Abstract
Metabotropic glutamate receptor 4 (mGluR4) possesses immune modulatory properties in vivo, such that a positive allosteric modulator (PAM) of the receptor confers protection on mice with relapsing-remitting experimental autoimmune encephalomyelitis (RR-EAE). ADX88178 is a newly-developed, one such mGluR4 modulator with high selectivity, potency, and optimized pharmacokinetics. Here we found that application of ADX88178 in the RR-EAE model system converted disease into a form of mild—yet chronic—neuroinflammation that remained stable for over two months after discontinuing drug treatment. In vitro, ADX88178 modulated the cytokine secretion profile of dendritic cells (DCs), increasing production of tolerogenic IL-10 and TGF-β. The in vitro effects required activation of a Gi-independent, alternative signaling pathway that involved phosphatidylinositol-3-kinase (PI3K), Src kinase, and the signaling activity of indoleamine 2,3-dioxygenase 1 (IDO1). A PI3K inhibitor as well as small interfering RNA targeting Ido1—but not pertussis toxin, which affects Gi protein-dependent responses—abrogated the tolerogenic effects of ADX88178-conditioned DCs in vivo. Thus our data indicate that, in DCs, highly selective and potent mGluR4 PAMs such as ADX88178 may activate a Gi-independent, long-lived regulatory pathway that could be therapeutically exploited in chronic autoimmune diseases such as multiple sclerosis.
Keywords: Neuroinflammation; Autoimmunity; mGluR4; Noncanonical GPCR signaling; PI3K; Src kinase; Tryptophan metabolism; Indoleamine 2,3-dioxygenase 1; Dendritic cells; Immune regulation
Abbreviations: DC, Dendritic cell; IDO1, indoleamine 2,3-dioxygenase 1; ITIM, immunoreceptor tyrosine-based inhibitory motif; mGluR, metabotropic glutamate receptor; PAM, positive allosteric modulator; PI3K, phosphatidylinositol-3-kinase; RR-EAE, relapsing-remitting experimental autoimmune encephalomyelitis; Treg, T regulatory
Highlights
-
•
ADX88178, a selective mGluR4 PAM, exerts long-term therapeutic effects in RR-EAE.
-
•
ADX88178 activates a noncanonical mGluR4 signaling in DCs.
-
•
ADX88178 induces a tolerogenic functional phenotype in DCs via immunoregulatory IDO1.
-
•
Highly selective mGluR4 PAMs may represent novel drugs in chronic neuroinflammation.
1. Introduction
Glutamate (Glu), the major excitatory neurotransmitter in the central nervous system (CNS), activates ligand-gated ion channels (ionotropic) receptors (iGluRs), as well as G-protein coupled (metabotropic) receptors (mGluRs). mGluRs form a family of eight subtypes, subdivided into three groups on the basis of their amino acid sequence, pharmacology, and G-protein coupling. Group I includes mGluR1 and mGluR5, which are coupled to Gq protein; group II includes mGluR2 and mGluR3, which are coupled to Gi and Go proteins; group III includes mGluR4, mGluR6, mGlur7 and mGluR8, which are also coupled to Gi and Go in heterologous expression systems (Conn and Pin, 1997).
Much like other neurotransmitters, and owing to the diverse nature and function of its receptors, Glu might affect immunity via specific effects on cells of the immune system (Franco et al., 2007, Volpi et al., 2012). Our previous studies indicated indeed that mice lacking mGluR4 are remarkably vulnerable to acute experimental autoimmune encephalomyelitis (EAE), in that they develop exacerbated neuroinflammatory responses, dominated by IL-17–producing T helper type 17 (Th17) cells (Fallarino et al., 2010, Hansen and Caspi, 2010, Volpi et al., 2012). Moreover, prophylactic but not therapeutic treatment with N-Phenyl-7- (hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC), an mGluR4 positive allosteric modulator (PAM), protected wild-type (WT) mice from acute EAE, an effect accompanied by reduced Th17 responses and increased numbers of CD4+Foxp3+ regulatory T (Treg) cells infiltrating CNS (Fallarino et al., 2010). Moreover, the drug did exert therapeutic effects, albeit short-lived, in an experimental model of relapsing-remitting EAE (RR-EAE), which more closely resembles human multiple sclerosis (Fallarino et al., 2010). We also found that constitutive expression of mGluR4 occurred in all dendritic cell (DC) subsets and in specific CD4+ T cell subtypes, including Treg but not inflammatory Th17 cells. However, the absence of mGluR4 in DCs—rather than in CD4+ T cells—would tip the balance of Th cell differentiation in favor of the inflammatory Th17 phenotype.
Although numerous studies have examined the biological effects of PHCCC, several major issues with the compound scaffold still remain open—PHCCC (a) is not very potent and highly selective, being an mGluR1 antagonist as well; (b) cannot be administered by the oral route; and (c) manifests limited brain penetration ability (Maj et al., 2003, Williams et al., 2010).
In the present study, we investigated the in vivo effects of ADX88178, a potent, highly selective, orally bioavailable, and brain-penetrant mGluR4 PAM in the model of RR-EAE as well as its mode of action in DC cultures. We found that ADX88178 is much more effective than PHCCC in controlling RR-EAE over the long term and, more interestingly, it activates an alternative, tolerogenic signaling in DCs that relies on PI3K, Src, noncanonical NF-κB, and immunoregulatory IDO1.
2. Materials & methods
2.1. Animals
Ten-week-old female SJL/J mice (Charles River Breeding laboratories) were used in RR-EAE experiments. For in vitro experiments, 8- to 12-week-old C57BL/6 mice, referred to as WT controls (purchased from Charles River Breeding laboratories), and Grm4−/− mice were used. Specifically, Grm4+/− (B6.129-Grm4tm1Hpn/J) mice, also on a C57/BL6 background, were purchased from The Jackson Laboratory (Bar Harbor, ME). The Grm4−/− offspring of heterozygotes was used to establish colonies of Grm4−/− mice in the animal facility of the University of Perugia. Although characterized by altered spatial learning and memory, Grm4−/− mice do not show any gross motor abnormalities or alterations of fine motor coordination (Pekhletski et al., 1996). All mice used in these studies were genotyped by PCR of DNA isolated from tail clippings. In the skin test assay, 8- to 12-week-old Balb/c (Charles River Breeding laboratories) were used. RR-EAE Exp. A and skin test assays were in compliance with national (Italian Approved Animal Welfare Assurance A-3143-01) and Perugia University Animal Care and Use Committee guidelines, and the overall study was approved by the Bioethics Committee of the University of Perugia. RR-EAE Exp. B, externally performed by MD Biosciences Ltd. (Neurology Discovery Services Division, Weizmann Science Park, Ness Ziona, Israel), was conducted in compliance with rules and regulations of the Israel Committee for Ethical Conduct in the Care and Use of Laboratory Animals. All animal studies complied with the ARRIVE guidelines, ensuring that all efforts were made to minimise animal suffering, to reduce the number of animals used, and to utilise alternatives to in vivo techniques, if available.
2.2. Drugs
ADX88178 (5-methyl-N-(4-methylpyrimidin-2yl)-4-(1H-pyrazol-4-yl)thiazol-2-amine; CAS: 1235318-89-4) (Kalinichev et al., 2014), ADX104608 (PCT Int. Appl. (2013) WO2013/107862), and ADX104583 were synthesized at Addex Therapeutics. PHCCC was purchased from Tocris Bioscience. Both ADX88178 and PHCCC were formulated in sesame oil for in vivo studies.
2.3. Induction of RR-EAE and in vivo treatments
RR-EAE was induced as described in both Exp. A and Exp. B (Fallarino et al., 2010). Briefly, SJL/J female mice were immunized with 100 μg of proteolipid protein peptide 139–151 (PLP139–151; HSLGKWLGHPDKF) emulsified in complete Freund adjuvant (CFA; Sigma–Aldrich) with 5 mg/ml Mycobacterium tuberculosis (BD Difco). Each mouse received s.c. injections of 200 μl emulsion, fractionated in two distinct sites draining axillary and inguinal lymph nodes. Pertussis toxin (PTX, 200 ng/mouse; List Biological Laboratories) was administered i.p. on the day of immunization and 48 h later. Mice were monitored daily blindly by two independent observers for clinical scores and body weights at least three times per week up to 80 d (Exp. A) or daily up to 60 d (Exp. B). EAE reactions were scored and recorded according to a 0–15 scale, as described (Weaver et al., 2005). In the scoring based on a 0–15 scale, the final score is the sum of the state of the tail and all of the four limbs. For the tail, a score of 0 reflects no signs, 1 represents a half paralyzed tail, while a score of 2 is given to a mouse with a fully paralyzed tail. For each of the hind- or fore-limbs, each assessed separately, 0 signifies no signs, a score of 1 is a weak or altered gait, 2 represents paresis, while a score of 3 denotes a fully paralyzed limb. Thus, a fully paralyzed quadriplegic animal would attain a score of 14, whereas mortality equals a score of 15. At the beginning of the first clinical attack (at 10–12 d in both experiments), mice were randomized into groups to be treated daily for two weeks and every other day on the third week with either vehicle alone (sesame oil), ADX88178 (at 10–30–60 mg/kg), or PHCCC (3 mg/kg; all administered s.c.). Dosing solutions in sesame oil (ready-to-administer) at 10 mL/kg were provided with blinded code. A 15-day stability of dosing solutions was performed and dosing solutions were stored at 4 °C until use. A relapse was defined as a sustained (≥2 d) increase in clinical score by at least 1 (or 3 in Exp. B) full grade after the animal had improved previously by at least 1 (or 3 in Exp. B) full grade and stabilized for at least 2 d as described (Theien et al., 2003). Mean peak clinical score was defined as the greatest clinical score reached during a specified phase of the treatment (on treatment or post treatment).
2.4. Collection of sera samples and ADX88178 plasma concentration analysis
Satellite groups to be used for pharmacokinetic studies were vaccinated with PLP and treated with either 10 or 60 mg/kg ADX88178, respectively, as described above. Blood samples were collected at days 1, 7 and 14 of drug administration by retro-orbital bleeding to produce approximately 50 μl of plasma on each bleeding. On each blood sampling day, plasma specimens were prepared immediately, frozen and shipped to Addex Therapeutics for analysis. For the dose of 30 mg/kg, plasma samples from naïve SJL/J mice were used.
