Abstract
Purpose
The dose intensity of doxorubicin (DID) is important to the survival of diffuse large B cell lymphoma (DLBCL) patients. However, due to expected toxicities, most elderly patients cannot receive full doses of anthracyclines. The purpose of this study was to evaluate the effect of DID on the survival of elderly DLBCL patients (age ≥ 70 years) in the rituximab era.
Materials and Methods
We analyzed 433 DLBCL patients who were treated with R-CHOP between December 2003 and October 2011 at the Seoul National University Hospital. Of these patients, 19.2% were aged ≥ 70 years. We analyzed the survival outcomes according to DID.
Results
Significantly poorer overall survival (OS) was observed for patients aged ≥ 70 years (2-year OS rate: 59.9% vs. 84.2%; p < 0.001). DID ≤ 10 mg/m2/wk had a significant effect on the OS and progression-free survival (PFS) in elderly patients (2-year OS rate: 40.0% in DID ≤ 10 mg/m2/wk vs. 62.6% in DID > 10 mg/m2/wk; p=0.031; 2-year PFS: 35.0% vs. 65.7%; p=0.036). The OS on each 1.7 mg/m2/wk doxorubicin increment above 10 mg/m2/wk in elderly patients was not significant among the groups (2-year OS rate: 75.0% in DID 10.0-11.7 mg/m2/wk vs. 66.7% in DID 15.0-16.7 mg/m2/wk; p=0.859). Treatment related mortality was not related to DID.
Conclusion
DID can be reduced up to 10 mg/m2/wk in elderly DLBCL patients in the rituximab era. Maintenance of DID > 10 mg/m2/wk and judicious selection of elderly patients who are tolerant to DID is necessary.
Keywords: Diffuse large B-cell lymphoma, Age groups, Doxorubicin
Introduction
The incidence of diffuse large B cell lymphoma (DLBCL) increases with age, with approximately 40% of patients aged over 70 years [1]. Further increased incidence of DLBCL is projected since life expectancy has grown worldwide. Combination chemotherapy has improved the prognosis of DLBCL; before the rituximab era the standard treatment included cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) [2]. While CHOP has improved the treatment outcome, many studies have shown that elderly patients with DLBCL had a low survival rate because of poor performance status, comorbidity or reduced dose of doxorubicin [3,4]. Whether DLBCL in elderly patients is associated with a specific genetic abnormality or histologic characteristic is not yet clear [5]. Elderly patients have a decreased ability to tolerate treatment and are more vulnerable to the toxic effects of chemotherapy such as doxorubicin than younger DLBCL patients. Dose reduction of doxorubicin may lead to different survival rates between young and old patients [6,7].
Rituximab, a chimeric monoclonal antibody against the CD20 protein has demonstrable efficacy in DLBCL patients [8,9]. Rituximab combination therapy has dramatically increased treatment outcomes. Several studies have indicated that the addition of rituximab to CHOP (R-CHOP) increased cure rates by 10%-15% in elderly patients, hence R-CHOP has become the new standard treatment [8,10]. Dose intensity of doxorubicin (DID) was important before the rituximab era [6,11]; however, few studies on proper DID are found in the literature.
To the best of our knowledge, few studies analyzing the effect of doxorubicin dose on treatment outcomes in elderly DLBCL patients in the rituximab era have been reported. The purpose of this study was to analyze the treatment outcomes of elderly patients who were treated with R-CHOP according to the DID.
Materials and Methods
1. Patients and treatment
We conducted a single center retrospective analysis of DLBCL patients treated with R-CHOP at the Seoul National University Hospital between December 2003 and October 2011. Inclusion criteria were (1) pathologically confirmed DLBCL patients according to World Health Organization criteria by specialized hematopathologists (Y.K.J. and C.W.K.) [12]; (2) patients who received first-line therapy with R-CHOP. Full dose R-CHOP consisted of 375 mg/m2 rituximab, 750 mg/m2 cyclophosphamide, 50 mg/m2 doxorubicin and 1.4 mg/m2 vincristine on day 1, and 100 mg oral prednisolone on days 1-5 of each cycle. The combination treatment was repeated at 3-week intervals. Patients who received radiotherapy, surgery, and chemotherapy other than R-CHOP were excluded. We analyzed 433 DLBCL patients who initially received R-CHOP. Patients aged ≥ 70 years were regarded as elderly. Response was assessed on the basis of the modified International Workshop criteria [13].
