Abstract
In this present research work, the aim was to develop ileo-colonic targeted matrix-mini-tablets-filled capsule system of Naproxen for chronotherapeutic treatment of Rheumatoid Arthritis. So Matrix-mini-tablets of Naproxen were prepared using microsomal enzyme dependent and pH-sensitive polymers by direct compression method which were further filled into an empty HPMC capsule. The compatibility was assessed using FT-IR and DSC studies for pure drug, polymers and their physical mixtures. The prepared batches were subjected to physicochemical studies, drug content estimation, in-vitro drug release and stability studies. When FTIR and DSC studies were performed, it was found that there was no interaction between Naproxen and polymers used. The physicochemical properties of all the prepared matrix-mini-tablets batches were found to be in limits. The drug content percentage in the optimized formulation F18 was found to be 99.24 ± 0.10%. Our optimized matrix-mini-tablets-filled-capsule formulation F18 releases Naproxen after a lag time of 2.45 ± 0.97 h and 27.30 ± 0.86%, 92.59 ± 0.47%, 99.38 ± 0.69% at the end of 5, 8, 12 h respectively. This formulation was also found to be stable as per the guidelines of International Conference on Harmonisation of Technical Requirements of Pharmaceuticals for Human Use. Thus, a novel ileo-colonic targeted delivery system of Naproxen was successfully developed by filling matrix-mini-tablets into an empty HPMC capsule shell for targeting early morning peak symptoms of rheumatoid arthritis.
Keywords: Matrix-mini-tablets, Naproxen, Microsomal enzyme dependent polymers, pH-sensitive polymers, Chronotherapy, HPMC capsule
1. Introduction
Rheumatoid arthritis is a chronic inflammatory syndrome which causes the destruction of joints integrity. The patients with this disease have joint pain and functional disability symptoms which mainly persists in the early morning hours (Cutolo, 2012). These symptoms occur due to diurnal variations in the levels of circulating proinflammatory cytokines, interleukin-6 and/or tumor necrosis factor-α (Arvidson et al., 1994). The concept of chronotherapy can be used for the better treatment of Rheumatoid arthritis so that the highest amount of drug can be maintained in the bloodstream during the early morning time (Buttgereit et al., 2011, Najmuddin et al., 2010). In this case, colon targeting of drug or intentionally delayed absorption can be preferable in order to have a uniform therapeutic effect. Because the drug can be delivered in more amount during its greatest need as the release of drug occurs after a lag time. Thus, the peak pain and stiffness symptoms of the disease can be overcome and good patient compliance can be achieved (Gothaskar et al., 2004, Krishainah and Satyanarayan, 2001). Dew et al., developed the first colonic targeted pH responsive drug delivery system and it is most specifically referred to as ‘ileo-colonic targeted drug delivery’ rather than colonic targeted drug delivery system (Dew et al., 1982, Evans et al., 1988, Ibekwe et al., 2008, Ibekwe et al., 2006).
A number of approaches can be used for targeting the drugs at the colonic junction. Some of them are by using enzyme and pH dependent approaches (Chickpetty et al., 2010). In the enzyme dependent approach, it makes use of such carriers or polymers which are degraded by enzymes produced by the colonic bacteria. Because the microflora is rich in colonic region and their energy needs are fulfilled by fermenting various substrates types that have been left undigested in the small intestine such as disaccharides, trisaccharides and polysaccharides. For their fermentation to take place, the microflora produces a vast number of enzymes such as azoreductase, arabinosidase, glucuronidase, galactomannase, galactosidase, nitroreductase, xylosidase, deaminase and ureadehydroxylase. As these enzymes are mainly present only in the colon, the use of enzyme degradable polymers such as natural polysaccharides from plant origin (for e.g. Guar gum) and algal origin (for e.g. Alginates) seems to be the most interested for colonic targeted drug delivery (Mohapatra et al., 2011). Whereas in the pH dependent approach, it depends on the increased pH of the gastrointestinal tract i.e. from stomach (pH 1.5–3) to terminal ileum (pH 7–8) Akhgari et al., 2005.
Mini-tablets are very small tablets whose diameter is equal to or smaller than 3 mm that can be either placed in sachets or filled into a capsule shell for easy administration (Lopes et al., 2006, Hadi et al., 2012, Rajabi Siaboomin, 2009, Mirela et al., 2010). They are having several benefits over single unit larger tablets such as consistent drug release, uniform clinical performance, more flexibility during the formulation development and maximum stability on storage (Rajabi Siaboomin, 2009, Mirela et al., 2010). Also mini-tablets are easier to prepare using direct compression method, which involves very less number of steps using simple equipments for their manufacture. Thus, the time and costs can be saved. Other benefits include regular shapes and excellent size uniformity (Lopes et al., 2006, Hadi et al., 2012, Rajabi Siaboomin, 2009, Mirela et al., 2010, Fegely, 2009, Maghsoodi and Kiafar, 2013). In the present work, the reason for designing matrix-mini-tablets-filled capsule formulation is to develop a more reliable dosage form which possess all the advantages of a single unit bigger tablet and yet the problems such as danger of dose dumping and alteration in release profile of drug due to unit to unit variation can be avoided.
Naproxen is a derivative of naphthylpropionic acid which belongs to the class of NSAID’s and it has been found to be effective in both experimental and clinical pain of rheumatoid arthritis (Uziel et al., 2000). So, an attempt was made to develop matrix-mini-tablets of Naproxen filled in a capsule for treating the early morning peak symptoms of rheumatoid arthritis.
2. Materials and methods
2.1. Materials
Naproxen was obtained as a gift sample from IPS Pharma Training Institute, Hyderabad, India. pH sensitive polymers (Eudragit® L-100 and Eudragit® S-100) were obtained as gift samples by Degussa India Pvt. Ltd., Mumbai, India. Sodium alginate, Guar gum, Microcrystalline cellulose Avicel PH102 and Aerosil® were purchased from SD Fine Chemicals, Mumbai, India. Magnesium stearate was purchased from Himedia Chem Lab, Mumbai, India. Empty HPMC capsules of almost all sizes were obtained as gift samples from ACG Associated capsules Pvt. Ltd. Mumbai, India. All other remaining materials used were of analytical grade.
2.2. Preformulation studies
2.2.1. Procedure for Fourier Transform Infrared (FTIR) spectral analysis
The compatibility for pure drug Naproxen, polymers and their physical mixtures used in this experimental procedure was evaluated by recording of spectra using FT-IR Spectrophotometer (Perkin Elmer, spectrum-100, Japan). The spectra were recorded by taking 5% of sample in potassium bromide (KBr) and after this mixture was grounded into a fine powder it was compressed into KBr pellets at 4000 Psi compaction pressure for a period of 2 min. The resolution was 1 cm−1 and the range of scanning was 400–4000 cm−1 (Hadi et al., 2014a, Hadi et al., 2014b).
