), respectively. The immunized animals did not show any overt clinical signs of infection or any loss of body weight throughout the study, indicating that the rNDVs were avirulent in the guinea pigs. The induction of NDV-specific serum antibodies was measured on days 35, 56, and 160 using a commercial NDV enzyme-linked immunosorbent assay (ELISA) kit. All four animal groups exhibited similar levels of NDV-specific IgG antibodies on these days (data not shown), suggesting that all the viruses replicated to the same extent in the immunized animals. The induction of HIV-1 Env-specific total IgG, IgG1, and IgG2 in serum was measured on days 21, 28, 35, 42, 49, 56, 70, 90, 120, and 160 by ELISA as described previously (16). Env-specific responses were detected on day 21 following the initial immunization in all of the groups except the parental rNDV group (Fig. 2). The rNDV-gp160 boost on day 21 was followed by increased immune responses in all the groups that peaked between days 28 to 42. The second rNDV-160 boost on day 49 resulted in marginal increases of total IgG and IgG1 titers. In contrast, when the rNDV-gp160+gp120 and rNDV-gp160+gp140 groups were boosted with gp120 and gp140 protein, respectively, the total IgG, IgG1, and IgG2a titers were increased significantly in these groups compared to the group immunized with only rNDV-gp160. The highest total IgG, IgG1, and IgG2a titers were observed in the rNDV-gp160+gp120 group, with increases of 17-fold, 39-fold to 68-fold, and 16-fold, respectively, compared to 11-fold, 5-fold to 10-fold, and 4-fold to 8-fold increases after the SOSIP gp140 protein boost from day 56 to day 70. Similar to the results reported by us previously (16), a strong IgG2a response and a weaker IgG1 response were seen in all the groups immunized with rNDV-160 that were consistent with a Th1-biased antibody response in these groups. Interestingly, after a boost with gp120 and SOSIP gp140 protein, the antibody responses remained Th1 biased in the rNDV-gp160+gp120 and rNDV-gp160+gp140 groups. The ratios of IgG1 to IgG2a measured on day 70 in the different groups ranged from 1:28 to 1:120. Induction of Th1-biased immune responses to HIV-1 is advantageous, as Th1 cells induce gamma interferon (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor alpha (TNF-α) cytokines, which are capable of stimulating CD8+ T cells.
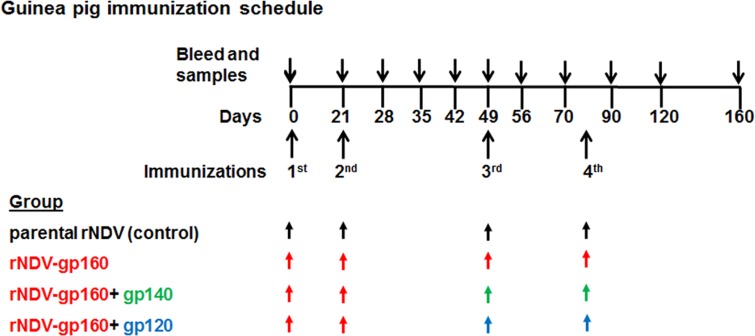
FIG 1.
Guinea pig immunization schedule. Twelve guinea pigs were assigned to 4 groups (n = 3/group). Animals in each group except the parental rNDV control group were immunized with two doses of recombinant NDV expressing gp160 on days 0 and 21 by the intranasal (i.n.) route of administration. Each i.n. dose consisted of 200 μl (100 μl in each nostril) of allantoic fluid containing 106 PFU/ml of virus. Animals in the rNDV-gp160 group were further boosted on days 49 and 79 with rNDV expressing gp160, whereas animals in the rNDV-160+gp120 group and the rNDV-gp160+gp140 group were boosted with 50 μg of purified gp120 protein and 50 μg purified SOSIP gp140 protein formulated in Montanide ISA 50 V2 adjuvant, respectively, via the intramuscular route. The animals in the parental rNDV control group were immunized on days 0, 21, 49, and 79 with empty rNDV vector without any insertion. Blood and vaginal washes were collected on the indicated days. All animals were sacrificed on day 160.
