ABSTRACT
Several reports have indicated that natural killer (NK) cells are of particular importance in the innate response against herpesvirus infections. As a consequence, herpesviruses have developed diverse mechanisms for evading NK cells, although few such mechanisms have been identified for the largest herpesvirus subfamily, the alphaherpesviruses. The antiviral activity of NK cells is regulated by a complex array of interactions between activating/inhibitory receptors on the NK cell surface and the corresponding ligands on the surfaces of virus-infected cells. Here we report that the US3 protein kinase of the alphaherpesvirus pseudorabies virus (PRV) displays previously uncharacterized immune evasion properties: it triggers the binding of the inhibitory NK cell receptor CD300a to the surface of the infected cell, thereby providing increased CD300a-mediated protection of infected cells against NK cell-mediated lysis. US3-mediated CD300a binding was found to depend on aminophospholipid ligands of CD300a and on group I p21-activated kinases. These data identify a novel alphaherpesvirus strategy for evading NK cells and demonstrate, for the first time, a role for CD300a in regulating NK cell activity upon contact with virus-infected target cells.
IMPORTANCE Herpesviruses have developed fascinating mechanisms to evade elimination by key elements of the host immune system, contributing to their ability to cause lifelong infections with recurrent reactivation events. Natural killer (NK) cells are central in the innate antiviral response. Here we report that the US3 protein kinase of the alphaherpesvirus pseudorabies virus displays a previously uncharacterized capacity for evasion of NK cells. Expression of US3 protects infected cells from NK cell-mediated lysis via increased binding of the inhibitory NK cell receptor CD300a. We show that this US3-mediated increase in CD300a binding depends on aminophospholipids and on cellular p21-activated kinases (PAKs). The identification of this novel NK cell evasion strategy may contribute to the design of improved herpesvirus vaccines and may also have significance for other PAK- and CD300a-modulating viruses and cancer cells.
INTRODUCTION
Natural killer (NK) cells are components of innate immunity and play a central role in the defense against viral infections and cancer development (1). For herpesviruses in particular, functional NK cells are crucial for limiting virus spread and disease symptoms. Indeed, impaired NK cell activity has been associated with life-threatening encephalitis caused by the human alphaherpesviruses herpes simplex virus 1 (HSV-1) and varicella-zoster virus (VZV) (2–4). Given the strong antiviral potential of NK cells against herpesviruses in particular, it comes as no surprise that several herpesvirus strategies for evading NK cells have been discovered (5). Interestingly, and paradoxically, such evasion strategies have been reported mainly for betaherpesviruses and gammaherpesviruses (5–17), while only three reports to date have described NK cell evasion strategies for the largest herpesvirus subfamily, the alphaherpesviruses (18–20).
NK cells display on their surfaces a diversity of activating and inhibiting germ line-encoded receptors that recognize specific ligands. This allows NK cells to sense a wide array of alterations in the surface profiles of target cells (21, 22). Alterations on the surfaces of virus-infected cells that may trigger NK cell activity include increased expression of stress-induced ligands for activating NK cell receptors and/or suppressed levels of ligands for inhibitory NK cell receptors. The latter is often a consequence of viral evasion of cytotoxic T lymphocytes. Indeed, to interfere with elimination by cytotoxic T lymphocytes, several viruses decrease levels of major histocompatibility complex class I (MHC I) molecules, which represent important ligands for the KIR family of inhibitory NK cell receptors, on the cell surface (23). To tilt the activating/inhibitory NK cell receptor balance to their own benefit, viruses may encode proteins that suppress the exposure of ligands for activating NK cell receptors and/or encode viral MHC I-like proteins that act as decoys for the inhibitory KIR receptors. Thus far, to our knowledge, there have been no reports on viral evasion of NK cells via increased binding of inhibitory NK cell receptors that do not recognize MHC class I.
A highly conserved type of inhibitory NK cell receptor that does not bind MHC class I is CD300a. CD300a, also known as IRp60, is a 60-kDa glycoprotein belonging to the immunoglobulin (Ig) superfamily and is characterized by a single V-type Ig-like domain in the extracellular domain and several immunoreceptor tyrosine-based inhibition motifs (ITIMs) in the cytoplasmic domain (24, 25). CD300a recognizes cell surface-exposed aminophospholipids, particularly phosphatidylserine (PS) and phosphatidylethanolamine (PE) (26, 27), and the interaction between CD300a and its ligands suppresses the cytolytic activity of NK cells (28). The inhibitory receptor CD300a and its lipid ligands are highly conserved across animal species and have been described in mammals, birds, and fish (29, 30). To date, no viral strategies for NK cell evasion that involve CD300a have been described.
Here we report that the US3 protein kinase of pseudorabies virus (PRV), a porcine alphaherpesvirus, contributes to NK cell evasion by inducing the binding of CD300a to the infected-cell surface. This novel alphaherpesvirus mechanism for NK cell evasion may shed new light on the role of CD300a and its ligands in NK cell and virus biology.
MATERIALS AND METHODS
Viruses and cells.
The wild-type (WT) virus PRV NIA3, its isogenic US3-null mutant, and the restored rescue virus have been described previously and were kindly provided by the ID-DLO, the Netherlands (31–33). The wild-type virus PRV Becker, its isogenic US3-null mutant, and a kinase-negative US3 mutant (D223A) have been described previously and were kindly provided by Greg Smith (Northwestern University, Chicago, IL) (34, 35). Porcine SK cells and porcine primary epithelial cells were obtained and cultivated as described previously (19, 36). Mouse P815 cells were maintained in RPMI medium supplemented with 10% fetal calf serum (FCS), l-glutamine, and antibiotics (penicillin and streptomycin) (37). Human HEK293 and HEK293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS, l-glutamine, and antibiotics (penicillin and streptomycin) (19).
Antibodies and reagents.
Antibodies directed against PRV glycoproteins gB (mouse IgG2a [mIgG2a]; 1C11) and gD (mIgG1; 13D12) were kindly provided by H. Nauwynck (Ghent University, Ghent, Belgium) and have been described previously (38). The mouse monoclonal antibody (MAb) raised against PRV US3 was kindly provided by L. Olsen and L. Enquist (Princeton University, Princeton, NJ). Antibodies KS153 (mIgM) and IT144 (mIgG1), directed against human CD300a, were generated in the Brescia and Genoa labs, respectively, by immunizing BALB/c mice with polyclonal interleukin-2 (IL-2)-activated NK cells. Splenocytes from immunized mice were fused with P3U1 cells, and hybridomas were selected for their abilities to produce MAbs specifically recognizing CD300a on human peripheral blood mononuclear cells (hPBMC) and on cell transfectants. The anti-human CD300a antibody E59/126 (IgG1) was generated and described previously (24). Mouse monoclonal antibodies against porcine markers CD3ε (mIgG1; PPT3), CD4 (mIgG2b; 72-14-4), CD8α (mIgG2a; 11/295/33), and CD172a (IgG1; 74-22-15) were kindly provided by E. Cox (Ghent University, Ghent, Belgium) and have all been described previously (39–41); they were used, and their titers were determined, on freshly isolated porcine PBMC. Primary antibodies raised against MHC I (PT85A [mIgG2a]; VMRD), phosphatidylserine (1H6 [mIgG]; Millipore), porcine CD16 (G7 [mIgG1]; AbD Serotec), and alpha-tubulin (DM1A [mIgG]; Abcam) were purchased. A recombinant CD300a-Fc chimera was produced as follows. The pcDNA3.1TOPO-CD300a plasmid, containing the sequence coding for the open reading frame (ORF) of CD300a, obtained by reverse transcription-PCR (RT-PCR) starting from human IL-2-activated polyclonal NK cells, was constructed. For this purpose, total RNA was extracted using an RNeasy minikit (Qiagen), and oligo(dT)-primed cDNA was prepared with a Transcriptor First Strand cDNA synthesis kit (Roche) according to the manufacturer's instructions. PCR amplification was carried out with Platinum Taq DNA polymerase (Invitrogen) by using the following primers: 5′-CAAGTGCCGCCTGTGCTG (CD300a ORF up) and 5′-TGGGGCCCATGAGAGCTC (CD300a ORF dw). Amplification was performed for 30 cycles (30 s at 95°C, 30 s at 58°C, and 1 min at 68°C). The 969-bp PCR product was subcloned into the pcDNA3.1/V5-His-TOPO expression vector (Invitrogen) to construct the pcDNA3.1TOPO-CD300a plasmid. The nucleotide sequence of the CD300a ORF was checked using a BigDye Terminator cycle-sequencing kit, version 3.1, and an ABI Prism 3100 genetic analyzer (Applied Biosystems). Starting from this pcDNA3.1TOPO-CD300a plasmid, the sequence encoding the extracellular portion of the human CD300a receptor was amplified using the following primers: 5′-CAGGGGAACTCGAGAACGGACCATGTGGCTGCCTTG (CD300a XhoI up) and 5′-GACTAGGATCCAAATGCTGTGAGTTCACCACCTC (CD300a BamHI dw). Amplification was performed with Platinum Taq DNA polymerase (high fidelity; Invitrogen) for 20 cycles (30 s at 95°C, 30 s at 58°C, and 1 min at 72°C), followed by a 7-min elongation step at 72°C. The PCR product was digested with the XhoI and BamHI restriction enzymes and was subcloned into the SalI-BamHI-digested pRB1-2B4Fcmut vector (kindly provided by M. Falco, Istituto Giannina Gaslini, Genoa, Italy) in frame with the sequence coding for the human IgG1 portion, which was mutagenized to produce a mutated Fc that does not bind to Fc receptors (mutations Leu234Ala, Leu235Glu, and Gly237Ala) (42). The pRB1-CD300aFcmut construct was stably transfected into the HEK293 human embryonic fibroblast cell line using FuGene 6 (Roche). Supernatants were collected from the cell transfectant cultured in Dulbecco's modified Eagle's medium supplemented with 10% ultralow IgG fetal bovine serum (Life Technologies) and 0.5 μg/ml G418 (Calbiochem), and the CD300a-Fc molecule was purified by affinity chromatography using protein A–Sepharose 4 Fast Flow (Amersham Biosciences). Purified protein was checked by SDS-PAGE, followed by silver staining and enzyme-linked immunosorbent assays (ELISA) using CD300a-specific MAbs. For flow cytometric analysis, R-phycoerythrin (R-PE)- or Alexa Fluor 647 (AF647)-labeled goat anti-human antibodies and R-PE- or Cy5-labeled goat anti-mouse antibodies (Life Technologies) were used. R-PE-labeled goat anti-mouse IgG1, AF647-labeled goat anti-mouse IgG2a, fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG2b (Life Technologies), and goat anti-mouse IgG MACS (magnetically activated cell sorting) beads (Miltenyi Biotec) were used for cell sorting. Horseradish peroxidase (HRP)-labeled polyclonal goat anti-mouse antibodies (Dako) were used for Western blot detection.
