Abstract
Purpose
The objective of this study was to explore the relationship between the circulating lymphocyte level during preoperative chemoradiotherapy (CRT) and pathologic complete response (pCR) in locally advanced rectal cancer.
Materials and Methods
From May 2010 to May 2013, 52 patients treated with preoperative CRT followed by surgery, were analysed. Patients received conventional fractionated radiotherapy (50-54 Gy) with fluorouracil-based chemotherapy. Surgical resection was performed at 4 to 8 weeks after the completion of preoperative CRT. Absolute blood lymphocyte counts and their relative percentage in total white blood cell counts were obtained from complete blood count tests performed prior to and after 4, 8, and 12 weeks of CRT. We analysed the association between achieving pCR and change in blood lymphocyte level during CRT, as well as clinical parameters.
Results
Among 52 patients, 14 (26.9%) had evidence of pCR. Sustaining the blood lymphocyte count during CRT (lymphocyte count at 4 weeks/baseline lymphocyte count > 0.35; odds ratio, 8.33; p=0.02) and initial carcinoembryonic antigen < 4.4 ng/mL (odds ratio, 6.71; p=0.03) were significantly associated with pCR in multivariate analyses.
Conclusion
Sustaining blood lymphocyte count during preoperative CRT was predictive for pCR in rectal cancer. Further studies are warranted to investigate the association between pathologic responses and circulating lymphocyte count with its subpopulation during preoperative CRT.
Keywords: Chemoradiotherapy, Rectal neoplasms, Neoadjuvant therapy, Lymphocyte count, Pathologic complete response, Predictive factor
Introduction
In a multimodality treatment of locally advanced rectal cancer, preoperative chemoradiotherapy (CRT) has been widely implemented because randomised trials indicated that its outcomes are superior to postoperative CRT in terms of local control, sphincter preservation and toxicity [1,2]. In addition to better outcomes of preoperative CRT, it also offers results of early response evaluation—pathologic response—that give clear information as to the degree of response to CRT and long-term prognosis. An excellent response to preoperative CRT, such as achieving a pathologic complete response (pCR), is associated with excellent long-term outcomes [3-5]. Since patients with evidence of pCR after CRT have a more favourable prognosis than those showing residual disease, achieving pCR can be a short-term goal in multimodal treatment of rectal cancer [4].
Many researchers have attempted to identify the predictive factors that influence the pathologic tumour response for rectal cancer. It has been reported that the pre-treatment level of carcinoembryonic antigen (CEA), distance from the anal verge, and biological tumour profiles were all associated with the pathologic response after preoperative CRT [6-9]. Recently, several investigations revealed that peripheral immune cell counts, such as lymphocytes or subtypes (regulatory T cell), are significantly associated with a favourable pathologic tumour response after CRT in rectal cancer [10-12]. The prognostic impact of treatment-related lymphopenia was also suggested in patients with non-small cell lung cancer and pancreatic cancer treated with definitive CRT [13,14].
In this study, we hypothesised that the peripheral lymphocyte level is correlated with the level of host immunity, which can influence the immune-mediated antitumor effect. Therefore, we explored whether the changes in blood lymphocyte count during preoperative CRT are associated with a pCR in surgical specimens for locally advanced rectal cancer.
Materials and Methods
Between May 2010 and May 2013, 52 patients underwent preoperative CRT in our institution. We retrospectively analysed the clinical data before and during CRT and evaluated its association with pathologic tumour response in surgical specimens. This study was reviewed and approved by the institutional review board of our institution. Patients underwent preoperative staging work-up, including complete blood count (CBC) with differential, biochemical tumour markers (CEA), colonoscopy, abdominal computed tomography (CT) scan, and pelvic magnetic resonance imaging. Patients with Eastern Cooperative Oncology Group (ECOG) performance status of 0-1 were eligible for preoperative CRT. Absolute blood lymphocyte counts and their relative percentage in total white blood cell (WBC) counts were obtained from CBC tests performed prior to and after 4, 8, and 12 weeks after initiation of CRT. Absolute numbers of WBC subtypes (lymphocyte, monocyte, and neutrophil) were calculated by multiplying the WBC number and each subtype ratio. CBC with differential count was analysed using Beckman Coulter LH 780 (Beckman Coulter, Miami, FL).
