Abstract
Malaysia is situated in Western Pacific region which bears 36.17% of total diabetes mellitus population. Pharmacist led diabetes interventions have been shown to improve the clinical outcomes amongst diabetes patients in various parts of the world. Despite high prevalence of disease in this region there is a lack of reported intervention outcomes from this region. The aim of this study was to evaluate the impact of a pharmacist led intervention on HbA1c, medication adherence, quality of life and other secondary outcomes amongst type 2 diabetes patients. Method: Type 2 diabetes mellitus patients (n = 73) attending endocrine clinic at Universiti Kebangsaan Malaysia Medical Centre (UKMMC) were randomised to either control (n = 36) or intervention group (n = 37) after screening. Patients in the intervention group received an intervention from a pharmacist during the enrolment, after three and six months of the enrolment. Outcome measures such as HbA1c, BMI, lipid profile, Morisky scores and quality of life (QoL) scores were assessed at the enrolment and after 6 months of the study in both groups. Patients in the control group did not undergo intervention or educational module other than the standard care at UKMMC. Results: HbA1c values reduced significantly from 9.66% to 8.47% (P = 0.001) in the intervention group. However, no significant changes were noted in the control group (9.64–9.26%, P = 0.14). BMI values showed significant reduction in the intervention group (29.34–28.92 kg/m2; P = 0.03) and lipid profiles were unchanged in both groups. Morisky adherence scores significantly increased from 5.83 to 6.77 (P = 0.02) in the intervention group; however, no significant change was observed in the control group (5.95–5.98, P = 0.85). QoL profiles produced mixed results. Conclusion: This randomised controlled study provides evidence about favourable impact of a pharmacist led diabetes intervention programme on HbA1c, medication adherence and QoL scores amongst type 2 diabetes patients at UKMMC, Malaysia.
Keywords: Diabetes mellitus, Pharmacist, Intervention, HbA1c, Medication adherence, Diabetes management, Quality of life
1. Introduction
Diabetes mellitus is now considered a global health priority due to increasing high prevalence, burden of co-morbidities and premature mortalities (Roglic et al., 2005, Wild et al., 2004). Malaysia is situated in the western pacific (WP) region which bears 138.2 million people (36.17%) with diabetes contributing to the total of 382 million diabetes population worldwide. The prevalence of diabetes amongst Malaysian adults aged between 20 and 79 years was reported to be 10.1% in 2013 (Federation, 2013). Prevalence of diabetes in the Asian population is predominant in the middle age group in contrast to the western countries; amongst all the regions WP region has the highest prevalence of diabetes in the age group of 40–59 year (Chan et al., 2009). The predominant prevalence of diabetes in the younger age in Asian population compared to their European counterparts is attributable to the changes in the epidemiological, socioeconomic and genetic characteristics of the Asian population over the past few decades (Ma and Chan, 2013).
Diabetes is a life-long disease and constantly increasing incidence of diabetes has drawn the attention of the healthcare community to the need of effective management programmes. In one such approach, the involvement of a pharmacist in diabetes management has been shown to improve patient outcomes in various healthcare settings across the world (Collins et al., 2011, Alhabib et al., 2014). Despite the high prevalence of diabetes in Asia, majority of the existing studies regarding the involvement of the pharmacists in diabetes originates from the Western countries. There is scarcity of relevant studies such as randomised controlled trials, from Malaysia. A prospective study was conducted to evaluate the role of the pharmacists in Diabetes Medication Therapy Adherence Clinic (DMTAC) (Lim and Lim, 2010). Therefore, there is a need to demonstrate the impact of a pharmacist intervention programme on diabetes care in Malaysia. Based on our knowledge, the present study is the first randomised control study in Malaysia measuring the impact of the pharmacist educational programme.
The aim of this study was to evaluate the impact of a pharmacist led diabetes management programme on type 2 diabetes patients on HbA1c, medication adherence and quality of life.
2. Methods
2.1. Study design and setting
This randomised controlled study was conducted within 6 months in a secondary endocrine clinic at Universiti Kebangsaan Malaysia Medical Centre (UKMMC). The recruitment and data collection were performed between August 2013 and August 2014. Setting for pharmacist intervention in this study consisted of a private counselling room at UKMMC. The study was approved by Universiti Kebangsaan Malaysia research ethics committee (Ref No. UKM 1.5.3.5/244/NF-018-2012).
2.2. Patients and randomisation
Based on a previous study, the sample size required for detecting the difference of 1% reduction with 1.2% standard deviation in A1c (power of study = 80%, significance = 5%) was 30 patients for a two tailed study (Kelly and Rodgers, 2000). Considering a dropout rate of 20% in the comparable intervention groups from the literature, 6 patients were added to each group with 36 patients for each arm.