2.2.2. Procedure for diffraction scanning calorimetric (DSC) studies
The DSC thermograms of pure drug Naproxen and their physical mixtures were recorded using Diffraction scanning calorimeter (DSC 60, Shimadzu, Japan). Their measurement was taken between 30 and 350 °C at a heating rate of 10 °C/min (Hadi et al., 2014a, Hadi et al., 2014b).
2.3. Formulation methods
2.3.1. Procedure for the preparation of matrix-mini-tablets of Naproxen
Matrix-mini-tablets of Naproxen were prepared using direct compression method as shown in Table 1. In the first step Naproxen, polymer or polymers and Microcrystalline cellulose were passed through the 60 mesh sieve and after weighing as per the formulation table they were mixed. Then in the second step, magnesium stearate and aerosil were passed separately through the same sieve and after weighing as per the formulation table they were added to the above mixture and blended thoroughly. This prepared blend was then compressed into matrix-mini-tablets by using 3 mm round concave punches in a rotary tablet press (Model RSB-4, Rimek mini-press, Karnavati Engineering, Ahmedabad) (Hadi et al., 2014a).
Table 1.
Composition of matrix-mini-tablets of Naproxen.
| F.C | Naproxen | Guar gum | Sodium alginate | Eudragit L-100 | Eudragit S-100 | Microcrystalline cellulose (Avicel PH102) | Magnesium stearate | Aerosil |
|---|---|---|---|---|---|---|---|---|
| F1 | 16.666 | 2 | – | – | – | 6.084 | 0.125 | 0.125 |
| F2 | 16.666 | 4 | – | – | – | 4.084 | 0.125 | 0.125 |
| F3 | 16.666 | 6 | – | – | – | 2.084 | 0.125 | 0.125 |
| F4 | 16.666 | 8 | – | – | – | 0.084 | 0.125 | 0.125 |
| F5 | 16.666 | 2 | 2 | – | – | 4.084 | 0.125 | 0.125 |
| F6 | 16.666 | 2 | 4 | – | – | 2.084 | 0.125 | 0.125 |
| F7 | 16.666 | 2 | 6 | – | – | 0.084 | 0.125 | 0.125 |
| F8 | 16.666 | 4 | 2 | – | – | 2.084 | 0.125 | 0.125 |
| F9 | 16.666 | 4 | 4 | – | – | 0.084 | 0.125 | 0.125 |
| F10 | 16.666 | 6 | 2 | – | – | 0.084 | 0.125 | 0.125 |
| F11 | 16.666 | – | – | 2 | – | 6.084 | 0.125 | 0.125 |
| F12 | 16.666 | – | – | 4 | – | 4.084 | 0.125 | 0.125 |
| F13 | 16.666 | – | – | 6 | – | 2.084 | 0.125 | 0.125 |
| F14 | 16.666 | – | – | 8 | – | 0.084 | 0.125 | 0.125 |
| F15 | 16.666 | – | – | – | 2 | 6.084 | 0.125 | 0.125 |
| F16 | 16.666 | – | – | – | 4 | 4.084 | 0.125 | 0.125 |
| F17 | 16.666 | – | – | – | 6 | 2.084 | 0.125 | 0.125 |
| F18 | 16.666 | – | – | – | 8 | 0.084 | 0.125 | 0.125 |
| F19 | 16.666 | – | – | 2 | 2 | 4.084 | 0.125 | 0.125 |
| F20 | 16.666 | – | – | 2 | 4 | 2.084 | 0.125 | 0.125 |
| F21 | 16.666 | – | – | 2 | 6 | 0.084 | 0.125 | 0.125 |
| F22 | 16.666 | – | – | 4 | 2 | 2.084 | 0.125 | 0.125 |
| F23 | 16.666 | – | – | 4 | 4 | 0.084 | 0.125 | 0.125 |
| F24 | 16.666 | – | – | 6 | 2 | 0.084 | 0.125 | 0.125 |
Note: 2 = 8%; 4 = 16%; 6 = 24%; 8 = 32% as total weight of each matrix-mini-tablet was 25 mg.
2.3.2. Procedure for the preparation of matrix-mini-tablets-filled capsule formulations
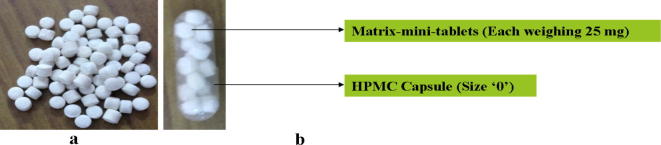
The capsule formulations were prepared by filling 15 matrix-mini-tablets equivalent to 250 mg of Naproxen into size ‘0’ HPMC capsule (as shown in Fig. 1) (Hadi et al., 2014a, Hadi et al., 2014b).
Figure 1.
(a) Matrix-mini-tablets and (b) matrix-mini-tablets-filled capsule formulation.
2.4. Evaluation methods
2.4.1. Procedure for pre-compression parameters
The prepared powder blends of formulation batches were evaluated for pre-compression parameters in order to study their flow properties and to maintain matrix-mini-tablets weight uniformity.
2.4.1.1. Angle of repose (θ)
The angle of repose was determined by taking accurately weighed quantity of powder blend into the funnel. The funnel height was adjusted such that the funnel tip should touch the apex of blend. This blend was then allowed to freely flow through the funnel onto the surface. From the formed powder cone, radius and height were measured and their angle of repose was calculated using the following equation (Hadi et al., 2014a, Hadi et al., 2014b).
where h and r are the height and radius of the formed powder cone respectively.
2.4.1.2. Loose Bulk density (LBD) and Tapped Bulk density (TBD)
Both the LBD and TBD were determined by accurately weighing 2gm of powder blend from each formulation batch which was previously shaken to break any agglomerates formation, and were introduced into 10 ml of measuring cylinder. After noting the initial volume, the measuring cylinder was made to fall under its own weight onto a hard surface from the height of 2.5 cm at 2 s time intervals. This process of tapping was continued until a further no change in volume of powder blend was noted and their LBD and TBD were calculated using the following equations (Hadi et al., 2014a, Hadi et al., 2014b).
LBD = Weight of the Granules /Untapped Volume of the packing
TBD = Weight of the Granules /Tapped Volume of the packing
2.4.1.3. Hausner’s ratio
Hausner’s ratio is an indirect index of ease of powder flow. It was calculated by using the following equation (Hadi et al., 2014a, Hadi et al., 2014b).
where, ρt is the tapped density and ρd is the bulk density.
2.4.1.4. Carr’s compressibility index
Carr’s Index (%) was calculated by using the following equation (Hadi et al., 2014a, Hadi et al., 2014b).