All patients underwent a simulation using a Philips Big Bore Brilliance CT (Philips Medical Systems, Madison, WI) with 5-mm slice intervals for three-dimensional radiotherapy planning (RTP). Delineation of the clinical target volume (CTV) for the whole pelvis included gross tumour volume, mesorectum, presacral space, and regional lymphatics. Boost CTV included gross tumour volume and mesorectum. The planning target volume (PTV) was determined by expanding CTV by a margin of 0.5-1.0 cm. Radiotherapy was delivered to the whole pelvis at a dose of 44.0-45.0 Gy, with a boost dose of 4.5-9.0 Gy to the primary tumour, up to total 50-54 Gy. Six-MV or 10-MV photon beams were used for a 3-field radiation treatment. We used the Varian Eclipse External Beam Planning System ver. 7.1 (Varian Medical System, Palo Alto, CA). Serum CEA was measured using the immunoassay on the Roche analytics E170 (Roche Diagnostics, Mennheim, Germany).
Patients received three cycles of chemotherapy, which was initiated on the first day of pelvic radiation and delivered concurrently with radiotherapy. Two cycles of bolus of 5-fluorouracil (400 mg/m2/day) and leucovorin (20 mg/m2/day) were administered for 5 days in the first and fifth week of radiotherapy. In the ninth week, the same chemotherapy regimen was repeated. At 4-8 weeks after the completion of preoperative CRT, patients underwent surgery. Surgical resection was performed with low anterior resection (LAR) or abdominoperineal resection (APR) at the discretion of surgeon.
Achieving pCR was defined as complete absence of any tumour cells, in both the primary site and the dissected lymph node in surgical specimens. Samples with gross or microscopic neoplastic residuals were defined as an incomplete response. To confirm the relation between variables with a pathological response, a chi-square test or Fisher exact test was used. The mean values were used as a cut-off for continuous variables. In the case of a significant continuous variable by a chi-square test or Fisher exact test, a receiver operating characteristic curve was drawn for determining the best chance of correlation with pCR. The variables selected by a univariate analysis (p < 0.1) were included in a multivariate analysis. For a multivariate analysis, a logistic regression with backward stepwise method was performed.
We examined the correlation between RTP-associated statistics and the change in lymphocyte level using a Pearson correlation coefficient analysis. The following parameters were included in RTP-associated statistics: volume of PTV, sum of monitor unit (MU), body volume received more than 5% of prescribed dose, and integral dose of body volume received more than 5% of prescribed dose. RTP-associated statistics were obtained from the radiotherapy plan of the whole pelvis. Two-sided p-values less than 0.05 were considered to indicate a significant difference. All statistical analyses were performed with IBM SPSS statistics software ver. 19.0 (IBM Co., Armonk, NY).
Results
Patient’s characteristics are summarised in Table 1. Thirty-eight patients were male (73.1%), and the age ranged from 34 to 77 (median, 56). All patients received surgical resection with negative resection margin. The types of surgery were LAR in 36 patients (69.2%) and APR in 16 patients (30.8%). A pCR was achieved in 14 patients (26.9%).
Table 1.
Patient characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Sex | |
| Male | 38 (73.1) |
| Female | 14 (26.9) |
| Median age (range, yr) | 34-77 (56) |
| Histologic type | |
| Adenocarcinoma | 51 (98.1) |
| Signet ring cell | 1 (1.9) |
| Clinical T stage | |
| T2 | 3 (5.8) |
| T3 | 41 (78.8) |
| T4 | 8 (15.4) |
| Clinical N stage | |
| N-negative | 5 (9.6) |
| N-positive | 47 (90.4) |
| Type of surgery | |
| LAR | 36 (69.2) |
| APR | 16 (30.8) |
| CEA level (ng/mL) | |
| ≥ 4.4 | 26 (50.0) |
| < 4.4 | 26 (50.0) |
LAR, lower anterior resection; APR, abdominoperineal resection; CEA, carcinoembryonic antigen.
