Abstract
The study was aimed at developing extended release matrix tablets of poorly water-soluble diclofenac sodium and highly water-soluble metformin hydrochloride by direct compression using cashew gum, xanthan gum and hydroxypropylmethylcellulose (HPMC) as release retardants. The suitability of light grade cashew gum as a direct compression excipient was studied using the SeDeM Diagram Expert System. Thirteen tablet formulations of diclofenac sodium (∼100 mg) and metformin hydrochloride (∼200 mg) were prepared with varying amounts of cashew gum, xanthan gum and HPMC by direct compression. The flow properties of blended powders and the uniformity of weight, crushing strength, friability, swelling index and drug content of compressed tablets were determined. In vitro drug release studies of the matrix tablets were conducted in phosphate buffer (diclofenac: pH 7.4; metformin: pH 6.8) and the kinetics of drug release was determined by fitting the release data to five kinetic models. Cashew gum was found to be suitable for direct compression, having a good compressibility index (ICG) value of 5.173. The diclofenac and metformin matrix tablets produced generally possessed fairly good physical properties. Tablet swelling and drug release in aqueous medium were dependent on the type and amount of release retarding polymer and the solubility of drug used. Extended release of diclofenac (∼24 h) and metformin (∼8–12 h) from the matrix tablets in aqueous medium was achieved using various blends of the polymers. Drug release from diclofenac tablets fitted zero order, first order or Higuchi model while release from metformin tablets followed Higuchi or Hixson-Crowell model. The mechanism of release of the two drugs was mostly through Fickian diffusion and anomalous non-Fickian diffusion. The study has demonstrated the potential of blended hydrophilic polymers in the design and optimization of extended release matrix tablets for soluble and poorly soluble drugs by direct compression.
Keywords: Cashew gum, Xanthan gum, HPMC, Direct compression, SeDeM Diagram Expert System, Diclofenac sodium, Metformin hydrochloride
1. Introduction
Hydrophilic polymers such as guar gum, pectin, chitosan, cashew gum, xanthan gum, HPMC and microcrystalline cellulose are pharmaceutical excipients which have been utilized individually or in blends to design hydrophilic matrix tablets to achieve controlled drug delivery (Maciel et al., 2006, Chivate et al., 2008, Nussinovitch, 2009, Vohra et al., 2012, Ali et al., 2013, Baviskar et al., 2013). Controlled-release dosage forms generally have reduced frequency of dosing, increased compliance, increased therapeutic effect, reduced side-effects, improved tolerability and reduced cost of treatment (Das and Das, 2003, Kamboj et al., 2009). Blending different hydrophilic polymers improves the physicochemical and release modifying properties of the resultant polymer leading to the design and formation of an optimized controlled-release product (Ofori-Kwakye et al., 2013) and the proper selection of polymers can help control the release profile of drugs (Fung and Saltzman, 1997). Hydrophilic matrix systems undergo swelling followed by gel formation, erosion and dissolution in aqueous media. In addition, such systems can sustain high drug loading and the excipients used are inexpensive and generally regarded as safe. They may however require optimal rate-controlling polymers for different active pharmaceutical ingredients (API’s) and there could be challenges with scale-up of manufacture (Aulton, 2007).
Hydrophilic matrix tablets can be manufactured by wet granulation or direct compression techniques (Colombo et al., 2000). Direct compression is a simple technique of tableting a blend of powdered ingredients without granule formation or agglomeration process (Thakkar et al., 2009, Thoorens et al., 2014) and involves two sequential operations of powder mixing and tableting. The procedure requires the use of API’s and or excipients with good flow and compressibility. As there is no involvement of heat and moisture in the process, direct compression is well-suited for heat and moisture-sensitive drugs and also enhances product stability (Aulton, 2007). The small number of operations involved in direct compression makes for reduced production cost making direct compression the most economical technique of manufacturing large batches of tablets (Thoorens et al., 2014). The major challenges associated with direct compression caused by the use of API’s and excipients with poor physical attributes include poor flowability of powder blends, variability in tablet weight, poor content uniformity, tablets with poor mechanical strength and poor dissolution properties (Hentzschel et al., 2012, Thoorens et al., 2014).
Diclofenac sodium is a poorly water soluble (pKa = 4), Biopharmaceutics Classification System (BCS) class II (low solubility and high permeability), non-steroidal anti-inflammatory drug. It is commonly used in the treatment of mild to moderate post-operative or post-traumatic pain, menstrual pain and endometritis (Dastidar et al., 2000). Extended release diclofenac formulations are required for the treatment of chronic conditions such as rheumatoid arthritis, osteoarthritis, chronic pain, ankylosing spondylitis and actinic keratosis.
Metformin hydrochloride is a highly water soluble (>300 mg/ml at 25 °C; pKa = 2.8 and 11.5), BCS class III (high solubility and low permeability) oral anti-diabetic drug in the biguanide class. It is a first line drug in the treatment of type-2 diabetes. Extended release metformin is needed for the long-term management and control of type-2 diabetes mellitus.
The aim of the current study was to design extended release oral matrix tablets of diclofenac sodium and metformin hydrochloride using varying blends of three hydrophilic polymers. The objective was to enhance the drug release modifying properties of the polymers leading to the formation of optimized formulations of the two model drugs with different water solubilities.
2. Materials and methods
2.1. Materials
Metformin HCl was a gift from Ernest Chemist Ltd. (Accra, Ghana). Diclofenac sodium BP was sourced from Hubei Prosperity Galaxy Chemical Co. Ltd. (China). Xanthan gum (SHL_PHXG980) was obtained from Luckystar Additives Co. Ltd. (Hong Kong). Microcrystalline cellulose (ACCEL 101) was sourced from Lavina Pharmaceuticals Pvt. Ltd., India. Glucophage® and voltaren retard® were purchased from retail pharmacies in Kumasi, Ghana. Potassium dihydrogen orthophosphate was obtained from Central Drug House Ltd. (New Delhi, India) and phosphoric acid and diethyl ether from Pokupharma Ltd. (Kumasi, Ghana). HPMC (Methocel E-15), magnesium stearate, ethanol, sodium hydroxide pellets, and hydrochloric acid were obtained from the Chemical store of the Pharmaceutics Department, KNUST, (Kumasi, Ghana). All other reagents used were of analytical grade. Crude cashew gum was collected from Bodokrom cashew plantation (Eastern Region, Ghana) and manually sorted into light and dark grades and the light grade cashew gum was purified as described elsewhere (Ofori-Kwakye et al., 2010). The purified light grade cashew gum (yield: 75.19%; moisture content 5.25 ± 0.35%, size range: 50–425 μm; swelling capacity: 3.33 (distilled water), 3.83 (phosphate buffer pH 6.8), 3.91 (phosphate buffer pH 7.4) (Mfoafo, 2013)) was employed as a direct compression excipient in the preparation of matrix tablets.