FIG 2.
Immune responses to HIV Env protein in sera from guinea pigs immunized with the rNDVs expressing gp160 (rNDV-gp160) by the i.n. route on days 0 and 21 in all the groups and boosted on days 49 and 79 with either rNDV-gp160 by the i.n. route in rNDV-gp160 group or purified gp120 and SOSIP gp140 proteins by the intramuscular (i.m.) route in the rNDV-gp160+gp120 and the rNDV-gp160+gp140 groups, respectively. HIV-1 gp120-specific total IgG (A), IgG1 (B), and IgG2a (C) serum antibody responses on days 0, 21, 28, 35, 42, 49, 56, 70, 90, 120, and 160 were analyzed by ELISA using purified gp120. ELISA titers were endpoint values and are defined as the last reciprocal serum dilutions at which the optical density was greater than 2-fold over the signal detected with the parental rNDV (control). Values plotted are the geometric mean titers ± standard errors of the means (SEM) for the 3 animals in each group. Antibodies specific to gp120 were not detected in any animal on any day in the parental rNDV control group. Arrows indicate times of rNDV and purified protein immunizations on days 0, 21, 49, and 79. Statistical differences between the results from all the groups were calculated by one-way analysis of variance (ANOVA). **, P value < 0.005; ***, P value < 0.0005.
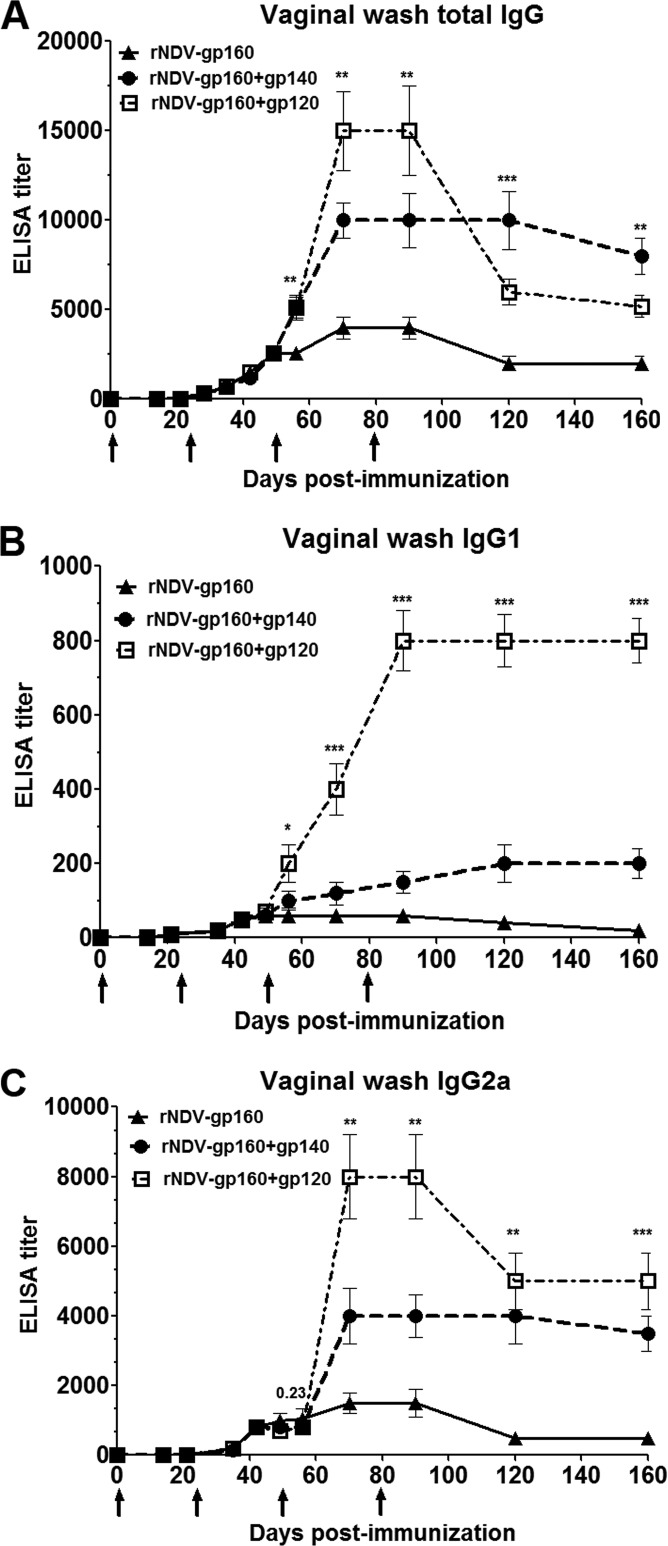
Next, we investigated whether the immunization regimens elicited virus-specific antibody responses at mucosal surfaces. Vaginal washes were collected from each animal at the indicated time points and evaluated for total IgG, IgG1, and IgG2a titers by ELISA (Fig. 3). A low titer was detected until day 49 in all the groups, but a boost on day 49 increased the titers significantly in the rNDV-gp160+gp120 and rNDV-gp160+gp140 groups compared to the results seen with the rNDV-gp160 group. The total IgG, IgG1, and IgG2a titers were increased by 2-fold to 4-fold, 3-fold to 13-fold, and 5-fold, respectively, in the rNDV-gp160+gp120 group compared to 2-fold to 2.5-fold, 1.6-fold to 2.5-fold, and 2.6-fold increases in the rNDV-gp160+gp140 group from day 56 to day 70. Similar to the serum IgG antibody isotypes described above, the IgG isotype analysis in vaginal washes provided evidence of a Th1-biased antibody response that remained Th1 biased even after protein boosts. The