Infections, transfections, and IPA-3 treatment.
SK cells were inoculated in suspension at a multiplicity of infection (MOI) of 10, seeded in suspension flasks (Sarstedt) at 1.2 × 106 cells/ml, and put on a rocking platform at 37°C basically as described previously (43). Porcine primary epithelial cells were grown in 6-well plates (Sarstedt) and were inoculated the next day at an MOI of 10, and the virus was washed away 2 h postinoculation (hpi), as described previously (19). The pcDNA3.1TOPO-CD300a construct and the corresponding empty plasmid were transiently transfected into the HEK293T human embryonic fibroblast cell line by using jetPEI (Polyplus) according to the manufacturer's instructions. Cells were treated with the group I PAK (p21-activated kinase) inhibitor IPA-3 (Tocris) or with dimethyl sulfoxide (DMSO) as a control, as described previously (35, 44).
Flow cytometric analysis.
Cells were harvested, incubated on ice for 40 min with mouse primary antibodies or recombinant CD300a-Fc (20 μg/ml), and subsequently washed and incubated for 40 min on ice with R-PE- or Cy5-labeled goat anti-mouse secondary antibodies or with R-PE- or AF647-labeled goat anti-human secondary antibodies (Life Technologies). Cells infected with PRV NIA3 strains were consistently stained using R-PE-labeled secondary antibodies. Cells infected with PRV Becker strains, which encode a monomeric red fluorescent protein (mRFP) expression cassette, were labeled with AF647 or Cy5 to avoid spectral overlap. Annexin V binding assays were performed according to the manufacturer's protocol (BD Biosciences). A total of 20,000 living cells were analyzed after washing by using a FACSAria III cell sorter and FACSDiva software (BD Biosciences). The live/dead stain Sytox Blue (Life Technologies) was used to identify living cells. Primary cells were analyzed similarly, but 10,000 living cells were used. For statistical analysis, the mean fluorescence intensity ratio (MFIR) was calculated by dividing the measured mean fluorescence intensity (MFI) by the MFI of the respective isotype control.
Western blotting.
Cell lysis was performed on a shaker at 4°C for 1 h with a lysis buffer containing NP-40 (Roche) and protease inhibitors (Sigma-Aldrich); nuclei were removed by centrifugation (13,000 × g, 10 min); and the protein content was measured using the bicinchoninic acid (BCA) protein assay kit (Thermo Scientific) (45). Per sample, 20 μg protein was loaded onto SDS-PAGE gels (10% acrylamide) and was transferred to a Hybond-P membrane (GE Healthcare), which was subsequently blocked using 5% milk powder diluted in PBS-T (phosphate-buffered saline [PBS] supplemented with 0.1% Tween 20 [Sigma-Aldrich]). Incubations with primary monoclonal antibodies or HRP-labeled secondary antibodies were performed for 1 h in 5% milk powder diluted in PBS-T at room temperature. Bands were detected by chemiluminescence using the ECL Prime kit (GE Healthcare) and were visualized by using a ChemiDoc MP imager (Bio-Rad) according to the manufacturer's instructions.
NK cells.
Human NK cells were isolated from PBMC using the RosetteSep NK cell enrichment kit (Stemcell Technologies), cultured in the presence of 100 U/ml human IL-2 (huIL2) (Chiron) as described previously (46), and used within 3 weeks. Porcine primary NK cells were isolated from porcine PBMC by negative MACS depletion and a fluorescence-activated cell sorter (FACS) purification step using antibodies against porcine CD172a, CD3, CD4, and CD8α, as described previously (19). After isolation, porcine NK cells were incubated for 18 h in the presence of 40 U/ml huIL2 (Life Technologies). CD16 expression on sorted cells confirmed ≥98% NK cell purity. The taking of blood for the isolation of porcine PBMC was approved by the Ethical Commission of the Faculty of Veterinary Medicine, Ghent University (EC2013/62).
Cytolytic and antibody redirected killing assays.
A flow cytometric propidium iodide–carboxyfluorescein succinimidyl ester-based assay was used to quantify NK cell-mediated lytic activity against infected target cells, as described previously (19). After incubation for 4 h at 37°C with NK cells, the viability of 5,000 target cells was evaluated by flow cytometry using propidium iodide (Life Technologies). Unless stated otherwise, cytolytic assays with human NK cells were performed at a target-to-effector cell ratio of 1:1 and assays with porcine NK cells at a ratio of 1:25. The percentage of NK cell-mediated lysis was calculated as (% dead targetNK − % dead targetspont)/(% dead targetmaximum − % dead targetspont), where % dead targetNK is the percentage of dead target cells in the presence of NK cells, % dead targetspont is the percentage of dead target cells without the addition of NK cells, and % dead targetmaximum is the maximal percentage of lysis of the target cells, which was determined by fixing and permeabilizing the cells, as described previously (19, 47). To determine the level of CD300a-dependent protection against NK cell-mediated killing, NK cell-mediated cytotoxicity was evaluated in the absence or presence of the CD300a-blocking IgM antibody KS153 (10 μg/ml). The percentage of CD300a-dependent protection from NK cell-mediated lysis was calculated by subtracting the percentage of NK cell-mediated lysis in the absence of KS153 from the percentage in the presence of KS153. Antibody redirected killing assays were performed by using the murine mastocytoma FcγR+ cell line P815 in the presence of either the medium alone, the anti-CD300a antibody IT144 (mIgG1), an IgG1 isotype control antibody, or anti-porcine CD16, and the percentage of NK cell-mediated lysis was calculated.
Statistics.
Statistical analysis was performed using Prism software (GraphPad) based on the means and standard errors of the means (SEM) for at least three independent replicates using one-way analysis of variance (ANOVA).
RESULTS
US3 reduces NK cell-mediated lysis of PRV-infected cells.
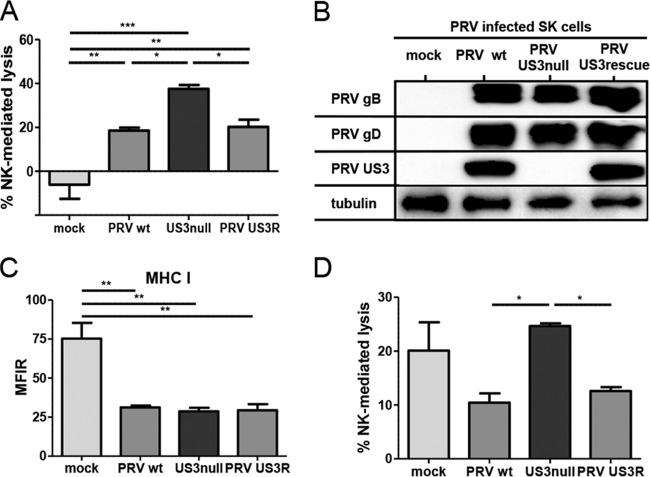
Using a variety of gene deletion mutants of pseudorabies virus (PRV), we recently discovered that PRV glycoprotein gD suppresses NK cell activity via downregulation of CD112, a ligand for the activating NK cell receptor DNAM-1 (19). Our initial NK cell cytotoxicity assays with different PRV mutants indicated that US3 may also display NK cell-evasive properties. To investigate whether PRV US3 indeed affects the susceptibility of infected cells to NK cell-mediated lysis, cytolytic assays were performed with SK cells infected with wild-type (WT) PRV, an isogenic US3-null virus, or an isogenic US3 rescue virus. At 10 hpi, mock-infected and PRV-infected cells were coincubated with IL-2-primed primary porcine NK cells for 4 h, and cells were subsequently assessed for viability by flow cytometry (Fig. 1A). Mock-infected SK cells did not elicit a significant cytolytic response from porcine NK cells; the percentage of NK cell-mediated lysis was not statistically different from zero, in line with earlier data (19). Also, as reported previously, PRV infection triggered porcine NK cell-mediated killing of SK cells (19). Cells infected with US3-null PRV showed higher susceptibility to NK cell-mediated lysis than SK cells infected with WT or US3 rescue PRV. The higher susceptibility of US3-null PRV-infected cells to NK cell-mediated cell lysis than of WT or US3 rescue PRV-infected cells was not due to differences in viral replication (Fig. 1B) or to differences in the abilities of these viruses to downregulate the expression of MHC I molecules (an important ligand for various inhibitory NK cell receptors) (Fig. 1C). In addition, NK cell cytotoxicity assays using a PRV strain expressing a kinase-inactive US3 mutant PRV harboring a point mutation (D223A) in the conserved aspartate in PRV US3 that constitutes the catalytic base required for phosphotransfer (34, 35) confirmed that kinase-intact US3 is required to increase the protection of infected cells against NK cell-mediated lysis (data not shown).
FIG 1.
