Abstract
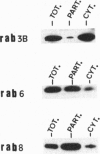
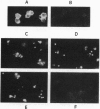
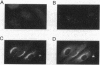
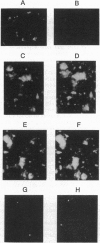
The activation of platelets by specific agonists is a tightly regulated mechanism that leads to the secretion of the dense- and alpha-granule contents. Platelets have been shown to possess small GTP-binding proteins thought to be involved in central biological processes; however, no rab proteins, which may regulate the exocytic process at different stages, have been reported. This study has shown that rab1, rab3B, rab4, rab6, and rab8 proteins, but not rab3A protein, were present in platelets and in endothelial cells. To probe their functional significance in platelets, rab3B, rab6, and rab8 proteins were further characterized with regard to their intracellular localization and their phosphorylation properties. Whereas rab3B protein was found to be mainly cytosolic, rab6 and rab8 proteins were preferentially targeted to the plasma membrane and to the alpha granules. The activation of platelets by thrombin, a potent inducer of secretion, resulted in the phosphorylation of rab3B, rab6, and rab8 proteins, whereas no phosphorylation was observed in the presence of prostaglandin E1, which stimulates cAMP-dependent protein kinase and inhibits the secretion process. These findings provide evidence that members of the subfamily of rab proteins, rab6 and rab8, are localized in platelets to one type of specific secretory vesicle, the alpha granule, and would suggest their possible implication in the secretion process through phosphorylation mechanisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barque J. P., Karniguian A., Brisson-Jeanneau C., Della-Valle V., Grelac F., Larsen C. J. Human autoantibodies identify a nuclear chromatin-associated antigen (PSL or p55) in human platelets. Eur J Cell Biol. 1990 Feb;51(1):183–187. [PubMed] [Google Scholar]
- Beckers C. J., Balch W. E. Calcium and GTP: essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989 Apr;108(4):1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar R. P., Chardin P., Haslam R. J. Identification of multiple ral gene products in human platelets that account for some but not all of the platelet Gn-proteins. FEBS Lett. 1990 Jan 15;260(1):48–52. doi: 10.1016/0014-5793(90)80063-o. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Woolkalis M. J., Manning D. R. Interactions in platelets between G proteins and the agonists that stimulate phospholipase C and inhibit adenylyl cyclase. J Biol Chem. 1988 Apr 15;263(11):5348–5355. [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Coller B. S., Beer J. H., Scudder L. E., Steinberg M. H. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989 Jul;74(1):182–192. [PubMed] [Google Scholar]
- Cramer E. M., Debili N., Martin J. F., Gladwin A. M., Breton-Gorius J., Harrison P., Savidge G. F., Vainchenker W. Uncoordinated expression of fibrinogen compared with thrombospondin and von Willebrand factor in maturing human megakaryocytes. Blood. 1989 Apr;73(5):1123–1129. [PubMed] [Google Scholar]
- Darchen F., Zahraoui A., Hammel F., Monteils M. P., Tavitian A., Scherman D. Association of the GTP-binding protein Rab3A with bovine adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5692–5696. doi: 10.1073/pnas.87.15.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Enouf J., Lompré A. M., Bredoux R., Bourdeau N., de La Bastie D., Levy-Toledano S. Different sensitivity to trypsin of the human platelet plasma and intracellular membrane Ca2+ pumps. J Biol Chem. 1988 Sep 25;263(27):13922–13929. [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Platelet tyrosine-specific protein phosphorylation is regulated by thrombin. Mol Cell Biol. 1988 Sep;8(9):3603–3610. doi: 10.1128/mcb.8.9.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G., Südhof T. C., Jahn R. A small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature. 1991 Jan 3;349(6304):79–81. doi: 10.1038/349079a0. [DOI] [PubMed] [Google Scholar]
- Fischer T. H., Gatling M. N., Lacal J. C., White G. C., 2nd rap1B, a cAMP-dependent protein kinase substrate, associates with the platelet cytoskeleton. J Biol Chem. 1990 Nov 15;265(32):19405–19408. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
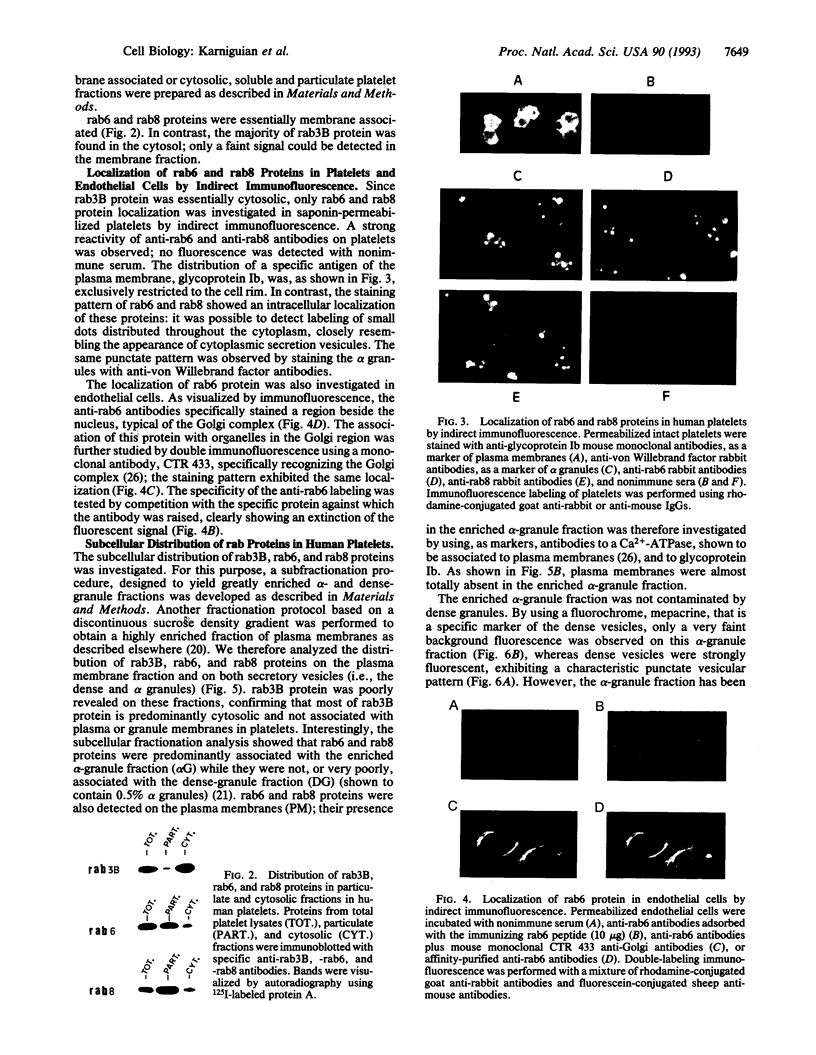