Patient registered for the day appointment and was diagnosed for poorly controlled diabetes mellitus type 2 (HbA1c ⩾ 8%) at UKMMC was recruited in this study. Patients were included in the study only if the record of their blood tests within the previous two months from the day of enrolment was available in the clinic database and understand either Malay or English language. Patients were excluded from the study if they were diagnosed with any concurrent endocrine disorder (such as thyroid disorders, obesity, and gestational diabetes), cardiac heart failure, end stage renal disease, hepatitis or cancer. Patients were also excluded from the study if they were enrolled in other concurrent educational programmes.
Simple random sampling was used in which a list of random numbers was generated using the patients’ hospital IDs and sample was collected from this list. After recruitment, simple randomisation technique was followed for assigning the subjects to either control or intervention group. Patients were asked to handpick an envelope from the basket indicating allocation to either control or intervention group.
2.3. Standard care
The standard care of diabetes patients at UKMMC consisted of a patient-physician meeting ranging from every four to nine months. Patients with poor glycaemic control were referred to a nurse diabetes educator for diabetes related education. In addition, patients received the standard pharmacy care upon their visit for medication refills every 2–3 months. To measure the effect of difference in the number of patient-physician meetings on the outcomes, chi square test was used in the data analysis. Patients in the control group received standard care only.
2.4. Intervention
Diabetes management programme in our study was called Patient Education by Pharmacist Programme (PEPP). The content of intervention was developed in accordance with ADA guidelines (Association, 2013) and the Malaysian Ministry of Health guidelines (Health, 2013) combined with the results from the previous interventions. The summary of the intervention content is attached as Appendix A. Patients in intervention group received standard care and PEPP intervention. The patients enrolled in the intervention group were required to visit the hospital at the baseline, three months and six months duration during the course of intervention. At the enrolment, patients received counselling about diabetes, its complications, diabetes medication, lifestyle modifications and selfmonitoring of the disease. On the second visit, pharmacist reinforced the intervention about the lifestyle modifications, medication adherence, and selfmonitoring. In addition, pharmacist assessed the knowledge of the patients about diabetes and complication components of education and repeated the intervention if the pharmacist felt the need for it after assessment. The pharmacist who performed the intervention was registered with the Malaysian Board of Pharmacy and did not take part in any specific training workshops before initiating the PEPP. Pharmacist delivered the intervention face to face meetings however to curb the drop-out rate, patients were contacted on the phone in case they were unable to turn up for the scheduled meeting due to time management. The data collection from the control group was performed by an independent researcher and pharmacist had no contact with the participants of control group.
2.5. Outcome Measures
Table 1 shows the summary of outcome assessments and intervention performed at each visit. Medication adherence and QoL questionnaires were completed by the patients in majority of cases. MMMAS is a self-administered instrument but sometimes pharmacist or researcher assisted the patients to overcome the non-responsiveness by some participants. This practice has been reported to be acceptable in the literature (Al-Qazaz et al., 2010). Additional information about interpretation of the questionnaires has been elaborated in Appendix B.
Table 1.
Summary of outcome measure assessment and intervention during study duration.
| Control group |
Intervention group |
|||||
|---|---|---|---|---|---|---|
| 0 Month | 3 Months | 6 Months | 0 Month | 3 Months | 6 Months | |
| Demographic data | ||||||
| HbA1c | ||||||
| Fasting blood glucose | ||||||
| Lipid profile (LDL, HDL, total cholesterol, triglycerides) | ||||||
| Body mass index | ||||||
| Medication adherence (modified Morisky medication adherence scale) | ||||||
| Quality of life (EQ5D-3L) | ||||||
| Review of patients’ knowledge about diabetes management | ||||||
| Standard care | ||||||
| PEPP intervention | ||||||
2.6. Statistical analyses
The Statistical Package for the Social Sciences (SPSS, Version 22 Inc., Chicago, IL, USA) for Windows was used in the data analysis for this study. Baseline characteristics of both groups were compared by using chi-square test for nominal/ordinal variables and suitable t-test for continuous variables. The assumptions for statistical tests such as normality and sample size were complied before applying the tests. The results were set to be significant at p < 0.05.