second dose of protein on day 79 did not result in an increase of humoral and mucosal immune responses, suggesting that a longer time interval may be required between the two protein boosts. We also checked the durability of humoral and mucosal immune responses until day 160 as shown in Fig. 2 and 3. Whereas the titer of Env-specific IgG in serum was sustained at the peak response level for at least 160 days, there was a marginal decline in the mucosal IgG titer in vaginal washes. Recently, we demonstrated that immunization of guinea pigs with rNDV expressing gp160 without any protein boost elicited long-lasting systemic and mucosal immune responses to HIV (14). These findings indicate that the long-lasting antibody response may be strongly influenced by the use of rNDV vector as a prime. In addition, various reports have shown that multiple immunizations with protein immunogens are required to induce a long-term immune response to HIV Env protein in guinea pigs and rabbits (3, 20, 21). In one of these reports, Shu et al. (3) showed that a single chimeric clade B Env (HxB2/Balgp140) protein immunization significantly boosted the antibody titer in guinea pigs primed with either DNA or recombinant adenovirus type 5 (rAd5) vaccine and attained a antibody titer similar to those achieved after three sequential protein immunizations. Compared with the mucosal immune response induced by SOSIP gp140 protein boost, the response induced by gp120 protein boost declined over time and plateaued near the level of the SOSIP gp140 boost response.
FIG 3.
Immune responses to HIV Env protein in vaginal washes from guinea pigs immunized with the indicated rNDVs and proteins as described for Fig. 1. HIV-1 gp120-specific total IgG (A), IgG1 (B), and IgG2a (C) responses in vaginal washes on different days were analyzed by ELISA using purified gp120. Graphs show the geometric mean titer ± SEM for the 3 animals in each group. Antibodies specific to gp120 were not detected in any animal on any day in the parental rNDV control group. Arrows indicate times of rNDV and purified protein immunizations on days 0, 21, 49, and 79. Statistical differences between the results from all the groups were calculated by one-way ANOVA. *, P value < 0.05; **, P value < 0.005; ***, P value < 0.0005.