2.4.2. Procedure for post-compression parameters
The prepared matrix-mini-tablets of all the formulation batches were evaluated for post-compression parameters in order to study their physicochemical properties.
2.4.2.1. Hardness test
The matrix-mini-tablets hardness was determined using Pfizer hardness tester. Three matrix-mini-tablets were randomly taken from each formulation batches and their values were calculated (Hadi et al., 2014a, Hadi et al., 2014b).
2.4.2.2. Friability test
The Friability test was evaluated by initially weighing (Winitial) twenty matrix-mini-tablets and finally transferring them into a Veego friabilator. The friabilator was operated at 25 rpm and run up to 100 revolutions. Then the matrix-mini-tablets were weighed again (Wfinal) Hadi et al., 2014a, Hadi et al., 2014b.The percentage friability was calculated by using the following equation.
2.4.2.3. Weight variation test
The Weight variation test was evaluated by randomly taking twenty matrix-mini-tablets from each formulation batch and weighing them individually to check their weight variation (Hadi et al., 2014a, Hadi et al., 2014b).
2.4.2.4. Uniformity of thickness
The Uniformity of thickness was evaluated by randomly taking six matrix-mini-tablets from each formulation batch and measuring them individually for thickness using screw gauge (Hadi et al., 2014a, Hadi et al., 2014b).
2.4.2.5. Drug content uniformity
The drug content uniformity was estimated by taking fifty matrix-mini-tablets and then crushing them into fine powder in the mortar. This fine powder equivalent to 250 mg of Naproxen was extracted into 7.2 pH phosphate buffer. The buffer solution was then filtered through a Millipore filter of 0.45 μm pore size. After suitable dilutions were done, drug content was determined at a wavelength of 331 nm using UV-Spectrophotometer (Hadi et al., 2014a, Hadi et al., 2014b).
2.4.3. Procedure for In-vitro dissolution testing of matrix-mini-tablets-filled capsule formulations
Dissolution testing evaluation was performed by using USP XXIII dissolution test apparatus (basket type). One matrix-mini-tablets-filled capsule formulation was immersed completely at a time. Three dissolution media with 1.2, 7.4 and 6.8 pH were used sequentially for formulations F1 to F10 and four dissolution media with 1.2, 6.5, 6.8 and 7.2 pH were used sequentially for formulations F11 to F24 to maintain the conditions of GI tract. These media represent the stomach (1.2 pH), proximal part of the small intestine (6.5 pH), lower part of the small intestine and colon (6.8 pH), terminal ileum (7.2 pH) and whole small intestine (7.4 pH). During dissolution testing for F1 to F10 formulations, 750 ml of 1.2 pH media was first used for two hours, then followed with 900 ml of 7.4 pH phosphate buffer for three hours and was continued further with 900 ml of 6.8 pH dissolution media containing 0.05 mg/ml of betagalactomannase enzyme for subsequent hours. Whereas during dissolution testing for F11 to F24 formulations, 750 ml of 1.2 pH media was first used for two hours, followed by 900 ml of 6.5, 6.8 and 7.2 pH phosphate buffers for one, two and subsequent hours respectively. Rotation speed and temperature were maintained at 100 rpm and 37 ± 0.5 °C respectively. At fixed time intervals (0, 1, 2, 3, 4, 5, 6, 7, 8, 10 and 12 h), 5 ml of dissolution media was withdrawn and was then replaced with fresh respective dissolution media. The samples withdrawn were analyzed at 230 nm for 1.2 pH media, 329 nm for 6.5 and 6.8 pH media and 331 nm for 7.2 and 7.4 pH media by UV absorption spectroscopy and the drug release percentage was calculated over the sampling time intervals (Hadi et al., 2014a, Hadi et al., 2014b, Kaur et al., 2010, Wong et al., 1997).
2.4.4. Stability studies
The stability studies for optimized formulation were performed at both room temperature and accelerated stability conditions. The room temperature storage conditions were kept at 30 ± 2 °C and 65 ± 5% relative humidity (RH) and for accelerated stability conditions were stored at 40 ± 2 °C and 75 ± 5% RH in a stability chamber. At regular time intervals of three and six months samples were withdrawn from the stability chamber and were tested for physical parameters such as Appearance, Weight variation, hardness, thickness, friability, drug content and In-vitro release profile of matrix-mini-tablets (Hadi et al., 2014a, Hadi et al., 2014b, ICH Guidelines, 2003, Cha et al., 2001).
3. Results and discussion
As the main objective of this research work was to develop a novel matrix-mini-tablets-filled capsule formulation which targets Naproxen at the ileo-colonic junction. So an attempt was tried by incorporating lowest to highest possible concentrations of individual or combined polymers in the mini-tablets. As the incorporating capability of size ‘0’ HPMC capsule (which is the most common largest size easily accepted by humans) was only 15 mini-tablets and the dose of Naproxen was also more i.e. 250 mg (so each matrix-mini-tablet weighing 25 mg contains 16.666 mg of Naproxen itself), so it was possible to use only up to maximum 32% concentration of polymers in individual matrix-mini-tablets.
The target release profile was based on the assumption that if the optimized formulation is administered at night 10:00 P.M (before going to bed) then maximum amount of Naproxen will be available in between 4:00 and 6:00 A.M. and this is the time when rheumatoid arthritis symptoms will be at its peak.