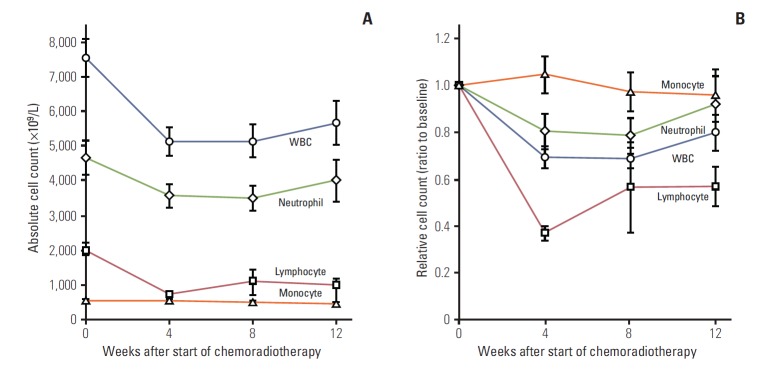
The baseline mean levels of haemoglobin, total WBC count, lymphocyte count, and differential count (%) were 13.2 g/dL (range, 9.1 to 16.0 g/dL), 7,537×109 /L (range, 3,900/L to 13,600/L), 2,036×109 /L (range, 1,007/L to 3,274/L), and 27.8% (range, 13.8% to 42.1%), respectively. The trend of changes in WBCs compared to the baseline level is illustrated in Fig. 1.
Fig. 1.
Change of blood cell count value over time. (A) Absolute cell count. (B) Relative cell count. Data points indicate mean value and error bars indicate 95% confidence interval. WBC, white blood cell.
In univariate analyses, clinical factors were examined to identify the association with pCR and baseline CEA (≥ 4.4 ng/mL vs. < 4.4 ng/mL, p < 0.01), and sustaining blood lymphocyte ratio at 4-week (lymphocyte count at 4-week/baseline lymphocyte count ≥ 0.35 vs. < 0.35, p=0.01) was statistically significant (Table 2). In multivariate analyses, the baseline level of CEA < 4.4 ng/mL (odds ratio, 6.71; p=0.03), and sustaining the lymphocyte ratio at 4-week ≥ 0.35 (odds ratio, 8.33; p=0.02) remained as statistically significant parameters (Table 3). Forty-four patients (84.6%) finished the planned three cycles of chemotherapy, and there was no significant difference of the complete response (CR) rate between the groups with/without completion of the planned chemotherapy (3 cycles vs. less than 3 cycles, 25.0% vs. 37.5%, p=0.66) (Table 2).
Table 2.