For analysis of ADX88178 plasma concentrations, 50 μl of plasma samples spiked with 10 μl DMSO for unknown samples or 10 μl of ADX88178 for calibration and quality control samples were precipitated with 150 μL of acetonitrile. After vortexing and centrifuging (15 min at 4 °C and 13,200 rpm), a sample portion (100 μL) was transferred into a 384-well analytical plate. Five μl of the supernatant were injected into an ultra performance liquid chromatography system (Waters) coupled with mass spectrometry (API 3200, Applied Biosystems), and signal was detected in an electrospray ionization positive mode. By using a 0.9 min gradient from 25% to 100% acetonitrile in formic acid/ammonium formate buffer at pH 3.5, the retention time of ADX88178 was 0.4 min.
2.5. Leukocyte isolation and Real-Time PCR
Purification of leukocytes from spinal cords (SC) were performed as described (Fallarino et al., 2010). Briefly, spinal cords were recovered from anesthetized mice perfused with cold PBS. After centrifugation of spinal cord homogenates, infiltrating leukocytes were separated on a discontinuous percoll gradient (Sigma–Aldrich).
Real-Time PCR (for Rorc, Foxp3, and Ido1) analyses were carried out as described (Fallarino et al., 2010, Pallotta et al., 2011) and expression of each gene was normalized to Gapdh expression, as determined by the relative quantification method (ΔΔCT) (mean ± SD of triplicate determination).
2.6. In vitro cell stimulation and treatments of purified DCs
Splenic DCs were purified by magnetic-activated cell sorting using CD11c MicroBeads and MidiMacs (Miltenyi Biotec), in the presence of EDTA to disrupt DC-T cell complexes, as described (Grohmann et al., 2007, Orabona et al., 2008, Pallotta et al., 2011). In sorting CD8− DCs, CD11c+ cells were further fractionated using CD8 MicroBeads (Miltenyi Biotec) (Belladonna et al., 2008, Fallarino et al., 2010, Pallotta et al., 2011). PHCCC, ADX88178, ADX104608, and ADX104583 were dissolved in DMSO at an initial concentration of 10 mM and diluted in complete cell culture medium in order to obtain the desired final concentration. DCs were incubated for 24 h with medium alone (unstimulated cells) or with 1 μg/ml LPS (strain 0:55; Sigma–Aldrich). Drugs were added to the cultures 30 min before LPS, until recovering supernatants at 24 h. In selected experiments, LY294002 (inhibitor of PI3K; Cell Signaling Technology), PP2 (an Src inhibitor), and PP3 (negative control for the Src inhibitor; both from Tocris Bioscience) were also used at the final concentration of 25 (LY294002) or 5 (PP2 and PP3) μM with the same timing of PAMs. PTX was added to DC cultures at the final concentration of 200 ng/ml 2 h before addition of PAMs.
2.7. Cytokine and kynurenine determination
Mouse cytokines (IL-6, IL-10, and TGF-β1) were measured in culture supernatants by ELISA using specific kits (eBioscience and Promega) or previously described reagents (Fallarino et al., 2010, Pallotta et al., 2011). The functional activity of IDO1 was measured in vitro in terms of the ability to metabolize tryptophan to l-kynurenine, whose concentration was measured by high-performance liquid chromatography in culture supernatants at 16 h after the addition of 100 μM tryptophan for the final 8 h (Grohmann et al., 2002, Grohmann et al., 2007).
2.8. cAMP determination
Measurements of intracellular cAMP levels were performed essentially as described (Fallarino et al., 2010) with some modifications. Briefly, DCs (0.5 × 106 cells/150 μl/sample) were pre-incubated in Locke's solution buffer (glutamate-free), pH 7.4, containing 0.5 mM isobutylmethylxanthine (a phosphodiesterase inhibitor) for 20 min to block the breakdown of cAMP. PHCCC and ADX compounds (dissolved in DMSO at the initial concentration of 10 mM and diluted in Locke's solution at different final concentrations) were added 30 s before the addition of 10 μM forskolin (Sigma–Aldrich) and the incubation was continued for additional 10 min. The reaction was stopped by incubating samples for 2 min on ice. Samples were then centrifuged at 5000 rpm for 5 min at 4 °C, washed in PBS, and intracellular cAMP levels were measured using the Direct Cyclic AMP enzyme immunoassay kit (Arbor Assays).
2.9. Western blotting
IDO1 and pIDO1 expressions were investigated in DCs by immunoblot with a rabbit monoclonal anti-mouse IDO1 antibody (cv152) or a rabbit polyclonal antibody to the phosphorylated ITIM2 motif of IDO1, respectively, both raised in our laboratory (Pallotta et al., 2011). Src and its phosphorylated form were revealed by specific anti-Src and -pSrc antibodies (Tyr416; Cell Signaling Technology). Anti-Akt and –pAkt were also from Cell Signaling Technology. Anti–β-actin antibody (Sigma–Aldrich) was used as a normalizer.
2.10. Skin test assay
A skin test assay was used for measuring major histocompatibility complex class I–restricted delayed-type hypersensitivity in response to challenge in the footpad with the IGRP synthetic peptide, as described (Grohmann et al., 2003a, Pallotta et al., 2014), using 12-wk-old Balb/c as DC donors and recipients. The H-2Kd-restricted IGRP peptide (KYNKANAFL) is a diabetogenic autoantigen in nonobese diabetic mice but is also recognized by H-2d−expressing BALB/c animals (Pallotta et al., 2011). The response to challenge in the footpad with the eliciting peptide was measured at 2 wk, and results are presented as the weight of peptide-injected footpad relative to vehicle-injected counterpart (Grohmann et al., 2003a, Grohmann et al., 2002, Grohmann et al., 2007, Pallotta et al., 2011, Volpi et al., 2013).
2.11. Statistical analyses
2.11.1. Many-to-one comparisons in repeated measures one-way ANOVA design
To assess whether treatment with ADX88178 and with PHCCC was effective in reducing disease severity as compared to vehicle treatment, we contrasted each treatment group with the respective control—PHCCC vs. vehicle, 10 mg/kg ADX88178 vs. vehicle, 30 mg/kg ADX88178 vs. vehicle and 60 mg/kg ADX88178 vs. vehicle (Table S2). An additional analysis was performed by comparing the PHCCC-treated group vs. ADX88178-treated groups (Table S3). Mean group EAE severity scores with standard errors are displayed in Fig. 2A. For each comparison, a two-sample Wilcoxon-Mann-Whitney one-sided test was performed at each measurement time. Global significance over time was obtained by using the maxT permutation-based familywise error rate controlling method of Westfall & Young (Goeman and Solari, 2014) to account for within experimental unit correlation existing in longitudinal data and for multiple time points. Finally, global P-values were multiplicity-adjusted for the four comparisons by using the Bonferroni-Holm method (Goeman and Solari, 2014).
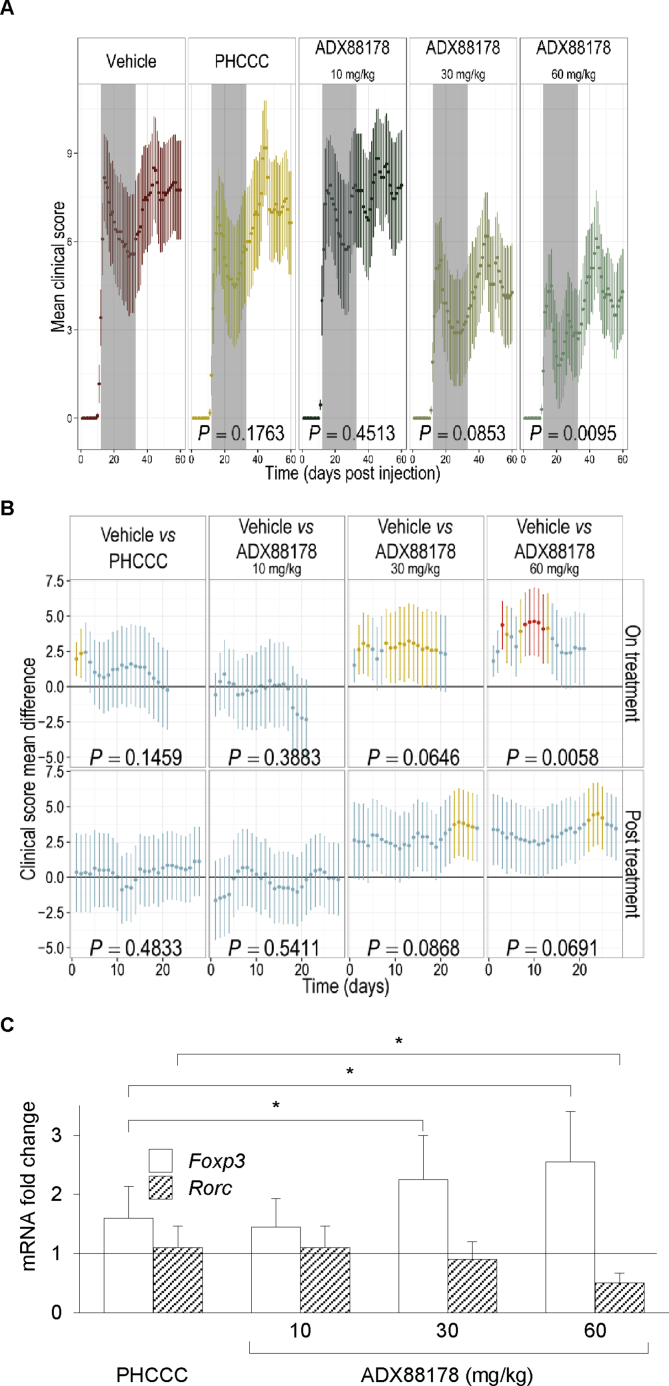
Fig. 2.
ADX88178 exerts long-term, therapeutics effects in RR-EAE. RR-EAE was induced in SJL/J mice by immunization with the PLP peptide on day 0. Two separated experiments, conducted as described in Materials and Methods, led to similar results. Mice were treated s.c. daily for 2 wk and every other day for an additional wk (indicated) with vehicle (control), PHCCC at 3 mg/kg or ADX88178 at 10, 30, or 60 mg/kg, starting from the disease onset. (A) Mean clinical scores over time. Grey boxes indicates the drug treatment period. Indicated P-values represent the global P-values (see also Table S3). (B) Clinical score mean differences over the time period encompassing (on treatment; day 0 corresponding to day 10 post-PLP sensitization) or after drug treatment (post treatment; day 0 corresponding to day 31 post-PLP sensitization). Indicated P-values represent the global P-values for each group in each defined time period. Blue bars (P > 0.05), yellow bars (P < 0.05), and red bars (P < 0.01). (C) Foxp3 and Rorc transcript levels in leukocytes purified from spinal cords of mice used in Exp. A at 60 d by Real Time-PCR, using Gapdh expression for normalization. Data are presented as fold change in normalized transcript expression in RR-EAE mice relative to vehicle-injected counterparts (in which fold change = 1; dotted line). All data (A–C) are presented as means ± SE. *P < 0.05.