3.1. FT-IR studies
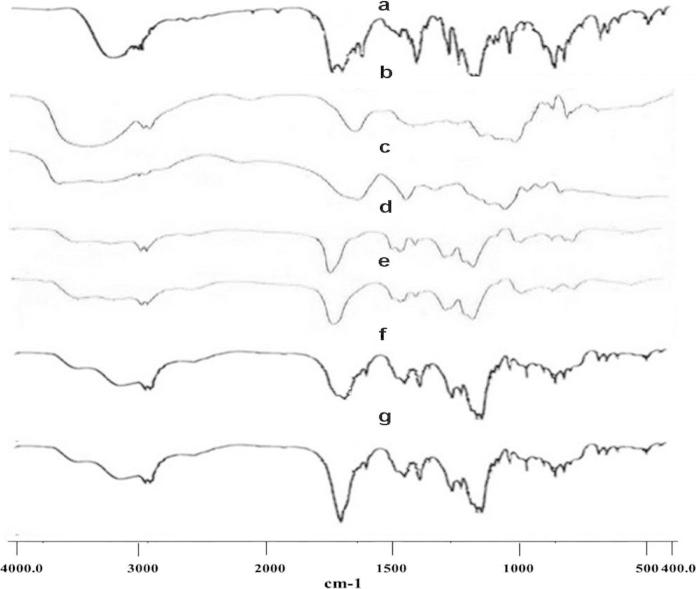
In order to evaluate the compatibility, the FT-IR spectra were recorded in between 400 and 4000 cm−1 for pure drug Naproxen, polymers and their physical mixtures (as shown in Fig. 2). For the present research Naproxen was used as the model drug. It has shown —OH, —CH3, —CH3 and —C O stretchings due to the presence of characteristic peaks at 3166 cm−1, 3002 cm−1, 2963 cm−1 and 1727 cm−1 respectively. These are all the characteristic peaks of Naproxen. The guar gum polymer spectrum has shown —OH, —CH and —CO stretchings due to the presence of characteristic peaks at 3382 cm−1, 2938 cm−1 and 1241 cm−1 respectively. Whereas, the Sodium alginate polymer spectrum has also shown similar —OH, —CH and —CO stretchings due to the presence of characteristic peaks at 3567 cm−1, 2943 cm−1 and 1302 cm−1 respectively. The Eudragit L100 polymer spectrum has shown —OH, —OCH3, —CH3 and —C O stretchings due to the presence of characteristic peaks at 3258 cm−1, 2997 cm−1, 2952 cm−1 and 1731 cm−1 respectively. Whereas, Eudragit S100 polymer spectrum also shows similar –OH, —OCH3, —CH3 and —C O stretchings due to the presence of characteristic peaks at 3225 cm−1, 2998 cm−1, 2953 cm−1 and 1727 cm−1 respectively. When the physical mixture spectra of Naproxen, guar gum, sodium alginate and Naproxen, Eudragit L100, Eudragit S100 were recorded, their respective higher spectrum has shown all the peaks corresponding to the three constituents. None of the peak was absent, as they were intact. Thus, it was observed that combination of pure drug Naproxen and the used polymers can be suitable for formulating matrix-mini-tablets meant for its desired therapeutic purpose (Hadi et al., 2014a, Hadi et al., 2014b).
Figure 2.
FTIR spectra of (a) pure drug, Naproxen, (b) Guar gum, (c) Sodium alginate, (d) Eudragit L100, (e) Eudragit S100, (f) physical mixture of Naproxen + Guar gum + Sodium alginate and (g) physical mixture of Naproxen + Eudragit L100 + Eudragit S100.
3.2. DSC studies
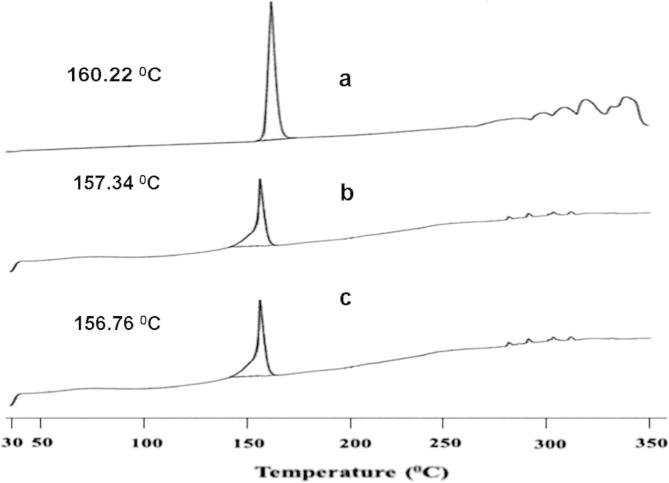
The above FTIR studies observation was further confirmed by DSC studies. The DSC thermograms of pure drug Naproxen and their physical mixtures with microsomal enzyme and pH sensitive polymers are shown in Fig. 3. The DSC thermogram of pure drug Naproxen, corresponding to its melting point gives a sharp exothermic peak at 160.22 °C. However, the DSC thermograms for physical mixtures of Naproxen, guar gum, sodium alginate and Naproxen, Eudragit L100, Eudragit S100 have not shown any significant shift in their exothermic peaks i.e. their peaks were found at 157.34 °C and 156.76 °C, respectively. Thus, the results of DSC thermograms also confirmed that the physical mixtures of drug and polymers used in the formulation batches were free from any chemical interaction (Hadi et al., 2014b).
Figure 3.
DSC spectra of (a) pure drug Naproxen, (b) physical mixture of Naproxen + Guar gum + Sodium alginate and (c) physical mixture of Naproxen + Eudragit L100 + Eudragit S100.
3.3. Evaluation of the powder blend of matrix-mini-tablets
The angle of repose values for the prepared blend of matrix-mini-tablets were found to range between 22°.25′ ± 0.17 and 24°.97′ ± 0.11. The LBD and TBD values were found to range between 0.503 ± 0.00 and 0.553 ± 0.00 and between 0.576 ± 0.00 and 0.628 ± 0.01 gm/cc respectively. The Carr’s compressibility index values were found to range between 10.23 ± 0.26 and 14.04 ± 0.86%. The Hausner’s ratio values were found to range between 1.11 ± 0.00 and 1.16 ± 0.01. As the values for angle of repose, Carr’s compressibility index and Hausner’s ratio were found to be less than 30°, 15% and 1.25 respectively it indicates good flow properties. Thus, the prepared powder blend of matrix-mini-tablets was found to exhibit good flow properties which are evident from the results shown in Table 2.
Table 2.
Physical evaluation results of pre-compression blend.