US3 suppresses the susceptibility of PRV-infected cells to porcine and human NK cell-mediated lysis. (A) SK cells were either mock infected or infected with WT, US3-null, or US3 rescue PRV (NIA3 strain) for 10 h and were subsequently incubated with IL-2-primed primary porcine NK cells at a target-to-effector cell ratio of 1:25 for 4 h. The viability of target cells was assessed by propidium iodide and flow cytometry, and the percentage of NK cell-mediated lysis was calculated. Data represent means + SEM for three independent experiments (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (B and C) SK cells were either mock infected or infected with WT, US3-null, or US3 rescue PRV for 12 h and were subsequently analyzed by Western blotting for the expression of PRV gB, PRV gD, PRV US3, and tubulin (B) or assessed for MHC I expression on the cell surface by flow cytometry (C). Data in panel C represent means + SEM for three independent repeats (**, P < 0.01). (D) SK cells were either mock infected or infected with WT, US3-null, or US3 rescue PRV for 10 h and were subsequently incubated with IL-2-cultivated human NK cells at a target-to-effector cell ratio of 1:1 for 4 h. The viability of target cells was assessed by propidium iodide and flow cytometry, and the percentage of NK cell-mediated lysis was calculated. Data represent means + SEM for three independent repeats (*, P < 0.05).
Because only a limited range of reagents and tools for the investigation of NK cell activation/inhibition in the porcine system is currently available, we investigated whether PRV US3 also generated a protective effect against human NK cells. To this end, the cytolytic activity of human IL-2-cultured NK cells against mock-infected SK cells or SK cells infected with WT, US3-null, or US3 rescue PRV was assessed (Fig. 1D). As observed previously, IL-2-cultured human NK cells lysed mock-infected SK cells to a significant extent, which is in line with the known xenogeneic response of human NK cells to porcine cells (48–51). US3-null PRV-infected cells again showed higher susceptibility to NK cell-mediated lysis than wild-type or US3 rescue PRV-infected cells. In conclusion, PRV US3 reduces the susceptibilities of infected cells to both porcine and human NK cells.
PRV US3 enhances resistance to NK cell-mediated killing by increasing the level of binding of the inhibitory NK cell receptor CD300a to infected cells.
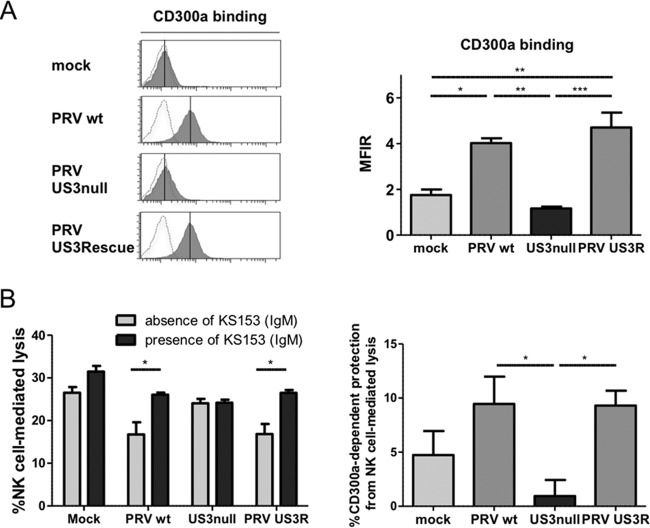
The protective effect of US3 against NK cell-mediated lysis may result from a modulation of the activating/inhibitory receptor balance on NK cells. Several inhibitory NK cell receptors (e.g., KIR receptors) recognize MHC class I molecules (52). Since we did not observe a difference in MHC class I levels on the cell surface between WT and US3-null PRV-infected cells (Fig. 1C), involvement of these NK cell receptors was unlikely. Still, the fact that US3 provided protection to PRV-infected cells against both porcine and human NK cells pointed to the potential involvement of highly conserved NK cell receptors and ligands. CD300a is a highly conserved inhibitory receptor that binds to the highly conserved ligands phosphatidylserine (PS) and phosphatidylethanolamine (PE) (29, 30). Therefore, we investigated whether US3 affects the binding of CD300a to PRV-infected cells. A CD300a-Fc soluble chimeric molecule was used in a flow cytometric binding assay. As shown in Fig. 2A, SK cells infected with WT PRV or the US3 rescue virus displayed substantially higher levels of binding of recombinant CD300a than US3-null PRV-infected or mock-infected cells. We then addressed whether this US3-dependent increase in the binding of CD300a to PRV-infected cells is involved in the US3-mediated protection from NK cell-mediated lysis. To this end, cytotoxicity assays were performed in the presence or absence of the anti-CD300a blocking MAb KS153. The reactivity and specificity of the KS153 antibody were confirmed by flow cytometric analysis on CD300a-transfected 293T cells, and the ability of KS153 to interfere with the binding of CD300a with its ligands was confirmed by blocking experiments using the recombinant CD300a-Fc protein (data not shown). Cytotoxicity assays in the presence or absence of KS153 allowed us to evaluate the percentage of CD300a-dependent protection of cells from human NK cell-mediated lysis (see Materials and Methods). Figure 2B shows that US3 increases the CD300a-mediated protection of infected cells against NK cell-mediated lysis. In conclusion, PRV-infected cells show a US3-dependent increase in the level of CD300a binding, and CD300a is involved in the protective effect of PRV US3 against NK cell-mediated lysis.
FIG 2.
PRV triggers US3-dependent increased binding of the inhibitory NK cell receptor CD300a to the infected-cell surface and increased CD300a-mediated protection of infected cells against NK cell-mediated lysis. (A) SK cells were infected with WT, US3-null, or US3 rescue PRV (NIA3 strain) for 12 h and were subsequently assessed by flow cytometry for the binding of recombinant CD300a-Fc (1 μg/sample). (Left) The x axes of histogram plots indicate fluorescence intensity, and vertical lines in histograms indicate median fluorescence intensity. Dotted-line histograms represent isotype-matched antibody control signals. (Right) The graph shows means + SEM for three independent repeats (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (B) SK cells were infected with WT, US3-null, or US3 rescue PRV (NIA3 strain) for 10 h and were subsequently incubated with IL-2-cultivated human NK cells at an effector-to-target cell ratio of 1:1 in the absence or presence of the anti-CD300a antibody KS153. The viability of target cells was assessed by propidium iodide and flow cytometry. (Left) Percentage of NK cell-mediated lysis; (right) percentage of CD300a-dependent protection against NK cell-mediated lysis. Data represent means + SEM for three independent repeats (*, P < 0.05).
The CD300a ligands PS and PE are involved in the PRV US3-mediated binding of CD300a.
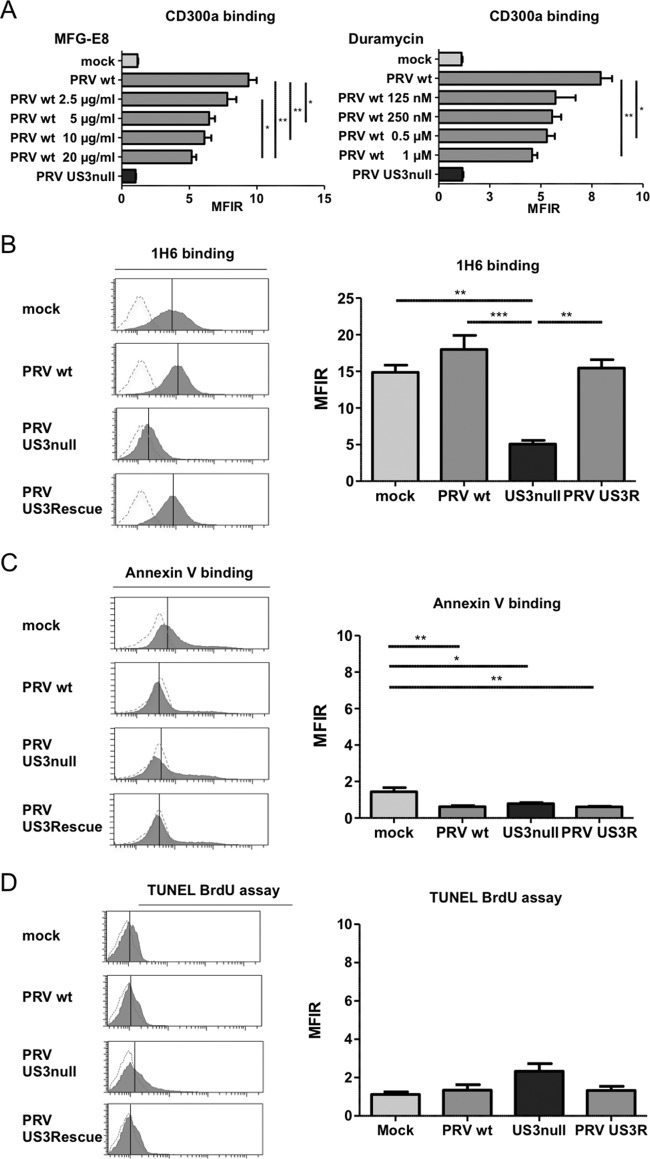
Two highly conserved cellular ligands, PS and PE, have been identified for the inhibitory NK cell receptor CD300a (26, 27). To investigate whether these cellular ligands are involved in the observed PRV US3-dependent increase in CD300a binding to infected cells, the binding of CD300a-Fc was assessed in the presence of increasing concentrations of milk fat globule–EGF factor 8 (MFG-E8; also known as lactadherin) or duramycin, agents that have been reported previously to interfere with the ability of CD300a to bind PS or PE, respectively (26). As shown in Fig. 3A, the addition of MFG-E8 resulted in a significant dose-dependent reduction in the level of CD300a-Fc binding to PRV-infected SK cells, indicating that PS is involved in the binding of CD300a to PRV-infected SK cells. Suppression of CD300a binding was also observed by using duramycin, although the effect appeared less dose dependent.
FIG 3.