Univariate analyses for variables associated with pathologic complete response
| Variable | Pathologic complete response |
p-valuea) | |
|---|---|---|---|
| Yes | No | ||
| Sex | |||
| Male | 8 | 30 | 0.16 |
| Female | 6 | 8 | |
| Age (yr) | |||
| < 53 | 5 | 16 | 0.75 |
| ≥ 53 | 9 | 22 | |
| Clinical T stage | |||
| T2 | 1 | 2 | > 0.99 |
| T3 | 11 | 30 | |
| T4 | 2 | 6 | |
| Clinical N stage | |||
| N-positive | 11 | 36 | 0.11 |
| N-negative | 3 | 2 | |
| Chemotherapy | |||
| < 3 cycles | 3 | 5 | 0.66 |
| ≥ 3 cycles | 11 | 33 | |
| CEA, baseline (ng/mL) | |||
| < 4.4 | 2 | 24 | < 0.01 |
| ≥ 4.4 | 12 | 14 | |
| Hemoglobin, baseline (g/dL) | |||
| < 13.2 | 9 | 15 | 0.20 |
| ≥ 13.2 | 5 | 22 | |
| WBC count, baseline (×109/L) | |||
| < 7,300 | 2 | 16 | 0.07 |
| ≥ 7,300 | 10 | 19 | |
| Lymphocyte, baseline (%) | |||
| < 27.2 | 6 | 20 | 0.76 |
| ≥ 27.2 | 8 | 18 | |
| Lymphocyte count, baseline (×109/L) | |||
| < 1,996 | 8 | 18 | 0.76 |
| ≥ 1,996 | 6 | 20 | |
| Lymphocyte, at 4-week (%) | |||
| < 14.8 | 7 | 19 | > 0.99 |
| ≥ 14.8 | 7 | 19 | |
| Lymphocyte count, at 4-week (×109/L) | |||
| < 671 | 8 | 18 | 0.76 |
| ≥ 671 | 6 | 20 | |
| Sustaining lymphocyte ratio, at 4-week | |||
| < 0.35 | 2 | 22 | 0.01 |
| ≥ 0.35 | 12 | 16 | |
| Lymphocyte, at 8-week (%)b) | |||
| < 18.0 | 6 | 12 | 0.21 |
| ≥ 18.0 | 3 | 16 | |
| Lymphocyte count, at 8-week (×109/L)b) | |||
| < 903 | 7 | 18 | 0.69 |
| ≥ 903 | 2 | 10 | |
| Sustaining lymphocyte ratio, at 8-weekb) | |||
| < 0.47 | 6 | 15 | 0.49 |
| ≥ 0.47 | 3 | 13 | |
| Lymphocyte, at 12-week (%)b) | |||
| < 19.3 | 5 | 12 | 0.86 |
| ≥ 19.2 | 4 | 11 | |
| Lymphocyte count, at 12-week (×109/L)b) | |||
| < 1,033 | 3 | 6 | 0.68 |
| ≥ 1,033 | 6 | 17 | |
| Sustaining lymphocyte ratio, at 12-weekb) | |||
| < 0.53 | 4 | 11 | 0.86 |
| ≥ 0.53 | 5 | 12 | |
CEA, carcinoembryonic antigen.
Chi-square test or Fisher exact test,
Analyses was done with available data at 8-week (n=37) and 12-week (n=32).
Table 3.
Multivariate analyses for predictors associated with a pathologic complete response
| Variable | OR (95% CI) | p-valuea) |
|---|---|---|
| CEA < 4.4 ng/dL | 6.71 (1.22-36.97) | 0.03 |
| Sustained lymphocyte ratio ≥ 0.35 | 8.33 (1.54-45.12) | 0.02 |
| Clinical N stage | 0.22 (0.02-2.57) | 0.22 |
OR, odds ratio; CI, confidence interval; CEA, carcinoembryonic antigen.
Logistic regression with backward stepwise method was performed.
RTP-associated statistics are summarised in Table 4. The correlation between RTP-associated statistics and sustaining the blood lymphocyte ratio at 4 weeks of CRT is illustrated in Fig. 2. The sum of the MU was significantly correlated with the sustained blood lymphocyte ratio at 4 weeks (Pearson’s coefficient, –0.341; p=0.01).
Table 4.
Radiotherapy planning–associated parameters
| Parameter | Median (range) |
|---|---|
| Radiation dose (Gy) | 50.0 (41.2-54.0) |
| Volume of PTV (mL) | 801 (556-1,266) |
| Sum of monitor unit | 378 (323-437) |
| Integral dose of body volume received > 5% of prescribed dose (L-Gy) | 20.6 (12.9-29.3) |
| Body volume received > 5% of prescribed dose (L) | 8.63 (4.84-11.93) |
PTV, planning target volume.
Fig. 2.