2. Measurement of dose intensity
The dose intensity of the agent was calculated by dividing the total received dose by the number of weeks of treatment [7,14]. The patients were stratified according to age and DID. The standard DID was 16.7 mg/m2/wk. All study patients received over 6.7 mg/m2/wk of doxorubicin (40% dose reduction from standard dose).
3. Statistical analysis
The chi-square test was used to compare the percentage between two groups by age or DID. Survival analysis was performed using the Kaplan-Meier method and tested using the log-rank test. Overall survival (OS) was defined as the time from diagnosis until death or the last follow-up. Progression-free survival (PFS) was defined as the time from first day of treatment until disease progression. We used multivariate Cox regression analysis to evaluate prognostic factors affecting survival. p-value of ≤ 0.05 was considered as difference. The study protocol was approved by the Institutional Review Board (IRB) of the Seoul National University Hospital (IRB No. H1-406-001-583). The study was conducted in accordance with the Declaration of Helsinki.
Results
1. Patient characteristics
Of the 433 patients, 83 (19.2%) were aged ≥ 70 years and 350 (80.8%) were younger than 70 years. The clinical features are shown in Table 1. There were no significant differences between the two groups, except for performance status (Eastern Cooperative Oncology Group [ECOG] 0-1 vs. ECOG 2 or more), International Prognostic Index (IPI), and bone marrow involvement.
Table 1.
Characteristics of DLBCL patients treated with R-CHOP
| Characteristic | No. of patients (%) | Age < 70 yr | Age ≥ 70 yr | p-value |
|---|---|---|---|---|
| Age | ||||
| No. (%) | 433 | 350 (80.8) | 83 (19.2) | |
| Median (range) | 58 (16-91) | 54 (16-69) | 74 (70-90) | |
| B Symptoms | ||||
| No | 327 (75.5) | 265 (75.7) | 62 (74.7) | 0.847 |
| Yes | 106 (24.5) | 85 (24.4) | 21 (25.3) | |
| Ann Arbor stage | ||||
| I/II | 233 (53.8) | 192 (54.9) | 41 (49.4) | 0.370 |
| III/IV | 200 (46.2) | 158 (45.1) | 42 (50.6) | |
| Performance status | ||||
| ECOG 0-1 | 357 (82.4) | 303 (86.6) | 54 (65.1) | < 0.001 |
| ECOG 2 or more | 76 (17.6) | 47 (13.4) | 29 (34.9) | |
| LDH level | ||||
| Normal | 179 (41.3) | 148 (42.5) | 31 (37.3) | 0.390 |
| Elevated | 252 (58.2) | 200 (57.5) | 52 (62.7) | |
| No. of extranodal sites | ||||
| 0-1 | 222 (51.3) | 181 (51.7) | 41 (49.4) | 0.704 |
| 2 or more | 211 (48.7) | 169 (48.3) | 42 (50.6) | |
| IPI score | ||||
| 0-1 | 208 (48.0) | 184 (52.6) | 24 (28.9) | < 0.001 |
| 2 | 105 (24.2) | 83 (23.7) | 22 (26.5) | |
| 3 | 71 (16.4) | 53 (15.1) | 18 (21.7) | |
| 4-5 | 49 (11.3) | 30 (8.6) | 19 (22.9) | |
| Bone marrow involvement | ||||
| Absence | 355 (82.0) | 295 (86.0) | 60 (76.9) | 0.046 |
| Presence | 66 (15.2) | 48 (14.0) | 18 (23.1) | |
| Bulky disease | ||||
| No | 340 (78.5) | 274 (78.3) | 66 (79.5) | 0.806 |
| Yes | 93 (21.5) | 76 (21.8) | 17 (20.5) |
DLBCL, diffuse large B cell lymphoma; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; IPI, International Prognostic Index.