The CD300a ligands PS and PE are involved in the US3-dependent increased binding of CD300a to the infected-cell surface, and US3 modulates PS cell surface exposure. (A) SK cells were either mock infected or infected with WT or US3-null PRV (NIA3 strain) for 12 h, incubated with MFG-E8 or duramycin at the concentrations given, and assessed by flow cytometry for the binding of recombinant CD300a-Fc (1 μg/sample). Data represent means + SEM for three independent repeats (*, P < 0.05; **, P < 0.01). (B to D) SK cells were either mock infected or infected with WT, US3-null, or US3 rescue PRV (NIA3 strain) for 12 h and were subsequently assessed by flow cytometry for cell surface exposure of PS by using antibody 1H6 (B) or annexin V (C) or for apoptotic DNA fragmentation by using a TUNEL bromodeoxyuridine (BrdU) assay (D). The x axes of histogram plots indicate fluorescence intensity, and vertical lines in histograms indicate median fluorescence intensity. Dotted-line histograms represent isotype-matched antibody control signals. Graphs show means + SEM for three independent repeats (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
The involvement of PS and PE in US3-mediated CD300a binding suggests that US3 may modulate the cell surface exposure of these CD300a ligands. No assays to specifically detect PE on the cell surface have been described. However, PS on the cell surface can be detected by using the PS-specific antibody 1H6 (28). To determine whether US3 modulates the cell surface exposure of PS, mock-infected SK cells or SK cells infected with WT, US3-null, or US3 rescue PRV were analyzed by flow cytometry using the anti-PS antibody 1H6. Figure 3B shows that SK cells infected with WT or US3 rescue PRV indeed expose PS at much higher levels than SK cells infected with US3-null PRV. Somewhat surprisingly, mock-infected cells also showed substantial PS exposure. Increased PS exposure is one of the hallmarks of apoptotic cells. In contrast to the results obtained with the PS-binding antibody 1H6, however, we did not observe detectable binding of annexin V, which is widely used to detect surface-exposed PS (for example, on apoptotic cells) (Fig. 3C). In support of the notion that the observed antibody 1H6 binding did not point to increased cell death, Sytox Blue live/dead staining indicated that none of the conditions (mock infection, WT PRV, US3-null PRV, or US3 rescue PRV) resulted in substantial or different quantities of dead cells. Also in line with this finding, TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) staining, which detects apoptotic DNA fragmentation, did not indicate substantial levels of apoptosis under any condition, or substantial differences in apoptosis among the conditions (Fig. 3D).
To better assess the potential biological significance of our findings, we investigated whether PRV US3 expression also led to increased CD300a binding to porcine primary epithelial cells and modulated PS exposure on the surfaces of these cells. Figure 4A and B show that the effects of PRV US3 on CD300a binding to infected porcine primary epithelial cells and PS cell surface exposure are very similar to the effects observed in the SK cell line. Again, Western blot analysis confirmed that this US3-induced phenotype is not caused by differences in virus replication levels (Fig. 4C). In conclusion, our findings show that PRV US3 triggers CD300a binding to infected cells and indicate that US3 modulates PS exposure on the surfaces of infected cells.
FIG 4.
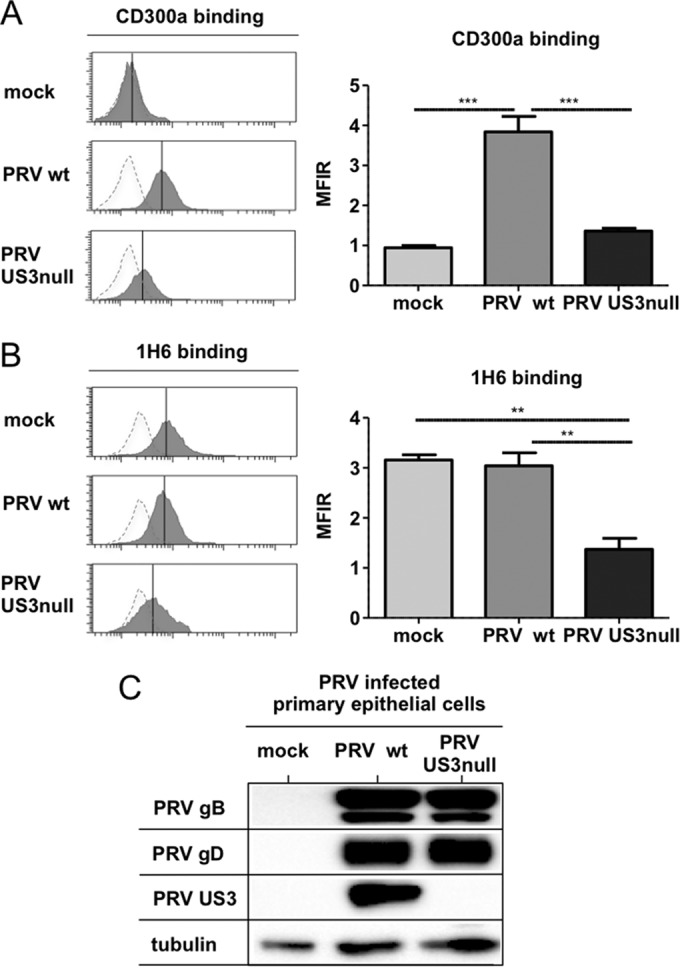
Modulation of CD300a binding and PS exposure by US3 also occurs in PRV-infected primary epithelial cells. Porcine primary epithelial cells were either mock infected or infected with WT or US3-null PRV (NIA3 strain) for 12 h. (A and B) Cells were assessed by flow cytometry for recombinant CD300a-Fc (1 μg/sample) binding (A) and PS exposure on the cell surface (by using antibody 1H6) (B). (Left) The x axes of histogram plots indicate fluorescence intensity, and vertical lines in histograms indicate median fluorescence intensity. Dotted-line histograms represent isotype-matched antibody control signals. (Right) Graphs show means + SEM for three independent repeats (**, P < 0.01; ***, P < 0.001). (C) Cells were assessed by Western blotting for the expression of PRV gB, PRV gD, PRV US3, and tubulin.
The kinase activity of PRV US3 and cellular PAKs are required for increased CD300a binding and modulation of PS exposure on the cell surface.
PRV US3 is a viral serine/threonine protein kinase and can directly phosphorylate and activate cellular group I p21-activated kinases (PAKs), which are central regulators in Rac1/CDC42 Rho GTPase signaling and comprise the closely related kinases PAK1, PAK2, and PAK3 (35, 53). Activation of group I PAKs has recently been reported to trigger increased PS exposure on the cell surface during thrombin-mediated activation of platelets (54). To investigate a possible involvement of the US3-PAK signaling axis in increased CD300a binding and the modulation of PS exposure, we first assessed whether the kinase activity of PRV US3 was required for the effects observed. For this purpose, the kinase-inactive US3 mutant PRV, which harbors a point mutation (D223A) in the conserved aspartate in PRV US3 that constitutes the catalytic base required for phosphotransfer, was used. SK cells were either mock infected or infected with WT PRV, isogenic US3 kinase-inactive PRV, or isogenic US3-null PRV and were assessed for the binding of recombinant CD300a and the modulation of cell surface PS. Figure 5A and B show that cells infected with PRV expressing kinase-inactive US3 display a phenotype similar to that of US3-null PRV-infected cells with regard to CD300a binding and PS exposure on the cell surface. Western blot analysis demonstrated similar infection efficiencies for cells infected with WT, US3-null, and US3 kinase-inactive PRVs (Fig. 5C). The effect of PRV on PS exposure in the assay for which results are shown in Fig. 5 is somewhat less pronounced than that in our earlier data (Fig. 3). This mild discrepancy can possibly be attributed to the fact that in the assays to determine the kinase involvement of US3, PRV strain Becker and isogenic mutants were used, whereas in the former experiments, the highly virulent field strain NIA3 (and isogenic mutants) was utilized.
FIG 5.
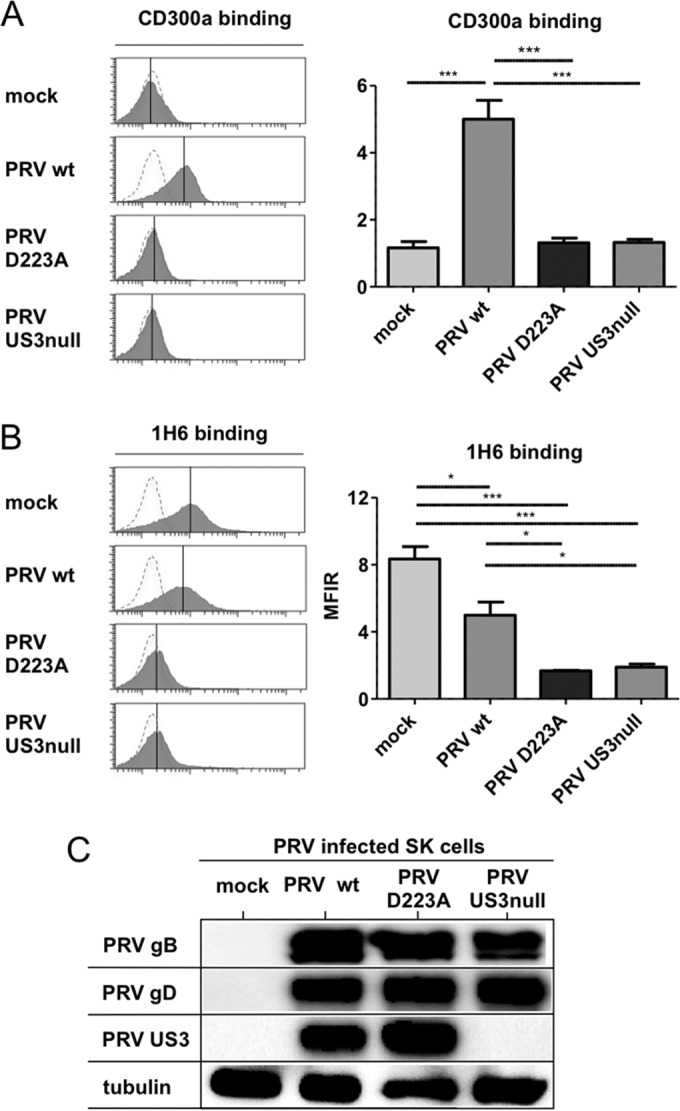
The kinase activity of PRV US3 is required for the modulation of CD300a binding and PS exposure. SK cells were either mock infected or infected with WT PRV, kinase-inactive D223A US3 PRV, or US3-null PRV (Becker strain) for 12 h. (A and B) Cells were assessed by flow cytometry for the binding of recombinant CD300a-Fc (1 μg/sample) (A) and for PS exposure on the cell surface (by using antibody 1H6) (B). (Left) The x axes of histogram plots indicate fluorescence intensity, and vertical lines in histograms indicate median fluorescence intensity. Dotted-line histograms represent isotype-matched antibody control signals. (Right) Graphs show means + SEM for three independent repeats (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (C) Cells were assessed by Western blotting for the expression of PRV gB, PRV gD, PRV US3, and tubulin.