2.2. Preparation of blended powders
The compositions of various diclofenac sodium and metformin HCl matrix tablet formulations are presented in Table 1, Table 2, respectively. Accurately weighed amounts of diclofenac sodium, microcrystalline cellulose, cashew gum, xanthan gum and HPMC were thoroughly mixed geometrically in a mortar for 15 min. The same procedure was employed in blending metformin HCl, microcrystalline cellulose, cashew gum, xanthan gum and HPMC. The powder blends prepared were lubricated with magnesium stearate for 2 min and stored for subsequent analysis and direct compression.
Table 1.
Composition of different formulations of diclofenac sodium matrix tablets (∼500 mg tablet).
| Formulation | Diclofenac sodium (mg) | Cashew gum (mg) | Xanthan gum (mg) | HPMC (mg) | Microcrystalline cellulose (mg) | Magnesium stearate (mg) |
|---|---|---|---|---|---|---|
| D1 | 100 | 200 | – | – | 195 | 5 |
| D2 | 100 | 100 | – | – | 295 | 5 |
| D3 | 100 | 150 | 50 | – | 195 | 5 |
| D4 | 100 | 100 | 100 | – | 195 | 5 |
| D5 | 100 | 50 | 150 | – | 195 | 5 |
| D6 | 100 | – | 200 | – | 195 | 5 |
| D7 | 100 | 150 | – | 50 | 195 | 5 |
| D8 | 100 | 100 | – | 100 | 195 | 5 |
| D9 | 100 | 50 | – | 150 | 195 | 5 |
| D10 | 100 | – | – | 200 | 195 | 5 |
| D11 | 100 | 100 | 50 | 50 | 195 | 5 |
| D12 | 100 | 50 | 100 | 50 | 195 | 5 |
| D13 | 100 | 50 | 50 | 100 | 195 | 5 |
Table 2.
Composition of different formulations of metformin hydrochloride matrix tablets (∼600 mg tablet).
| Formulation | Metformin HCl (mg) | Cashew gum (mg) | Xanthan gum (mg) | HPMC (mg) | Microcrystalline cellulose (mg) | Magnesium stearate (mg) |
|---|---|---|---|---|---|---|
| M1 | 200 | 100 | – | – | 294 | 6 |
| M2 | 200 | 200 | – | – | 194 | 6 |
| M3 | 200 | – | 100 | – | 294 | 6 |
| M4 | 200 | – | 200 | – | 194 | 6 |
| M5 | 200 | – | – | 100 | 294 | 6 |
| M6 | 200 | – | – | 200 | 194 | 6 |
| M7 | 200 | 150 | 50 | - | 194 | 6 |
| M8 | 200 | 100 | 100 | - | 194 | 6 |
| M9 | 200 | 100 | 50 | 50 | 194 | 6 |
| M10 | 200 | 150 | – | 50 | 194 | 6 |
| M11 | 200 | 100 | – | 100 | 194 | 6 |
| M12 | 200 | 50 | 100 | 50 | 194 | 6 |
| M13 | 200 | 50 | 50 | 100 | 194 | 6 |
2.3. Bulk density measurements of cashew gum and blended powders
Ten grams of purified cashew gum was weighed and poured through a funnel into a 100 ml measuring cylinder. The cylinder was lightly tapped twice to collect all the granules sticking on the wall of the cylinder and the initial volume, Vo was recorded. The cylinder was tapped from a height of 2.0 cm on a wooden bench top to a constant volume of powder denoted as Vf. The initial density was calculated as the initial bulk density, Do = mass/Vo. The final density was calculated as the final bulk density or tapped bulk density, Df = mass/Vf. The ratio Df/Do was calculated as the Hausner ratio while Carr’s index was calculated as (Df − Do/Df) × 100% and the interparticle porosity was calculated as [(Df − Do)/(Df × Do)] (Aulton, 2007). The procedures were repeated in determining the Hausner ratio, Carr index and interparticle porosity of blended powders of diclofenac and metformin formulations.
2.4. Angle of repose of cashew gum and blended powders
The angles of repose (θ) of purified cashew gum and blended powders of diclofenac and metformin HCl formulations were determined using the fixed height method (Aulton, 2007). Each powder formulation was allowed to flow freely from a funnel at a fixed height onto a horizontal surface to form a cone. The base of the cone was marked and the height of the orifice of the funnel from the horizontal surface was measured. The height of the cone was measured and the angle of repose was calculated from the height of the cone (h) and the radius (r) of its base using the equation, θ = tan−1 h/r.
2.5. Determination of suitability of cashew gum for direct compression
Eight out of the twelve physicochemical tests (minimum reliability) used in the SeDeM Diagram method (Pérez-Lozano et al., 2006, Suñé-Negre et al., 2008, Suñe-Negre et al., 2011) were applied to purified light grade cashew gum to determine its suitability for direct compression. The Dimensional Parameters (bulk density and tapped density), Compressibility Parameters (inter-particle porosity and Carr’s index), Flowability Parameters (Hausner ratio and angle of repose), Stability Parameter (loss on drying) and Dosage Parameter (% particle < 75 μm) of light grade cashew gum were determined using pharmacopoeial and other accepted experimental procedures. Each result was converted to a radius on a 0–10 scale and an eight-sided polygon drawn from it after which the Parameter Index (nP ⩾ 5/nPt, where nP ⩾ 5 = number of parameters that have values equal to or greater than 5, and nPt = total number of parameters studied), Parameter Profile Index (average of radii of all parameters examined) and Reliability Factor (ratio of area of polygon and area of circle) were calculated (Suñe-Negre et al., 2011). The Good Compressibility Index (IGC) which is a measure of the suitability of a powdered material for direct compression was calculated by multiplying the Parameter Profile Index and the Reliability Factor. Powders with IGC values ⩾ 5 are considered suitable for use in direct compression (Pérez-Lozano et al., 2006, Suñé-Negre et al., 2008, Aguilar-Díaz et al., 2009).
2.6. Direct compression of tablets
Thirteen different diclofenac blended powder formulations were directly compressed into matrix tablets each containing ∼100 mg diclofenac with a nominal tablet weight of 500 mg, using a DP 30 Single-punch tablet Press (Pharmao Industries Co. Ltd., India) fitted with a concave punch and die set. The procedure was repeated to directly compress thirteen metformin HCl powder formulations into matrix tablets each containing ∼200 mg metformin with a nominal tablet weight of 600 mg.
2.7. Evaluation of tablet properties
Quality parameters of diclofenac sodium and metformin HCl matrix tablets, namely tablet thickness, uniformity of weight, crushing strength and friability were evaluated. A vernier caliper was used to measure the thickness of five (5) matrix tablets from each batch and the mean and standard deviation determined. The uniformity of weight of twenty tablets was determined according to the British Pharmacopoeia (2012) method. The crushing strength of ten tablets was determined by diametrical compression using a dr Schleuniger Hardness Tester (model 5Y, Pharmatron, Switzerland). All measurements were made in triplicate and the average calculated. The friability of the tablets was determined with an Erweka friabilator (Type TA20, Heusenstamm, Germany) according to the procedure of the United States Pharmacopoeia and National Formulary (2007). The ratio of the crushing strength to friability of a batch of tablets was determined as the crushing strength friability ratio (CSFR).
