Abstract
Purpose
Anorectal malignant melanomas (AMM) are rare and have poor survival. The study aims to evaluate the clinicopathologic characteristics and outcomes of patients with AMM, and to devise a staging system predictive of survival outcome.
Materials and Methods
This was a retrospective study of 28 patients diagnosed with, and treated for AMM. Patients classified by clinical staging of mucosal melanoma (MM) were reclassified via rectal and anal TNM staging. Survival outcomes were compared among patients grouped by the three different staging systems.
Results
The three staging systems were equated with similar figures for 5-year overall survival (OS) and 5-year disease-free survival (DFS) of patients diagnosed with stage I disease. Patients (n=19) diagnosed with MM stage II disease were reclassified by rectal TNM staging into three subgroups: IIIA, IIIB, and IIIC. For these patients, both 5-year OS and 5-year DFS differed significantly between the subgroups IIIA and IIIC (OS: IIIA vs. IIIC, 66.7% vs. 0%, p=0.002; DFS: IIIA vs. IIIC, 51.4% vs. 0%, p < 0.001).
Conclusion
The accuracy of prognosis in patients diagnosed with AMM and lymph node metastasis has improved by using rectal TNM staging, which includes information regarding the number of lymph node metastases.
Keywords: Anus canal, Rectum, Melanoma, Neoplasm staging
Introduction
Primary malignant melanomas of the anus and rectum are rare neoplasms with aggressive behavior, accounting for 0.1%-4.6% of anal canal tumors [1]. Mucosal melanomas (MMs) account for approximately 1.2% of all melanomas, of which, fewer than 25% are anorectal [2]. The 5-year survival rate for anorectal malignant melanomas (AMM) was reported to be as low as < 20%, in contrast to the value of approximately 80% for cutaneous melanomas [2]. Furthermore, up to 67% of patients are found to have distant metastases at the time of their initial diagnosis with AMM [3].
Due to its rarity in incidence and diagnostic variability, misdiagnosis of AMM is common [4]; currently, there is no pathologic staging system specific to the disease. Accurate tumor staging at the time of diagnosis is essential for determining both prognosis and treatment. Several retrospective studies have suggested a clinical staging system for mucosal malignant melanomas, namely, stage I as localized disease only, stage II as regional lymph node (LN) involvement, and stage III as distant metastases [5-7]. Two alternatives, based on the 7th American Joint Committee on Cancer (AJCC) staging system, might be applicable to AMM: tumor node metastasis (TNM) staging of rectal cancer (rectal TNM), and of anal canal cancer (anal TNM). Rectal TNM is based on the depth of tumor invasion into or beyond the wall of the rectum (T), number of regional lymph nodes involved (N), and status of distant metastasis (M). Anal TNM differs from rectal TNM in terms of tumor size (T) and status of regional or systemic LN involvement (N) [8]. Given the rarity of AMM, most studies have been exclusively confined to clinical outcomes [9-12].
This study aimed to evaluate the clinicopathologic characteristics and survival outcome of patients with AMM who underwent surgery. Additionally, we compared the survival rates of AMM patients grouped in accordance to three different staging systems to identify a staging system that most efficiently predicted the outcome.
Materials and Methods
1. Patients
Patients who were diagnosed with and treated for AMM at the Asan Medical Center (Seoul, Korea) were enrolled for this retrospective case-series analysis, between June 1989 and July 2013. A total of 29 patients were recruited; however, one patient with systemically fulminant metastases, who was treated by colostomy alone, was excluded from the study. Therefore, a total of 28 patients were finally included, and their medical records and archived tissues were reviewed and re-examined, respectively.
The clinical variables obtained were age, gender, clinical symptoms and signs, operation type, presence or absence of adjuvant treatment, and follow-up features. The pathologic variables examined were tumor size, depth of tumor invasion, LN status, lympho-vascular invasion (LVI), peri-neural invasion (PNI), and status of amelanosis. Individual surgeons, based on each patient’s clinical features and preoperative imaging studies, determined the type of operation at the time of diagnosis. Five patients with no evidence of LN involvement from imaging studies received local excision (LE) alone, and their LN status was considered to be as N0.