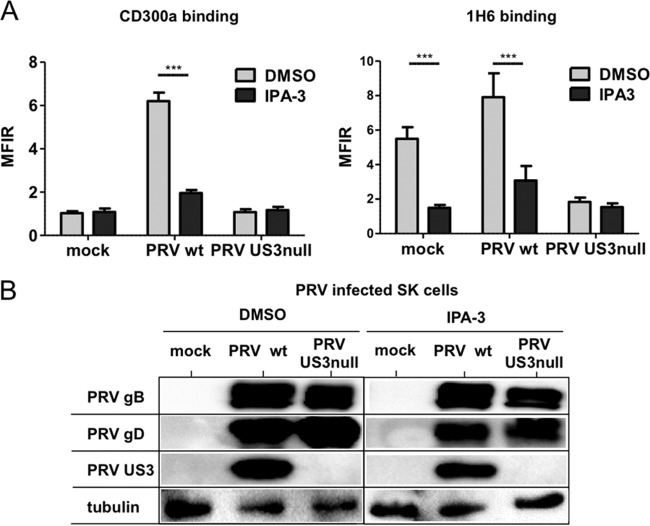
To investigate whether group I PAKs are involved in PRV US3-mediated effects on CD300a binding and PS exposure on the cell surface, group I PAKs were inhibited using the selective allosteric group I PAK inhibitor IPA-3 (44, 55). As shown in Fig. 6A, treatment with IPA-3 abrogated the increase in CD300a binding and reduced PS cell surface exposure in WT PRV-infected cells and mock-infected cells to the levels observed in US3-null PRV-infected cells. The inhibitory effects of IPA-3 are not caused by suppressive effects on PRV infection or the expression level of US3, as indicated by the Western blots shown in Fig. 6B. In conclusion, these experiments indicate that the PRV US3-mediated increase in CD300a binding and the modulation of PS exposure on the cell surface depend on the kinase activity of US3 and on the group I PAK cell-signaling pathway.
FIG 6.
Group I PAKs are involved in US3-mediated modulation of CD300a binding and PS exposure. SK cells were either mock infected or infected with WT or US3-null PRV (NIA3 strain). At 2 hpi, the group I PAK inhibitor IPA-3 (10 μM) or DMSO (as a diluent control) was added. (A) At 12 hpi, cells were assessed by flow cytometry for the binding of recombinant CD300a-Fc (1 μg/sample) (left) and for PS exposure on the cell surface (by using antibody 1H6) (right). Graphs show means + SEM for three independent repeats (***, P < 0.001). (B) At 12 hpi, cells were assessed by Western blotting for the expression of PRV gB, PRV gD, PRV US3, and tubulin.
CD300a in primary porcine NK cells.
The CD300a receptor is highly conserved across mammals and recently was also characterized as an inhibitory receptor in birds (29, 30). The natural host of PRV is the pig, and currently, porcine NK cell receptors are poorly characterized. The function of CD300a in porcine NK cells has not yet been addressed.
To assess whether CD300a also serves as an inhibitory receptor in porcine NK cells, a P815-based antibody redirected killing assay using porcine NK cells as effector cells was performed. This assay is typically used to determine the activating or inhibitory nature of a given NK cell receptor and has been used to demonstrate that CD300a serves as an inhibitory receptor in human NK cells (24, 28, 56). Murine P815 cells express receptors for the constant (Fc) domain of IgG antibodies (Fcγ receptors) on their surfaces. Hence, when monoclonal IgG antibodies against particular NK cell receptors are added to the assay mixture, the antibodies will bind to their respective receptors on the NK cells via their antigen binding (Fab) domains and, at the same time, to the Fcγ receptors on the P815 cells via their Fc domains, thereby bridging the NK cells and P815 cells. Depending on whether the antibody recognizes an activating or an inhibitory NK cell receptor, this antibody bridging will trigger increased or decreased NK cell-mediated lysis of P815 cells, respectively. In the case of IgG antibodies directed against CD300a, this assay results in reduced killing of P815 cells by human NK cells (24, 28, 56).
Here (Fig. 7) a similar redirected killing assay was performed using primary porcine NK cells instead of human NK cells to evaluate the cross-reactivity of the anti-human CD300a antibody IT144 with the porcine CD300a homologue and to determine whether, as in human NK cells, CD300a serves as an inhibitory receptor in porcine NK cells. The redirected killing assay was performed in the presence of either mouse monoclonal IgG1 antibody IT144, directed against huCD300a, an isotype-matched mouse IgG1 control antibody, a mouse monoclonal IgG1 anti-porcine CD16 antibody (which generates an activating effect on NK cells), or medium alone, and the percentage of NK cell-mediated lysis of P815 cells was calculated. Figure 7 shows that, as reported previously (57), the porcine CD16 receptor serves as an activating NK cell receptor, since triggering of CD16 resulted in substantially increased porcine NK cell-mediated killing of P815 cells. Importantly, the CD300a-directed antibody triggered significant inhibition of porcine NK cell-mediated lysis of P815 cells compared to lysis with the isotype-matched control or medium alone, indicating that in the porcine system also, CD300a serves as an inhibitory NK cell receptor.
FIG 7.
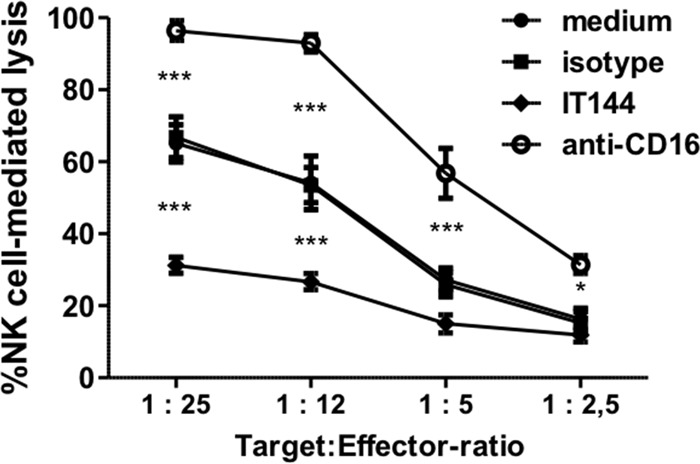
Porcine NK cells express a functional homologue of the inhibitory NK cell receptor CD300a. An antibody redirected killing assay using Fc receptor-bearing P815 cells was performed with IL-2-activated porcine primary NK cells, at the indicated target-to-effector cell ratios, in the presence of either medium, anti-CD300a antibody IT144, an anti-porcine CD16 antibody, or an isotype-matched control antibody. The viability of target cells was assessed by propidium iodide and flow cytometry, and the percentage of NK cell-mediated lysis was calculated. Data represent means + SEM for three independent repeats (*, P < 0.05; ***, P < 0.001).
DISCUSSION
In the current report, we describe a previously uncharacterized viral strategy for evading NK cells and reveal the involvement of CD300a in the recognition of virus-infected cells. By using PRV mutants and specific inhibitors, we demonstrate that this trait depends on the CD300a ligands phosphatidylserine (PS) and phosphatidylethanolamine (PE), on the expression of catalytically intact US3, and on the activation of group I PAKs. Interestingly, different tumor cell lines have recently been reported to show increased PS exposure and CD300a binding (28). In addition, blocking of PS using MFG-E8 enhanced NK cell-mediated killing of tumor cells, leading to the hypothesis that tumor cells may subvert NK cell-mediated lysis via increased CD300a binding (28). Our data, obtained by using CD300a-blocking antibodies, demonstrate directly, for the first time, that manipulation of CD300a may indeed represent a bona fide NK cell evasion strategy, indicating that viruses as well as tumor cells may manipulate this NK cell-inhibitory pathway for their own benefit.
CD300a is expressed not only on NK cells but also on several other immune cell populations, where it typically functions as an inhibitory receptor. Thus, CD300a has been implicated in the inhibition not only of NK cell activity (24, 28) but also of the activities of mast cells (58), neutrophils (59), eosinophils (60), and B and T cells (61, 62). As such, the consequences of viral triggering of the CD300a inhibitory receptor may stretch beyond the effects on NK cells described here. On the other hand, CD300a displays significant overlap regarding ligand specificity with the closely related activating receptor CD300c, which is specifically expressed on monocytes and mast cells (63, 64). Hence, in future research, it will be interesting to study whether the US3-mediated modulation of CD300a binding has consequences for other immune cells and whether or not US3 also affects CD300c binding.