2.8. Determination of swelling index
The swelling behavior of diclofenac sodium and metformin HCl matrix tablets was determined at 37 ± 0.5 °C in phosphate buffer pH 7.4 and phosphate buffer pH 6.8, respectively, over 18 h. Five tablets from each formulation were individually kept in a petri dish containing 50 ml of the buffer solution. At the end of the stipulated period the tablet was removed, blotted with a tissue paper and weighed. The extent of swelling was calculated as: Swelling index = [(Mt − Mo)/Mo] × 100, where, Mt and Mo are the weight of tablet at time = t and time = 0, respectively.
2.9. Determination of drug content
The amount of diclofenac sodium in twenty randomly selected diclofenac sodium matrix tablets was determined spectrophotometrically (T90 UV/VIS Spectrometer, PG Instruments Ltd., England) at 276 nm as reported elsewhere (Ofori-Kwakye et al., 2013). Also, the amount of metformin HCl in ten randomly selected metformin HCl matrix tablets was determined spectrophotometrically at 233 nm (Narasimharao et al., 2011).
2.10. In vitro drug release studies
In vitro drug release studies were carried out using Voltaren retard® and Glucophage® as reference tablets for diclofenac and metformin, respectively. A USP type II dissolution apparatus (paddle method) (Erweka dissolution machine, Type DT6, GmbH, Heusenstamm, Germany) was used for the studies. The test conditions for diclofenac sodium matrix tablets were as follows: dissolution medium: 900 ml phosphate buffer pH 7.4; paddle speed: 50 rpm; temperature: 37 ± 0.5 °C; sampling period: 24 h. The same test conditions were used for metformin HCl matrix tablets except that the pH, paddle speed and sampling period were 6.8, 100 rpm and 12 h, respectively. At specified time intervals, 10 ml samples were withdrawn and replaced with the same volume of fresh dissolution medium maintained at 37 ± 0.5 °C. The withdrawn samples were rapidly filtered using a Whatman filter paper and diluted appropriately with the dissolution medium. The diluted filtrates were analyzed by UV spectrophotometry (T90 UV/VIS Spectrometer, PG Instruments Ltd., England) at 276 nm and 233 nm for diclofenac sodium and metformin HCl samples respectively using the respective dissolution media as reference solutions. A 1 cm quartz cuvette was used throughout the study. The amount of diclofenac in diclofenac sodium matrix tablets was determined using regression data (y = 345.51x + 0.1929, R2 = 0.9983) obtained from calibration plot of diclofenac in phosphate buffer pH 7.4 (0.75–2.5 mg/100 ml). The amount of metformin in metformin HCl matrix tablets was determined using regression data (y = 743x + 0.2872, R2 = 0.9947) obtained from a calibration plot of metformin in phosphate buffer pH 6.8 (0.2–0.6 mg/100 ml). From these, plots of percentage drug released from the tablet formulations (mean ± S.D., n = 3) versus time were established.
2.11. Kinetics of drug release
The kinetics of drug release from the matrix tablets was determined by fitting the appropriate drug release data to zero order (Varelas et al., 1995), first order (Gibaldi and Feldman, 1967, Wagner, 1969), Higuchi equation (Higuchi, 1963), Hixson-Crowell equation (Hixson and Crowell, 1931) and the Korsmeyer-Pepas model (Korsmeyer et al., 1983, Peppas, 1985).
where Q is amount of drug release at time t, Q0 is the initial amount of drug, QR is the amount of drug remaining at time t, and QT is the total amount of drug release. k0, k1, kH, ks and kkp are the kinetic constants for zero order, first order, Higuchi, Hixson-Crowell and Korsmeyer-Peppas models, respectively, and n is the release exponent.
2.12. Statistical analysis
The drug release data generated for the two drugs were subjected to one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California, USA, www.graphpad.com). Differences between batches of tablets were considered significant when p < 0.5.
3. Results and discussions
Light grade cashew gum obtained locally in Ghana was purified and the suitability of the powder as a direct compression excipient was evaluated based on the SeDeM Diagram Expert System. The SeDeM method uses twelve parameters to examine whether or not a powder is suitable for direct compression and a minimum of eight parameters can be used (Pérez-Lozano et al., 2006). Table 3 presents the physicochemical properties of purified cashew gum and the derived data based on eight parameters of the SeDeM Expert System. The SeDeM diagram is a useful tool used in evaluating the physical characteristics of pharmaceutical excipients (Aguilar-Díaz et al., 2009), and to determine the suitability of API’s and excipients for direct compression. The technique is also used in determining the amount of excipient required for the compression of an API which is not apt for direct compression. According to the SeDeM model, an API or excipient is suitable for direct compression when the good compressibility index (IGC) determined from the SeDeM equation is ⩾5.0 (Suñe-Negre et al., 2011). The IGC obtained for cashew gum in the current study was 5.173, indicating its suitability as a direct compression excipient.
Table 3.
Physicochemical properties of cashew gum and derived data based on the SeDeM Diagram Expert System.
| Parameter | Experimental results | Limit value (V) | Factor applied to V | Radius |
|---|---|---|---|---|
| Bulk density (g/ml) | 0.71 | 0–1 | 10V | 7.1 |
| Tapped density (g/ml) | 0.83 | 0–1 | 10V | 8.3 |
| Interparticle porosity | 0.204 | 0–1.2 | 10V/1.2 | 1.7 |
| Carr’s index | 14.46 | 0–50 | V/5 | 2.9 |
| Hausner ratio | 1.17 | 3–1 | (30–10V)/2 | 9.15 |
| Angle of repose (°) | 38.7 | 50–0 | 10 − (V/5) | 2.26 |
| Loss on drying (%) | 5.25 | 10–0 | 10 − V | 4.75 |
| Particles < 75 μm (%) | 0.9 | 50–0 | 10 − (V/5) | 9.82 |
Parameter Index (PI) = 0.5; Parameter profile index = 5.7475; Reliability factor = 0.9; Good compressibility index = 5.173.
Thirteen different powder formulations of diclofenac sodium matrix tablets and metformin HCl matrix tablets were prepared employing varying ratios of cashew gum, xanthan gum and HPMC as drug release retardants/modifiers. The objective was to develop matrix tablet formulations of the two drugs having the requisite extended release characteristics. The flowability of the blended powders was characterized using indirect methods of Hausner ratio, Carr index and angle of repose (Staniforth, 2002). Hausner ratio values >1.6 indicate poor flow properties while values ∼1.25 demonstrate good flow properties. Carr compressibility index ⩽16% is indicative of good flow properties while values > 23% exhibit poor flowability. In terms of angle of repose, values closer to 25° have good flow properties while values closer to 40° have poor flow properties. Blended powder formulations of diclofenac sodium had Hausner ratio, Carr index, interparticle porosity and angle of repose values of 1.42–1.58, 29.58–36.62%, 0.592–0.833 and 28.5–42.3°, respectively. While Carr index values of diclofenac sodium powder formulations appeared to indicate poor flow, the Hausner ratio and angle of repose values showed the blended powders in general possessed average to good flowability. Blended powder formulations of metformin had Hausner ratio (1.31–1.49), Carr index (23.38–35.06%), interparticle porosity (0.396–0.730), and angle of repose (29.2–32.3°). Metformin HCl powder formulations generally possessed good flow properties and exhibited lower interparticle porosity than diclofenac formulations. Magnesium stearate, a glidant was employed to enhance the flowability of the blended powders for direct compression.