A total of 28 patients who were classified by clinical staging of MM were reclassified by rectal and anal TNM, according to the 7th AJCC staging system. The 5-year overall survival (OS) and disease-free survival (DFS) were determined for all patients. The ability of rectal and anal TNM staging to predict survival was assessed by comparing the 5-year OS and DFS figures for patients grouped by these systems against OS and DFS figures for patients grouped according to a simple stage system for MM staging.
Patients received postoperative follow-up for at least 5 years, including history-taking, physical examination, complete blood counts, blood chemistry, and plain chest radiography every 3 months for the first 2 years and every 6 months thereafter. In addition, patients were evaluated by abdominopelvic computed tomography (CT) and/or magnetic resonance imaging (MRI) every 6 months, and chest CT every 6 or 12 months in accordance to the patient’s condition. Colonofiberscopy was performed at 6-12 months after surgery and then every 2-3 years. Recurrence was generally determined by abdominopelvic CT or MRI, and concurrently proven by CT–positron emission tomography and biopsy whenever possible. Recurrence was defined as either local or metastatic disease detected by pathologic evidence or imaging studies showing sequential enlargement of the tumor during follow-up period.
Adjuvant treatments were comprised of chemotherapy, immunotherapy, or radiotherapy. Cisplatin-based chemotherapy was used in our institution for adjuvant chemotherapy, and most of those who received adjuvant chemotherapy received combination chemotherapy, using cisplatin, vincristine, or dacarbazine. For immunotherapy, interferon-α as single agent was administered to three patients in our institution by subcutaneous injection. One patient received adjuvant radiotherapy, with a total radiation dose of 50 Gy in 25 fractions over a month.
The study protocol was approved by the Institutional Review Board of Asan Medical Center (registration No. 2014-0909), in accordance with the Declaration of Helsinki.
2. Pathologic evaluation
Histological features were reviewed by two pathologists (J.R. and C.S.P.). Staining of biopsy samples or resected specimens, taken at the time of diagnosis, was carried out with at least one of the following immunohistochemical (IHC) stains: S100 (1:200, Zymed, San Francisco, CA), Melan A (1:50, Novo, Newcastle upon Tyne, UK), SOX 10 (1:25, Zymed), or human melanoma black 45 (HMB45; 1:50, Dako, Glostrup, Denmark). All patients were sub-classified as having either amelanotic or melanotic melanoma; patients with no melanin pigments in gross examination and no, or sparse, pigments in histologic examination were categorized as having amelanotic melanoma (Fig. 1).
Fig. 1.
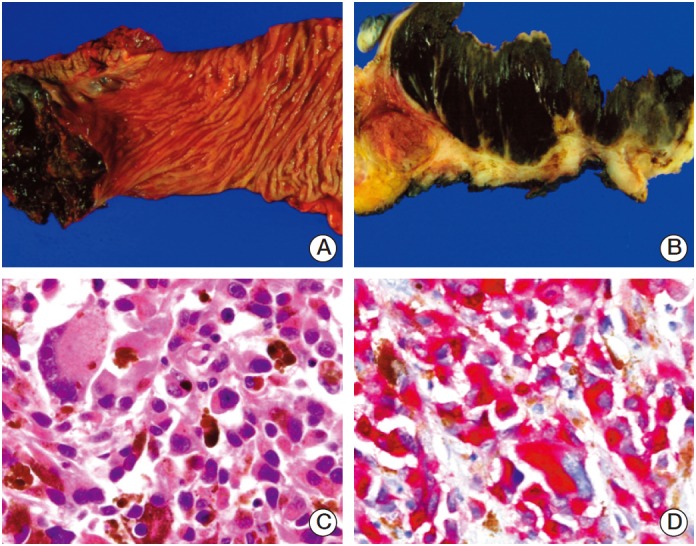
Representative images of anorectal melanoma. (A) Case 25 shows 7.3-cm-sized well-demarcated black ulcerofungating mass in the rectum. (B) The cut surface of the mass is homogeneously black and soft. It extends to the proper muscle layer and invades dentate line. (C) Microscopically, tumor contains highly anaplastic discohesive cells without specific growth pattern. Individual cells have abundant acidophilic, finely granular dense cytoplasm. Prominent macronucleoli is also identified. Occasionally, multinucleated giant cells and cytoplasmic melanin pigments are present (H&E staining, ×400). (D) Immunohistochemically, tumor cells are reactive for Melan-A (×400).