3. Results
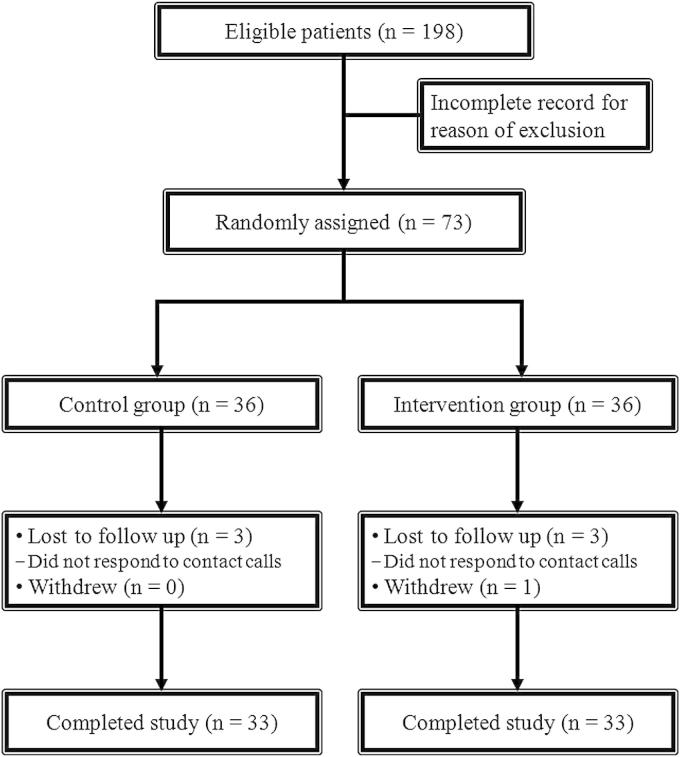
Of the 73 eligible patients, 37 patients were randomised to the intervention group and 36 patients were randomised to the control group. A total of 66 patients completed the study. Fig. 1 shows the trial flow diagram prepared in accordance with the CONSORT guidelines. The baseline characteristics of both groups are presented in Table 2. There was no significant difference in the baseline demographic and clinical characteristics of the participants. The first session of the intervention took approximately 35–45 min and second session was completed in approximately 20–30 min.
Figure 1.
Trial flow diagram in accordance with CONSORT guidelines (CONSORT, Consolidated Standards of Reporting Trials).
Table 2.
Baseline demographic and clinical characteristics of participants (n = 66).
| Control (n = 33) | Intervention (n = 33) | Total | p-value | |
|---|---|---|---|---|
| Age mean ± SD | 57.12 ± 10.78 | 57.42 ± 7.17 | 0.89 | |
| Marital status | ||||
| Married (%) | 93.9 | 90.9 | 0.64a | |
| Gender | ||||
| Male | 14 (42.4%) | 13 (39.4%) | 27 (40.9%) | |
| Female | 19 (57.6%) | 20 (60.6%) | 39 (59.1%) | 0.80a |
| Race | ||||
| Malay | 20 (60.6%) | 18 (54.5%) | 38 (57.6%) | |
| Chinese | 8 (24.2%) | 7 (21.2%) | 15 (22.7%) | |
| Indian | 5 (15.2%) | 8 (24.2%) | 13 (19.7%) | 0.65a |
| Duration of diabetes (years) | ||||
| 1–5 | 7 (24.1%) | 7 (23.3%) | 14 (23.7%) | 0.90a |
| 5–10 | 10 (34.5%) | 12 (40%) | 22 (37.3%) | |
| >10 | 12 (41.4%) | 11 (36.7%) | 23 (39.0%) | |
| A1c (%) Mean ± SD | 9.64 ± 1.41 | 9.66 ± 1.57 | 0.94 | |
| Medication | ||||
| Oral | 17 (53.1%) | 12 (37.5%) | 29 (45.3%) | 0.34b |
| Insulin only | 1 (3.1%) | 3 (9.4%) | 4 (6.3%) | |
| Oral + insulin | 14 (43.2%) | 17 (53.1%) | 31 (48.4%) | |
| Adherence level | ||||
| Poor | 16 (48.5%) | 19 (57.6%) | 35 (53%) | 0.45a |
| Medium/high | 17 (51.5%) | 14 (42.6%) | 31 (47%) |
Chi-square test.
3.1. Clinical outcomes
The changes in the primary and secondary clinical outcomes of the study are presented in Table 3. Measurement of HbA1c was the primary outcome in our study; mean HbA1c values showed significant decline in the intervention group compared to the control group (p = 0.04). BMI decreased significantly in the intervention group participants but the change was non-significant compared to the change in the control group (p > 0.05). Lipid profiles of the patients showed no significant changes in both groups over the study period. Table 4 shows the percentage of patients who met ADA target of 7% HbA1c for glycaemic control and Fig. 2 is the graphical representation of the changes in glycaemic control before and after intervention in the control and the intervention groups.
Table 3.