Microcrystalline cellulose (MCC) was employed in the formulation of matrix tablets of the two drugs because of its versatility as a direct compression excipient. MCC improves the compactibility or tabletability of the compression mix of tablet formulations (Thoorens et al., 2014). Table 4, Table 5 show the physical characteristics of diclofenac sodium (∼100 mg) and metformin HCl matrix tablets (∼200 mg), respectively. The average weight of diclofenac tablets ranged from 496.3 to 519.5 mg while that of metformin tablet was 595.3–627.4 mg. All tablet formulations of the two drugs passed the British Pharmacopoeia (2012) uniformity of weight test (<2 tablets ± 5% mean weight, none ± 10% mean weight, n = 20). Tablets of the two drugs exhibited good mechanical strength with crushing strength values ⩾ 39.2 N, except diclofenac formulation D4 and metformin formulation M7. The friability test measures the ability of tablets to withstand abrasion and chipping during handling, packaging and transportation and should not exceed 1%. Five diclofenac tablet formulations, D1, D2, D4, D5 and D11 and five metformin tablet formulations M1, M3, M5, M7 and M13 failed the friability test.
Table 4.
Physical characteristics of diclofenac sodium matrix tablets prepared by direct compression.
| Formulation | Weight (mg) | Thickness (mm) | Crushing strength (N) | Friability (%) | CSFR | Drug content (%) |
|---|---|---|---|---|---|---|
| D1 | 506.0 ± 10.0 | 5.06 ± 0.20 | 44.5 ± 12.8 | 3.1 | 14.35 | 90.34 ± 1. 29 |
| D2 | 519.5 ± 10.2 | 4.91 ± 0.06 | 66.3 ± 19.6 | 1.5 | 44.20 | 80.99 ± 2.34 |
| D3 | 508.8 ± 9.5 | 5.12 ± 0.08 | 114.6 ± 17.5 | 0.7 | 163.71 | 89.75 ± 4.32 |
| D4 | 499.3 ± 10.0 | 5.24 ± 0.12 | 28.3 ± 8.2 | 6.0 | 4.72 | 59.25 ± 3.88 |
| D5 | 496.3 ± 8.4 | 5.39 ± 0.11 | 65.3 ± 16.9 | 3.1 | 21.06 | 85.23 ± 2.81 |
| D6 | 498.4 ± 10.1 | 5.18 ± 0.09 | 102.6 ± 31.0 | 0.7 | 146.57 | 60.33 ± 4.32 |
| D7 | 503.5 ± 10.0 | 5.13 ± 0.12 | 85.8 ± 17.1 | 0.8 | 107.25 | 92.59 ± 1.99 |
| D8 | 508.9 ± 19.3 | 5.06 ± 0.11 | 112.8 ± 19.0 | 0.7 | 161.14 | 95.51 ± 2.65 |
| D9 | 510.6 ± 10.4 | 5.72 ± 0.11 | 81.8 ± 16.1 | 0.8 | 102.25 | 96.51 ± 3.22 |
| D10 | 510.7 ± 8.7 | 5.59 ± 0.08 | 108.2 ± 38.1 | 0.7 | 154.57 | 98.20 ± 1.29 |
| D11 | 500.2 ± 9.1 | 5.40 ± 0.13 | 56.6 ± 14.9 | 1.5 | 37.73 | 91.17 ± 3.29 |
| D12 | 501.1 ± 11.1 | 5.26 ± 0.08 | 91.5 ± 1.1 | 0.9 | 101.67 | 90.80 ± 2.73 |
| D13 | 498.3 ± 7.9 | 5.18 ± 0.13 | 108.9 ± 20.9 | 0.9 | 121.00 | 99.80 ± 1.20 |
Table 5.
Physical characteristics of metformin hydrochloride matrix tablets prepared by direct compression.
| Formulation | Weight (mg) | Thickness (mm) | Crushing strength (N) | Friability (%) | CSFR | Drug content (%) |
|---|---|---|---|---|---|---|
| M1 | 619.2 ± 20.0 | 6.92 ± 0.12 | 65.9 ± 21.3 | 1.30 | 50.69 | 106.64 ± 2.73 |
| M2 | 627.4 ± 17.2 | 6.73 ± 0.16 | 136.5 ± 21.6 | 0.47 | 290.43 | 96.24 ± 2.55 |
| M3 | 601.7 ± 16.0 | 7.05 ± 0.17 | 41.0 ± 10.0 | 2.71 | 15.13 | 96.38 ± 1.78 |
| M4 | 598.8 ± 15.3 | 6.68 ± 0.33 | 193.0 ± 38.1 | 0.70 | 275.71 | 90.12 ± 3.20 |
| M5 | 602.2 ± 18.1 | 7.13 ± 0.32 | 65.3 ± 33.6 | 1.34 | 48.73 | 96.18 ± 1.94 |
| M6 | 596.2 ± 10.0 | 7.06 ± 0.04 | 128.7 ± 17.7 | 0.64 | 201.09 | 99.15 ± 3.38 |
| M7 | 612.6 ± 9.7 | 7.31 ± 0.06 | 32.7 ± 8.6 | 3.23 | 10.12 | 103.29 ± 2.94 |
| M8 | 616.8 ± 16.9 | 6.66 ± 0.04 | 97.5 ± 18.2 | 0.75 | 130.00 | 104.68 ± 1.78 |
| M9 | 602.2 ± 8.7 | 6.41 ± 0.09 | 180.3 ± 18.1 | 0.67 | 269.10 | 105.17 ± 4.23 |
| M10 | 595.3 ± 7.8 | 6.68 ± 0.02 | 80.4 ± 18.7 | 0.46 | 174.78 | 99.27 ± 2.56 |
| M11 | 600.0 ± 10.1 | 6.83 ± 0.20 | 139.6 ± 20.3 | 0.61 | 228.85 | 106.35 ± 2.44 |
| M12 | 620.4 ± 9.0 | 7.11 ± 0.14 | 56.4 ± 21.7 | 0.84 | 67.14 | 109.30 ± 1.98 |
| M13 | 615.8 ± 15.3 | 6.93 ± 0.22 | 51.9 ± 31.3 | 2.72 | 19.08 | 102.93 ± 3.26 |
The crushing strength-friability ratio (CSFR) is a parameter used to evaluate the quality of tablets (Odeku, 2005, Bakre and Jaiyeoba, 2009). The CSFR is a ratio of tablet strength (crushing strength) and tablet weakness (friability) and high CSFR values are indicative of tablets of high quality. The CSFR values of diclofenac tablets ranged from 4.72 to 163.71 while that of metformin tablets ranged from 10.12 to 290.43. Metformin tablet formulations generally had higher CSFR values than diclofenac tablets and could indicate tablets of higher quality. The diclofenac content in diclofenac matrix tablets ranged from 59.25% to 99.80% while metformin content in metformin HCl matrix tablets was 90.12–109.30%. Diclofenac and metformin tablets should contain 90–110% diclofenac and 95–105% metformin of the drug content on assay (USP, 2007). Nine diclofenac tablet formulations satisfied this pharmacopoeia specification except formulations D2, D4, D5 and D6 which were underdose. Also, nine metformin tablet formulations conformed to the pharmacopoeia specification but while M1, M11 and M12 were overdose, M4 was underdose. The failure of some tablet formulations to pass the assay test could be due to improper flow of the blended powders as well as non-uniform filling of the die.
