Abstract
Purpose
The purpose of this study is to investigate the role of fibroblast growth factor receptor 4 (FGFR4) polymorphism in esophageal cancer after chemoradiotherapy (CRT).
Materials and Methods
Peripheral blood samples from 244 patients treated with CRT for esophageal squamous cell carcinoma were assessed for the role of FGFR4 genotype on treatment response and survival.
Results
A total of 94 patients were homozygous for the Gly388 allele, and 110 were heterozygous and 40 homozygous for the Arg388 allele. No significant association was found between the FGFR4 genotype and clinicopathological parameters. However, patients carrying the Gly388 allele showed a better overall response rate than Arg388 carriers (p=0.038). In addition, Gly388 allele patients at an earlier stage showed better overall survival (OS) and progression-free survival than Arg388 carriers. Among these, the Gly388 allele showed significantly improved OS compared to Arg388 carriers in the lymph node (LN) metastasis group (p=0.042) compared to the no LN metastasis group (p=0.125). However, similar survival outcomes were observed for advanced-stage disease regardless of genotype.
Conclusion
This result suggests that the role of FGFR4 Gly388 in treatment outcomes differs according to esophageal cancer stage. It showed a predictive role in the response of esophageal cancer patients to CRT with a better trend for OS in Gly388 than Arg388 carriers in the early stages. In particular, LN-positive early-stage patients carrying the Gly388 allele showed improved OS compared to those carrying Arg388.
Keywords: Biological markers, Chemoradiotherapy, Esophageal neoplasms, Fibroblast growth factor receptor 4
Introduction
Treatments for esophageal cancer have been studied intensively in recent decades. However, the clinical outcomes remain unsatisfactory, with 5-year survival rates of 49.3% for localized disease and 2.8% for metastatic disease [1]. Until recently, the standard treatment for early or locally advanced esophageal cancer was surgery. However, definitive chemoradiotherapy (CRT) is another possibility in cases where surgery is not an option due to advanced-stage disease or the presence of comorbidities [2]. To enhance the efficacy of CRT, clinical trials of various drugs have been conducted, including trials for cisplatin and 5-fluorouracil (5-FU), as well as docetaxel [3], paclitaxel [4], and cetuximab [5].
In addition, many recent studies have focused on identification of new biomarkers to help in prediction of the outcome after CRT and to find additional therapeutic targets. Although the underlying pathogenic mechanisms of lung cancer, colorectal cancer, and breast cancer have been investigated extensively and targeted therapies have since been developed, relatively little is known about esophageal cancer.
Esophageal cancer can metastasize readily, even at early disease stages, due to the absence of a serosa and a rich lymphatic system adjacent to the major organ, resulting in a tendency for metastasis and poor outcomes. Thus, to control the development of esophageal cancer, it is important to not only kill the tumor cells but also to inhibit invasion and metastasis. Recently, exploiting the relationship between tumor cells and the stroma was highlighted as a possible way to prevent the initiation and progression of tumors.
Fibroblasts play an important role in the tumor microenvironment. Carcinoma-associated fibroblasts in the tumor microenvironment influence tumor cell survival, growth, angiogenesis, invasion, inflammation, migration, and metastasis [6]. Fibroblast growth factors (FGFs) are divided into four groups (FGF1-4), and dysregulation of their receptors (FGFRs) plays an important role in tumorigenesis. Our understanding of the biological properties of FGFRs is increasing, and several reports have described their roles in cancer progression and drug sensitivity [7,8]. Among them, FGFR4 is expressed in myofibroblasts during regeneration following injury, but not in mature skeletal muscle. An FGFR4 Gly388Arg polymorphism (rs351855), which causes the substitution of arginine for glycine (FGFR4 Arg388) in the transmembrane domain of the receptor, was reported to increase cancer risk [9], aggressiveness, metastasis [10], and drug resistance [11]. Ansell et al. [12] recently reported a significantly increased risk of head and neck squamous cell carcinoma (HNSCC) with the FGFR4 Gly388 allele, whereas Arg388 resulted in cisplatin sensitivity (p=0.141) in an in vitro assay. In addition, Arg388 carriers showed significantly poor survival outcome in head and neck or lung cancer patients [10,13]. Considering the similar causal factors such as alcohol or smoking and treatment modality of head and neck, lung or esophageal cancer, FGFR4 polymorphism could be a targetable prognostic marker in esophageal cancer, for which there has been no reliable biomarker until now.
Thus, we investigated the role of FGFR4 polymorphisms in esophageal squamous cell carcinoma patients who were treated with CRT.