Changes in outcome parameters at the start and end of the study in control and intervention groups.
| Outcome | Control group (n = 33) |
Intervention group (n = 33) |
p-value (control vs. intervention) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Final | p-value | Baseline | Final | p-value | ||
| HbA1c (%) | 9.64 ± 1.45 | 9.26 ± 1.61 | 0.14 | 9.66 ± 1.57 | 8.47 ± 1.61 | 0.001a | 0.04a |
| FBS (mmol/l) | 9.63 ± 3.17 | 9.71 ± 4.24 | 0.94 | 10.58 ± 3.21 | 9.5 ± 3.50 | 0.15 | 0.86 |
| BMI (kg/m2) | 28.17 ± 4.57 | 27.97 ± 4.44 | 0.07 | 29.34 ± 5.22 | 28.92 ± 5.16 | 0.03a | 0.44 |
| TC (mmol/l)b | 4.95 ± 1.26 | 4.99 ± 1.41 | 0.81 | 5.44 ± 1.69 | 5.66 ± 1.59 | 0.46 | 0.10 |
| HDL-C (mmol/l)b | 1.28 ± .32 | 1.34 ± 0.35 | 0.10 | 1.39 ± 0.47 | 1.44 ± 0.48 | 0.40 | 0.36 |
| LDL-C (mmol/l)b | 2.77 ± 1.01 | 2.86 ± 1.10 | 0.52 | 3.24 ± 1.63 | 3.27 ± 1.38 | 0.88 | 0.35 |
| Triglycerides (mmol/l)b | 1.53 ± 0.55 | 1.51 ± 0.86 | 0.90 | 1.66 ± 0.78 | 1.88 ± 1.09 | 0.28 | 0.13 |
| Poor adherence (%) | 16 (48.5%) | 17 (51.5%) | 0.8 | 19 (57.6%) | 10 (30.3%) | 0.02a | 0.08 |
| MMMAS scores | 5.95 ± 1.51 | 5.98 ± 1.50 | 0.85 | 5.83 ± 1.84 | 6.77 ± 1.76 | 0.02a | 0.03a |
All values are presented in Mean ± SD except poor adherence which is shown in percentage; FBS, fasting blood sugar; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; MMMAS, modified Morisky medication adherence scale.
Shows statistical significance.
Some data missing.
Table 4.
Number of participants who achieved ADA target of glycaemic control.
| Outcome | Control | PEPP | X2 (p-value) |
|---|---|---|---|
| No. of patients | |||
| <7% HbA1c | 4 (12.1%) | 6 (18.2%) | 0.47 (0.49) |
| <8% HbA1c | 7 (21.2%) | 15 (45.2%) | 4.36 (0.037a) |
Significant at p < 0.05.
Figure 2.
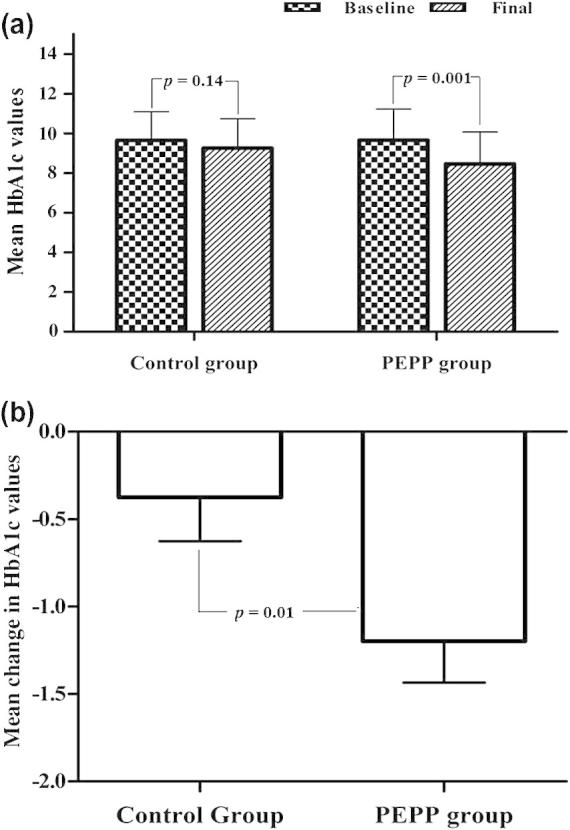
(a) Mean baseline and final HbA1c values in control and intervention groups and (b) mean change in HbA1c values during study duration.
3.2. Medication adherence (MMMAS)
Medication adherence was significantly improved in the intervention group (p = 0.03) unlike the corresponding non-significant improvement in the control group (p > 0.05). The percentage of patients with poor adherence significantly decreased from the baseline to the end of the study in the intervention group (p = 0.02); however this change was non-significant between the control and the intervention groups (p = 0.08).
3.3. Quality of life (EQ-5D-3L)
There was no baseline difference in the QoL profiles in both groups. Amongst the total five dimensions, mobility (p = 0.03) and anxiety (p < 0.0001) profiles showed significant changes within the intervention group. However no significant changes between profiles of both groups were reported. Analysis of EQ-5D-3L VAS scores showed no significant changes between two groups despite the significant improvement in the VAS scores of the intervention group (p = 0.03) at the end of study (Table 5).
Table 5.