IHC staining was performed on selected formalin-fixed, paraffin-embedded tissue blocks. Each stain was carried out on an auto-immune stainer (Benchmark XT, Ventana Medical Systems, Tucson, AZ) in accordance to the manufacturer’s instructions. In brief, sections of 4 μm were mounted on silanized charged slides and allowed to dry for 10 minutes at room temperature, followed by 20 minutes in an incubator at 65°C. After deparaffinization, heat-induced epitope retrieval, using standard Cell Conditioning 1, was performed for 24 minutes. Subsequently, primary anti-S100 (1:200, Zymed), anti-Melan A (1:50, Novo), anti-SOX 10 (1:25, Zymed), and HMB45 (1:50, Dako) were labeled using an automated immune staining system, using a detection kit (Ventana Medical Systems). Immune-stained sections were counter-stained with hematoxylin, dehydrated in ethanol, and cleared in xylene (Fig. 1).
3. Statistical methods
The patient groups were compared using a chi-square test for discrete variables and unpaired Student’s t tests for continuous variables. OS and DFS curves were plotted using a Kaplan-Meier method and compared using the log rank test. Statistical significance was defined as p < 0.05, and all analyses were performed using SPSS ver. 21 (SPSS Inc., Chicago, IL).
Results
1. Clinico-pathologic variables
The incidence of AMM was 0.15% (28 patients) among the 18,784 colorectral malignancies treated at the Asan Medical Center during the study period. The median age at diagnosis was 57 (interquartile range [IQR], 38 to 71 years), and the female-to-male ratio was 1:0.47. The most common symptoms encountered were hematochezia (50%), difficult passage of stool (18%), and bowel habit change and anal pain (both 11%), in descending order of frequency. The median duration of symptoms before surgery was 5 (IQR, 2 to 7) months. For operative types, LE was performed in five patients (18%) and abdomino-perineal resection (APR) in 23 patients (82%). Twelve patients (43%) received adjuvant treatment after their operation. The median follow-up period was 42 months (IQR, 7 to 81 months) (Table 1).
Table 1.
Demographic and clinical findings
| Feature | No. of patients (%) |
|---|---|
| Sex (female:male) | 19:9 (67.9:32.1) |
| Median age (IQR, yr) | 57 (38-71) |
| Clinical symptoms and signs | |
| Hematochezia | 14 (50.0) |
| Difficult passage of stool | 5 (17.9) |
| Bowel habit change | 3 (10.7) |
| Anal pain | 3 (10.7) |
| Anal mass | 1 (3.6) |
| Incontinence | 1 (3.6) |
| Incidental finding | 1 (3.6) |
| Symptom duration (mo) | 5 (0-12) |
| Operative type | |
| WLE | 5 (17.9) |
| APR | 23 (82.1) |
| Adjuvant treatment | |
| Chemotherapy | 8 (28.6) |
| Immunotherapy | 2 (7.1) |
| Chemotherapy+immunotherapy | 1 (3.6) |
| Radiotherapy | 1 (3.6) |
| Follow-up duration (mo) | 42 (7-81) |
IQR, interquartile range; WLE, wide local excision; APR, abdominoperineal resection.
The median size of the tumor was 4.3 cm (IQR, 2.1 to 5.1 cm). Amelanotic melanoma was found in nine patients (32%). The presence of LVI and PNI was identified in seven (25%) and two patients (7%), respectively (Table 2). According to a simple staging system for mucosal malignant melanomas (MM stage), nine patients (32%) and 19 patients (68%) were diagnosed as stage I and II, respectively (Table 2). Among the former, nine patients with MM stage I disease, reclassification by AJCC-based anal TNM stage indicated that five of these patients were at stage II; by contrast, reclassification by rectal TNM stage was consistent. Among the 19 patients with MM stage II disease, reclassification by anal TNM stage indicated that 15 patients were at stage IIIA and four patients at stage IIIB; whereas, reclassification in accordance to rectal TNM stage indicated that seven patients were at stage IIIA, 10 patients were at stage IIIB, and two patients were at stage IIIC (Fig. 2).