Materials and Methods
1. Patients and treatment
This retrospective study was conducted to assess the relationship between FGFR4 polymorphisms and treatment outcomes in patients receiving CRT for esophageal squamous cell carcinoma. The inclusion criteria were (1) a diagnosis of squamous esophageal carcinoma, (2) treatment with concurrent CRT as a preoperative or curative-aim first-line therapy with at least 4,000 cGy of radiation, (3) evaluation of the response after CRT, (4) a Karnofsky performance status ≥ 70 at diagnosis, (5) preserved organ function sufficient to receive CRT, and (6) a blood sample available for DNA analysis. Patients were excluded for (1) other confirmed or suspected malignancies, (2) types of cancer other than squamous cell carcinoma (e.g., adenocarcinoma), (3) gastroesophageal junction carcinoma, and (4) postoperative CRT.
During radiotherapy, the patients received combination chemotherapy with 5-FU (1,000 mg/m2 on days 1-4) and cisplatin (75 mg/m2 on day 1) every 4 weeks or weekly docetaxel (30 mg/m2 on days 1 and 8) and cisplatin (30 mg/m2 on days 1 and 8) every 3 weeks. Blood samples for genotyping were drawn before the start of CRT.
Evaluation of the response was based on the Response Evaluation Criteria in Solid Tumors (RECIST ver. 1.1), and was assessed 8 weeks after completion of CRT. After CRT, surgery was performed for the preoperative CRT cases. Additional chemotherapy was performed in cases where any disease remained without progression, or if surgery was not possible. Second-line chemotherapy was administered in cases where the disease had progressed.
Blood samples for polymorphism study were provided by the Chonnam National University Hwasun Hospital National Biobank of Korea, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs. The study protocol was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (CNUHH-2014-016).
2. Genotyping
Genomic DNA was extracted from peripheral blood using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA), according to the manufacturer’s protocol. FGFR4 Gly388Arg genotyping was performed using high-resolution melting (HRM) analysis with a Rotor Gene 6000 (Corbett Research, Sydney, Australia). The primers were as follows: forward, 5'-ggagagcttctgcacagtgg-3'; and reverse, 5'-cttggctgtgctcctgct-3'. The reaction mixture for HRM included 200 nM polymerase chain reaction primers, 1 μM SYTO 9 fluorescent dye (Invitrogen, Carlsbad, CA), 0.5 U of f-Taq polymerase, and 40 ng of genomic DNA in 10-μL reaction volumes. The cycling conditions included an initial 5-minute hold at 95°C, followed by 40 cycles at 95°C for 5 seconds, 65°C for 30 seconds, and 72°C for 20 seconds, with an increase in the melting temperature from 78°C to 92°C at 0.1°C/sec. The genotyping results were validated using direct sequencing (ABI PRISM 3100 Genetic Analyzer, Life Technologies, Carlsbad, CA) of 16 subjects (6%), and the results showed 100% concordant. Appropriate positive/negative and internal controls were included.
3. Statistical analyses
The progression-free survival (PFS) time was calculated from the start of CRT to the appearance of disease progression or recurrence after surgery. The overall survival (OS) time was calculated from the start of CRT to death from any cause; patients who were alive at the last follow-up were recorded at that time. Chi-square tests were used to evaluate associations between genotypes and clinicopathological characteristics and chemotherapy responses. The Cox proportional hazards model was used to evaluate the effect of variables on OS and PFS, and to produce survival curves. The proportional hazards assumption was assessed by graphical evaluation of log-log plots. All statistical analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL). A p-value of < 0.05 was considered to indicate statistical significance.
Results
1. Study population
Between May 2004 and December 2012, 264 patients were treated for esophageal cancer using CRT, and 244 patients suitable for inclusion were analyzed in this study. The median dose of radiation was 5,400 cGy (range, 4,000 to 6,600 cGy); 187 patients (77%) received radiation doses ≥ 5,000 cGy, and 57 (23%) received doses < 5,000 cGy due to preoperative aims or poor general conditions.
The chemotherapy regimens during radiation treatment were as follows: 5-FU and cisplatin in 204 (84%) and docetaxel and cisplatin in 40 patients (16%). After CRT, 58 patients (24%) underwent surgery with a curative aim. In total, 143 patients (58.6%) showed disease progression after CRT or surgery, and 88 patients (36%) received second-line chemotherapy (Table 1).
Table 1.