Summary of EQ-5D-3L data for quality of life domains.
| Outcome | Patients reporting problems in control group |
Patients reporting problems in intervention group |
X2 (p-value) (control vs. intervention) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Final | X2 (p-value) | Baseline | Final | X2 (p-value) | ||
| Mobility | 8 (24.2%) | 5 (15.2%) | 0.86 (0.35) | 14 (42.4%) | 6 (18.2%) | 4.52 (0.03∗) | 1.16 (0.28) |
| Selfcare | 2 (6.1%) | 0 | 2.06 (0.15) | 2 (6.1%) | 0 | 2.06 (0.15) | 1.01 (0.31) |
| Usual activities | 4 (12.1%) | 4 (12.1%) | 0.00 (1.00) | 3 (9.1%) | 1 (3%) | 1.06 (0.30) | 3.14 (0.07) |
| Pain/discomfort | 14 (42.4%) | 8 (24.2%) | 2.45 (0.11) | 14 (42.4%) | 9 (27.3%) | 1.66 (0.19) | 0.77 (0.37) |
| Anxiety/depression | 13 (39.4%) | 9 (27.3%) | 1.20 (0.30) | 12 (36.4%) | 0 | 14.66 (0.0001∗) | 3.26 (0.07) |
| EQ-5D VAS | |||||||
| Mean score ± SD | 70.60 ± 18.31 | 77.12 ± 11.52 | 0.04∗ | 75.06 ± 16.62 | 82.45 ± 10.26 | 0.007∗ | 0.05∗, a |
Analysis using t-test.
Shows statistical significance.
3.4. Other outcomes
Chi-square analysis showed there was no meaningful difference in the standard care between the control and the intervention groups at the end of the study (p > 0.05).
3.5. Sustainability of outcomes
The comparison of mean HbA1c values after 6 months of second intervention demonstrated that there was no significant difference between participants who were enrolled in either intervention or control group (intervention group mean HbA1c = 8.79 ± 1.45, control group mean HbA1c = 8.72 ± 1.46; p = 0.88). However the dropout rate of participant was increased at this follow up and data of 25 participants in the intervention group and 22 participants in the control group were available.
4. Discussion
Our study was designed to measure the impact of pharmacist intervention on HbA1c levels in type 2 diabetes patients. Outcomes of the study showed reduction in HbA1c levels in the intervention group were significantly better than the reduction in the control group. The HbA1c reduction of 1.19% (SD = 1.36) in our study is clinically relevant as well as statistically significant. Our results were comparable to the findings of a meta-analysis by Collins et al. (2011). It was reported that randomised controlled trials within 6 month duration of pharmacist intervention produced a mean change of −0.95% in HbA1c levels with confidence interval of 95% (Collins et al., 2011). Another finding of the meta-analysis showed a qualitative trend towards higher mean of HbA1c reductions in studies with pharmacist intervention longer than 6 months. Our study was restricted to 6 months of duration therefore we compared the results of our study primarily with RCTs spanning six months.
The glycaemic control results in our study are comparable to other findings. A randomised controlled trial reported significant reduction of 0.6% in the intervention group after six months of pharmacist intervention (Mehuys et al., 2011). Smaller baseline HbA1c values in this study explained the small final changes in the HbA1c compared to our study; patients with higher HbA1c values showed greater improvement in the final values (Choe et al., 2005, Ragucci et al., 2005). In a second six month randomised control trial, the HbA1c values in the intervention group decreased significantly by 0.8% in contrast to increase in the control group (Jarab et al., 2012). Another study with 6 month pharmacist intervention reported 0.8% reduction of HbA1c values in the intervention group. This reduction was clinically significant but was reported to be statistically non-significant against control group. The non-significant changes were explained by the structure of the intervention leading to the contamination of both groups in addition to the relatively smaller baseline HbA1 values (Phumipamorn et al., 2008). Apart from RCTs, studies with other experimental designs also produced similar results after six months of pharmacist intervention. A prospective 6 month study produced 1.9% decrease in HbA1c levels from the baseline to the end of the study period. Intervention was intensive with a high baseline HbA1c value of 10.8% (Rothman et al., 2003). In a 6 month retrospective chart review, patients were reported to have 1.4% reduction at the end of the study after frequent and indefinite meetings between patients and pharmacist (McCord, 2006). Results of our study showed significant benefits of pharmacist intervention in Malaysian type 2 diabetes patients and further support the findings of the previous studies from the other parts of the world.
Currently, the target HbA1c value for diabetes is set at below 7% by ADA and below 6.5% by the Malaysian Ministry of Health. In our study, 18.2% participants in the intervention group achieved this target. The percentage of patients who achieved ADA target in our study is lesser than other RCTs; 28% (Phumipamorn et al., 2008), 23.4% (Jarab et al., 2012), 38.4% (McWhorter and GM, 2005) patients achieved the ADA target in other studies. This can be explained by the higher baseline HbA1c values in our study. To meet the ADA target, our study participants were required to produce a greater change in the HbA1c. Fasting blood sugar measurements were excluded from the final analysis due to the noncompliance amongst patients with fasting before the test. Thus the results were considered inappropriate for the analysis.