2. OS by age group
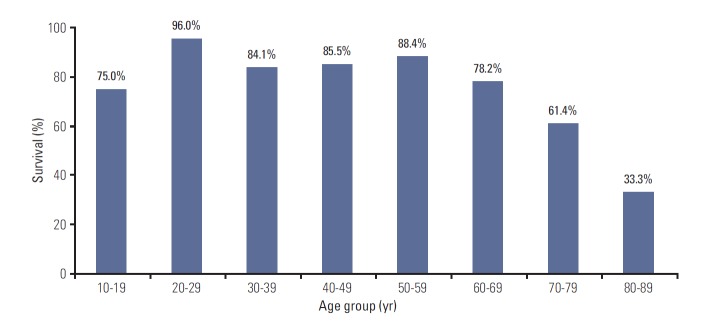
OS of the patients with DLBCL who received R-CHOP was analyzed according to age group. Age-specific 2-year OS rate was decreased with age in patients aged ≥ 70 years, below 70% (Fig. 1).
Fig. 1.
Age-specific 2-year overall survival rate.
3. Treatment outcomes by DID
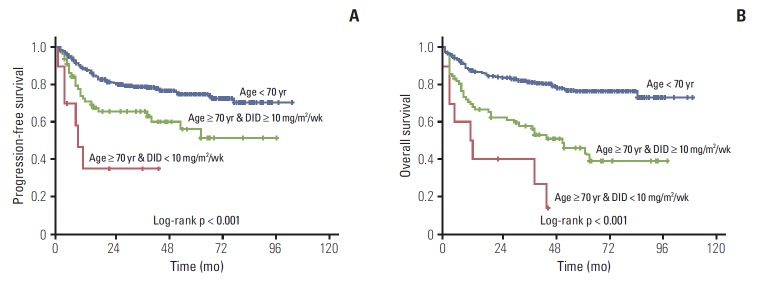
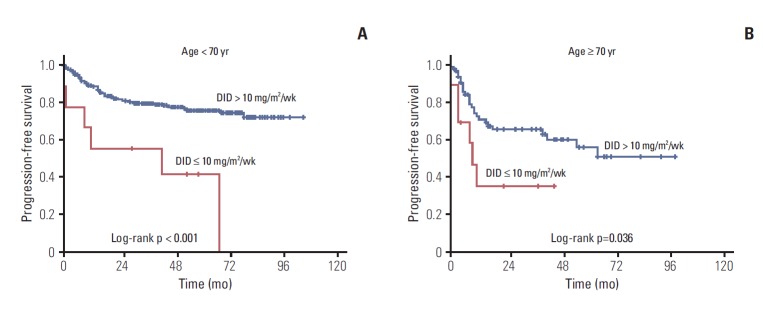
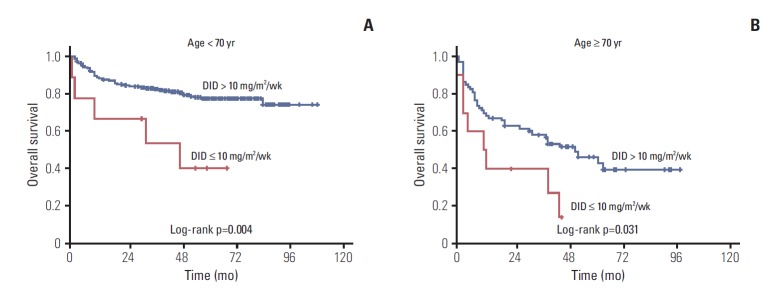
Approximately 53% of elderly patients received doxorubicin at 11.7 mg/m2/wk < DID ≤ 15.0 mg/m2/wk (Table 2). Twenty-four patients (aged ≥ 70) used prophylactic granulocyte-colony stimulating factors, 87.5% of them received DID ≥ 10 mg/m2/wk. We analyzed treatment outcomes according to DID in the two age groups as shown in Table 3 and Fig. 2. DID did not have an effect on complete remission (CR) rate, disease progression and death rate in patients aged ≥ 70 years, however significant differences were observed in the younger than 70 year age group. Two-year PFS and OS showed significant difference according to doxorubicin dose intensity (DID ≤ 10 mg/m2/wk and DID > 10 mg/m2/wk) in the young and elderly patients groups. The difference of survival was more significant in younger patients (Figs. 3 and 4). Survival outcomes in elderly DLBCL patients remained with no significant difference when DID was over 10 mg/m2/wk.