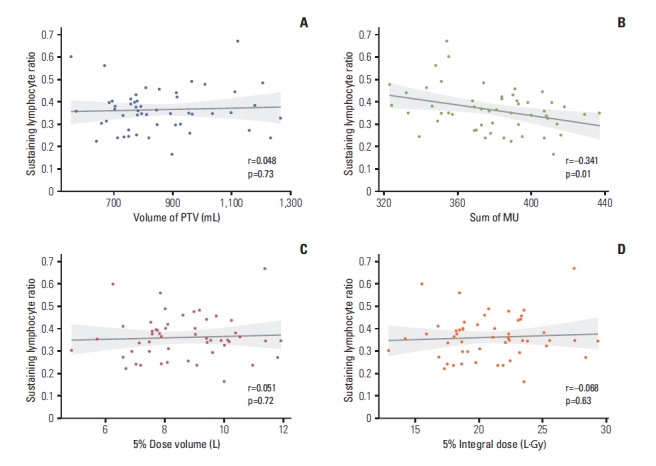
Correlations between radiotherapy planning–associated parameters and the sustained lymphocyte ratio at 4 weeks of chemoradiotherapy. (A) Volume of planning target volume (PTV). (B) Sum of monitor unit (MU). (C) Body volume received more than 5% of prescribed dose. (D) Integral dose of body volume received more than 5% of prescribed dose.
Discussion
The results of this study showed that a high ratio of sustained blood lymphocyte count during CRT (sustaining ratio > 0.35 at 4 weeks) was predictive for achieving pCR in rectal cancer. While it is assumed that the high level of sustained blood lymphocyte count represents the maintenance of host immunity during CRT, this result may suggest that sustaining the blood lymphocyte count during CRT enhances the immune-mediated anti-tumour effect and leads to higher values for pCR. Lymphocytes play a major role in the host anti-tumour response of dendritic cells. Denkert et al. [15] reported that tumour infiltration of lymphocytes was a significant independent parameter for pCR in surgical specimens after neoadjuvant chemotherapy in breast cancer. Data from colorectal cancer has confirmed the prognostic value of tumour infiltration by lymphocytes [16,17]. Although the peripheral level of lymphocytes is not directly correlated with the level of tumour-infiltrating lymphocytes, circulating lymphocytes can be an indirect but easily accessible surrogate for evaluating lymphocyte-mediated immunity. A direct correlation between the levels of peripheral lymphocytes and tumour-infiltrating lymphocytes should be studied by more detailed pathologic examinations of surgical specimens.
We focused on the level of peripheral blood lymphocytes to determine its clinical implication for the early prediction of tumour response, which may be useful for a tailored patient approach in rectal cancer [18]. Schmidt et al. [12] showed that higher levels of circulating regulatory T cells (Treg) after 5 days of CRT are associated with a favourable pathologic tumour stage (< pT3a) in surgical specimens in colorectal cancer patients. They suggested that the levels of Treg during CRT might be a good indicator for necessary dose adjustments in CRT. Although we analysed the whole lymphocyte count and not the subgroup differential counts, compared to the study of Schmidt et al. [12], we showed that whole lymphocyte counts during CRT also have a significant association with a favourable tumour stage (pCR). Based on these two results, there seems to be a close correlation between the levels of total blood lymphocytes and Treg cells during CRT. However, the change of total lymphocytes and its subgroups did not show a consistent pattern over a period of radiotherapy. The results of Schmidt et al. [12] showed that the percentage of CD4+ cells among lymphocytes remained relatively constant and the percentage of Treg decreased at 5 days of CRT. Lissoni et al. [19] studied the effects of conventional antitumor therapies on circulating Treg lymphocytes, including whole lymphocyte count, and showed that the mean values of whole lymphocyte and CD4+ lymphocyte count dropped dramatically at the end of pelvic irradiation. However, the Treg (CD4+CD25+) lymphocyte level showed no substantial changes. Schuler et al. [20] reported that the Treg level increased in some patients while it decreased in others after CRT for head and neck cancer. This variability among studies may be due to different timing of blood sampling, heterogeneous immune responses by various hosts and tumour conditions. For elucidating the role of total lymphocyte levels and its interplay with other immune cells during CRT, an analysis of the association between pathologic responses and the level of subpopulations of blood lymphocytes sampled at multiple times is needed, also in combination with cytokine milieu analyses.