Figure 1, Figure 2 are plots of swelling indices of diclofenac tablets in phosphate buffer pH 7.4 and metformin matrix tablets in phosphate buffer pH 6.8, respectively, over 18 h. The swelling index of diclofenac tablet formulations ranged from 32.29% to 291.85% while that of metformin ranged from 28.0% to 184.09%. Tablet formulations of the two drugs containing xanthan gum alone or in combination with other release retardants exhibited high swelling indices (D3, D4, D5, D6, D11, D12, D13 and M3, M4, M7, M8, M9, M12, M13). On the other hand, formulations containing HPMC alone or in combination with cashew gum exhibited low swelling indices (D7, D8, D9, D10 and M5, M6, M10, M11). The swelling index is used to characterize the hydrophilic character of formulations. The polymers employed in the preparation of the matrix tablets are hydrophilic in nature and in aqueous media the tablets absorb water, swell and increase in size. The swelling index is an indication of the ability of the polymer material used to absorb water while maintaining the integrity of the tablets.
Figure 1.
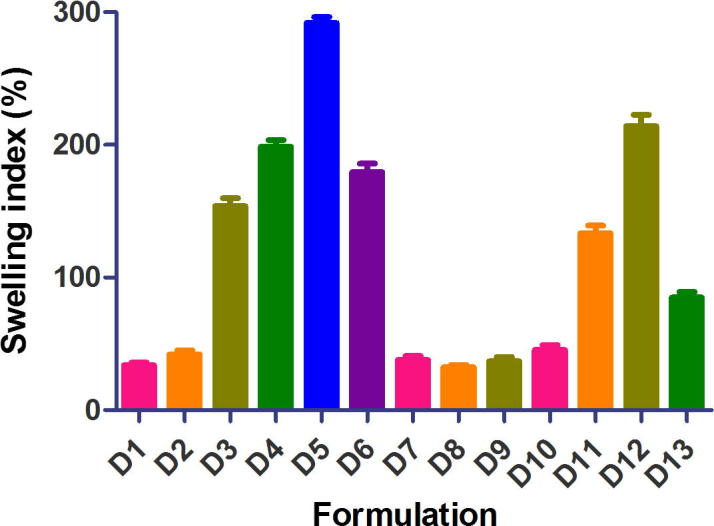
Swelling index of extended release diclofenac sodium matrix tablets in phosphate buffer pH 7.4 (mean ± S.D, n = 5).
Figure 2.
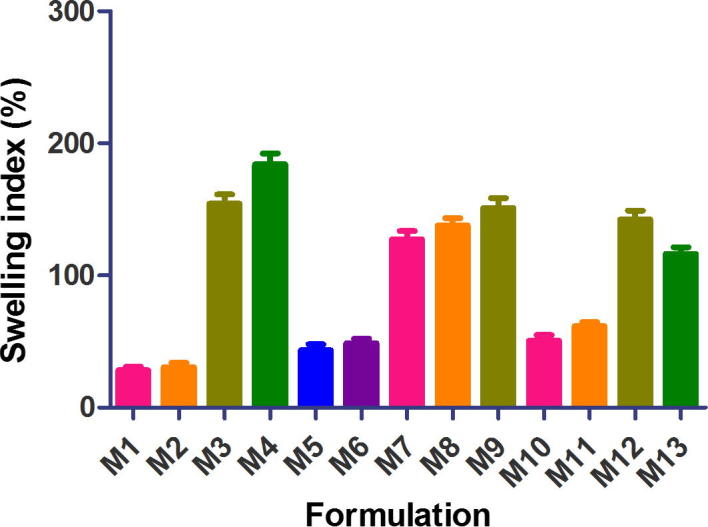
Swelling index of extended release metformin hydrochloride matrix tablets in phosphate buffer pH 6.8 (mean ± S.D, n = 5).