| Formulation code | Angle of repose (°) ±SD, n = 3 | Bulk density (gm/cc) ±SD, n = 3 | Tapped density (gm/cc) ±SD, n = 3 | Carr’s index (%) ±SD, n = 3 | Hausner’s ratio ±SD, n = 3 |
|---|---|---|---|---|---|
| F1 | 23°.67′ ± 0.14 | 0.510 ± 0.01 | 0.583 ± 0.01 | 12.56 ± 0.88 | 1.14 ± 0.01 |
| F2 | 22°.25′ ± 0.17 | 0.513 ± 0.01 | 0.586 ± 0.01 | 12.50 ± 1.12 | 1.14 ± 0.01 |
| F3 | 24°.16′ ± 0.20 | 0.516 ± 0.00 | 0.593 ± 0.00 | 12.91 ± 0.92 | 1.14 ± 0.01 |
| F4 | 24°.32′ ± 0.29 | 0.536 ± 0.01 | 0.606 ± 0.01 | 12.25 ± 1.34 | 1.14 ± 0.01 |
| F5 | 23°.65′ ± 0.12 | 0.523 ± 0.01 | 0.596 ± 0.01 | 12.28 ± 0.74 | 1.14 ± 0.00 |
| F6 | 22°.35′ ± 0.22 | 0.520 ± 0.01 | 0.586 ± 0.00 | 11.36 ± 1.04 | 1.12 ± 0.01 |
| F7 | 22°.57′ ± 0.28 | 0.540 ± 0.01 | 0.613 ± 0.01 | 11.94 ± 0.65 | 1.13 ± 0.00 |
| F8 | 22°.89′ ± 0.19 | 0.546 ± 0.00 | 0.620 ± 0.01 | 11.82 ± 0.76 | 1.13 ± 0.00 |
| F9 | 24°.60′ ± 0.22 | 0.536 ± 0.01 | 0.610 ± 0.01 | 12.02 ± 0.96 | 1.13 ± 0.01 |
| F10 | 23°.61′ ± 0.12 | 0.520 ± 0.01 | 0.593 ± 0.01 | 14.04 ± 0.86 | 1.16 ± 0.01 |
| F11 | 23°.38′ ± 0.33 | 0.546 ± 0.00 | 0.613 ± 0.00 | 10.86 ± 0.89 | 1.12 ± 0.01 |
| F12 | 22°.68′ ± 0.24 | 0.526 ± 0.01 | 0.600 ± 0.01 | 12.22 ± 0.97 | 1.13 ± 0.01 |
| F13 | 23°.99′ ± 0.10 | 0.546 ± 0.01 | 0.623 ± 0.00 | 12.29 ± 0.87 | 1.14 ± 0.01 |
| F14 | 24°.22′ ± 0.24 | 0.520 ± 0.01 | 0.593 ± 0.00 | 12.36 ± 1.04 | 1.14 ± 0.01 |
| F15 | 24°.02′ ± 0.18 | 0.533 ± 0.00 | 0.620 ± 0.01 | 10.74 ± 0.79 | 1.12 ± 0.00 |
| F16 | 24°.75′ ± 0.20 | 0.503 ± 0.00 | 0.576 ± 0.00 | 12.71 ± 0.94 | 1.14 ± 0.01 |
| F17 | 23°.11′ ± 0.16 | 0.540 ± 0.01 | 0.610 ± 0.01 | 11.47 ± 0.18 | 1.12 ± 0.00 |
| F18 | 23°.93′ ± 0.22 | 0.553 ± 0.01 | 0.620 ± 0.01 | 10.74 ± 0.64 | 1.12 ± 0.00 |
| F19 | 23°.84′ ± 0.18 | 0.526 ± 0.01 | 0.609 ± 0.01 | 10.23 ± 0.26 | 1.11 ± 0.00 |
| F20 | 24°.48′ ± 0.15 | 0.550 ± 0.01 | 0.628 ± 0.01 | 11.29 ± 0.18 | 1.12 ± 0.00 |
| F21 | 24°.97′ ± 0.11 | 0.513 ± 0.01 | 0.576 ± 0.01 | 10.97 ± 0.79 | 1.12 ± 0.01 |
| F22 | 23°.07′ ± 0.20 | 0.520 ± 0.01 | 0.593 ± 0.00 | 12.36 ± 1.04 | 1.14 ± 0.01 |
| F23 | 22°.89′ ± 0.24 | 0.510 ± 0.01 | 0.583 ± 0.01 | 12.56 ± 0.88 | 1.14 ± 0.01 |
| F24 | 22°.64′ ± 0.17 | 0.533 ± 0.00 | 0.606 ± 0.01 | 12.07 ± 0.71 | 1.13 ± 0.00 |
3.4. Evaluation of matrix-mini-tablets
The weight variation values of the matrix-mini-tablets were found to range between 24 ± 0.10 and 27 ± 0.18 mg. The pharmacopoeial limit for percentage deviation of tablets of 130 mg or less is ± 10% and all the formulation batches were found to be passed according to the specifications given in Indian pharmacopoeia (IP). The values for hardness were found to be uniform and were found to range between 2.20 ± 0.09 and 2.39 ± 0.08 kg. Friability values have also shown that matrix-mini-tablets have got sufficient strength and were found to range between 0.26 ± 0.09 and 0.61 ± 0.08%. The values for thickness were found to range between 2.04 ± 0.01 and 2.11 ± 0.01 mm. Excellent drug content uniformity was also found in matrix-mini-tablets, as their values were found to range between 97.40 ± 0.13 and 99.98 ± 0.08% which is more than 95%. Thus, all the post-compressional parameters of matrix-mini-tablets were found to be satisfactory as evident from the results shown in Table 3.
Table 3.
Evaluation results of matrix-mini-tablets.
| Formulation code | Weight variation (mg) (±SD), n = 20 | Hardness (kg) (±SD), n = 6 | Thickness (mm) (±SD), n = 6 | Friability (%) (±SD), n = 20 | % Drug content (±SD), n = 3 |