- Goud B., Zahraoui A., Tavitian A., Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990 Jun 7;345(6275):553–556. doi: 10.1038/345553a0. [DOI] [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Jasmin B. J., Cartaud J., Bornens M., Changeux J. P. Golgi apparatus in chick skeletal muscle: changes in its distribution during end plate development and after denervation. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7218–7222. doi: 10.1073/pnas.86.18.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M., Kikuchi A., Hoshijima M., Yamamoto K., Hashimoto E., Yamamura H., Takai Y. Phosphorylation of smg p21, a ras p21-like GTP-binding protein, by cyclic AMP-dependent protein kinase in a cell-free system and in response to prostaglandin E1 in intact human platelets. J Biol Chem. 1989 Sep 15;264(26):15688–15695. [PubMed] [Google Scholar]
- Kremer N. E., D'Arcangelo G., Thomas S. M., DeMarco M., Brugge J. S., Halegoua S. Signal transduction by nerve growth factor and fibroblast growth factor in PC12 cells requires a sequence of src and ras actions. J Cell Biol. 1991 Nov;115(3):809–819. doi: 10.1083/jcb.115.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Lacal J. C., Reep B. R., Molina y Vedia L. A ras-related protein is phosphorylated and translocated by agonists that increase cAMP levels in human platelets. Proc Natl Acad Sci U S A. 1989 May;86(9):3131–3134. doi: 10.1073/pnas.86.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti K. B., Lapetina E. G. Epinephrine suppresses rap1B.GAP-activated GTPase activity in human platelets. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2784–2788. doi: 10.1073/pnas.89.7.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya K. L., Tavitian B., Zahraoui A., Tavitian A. Localization of the ras-like rab3A protein in the adult rat brain. Brain Res. 1992 Sep 11;590(1-2):118–127. doi: 10.1016/0006-8993(92)91087-u. [DOI] [PubMed] [Google Scholar]
- Nigam S. K. Subcellular distribution of small GTP binding proteins in pancreas: identification of small GTP binding proteins in the rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1296–1299. doi: 10.1073/pnas.87.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori T., Kikuchi A., Yamamoto K., Kim S., Takai Y. Small molecular weight GTP-binding proteins in human platelet membranes. Purification and characterization of a novel GTP-binding protein with a molecular weight of 22,000. J Biol Chem. 1989 Jan 25;264(3):1877–1881. [PubMed] [Google Scholar]
- Ohmstede C. A., Farrell F. X., Reep B. R., Clemetson K. J., Lapetina E. G. RAP2B: a RAS-related GTP-binding protein from platelets. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6527–6531. doi: 10.1073/pnas.87.17.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991 Oct;115(1):1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. G., Weber R. F., Nevins B., Didsbury J. R., Evans T., Snyderman R. Identification of the ral and rac1 gene products, low molecular mass GTP-binding proteins from human platelets. J Biol Chem. 1989 Oct 5;264(28):16383–16389. [PubMed] [Google Scholar]
- Rendu F., Lebret M., Danielian S., Fagard R., Levy-Toledano S., Fischer S. High pp60c-src level in human platelet dense bodies. Blood. 1989 May 1;73(6):1545–1551. [PubMed] [Google Scholar]
- Rendu F., Lebret M., Nurden A. T., Caen J. P. Initial characterization of human platelet mepacrine-labelled granules isolated using a short metrizamide gradient. Br J Haematol. 1982 Oct;52(2):241–251. doi: 10.1111/j.1365-2141.1982.tb03886.x. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Brugge J. S. Protein tyrosine phosphorylation and the adhesive functions of platelets. Curr Opin Cell Biol. 1991 Oct;3(5):869–879. doi: 10.1016/0955-0674(91)90062-4. [DOI] [PubMed] [Google Scholar]
- Staatz W. D., Rajpara S. M., Wayner E. A., Carter W. G., Santoro S. A. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J Cell Biol. 1989 May;108(5):1917–1924. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Sluijs P., Hull M., Zahraoui A., Tavitian A., Goud B., Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Olmsted J. B., Marder V. J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982 Oct;95(1):355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth N. C., Brennwald P., Kabcenell A. K., Garrett M., Novick P. Hydrolysis of GTP by Sec4 protein plays an important role in vesicular transport and is stimulated by a GTPase-activating protein in Saccharomyces cerevisiae. Mol Cell Biol. 1992 May;12(5):2017–2028. doi: 10.1128/mcb.12.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahraoui A., Touchot N., Chardin P., Tavitian A. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem. 1989 Jul 25;264(21):12394–12401. [PubMed] [Google Scholar]