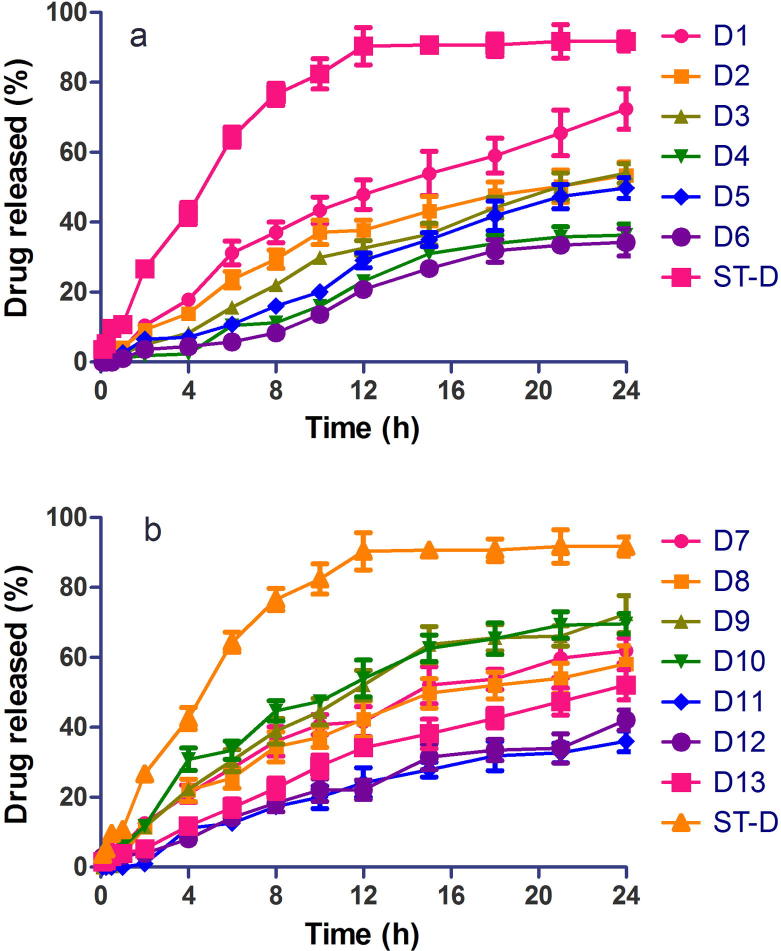
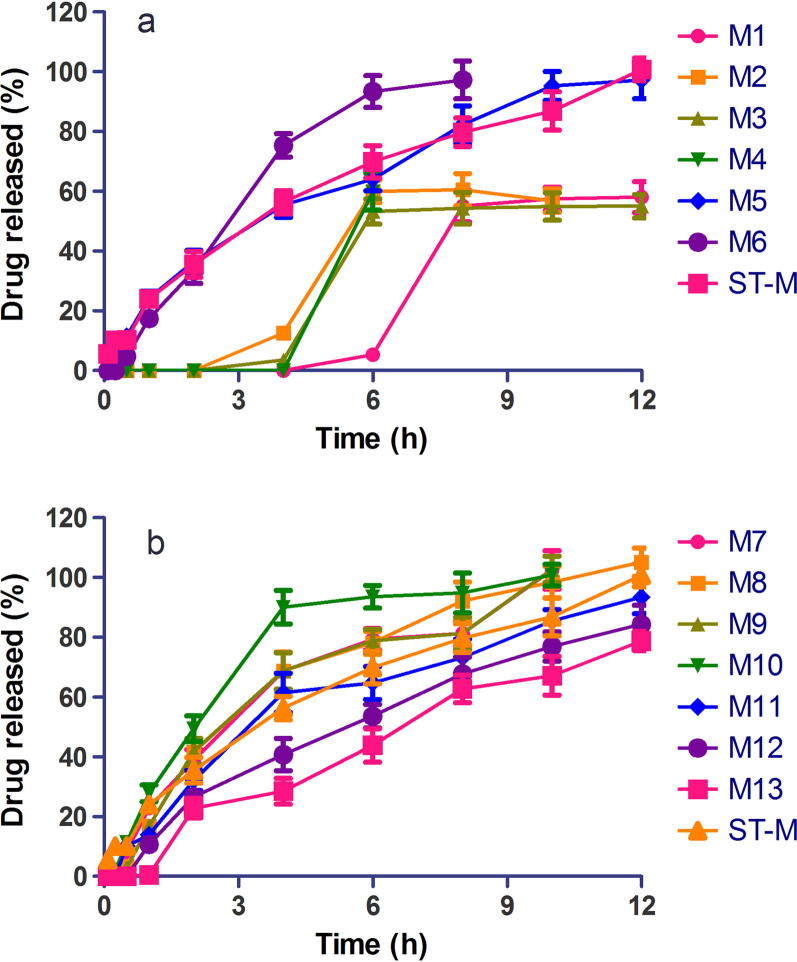
Figure 3, Figure 4 are release profiles of diclofenac and metformin matrix tablets, respectively, in comparison with the reference drug samples. Extended drug release profiles for 24 h were obtained for diclofenac matrix tablets while extended release for 8–12 h was achieved for metformin matrix tablets. Thus, the water insoluble diclofenac achieved a longer drug release than the water-soluble metformin, confirming the finding that more release-retarding polymer is required to sustain drug release from water soluble drugs than water insoluble drugs (Chakraborty et al., 2009). Drug solubility is one of the key factors considered in the fabrication of controlled release delivery systems (Sudha et al., 2010). Drug release from diclofenac tablet formulations D2, D3, D4, D5, D6, D11, D12 and D13 was significantly different (p < 0.05) from voltaren retard, the reference drug, while drug release from D1, D7, D8, D9 and D10 was similar (p > 0.05) to that of Voltaren retard. Drug release from the various metformin matrix tablet formulations was similar (p > 0.05) to that of Glucophage SR, the reference drug.
Figure 3.
Dissolution profiles of extended release diclofenac sodium matrix tablets in phosphate buffer pH 7.4 (mean ± S.D, n = 3), (a) formulations D1-D6, ST-D, (b) formulations D7-D13, ST-D.
Figure 4.
Dissolution profiles of extended release metformin hydrochloride matrix tablets in buffer pH 6.8 (mean ± S.D, n = 3), (a) formulations M1-D6, ST-M, (b) formulations M7-M13, ST-M.
Release kinetics is an essential aspect of drug formulation development and kinetic data are also employed in setting in vivo–in vitro correlation (IVIVC) of dosage forms (Thakkar et al., 2009). Table 6, Table 7 present the kinetics of release of extended release diclofenac and metformin matrix tablets, respectively. The release kinetics was evaluated by fitting the drug release data to five kinetic models. The kinetic model with the highest correlation coefficient value (R2) was selected as the model that best described the dissolution data. Drug release from diclofenac and metformin tablet formulations followed zero order, first order, Higuchi or Hixson-Crowell models but none followed the Korsmeyer-Peppas model. In general, most of the poorly water-soluble diclofenac tablet formulations followed the first order (R2 = 0.9845–0.9964, for D1, D3, D12, D13) or Higuchi kinetic model (R2 = 0.9187–0.9908, for D2, D7, D8, D9, D10, D11, ST-D) while water-soluble metformin tablet formulations followed Higuchi (R2 = 0.7937–0.9618, for M1, M2, M3, M5, M9, M13) or Hixson-Crowell model (R2 = 0.9491–0.9899, for M6, M8, M8, M10, M11, M13). This current study confirms previous findings that drug solubility affects drug dissolution, kinetics and mechanism of drug release from matrix tablet preparations (Li et al., 2008, Chakraborty et al., 2009, Prasanthi et al., 2010). First order kinetics refers to drug release which is concentration-dependent while the Higuchi model is used to describe drug dissolution from several types of modified release pharmaceutical dosage forms, including matrix tablets and some transdermal systems (Singhvi and Singh, 2011). The Hixson-Crowell model describes drug release from dosage forms including tablets, whereby there is a change in surface area and diameter of the dosage form in dissolution media. Drug dissolution in this model occurs in planes which are parallel to the drug surface if the tablet dimensions diminish proportionally; in a manner that the initial geometrical form is kept constant at all times (Hixson and Crowell, 1931). Drug release from most of the extended release tablet formulations of diclofenac and metformin was by Fickian diffusion or diffusion-controlled release (first order or Higuchi model) while drug release from the remaining tablet formulations occurred by anomalous or non-Fickian diffusion.
Table 6.