|---|---|---|---|---|---|
| F1 | 25 ± 0.18 | 2.24 ± 0.08 | 2.09 ± 0.02 | 0.57 ± 0.07 | 99.56 ± 0.12 |
| F2 | 26 ± 0.06 | 2.35 ± 0.12 | 2.05 ± 0.02 | 0.39 ± 0.05 | 99.62 ± 0.08 |
| F3 | 25 ± 0.26 | 2.28 ± 0.07 | 2.02 ± 0.01 | 0.44 ± 0.08 | 99.11 ± 0.16 |
| F4 | 24 ± 0.17 | 2.32 ± 0.15 | 2.08 ± 0.01 | 0.33 ± 0.04 | 99.85 ± 0.10 |
| F5 | 23 ± 0.25 | 2.26 ± 0.10 | 2.06 ± 0.02 | 0.64 ± 0.09 | 97.40 ± 0.13 |
| F6 | 25 ± 0.15 | 2.25 ± 0.08 | 2.05 ± 0.01 | 0.32 ± 0.05 | 99.31 ± 0.06 |
| F7 | 26 ± 0.26 | 2.31 ± 0.10 | 2.08 ± 0.01 | 0.47 ± 0.05 | 98.91 ± 0.10 |
| F8 | 27 ± 0.20 | 2.36 ± 0.08 | 2.05 ± 0.02 | 0.36 ± 0.08 | 99.84 ± 0.08 |
| F9 | 25 ± 0.10 | 2.38 ± 0.11 | 2.06 ± 0.02 | 0.50 ± 0.09 | 98.96 ± 0.10 |
| F10 | 27 ± 0.18 | 2.33 ± 0.16 | 2.04 ± 0.01 | 0.43 ± 0.06 | 99.77 ± 0.06 |
| F11 | 24 ± 0.16 | 2.36 ± 0.14 | 2.09 ± 0.01 | 0.61 ± 0.08 | 99.98 ± 0.08 |
| F12 | 25 ± 0.19 | 2.29 ± 0.10 | 2.05 ± 0.01 | 0.59 ± 0.07 | 99.06 ± 0.10 |
| F13 | 24 ± 0.21 | 2.30 ± 0.13 | 2.04 ± 0.02 | 0.57 ± 0.06 | 99.77 ± 0.12 |
| F14 | 24 ± 0.10 | 2.32 ± 0.07 | 2.10 ± 0.02 | 0.35 ± 0.05 | 97.65 ± 0.07 |
| F15 | 26 ± 0.11 | 2.36 ± 0.05 | 2.08 ± 0.02 | 0.47 ± 0.05 | 98.93 ± 0.10 |
| F16 | 26 ± 0.16 | 2.27 ± 0.14 | 2.06 ± 0.01 | 0.26 ± 0.09 | 99.30 ± 0.08 |
| F17 | 25 ± 0.29 | 2.32 ± 0.12 | 2.08 ± 0.01 | 0.41 ± 0.07 | 99.58 ± 0.05 |
| F18 | 27 ± 0.12 | 2.20 ± 0.09 | 2.06 ± 0.02 | 0.59 ± 0.06 | 99.24 ± 0.10 |
| F19 | 25 ± 0.23 | 2.25 ± 0.06 | 2.11 ± 0.01 | 0.29 ± 0.07 | 98.88 ± 0.06 |
| F20 | 25 ± 0.15 | 2.37 ± 0.10 | 2.03 ± 0.02 | 0.32 ± 0.05 | 99.15 ± 0.06 |
| F21 | 24 ± 0.10 | 2.39 ± 0.08 | 2.07 ± 0.01 | 0.48 ± 0.08 | 98.02 ± 0.14 |
| F22 | 26 ± 0.28 | 2.22 ± 0.12 | 2.08 ± 0.01 | 0.31 ± 0.09 | 99.69 ± 0.10 |
| F23 | 25 ± 0.20 | 2.34 ± 0.06 | 2.09 ± 0.01 | 0.28 ± 0.10 | 99.40 ± 0.09 |
| F24 | 25 ± 0.12 | 2.28 ± 0.12 | 2.05 ± 0.01 | 0.57 ± 0.06 | 99.32 ± 0.12 |
3.5. In-vitro dissolution testing of matrix-mini-tablets-filled capsule systems
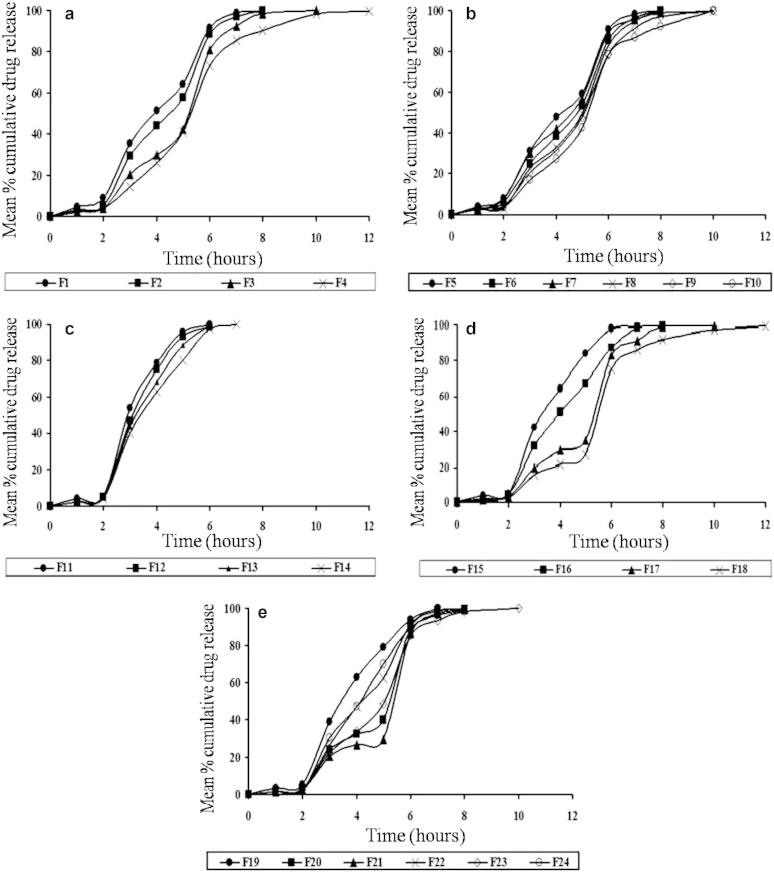
During dissolution testing of formulations, it was found that the HPMC capsule disintegrated and the matrix-mini-tablets were released within 9 min in 1.2 pH dissolution media. In our attempt to target Naproxen at the ileo-colonic junction, we prepared twenty-four formulations of matrix-mini-tablets by using Guar gum, Sodium alginate, Eudragit L100 and Eudragit S100 polymers both in individual and combined concentrations (i.e. 8%, 16%, 24%, 32%). From the results of dissolution testing, it was found that all the formulations except F18 could only prevent the Naproxen release in pH 1.2 buffer but could not prevent the release in. pH 6.5 and 6.8 buffers. However, when compared to all the formulations, only the release from formulations F18 was found to release very less amount of naproxen at acidic and intestinal pH and maximum portion of Naproxen at ileo-colonic pH. Because it releases Naproxen after a lag time of 2.45 ± 0.97 h and 27.30 ± 0.86%, 92.59 ± 0.47%, 99.38 ± 0.69% at the end of 5, 8, 12 h respectively. Thus, it was considered to be the best formulation for matrix-mini-tablets-filled-capsule formulations of Naproxen. The in-vitro dissolution testing results of all the matrix-mini-tablets-filled capsule formulations are shown in Table 4 and represented in Fig. 4.
Table 4.
Results of in-vitro release studies of Naproxen.