2.11.2. Other analyses
Unpaired Student's t-test was used for in vitro analyses, using at least three values from 1 to 3 experiment per group, and for the skin test assay (using at least 6 mice per group). For cell recovery, one way ANOVA associated with Bonferroni's analysis between specific pairs of samples was used. Differences were considered significant with P < 0.05.
3. Results
3.1. ADX88178 exerts long-term therapeutic effects in RR-EAE
Our previous studies indicated that PHCCC significantly reduces the number and severity of relapses in mouse RR-EAE, but only if continuously administered s.c. until the end of the experiment (daily dose of 3 mg/kg) (Fallarino et al., 2010). We here compared the efficacy of a short administration regimen of the previously used dose of PHCCC and ADX88178 (the most potent and selective mGluR4 PAM as revealed by pharmacological analyses; SI Results) at the dose of 10, 30, and 60 mg/kg in the same disease setting (Fig. 1, Fig. 2). Either drug was administered s.c. daily for the first two weeks and every other day for the third week, starting approximately at 10 days post vaccination (i.e., at the first clinical attack) with the proteolipid protein peptide (PLP), and clinical scores were measured daily up to 60 days, when mice were sacrificed. For pharmacokinetic analyses, satellite groups of mice were administered ADX88178 at either 10 or 60 mg/kg and blood samples collected at days 1, 7 and 14 of drug administration. The administration of ADX88178 at the dose of 60 but not 10 mg/kg maintained a total plasma concentration close to, or greater than, 55 ng/ml (Table S1), that is, above the minimal effective concentration (9 nM) in vitro that ensues potentiation of mGluR4 activation (Kalinichev et al., 2014).
Fig. 1.
Chemical structures of mGluR4 PAMs evaluated in the mouse RR-EAE model. PHCCC (N-Phenyl-7- (hydroxyimino)cyclopropa[b]chromen-1a-carboxamide) and ADX88178 (5-methyl-N-(4-methylpyrimidin-2-yl)-4-(1H-pyrazol-4-yl)thiazol-2-amine).
Analysis of the disease profile over the entire duration of the experiment indicated that all animal groups exhibited approximately two episodes (or three, for mice receiving the lowest dose of ADX88178) of RR-EAE followed by a chronic phase of the disease (Fig. 2A). The overall drug effect in each experimental group was evaluated by determining the global significance (i.e., global P value) of daily mean clinical scores over the entire period of observation (from day 10 to day 60 of post-sensitization with PLP) on contrasting drug vs. vehicle administration by a robust statistical analysis as described in Material and Methods. Mice on PHCCC or ADX88178 at 10 mg/kg did not reach the global significance threshold when compared to the vehicle group (Fig. 2A and Table S2; global P = 0.1763 and 0.4513, respectively). In contrast, the global therapeutic effect over time of ADX88178 at the doses of 30 and 60 mg/kg was almost (P = 0.0853) or highly significant (P = 0.0095), respectively (Fig. 2A and Table S2).
To evaluate whether the global analysis could have masked significant therapeutic effects occurring during or after drug treatment, the statistical analysis was re-run on specific time frames, i.e., on treatment (including the three weeks of drug treatment) and post treatment (after day 31 of post-sensitization with PLP). On comparing the clinical score mean differences of drug v. vehicle administration at such specific periods of time, we found that, again, treatment with either PHCCC or ADX88178 at the lowest dose did not reach the global significance value during or after drug treatment, although significant differences (P < 0.05) with the vehicle group could be observed for PHCCC treatment at two early time points (Fig. 2B and Table S2). In contrast, the highest dose of the selective PAM did yield global significance effects during the entire timeframe of treatment (P = 0.0058). The same dose during the post-treatment period and the median dose during both time periods yielded results very close to global significance (P = 0.0691 and P = 0.0868, respectively). Moreover, in the ADX88178 at 30 and 60 mg/kg groups, mean clinical scores were significantly different (P < 0.05 and P < 0.01) from those of the vehicle group at several, specific time points, at either on or post treatment times (Fig. 2B). No significant effects could be observed for any treatments on mean body weights (Fig. S1) as compared to the control group.
To get some insight into the immunoregulatory effects possibly exerted by the two mGluR4 PAMs in RR-EAE, mice were sacrificed at the end of the experiment to evaluate histopathology and nature of infiltrating lymphocytes in spinal cords. In line with previous results by others in the mouse RR-EAE model (Theien et al., 2003), low-grade inflammation and limited damage of myelin-containing nervous fibers could be detected in all groups, with some improvements in mice given ADX88178 at 30 and 60 but not 10 mg/kg as well as PHCCC (Fig. S2). On performing Real-Time PCR experiments, we found a significant increase in the expression of Foxp3 (encoding the Treg specification factor) in transcripts of infiltrating leukocytes purified from the spinal cords of PHCCC- or ADX88178-treated mice as compared to control animals (Fig. 2C). Interestingly, the increase in Foxp3 expression was significantly higher in ADX88178-treated (at 30 and 60 mg/kg) than PHCCC-treated mice. Moreover, Rorc transcripts (encoding the Th17 specification factor) were significantly downregulated in ADX88178-but not PHCCC-treated animals.
Overall, our data, besides further confirming the therapeutic value of mGluR4 PAMs in neuroinflammation, indicate that ADX88178, possibly by virtue of its stringent mGluR4 selectivity, high potency and optimal bioavailability, exhibits higher efficacy in RR-EAE than PHCCC (Table S3). Perhaps more importantly, the therapeutic effects of ADX88178—at variance with PHCCC (Fallarino et al., 2010)—could be still observed after drug discontinuation, albeit to a lower extent, as suggested by a reduced significance level (P = 0.0691; Fig. 2 and Table S2), and its disappearance from plasma (Table S1), suggesting the involvement of mechanisms that, once activated, are capable of self-sustaining immunoregulatory effects over the long term.
3.2. ADX88178 and ADX104608 induce an immunoregulatory cytokine profile in DCs in an mGluR4-dependent fashion
Our previous studies indicated that the immunoregulatory effects of mGluR4 activation are wholly dependent on DCs. In fact, in DC-T cell co-cultures, the absence of Grm4 (the gene coding for the receptor) in DCs but not in CD4+ T cells favored the emergence of Th17 cells (Fallarino et al., 2010). In cultures with DCs alone activated with the TLR4 agonist LPS, lack of mGluR4 increased the production of IL-6 and IL-23 (both cytokines being required for the induction and/or expansion of Th17 cells) and of IL-10 as well, and it decreased the levels of secreted IL-12 (the primary stimulus for Th1 cell induction) and of immunoregulatory TGF-β. Conversely, in vitro treatment of WT DCs with PHCCC reduced the production of IL-6, IL-10 and IL-23, and it increased IL-12 and TGF-β, but only when cells had been activated with LPS. As a whole, our previous studies suggested that, under inflammatory conditions, PHCCC can significantly bias the cytokine secretion profile of DCs towards a less proinflammatory and more immunoregulatory profile, although the reduction in IL-10, a typically anti-inflammatory mediator (Saraiva and O'Garra, 2010), would not fit properly into such a conceptual framework.
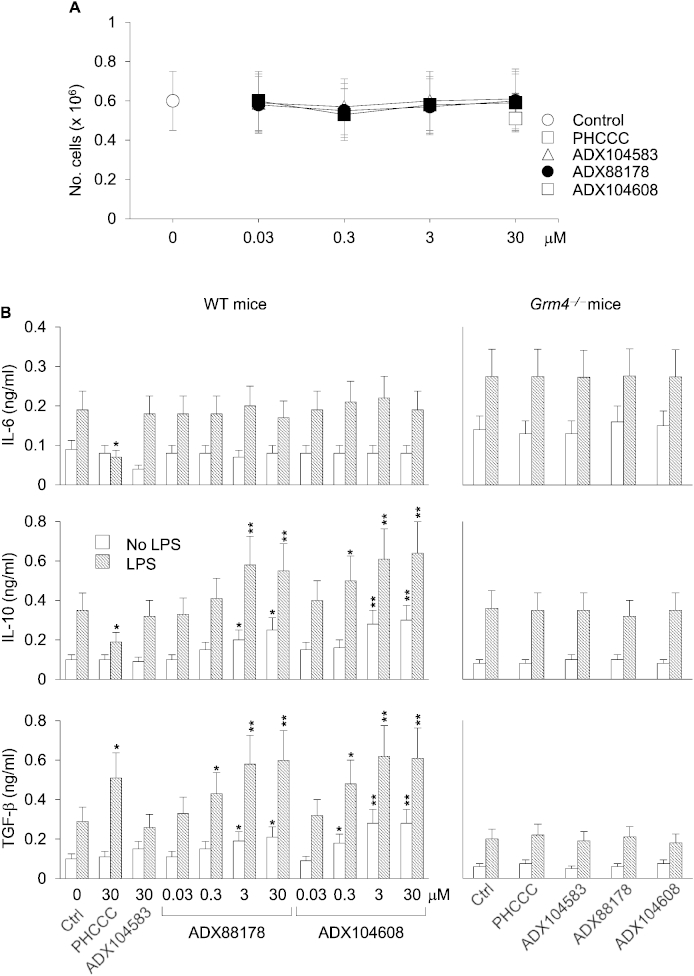
Because DCs are non-proliferating cells and several maneuvers, including drug treatments, are known to decrease their viability in vitro (Hackstein and Thomson, 2004), we first evaluated whether ADX88178 and the novel mGlu4R PAM ADX104608 (SI Results) would affect viability in DCs. We measured cell viability in terms of absolute number of cells recovered after a 24-h culture of purified conventional DCs, i.e., expressing the DC marker CD11c, with or without mGluR4 PAMs at different concentrations (30 nM–30 μM), in the presence or absence of LPS. PHCCC and ADX104583 (inactive at mGluR4) were also assayed (both at 30 μM). No significant effects on the recovery of DCs after a 24-h culture could be observed for all of the tested compounds (Fig. 3A). Thus these data suggest that selective mGluR4 PAMs will not significantly affect cell viability in DCs.
Fig. 3.