Table 2.
Number and survival outcomes of patients from each subgroup according to DID in elderly DLBCL patients (age ≥ 70 years)
| DID (mg/m2/wk) | No. of patients subgroup (%) |
2-Year PFS/OS rate (%) according to DID subgroup in aged ≥ 70 yr |
||
|---|---|---|---|---|
| Age < 70 yr | Age ≥ 70 yr | 2-Year PFS rate | 2-Year OS rate | |
| (a) 6.7 < DID ≤ 8.3 | 5 (1.4) | 5 (6.0) | 30.0 | 20.0 |
| (b) 8.3 < DID ≤ 10.0 | 3 (0.9) | 5 (6.0) | 40.0 | 60.0 |
| (c) 10.0 < DID ≤ 11.7 | 6 (1.7) | 8 (9.6) | 85.7 | 75.0 |
| (d) 11.7 < DID ≤ 13.4 | 33 (9.5) | 35 (42.2) | 61.1 | 50.4 |
| (e) 13.4 < DID ≤ 15.0 | 36 (10.3) | 9 (10.8) | 66.7 | 88.9 |
| (f) 15.0 < DID ≤ 16.7 | 266 (76.2) | 21 (25.3) | 63.8 | 66.7 |
p-value for 2-year PFS rate: (a+b) vs. (c+d+e+f)=0.036; (a) vs. (b)=0.271; (c) vs. (d)=0.362; (c) vs. (e)=0.462; (c) vs. (f)=0.392; (d) vs. (e)=0.782; (d) vs. (f)=0.913; (e) vs. (f)=0.815. p-value for 2-year OS rate: (a+b) vs. (c+d+e+f)=0.031; (a) vs. (b)=0.174; (c) vs. (d)=0.216; (c) vs. (e)=0.577; (c) vs. (f)=0.859; (d) vs. (e)=0.065; (d) vs. (f)=0.103; (e) vs. (f)=0.637. DID, dose intensity of doxorubicin; DLBCL, diffuse large B cell lymphoma; PFS, progression-free survival; OS, overall survival.
Table 3.
Treatment outcomes according to the dose intensity
| Clinical outcome | Dose intensity of doxorubicin (mg/m2/wk) |
|||||
|---|---|---|---|---|---|---|
| Age < 70 yr |
Age ≥ 70 yr |
|||||
| ≤ 10 | > 10 | p-value | ≤ 10 | > 10 | p-value | |
| Attainment of CR | ||||||
| No | 4 (44.4) | 59 (17.3) | 0.059 | 4 (40.0) | 26 (35.6) | 1.000 |
| Yes | 5 (55.6) | 282 (82.7) | 6 (60.0) | 47 (64.4) | ||
| Disease progression | ||||||
| No | 3 (33.3) | 268 (78.6) | 0.005 | 4 (40.0) | 48 (65.8) | 0.164 |
| Yes | 6 (66.7) | 73 (21.4) | 6 (60.0) | 25 (34.2) | ||
| Death | ||||||
| No | 4 (44.4) | 272 (79.8) | 0.023 | 2 (20.0) | 35 (47.9) | 0.173 |
| Yes | 5 (55.6) | 69 (20.2) | 8 (80.0) | 38 (52.1) | ||
| 2-Year PFS rate (%) | 55.6 | 82.0 | < 0.001 | 35.0 | 65.7 | 0.036 |
| HR for PFS (95% CI) | 4.043 (1.756-9.305) | 0.001 | 2.520 (1.021-6.224) | 0.045 | ||
| 2-Year OS rate (%) | 66.7 | 84.7 | 0.004 | 40.0 | 62.6 | 0.031 |
| HR for OS (95% CI) | 3.459 (1.394-8.579) | 0.007 | 2.242 (1.036-4.854) | 0.040 | ||
Values are presented as number (%) unless otherwise indicated. CR, complete remission; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; OS, overall survival.