The polar lipid PS and the neutral lipid PE have been described as the main CD300a ligands (26). Both PS and PE are typically distributed asymmetrically in the plasma membrane lipid bilayer and are enriched at the inner, cytoplasmic leaflet (65). Although externalization of PS is a hallmark of early stages of apoptosis, there is increasing evidence that cells may also show PS exposure independently of programmed cell death, as exemplified by reports on apoptosis-independent PS exposure on tumor cells and during activation of mast cells, B cells, T cells, and platelets (28, 66–70). Also along these lines, apoptosis is considered to trigger an eat-me signal for phagocytosis, and PS exposure was found not to be sufficient to induce this process in vivo (71). Our results are in line with the possibility that under certain circumstances, PS exposure may be independent of apoptotic cell death. Although we observed PS exposure by using the PS-specific antibody 1H6, this was not accompanied by an obvious apoptotic cellular phenotype, since we did not detect differences in viability (using the Sytox Blue dead/live marker) and apoptotic DNA fragmentation (using the TUNEL assay). Remarkably, antibody 1H6 reactivity was not accompanied by increased binding of annexin V, a reagent commonly used to detect PS exposure. The reasons underlying the differential reactivity between annexin V and 1H6 are currently unclear. One could argue that 1H6 may not specifically bind PS. However, several reports indicate that antibody 1H6 specifically binds to PS and not to other lipids (72–74). Nevertheless, we cannot formally rule out the possibility that 1H6 binds another lipid(s) in the cell membrane that is modulated by PRV US3 during infection. Irrespective of whether this is the case, we found that exposure of 1H6-reactive lipids is not sufficient to trigger substantial recombinant CD300a binding. Indeed, mock-infected cells showed a level of 1H6 reactivity comparable to (and sometimes even higher than) that observed for PRV-infected cells but, unlike PRV-infected cells, did not show significant CD300a binding. Hence, additional CD300a ligands appear to be involved in US3-triggered CD300a binding. This is in line with the findings of our blocking assays, which showed that not only the PS-blocking reagent MFG-E8, but also the PE-blocking agent duramycin, suppressed the US3-mediated increase in CD300a binding. Also in line with the view that additional ligands may be involved in the binding of CD300a to cells, we found that US3-null PRV-infected cells, which do not show obvious reactivity with the anti-PS antibody 1H6, display a level of CD300a binding comparable to that of mock-infected cells. Functional recognition reporter assays indicated that CD300a may bind more strongly to PE than to PS (26, 64, 75). This could account for the discrepancies in the observed correlation of CD300a binding and PS exposure. Unfortunately, due to the lack of reagents for the detection of PE, potential differences in PE exposure between mock-, WT PRV-, and US3-null PRV-infected cells could not be assessed. In any case, our data suggest that US3 expression affects the cell surface exposure of CD300a ligands, which are associated with substantially increased CD300a binding.
We showed previously that PRV US3 directly phosphorylates and thereby activates group I p21-activated kinases, critical downstream effectors of the Cdc42/Rac1 signaling pathways (35). Here we report that inhibition of group I PAK activity inhibits the ability of US3 to trigger increased CD300a binding or modulate PS exposure. Group I PAK activity has been reported previously to be critically involved in PS exposure during platelet activation (54). Our current data therefore suggest that group I PAKs may be linked to PS exposure/CD300a binding in different cell types. In this context, it is interesting that several viruses, including HIV, have been reported to trigger group I PAK activity (76) and may therefore modulate effects similar to those we describe here. In line with this, several viruses have been reported to trigger the exposure of aminophospholipids, such as PS (77). This has been speculated to enable viruses to evade immune recognition and dampen inflammatory responses to infection (77). Our current report demonstrates that viral manipulation of the exposure of phospholipids, such as PS, may indeed allow viruses to subvert important components of the antiviral immune response. Targeting of aminophospholipid exposure and signaling pathways, such as those mediated by group I PAKs, and identification of the viral factors that trigger these events may therefore hold promise as therapeutic strategies for viral diseases (76, 77). The viral US3 protein kinase may be of particular interest in this respect, since it is an important alphaherpesvirus virulence factor, and since US3 does not appear to be closely related genetically to any known cellular kinase, which may make it an attractive candidate to target for the development of antiviral drugs (78).
Interestingly, PRV strains lacking the US3 protein kinase show substantially reduced virulence in pigs (79–81). Despite this attenuation, pigs infected with US3-null PRV were protected against clinical signs upon challenge infection with a virulent wild-type virus (79, 80). Although further studies will be needed to elucidate how attenuated US3-null PRV may generate a protective immune response, our current findings on the immune evasion properties of the US3 protein may be significant in this context.
Our findings may also have relevance for cancer therapy, since several types of cancer have been associated with upregulated group I PAK activity and nonapoptotic PS exposure (82–84). Our observation that noninfected primary epithelial cells are substantially recognized by the PS-binding antibody 1H6 may indicate that perhaps caution should be exercised in targeting PS for anticancer or antiviral therapy (82). We found that, despite showing similar binding of 1H6, PRV-infected cells show substantially higher levels of CD300a binding than mock-infected cells. Combined with the observation that several types of cancer cells also display substantially increased CD300a binding (28, 77), this may indicate that targeting CD300a binding may be a more stringent strategy for identifying virus-infected or tumor cells than targeting PS exposure.
Finally, our data indicate that, as in humans and other mammals, CD300a serves as an inhibitory receptor in swine. Several studies have reported that the CD300 receptor family is highly conserved across multiple species (29, 30). This is particularly true for CD300a, as illustrated by its recent identification and characterization in chickens, where it shows inhibitory activity and affinity for PS and PE, as described for mammals (30). Our data also indicate that the human inhibitory NK cell receptor CD300a recognizes porcine cells, suggesting that, under certain circumstances, human CD300a can be involved in the recognition of porcine cells, which may be relevant to the study of the human NK cell response to pig xenografts.
In conclusion, we report a novel alphaherpesvirus strategy for evading NK cells, consisting of US3-dependent increased binding of the inhibitory NK cell receptor CD300a, which is orchestrated by group I PAK activity and phospholipids such as PS and PE. Our data provide novel insights in alphaherpesvirus and CD300a biology and may have implications for antiviral and antitumor therapies.
ACKNOWLEDGMENTS
We thank C. Van Waesberghe, S. Brabant, H. Vereecke, C. Helsmoortel, and L. Sys for excellent technical assistance, R. Cooman for animal management, and G. Smith (Northwestern University, Chicago, IL), L. Enquist (Princeton University, Princeton, NJ), ID-DLO (Lelystad, the Netherlands), and H. Nauwynck and E. Cox (Ghent University, Ghent, Belgium) for reagents.
This research was supported by grants from the Special Research Fund of Ghent University (grants 01J29110, 01J11611, and 01G01311) and F.W.O.-Vlaanderen (grants 1.5.077.11N and G.0176.15N), Hercules Foundation grant AUGE-035, and AIRC:IG project 15428 (to M.V.).
REFERENCES
- 1.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. 2011. Innate or adaptive immunity? The example of natural killer cells. Science 331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etzioni A, Eidenschenk C, Katz R, Beck R, Casanova JL, Pollack S. 2005. Fatal varicella associated with selective natural killer cell deficiency. J Pediatr 146:423–425. doi: 10.1016/j.jpeds.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 3.Almerigogna F, Fassio F, Giudizi MG, Biagiotti R, Manuelli C, Chiappini E, Galli L, Romagnani S, De Martino M. 2011. Natural killer cell deficiencies in a consecutive series of children with herpetic encephalitis. Int J Immunopathol Pharmacol 24:231–238. [DOI] [PubMed] [Google Scholar]