ADX88178 and ADX104608 increase the production of IL-10 and TGF-β in mGluR4-expressing DCs. (A) Viability of splenic DCs treated with ADX compounds. DCs (106/well) purified from the spleens of C57BL/6 mice were either left unstimulated or stimulated with LPS in the presence or absence (Ctrl, control) of different concentrations (indicated) of ADX88178 and ADX104608. PHCCC and the negative control (ADX104583), both at 30 μM, were also used. After 24-h of culture, cells were harvested and counted. (B) IL-6, IL-10, and TGF-β production by DCs treated with mGluR4 PAMs. DCs from WT or Grm4−/− C57BL/6 mice were either left unstimulated or stimulated with LPS in the presence or absence of different concentrations (WT mice) or 3 μM (Grm4−/− mice) of ADX88178 and ADX104608. PHCCC and ADX104583 were also assayed as in (A). After 24 h of culture, cell supernatants were harvested and assayed for cytokine contents by ELISA. All data (A, B), presented as means ± SD from triplicate samples, are shown for one representative experiment of three. *P < 0.05 and **P < 0.01 (drug-treated samples vs control).
We then evaluated the in vitro effects of ADX88178 and ADX104608 at different concentrations on the production of IL-6, IL-10, and TGF-β1 by DCs, either unstimulated or stimulated with LPS. PHCCC and ADX104583 at 30 μM were used as controls. In accordance with our previous data, PHCCC significantly reduced production of IL-6 and IL-10 and increased the production of TGF-β1 in DCs activated by LPS but not in unstimulated cells (Fig. 3B). Although no change could be observed in the levels of proinflammatory IL-6, ADX88178 and ADX104608 significantly increased production of all the anti-inflammatory cytokines being tested, namely TGF-β1 and IL-10 as well, an effect that was evident in both LPS-activated and non-activated DCs (Fig. 3B). No significant modulatory effect was found in the production of any cytokine upon treatment with the negative control compound. All cytokines found to be modulated significantly by mGluR4 PAMs were also measured in culture supernatants from DCs purified from the spleens of Grm4−/− mice, using the same drug concentrations as above, but no significant modulations were found (Fig. 3B).
These data indicate that selective and potent mGluR4 PAMs such as ADX88178 and ADX104608 exert important effects on DCs, promoting a clear immunoregulatory cytokine profile that, however, is distinct from that induced by PHCCC. Thus ADX88178 and ADX104608 may have a different—yet mGluR4-dependent—mode of action, as compared to PHCCC.
3.3. Selective mGluR4 PAMs activate Gi-dependent and -independent signaling pathways in DCs
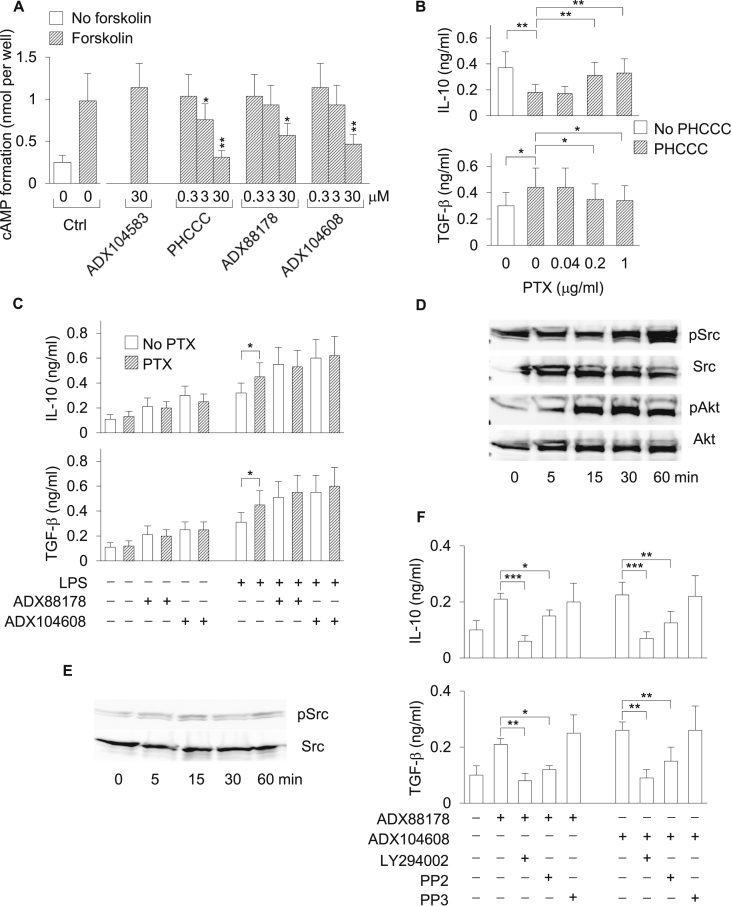
In presynaptic nerve terminals and microglia, mGluR4 activation lowers intracellular cAMP formation in a Gi protein-dependent fashion (Conn and Pin, 1997). In our previous work (Fallarino et al., 2010), we demonstrated that PHCCC reduces formation of cAMP in DCs stimulated with forskolin, a direct activator of adenylyl cyclase. We therefore measured cAMP levels in DCs stimulated with forskolin in the presence or absence of ADX88178 or ADX104608 at different concentrations. PHCCC and ADX104583 were used as a positive and negative control, respectively. In accordance with our previous data, PHCCC at 3 and 30 μM significantly reduced the cAMP formation in DCs. ADX88178 and ADX104608 also reduced cAMP levels as induced by forskolin in DCs, but only when used at the highest concentration, i.e., 30 μM (Fig. 4A).
Fig. 4.
PI3K/Akt- and Src- but not Gi-dependent signaling pathways are required for the production of IL-10 and TGF-β by DCs treated with ADX88178 and ADX104608. (A) Intracellular cAMP formation in DCs treated with mGluR4 PAMs. Cells were stimulated with forskolin (or left unstimulated as control) at 10 μM for 10 min in the presence or absence of ADX88178, ADX104608, and PHCCC at different concentrations. Ctrl, control. (B) Effect of PTX on the inhibition of IL-10 and induction of TGF-β by PHCCC in LPS-stimulated DCs. DCs treated with PHCCC at 30 μM were stimulated with LPS in the presence or absence of PTX at different concentrations and cytokine contents were measured in 24-h culture supernatants. (C) Effect of PTX on the induction of IL-10 and TGF-β by ADX88178 and ADX104608. DCs treated with ADX88178 or ADX104608 at 3 μM were left unstimulated or stimulated with LPS in the presence or absence of PTX at 0.2 μM and cytokine contents were measured in 24-h culture supernatants. (D) Kinetics of Akt and Src phosphorylation in WT DCs treated with ADX88178 at 3 μM. Lysates from drug-treated DCs (not subjected to LPS stimulation) were analyzed by sequential immunoblotting with antibody to phosphorylated Akt (pAkt) and anti-Akt or to phosphorylated Src (pSrc) and anti-Src. (E) Kinetics of Src phosphorylation in Grm4−/− DCs treated as in D. (F) Effect of PI3K (LY294002; 25 μM) and Src (PP2; 5 μM) inhibitors on the induction of IL-10 and TGF-β by ADX88178 and ADX104608 in WT DCs. PP3 (5 μM) was used as PP2 negative control. DCs (not subjected to LPS stimulation) were treated with selective mGluR4 PAMs at 3 μM in the presence or absence of enzyme inhibitors and cytokine contents were measured in 24-h culture supernatants. Data (A-C and F), presented as means ± SD from triplicate samples, are shown for one representative experiment of three. In D and E, one experiment of two. *P < 0.05, **P < 0.01, and ***P < 0.001.
Because the in vitro effects of selective mGluR4 PAMs on the production of immunoregulatory cytokines by DCs were observable at 3 μM (and also at 0.3 μM; Fig. 3B) and no modulation of cAMP levels was evident at this concentration, we asked whether ADX88178 and ADX104608 effects could be, at least at low concentrations, Gi-independent. To verify our hypothesis, TGF-β and IL-10 levels were measured in culture supernatants of DCs preincubated for 2 h with pertussis toxin (PTX), a standard investigative tool that uncouples metabotropic receptors from Gi/Go proteins (Kurose and Ui, 1983), prior to the 24 h-incubation with mGluR4 PAMs in the presence or absence of LPS. Pre-incubation with PTX significantly decreased and increased the levels of IL-10 and TGF-β, respectively, induced by PHCCC in LPS-stimulated cells (Fig. 4B). In contrast, upregulation of TGF-β and IL-10 as induced by ADX88178 and ADX104608 in either unstimulated or LPS-stimulated DCs were not significantly affected by PTX (Fig. 4C). Although PTX alone upregulated TGF-β and IL-10 in LPS-treated DCs (Fig. 4B,C), an effect previously reported as being due to toxin synergism with TLR4 signaling (Nishida et al., 2010), these data seemed to suggest that the immunoregulatory effects of selective mGluR4 PAMs in DCs are Gi-independent.
G-protein activation is considered to be a key event in mGluR-dependent responses. Nevertheless, alternative pathways can also be at work (Enz, 2007, Gerber et al., 2007). In particular, stimulation of group III mGluRs by l-AP-4, a nonselective orthosteric agonist, has been shown to induce activation of Src kinases in midbrain neurons (Jiang et al., 2006). Moreover, in cerebellar granule cells, group III mGluRs are functionally coupled to the PI3K/Akt pathway, which is required for the neuroprotective effects of l-AP-4 (Iacovelli et al., 2002).
We therefore investigated whether ADX88178 and ADX104608, at concentrations reflecting functional effects in DCs, could activate a signaling pathway mediated by PI3K/Akt and/or Src kinase in DCs. Phosphorylation of Akt and Src was evaluated by immunoblotting in DC lysates from WT mice and incubated with mGluR4 PAMs for 5–60 min in the absence of TLR4 activation (Fig. 4D). Akt and Src phosphorylation occurred at 15 min of treatment of WT DCs with ADX88178 at 3 μM and remained at sustained levels up to 1 h (Fig. 4D). In contrast, no phosphorylation of Src could be detected in Grm4−/− DCs at any time points (Fig. 4E). Although similar results were obtained with ADX104608, PHCCC did not induce Akt and Src phosphorylation in DCs (data not shown).
TGF-β and IL-10 levels were next measured in culture supernatants of DCs treated with the selective mGluR4 PAMs for 24 h after preincubation with LY294002 (a PI3K inhibitor) or PP2 (a Src inhibitor) for 2 h. PP3 was used as a PP2 negative control. The PI3K inhibitor completely ablated both TGF-β and IL-10 induction by ADX88178 and ADX104608, whereas inhibition by the Src antagonist was only partial (Fig. 4F). No significant effects could be observed for PP3.