This study provides the evidence of benefits of pharmacist intervention in Malaysian population. Previous studies have shown that care of quality and access for diabetes varies across socioeconomic conditions and there are differences in diabetes complications, end points and quality of care amongst different ethnicities (Lanting et al., 2005, Oyetayo et al., 2011). Therefore, results obtained from the other parts of the world with different ethnic and socioeconomic compositions are not generalisable for the local population. Our results demonstrate the positive impact on the quality of the pharmacist care in diabetes in Malaysia. Malaysian population is multi-ethnic; however our study did not evaluate the characteristics of individual ethnicities. Future studies that evaluate the impact of ethnic difference in Malaysian population on glycaemic outcomes can be conducted. Currently there is an ongoing pharmacist managed Medication Therapy Adherence Clinic (MTAC) for diabetes in Malaysia. Patients are required to meet the pharmacist after every one or two months once they are enrolled in the programme. Findings of this study showed significant changes in HbA1c with patient pharmacist meetings being held after three months of enrolment. Therefore it may be suggested to eliminate the frequent meetings between patient and pharmacist in MTAC. Eliminating the excess meetings will facilitate the more time efficient use of pharmacist manpower in MTAC. However future studies that investigate and compare the impact of meeting schedule on glycaemic control for longer period are needed before drawing a definite conclusion.
In our study at 3 months of follow up there was significant difference between both groups however the difference waned off at 6 months. This result appears to be in agreement with the trend noticed in the literature (Sarkadi and Rosenqvist, 2004, Mehuys et al., 2011). It proposes that pharmacist intervention may need to be reinforced after a gap longer than 3 months for sustainability of beneficial effect.
In our study there were no significant changes in the lipid profile before and after the intervention. Likewise, one study on pharmacist managed diabetes service found that the lipid profiles showed no significant changes from the baseline to the final examination (Kelly and Rodgers, 2000). However there is ample evidence of significant improvement in lipid profiles along with the glycaemic control after pharmacist intervention (Phumipamorn et al., 2008, Al Mazroui et al., 2009, Oyetayo et al., 2011). Although there was significant improvement in the lipid profiles of these studies, the final values did not meet the target ADA values. The lack of improvement in lipid profiles despite improved glycaemic control in our study may be attributable to the ADA findings about dyslipidaemia. It was reported that glycaemic control alone will not result in the complete recovery of diabetes related dyslipidaemia and initiation of lipid lowering agents becomes necessary (Association, 2002, Association, 2004). Our study lacks the data on lipid lowering therapy of the participants and therefore a definite conclusion cannot be drawn. The baseline glycaemic values showed a significant statistical correlation with the final changes in the HbA1c values (p = 0.01). This observation is consistent with the previous evidence (Choe et al., 2005, Ragucci et al., 2005, Oyetayo et al., 2011).
Despite the significant changes in adherence scores reported in the intervention group, the status of average mean adherence (poor, moderate or high) remained unchanged before and after the intervention. The participants were moderately adherent to their medication before and after the intervention (5.83 vs 6.77; p = 0.03). Comparing the results to the previous studies, most studies showed the positive impact of pharmacist intervention amongst diabetes patients (Al Mazroui et al., 2009, Borges et al., 2010, Mitchell et al., 2011, Jarab et al., 2012) however, an absence of any improvement in adherence after pharmacist intervention was also reported (Odegard et al., 2005). A lack of gold standard to measure medication adherence hinders the quantification of adherence scores. It is not possible to compare the degree of improvement in medication adherence across various studies.
The improvement in QoL anxiety profile of our study is consistent with some previously published studies. Scott and colleagues demonstrated there was an improvement in QoL anxiety measure amongst pharmacist managed diabetes patients (Scott et al., 2006). In another 12 month study, mental component summary (MCS) of QoL questionnaire showed improvement after pharmacist intervention but physical component was not significantly affected (Johnson et al., 2008). An interesting finding showed a high significant (p = 0.007) improvement in EQ-5D of VAS scores in the intervention group over 6 months. This may have been facilitated by a significant improvement in the anxiety profile of participants as a result of intervention. VAS scores represent selfrated health state of patients and patients will likely rate their health state better when depression is removed or alleviated from their lives. A high percentage of patients in the intervention group presented with problems in mobility at the baseline, therefore probability of improvement during the study period was high with significant improvement was reported.
Majority (>87.9%) of the patients in both groups presented with no problems in the self-care and usual activity dimension at the baseline; therefore chances of meaningful improvement were either very slim or null. In case of pain/discomfort there was substantial improvement amongst patients at the end of the study however this did not reach the statistical significance.