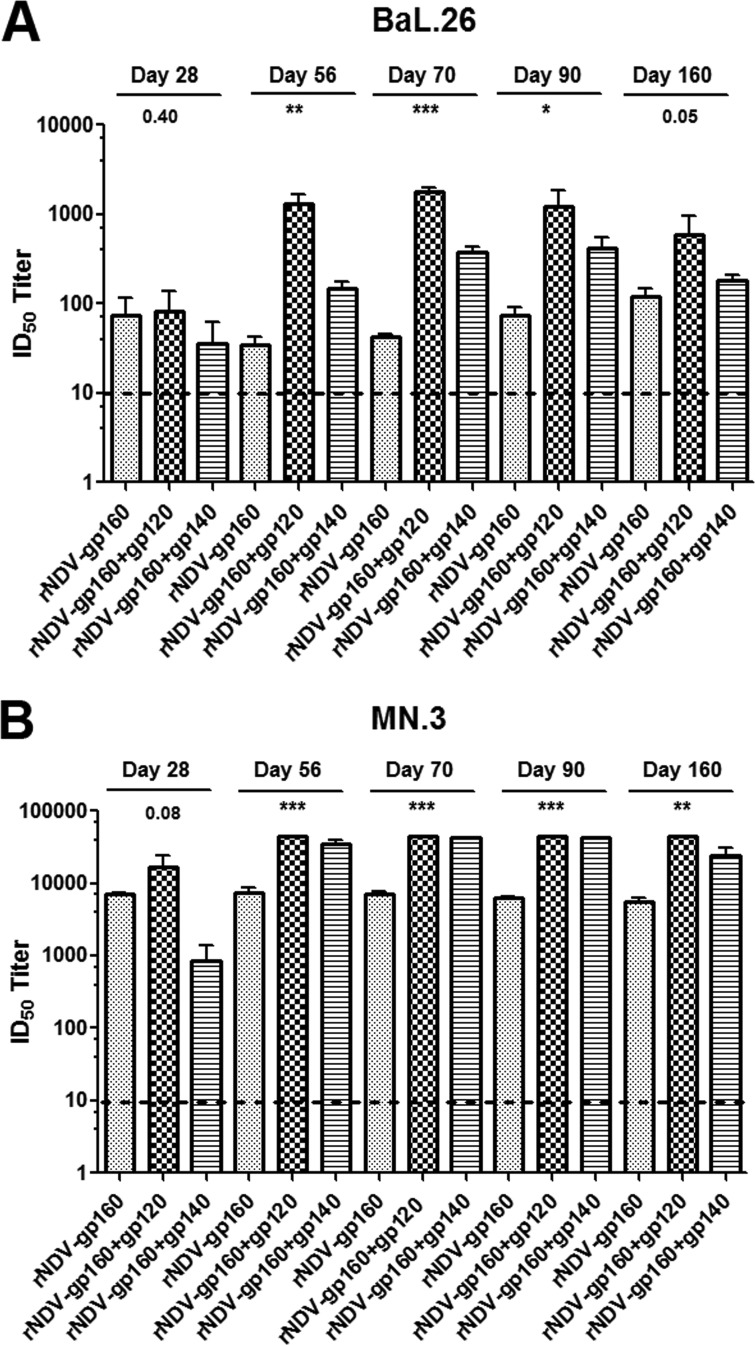
Sera collected on days 28, 56, 70, 90, and 160 were evaluated by the TZM.bl assay (22) for their ability to neutralize homologous clade B tier 1B HIV-1 strain BaL.26 (Fig. 4A) and heterologous clade B tier 1A HIV-1 strain MN.3 (Fig. 4B). Neutralizing Ab (NAb) activity (expressed as ID50 values) against BaL.26 was detected in sera from all groups of animals. However, after a first protein boost on day 49, the NAb response increased significantly compared to the response in the rNDV-gp160 group, with the increases ranging from 16-fold to 38-fold and from 4.3-fold to 9-fold from day 56 to day 90 following a boost with gp120 and SOSIP gp140 protein, respectively. NAb titers persisted at higher levels, with 5-fold and 1.5-fold increases on day 160 in the rNDV-gp160+gp120 and rNDV-gp160+gp140 groups, respectively, compared to the rNDV-gp160 group. The NAb response to HIV-1 strain MN.3 in immunized guinea pigs was significantly higher in the different groups than the response to BaL.26. Compared to the rNDV-gp160-immunized group, the rNDV-gp160+gp120 group showed the strongest NAb response from day 56 to day 160, with increases of 5.9-fold to 7.9-fold in mean ID50 values, followed by the rNDV-gp160+gp140 group, with 4.2-fold to 6.5-fold increases. NAb activity was also evaluated using tier 2 clade B virus strain TRO.11. No response to TRO.11 was observed in any of the immunization groups (data not shown).