Overall, these data indicated that, as previously reported for other metabotropic receptors (Jones et al., 1991), different concentrations of mGluR4 ligands can activate distinct mechanisms, i.e., Gi-dependent and -independent in nature. Perhaps most importantly, a PI3K/Src-rather than Gi-mediated signaling pathway appears to be indispensable for the immunoregulatory efficacy of ADX88178 and ADX104608 in DCs.
3.4. Selective mGluR4 PAMs induce IDO1 expression and signaling in DCs
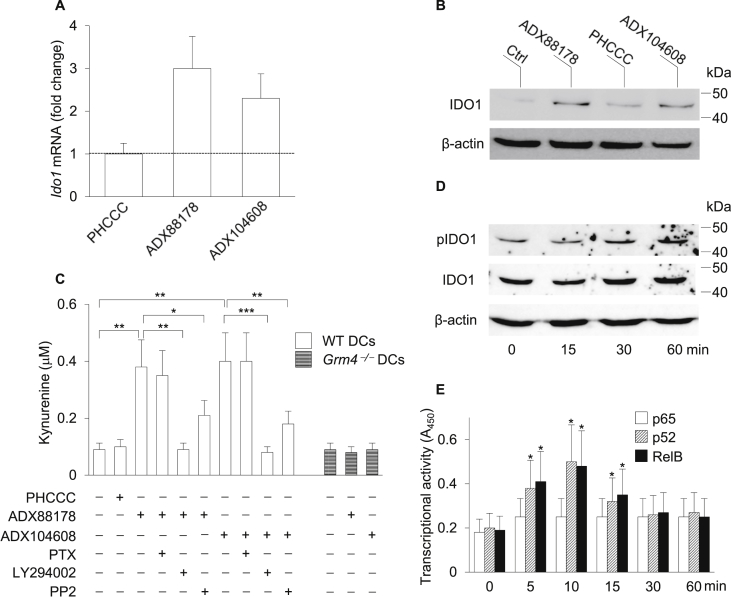
Indoleamine 2,3-dioxygenase 1 (IDO1) is a natural immunoregulatory mechanism that contributes to immune suppression and tolerance in a variety of settings (Grohmann et al., 2003b, Mellor and Munn, 2004). IDO1 immunoregulatory effects are mainly mediated by DCs and involve tryptophan deprivation and production of immunoactive kynurenines. As a result, IDO1-expressing DCs mediate multiple effects on T lymphocytes, including inhibition of proliferation, apoptosis, and differentiation towards a regulatory phenotype (Grohmann and Bronte, 2010, Puccetti and Grohmann, 2007). IDO1-dependent effects also include non-enzymic functions, namely intracellular signaling events that, initiated by phosphorylation of specific domains (i.e., immunoreceptor tyrosine-based inhibitory motifs or ITIMs) in the enzyme, are involved in reprogramming gene expression and in the induction of a stably regulatory phenotype in splenic DCs (Orabona et al., 2012, Pallotta et al., 2011). In particular, IDO1 ITIM phosphorylation is triggered in DCs by TGF-β, via a pathway that requires PI3K and a tyrosine kinase of the Src family (specifically, Fyn in plasmacytoid DCs (Pallotta et al., 2011) or Src in conventional DCs (Bessede et al., 2014)) that phosphorylates IDO1 ITIMs, thus creating docking sites for protein phosphatases such as SHP-1 and SHP-2. These events lead to the activation of an immunoregulatory signaling pathway in DCs that relies on the noncanonical NF-κB pathway, endogenous production of TGF-β, and induction of the Ido1 gene, perpetuating the IDO1 signaling events and the consequent immunosuppressive effects over the long term (Bessede et al., 2014, Pallotta et al., 2014, Pallotta et al., 2011).
Considering that ADX88178 exerts long-term immunosuppressive effects in the RR-EAE model (Fig. 2) and, similarly to ADX104608, induces the production of TGF-β as well as IL-10 by conventional DCs via a pathway mediated by PI3K and Src (Fig. 4), we investigated whether selective mGluR4 PAMs could also induce IDO1 and its immunoregulatory signaling. WT DCs were stimulated with ADX88178 or ADX104608 at 3 μM or PHCCC at 30 μM and, after 24 h, IDO1 expression in terms of both transcript (Fig. 5A) and protein (Fig. 5B) was evaluated by means of Real-Time PCR and Western blot analysis, respectively. No evident increase in IDO1 transcripts or protein could be observed in DCs incubated with PHCCC. In contrast, significant up-regulation of Ido1 transcription and IDO1 protein expression was detected in cells treated with either ADX88178 or ADX104608 (Fig. 5A,B). Similar results were obtained on evaluating the levels of l-kynurenine, the main product of IDO1's catalytic activity, which significantly increased in culture supernatants of WT but not Grm4−/− DCs treated with mGluR4 PAMs, an effect completely (LY294002), partially (PP2), or not at all (PTX) (data not shown for PP3) negated by the inhibitors (Fig. 5C).
Fig. 5.
ADX88178 and ADX104608 induce IDO1 expression and activate IDO1 signaling in DCs. (A) Expression of Ido1 transcripts in DCs treated with mGluR4 PAMs. WT DCs were treated with ADX88178 or ADX104608 at 3 μM, or PHCCC at 30 μM, for 24 h and analyzed for Ido1 expression by Real-Time PCR, using expression of the Gapdh gene for normalization. Data are presented as normalized transcript expression in the samples relative to normalized transcript expression in the control (i.e., untreated DCs) culture (that is, fold change = 1, dotted line). (B) Expression of the IDO1 protein in WT DCs treated with mGluR4 PAMs. Cell lysates from DCs treated as in A for 24 h were subjected to sequential immunoblotting analysis by using specific anti–IDO1 and –β-actin antibodies. (C) IDO1 catalytic activity in WT and Grm4−/− DCs treated with mGluR4 PAMs and modulation thereof by PTX or PI3K and Src inhibitors. DCs were treated as in (B) and IDO1 catalytic activity was assessed as l-kynurenine in culture supernatants. (D) Kinetics of phosphorylation of IDO1 ITIM in WT DCs treated with ADX88178. DC cell lysates were analyzed by sequential immunoblotting with anti–pIDO1, –IDO1, and –β-actin specific antibodies. (E) Activation of p65, p52 and RelB in nuclear extracts of WT DCs treated or untreated (time 0) with ADX88178 at 3 μM for different times measured by ELISA. Results are presented as absorbance at 450 nm (A450). One representative experiment out of two (D, E) or three (A–C) is shown. Data (A, C, and E) are presented as means ± SD from triplicate samples. *P < 0.05, **P < 0.01, and **P < 0.001.
To investigate whether IDO1 signaling, in addition to IDO1 expression and catalytic activity, could also be induced by selective mGluR4 PAMs, we evaluated IDO1 phosphorylation, using an antibody specific for the phosphorylated form of the enzyme (pIDO1) (Pallotta et al., 2011). We also investigated the activation of noncanonical NF-κB in response to DC stimulation with ADX88178. In fact, molecular dissection of NF-κB activation has shown that NF-κB can be induced by the so-called canonical (classical; IκB (IKK)β-dependent) and noncanonical (alternative; IKKα-dependent) signaling pathways, leading to distinct patterns in the individual NF-κB subunits that are activated and distinct downstream genetic responses. While the canonical NF-κB pathway involves the nuclear translocation of p50-p65 dimers and proinflammatory effects, the noncanonical NF-κB signaling relies on the proteasomal degradation of p100 into p52 and formation of p52-RelB dimers that translocate to the nucleus and activate an anti-inflammatory gene program (Bonizzi and Karin, 2004, Puccetti and Grohmann, 2007). Particularly relevant in this context is the fact that IDO1 expression and signaling are contingent on activation of the noncanonical NF-κB pathway (Bessede et al., 2014, Manches et al., 2012, Pallotta et al., 2014, Pallotta et al., 2011, Puccetti and Grohmann, 2007, Volpi et al., 2013). DC treatment with ADX88178 (Fig. 5D) but not PHCCC (data not shown) induced the appearance of pIDO1 at 30–60 min of drug incubation. Moreover, the selective mGluR4 PAM (Fig. 5E) but not PHCCC (data not shown) significantly increased the nuclear translocation of p52 and RelB but not p65 NF-κB subunits, which peaked at 10 min of drug exposure.
Overall, our data indicated that selective and potent mGluR4 PAMs can be considered as novel inducers of the expression, catalytic activity, and signaling ability as well of immunoregulatory IDO1 via a mechanism that requires mGluR4, PI3K, Src, and noncanonical NF-κB but not Gi/Go proteins.
3.5. Selective mGluR4 PAMs induce an IDO1-dependent, tolerogenic phenotype in DCs
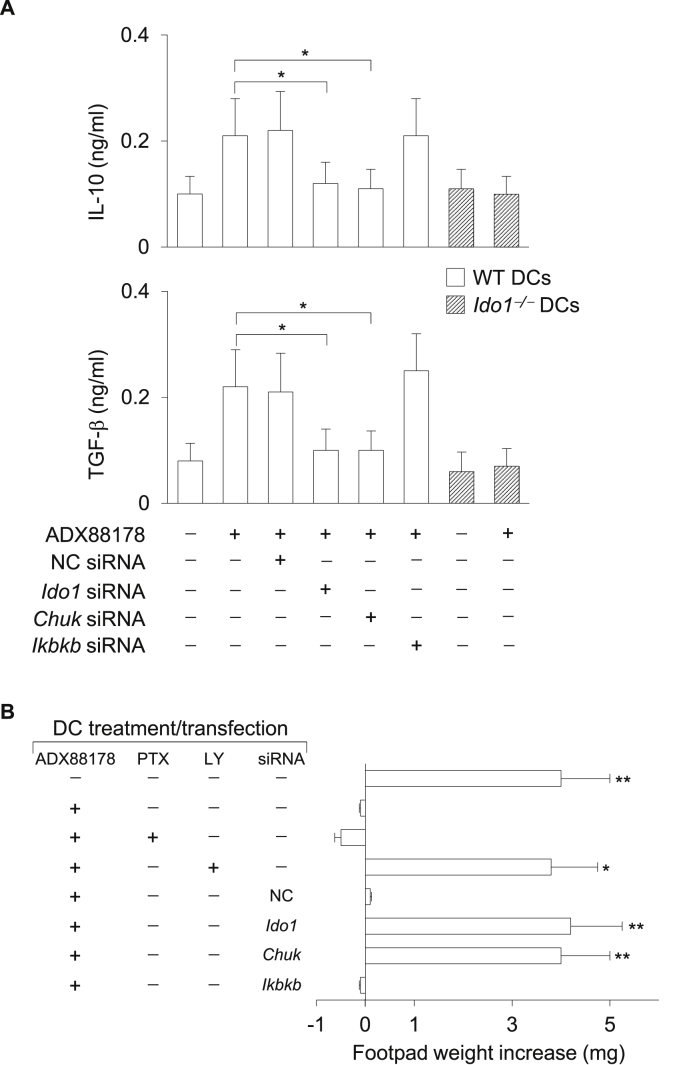
Because ADX88178 and ADX104608 but not PHCCC induce expression and signaling activity of immunoregulatory IDO1, we evaluated the possible role of IDO1 in the in vitro and in vivo regulatory effects induced by selective mGluR4 PAMs in DCs. We used DCs lacking Ido1 expression either as a consequence of gene knock-out (i.e., Ido1−/− cells) or knock-in maneuvers (cells treated with Ido1-specific small interfering RNA; siRNA). Moreover, to evaluate the possible functional role of the noncanonical versus canonical NF-κB pathway, siRNAs specific for Chuk (the IKKα-encoding gene) or Ikbkb (the IKKβ-encoding gene) were also used, respectively. In the in vitro setting, IL-10 and TGF-β levels were measured in culture supernatants of Ido1−/− and WT DCs, the latter after gene silencing with an Ido1-, Chuk-, Ikbkb-specific or control siRNA (Pallotta et al., 2014, Pallotta et al., 2011) prior to incubation with ADX88178 for 24 h. Induction of both cytokines by the mGluR4 PAM was severely impaired in Ido1−/−cells (or however reduced in Ido1 siRNA-transfected cells) (Pallotta et al., 2014, Pallotta et al., 2011), as compared to DCs competent for the enzyme (Fig. 6A). Moreover, Chuk-specific siRNA also greatly and significantly inhibited the production of IL-10 and TGF-β, whereas no modulatory effect was exerted by either Ikbkb-specific or control siRNAs (Fig. 6A).