5. Limitations
Findings of our study have highlighted the favourable impact of the pharmacist intervention on diabetes management. Nevertheless, these findings should be interpreted with caution due to several limitations. An important limitation was that our study might not have been fully representative of general diabetes population since the participation in the study was voluntary. There was incomplete record about the total number of patients who were approached to participate in the study and reason they were excluded from the study. The short duration of the study was a limitation to measure the sustainability of the favourable outcomes. There was lack of a strict protocol for the baseline clinical values; we used the values in range extending from past week to past two months. The values measured during that interval may have been changed at the baseline visit. Further studies which measure outcomes at the lesser flexibility will increase reliability of the results. Another limitation is the exclusion of those patients who could not understand either Malay or English language. Excluded patients may have had different clinical characteristics and presentation. Future studies with similar design at multiple centres can be conducted to produce more reliable and generalisable results.
6. Conclusion
A pharmacist led diabetes mellitus management programme facilitated the improvement in glycaemic control, medication adherence and quality of life amongst type 2 diabetes mellitus patients. The results of the present study support the evidence on the benefits of pharmacy related management programmes for diabetes from the other parts of the world.
Acknowledgement
The study was funded by Universiti Kebangsaan Malaysia grant UKM-GGPM-048-2010.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mubashra Butt, Email: mestar25@yahoo.com.
Adliah Mhd Ali, Email: adliah@ukm.edu.my.
Mohd Makmor Bakry, Email: mohdclinpharm@ukm.edu.my.
Norlaila Mustafa, Email: norlaila@ppukm.edu.my.
Appendix A
PEPP consisted of a flip chart with multiple units. The highlights of each unit are listed below.
See Table A1
Table A1.
Constituents of intervention programme (PEPP) used in the study.
| Unit | Description |
|---|---|
| Diabetes mellitus and complications of diabetes | • Pictorial illustration of diabetes signs and symptoms |
| • Concise pictorial illustration of diabetes complications | |
| • Management of diabetes complications | |
| Hypoglycaemia and hyperglycaemia | • Pictorial illustration of signs and symptoms |
| • Management of hypoglycaemia and hyperglycaemia | |
| • Target levels | |
| Diabetes medication | • Pictorial illustration of correct insulin administration sites and technique |
| • Emphasis on medication adherence | |
| • Pharmacist was not authorised for pharmacotherapy modifications | |
| Lifestyle modifications | Section A |
| • Pictorial illustration of suitable exercises for diabetes patients | |
| • Preparation before physical activity and warning signs in case of emergency | |
| Section B | |
| • Pictorial illustration of plate method by ADA | |
| • Interactive session about food groups | |
| Selfmonitoring and follow up | • Importance of selfmonitoring |
| • How to keep the record of blood glucose in the diaries | |
| • When to contact the pharmacist for next appointment | |
.
Appendix B
B.1. Interpretation of questionnaires
B.1.1. Modified Morisky medication adherence scale
Scoring of Morisky scale was performed according to the validated Malay version of the questionnaire. Interpretation of scores is given below:
Low adherence (<6).
Medium adherence (6 to <8).
High adherence (=8).
B.1.2. Quality of life (EQ-5D-3L)
Each dimension on the questionnaire has 3 levels: no problems, some problems, extreme problems coded as 1, 2, and 3 respectively. For interpretation, Level 2 and 3 were combined into one value and labelled ‘problems’. The EQ VAS records the respondent’s self-rated health on a vertical, visual analogue scale where the endpoints are labelled as ‘best imaginable health state’ and ‘worst imaginable health state’.
References
- Al Mazroui N.R., Kamal M.M. Influence of pharmaceutical care on health outcomes in patients with Type 2 diabetes mellitus. Br. J. Clin. Pharmacol. 2009;67(5):547–557. doi: 10.1111/j.1365-2125.2009.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhabib S., Aldraimly M. An evolving role of clinical pharmacists in managing diabetes: evidence from the literature. Saudi Pharm. J. 2014 doi: 10.1016/j.jsps.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qazaz H.K., Hassali M.A. The eight-item Morisky medication adherence scale MMAS: translation and validation of the Malaysian version. Diabetes Res. Clin. Practice. 2010;90(2):216–221. doi: 10.1016/j.diabres.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association A.D. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2002;25(Suppl 1):s74–s77. [Google Scholar]
- Association A.D. Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27(Suppl 1):s68–s71. doi: 10.2337/diacare.27.2007.s68. [DOI] [PubMed] [Google Scholar]
- Association A.D. Standards of medical care in diabetes. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges A.P.d.S., Guidoni C.M. The pharmaceutical care of patients with type 2 diabetes mellitus. Pharm. World Sci. 2010;32(6):730–736. doi: 10.1007/s11096-010-9428-3. [DOI] [PubMed] [Google Scholar]
- Chan J.C.N., Malik V. Diabetes in Asia; epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- Choe H.M., Mitrovich S. Proactive case management of high-risk patients with Type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial. Am. J. Manage. Care. 2005;11:253–260. [PubMed] [Google Scholar]
- Collins C., Limone B.L. Effect of pharmacist intervention on glycemic control in diabetes. Diabetes Res. Clin. Practice. 2011;92(2):145–152. doi: 10.1016/j.diabres.2010.09.023. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. 2013. IDF Diabetes Atlas, sixth ed. International Diabetes Federation, Brussels, Belgium <http://www.idf.org/diabetesatlas>.