Release kinetics of diclofenac sodium matrix tablets in phosphate buffer pH 7.4 at 37 °C.
| Formulation | Zero order R2 | First order R2 | Higuchi R2 | Hixson-Crowell R2 | Korsmeyer-Peppas R2 |
|---|---|---|---|---|---|
| D1 | 0.9593 | 0.9947 | 0.9897 | 0.9889 | 0.9337 |
| D2 | 0.9461 | 0.9748 | 0.9855 | 0.9667 | 0.9566 |
| D3 | 0.9853 | 0.9964 | 0.9738 | 0.9939 | 0.8863 |
| D4 | 0.9657 | 0.9683 | 0.9330 | 0.9685 | 0.9338 |
| D5 | 0.9886 | 0.9862 | 0.9453 | 0.9887 | 0.9821 |
| D6 | 0.9696 | 0.9634 | 0.9201 | 0.9666 | 0.9614 |
| D7 | 0.9417 | 0.9819 | 0.9908 | 0.9718 | 0.9208 |
| D8 | 0.9223 | 0.9690 | 0.9883 | 0.9548 | 0.9742 |
| D9 | 0.9349 | 0.9806 | 0.9895 | 0.9703 | 0.8598 |
| D10 | 0.9013 | 0.9654 | 0.9807 | 0.9479 | 0.9573 |
| D11 | 0.9558 | 0.9696 | 0.9848 | 0.9682 | 0.8391 |
| D12 | 0.9786 | 0.9845 | 0.9624 | 0.9829 | 0.9290 |
| D13 | 0.9806 | 0.9954 | 0.9827 | 0.9903 | 0.9521 |
| ST-D | 0.7831 | 0.8953 | 0.9187 | 0.8655 | 0.8964 |
ST-D = Voltaren retard® (standard diclofenac tablet).
Table 7.
Release kinetics of metformin hydrochloride matrix tablets in phosphate buffer pH 6.8 at 37 °C.
| Formulation | Zero order R2 | First order R2 | Higuchi R2 | Hixson-Crowell R2 | Korsmeyer-Peppas R2 |
|---|---|---|---|---|---|
| M1 | 0.6775 | 0.6839 | 0.8008 | 0.6807 | 0.4395 |
| M2 | 0.6682 | 0.6642 | 0.8019 | 0.6712 | 0.5701 |
| M3 | 0.6726 | 0.6795 | 0.7937 | 0.6703 | 0.4326 |
| M4 | ND | ND | ND | ND | ND |
| M5 | 0.7511 | 0.8664 | 0.9013 | 0.8440 | 0.8346 |
| M6 | 0.8783 | 0.9579 | 0.9410 | 0.9863 | 0.9396 |
| M7 | 0.8793 | 0.9532 | 0.9452 | 0.9308 | 0.9477 |
| M8 | 0.8959 | 0.9558 | 0.9640 | 0.9899 | 0.8981 |
| M9 | 0.9035 | 0.9528 | 0.9618 | 0.9332 | 0.8865 |
| M10 | 0.8129 | 0.9354 | 0.9029 | 0.9491 | 0.9099 |
| M11 | 0.9119 | 0.9667 | 0.9671 | 0.9759 | 0.9682 |
| M12 | 0.9580 | 0.9942 | 0.9893 | 0.9939 | 0.9754 |
| M13 | 0.9713 | 0.9822 | 0.9753 | 0.9871 | 0.9557 |
| ST-M | 0.9434 | 0.9937 | 0.9785 | 0.9852 | 0.8532 |
ND = Not determined due to insufficient data points; ST-M = Glucophage® (standard metformin tablet).
4. Conclusions
Results from the study have shown that light grade cashew gum powder possesses the requisite physicochemical properties for use as a direct compression excipient. Extended release matrix tablets of diclofenac sodium and metformin HCl were successfully produced using various combinations/blends of three hydrophilic polymers. Xanthan gum containing matrix tablets of the two drugs exhibited enhanced swelling and extended drug release. Drug release from highly water-soluble metformin occurred over a shorter time period than the poorly water-soluble diclofenac sodium. The study emphasizes the complex nature of a pharmaceutical formulation and that there is no such thing as a universal formulation and each drug must be considered on a case-by-case basis.
Acknowledgement
The authors gratefully acknowledge the technical assistance of Technicians of the Department of Pharmaceutics, KNUST, Ghana. Our sincere thanks go to the Management and Staff of Bodokrom Cashew Plantation, Bodokrom, Ghana, for providing cashew gum for the study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aguilar-Díaz J.E., García-Montoya E., Pérez-Lozano P., Suñe-Negre J.M., Miñarro M., Ticó J.R. The use of the SeDeM Diagram expert system to determine the suitability of diluents-disintegrants for direct compression and their use in formulation of ODT. Eur. J. Pharm. Biopharm. 2009;73(3):414–423. doi: 10.1016/j.ejpb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Ali A., Iqbal M., Akhtar N., Khan H.M., Ullah A., Uddin M., Khan M.T. Assessment of xanthan gum based sustained release matrix tablets containing highly water-soluble propranolol HCl. Acta Pol. Pharm. 2013;70(2):283–289. [PubMed] [Google Scholar]
- Aulton M.E. third ed. Churchill Livingstone; London: 2007. Pharmaceutics: The Design and Manufacture of Medicines. [Google Scholar]
- Bakre L.G., Jaiyeoba K.T. Evaluation of a new tablet disintegrant from dried pods of Abelmuscus esculentus L. (okra) Asian J. Pharm. Clin. Res. 2009;2(3):82–91. [Google Scholar]
- Baviskar D., Sharma R., Jain D. Modulation of drug release by utilizing pH-independent matrix system comprising water soluble drug verapamil hydrochloride. Pak. J. Pharm. Sci. 2013;26(1):137–144. [PubMed] [Google Scholar]
- Chakraborty S., Khandai M., Sharma A., Niranjan Patra C.H., Jagannath Patro V., Kumar Sen K. Effects of drug solubility on the release kinetics of water soluble and insoluble drugs from HPMC based matrix formulations. Acta Pharm. 2009;59:313–323. doi: 10.2478/v10007-009-0025-8. [DOI] [PubMed] [Google Scholar]
- Chivate A.A., Poddar S.S., Abdul S., Savant G. Evaluation of Sterculia foetida gum as controlled release excipient. AAPS PharmSciTech. 2008;9:197–204. doi: 10.1208/s12249-008-9039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo P., Bettini R., Santi P., Peppas N.A. Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optimal performance. Pharm. Sci. Technol. Today. 2000;3(6):198–204. doi: 10.1016/s1461-5347(00)00269-8. [DOI] [PubMed] [Google Scholar]
- Das, N. G., Das, S. K., 2003. Controlled-release of oral dosage forms. Formulation, Fill & Finish, pp. 10–16. <www. Pharmtech.com>.