Fig. 6.
Immunoregulatory effects of ADX88178 in DCs are mediated by IDO1 expression and signaling. (A) Effect of lack of IDO1 expression and signaling in the induction of IL-10 and TGF-β by ADX88178 in DCs. WT and Ido1−/− DCs were treated with ADX88178 at 3 μM and cytokine contents were measured in 24-h culture supernatants. WT DCs were used as such or after transfection with Ido1, Chuk, Ikbkb, or negative control (NC) siRNA. Data, presented as means ± SD from triplicate samples, are shown for one representative experiment of three. *P < 0.01. (B) Effect of lack of IDO1 expression and signaling in the in vivo suppressive ability of ADX88178-conditioned DCs. Purified CD8–CD11c+ DCs were pulsed for 2 h with the IGRP peptide and transferred into recipient mice to be assayed for skin reactivity to the eliciting peptide. The CD8–CD11c+ DC fraction was used in combination with 5% CD11c+ DCs, either untreated or preconditioned in vitro with ADX88178 in the presence or absence of PTX or LY294002 (LY) as in Fig. 4F. Cells were left untransfected or transfected with siRNA as in A. Analysis of skin reactivity of recipient mice to the eliciting peptide at 15 d is presented as change in footpad weight. Results (mean ± SD) are representative of three experiments. *P < 0.05 and **P < 0.01.
To better appreciate the tolerogenic potential of DCs treated with selective mGluR4 PAMs, we resorted to the skin test assay, an established protocol for measuring the in vivo induction of antigen-specific immunoreactivity versus tolerance by DCs (Grohmann et al., 2007, Orabona et al., 2004, Volpi et al., 2013). Balb/c mice were sensitized with the IGRP peptide (containing the immunodominant epitope of a diabetogenic autoantigen that can also prime nondiabetic conventional mice such as Balb/c animals (Pallotta et al., 2014)) (Pallotta et al., 2011) presented by highly immunostimulatory conventional DCs characterized by the absence of the CD8α marker (i.e., CD8−CD11c+ DCs) in combination with a minority fraction (approximately 5% of the entire population) of the same cells that had been pretreated in vitro with ADX88178 or medium alone for 24 h and pulsed with the same Ag. After priming the mice, we assessed immune reactivity at 2 wk by intrafootpad challenge with the IGRP peptide in the absence of DCs. As expected, the priming ability of CD8− DCs was not affected by the presence of untreated cells, yet sensitization together with ADX88178-pretreated DCs caused suppression of IGRP-specific reactivity, an effect abrogated by co-treatment of DCs with the PI3K inhibitor but not PTX (Fig. 6B). Moreover, the suppressive effect exerted by ADX88178-pretreated DCs on the sensitization by IGRP-pulsed CD8− DCs was also lost when cells had been pre-incubated with Ido1 or Chuk but not Ikbkb or control siRNA prior to conditioning with the mGluR4 PAM (Fig. 6B).
Therefore, our data suggest that the immunoregulatory effects of selective mGluR4 PAMs rely on IDO1 but not Gi signaling in DCs and could be exploited therapeutically in several chronic autoimmune diseases, including MS and type 1 diabetes (T1D).
4. Discussion
MS is a disease both inflammatory and autoimmune in nature (McFarland and Martin, 2007, Steinman et al., 2002). IDO1, by virtue of its anti-inflammatory and immunoregulatory effects, could thus represent a unique therapeutic target in inflammatory/autoimmune diseases such as MS. Interestingly, the reduced relapse rate of MS observed during pregnancy has been proposed to be due to the up-regulation of IDO1 by estrogens in monocyte-derived DCs (Zhu et al., 2007). Moreover, in vitro stimulation of monocyte-derived DCs from MS patients with 1,25(OH)2D3, the biologically active form of vitamin D, greatly upregulates expression and activity of IDO1, an effect that results in increased numbers of Treg cells in DC-T cell cocultures (Correale et al., 2009). Intriguingly, kynurenine levels are significantly increased in the plasma of patients undergoing effective therapy with IFN-β, a standard treatment for MS (Amirkhani et al., 2005).
Tryptophan metabolism by IDO1 in DCs is a highly versatile regulator of innate and adaptive immune responses (Grohmann et al., 2003b, Orabona et al., 2012, Puccetti and Grohmann, 2007). When induced by proinflammatory cytokines such as IFN-γ, the IDO1 enzyme degrades tryptophan and yields a series of catabolites (Grohmann et al., 2000, Grohmann et al., 2002)—collectively known as kynurenines—regulating immune homeostasis by acting as ligands of the aryl hydrocarbon receptor (AhR) and allowing the generation of Treg cells that protect from hyperinflammatory responses (Bessede et al., 2014, Romani et al., 2008). IDO1 does not merely degrade tryptophan and produce immunoregulatory kynurenines but also acts as a signal-transducing molecule independently of its enzyme activity (Orabona et al., 2012). This additional function is induced in DCs by TGF-β, is mediated by PI3K, and culminates in IDO1 phosphorylation by kinases belonging to the Src family. Because AhR–associated Src activity has recently been found to be responsible for IDO1 phosphorylation and TGF-β production by IDO1-competent cells in endotoxin tolerance (Bessede et al., 2014), the concomitant activation of enzymatic and signaling activities appears to be mandatory for the implementation of IDO1's immunoregulatory potential.
Direct evidence for a protective role of IDO1 in MS has been obtained in mice with different forms of EAE, i.e., acute, relapsing-remitting, or adoptively transferred disease. Administration of 1-MT, the standard inhibitor of IDO1 catalytic activity, exacerbates clinical course of the disease, either relapsing-remitting (Kwidzinski et al., 2005) or adoptively transferred (Sakurai et al., 2002). IDO1-deficient mice develop exacerbated acute EAE with enhanced encephalitogenic Th1 and Th17 cell responses and reduced Treg cell numbers (Yan et al., 2010). Conversely, administration of 3-hydroxyanthranilic acid (3-HAA) (Yan et al., 2010), a tryptophan catabolite downstream of IDO1 in the kynurenine pathway, or of an orally active synthetic derivative thereof (Platten et al., 2005), ameliorates neuroinflammation and paralysis in mice with acute EAE via inhibition of autoreactive Th1/Th17 cells and the induction of Treg cells. Moreover, cinnabarinic acid (CA), a compound derived from the in vivo spontaneous condensation of two 3-HAA molecules, also exerts protective effects in acute EAE when administered continuously commencing on the day of immunization with the myelin oligodendrocyte glycoprotein peptide (Fazio et al., 2014). CA therapeutic effects in vivo are accompanied by an immune response dominated by Treg cells at the expense of Th17 cells and, in vitro, by TGF-β production from DCs. Of particular interest in the present context, the therapeutic action of CA in acute EAE associates with the up-regulation of Ido1 and Kynu genes (the latter coding for kynureninase, an enzyme downstream of IDO1 along the kynurenine pathway that produces 3-HAA) and is dampened, at least in part, in mice lacking mGluR4 expression (Fazio et al., 2014). In previous experiments with heterologous expression systems, CA was indeed shown to act as a selective, though weak, orthosteric agonist of mGluR4 (Fazio et al., 2012). Taken together, the available literature points to the possible existence of an mGluR4-IDO1 loop protecting mice from neuroinflammation.
In our present study, we found that the selective and potent mGluR4 PAM ADX88178 converted experimental RR-EAE into a milder form of neuroinflammation that remained stable for over two months after discontinuing drug treatment. In vitro, the drug appeared to act through mechanisms involving PI3K, Src kinase, and the signaling activity of IDO1, thus highlighting a major difference with PHCCC, whose effects on DCs are mediated by a Gi protein, and thus by the canonical mGluR4 pathway (Conn and Pin, 1997).
The durability of the in vivo therapeutic effects of ADX88178, as well as the induction of TGF-β production, and the activation of PI3K and Src kinases in DCs prompted us to further investigate the IDO1-dependent signaling events. We performed a series of in vitro and in vivo experiments with DCs and found that ADX88178 and ADX104608 alone, i.e., in the absence of LPS, significantly up-regulated IDO1 expression and catalytic activity and, perhaps more importantly, triggered the IDO1 signaling pathway mediated by phosphorylated IDO1 and noncanonical NF-κB in an mGluR4-dependent fashion. In contrast, PHCCC was unable to induce either IDO1 expression and/or signaling under any conditions and concentrations. Activation of the IDO1 signaling by selective mGluR4 PAMs was required for TGF-β and IL-10 production and, more importantly, for in vivo immunosuppression on testing a diabetogenic autoantigen by in vitro-treated DCs. Because ADX88178 also upregulated Kynu expression in DCs (Iacono A., unpublished observation), our data would suggest that the immunoregulatory effects of selective mGluR4 PAMs could be due, at least in part, to a positive feedback activity of CA, possibly formed from the condensation of 3-HAA in vitro and/or in vivo, on mGluR4 and thus to the activation of a positive and protective mGluR4-IDO1 loop.