A negative prognostic value for the initial lymphopenia and treatment-related decrease in the number of blood lymphocytes has been demonstrated in various settings and malignancies [13,14,21,22]. Baseline blood lymphocyte counts were not significantly associated with pCR in our study because patients with lymphopenia were excluded at the time of patient selection for preoperative CRT. Although a marked decrease in lymphocyte count was observed in most patients (Fig. 1), treatment-related lymphopenia (lymphocyte count at 4 weeks < 500×109 cells/L) had developed in only four patients (8.5%). In our study, a significant predictor for pCR was not the absolute lymphocyte count, but the relative ratio of lymphocyte count to the initial lymphocyte count (sustained ratio). This fact suggests that relative ratio of treatment-related decrease of lymphocyte may have a negative prognostic value, as well as treatment-related lymphopenia in preoperative CRT for rectal cancer.
It has been investigated in several studies that peripheral lymphocyte count is associated with pathologic response after preoperative CRT. The hypothesis of these investigations was that the level of host immunity related to lymphocyte could facilitate the pathologic responses. High pretreatment lymphocyte number [10,11] or subtype of lymphocyte (regulatory T cell) [12] can be considered to reflect the level of lymphocyte-mediated immunity and demonstrated in previous researches. In these contexts, it can be assumed that the sustained level of lymphocyte during CRT can be also a predictive marker for pCR as we showed, although the predictive value of pre-treatment lymphocyte count was not demonstrated in this study.
Compliance of chemotherapy may be a confounding factor in the association between sustained level of lymphocyte and achieving pCR because the level of lymphocyte during treatment may be a mere reflection of chemotherapy tolerance. Those who were maintaining the level of lymphocyte had better performance and were likely to withstand preoperative CRT. However, the CR rates between the groups with/without completion of planned chemotherapy did not show significant difference (25.0% vs. 37.5%, p=0.66) (Table 2).
We analysed the correlation between RTP-associated parameters and the sustained ratio of blood lymphocytes at 4 weeks of CRT (Fig. 2). The volume of PTV, integral dose of body volume receiving more than 5% of the prescribed dose, and body volume receiving more than 5% of the prescribed dose were not correlated with the sustained lymphocyte ratio. Only the sum of the monitor unit was significantly correlated with the decreased ratio of lymphocytes (r=–0.341, p=0.01) (Fig. 2B). The dose rate, implemented radiation techniques, volume of PTV (field size), and integral dose can influence lymphocyte counts. Yovino et al. [23] undertook a simulation study of the effect of factors related to radiation technique on the irradiated volume of the circulating blood pool. They showed that a potentially lymphotoxic dose (> 0.5 Gy) could be delivered to the circulating blood in a standard treatment plan for brain tumours, and that a larger PTV volume and low dose rate sped up the saturation rate of the irradiated blood volume. However, in our study, RTP-associated parameters were not different among patients due to a very homogenous study population. In all patients, identical landmarks for the borders of the radiation field and similar techniques were used. These homogeneities resulted in no definite correlations with decreases in lymphocytes during CRT except for the sum of the monitor unit. In the case of the monitor unit, because we fixed the dose rate at 300 MU/min, the sum of the monitor unit is correlated exactly with the irradiated time. A longer beam-on time induced a longer exposure of the blood pool to radiation, and it is thought that this longer exposure resulted in a lower sustained lymphocyte ratio. However, although there was a moderate correlation between the sum of MU and sustained level of lymphocyte, it was not enough to draw a conclusion that less MU use in radiotherapy would lead to more pCR.