Fig. 2.
Kaplan-Meier plots of progression-free survival (A) and overall survival (B) of young patients, elderly patients with dose intensity of doxorubicin (DID) ≤ 10 mg/m2/wk, and elderly patients with DID > 10 mg/m2/wk.
Fig. 3.
(A, B) Kaplan-Meier plots of progression-free survival by age and dose intensity of doxorubicin (DID).
Fig. 4.
(A, B) Kaplan-Meier plots of overall survival according to age and dose intensity of doxorubicin (DID).
4. Prognostic factors and cause of death in elderly
We utilized univariate and multivariate analyses to determine the effect of prognostic factors (B symptom, Ann Arbor stage, performance status, lactate dehydrogenase [LDH] level, number of extranodal sites, IPI score, bone marrow involvement, bulky tumor, and DID) on treatment outcome. Performance status, B symptom, IPI score, LDH level, and Ann Arbor stage, DID were identified as significant prognostic factors in univariate analysis. Performance status was the only prognostic factor associated with poor survival rate in multivariate analysis (Table 4).
Table 4.
Cox regression analysis for overall survival in elderly patients
| Variable | Multivariate |
Univariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| B Symptom (yes vs. no) | 0.709 | 0.311-1.615 | 0.413 | 0.403 | 0.218-0.746 | 0.004 |
| Ann Arbor stage (I/II vs. III/IV) | 0.601 | 0.204-1.765 | 0.354 | 1.423 | 1.008-1.861 | 0.010 |
| Performance status (ECOG 0-1 vs. ≥ 2) | 0.454 | 0.223-0.922 | 0.029 | 2.867 | 1.586-5.182 | < 0.001 |
| LDH level (normal vs. elevated) | 0.616 | 0.254-1.492 | 0.283 | 2.390 | 1.212-4.711 | 0.012 |
| No. of extranodal sites (0-1 vs. ≥ 2) | 0.864 | 0.426-1.730 | 0.684 | 1.825 | 1.007-3.308 | 0.047 |
| IPI score (0-2 vs. 3-5) | 0.596 | 0.156-2.227 | 0.449 | 3.137 | 1.715-5.738 | < 0.001 |
| Bone marrow involvement (yes vs. no) | 1.922 | 0.848-4.353 | 0.117 | 1.236 | 0.632-2.418 | 0.536 |
| Bulky tumor (yes vs. no) | 1.970 | 0.763-5.089 | 0.161 | 0.782 | 0.363-1.684 | 0.529 |
| DID (< 10 mg/m2/wk vs. ≥ 10 mg/m2/wk) | 1.597 | 0.607-4.202 | 0.343 | 0.446 | 0.206-0.965 | 0.040 |
HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; IPI, International Prognostic Index; DID, dose intensity of doxorubicin.
Causes of death in elderly patients are shown in Table 5. Disease progression and treatment related mortality such as sepsis and bleeding were the major causes of death in elderly DLBCL patients, regardless of DID.
Table 5.
Cause of death in elderly DLBCL patients (age ≥ 70 years)
| Cause | DID ≤ 10 mg/m2/wk | DID > 10 mg/m2/wk |
|---|---|---|
| Treatment related mortality | 4 (50.0) | 11 (28.9) |
| Disease progression | 3 (37.5) | 14 (36.8) |
| Unknown | 1 (12.5) | 9 (23.7) |
| Secondary malignancy | 0 | 4 (10.5) |
Values are presented as number (%). DLBCL, diffuse large B cell lymphoma; DID, dose intensity of doxorubicin.