As their name suggests, activation of heterotrimeric G proteins has normally been considered as the main signaling pathway of GPCRs. However, a multitude of studies has provided evidence for a G-protein−independent signaling occurring in the transduction of several GPCRs (reviewed in (Baker and Hill, 2007)). The signaling diversity of GPCRs arises from numerous factors, the most important being the ability to adopt multiple (and not merely two, as previously thought) active states with different effector-coupling profiles (Maudsley et al., 2005). Distinct GPCR ligands, either orthosteric or allosteric, may thus select a different choice from a menu of active receptor conformations by virtue of their binding affinity and efficacy and favor specific signaling pathways. The recent obtained crystal structure of mGluR1 bound to a negative allosteric modulator has unveiled critical residues operating in the communication between the orthosteric and allosteric site of the receptor and also directing interaction with other protein partners (Wu et al., 2014), paving the way to the identification of additional mGluR conformations selected by allosteric ligands. In addition to the role played by receptor ligands, the cell type may also significantly contribute to the choice of signaling pathways by means of a cell-specific scaffolding and preorganization of GPCR molecular partners (Baker and Hill, 2007, Maudsley et al., 2005).
The signaling pathway of mGluR4 has been investigated primarily in heterologous expression systems mostly using nonselective orthosteric agonists (i.e., l-AP-4) and weak PAMs (PHCCC), with few studies done on nervous and tumor cells. As a whole, the bulk of observations, including our previous data in DCs stimulated with LPS and treated with PHCCC (Fallarino et al., 2010), would point to a Gi-mediated signal transduction (Conn and Pin, 1997, Tanabe et al., 1993), although alternative pathways mediated by kinases such as ERK, MAPK, PI3K/Akt, and Src, have also been reported (Iacovelli et al., 2002, Jiang et al., 2006). In general, evidence for a Gi-mediated signaling can be obtained in the presence of signals capable of activating adenylyl cyclase either directly (i.e., forskolin) or indirectly (Gs-dependent receptors and LPS). As a matter of fact, PHCCC reduced cAMP levels in DCs, but only in the presence of forskolin or LPS, and, more importantly, modified the DC cytokine profile only in the presence of the TLR4 ligand (as per our previous (Fallarino et al., 2010) and current data). In contrast, the in vitro effects of ADX88178 and ADX104608 in DCs, including TGF-β, IL-10, and IDO1 induction, occurred in the absence of any additional signal, were PTX-insensitive, and could be inhibited by PI3K and Src inhibitors. Moreover, the effects were evident at such low compound concentrations (≤3 μM) that were insufficient to inhibit cAMP levels in DCs stimulated with forskolin. Interestingly, in cerebellar granule cells stimulated in vitro with forskolin, CA inhibited cAMP formation but only at concentrations ≥30 μM (Fazio et al., 2012), thus suggesting that alternative signaling pathways could be still activated at lower concentrations also by CA. Taken together, our data would suggest that, at least in DCs, different PAMs may select distinct mGluR4 conformations and thus activate distinct effector-coupled signaling pathways, further confirming the great plasticity of mGluRs (Nakanishi, 1994).
5. Conclusions
The discovery of positive allosteric modulators (PAMs) selectively activating distinct mGluRs has clarified that mGluR4 may represent a promising drug target not only in neuropsychiatric disorders (Conn et al., 2009) but also in peripheral pathologies such as chronic inflammatory/autoimmune diseases (Celanire and Campo, 2012, Fallarino et al., 2010, Hansen and Caspi, 2010), where small-molecule compounds would represent a convenient alternative to current effective therapies, mainly represented by expensive biodrugs. ADX88178 and ADX104608, the mGluR4 PAMs investigated in the current study, are endowed not only with a pronounced selectivity and high potency, but also with an optimal pharmacokinetics, good brain-penetrance ability, and almost absent toxicity (Kalinichev et al., 2014). Taken into consideration the critical role played by IDO1 in several inflammatory/autoimmune conditions, including rheumatoid arthritis (Seo et al., 2004, Szanto et al., 2007) and inflammatory bowel diseases (Ciorba et al., 2010, Gurtner et al., 2003, Matteoli et al., 2010) in addition to MS (Amirkhani et al., 2005, Correale et al., 2009, Fazio et al., 2014, Kwidzinski et al., 2005, Platten et al., 2005, Sakurai et al., 2002, Yan et al., 2010, Zhu et al., 2007) and T1D (Fallarino et al., 2009, Grohmann et al., 2003a, Pallotta et al., 2014), these compounds may represent the first drugs capable of activating the entire spectrum of functions of such an important target, thus allowing an efficient resetting of a natural immunoregulatory pathway controlling several immune pathologies.
Acknowledgments
We thank G. Andrielli for digital art and image editing.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neuropharm.2015.10.036.
Funding
This work was supported by Addex Therapeutics and the European Research Council (338954-DIDO to U.G.).
Author contributions
C.O., F.F., L.G., and U.G. designed research; C. Volpi, G.M., M.T.P., C. Vacca, A.I., M.G., E.A., R.B., M.L.B., I.R.-U., M.S., M.C., and C.A. performed experiments; C.M. and S.C. contributed to new reagents/analytic tools; A.S., S.B., P.P., P.-A.V., M.C., S.-M.P., and U.G. analyzed data; S.C. and S.-M.P. contributed writing parts of the manuscript; U.G. wrote the paper. All Authors approved the final version of the manuscript.
Conflict of interest
All authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Amirkhani A., Rajda C., Arvidsson B., Bencsik K., Boda K., Seres E., Markides K.E., Vecsei L., Bergquist J. Interferon-beta affects the tryptophan metabolism in multiple sclerosis patients. Eur. J. Neurol. 2005;12:625–631. doi: 10.1111/j.1468-1331.2005.01041.x. [DOI] [PubMed] [Google Scholar]
- Baker J.G., Hill S.J. Multiple GPCR conformations and signalling pathways: implications for antagonist affinity estimates. Trends Pharmacol. Sci. 2007;28:374–381. doi: 10.1016/j.tips.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belladonna M.L., Volpi C., Bianchi R., Vacca C., Orabona C., Pallotta M.T., Boon L., Gizzi S., Fioretti M.C., Grohmann U., Puccetti P. Cutting edge: autocrine TGF-β sustains default tolerogenesis by IDO-competent dendritic cells. J. Immunol. 2008;181:5194–5198. doi: 10.4049/jimmunol.181.8.5194. [DOI] [PubMed] [Google Scholar]
- Bessede A., Gargaro M., Pallotta M.T., Matino D., Servillo G., Brunacci C., Bicciato S., Mazza E.M., Macchiarulo A., Vacca C., Iannitti R., Tissi L., Volpi C., Belladonna M.L., Orabona C., Bianchi R., Lanz T.V., Platten M., Della Fazia M.A., Piobbico D., Zelante T., Funakoshi H., Nakamura T., Gilot D., Denison M.S., Guillemin G.J., DuHadaway J.B., Prendergast G.C., Metz R., Geffard M., Boon L., Pirro M., Iorio A., Veyret B., Romani L., Grohmann U., Fallarino F., Puccetti P. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G., Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Celanire S., Campo B. Recent advances in the drug discovery of metabotropic glutamate receptor 4 (mGluR4) activators for the treatment of CNS and non-CNS disorders. Expert Opin. Drug Discov. 2012;7:261–280. doi: 10.1517/17460441.2012.660914. [DOI] [PubMed] [Google Scholar]
- Ciorba M.A., Bettonville E.E., McDonald K.G., Metz R., Prendergast G.C., Newberry R.D., Stenson W.F. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J. Immunol. 2010;184:3907–3916. doi: 10.4049/jimmunol.0900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn P.J., Christopoulos A., Lindsley C.W. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn P.J., Pin J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Correale J., Ysrraelit M.C., Gaitan M.I. Immunomodulatory effects of vitamin D in multiple sclerosis. Brain. 2009;132:1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- Enz R. The trick of the tail: protein-protein interactions of metabotropic glutamate receptors. Bioessays. 2007;29:60–73. doi: 10.1002/bies.20518. [DOI] [PubMed] [Google Scholar]
- Fallarino F., Volpi C., Fazio F., Notartomaso S., Vacca C., Busceti C., Bicciato S., Battaglia G., Bruno V., Puccetti P., Fioretti M.C., Nicoletti F., Grohmann U., Di Marco R. Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nat. Med. 2010;16:897–902. doi: 10.1038/nm.2183. [DOI] [PubMed] [Google Scholar]
- Fallarino F., Volpi C., Zelante T., Vacca C., Calvitti M., Fioretti M.C., Puccetti P., Romani L., Grohmann U. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J. Immunol. 2009;183:6303–6312. doi: 10.4049/jimmunol.0901577. [DOI] [PubMed] [Google Scholar]
- Fazio F., Lionetto L., Molinaro G., Bertrand H.O., Acher F., Ngomba R.T., Notartomaso S., Curini M., Rosati O., Scarselli P., Di Marco R., Battaglia G., Bruno V., Simmaco M., Pin J.P., Nicoletti F., Goudet C. Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors. Mol. Pharmacol. 2012;81:643–656. doi: 10.1124/mol.111.074765. [DOI] [PubMed] [Google Scholar]
- Fazio F., Zappulla C., Notartomaso S., Busceti C., Bessede A., Scarselli P., Vacca C., Gargaro M., Volpi C., Allegrucci M., Lionetto L., Simmaco M., Belladonna M.L., Nicoletti F., Fallarino F. Cinnabarinic acid, an endogenous agonist of type-4 metabotropic glutamate receptor, suppresses experimental autoimmune encephalomyelitis in mice. Neuropharmacology. 2014;81:237–243. doi: 10.1016/j.neuropharm.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Franco R., Pacheco R., Lluis C., Ahern G.P., O'Connell P.J. The emergence of neurotransmitters as immune modulators. Trends Immunol. 2007;28:400–407. doi: 10.1016/j.it.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Gerber U., Gee C.E., Benquet P. Metabotropic glutamate receptors: intracellular signaling pathways. Curr. Opin. Pharmacol. 2007;7:56–61. doi: 10.1016/j.coph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Goeman J.J., Solari A. Multiple hypothesis testing in genomics. Stat. Med. 2014;33:1946–1978. doi: 10.1002/sim.6082. [DOI] [PubMed] [Google Scholar]
- Grohmann U., Bianchi R., Belladonna M.L., Silla S., Fallarino F., Fioretti M.C., Puccetti P. IFN-γ inhibits presentation of a tumor/self peptide by CD8 alpha- dendritic cells via potentiation of the CD8α+ subset. J. Immunol. 2000;165:1357–1363. doi: 10.4049/jimmunol.165.3.1357. [DOI] [PubMed] [Google Scholar]