Patient clinicopathologic characteristics
| Total | G/G | G/A | A/A | p-value | G/A or AA | p-value | |
|---|---|---|---|---|---|---|---|
| No. (%) | 244 | 94 (39) | 110 (45) | 40 (16) | 150 (61) | ||
| Median age (range, yr) | 66 (41-87) | 64 (41-79) | 66 (47-87) | 66 (49-81) | |||
| ≤ 60 | 75 (31) | 32 (34) | 31 (28) | 12 (30) | 0.66 | 43 (29) | 0.228 |
| > 60 | 169 (69) | 62 (66) | 79 (72) | 28 (70) | 107 (71) | ||
| Sex | |||||||
| Male | 237 (97) | 91 (97) | 106 (96) | 40 (100) | 0.485 | 146 (97) | 0.549 |
| Female | 7 (7) | 3 (3) | 4 (4) | 0 | 4 (3) | ||
| Differentiation | |||||||
| Well | 102 (42) | 37 (39) | 46 (42) | 19 (47) | 0.95 | 65 (43) | 0.75 |
| Moderate | 85 (35) | 32 (34) | 40 (36) | 13 (33) | 53 (35) | ||
| Poorly | 32 (13) | 15 (16) | 13 (12) | 4 (10) | 17 (12) | ||
| Unknown | 25 (10) | 10 (11) | 11 (10) | 4 (10) | 15 (10) | ||
| Tumor location | |||||||
| Upper | 63 (26) | 27 (29) | 21 (19) | 15 (38) | 0.123 | 36 (24) | 0.712 |
| Mid | 133 (54) | 49 (52) | 68 (62) | 16 (40) | 84 (56) | ||
| Lower | 48 (20) | 18 (19) | 21 (19) | 9 (23) | 30 (20) | ||
| TNM stage | |||||||
| I | 11 (5) | 3 (3) | 7 (6) | 1 (3) | 0.859 | 8 (5) | 0.839 |
| II | 54 (22) | 21 (21) | 23 (21) | 11 (27) | 34 (23) | ||
| III | 101 (41) | 39 (42) | 45 (41) | 17 (43) | 62 (41) | ||
| IV | 78 (32) | 32 (34) | 35 (32) | 1 (27) | 46 (31) | ||
| Chemotherapy | |||||||
| FP | 204 (84) | 76 (81) | 93 (85) | 36 (87) | 0.596 | 128 (85) | 0.228 |
| DP | 40 (16) | 18 (19) | 17 (15) | 5 (13) | 22 (15) | ||
| Operation | |||||||
| Yes | 58 (24) | 73 (78) | 82 (74) | 32 (80) | 0.661 | 113 (75) | 0.399 |
| No | 186 (76) | 21 (22) | 29 (26) | 8 (20) | 37 (25) | ||
| Second-line chemotherapy | |||||||
| Yes | 88 (36) | 58 (62) | 71 (65) | 27 (67) | 0.802 | 98 (65) | 0.33 |
| No | 156 (64) | 36 (38) | 39 (35) | 13 (33) | 52 (35) |
Values are presented as number (%) unless otherwise indicated. FP, fluoropyrimidine and cisplatin; DP, docetaxel and cisplatin.
2. Chemotherapy responses and survival
Fifty-two patients (21%; 95% confidence interval [CI], 16 to 27) showed a complete response, and 158 patients (65%; 95% CI, 58 to 70) showed a partial response after CRT.
Patients at an earlier stage of disease showed a significantly better response. The chemotherapy regimen during radiation therapy (5-FU+cisplatin vs. docetaxel+cisplatin) had no effect on the overall response rate (p=0.307). The median OS and PFS for stage I disease were not reached in this analysis. The median OS for stages II, III, and IV were 44.0 months (95% CI, 22.6 to 46.2), 25.6 months (95% CI, 14.0 to 32.6), and 21.9 months (95% CI, 11.3 to 27.3), respectively, and statistically significant differences were observed among the stages (p=0.001). The median PFS rates of stages II, III, and IV were also significantly different at 42.0 months (95% CI, 0.8 to 54.8), 12.7 months (95% CI, 10.0 to 15.6), and 9.3 months (95% CI, 12.1 to 16.9), respectively (p < 0.001). These results were similar after grouping as early-stage (stages I and II) versus advanced-stage (stages III and IV) disease in PFS (p < 0.001) and OS (p=0.001). Although 58 patients (23.7%) underwent surgery after CRT, no significant difference in survival was observed between those who underwent surgery (PFS, 17.3 months; 95% CI, 5.4 to 29.2 and OS, 30.2 months; 95% CI, 15.5 to 44.9) and those who did not (PFS, 13.6 months; 95% CI, 10.6 to 16.6; p=0.293 and OS, 24.3 months; 95% CI, 18.5 to 30.1; p=0.434).