FIG 4.
Virus neutralizing antibody activity (50%-inhibitory-dilution [ID50] titers) against homologous HIV-1 clade B tier 1B strains (BaL.26) and heterologous clade B tier 1A strain MN.3 in sera from guinea pigs immunized with the indicated rNDVs and proteins. Guinea pig sera obtained on days 28, 56, 70, 90, and 160 were tested against BaL.26 (A) and MN.3 (B) pseudoviruses by the TZM-bl assay. Preimmune sera were used to establish the baseline neutralizing activity in each individual guinea pig, and these values were subtracted from the values shown. The dashed lines denote background titers of <10 against the murine leukemia virus (MLV) negative-control pseudovirus or pooled preimmune sera. The samples that did not neutralize the target virus (titers of <20) were given a value of 10 for plotting purposes; therefore, all samples registering an ID50 titer of 10 represent negative results. Some of the ID50 values against MN.3 induced after a boost with gp120 and SOSIP gp140 proteins on days 56, 70, 90, and 160 were off the scale and were at least 43,740. Statistical differences between the results from all the groups were calculated by one-way ANOVA. *, P value < 0.05; **, P value < 0.005; ***, P value < 0.0005.
In summary, we compared rNDV-gp160 prime and boost, rNDV-160 prime and gp120 protein boost, and rNDV-160 prime and SOSIP gp140 protein boost regimens for induction of systemic and mucosal antibody responses to HIV-1 clade B strain BaL.1. Our results showed that the regimen consisting of an rNDV prime followed by protein immunization stimulated NAb in response to laboratory-adapted MN.3 and homologous Bal.26 strains at a higher magnitude than the regimen consisting of repeated rNDV prime and boost in guinea pigs. In addition, boosting with gp120 protein induced a higher NAb titer than boosting with SOSIP gp140 protein in response to both these strains. However, previous studies have shown that gp140 trimers, including SOSIP trimers, are superior to gp120 monomers at inducing NAb (23–27). The differences in immune responses in our study compared to previous studies may be due to use of different HIV strains, use of different adjuvants, priming with DNA or without any DNA and viral vector, and study of immune responses in different animal species. Another reason for the lower immune response induced by SOSIP gp140 protein may have been the presence of a heterogeneous population of trimers, dimers, and monomers in the SOSIP gp140 protein used in this study. Further, we found that a rNDV prime and protein boost regimen is valuable for stimulating stronger serum and mucosal IgG responses. Our data demonstrate that an rNDV prime followed by a protein boost represents a promising strategy to enhance immunogenicity against HIV.
ACKNOWLEDGMENTS
This research was supported by NIH R21 grant AI-093198 awarded to S.K.S., A.L.D., and S.K.K. and by NIAID Primate Central Immunology Laboratory contract HHSN27201100016C awarded to D.C.M.