- 4.Biron CA, Byron KS, Sullivan JL. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med 320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 5.Babić M, Krmpotić A, Jonjić S. 2011. All is fair in virus-host interactions: NK cells and cytomegalovirus. Trends Mol Med 17:677–685. doi: 10.1016/j.molmed.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fielding CA, Aicheler R, Stanton RJ, Wang EC, Han S, Seirafian S, Davies J, McSharry BP, Weekes MP, Antrobus PR, Prod'homme V, Blanchet FP, Sugrue D, Cuff S, Roberts D, Davison AJ, Lehner PJ, Wilkinson GW, Tomasec P. 2014. Two novel human cytomegalovirus NK cell evasion functions target MICA for lysosomal degradation. PLoS Pathog 10:e1004058. doi: 10.1371/journal.ppat.1004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson GW, Tomasec P, Stanton RJ, Armstrong M, Prod'homme V, Aicheler R, McSharry BP, Rickards CR, Cochrane D, Llewellyn-Lacey S, Wang EC, Griffin CA, Davison AJ. 2008. Modulation of natural killer cells by human cytomegalovirus. J Clin Virol 41:206–212. doi: 10.1016/j.jcv.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 9.Dunn C, Chalupny NJ, Sutherland CL, Dosch S, Sivakumar PV, Johnson DC, Cosman D. 2003. Human cytomegalovirus glycoprotein UL16 causes intracellular sequestration of NKG2D ligands, protecting against natural killer cell cytotoxicity. J Exp Med 197:1427–1439. doi: 10.1084/jem.20022059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasec P, Wang EC, Davison AJ, Vojtesek B, Armstrong M, Griffin C, McSharry BP, Morris RJ, Llewellyn-Lacey S, Rickards C, Nomoto A, Sinzger C, Wilkinson GW. 2005. Downregulation of natural killer cell-activating ligand CD155 by human cytomegalovirus UL141. Nat Immunol 6:181–188. doi: 10.1038/ni1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. 2009. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe 5:376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Smith W, Tomasec P, Aicheler R, Loewendorf A, Nemcovicova I, Wang EC, Stanton RJ, Macauley M, Norris P, Willen L, Ruckova E, Nomoto A, Schneider P, Hahn G, Zajonc DM, Ware CF, Wilkinson GW, Benedict CA. 2013. Human cytomegalovirus glycoprotein UL141 targets the TRAIL death receptors to thwart host innate antiviral defenses. Cell Host Microbe 13:324–335. doi: 10.1016/j.chom.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madrid AS, Ganem D. 2012. Kaposi's sarcoma-associated herpesvirus ORF54/dUTPase downregulates a ligand for the NK activating receptor NKp44. J Virol 86:8693–8704. doi: 10.1128/JVI.00252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas M, Boname JM, Field S, Nejentsev S, Salio M, Cerundolo V, Wills M, Lehner PJ. 2008. Down-regulation of NKG2D and NKp80 ligands by Kaposi's sarcoma-associated herpesvirus K5 protects against NK cell cytotoxicity. Proc Natl Acad Sci U S A 105:1656–1661. doi: 10.1073/pnas.0707883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krmpotić A, Busch DH, Bubić I, Gebhardt F, Hengel H, Hasan M, Scalzo AA, Koszinowski UH, Jonjić S. 2002. MCMV glycoprotein gp40 confers virus resistance to CD8+ T cells and NK cells in vivo. Nat Immunol 3:529–535. doi: 10.1038/ni799. [DOI] [PubMed] [Google Scholar]
- 16.Zarama A, Pérez-Carmona N, Farré D, Tomic A, Borst EM, Messerle M, Jonjić S, Engel P, Angulo A. 2014. Cytomegalovirus m154 hinders CD48 cell-surface expression and promotes viral escape from host natural killer cell control. PLoS Pathog 10:e1004000. doi: 10.1371/journal.ppat.1004000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanton RJ, Prod'homme V, Purbhoo MA, Moore M, Aicheler RJ, Heinzmann M, Bailer SM, Haas J, Antrobus R, Weekes MP, Lehner PJ, Vojtesek B, Miners KL, Man S, Wilkie GS, Davison AJ, Wang EC, Tomasec P, Wilkinson GW. 2014. HCMV pUL135 remodels the actin cytoskeleton to impair immune recognition of infected cells. Cell Host Microbe 16:201–214. doi: 10.1016/j.chom.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schepis D, D'Amato M, Studahl M, Bergstrom T, Karre K, Berg L. 2009. Herpes simplex virus infection downmodulates NKG2D ligand expression. Scand J Immunol 69:429–436. doi: 10.1111/j.1365-3083.2009.02241.x. [DOI] [PubMed] [Google Scholar]
- 19.Grauwet K, Cantoni C, Parodi M, De Maria A, Devriendt B, Pende D, Moretta L, Vitale M, Favoreel HW. 2014. Modulation of CD112 by the alphaherpesvirus gD protein suppresses DNAM-1-dependent NK cell-mediated lysis of infected cells. Proc Natl Acad Sci U S A 111:16118–16123. doi: 10.1073/pnas.1409485111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell TM, McSharry BP, Steain M, Slobedman B, Abendroth A. 20 May 2015. Varicella-zoster virus and herpes simplex virus 1 differentially modulate NKG2D ligand expression during productive infection. J Virol doi: 10.1128/JVI.00292-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. 2008. Functions of natural killer cells. Nat Immunol 9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 22.Sivori S, Carlomagno S, Pesce S, Moretta A, Vitale M, Marcenaro E. 2014. TLR/NCR/KIR: which one to use and when? Front Immunol 5:105. doi: 10.3389/fimmu.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Weijer ML, Luteijn RD, Wiertz EJ. 2015. Viral immune evasion: lessons in MHC class I antigen presentation. Semin Immunol 27:125–137. doi: 10.1016/j.smim.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Cantoni C, Bottino C, Augugliaro R, Morelli L, Marcenaro E, Castriconi R, Vitale M, Pende D, Sivori S, Millo R, Biassoni R, Moretta L, Moretta A. 1999. Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human NK cells. Eur J Immunol 29:3148–3159. doi:. [DOI] [PubMed] [Google Scholar]
- 25.Green BJ, Clark GJ, Hart DN. 1998. The CMRF-35 mAb recognizes a second leukocyte membrane molecule with a domain similar to the poly Ig receptor. Int Immunol 10:891–899. doi: 10.1093/intimm/10.7.891. [DOI] [PubMed] [Google Scholar]
- 26.Simhadri VR, Andersen JF, Calvo E, Choi SC, Coligan JE, Borrego F. 2012. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood 119:2799–2809. doi: 10.1182/blood-2011-08-372425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakahashi-Oda C, Tahara-Hanaoka S, Honda S, Shibuya K, Shibuya A. 2012. Identification of phosphatidylserine as a ligand for the CD300a immunoreceptor. Biochem Biophys Res Commun 417:646–650. doi: 10.1016/j.bbrc.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Lankry D, Rovis TL, Jonjić S, Mandelboim O. 2013. The interaction between CD300a and phosphatidylserine inhibits tumor cell killing by NK cells. Eur J Immunol 43:2151–2161. doi: 10.1002/eji.201343433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon JP, O'Driscoll M, Litman GW. 2012. Specific lipid recognition is a general feature of CD300 and TREM molecules. Immunogenetics 64:39–47. doi: 10.1007/s00251-011-0562-4. [DOI] [PubMed] [Google Scholar]
- 30.Sperling B, Viertlboeck BC, Gobel TW. 2015. Chicken CD300a homolog is found on B lymphocytes, various leukocytes populations and binds to phospholipids. Dev Comp Immunol 50:121–128. doi: 10.1016/j.dci.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Baskerville A. 1973. Ultrastructural changes in the lungs of pigs infected with Aujeszky's disease virus. Res Vet Sci 14:229–233. [PubMed] [Google Scholar]
- 32.Kimman TG, Pol JM, de Wind N, Oei-Lie N, Berns AJ, Gielkens AL. 1992. Role of different genes in the virulence and pathogenesis of Aujeszky's disease virus. Vet Microbiol 33:45–52. doi: 10.1016/0378-1135(92)90034-Q. [DOI] [PubMed] [Google Scholar]
- 33.van Zijl M, van der Gulden H, de Wind N, Gielkens A, Berns A. 1990. Identification of two genes in the unique short region of pseudorabies virus; comparison with herpes simplex virus and varicella-zoster virus. J Gen Virol 71(Part 8):1747–1755. doi: 10.1099/0022-1317-71-8-1747. [DOI] [PubMed] [Google Scholar]
- 34.Coller KE, Smith GA. 2008. Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus. Traffic 9:1458–1470. doi: 10.1111/j.1600-0854.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Broeke C, Radu M, Deruelle M, Nauwynck H, Hofmann C, Jaffer ZM, Chernoff J, Favoreel HW. 2009. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc Natl Acad Sci U S A 106:8707–8712. doi: 10.1073/pnas.0900436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geenen K, Favoreel HW, Nauwynck HJ. 2005. Higher resistance of porcine trigeminal ganglion neurons towards pseudorabies virus-induced cell death compared with other porcine cell types in vitro. J Gen Virol 86:1251–1260. doi: 10.1099/vir.0.80760-0. [DOI] [PubMed] [Google Scholar]
- 37.Takeda K, Oshima H, Hayakawa Y, Akiba H, Atsuta M, Kobata T, Kobayashi K, Ito M, Yagita H, Okumura K. 2000. CD27-mediated activation of murine NK cells. J Immunol 164:1741–1745. doi: 10.4049/jimmunol.164.4.1741. [DOI] [PubMed] [Google Scholar]
- 38.Nauwynck HJ, Pensaert MB. 1995. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch Virol 140:1137–1146. doi: 10.1007/BF01315422. [DOI] [PubMed] [Google Scholar]
- 39.Yang H, Oura CA, Kirkham PA, Parkhouse RM. 1996. Preparation of monoclonal anti-porcine CD3 antibodies and preliminary characterization of porcine T lymphocytes. Immunology 88:577–585. doi: 10.1046/j.1365-2567.1996.d01-682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pescovitz MD, Lunney JK, Sachs DH. 1984. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol 133:368–375. [PubMed] [Google Scholar]
- 41.Jonjić S, Koszinowski UH. 1984. Monoclonal antibodies reactive with swine lymphocytes. I. Antibodies to membrane structures that define the cytolytic T lymphocyte subset in the swine. J Immunol 133:647–652. [PubMed] [Google Scholar]
- 42.Canfield SM, Morrison SL. 1991. The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J Exp Med 173:1483–1491. doi: 10.1084/jem.173.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Favoreel HW, Nauwynck HJ, Van Oostveldt P, Mettenleiter TC, Pensaert MB. 1997. Antibody-induced and cytoskeleton-mediated redistribution and shedding of viral glycoproteins, expressed on pseudorabies virus-infected cells. J Virol 71:8254–8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. 2008. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol 15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deruelle M, Geenen K, Nauwynck HJ, Favoreel HW. 2007. A point mutation in the putative ATP binding site of the pseudorabies virus US3 protein kinase prevents Bad phosphorylation and cell survival following apoptosis induction. Virus Res 128:65–70. doi: 10.1016/j.virusres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Balsamo M, Vermi W, Parodi M, Pietra G, Manzini C, Queirolo P, Lonardi S, Augugliaro R, Moretta A, Facchetti F, Moretta L, Mingari MC, Vitale M. 2012. Melanoma cells become resistant to NK-cell-mediated killing when exposed to NK-cell numbers compatible with NK-cell infiltration in the tumor. Eur J Immunol 42:1833–1842. doi: 10.1002/eji.201142179. [DOI] [PubMed] [Google Scholar]