3. FGFR4 genotype and treatment outcome
The frequencies of the FGFR4 Gly388Arg polymorphic genotypes were as follows: 94 patients (38.5%) had the Gly388 allele (G/G), 110 (45.4%) were heterozygous for G/A, and 40 (16.4%) had the Arg388 allele (A/A). Patients with the G/G allele (91.5%; 95% CI, 82.9 to 95.6) showed significantly better responses to CRT than Arg carriers (82.7%; 95% CI, 75.4 to 88.0; p=0.038) (Table 2). However, no significant association was found between the FGFR4 genotype and any clinicopathological parameter examined, including tumor stage, differentiation, and tumor location (Table 1). No difference between heterozygous and homozygous Arg388 carriers was observed in this study.
Table 2.
Response after chemoradiotherapy according to FGFR4 Gly388Arg polymorphism
| Genotype | Responder (CR+PR, n=210) | Non-responder (SD+PD, n=34) | p-value |
|---|---|---|---|
| G/G (n=94) | 86 (91.5) | 8 (8.5) | 0.090 |
| G/A (n=110) | 89 (80.9) | 21 (19.1) | |
| A/A (n=40) | 35 (87.5) | 5 (12.5) | |
| G/G (n=94) | 86 (91.5) | 8 (8.5) | 0.038 |
| G/A+A/A (n=150) | 124 (82.7) | 26 (17.3) |
Values are presented as number (% of each gene group). FGFR4, fibroblast growth factor receptor 4; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
In survival analyses, no significant difference in OS or PFS was observed among genotypes. For comprehensive evaluation of the role of genotype in treatment outcome, the patients were divided into early (stages I and II; n=65) and advanced stage groups (stages III and IV; n=179). In the early stages, patients with the Gly388 allele tended to have somewhat better PFS (unreached median OS [mOS] in G/G, 38 months in Arg388 carriers; 95% CI, 11.9 to 63.9; p=0.506) and OS rates (unreached mOS in G/G, 45 months; 95% CI, 26.1 to 63.5; p=0.773) (Fig. 1A and C) than the Arg388 carriers. However, patients with advanced stage disease showed similar patterns of PFS (9.6 months in G/G; 95% CI, 6.1 to 13 and 12.6 months in Arg388 carriers; 95% CI, 10.2 to 14.9; p=0.678) and OS (24.7 months in G/G; 95% CI, 19.5 to 29.90 and 22.4 months in Arg388 carriers, 95% CI, 15.3 to 29.5; p=0.668) (Fig. 1B and D) regardless of genotype. In particular, patients with the Gly388 allele showed a significantly better OS rate than Arg388 carriers in the lymph node (LN) metastasis group (p=0.042) compared to the no LN metastasis group (p=0.125) (Fig. 2) in the early stages.
Fig. 1.
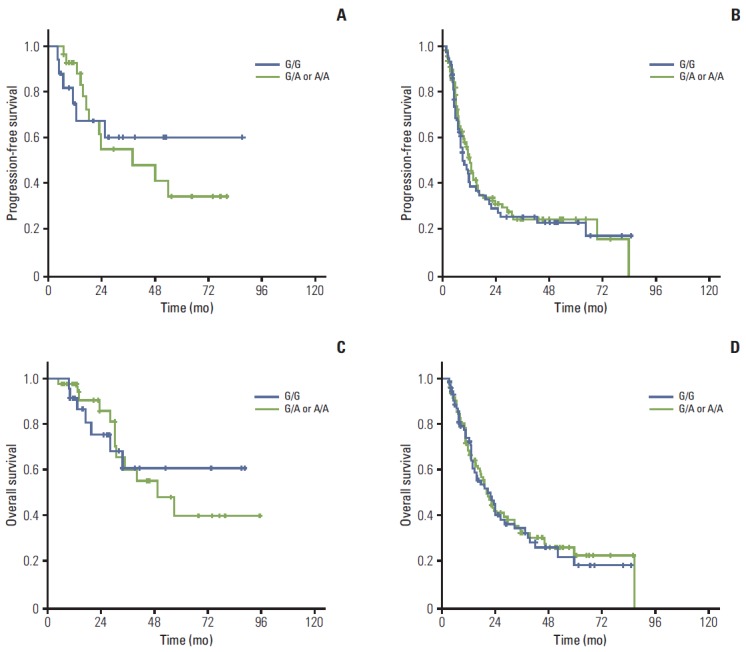
Association between genotype and survival outcome. In early stage of esophageal cancer, FGFR4 Gly388 allele (G/G) patients show better trends of progression-free survival (A) and overall survival (C) than FGFR4 Arg388 carriers (G/A or A/A) without statistical significance. However, the progression-free survival (B) and overall survival (D) in advanced stage of esophageal cancer patients show comparable outcomes regardless of genotypes.