| Formulation code | Lag time in hours (i.e. time taken for less than 10% of Naproxen release) | Mean % cumulative drug release at the end of 8 h | Mean % cumulative drug release at the end of 12 h |
|---|---|---|---|
| F1 | 2.01 ± 0.89 | 99.52 ± 0.82 | – |
| F2 | 2.12 ± 0.70 | 99.87 ± 0.90 | – |
| F3 | 2.36 ± 0.93 | 98.39 ± 0.44 | 99.86 ± 0.94+ |
| F4 | 2.42 ± 0.80 | 90.16 ± 0.76 | 99.73 ± 0.52 |
| F5 | 2.03 ± 0.66 | 99.95 ± 0.59 | – |
| F6 | 2.20 ± 1.07 | 99.80 ± 1.14 | – |
| F7 | 2.08 ± 0.59 | 99.74 ± 0.78 | – |
| F8 | 2.25 ± 1.15 | 98.60 ± 0.61 | 99.46 ± 0.70+ |
| F9 | 2.34 ± 0.61 | 97.10 ± 0.98 | 99.85 ± 0.83+ |
| F10 | 2.40 ± 0.98 | 91.92 ± 0.39 | 99.90 ± 0.95+ |
| F11 | 2.03 ± 0.74 | 99.97 ± 0.89∗ | – |
| F12 | 2.06 ± 0.96 | 98.61 ± 0.71∗ | – |
| F13 | 2.10 ± 0.80 | 99.57 ± 0.40∗ | – |
| F14 | 2.15 ± 0.79 | 99.86 ± 0.95∗ | – |
| F15 | 2.09 ± 0.94 | 99.10 ± 0.62∗ | – |
| F16 | 2.14 ± 0.67 | 99.47 ± 0.37 | – |
| F17 | 2.34 ± 1.10 | 99.69 ± 1.18 | – |
| F18 | 2.45 ± 0.97 | 92.59 ± 0.47 | 99.38 ± 0.69 |
| F19 | 2.11 ± 0.74 | 99.94 ± 1.07∗ | – |
| F20 | 2.27 ± 0.90 | 99.47 ± 0.36 | – |
| F21 | 2.32 ± 0.96 | 99.20 ± 0.54 | – |
| F22 | 2.24 ± 0.79 | 99.74 ± 0.86 | – |
| F23 | 2.26 ± 0.88 | 98.26 ± 0.73 | 99.88 ± 0.92+ |
| F24 | 2.21 ± 0.94 | 99.15 ± 0.66 | – |
Note: ∗Mark indicates that the Naproxen was released before 8 h.
+Mark indicates that the Naproxen was released before 12 h.
Figure 4.
In-vitro release profile of matrix-mini-tablets-filled capsule formulations prepared with (a) Guar gum, (b) combination of Guar gum and Sodium alginate, (c) Eudragit L100, (d) Eudragit S100 and (e) combination of Eudragit L100 and Eudragit S100.
3.5.1. Effect of microsomal enzyme dependent polymers (Guar gum and sodium alginate)
From the in-vitro dissolution profile, it was found that as the guar gum polymer concentration was increasing the lag time (i.e. time taken for less than 10% of Naproxen release) was increased and release rate of Naproxen was decreased. This is due to the reason that guar gum polymer because of its microsomal enzyme dependent characteristics has retarded the release of Naproxen in acidic and intestinal pH buffers and increased the release in colonic buffer containing 0.05 mg/ml of betagalactomannase enzyme. But when guar gum and sodium alginate were used as combination polymers, it was found that the Naproxen release rate was increased while compared to batches formulated with guar gum alone. This is due to the reason that sodium alginate has increased the permeation of Naproxen from guar gum polymer because of its quickly water absorbing capacity.
3.5.2. Effect of pH dependent polymers (Eudragit L100 and Eudragit S100)
From the in-vitro dissolution profile, it was found that as the individual concentrations of both the polymers in matrix-mini-tablets were increasing the lag time was increased and release rate of Naproxen was decreased. This is due to the reason that Eudragit polymers because of their pH dependent solubility characteristics have retarded the release of Naproxen in acidic and intestinal pH buffers and increased the release in ileo-colonic buffer. But when both the Eudragit L100 and Eudragit S100 polymers were used in combination, it was found that the Naproxen release rate was increased from matrix-mini-tablets while compared to batches formulated with Eudragit S100 alone. This is due to the reason that the Eudragit L100 polymer because of its good solubility in 6.5 and 6.8 pH buffers increased the Naproxen permeation from Eudragit S100 polymer which dissolves at 7.2 pH buffer.
3.6. Stability studies
The obtained results of stability studies at both room temperature and accelerated stability revealed that the optimized matrix-mini-tablets-filled capsule formulation did not shown any significant changes in physical stability parameters such as Appearance, Weight variation, Hardness, Thickness, Friability, Drug content estimation and In-vitro release profile of Naproxen during the period of study (as shown in Table 5). Thus, for the optimized formulation stability was found as per ICH guidelines.
Table 5.
Results of physical stability studies for optimized formulation F18.
| Parameters | Storage conditions and time (months) |
||||
|---|---|---|---|---|---|
| Initial results | Room temperature | Accelerated stability | |||
| 30 ± 2 °C and 65 ± 5% RH |
40 ± 2 °C and 75 ± 5% RH |
||||
| 0 | 3 | 6 | 3 | 6 | |
| Appearance | White circular mini-tablets | No change in appearance | No change in appearance | No change in appearance | No change in appearance |
| Weight variation (mg) (±SD), n = 20 | 27 ± 0.12 | 27 ± 0.18 | 26 ± 0.15 | 26 ± 0.14 | 25 ± 0.10 |
| Hardness (kg) (±SD), n = 6 | 2.20 ± 0.09 | 2.19 ± 0.03 | 2.18 ± 0.06 | 2.20 ± 0.05 | 2.20 ± 0.08 |
| Thickness (mm) (±SD), n = 6 | 2.06 ± 0.02 | 2.06 ± 0.02 | 2.06 ± 0.01 | 2.06 ± 0.01 | 2.06 ± 0.01 |
| Friability (%) (±SD), n = 6 | 0.59 ± 0.06 | 0.43 ± 0.08 | 0.50 ± 0.05 | 0.54 ± 0.04 | 0.52 ± 0.07 |
| % Drug content (±SD), n = 3 | 99.24 ± 0.10 | 98.79 ± 0.17 | 98.43 ± 0.15 | 99.13 ± 0.14 | 98.81 ± 0.20 |
| Lag time in hours (i.e. time taken for less than 10% of Naproxen release) | 2.45 ± 0.97 | 2.40 ± 0.75 | 2.37 ± 0.98 | 2.44 ± 0.83 | 2.41 ± 0.67 |
| Mean % cumulative drug release at the end of 8 h | 92.59 ± 0.47 | 92.37 ± 0.96 | 92.01 ± 0.65 | 92.16 ± 0.82 | 91.77 ± 0.70 |
| Mean % cumulative drug release at the end of 12 h | 99.38 ± 0.69 | 98.97 ± 0.55 | 98.94 ± 0.87 | 99.24 ± 0.71 | 98.73 ± 0.91 |
4. Conclusion
As the aim was to target Naproxen for the treatment of rheumatoid arthritis according to chronotherapeutic pattern, so a novel ileo-colonic targeted delivery system of Naproxen was developed by filling fifteen matrix-mini-tablets into an empty HPMC capsule. By using microsomal enzyme dependent and pH dependent polymers a total number of twenty-four formulations were prepared and formulation F18 was considered as the best formulation as it released Naproxen after a lag time of 2.45 ± 0.97 h and 27.30 ± 0.86%, 92.59 ± 0.47%, 99.38 ± 0.69% at the end of 5, 8, 12 h respectively. This formulation was also found to be stable as per the ICH guidelines.