Discussion
Significantly shorter survival was observed for DID ≤ 10 mg/m2/wk than for DID > 10 mg/m2/wk in elderly patients with DLBCL. When more than 10 mg/m2/wk of doxorubicin was administered, no significant differences were observed in treatment outcomes according to DID (2-year OS rate, 75.0%; 10.0 mg/m2/wk < DID ≤ 11.7 mg/m2/wk [60%-70% of standard DID], 66.7%; 15.0 mg/m2/wk < DID ≤ 16.7 mg/m2/wk [90%-100% of standard DID]; p=0.815). Each subgroup divided by 1.7 mg/m2/wk of DID showed insignificant difference in PFS and OS. CR rate and disease progression rate were similar regardless of DID; however, survival outcomes improved with more than 10 mg/m2/wk of doxorubicin in patients aged ≥ 70 years. Patients younger than 70 showed significant difference in CR rate, disease progression rate, and treatment outcomes by DID.
DID was not associated with treatment related mortality. The reason for this finding was assumed to be the small number of expired patients (n=46). The cause of death was unknown in 21.3%. Performance status was an independent prognostic factor in multivariative analysis, and the elderly patients had a poorer performance status than young patients, which was in agreement with past studies [15,16].
A previous study which analyzed treatment outcomes according to DID before the rituximab era reported that patients with DID ≥ 10 mg/m2/wk had better treatment outcomes than DID < 10 mg/m2/wk in elderly patients with DLBCL [7]. A recent study showed that low-dose CHOP (mini-CHOP) plus rituximab was effective and safe in DLBCL patients aged ≥ 80 years [17]. Our study showed that DID can be reduced; however, maintenance of DID ≥ 10 mg/m2/wk was necessary in elderly DLBCL patients in the rituximab era.
Doxorubicin is essential to CHOP or R-CHOP. However, elderly patients could not receive full dose doxorubicin due to myocardial toxicity. A recent study showed that elderly DLBCL with CHOP or R-CHOP had an increased risk of cardiovascular disease, such as congestive heart failure, cardiomyopathy and acute myocardial infarction [18]. Several studies were conducted with additional alternate anti-cancer drugs such as bleomycin, and pixantrone, to reduce the myocardial damage of doxorubicin-based chemotherapy in elderly patients. However, they were less effective or no more beneficial than CHOP [19-22]. It is thus important to determine the tolerable dose of doxorubicin that achieves comparable treatment outcomes.
Our study had some limitations. First, it was a retrospective, single center study on a small patient population. Additional studies are required to differentiate between elderly and young patients. Second, there is no consensus on the definition of ‘elderly’ among DLBCL patients. According to the IPI, age ≥ 60 years was a poor prognostic factor [23]. Several studies evaluating the effect of age on prognosis showed that > 70 years was a significant adverse factor [24]. We analyzed treatment outcomes according to the 70 year definition. As life expectancy increases, a consensus on the definition of ‘elderly’ is necessary and follow-up study is needed. However the strength of our study lay in the comparison of treatment outcomes according to DID in several subgroups.
Conclusion
DID was required at a minimum of 10 mg/m2 per week in elderly patients with DLBCL in the rituximab era. Despite improved survival outcomes by the introduction of rituximab, maintenance of DID was still important to the treatment of elderly DLBCL patients.
Acknowledgments
This study was supported by grants from the Innovative Research Institute for Cell Therapy, Republic of Korea (A062260).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–76. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage JO. Treatment of non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1023–30. doi: 10.1056/NEJM199304083281409. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040–5. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tirelli U, Errante D, Van Glabbeke M, Teodorovic I, Kluin-Nelemans JC, Thomas J, et al. CHOP is the standard regimen in patients > or = 70 years of age with intermediate-grade and high-grade non-Hodgkin's lymphoma: results of a randomized study of the European Organization for Research and Treatment of Cancer Lymphoma Cooperative Study Group. J Clin Oncol. 1998;16:27–34. doi: 10.1200/JCO.1998.16.1.27. [DOI] [PubMed] [Google Scholar]