Fig. 2.
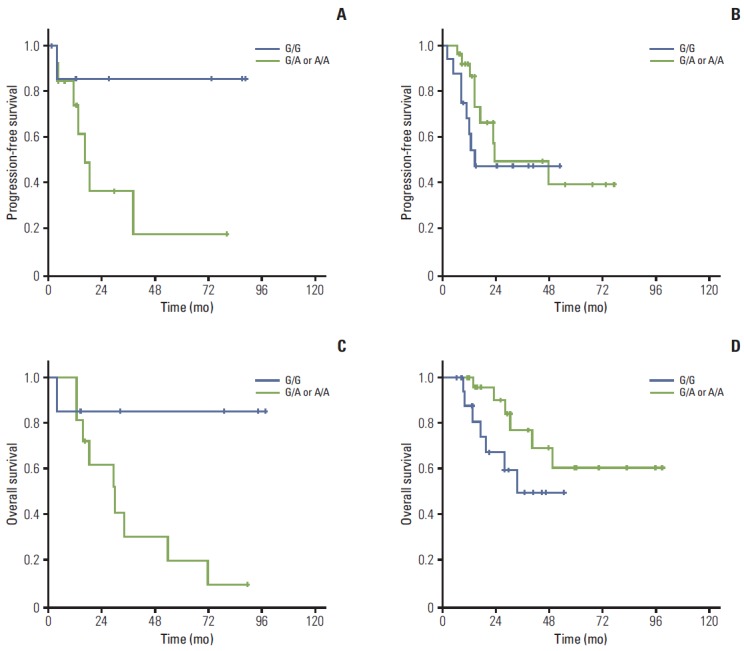
Survival according to genotype in early esophageal cancer. In early esophageal cancer, FGFR4 Gly388 allele patients showed a better trend of progression-free survival (A) (p=0.079) and significantly better overall survival (C) (p=0.042) than FGFR4 Arg388 carriers in lymph node (LN)-positive patients. However, there was no difference in LN-negative patients according to genotypes (B, D).
4. Univariate and multivariate analyses
To explore the prognostic factors for esophageal cancer patients, a univariate analysis was performed with clinicopathologic variables and genotypes. A multivariate analysis using the Cox proportional hazards model was used for evaluation of the statistically significant variables found in the univariate analysis. As shown in Table 3, age > 60 years, early-stage disease, and positive responder status (showing a complete or partial response) were significantly favorable prognostic factors for PFS, as were female sex, early-stage disease, and positive responder status for OS in the univariate analysis. Among these factors, stage and responder status were significant prognostic factors for survival in the multivariate analysis.
Table 3.
Univariate analysis for survival
| PFS (median, mo) | p-value | OS (median, mo) | p-value | |
|---|---|---|---|---|
| Age (yr) | ||||
| ≤ 60 | 9.9±2.12 | 0.003 | 24.4±3.15 | 0.139 |
| > 60 | 21.9±4.03 | |||
| Sex | ||||
| Male | 8.1±7.10 | 0.472 | 16.3±6.10 | 0.044 |
| Female | 15.4±1.69 | 33.3±6.55 | ||
| Differentiation | ||||
| Well | 20.9±5.47 | 0.055 | 34.5±11.12 | 0.318 |
| Moderate+poorly | 12.8±1.30 | 23.7±4.26 | ||
| Tumor location | ||||
| Upper | 18.3±3.64 | 0.28 | 38.3±7.94 | 0.279 |
| Middle | 14.3±3.38 | 34.5±7.03 | ||
| Lower | 12.3±2.55 | 19.0±1.82 | ||
| Clinical stage | ||||
| I-II | NR | < 0.001 | NR | 0.001 |
| III-IV | 11.6±1.13 | 23.5±2.29 | ||
| CRT response | ||||
| Responder (CR+PR) | 20.9±2.93 | < 0.001 | 38.3±4.52 | < 0.001 |
| Non responder (SD+PD) | 6.8±1.11 | 11.8±1.56 | ||
| Operation | ||||
| Yes | 21.7±5.97 | 0.239 | 56.7±15.49 | 0.14 |
| No | 14.3±1.43 | 29.8±4.79 | ||
| RT dose | ||||
| Stage I-II | ||||
| ≥ 5,000 cGy | 58.9±7.52 | 0.353 | 66.3±7.19 | 0.318 |
| < 5,000 cGy | 63.7±13.63 | 75.8±13.29 | ||
| Stage III-IV | ||||
| ≥ 5,000 cGy | 26.8±2.81 | 0.426 | 48.3±6.66 | 0.415 |
| < 5,000 cGy | 38.2±6.38 | 40.2±3.37 | ||
| Chemoregimen | ||||
| FP | 15.0±1.66 | 0.637 | 32.4±4.57 | 0.664 |
| DP | 18.0±6.86 | 34.5±9.70 | ||
| Second-line chemotherapy | ||||
| Yes | 8.8±0.82 | 0.382 | 21.6±1.99 | 0.692 |
| No | 9.0±1.14 | 17.8±2.64 | ||
| Genotype | ||||
| Stage I-II | ||||
| G/G | NR | 0.506 | NR | 0.773 |
| G/A+A/A | 44.8±9.54 | 37.9±13.25 | ||
| Stage III-IV | ||||
| G/G | 9.6±1.75 | 0.678 | 24.7±2.65 | 0.668 |
| G/A+A/A | 12.5±1.22 | 22.4±3.63 |
PFS, progression-free survival; OS, overall survival; NR, not reached until this analysis; CRT, chemoradiotherapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease, RT, radiotherapy; FP, 5-fluorouracil+cisplatin; DP, docetaxel+cisplatin.