- 47.Pintaric M, Gerner W, Saalmuller A. 2008. Synergistic effects of IL-2, IL-12 and IL-18 on cytolytic activity, perforin expression and IFN-γ production of porcine natural killer cells. Vet Immunol Immunopathol 121:68–82. doi: 10.1016/j.vetimm.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Forte P, Lilienfeld BG, Baumann BC, Seebach JD. 2005. Human NK cytotoxicity against porcine cells is triggered by NKp44 and NKG2D. J Immunol 175:5463–5470. doi: 10.4049/jimmunol.175.8.5463. [DOI] [PubMed] [Google Scholar]
- 49.Sommaggio R, Cohnen A, Watzl C, Costa C. 2012. Multiple receptors trigger human NK cell-mediated cytotoxicity against porcine chondrocytes. J Immunol 188:2075–2083. doi: 10.4049/jimmunol.1100433. [DOI] [PubMed] [Google Scholar]
- 50.Tran PD, Christiansen D, Winterhalter A, Brooks A, Gorrell M, Lilienfeld BG, Seebach JD, Sandrin M, Sharland A. 2008. Porcine cells express more than one functional ligand for the human lymphocyte activating receptor NKG2D. Xenotransplantation 15:321–332. doi: 10.1111/j.1399-3089.2008.00489.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim TJ, Kim N, Kim EO, Choi JR, Bluestone JA, Lee KM. 2010. Suppression of human anti-porcine natural killer cell xenogeneic responses by combinations of monoclonal antibodies specific to CD2 and NKG2D and extracellular signal-regulated kinase kinase inhibitor. Immunology 130:545–555. doi: 10.1111/j.1365-2567.2010.03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parham P, Moffett A. 2013. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol 13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pacheco A, Chernoff J. 2010. Group I p21-activated kinases: emerging roles in immune function and viral pathogenesis. Int J Biochem Cell Biol 42:13–16. doi: 10.1016/j.biocel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aslan JE, Baker SM, Loren CP, Haley KM, Itakura A, Pang J, Greenberg DL, David LL, Manser E, Chernoff J, McCarty OJ. 2013. The PAK system links Rho GTPase signaling to thrombin-mediated platelet activation. Am J Physiol Cell Physiol 305:C519–C528. doi: 10.1152/ajpcell.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van den Broeke C, Deruelle M, Nauwynck HJ, Coller KE, Smith GA, Van Doorsselaere J, Favoreel HW. 2009. The kinase activity of pseudorabies virus US3 is required for modulation of the actin cytoskeleton. Virology 385:155–160. doi: 10.1016/j.virol.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 56.Lankry D, Simic H, Klieger Y, Levi-Schaffer F, Jonjić S, Mandelboim O. 2010. Expression and function of CD300 in NK cells. J Immunol 185:2877–2886. doi: 10.4049/jimmunol.0903347. [DOI] [PubMed] [Google Scholar]
- 57.Mair KH, Essler SE, Patzl M, Storset AK, Saalmuller A, Gerner W. 2012. NKp46 expression discriminates porcine NK cells with different functional properties. Eur J Immunol 42:1261–1271. doi: 10.1002/eji.201141989. [DOI] [PubMed] [Google Scholar]
- 58.Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F. 2005. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J Immunol 175:7989–7995. doi: 10.4049/jimmunol.175.12.7989. [DOI] [PubMed] [Google Scholar]
- 59.Alvarez Y, Tang X, Coligan JE, Borrego F. 2008. The CD300a (IRp60) inhibitory receptor is rapidly up-regulated on human neutrophils in response to inflammatory stimuli and modulates CD32a (FcγRIIa) mediated signaling. Mol Immunol 45:253–258. doi: 10.1016/j.molimm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munitz A, Bachelet I, Eliashar R, Moretta A, Moretta L, Levi-Schaffer F. 2006. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood 107:1996–2003. doi: 10.1182/blood-2005-07-2926. [DOI] [PubMed] [Google Scholar]
- 61.Silva R, Moir S, Kardava L, Debell K, Simhadri VR, Ferrando-Martinez S, Leal M, Pena J, Coligan JE, Borrego F. 2011. CD300a is expressed on human B cells, modulates BCR-mediated signaling, and its expression is down-regulated in HIV infection. Blood 117:5870–5880. doi: 10.1182/blood-2010-09-310318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simhadri VR, Mariano JL, Zhou Q, DeBell KE, Borrego F. 2011. Differential expression of CD300a/c on human TH1 and TH17 cells. BMC Immunol 12:62. doi: 10.1186/1471-2172-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simhadri VR, Mariano JL, Gil-Krzewska A, Zhou Q, Borrego F. 2013. CD300c is an activating receptor expressed on human monocytes. J Innate Immun 5:389–400. doi: 10.1159/000350523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi M, Izawa K, Kashiwakura J, Yamanishi Y, Enomoto Y, Kaitani A, Maehara A, Isobe M, Ito S, Matsukawa T, Nakahara F, Oki T, Kajikawa M, Ra C, Okayama Y, Kitamura T, Kitaura J. 2013. Human CD300C delivers an Fc receptor-γ-dependent activating signal in mast cells and monocytes and differs from CD300A in ligand recognition. J Biol Chem 288:7662–7675. doi: 10.1074/jbc.M112.434746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hankins HM, Baldridge RD, Xu P, Graham TR. 2015. Role of flippases, scramblases and transfer proteins in phosphatidylserine subcellular distribution. Traffic 16:35–47. doi: 10.1111/tra.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lentz BR. 2003. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res 42:423–438. doi: 10.1016/S0163-7827(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 67.Dillon SR, Mancini M, Rosen A, Schlissel MS. 2000. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol 164:1322–1332. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- 68.Elliott JI, Surprenant A, Marelli-Berg FM, Cooper JC, Cassady-Cain RL, Wooding C, Linton K, Alexander DR, Higgins CF. 2005. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol 7:808–816. doi: 10.1038/ncb1279. [DOI] [PubMed] [Google Scholar]
- 69.Elliott JI, Sardini A, Cooper JC, Alexander DR, Davanture S, Chimini G, Higgins CF. 2006. Phosphatidylserine exposure in B lymphocytes: a role for lipid packing. Blood 108:1611–1617. doi: 10.1182/blood-2005-11-012328. [DOI] [PubMed] [Google Scholar]
- 70.Rysavy NM, Shimoda LM, Dixon AM, Speck M, Stokes AJ, Turner H, Umemoto EY. 2014. Beyond apoptosis: the mechanism and function of phosphatidylserine asymmetry in the membrane of activating mast cells. Bioarchitecture 4:127–137. doi: 10.1080/19490992.2014.995516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Segawa K, Suzuki J, Nagata S. 2011. Constitutive exposure of phosphatidylserine on viable cells. Proc Natl Acad Sci U S A 108:19246–19251. doi: 10.1073/pnas.1114799108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mourdjeva M, Kyurkchiev D, Mandinova A, Altankova I, Kehayov I, Kyurkchiev S. 2005. Dynamics of membrane translocation of phosphatidylserine during apoptosis detected by a monoclonal antibody. Apoptosis 10:209–217. doi: 10.1007/s10495-005-6076-5. [DOI] [PubMed] [Google Scholar]
- 73.Prudovsky I, Vary CP, Markaki Y, Olins AL, Olins DE. 2012. Phosphatidylserine colocalizes with epichromatin in interphase nuclei and mitotic chromosomes. Nucleus 3:200–210. doi: 10.4161/nucl.19662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mandinov L, Mandinova A, Kyurkchiev S, Kyurkchiev D, Kehayov I, Kolev V, Soldi R, Bagala C, de Muinck ED, Lindner V, Post MJ, Simons M, Bellum S, Prudovsky I, Maciag T. 2003. Copper chelation represses the vascular response to injury. Proc Natl Acad Sci U S A 100:6700–6705. doi: 10.1073/pnas.1231994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zenarruzabeitia O, Vitalle J, Eguizabal C, Simhadri VR, Borrego F. 2015. The biology and disease relevance of CD300a, an inhibitory receptor for phosphatidylserine and phosphatidylethanolamine. J Immunol 194:5053–5060. doi: 10.4049/jimmunol.1500304. [DOI] [PubMed] [Google Scholar]
- 76.Van den Broeke C, Radu M, Chernoff J, Favoreel HW. 2010. An emerging role for p21-activated kinases (Paks) in viral infections. Trends Cell Biol 20:160–169. doi: 10.1016/j.tcb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soares MM, King SW, Thorpe PE. 2008. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat Med 14:1357–1362. doi: 10.1038/nm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deruelle MJ, Favoreel HW. 2011. Keep it in the subfamily: the conserved alphaherpesvirus US3 protein kinase. J Gen Virol 92:18–30. doi: 10.1099/vir.0.025593-0. [DOI] [PubMed] [Google Scholar]
- 79.Kimman TG, de Wind N, Oei-Lie N, Pol JM, Berns AJ, Gielkens AL. 1992. Contribution of single genes within the unique short region of Aujeszky's disease virus (suid herpesvirus type 1) to virulence, pathogenesis and immunogenicity. J Gen Virol 73(Part 2):243–251. doi: 10.1099/0022-1317-73-2-243. [DOI] [PubMed] [Google Scholar]
- 80.Kimman TG, De Wind N, De Bruin T, de Visser Y, Voermans J. 1994. Inactivation of glycoprotein gE and thymidine kinase or the US3-encoded protein kinase synergistically decreases in vivo replication of pseudorabies virus and the induction of protective immunity. Virology 205:511–518. doi: 10.1006/viro.1994.1672. [DOI] [PubMed] [Google Scholar]
- 81.Mulder WA, Pol JM, Gruys E, Jacobs L, De Jong MC, Peeters BP, Kimman TG. 1997. Pseudorabies virus infections in pigs. Role of viral proteins in virulence, pathogenesis and transmission. Vet Res 28:1–17. [PubMed] [Google Scholar]
- 82.Riedl S, Rinner B, Asslaber M, Schaider H, Walzer S, Novak A, Lohner K, Zweytick D. 2011. In search of a novel target—phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochim Biophys Acta 1808:2638–2645. doi: 10.1016/j.bbamem.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Riedl S, Rinner B, Schaider H, Lohner K, Zweytick D. 2014. Killing of melanoma cells and their metastases by human lactoferricin derivatives requires interaction with the cancer marker phosphatidylserine. Biometals 27:981–997. doi: 10.1007/s10534-014-9749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radu M, Semenova G, Kosoff R, Chernoff J. 2014. PAK signalling during the development and progression of cancer. Nat Rev Cancer 14:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]