The views expressed here do not necessarily reflect the official policies of the Department of Health and Human Services, nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. government.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Forsell MN, Li Y, Sundback M, Svehla K, Liljestrom P, Mascola JR, Wyatt R, Karlsson Hedestam GB. 2005. Biochemical and immunogenic characterization of soluble human immunodeficiency virus type 1 envelope glycoprotein trimers expressed by Semliki Forest virus. J Virol 79:10902–10914. doi: 10.1128/JVI.79.17.10902-10914.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooney EL, McElrath MJ, Corey L, Hu SL, Collier AC, Arditti D, Hoffman M, Coombs RW, Smith GE, Greenberg PD. 1993. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc Natl Acad Sci U S A 90:1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shu Y, Winfrey S, Yang ZY, Xu L, Rao SS, Srivastava I, Barnett SW, Nabel GJ, Mascola JR. 2007. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine 25:1398–1408. doi: 10.1016/j.vaccine.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu R, Srivastava IK, Greer CE, Zarkikh I, Kraft Z, Kuller L, Polo JM, Barnett SW, Stamatatos L. 2006. Characterization of immune responses elicited in macaques immunized sequentially with chimeric VEE/SIN alphavirus replicon particles expressing SIVGag and/or HIVEnv and with recombinant HIVgp140Env protein. AIDS Res Hum Retroviruses 22:1022–1030. doi: 10.1089/aid.2006.22.1022. [DOI] [PubMed] [Google Scholar]
- 5.Fultz PN, Stallworth J, Porter D, Novak M, Anderson MJ, Morrow CD. 2003. Immunogenicity in pig-tailed macaques of poliovirus replicons expressing HIV-1 and SIV antigens and protection against SHIV-89.6P disease. Virology 315:425–437. doi: 10.1016/S0042-6822(03)00546-4. [DOI] [PubMed] [Google Scholar]
- 6.Barnett SW, Burke B, Sun Y, Kan E, Legg H, Lian Y, Bost K, Zhou F, Goodsell A, Zur Megede J, Polo J, Donnelly J, Ulmer J, Otten GR, Miller CJ, Vajdy M, Srivastava IK. 2010. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J Virol 84:5975–5985. doi: 10.1128/JVI.02533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, Shields J, Parenteau L, Whitney JB, Abbink P, Ng‘ang'a DM, Seaman MS, Lavine CL, Perry JR, Li W, Colantonio AD, Lewis MG, Chen B, Wenschuh H, Reimer U, Piatak M, Lifson JD, Handley SA, Virgin HW, Koutsoukos M, Lorin C, Voss G, Weijtens M, Pau MG, Schuitemaker H. 2015. HIV-1 vaccines. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349:320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 9.Bukreyev A, Huang Z, Yang L, Elankumaran S, St Claire M, Murphy BR, Samal SK, Collins PL. 2005. Recombinant Newcastle disease virus expressing a foreign viral antigen is attenuated and highly immunogenic in primates. J Virol 79:13275–13284. doi: 10.1128/JVI.79.21.13275-13284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiNapoli JM, Kotelkin A, Yang L, Elankumaran S, Murphy BR, Samal SK, Collins PL, Bukreyev A. 2007. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc Natl Acad Sci U S A 104:9788–9793. doi: 10.1073/pnas.0703584104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiNapoli JM, Nayak B, Yang L, Finneyfrock BW, Cook A, Andersen H, Torres-Velez F, Murphy BR, Samal SK, Collins PL, Bukreyev A. 2010. Newcastle disease virus-vectored vaccines expressing the hemagglutinin or neuraminidase protein of H5N1 highly pathogenic avian influenza virus protect against virus challenge in monkeys. J Virol 84:1489–1503. doi: 10.1128/JVI.01946-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiNapoli JM, Yang L, Samal SK, Murphy BR, Collins PL, Bukreyev A. 2010. Respiratory tract immunization of non-human primates with a Newcastle disease virus-vectored vaccine candidate against Ebola virus elicits a neutralizing antibody response. Vaccine 29:17–25. doi: 10.1016/j.vaccine.