Discussion
Treatment outcomes for various cancers have improved over recent decades. This can be attributed largely to the use of new chemotherapeutic agents, as well as enhanced technologies for surgery and radiation therapy. However, despite development of more drugs that target tumor cells, many obstacles to successful treatment remain. Accurate control of tumor cells and the tumor microenvironment is important in eradicating cancer. Based on this rationale, FGFRs have been identified as potential therapeutic targets known to be associated with drug resistance. Although FGFR1-3 has been extensively investigated [14-16], the role of FGFR4 and its potential as a therapeutic target is less understood. FGFR4 is overexpressed in human prostate, breast, colon, rhabdomyosarcoma, gastric, and hepatocellular cancers, where it may be associated with tumor progression and invasion via a variety of pathways, and with a poor prognosis [17,18]. Recent studies found that FGFR4 promotes the epithelialmesenchymal transition (EMT) in cancer cells [19,20].
Studies of FGFR polymorphisms have reported association of Arg388 with increased cancer incidence, tumor size [17,21], and recurrence after adjuvant treatment [11,12,22]. In addition, FGFR affects radiation therapy outcomes. The current study was conducted in order to assess the role of FGFR4 Arg388 in patients treated with CRT for esophageal cancer, which is treated primarily by CRT in inoperable cases. We found that patients with the Gly388 allele showed a significantly better response to CRT than Arg388 carriers, regardless of the cancer stage. Interestingly, the PFS and OS rates in early esophageal cancer (stages I and II) showed improved survival trends with the Gly388 versus the Arg388 allele. However, comparable patterns of PFS and OS in advanced esophageal cancer (stages III and IV) were observed between the groups.
In the study reported by Thussbas et al. [11] in breast cancer patients, disease-free survival with the Gly388 allele was prolonged significantly compared with the Arg388 allele when patients received adjuvant chemotherapy; however, no difference was observed among patients who underwent adjuvant endocrine therapy. Thus, FGFR4 could be involved in efficacy of chemotherapy, but not endocrine therapy. The current study suggests that FGFR4 affects the response to chemotherapy and adjuvant therapy outcomes. A possible mechanism that would fit this result is the EMT, an important step in disease recurrence after surgery [23]. In a previous report, FGFR4 was shown to participate in a complex with membrane-type 1 matrix metalloproteinase (MT1-MMP), decreasing MT1-MMP lysosomal degradation and leading to increased invasion. In the current study, we extended the focus by assessing the functional role of FGFR4 according to stage. Although the difference was not statistically significant, the pattern of survival differed according to stage, showing a tendency to be prolonged in Gly388 versus Arg388 carriers. In addition, in early esophageal cancer patients with LN invasion, significantly improved OS was observed in patients with the Gly388 allele compared to Arg388 carriers. However, this result was not observed in patients receiving palliative care for advanced-stage disease. Progression in advanced tumors is more complex than in early stage tumors through the activation of mesenchymal and epithelial signaling pathways. Thus the function of a single gene can be difficult to assess during treatment in advanced cancer. Taken together, the current data suggest that targeted therapy for FGFR4 could be more effective at earlier rather than more advanced stages of esophageal cancer, and that another treatment approach is needed for patients carrying Arg388 in order to improve the treatment outcome. Unexpectedly, a better trend of OS was observed in Gly388 with LN invasion patients compared to Gly388 without LN invasion patients. Further study with a large number of patients will be needed in order to provide additional evidence to support this result.