- 5.Armitage JO. Is lymphoma occurring in the elderly the same disease? Leuk Lymphoma. 2008;49:14–6. doi: 10.1080/10428190701742522. [DOI] [PubMed] [Google Scholar]
- 6.Huntington SF, Talbott MS, Greer JP, Morgan DS, Reddy N. Toxicities and outcomes among septuagenarians and octogenarians with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone therapy. Leuk Lymphoma. 2012;53:1461–8. doi: 10.3109/10428194.2012.658793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KW, Kim DY, Yun T, Kim DW, Kim TY, Yoon SS, et al. Doxorubicin-based chemotherapy for diffuse large B-cell lymphoma in elderly patients: comparison of treatment outcomes between young and elderly patients and the significance of doxorubicin dosage. Cancer. 2003;98:2651–6. doi: 10.1002/cncr.11846. [DOI] [PubMed] [Google Scholar]
- 8.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 9.Shin HJ, Chung JS, Song MK, Kim SK, Choe S, Cho GJ. Addition of rituximab to reduced-dose CHOP chemotherapy is feasible for elderly patients with diffuse large B-cell lymphoma. Cancer Chemother Pharmacol. 2012;69:1165–72. doi: 10.1007/s00280-011-1814-6. [DOI] [PubMed] [Google Scholar]
- 10.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–26. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 11.Bastion Y, Blay JY, Divine M, Brice P, Bordessoule D, Sebban C, et al. Elderly patients with aggressive non-Hodgkin's lymphoma: disease presentation, response to treatment, and survival: a Groupe d'Etude des Lymphomes de l'Adulte study on 453 patients older than 69 years. J Clin Oncol. 1997;15:2945–53. doi: 10.1200/JCO.1997.15.8.2945. [DOI] [PubMed] [Google Scholar]
- 12.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–32. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 14.Hryniuk WM, Goodyear M. The calculation of received dose intensity. J Clin Oncol. 1990;8:1935–7. doi: 10.1200/JCO.1990.8.12.1935. [DOI] [PubMed] [Google Scholar]
- 15.Arcaini L, Rattotti S, Gotti M, Luminari S. Prognostic assessment in patients with indolent B-cell lymphomas. Scientific World Journal. 2012;2012:107892. doi: 10.1100/2012/107892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 17.Peyrade F, Jardin F, Thieblemont C, Thyss A, Emile JF, Castaigne S, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12:460–8. doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 18.Tsai HT, Pfeiffer RM, Warren J, Wilson W, Landgren O. The effects of cardiovascular disease on the clinical outcome of elderly patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2015;56:682–7. doi: 10.3109/10428194.2014.921914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonneveld P, de Ridder M, van der Lelie H, Nieuwenhuis K, Schouten H, Mulder A, et al. Comparison of doxorubicin and mitoxantrone in the treatment of elderly patients with advanced diffuse non-Hodgkin's lymphoma using CHOP versus CNOP chemotherapy. J Clin Oncol. 1995;13:2530–9. doi: 10.1200/JCO.1995.13.10.2530. [DOI] [PubMed] [Google Scholar]
- 20.Tilly H, Lepage E, Coiffier B, Blanc M, Herbrecht R, Bosly A, et al. A randomized comparison of ACVBP and CHOP in the treatment of advanced aggressive non-Hodgkin's lymphoma: the LNH93-5 study. Blood. 2000;96:832A. [Google Scholar]
- 21.Herbrecht R, Cernohous P, Engert A, Le Gouill S, Macdonald D, Machida C, et al. Comparison of pixantrone-based regimen (CPOP-R) with doxorubicin-based therapy (CHOP-R) for treatment of diffuse large B-cell lymphoma. Ann Oncol. 2013;24:2618–23. doi: 10.1093/annonc/mdt289. [DOI] [PubMed] [Google Scholar]
- 22.Kreher S, Lammer F, Augustin D, Pezzutto A, Baldus CD. R-split-CHOP chemotherapy for elderly patients with diffuse large B-cell lymphoma. Eur J Haematol. 2014;93:70–6. doi: 10.1111/ejh.12304. [DOI] [PubMed] [Google Scholar]
- 23.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 24.Advani RH, Chen H, Habermann TM, Morrison VA, Weller EA, Fisher RI, et al. Comparison of conventional prognostic indices in patients older than 60 years with diffuse large B-cell lymphoma treated with R-CHOP in the US Intergroup Study (ECOG 4494, CALGB 9793): consideration of age greater than 70 years in an elderly prognostic index (E-IPI) Br J Haematol. 2010;151:143–51. doi: 10.1111/j.1365-2141.2010.08331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]