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiNapoli JM, Yang L, Suguitan A Jr, Elankumaran S, Dorward DW, Murphy BR, Samal SK, Collins PL, Bukreyev A. 2007. Immunization of primates with a Newcastle disease virus-vectored vaccine via the respiratory tract induces a high titer of serum neutralizing antibodies against highly pathogenic avian influenza virus. J Virol 81:11560–11568. doi: 10.1128/JVI.00713-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khattar SK, Manoharan V, Bhattarai B, LaBranche CC, Montefiori DC, Samal SK. 2015. Mucosal immunization with Newcastle disease virus vector coexpressing HIV-1 Env and Gag proteins elicits potent serum, mucosal, and cellular immune responses that protect against vaccinia virus Env and Gag challenges. mBio 6:e01005. doi: 10.1128/mBio.01005-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khattar SK, Palaniyandi S, Samal S, LaBranche CC, Montefiori DC, Zhu X, Samal SK. 2015. Evaluation of humoral, mucosal, and cellular immune responses following co-immunization of HIV-1 Gag and Env proteins expressed by Newcastle disease virus. Hum Vaccin Immunother 11:504–515. doi: 10.4161/21645515.2014.987006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khattar SK, Samal S, Devico AL, Collins PL, Samal SK. 2011. Newcastle disease virus expressing human immunodeficiency virus type 1 envelope glycoprotein induces strong mucosal and serum antibody responses in Guinea pigs. J Virol 85:10529–10541. doi: 10.1128/JVI.05050-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Khattar SK, Samal S, LaBranche CC, Montefiori DC, Collins PL, Samal SK. 2013. Comparative immunogenicity of HIV-1 gp160, gp140 and gp120 expressed by live attenuated Newcastle disease virus vector. PLoS One 8:e78521. doi: 10.1371/journal.pone.0078521. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Sajadi MM, Lewis GK, Seaman MS, Guan Y, Redfield RR, DeVico AL. 2012. Signature biochemical properties of broadly cross-reactive HIV-1 neutralizing antibodies in human plasma. J Virol 86:5014–5025. doi: 10.1128/JVI.06547-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dey AK, David KB, Klasse PJ, Moore JP. 2007. Specific amino acids in the N-terminus of the gp41 ectodomain contribute to the stabilization of a soluble, cleaved gp140 envelope glycoprotein from human immunodeficiency virus type 1. Virology 360:199–208. doi: 10.1016/j.virol.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sneha Priya R, Veena M, Kalisz I, Whitney S, Priyanka D, LaBranche CC, Sri Teja M, Montefiori DC, Pal R, Mahalingam S, Kalyanaraman VS. 2015. Antigenicity and immunogenicity of a trimeric envelope protein from an Indian clade C HIV-1 isolate. J Biol Chem 290:9195–9208. doi: 10.1074/jbc.M114.621185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Svehla K, Mathy NL, Voss G, Mascola JR, Wyatt R. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J Virol 80:1414–1426. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang YK, Andjelic S, Binley JM, Crooks ET, Franti M, Iyer SP, Donovan GP, Dey AK, Zhu P, Roux KH, Durso RJ, Parsons TF, Maddon PJ, Moore JP, Olson WC. 2009. Structural and immunogenicity studies of a cleaved, stabilized envelope trimer derived from subtype A HIV-1. Vaccine 27:5120–5132. doi: 10.1016/j.vaccine.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beddows S, Franti M, Dey AK, Kirschner M, Iyer SP, Fisch DC, Ketas T, Yuste E, Desrosiers RC, Klasse PJ, Maddon PJ, Olson WC, Moore JP. 2007. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology 360:329–340. doi: 10.1016/j.virol.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Wyatt R, Sodroski J. 2001. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J Virol 75:1165–1171. doi: 10.1128/JVI.75.3.1165-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bower JF, Yang X, Sodroski J, Ross TM. 2004. Elicitation of neutralizing antibodies with DNA vaccines expressing soluble stabilized human immunodeficiency virus type 1 envelope glycoprotein trimers conjugated to C3d. J Virol 78:4710–4719. doi: 10.1128/JVI.78.9.4710-4719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramirez M, Back JW, Koff WC, Julien JP, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP. 2015. HIV-1 vaccines. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349:aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]