FGFs play important roles in the tumor microenvironment, where there is a protective niche for therapeutic escape, through the secretion of various growth factors [6]. The molecular mechanism by which the FGFR4 Arg388 polymorphism leads to a more aggressive clinical phenotype is not yet fully understood. In a colorectal cancer model, Heinzle et al. [21] showed that the FGFR4 Arg388 polymorphism induced overexpression of FGFR and that this could attenuate the response to chemotherapy [22]. A similar result was reported in a gastric cancer model showing that an FGFR4 inhibitor and 5-FU reduced proliferation and promoted apoptosis [24]. For HNSCC, the association between CRT and FGFR4 was evaluated in vitro, showing increased sensitivity of cells containing the Arg388 allele to cisplatin, which differs from our observations [12].
In contrast to various results primarily from in vitro studies, there is a lack of clear evidence for any relationship between the FGFR4 Arg388 genotype and protein expression or clinicopathological parameters in cancer patients [11,18] However, the FGFR4 Gly388 allele has been reported as a prognostic marker for survival in breast and gastric cancer patients. The lack of correlation between genotype and phenotype for FGFR4 may be explained in part by the lack of elevated tyrosine phosphorylation of FGFR4 Arg388 compared with FGFR4 Gly388, the use of different intracellular signal transduction pathway(s) or interactions with different cell surface protein(s), or linkage disequilibrium with other genetic changes that also contribute to a poor prognosis [11]. To the best of our knowledge, this is the first report describing a predictive role for FGFR4 Arg388 after CRT. The biochemical effects of FGFR4 Arg388 with regard to chemotherapy should be evaluated in future studies including other biomarkers.
Based on our results, clinical factors including early-stage disease and chemotherapy responders were favorable prognostic factors for PFS and OS in a multivariate analysis. Interestingly, patients > 60 years of age had longer PFS than younger patients. The proportions of TNM stages were similar between the two groups (p=0.324); thus, understanding the biology during aging may be another approach to improving treatment outcomes.
Conclusion
Although the FGFR4 polymorphism examined in this study is not a significant prognostic factor in esophageal cancer treated with CRT, esophageal cancer patients carrying FGFR4 Gly388 showed a good response after CRT. Especially in early esophageal cancer, the Gly388 allele resulted in increased OS and PFS, and select patients showing LN invasion showed significant prolonged OS compared to Arg388 carriers. These findings support the idea that the effect of FGFR4 on treatment outcomes after CRT differs according to stage and further prospective study is needed in order to validate these findings.
Acknowledgments
This research was sponsored by a grant (HCRI14027-21) from the Chonnam National University Hwasun Hospital Institute for Biomedical Science.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kim DE, Kim UJ, Choi WY, Kim MY, Kim SH, Kim MJ, et al. Clinical prognostic factors for locally advanced esophageal squamous carcinoma treated after definitive chemoradiotherapy. Cancer Res Treat. 2013;45:276–84. doi: 10.4143/crt.2013.45.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shim HJ, Kim DE, Hwang JE, Bae WK, Nam TK, Na KJ, et al. A phase II study of concurrent chemoradiotherapy with weekly docetaxel and cisplatin in advanced oesophageal cancer. Cancer Chemother Pharmacol. 2012;70:683–90. doi: 10.1007/s00280-012-1962-3. [DOI] [PubMed] [Google Scholar]
- 4.Kelsey CR, Chino JP, Willett CG, Clough RW, Hurwitz HI, Morse MA, et al. Paclitaxel-based chemoradiotherapy in the treatment of patients with operable esophageal cancer. Int J Radiat Oncol Biol Phys. 2007;69:770–6. doi: 10.1016/j.ijrobp.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Hong TS, Wo JY, Kwak EL. Targeted therapies with chemoradiation in esophageal cancer: development and future directions. Semin Radiat Oncol. 2013;23:31–7. doi: 10.1016/j.semradonc.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Paraiso KH, Smalley KS. Fibroblast-mediated drug resistance in cancer. Biochem Pharmacol. 2013;85:1033–41. doi: 10.1016/j.bcp.