- Jarab A.S., Alqudah S.G. Randomized controlled trial of clinical pharmacy management of patients with Type 2 diabetes in an outpatient diabetes clinic in Jordan. J. Manage. Care Pharm. 2012;18:516–526. doi: 10.18553/jmcp.2012.18.7.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.L., Nicholas A. Outcomes from DiabetesCARE: a pharmacist-provided diabetes management service. J. Am. Pharm. Assoc. 2008;48:722–730. doi: 10.1331/JAPhA.2008.07133. [DOI] [PubMed] [Google Scholar]
- Kelly C., Rodgers P.T. Implementation and evaluation of a pharmacist-managed diabetes service. J. Manage. Care Pharm. 2000;6:488–493. [Google Scholar]
- Lanting L.C.J., Inez M.A., Mackenbach J.P. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients; a review. Diabetes Care. 2005;28(9):2280–2288. doi: 10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- Lim P.C., Lim K. Evaluation of a pharmacist-managed diabetes medication therapy adherence clinic. Pharm. Practice. 2010;8(4):250–254. doi: 10.4321/s1886-36552010000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R.C.W., Chan J.C.N. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann. N.Y Acad. Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaysian Ministry of Health. 2013. Management of Type 2 diabetes mellitus <http://www.moh.gov.my/attachments/3878.pdf> (Last accessed on May 2013).
- McCord A.D. Clinical impact of a pharmacist-managed diabetes mellitus drug therapy management service. Pharmacotherapy. 2006;26:248–253. doi: 10.1592/phco.26.2.248. [DOI] [PubMed] [Google Scholar]
- McWhorter L., GM O. Providing diabetes education and care to underserved patients in a collaborative practice at a Utah community health center. Pharmacotherapy. 2005;25:96–109. doi: 10.1592/phco.25.1.96.55623. [DOI] [PubMed] [Google Scholar]
- Mehuys E., Van Bortel L. Effectiveness of a community pharmacist intervention in diabetes care: a randomized controlled trial. J. Clin. Pharm. Ther. 2011;36(5):602–613. doi: 10.1111/j.1365-2710.2010.01218.x. [DOI] [PubMed] [Google Scholar]
- Mitchell B., Armoura C. Diabetes medication assistance service: the pharmacist’s role in supporting patient self-management of type 2 diabetes (T2DM) in Australia. Patient Educ. Counselling. 2011;83(3):288–294. doi: 10.1016/j.pec.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Odegard P.S., Goo A. Caring for poorly controlled diabetes mellitus: a randomized pharmacist intervention. Ann. Pharmacother. 2005;39(3):433–440. doi: 10.1345/aph.1E438. [DOI] [PubMed] [Google Scholar]
- Oyetayo O.O., James C. The Hispanic diabetes management program: impact of community pharmacists on clinical outcomes. J. Am. Pharm. Assoc. 2011;51(5):623–626. doi: 10.1331/JAPhA.2011.09229. [DOI] [PubMed] [Google Scholar]
- Phumipamorn S., Pongwecharak J. Effects of the pharmacist’s input on glycaemic control and cardiovascular risks in Muslim diabetes. Prim Care Diabetes. 2008;2(1):31–37. doi: 10.1016/j.pcd.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ragucci K.R., Fermo J.D. Effectiveness of pharmacist-administered diabetes mellitus education and management services. Pharmacotherapy. 2005;25(12):1809–1816. doi: 10.1592/phco.2005.25.12.1809. [DOI] [PubMed] [Google Scholar]
- Roglic G., Unwin N. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005;28(9):2130–2135. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- Rothman R., Malone R. Pharmacist-led, primary care-based disease management improves hemoglobin A1c in high-risk patients with diabetes. Am. J. Med. Qual. 2003;18(2):51–58. doi: 10.1177/106286060301800202. [DOI] [PubMed] [Google Scholar]
- Sarkadi A., Rosenqvist U. Experience-based group education in type 2 diabetes: a randomised controlled trial. Patient Educ. Counselling. 2004;53:291–298. doi: 10.1016/j.pec.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Scott D.M., Boyd S.T. Outcomes of pharmacist-managed diabetes care services in a community health center. Am. J. Health Syst. Pharm. 2006;63(21):2116–2122. doi: 10.2146/ajhp060040. [DOI] [PubMed] [Google Scholar]
- Wild S., Roglic G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]