- Dastidar S.G., Ganguly K., Chaudhuri K., Chakrabarty A.N. The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis. Int. J. Antimicrob. Ag. 2000;14:249–251. doi: 10.1016/s0924-8579(99)00159-4. [DOI] [PubMed] [Google Scholar]
- Fung L.K., Saltzman W.M. Polymeric implants for cancer chemotherapy. Adv. Drug Deliv. Rev. 1997;26:209–230. doi: 10.1016/s0169-409x(97)00036-7. [DOI] [PubMed] [Google Scholar]
- Gibaldi M., Feldman S. Establishment of sink conditions in dissolution rate determinations – theoretical considerations and application to nondisintegrating dosage forms. J. Pharm. Sci. 1967;56:1238–1242. doi: 10.1002/jps.2600561005. [DOI] [PubMed] [Google Scholar]
- Hentzschel C.M., Sakmann A., Leopold C.S. Comparison of traditional and novel tableting excipients: physical and compaction properties. Pharm. Dev. Technol. 2012;17:649–653. doi: 10.3109/10837450.2011.572897. [DOI] [PubMed] [Google Scholar]
- Higuchi T. Mechanism of sustained action medication: theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963;52:1145–1148. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- Hixson A.W., Crowell J.H. Dependence of reaction velocity upon surface and agitation: theoretical considerations. Ind. Eng. Chem. 1931;23:923–931. [Google Scholar]
- Kamboj, S., Gupta, G. D., Oberoy, J., 2009. Matrix tablets: An important tool for oral controlled-release dosage forms – A review. Pharminfo.net 7.
- Korsmeyer R.W., Gurny R., Doelker E., Buri P., Peppas N.A. Mechanism of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15:25–35. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- Li H., Hardy R.J., Gu X. Effect of drug solubility on polymer hydration and drug dissolution from polyethylene oxide (PEO) matrix tablets. AAPS PharmSciTech. 2008;9(2):437–443. doi: 10.1208/s12249-008-9060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel J.S., Paula H.C.B., Miranda M.A.R., Sasaki J.M., de Paula R.C.M. Reacetylated chitosan/cashew gum gel: preliminary study for potential utilization as drug release matrix. J. Appl. Polym. Sci. 2006;99(1):326–334. [Google Scholar]
- Mfoafo K.A. Kwame Nkrumah University of Science and Technology, Kumasi; Ghana: 2013. Evaluation of cashew gum as a direct compression excipient for controlled drug delivery using diclofenac sodium and metformin hydrochloride as model drugs; p. 182. (MPhil (Pharmaceutics) thesis) [Google Scholar]
- Narasimharao R., Anusha Reddy M., Swetha Reddy N., Divyasagar P., Keerthana K. Design and evaluation of metformin hydrochloride extended release tablets by direct compression. IJRPBS. 2011;2:1118–1133. [Google Scholar]
- Nussinovitch A. Plant Gum Exudates of the World. CRC Press; 2009. Major plant exudates of the world. [Google Scholar]
- Odeku O.A. Assessment of Albizia zygia gum as a binding agent in tablet formulations. Acta Pharm. 2005;55:263–276. [PubMed] [Google Scholar]
- Ofori-Kwakye K., Asantewaa Y., Kipo S.L. Physicochemical and binding properties of cashew tree gum in metronidazole tablet formulations. Int. J. Pharm. Pharm. Sci. 2010;2(Suppl. 4):105–109. [Google Scholar]
- Ofori-Kwakye K., Obese E., Boakye-Gyasi E. Formulation and in-vitro evaluation of sustained release diclofenac sodium matrix tablets using blends of cashew gum, xanthan gum and hydroxypropylmethylcellulose as hydrophilic drug release modifiers. IJNDD. 2013;5(4):187–197. [Google Scholar]
- Peppas N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985;60(4):110–111. [PubMed] [Google Scholar]
- Pérez-Lozano P., Suñé-Negre J.M., Miñarro M., Roig M., Fuster R., García-Montoya E., Hernández C., Ruhí R., Ticó J.R. A new expert systems (SeDeM diagram) for control batch powder formulation and preformulation drug products. Eur. J. Pharm. Biopharm. 2006;64(3):351–359. doi: 10.1016/j.ejpb.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Pharmacopoeia British. Her Majesty’s Stationery Office; London: 2012. British Pharmacopoeia Commission. [Google Scholar]
- Prasanthi N.L., Manikiran S.S., Rama Rao N. Effect of solubility of the drug on the release kinetics from hydrophilic matrices. Int. J. PharmTech. Res. 2010;2(4):2506–2511. [Google Scholar]
- Singhvi G., Singh M. Review: in-vitro drug release characterization models. IJPSR. 2011;2:1–8. [Google Scholar]
- Staniforth J. Powder flow. In: Aulton M.E., editor. Pharmaceutics – the Science of Dosage form Design. second ed. Churchill Livingstone; London: 2002. pp. 197–210. [Google Scholar]
- Sudha B.S., Sridhar B.K., Srinatha A. Modulation of tramadol release from a hydrophilic matrix: implications of formulations and processing variables. AAPS PharmSciTech. 2010;II:433–440. doi: 10.1208/s12249-010-9400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suñé-Negre J.M., Pérez-Lozano P., Miñarro M., Roig M., Fuster R., Hernández C., Ruhí R., García-Montoya E., Ticó J.R. Application of the SeDeM diagram and a new mathematical equation in the design of direct compression tablet formulation. Eur. J. Pharm. Biopharm. 2008;69(3):1029–1039. doi: 10.1016/j.ejpb.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Suñe-Negre, J.M., García-Montoya, E., Pérez-Lozano, P., Aguilar-Díaz, J.E., Carreras, M.R., Garcia, R.F., Carmona, M.M., Tico-Grau, J.R., 2011. SeDeM diagram: a new expert system for the formulation of drugs in solid form. In: Vizureanu P. (Ed.), Expert Systems for Human, Materials and Automation, InTech. <http://www.intechopen.com/books/expert-systems-for-human-materials-and-automation/sedem-diagram-anew-expert-system-for-the-formulation-of-drugs-in-solid-form> [ISBN 978-953-307-334-7].
- Thakkar V.T., Shah P.A., Soni T.G., Parmar M.Y., Gohel M.C., Gandhi T.R. Goodness-of-fit model-dependent approach for release kinetics of levofloxacin hemihydrates floating tablet. Dissolut. Technol. 2009:35–39. [Google Scholar]
- Thoorens G., Krier F., Leclercq B., Carlin B., Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment – a review. Int. J. Pharm. 2014;473(1):64–72. doi: 10.1016/j.ijpharm.2014.06.055. [DOI] [PubMed] [Google Scholar]
- United States Pharmacopoeia and National Formulary, 2007. United States Pharmacopoeia XXIII: Rockville U.S.P Convention Inc.
- Varelas C.G., Dixon D.G., Steiner C. Zero-order release from biphasic polymer hydrogels. J. Control. Release. 1995;34:185–192. doi: 10.1016/s0168-3659(97)00182-x. [DOI] [PubMed] [Google Scholar]
- Vohra D.D., Pagi K.S., Rajesh K.S. Losartan potassium loaded sustained release matrix tablets: influence of various hydrophilic and hydrophobic polymers on drug release behavior. J. Pharm. Bioallied Sci. 2012;4(Suppl 1):S79–80. doi: 10.4103/0975-7406.94147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J.G. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J. Pharm. Sci. 1969;58:1253–1257. doi: 10.1002/jps.2600581021. [DOI] [PubMed] [Google Scholar]