Acknowledgment
The authors are very much thankful to the Chairman of JB group of Educational Institutions Shri. J. V. Krishna Rao Garu for his constant help, support and encouragement to the academics generally and research particularly. The authors are also thankful to him for providing suitable research laboratory facilities at Bhaskar Pharmacy College, R.R.District, Hyderabad. The results described in this paper are a part of academic thesis.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akhgari A., Afrasiabi Garekani H., Sadeghi F., Azimaie M. Statistical optimization of indomethacin pellets coated with pH-dependent methacrylic polymers for possible colonic drug delivery. Int. J. Pharm. 2005;305:22–30. doi: 10.1016/j.ijpharm.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Arvidson N.G., Gudbjornsson B., Elfman L., Ryden A.C., Totterman T.H., Hallgren R. Circadian rhythm of serum interleukin-6 in rheumatoid arthritis. Ann. Rheum. Dis. 1994;53:521–524. doi: 10.1136/ard.53.8.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F. Chronotherapy with glucorticoids in rheumatoid arthritis. Rheumatologist. 2011;(January) [Google Scholar]
- Cha J., Gilmor T., Lane P., Ranweiler J.S. Stability studies in Handbook of modern pharmaceutical analysis. Elsevier; 2001. pp. 459–505. (Separation Science and Technology). [Google Scholar]
- Chickpetty S.M., Baswaraj R., Nanjwade B.K. Studies on development of novel combined time and ph dependent solventless compression coated delivery systems for colonic delivery of diclofenac sodium. Asian J. Pharm. Clin. Res. 2010;3:110–113. [Google Scholar]
- Cutolo M. Rheumatoid arthritis chronotherapy. Eur. Musculoskeletal. Rev. 2012;7:29–32. [Google Scholar]
- Dew M.J., Hughes P.J., Lee M.G., Evans B.K., Rhodes J. An oral preparation to release drugs in the human colon. Br. J. Clin. Pharmacol. 1982;14:405–408. doi: 10.1111/j.1365-2125.1982.tb01999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D.F., Pye G., Bramley R., Clark A.G., Dyson T.J., Hardcastle J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegely Kurt. The solid dose. Colorcon News. 2009:1–5. [Google Scholar]
- Gothaskar A.V., Joshi A.M., Joshi N.H. Pulsatile drug delivery system – a review. Drug Deliv. Technol. 2004;4 < http://www.drugdeliverytech.com>. [Google Scholar]
- Hadi Mohd Abdul, Raghavendra Rao N.G., Sunil F. Mini-tablets technology: an overview. Am. J. Pharm. Tech. Res. 2012;2(2):128–150. [Google Scholar]
- Hadi Mohd Abdul, Raghavendra Rao N.G., Srinivasa Rao A. Matrix-mini-tablets of lornoxicam for targeting early morning peak symptoms of rheumatoid arthritis. Iran. J. Bas. Med. Sci. 2014;17:357–369. [PMC free article] [PubMed] [Google Scholar]
- Hadi Mohd Abdul, Raghavendra Rao N.G., Srinivasa Rao A. Formulation and evaluation of pH responsive mini-tablets for ileo-colonic targeted drug delivery. Trop. J. Pharm. Res. 2014;13(7):1021–1029. [Google Scholar]
- Ibekwe V.C., Liu F., Fadda H.M., Khela M.K., Evans D.F., Parsons G.E., Basit A.W. An investigation into the in vivo performance variability of pH responsive polymers for ileo-colonic drug delivery using gamma scintigraphy in humans. J. Pharm. Sci. 2006;95:2760–2766. doi: 10.1002/jps.20742. [DOI] [PubMed] [Google Scholar]
- Ibekwe V.C., Fadda H., Mc Connell E.L., Khela M.K., Evans D.F., Basit A.W. Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharm. Res. 2008;25:1828–1835. doi: 10.1007/s11095-008-9580-9. [DOI] [PubMed] [Google Scholar]
- ICH Guidelines, 2003. Stability testing of new drug substances and products. Q1A (R2) Step 4 versions. <http://www.ich.org/cache/compo/363-272- html #Q1C, http://www.isppharmaceuticals.com Literature/SPPH5951_Advantia_Perf_Case_Study_VF.pdf>.
- Kaur G., Rana V., Jain S., Tiwary A.k. Colon delivery of budesonide: evaluation of chitosan-chondroitin sulfate interpolymer complex. AAPS. Pharm. Sci. Tech. 2010;11:36–45. doi: 10.1208/s12249-009-9353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishainah Y.S.R., Satyanarayan S. In: Advances in Controlled and Novel Drug Delivery. first ed. Jain N.K., editor. CBS Publishers and Distributors; New Delhi: 2001. Colon-specific drug delivery system; pp. 89–119. [Google Scholar]
- Lopes C.M., Lobo J.M., Pinto J.F., Costa P. Compressed mini-tablet as a biphasic drug delivery system. Int. J. Pharm. 2006;323:93–100. doi: 10.1016/j.ijpharm.2006.05.063. [DOI] [PubMed] [Google Scholar]
- Maghsoodi M., Kiafar F. Co-precipitation with PVP and agar to improve physicomechanical properties of ibuprofen. Iran. J. Basic. Med. Sci. 2013;16:627–634. [PMC free article] [PubMed] [Google Scholar]
- Mirela B., Tomuta I., Leucuta S. Identifica-tion of critical formulation variables for obtaining metoprolol tartrate mini-tablets. Farmacia. 2010;58:719–727. [Google Scholar]
- Mohapatra S.K., Kshirsagar S.J., Bhalekar M.R., Shukla G.N., Patil A.V. Development and evaluation of enzymatically triggered multiparticulate colon targeted drug delivery system. Int. J. Res. Ayurveda Pharm. 2011;2:211–215. [Google Scholar]
- Najmuddin M., Ashok Patel V., Azgar A., Shelar S., Tousif K. Development and evaluation of pulsatile drug delivery system of flurbiprofen. Res. J. Pharm. Biol. Chem. Sci. 2010;1:289–290. [Google Scholar]
- Rajabi Siaboomin A. The solid dose. Colorcon News. 2009:1–4. [Google Scholar]
- Uziel Y., Haskhes E., Kaseem S., Padeh R., Goldman Vollach B. The use of naproxen in the treatment of children with rheumatic fever. J. Pediatr. 2000;137:269–271. doi: 10.1067/mpd.2000.107158. [DOI] [PubMed] [Google Scholar]
- Wong D., Larrabee S., Clifford K., Tremblay J., Friend D.R. USP dissolution apparatus III (reciprocating cylinder) for screening of guar-based colonic delivery formulations. J. Control. Release. 1997;47:173–179. [Google Scholar]