- Grohmann U., Bronte V. Control of immune response by amino acid metabolism. Immunol. Rev. 2010;236:243–264. doi: 10.1111/j.1600-065X.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- Grohmann U., Fallarino F., Bianchi R., Orabona C., Vacca C., Fioretti M.C., Puccetti P. A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice. J. Exp. Med. 2003;198:153–160. doi: 10.1084/jem.20030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann U., Fallarino F., Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- Grohmann U., Orabona C., Fallarino F., Vacca C., Calcinaro F., Falorni A., Candeloro P., Belladonna M.L., Bianchi R., Fioretti M.C., Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- Grohmann U., Volpi C., Fallarino F., Bozza S., Bianchi R., Vacca C., Orabona C., Belladonna M.L., Ayroldi E., Nocentini G., Boon L., Bistoni F., Fioretti M.C., Romani L., Riccardi C., Puccetti P. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat. Med. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- Gurtner G.J., Newberry R.D., Schloemann S.R., McDonald K.G., Stenson W.F. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- Hackstein H., Thomson A.W. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat. Rev. Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- Hansen A.M., Caspi R.R. Glutamate joins the ranks of immunomodulators. Nat. Med. 2010;16:856. doi: 10.1038/nm0810-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L., Bruno V., Salvatore L., Melchiorri D., Gradini R., Caricasole A., Barletta E., De Blasi A., Nicoletti F. Native group-III metabotropic glutamate receptors are coupled to the mitogen-activated protein kinase/phosphatidylinositol-3-kinase pathways. J. Neurochem. 2002;82:216–223. doi: 10.1046/j.1471-4159.2002.00929.x. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Yan Z., Feng J. Activation of group III metabotropic glutamate receptors attenuates rotenone toxicity on dopaminergic neurons through a microtubule-dependent mechanism. J. Neurosci. 2006;26:4318–4328. doi: 10.1523/JNEUROSCI.0118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.B., Halenda S.P., Bylund D.B. Alpha 2-adrenergic receptor stimulation of phospholipase A2 and of adenylate cyclase in transfected chinese hamster ovary cells is mediated by different mechanisms. Mol. Pharmacol. 1991;39:239–245. [PubMed] [Google Scholar]
- Kalinichev M., Le Poul E., Bolea C., Girard F., Campo B., Fonsi M., Royer-Urios I., Browne S.E., Uslaner J.M., Davis M.J., Raber J., Duvoisin R., Bate S.T., Reynolds I.J., Poli S., Celanire S. Characterization of the novel positive allosteric modulator of the metabotropic glutamate receptor 4 ADX88178 in rodent models of neuropsychiatric disorders. J. Pharmacol. Exp. Ther. 2014;350:495–505. doi: 10.1124/jpet.114.214437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose H., Ui M. Functional uncoupling of muscarinic receptors from adenylate cyclase in rat cardiac membranes by the active component of islet-activating protein, pertussis toxin. J. Cycl. Nucleotide Protein Phosphor. Res. 1983;9:305–318. [PubMed] [Google Scholar]
- Kwidzinski E., Bunse J., Aktas O., Richter D., Mutlu L., Zipp F., Nitsch R., Bechmann I. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- Maj M., Bruno V., Dragic Z., Yamamoto R., Battaglia G., Inderbitzin W., Stoehr N., Stein T., Gasparini F., Vranesic I., Kuhn R., Nicoletti F., Flor P.J. (-)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology. 2003;45:895–906. doi: 10.1016/s0028-3908(03)00271-5. [DOI] [PubMed] [Google Scholar]
- Manches O., Fernandez M.V., Plumas J., Chaperot L., Bhardwaj N. Activation of the noncanonical NF-κB pathway by HIV controls a dendritic cell immunoregulatory phenotype. Proc. Natl. Acad. Sci. U. S. A. 2012;109:14122–14127. doi: 10.1073/pnas.1204032109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G., Mazzini E., Iliev I.D., Mileti E., Fallarino F., Puccetti P., Chieppa M., Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- Maudsley S., Martin B., Luttrell L.M. The origins of diversity and specificity in G protein-coupled receptor signaling. J. Pharmacol. Exp. Ther. 2005;314:485–494. doi: 10.1124/jpet.105.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland H.F., Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- Mellor A.L., Munn D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Nishida M., Suda R., Nagamatsu Y., Tanabe S., Onohara N., Nakaya M., Kanaho Y., Shibata T., Uchida K., Sumimoto H., Sato Y., Kurose H. Pertussis toxin up-regulates angiotensin type 1 receptors through Toll-like receptor 4-mediated rac activation. J. Biol. Chem. 2010;285:15268–15277. doi: 10.1074/jbc.M109.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orabona C., Grohmann U., Belladonna M.L., Fallarino F., Vacca C., Bianchi R., Bozza S., Volpi C., Salomon B.L., Fioretti M.C., Romani L., Puccetti P. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat. Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- Orabona C., Pallotta M.T., Grohmann U. Different partners, opposite outcomes: a new perspective of the immunobiology of indoleamine 2,3-dioxygenase. Mol. Med. 2012;18:834–842. doi: 10.2119/molmed.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orabona C., Pallotta M.T., Volpi C., Fallarino F., Vacca C., Bianchi R., Belladonna M.L., Fioretti M.C., Grohmann U., Puccetti P. SOCS3 drives proteasomal degradation of indoleamine 2,3- dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20828–20833. doi: 10.1073/pnas.0810278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta M.T., Orabona C., Bianchi R., Vacca C., Fallarino F., Belladonna M.L., Volpi C., Mondanelli G., Gargaro M., Allegrucci M., Talesa V.N., Puccetti P., Grohmann U. Forced IDO1 expression in dendritic cells restores immunoregulatory signalling in autoimmune diabetes. J. Cell. Mol. Med. 2014;18:2082–2091. doi: 10.1111/jcmm.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta M.T., Orabona C., Volpi C., Vacca C., Belladonna M.L., Bianchi R., Servillo G., Brunacci C., Calvitti M., Bicciato S., Mazza E.M., Boon L., Grassi F., Fioretti M.C., Fallarino F., Puccetti P., Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- Pekhletski R., Gerlai R., Overstreet L.S., Huang X.P., Agopyan N., Slater N.T., Abramow-Newerly W., Roder J.C., Hampson D.R. Impaired cerebellar synaptic plasticity and motor performance in mice lacking the mGluR4 subtype of metabotropic glutamate receptor. J. Neurosci. 1996;16:6364–6373. doi: 10.1523/JNEUROSCI.16-20-06364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten M., Ho P.P., Youssef S., Fontoura P., Garren H., Hur E.M., Gupta R., Lee L.Y., Kidd B.A., Robinson W.H., Sobel R.A., Selley M.L., Steinman L. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- Puccetti P., Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-κB activation. Nat. Rev. Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- Romani L., Fallarino F., De Luca A., Montagnoli C., D'Angelo C., Zelante T., Vacca C., Bistoni F., Fioretti M.C., Grohmann U., Segal B.H., Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- Sakurai K., Zou J.P., Tschetter J.R., Ward J.M., Shearer G.M. Effect of indoleamine 2,3- dioxygenase on induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2002;129:186–196. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- Saraiva M., O'Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Seo S.K., Choi J.H., Kim Y.H., Kang W.J., Park H.Y., Suh J.H., Choi B.K., Vinay D.S., Kwon B.S. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat. Med. 2004;10:1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- Steinman L., Martin R., Bernard C., Conlon P., Oksenberg J.R. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu. Rev. Neurosci. 2002;25:491–505. doi: 10.1146/annurev.neuro.25.112701.142913. [DOI] [PubMed] [Google Scholar]
- Szanto S., Koreny T., Mikecz K., Glant T.T., Szekanecz Z., Varga J. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Res. Ther. 2007;9:R50. doi: 10.1186/ar2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y., Nomura A., Masu M., Shigemoto R., Mizuno N., Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J. Neurosci. 1993;13:1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theien B.E., Vanderlugt C.L., Nickerson-Nutter C., Cornebise M., Scott D.M., Perper S.J., Whalley E.T., Miller S.D. Differential effects of treatment with a small-molecule VLA-4 antagonist before and after onset of relapsing EAE. Blood. 2003;102:4464–4471. doi: 10.1182/blood-2003-03-0974. [DOI] [PubMed] [Google Scholar]
- Volpi C., Fallarino F., Pallotta M.T., Bianchi R., Vacca C., Belladonna M.L., Orabona C., De Luca A., Boon L., Romani L., Grohmann U., Puccetti P. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat. Commun. 2013;4:1852. doi: 10.1038/ncomms2874. [DOI] [PubMed] [Google Scholar]
- Volpi C., Fazio F., Fallarino F. Targeting metabotropic glutamate receptors in neuroimmune communication. Neuropharmacology. 2012;63:501–506. doi: 10.1016/j.neuropharm.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Weaver A., Goncalves da Silva A., Nuttall R.K., Edwards D.R., Shapiro S.D., Rivest S., Yong V.W. An elevated matrix metalloproteinase (MMP) in an animal model of multiple sclerosis is protective by affecting Th1/Th2 polarization. FASEB J. 2005;19:1668–1670. doi: 10.1096/fj.04-2030fje. [DOI] [PubMed] [Google Scholar]
- Williams R., Zhou Y., Niswender C.M., Luo Q., Conn P.J., Lindsley C.W., Hopkins C.R. Re- exploration of the PHCCC scaffold: discovery of improved positive allosteric modulators of mGluR4. ACS Chem. Neurosci. 2010;1:411–419. doi: 10.1021/cn9000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Wang C., Gregory K.J., Han G.W., Cho H.P., Xia Y., Niswender C.M., Katritch V., Meiler J., Cherezov V., Conn P.J., Stevens R.C. Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science. 2014;344:58–64. doi: 10.1126/science.1249489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Zhang G.X., Gran B., Fallarino F., Yu S., Li H., Cullimore M.L., Rostami A., Xu H. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W.H., Lu C.Z., Huang Y.M., Link H., Xiao B.G. A putative mechanism on remission of multiple sclerosis during pregnancy: estrogen-induced indoleamine 2,3-dioxygenase by dendritic cells. Mult. Scler. 2007;13:33–40. doi: 10.1177/1352458506071171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.