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Zhang Y, Ressler SJ, Ittmann MM, Ayala GE, Dang TD, et al. FGFR1 is essential for prostate cancer progression and metastasis. Cancer Res. 2013;73:3716–24. doi: 10.1158/0008-5472.CAN-12-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran TN, Selinger CI, Kohonen-Corish MR, McCaughan BC, Kennedy CW, O'Toole SA, et al. Fibroblast growth factor receptor 1 (FGFR1) copy number is an independent prognostic factor in non-small cell lung cancer. Lung Cancer. 2013;81:462–7. doi: 10.1016/j.lungcan.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Ma Z, Tsuchiya N, Yuasa T, Inoue T, Kumazawa T, Narita S, et al. Polymorphisms of fibroblast growth factor receptor 4 have association with the development of prostate cancer and benign prostatic hyperplasia and the progression of prostate cancer in a Japanese population. Int J Cancer. 2008;123:2574–9. doi: 10.1002/ijc.23578. [DOI] [PubMed] [Google Scholar]
- 10.Spinola M, Leoni V, Pignatiello C, Conti B, Ravagnani F, Pastorino U, et al. Functional FGFR4 Gly388Arg polymorphism predicts prognosis in lung adenocarcinoma patients. J Clin Oncol. 2005;23:7307–11. doi: 10.1200/JCO.2005.17.350. [DOI] [PubMed] [Google Scholar]
- 11.Thussbas C, Nahrig J, Streit S, Bange J, Kriner M, Kates R, et al. FGFR4 Arg388 allele is associated with resistance to adjuvant therapy in primary breast cancer. J Clin Oncol. 2006;24:3747–55. doi: 10.1200/JCO.2005.04.8587. [DOI] [PubMed] [Google Scholar]
- 12.Ansell A, Farnebo L, Grenman R, Roberg K, Thunell LK. Polymorphism of FGFR4 in cancer development and sensitivity to cisplatin and radiation in head and neck cancer. Oral Oncol. 2009;45:23–9. doi: 10.1016/j.oraloncology.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Dutra RL, de Carvalho MB, Dos Santos M, Mercante AM, Gazito D, de Cicco R, et al. FGFR4 profile as a prognostic marker in squamous cell carcinoma of the mouth and oropharynx. PLoS One. 2012;7: doi: 10.1371/journal.pone.0050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J. 2011;437:199–213. doi: 10.1042/BJ20101603. [DOI] [PubMed] [Google Scholar]
- 15.Greulich H, Pollock PM. Targeting mutant fibroblast growth factor receptors in cancer. Trends Mol Med. 2011;17:283–92. doi: 10.1016/j.molmed.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34:280–300. doi: 10.1002/med.21288. [DOI] [PubMed] [Google Scholar]
- 17.Taylor JG 6th, Cheuk AT, Tsang PS, Chung JY, Song YK, Desai K, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest. 2009;119:3395–407. doi: 10.1172/JCI39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Y, Shi Y, Zhou Y, Du C, Wang C, Zhan H, et al. The fibroblast growth factor receptor-4 Arg388 allele is associated with gastric cancer progression. Ann Surg Oncol. 2010;17:3354–61. doi: 10.1245/s10434-010-1323-6. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Li J, Xie K, Zhang T, Lei Y, Chen Y, et al. FGFR4 promotes stroma-induced epithelial-to-mesenchymal transition in colorectal cancer. Cancer Res. 2013;73:5926–35. doi: 10.1158/0008-5472.CAN-12-4718. [DOI] [PubMed] [Google Scholar]
- 20.Pelaez-Garcia A, Barderas R, Torres S, Hernandez-Varas P, Teixido J, Bonilla F, et al. FGFR4 role in epithelial-mesenchymal transition and its therapeutic value in colorectal cancer. PLoS One. 2013;8: doi: 10.1371/journal.pone.0063695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinzle C, Gsur A, Hunjadi M, Erdem Z, Gauglhofer C, Stattner S, et al. Differential effects of polymorphic alleles of FGF receptor 4 on colon cancer growth and metastasis. Cancer Res. 2012;72:5767–77. doi: 10.1158/0008-5472.CAN-11-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon MA, Gil J, Lu B, Zhang W, Yang D, Yun J, et al. Genomic profiling associated with recurrence in patients with rectal cancer treated with chemoradiation. Pharmacogenomics. 2006;7:67–88. doi: 10.2217/14622416.7.1.67. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama N, Varjosalo M, Meller P, Lohi J, Chan KM, Zhou Z, et al. FGF receptor-4 (FGFR4) polymorphism acts as an activity switch of a membrane type 1 matrix metalloproteinase-FGFR4 complex. Proc Natl Acad Sci U S A. 2010;107:15786–91. doi: 10.1073/pnas.0914459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye YW, Hu S, Shi YQ, Zhang XF, Zhou Y, Zhao CL, et al. Combination of the FGFR4 inhibitor PD173074 and 5-fluorouracil reduces proliferation and promotes apoptosis in gastric cancer. Oncol Rep. 2013;30:2777–84. doi: 10.3892/or.2013.2796. [DOI] [PubMed] [Google Scholar]