The results of this study are limited due to relatively small sample size and single institutional retrospective design. Moreover, detailed analyses with subpopulation of lymphocyte could not be evaluated in this exploratory study. However, this study suggests the potential association between pathologic response and sustained ratio of peripheral lymphocyte level during CRT in rectal cancer. Larger scale retrospective analyses would be needed to confirm the predictive significance of the peripheral lymphocyte changes at various timings during CRT. The effect of the peripheral lymphocyte level on long-term survival should also be investigated in these retrospective analyses.
Conclusion
In conclusion, sustaining blood lymphocyte count during preoperative CRT was predictive for pCR in rectal cancer. Further studies are warranted to investigate the association between pathologic responses and circulating lymphocyte count with its subpopulation during preoperative CRT.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Kong M, Hong SE, Choi WS, Kim SY, Choi J. Preoperative concurrent chemoradiotherapy for locally advanced rectal cancer: treatment outcomes and analysis of prognostic factors. Cancer Res Treat. 2012;44:104–12. doi: 10.4143/crt.2012.44.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–28. doi: 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- 4.Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 5.Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01) Ann Surg. 2010;252:998–1004. doi: 10.1097/SLA.0b013e3181f3f1b1. [DOI] [PubMed] [Google Scholar]
- 6.Yoon SM, Kim DY, Kim TH, Jung KH, Chang HJ, Koom WS, et al. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2007;69:1167–72. doi: 10.1016/j.ijrobp.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Restivo A, Zorcolo L, Cocco IM, Manunza R, Margiani C, Marongiu L, et al. Elevated CEA levels and low distance of the tumor from the anal verge are predictors of incomplete response to chemoradiation in patients with rectal cancer. Ann Surg Oncol. 2013;20:864–71. doi: 10.1245/s10434-012-2669-8. [DOI] [PubMed] [Google Scholar]
- 8.Rodel C, Grabenbauer GG, Papadopoulos T, Bigalke M, Gunther K, Schick C, et al. Apoptosis as a cellular predictor for histopathologic response to neoadjuvant radiochemotherapy in patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:294–303. doi: 10.1016/s0360-3016(01)02643-8. [DOI] [PubMed] [Google Scholar]
- 9.Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673–88. doi: 10.1016/j.ijrobp.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Choi CH, Kim WD, Lee SJ, Park WY. Clinical predictive factors of pathologic tumor response after preoperative chemoradiotherapy in rectal cancer. Radiat Oncol J. 2012;30:99–107. doi: 10.3857/roj.2012.30.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 2010;5:47. doi: 10.1186/1748-717X-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt MA, Fortsch C, Schmidt M, Rau TT, Fietkau R, Distel LV. Circulating regulatory T cells of cancer patients receiving radiochemotherapy may be useful to individualize cancer treatment. Radiother Oncol. 2012;104:131–8. doi: 10.1016/j.radonc.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Campian JL, Ye X, Brock M, Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013;31:183–8. doi: 10.3109/07357907.2013.767342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2013 May 2;31 doi: 10.1097/COC.0b013e3182940ff9. [Epub]. http://dx.doi.org/10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 16.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–4. [PubMed] [Google Scholar]
- 17.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–24. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Haustermans K, Debucquoy A, Lambrecht M. The ESTRO Breur Lecture 2010: toward a tailored patient approach in rectal cancer. Radiother Oncol. 2011;100:15–21. doi: 10.1016/j.radonc.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Lissoni P, Brivio F, Fumagalli L, Messina G, Meregalli S, Porro G, et al. Effects of the conventional antitumor therapies surgery, chemotherapy, radiotherapy and immunotherapy on regulatory T lymphocytes in cancer patients. Anticancer Res. 2009;29:1847–52. [PubMed] [Google Scholar]
- 20.Schuler PJ, Borger V, Bolke E, Habermehl D, Matuschek C, Wild CA, et al. Dendritic cell generation and CD4+ CD25high FOXP3+ regulatory t cells in human head and neck carcinoma during radio-chemotherapy. Eur J Med Res. 2011;16:57–62. doi: 10.1186/2047-783X-16-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–91. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–80. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–4. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]