Table 2.
Pathologic characteristics and reclassified by rectal TNM and anal TNM systems of 28 patients diagnosed with anorectal malignant melanoma
| Feature | No. of patients (%) |
|---|---|
| Median tumor size (IQR, cm) | 4.3 (0.2-13.5) |
| Amelanosis | 9 (32.1) |
| LVI | |
| Presence | 7 (25.0) |
| Absence | 21 (75.0) |
| PNI | |
| Presence | 2 (7.1) |
| Absence | 26 (92.9) |
| Mucosal melanoma stage | |
| Stage I | 9 (32.1) |
| Stage II | 19 (67.9) |
| T category of rectal TNM | |
| T1 | 12 (42.9) |
| T2 | 9 (32.1) |
| T3 | 6 (21.4) |
| T4 | 1 (3.6) |
| N category of rectal TNM | |
| N0 | 9 (32.1) |
| N1 | 11 (39.3) |
| N2 | 8 (28.6) |
| Stage of rectal TNM | |
| Stage I | 8 (28.6) |
| Stage II | 1 (3.6) |
| Stage III | 19 (67.9) |
| T category of anal TNM | |
| T1 | 6 (21.4) |
| T2 | 12 (42.9) |
| T3 | 9 (32.1) |
| T4 | 1 (3.6) |
| N category of anal TNM | |
| N0 | 9 (32.1) |
| N1 | 16 (57.1) |
| N2 | 2 (7.1) |
| N3 | 1 (3.6) |
| TNM stage of anal TNM | |
| Stage I | 4 (14.3) |
| Stage II | 5 (17.9) |
| Stage III | 19 (67.9) |
IQR, interquartile range; LVI, lympho-vascular invasion; PNI, peri-neural invasion; rectal TNM, tumor node metastasis (TNM) staging of the rectal cancer based on the 7th American Joint Committee on Cancer (AJCC) staging system; anal TNM, TNM staging of the anal cancer based on the 7th AJCC staging system.
Fig. 2.
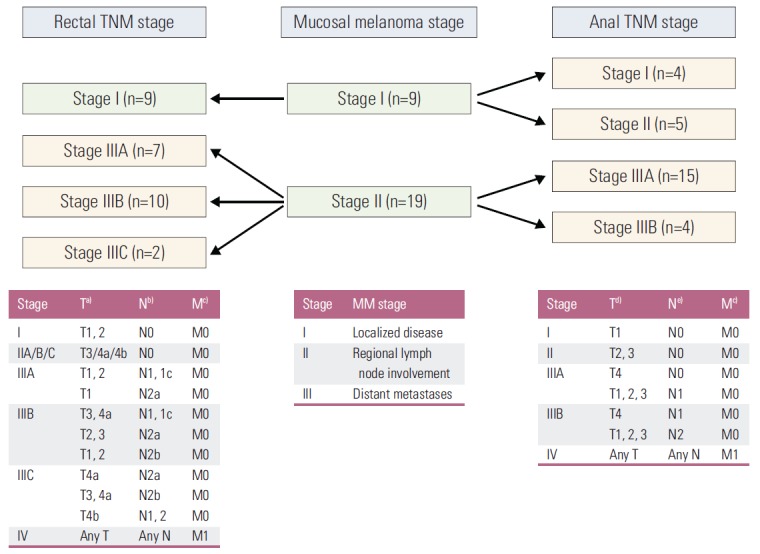
Detailed changes in 28 patients with clinical stage I or stage II anorectal malignant melanoma, as classified by rectal TNM and anal TNM. Rectal TNM, tumor node metastasis (TNM) staging of rectal cancer based on the 7th American Joint Committee on Cancer (AJCC) staging system; anal TNM, TNM staging of anal cancer based on the 7th AJCC staging system. a)T1 invades submucosa, T2 invades muscularis propria, T3 invades into perirectal tissues, T4a penetrates to the surface of the visceral peritoneum, T4b invades to other organs, b)N1a, metastasis in 1 regional lymph nodes; N1b, metastasis in 2-3 regional lymph nodes; N1c, tumor deposit; N2a, metastasis in 4-6 regional lymph nodes; N2b, metastasis in 7 or more regional lymph nodes, c)M, distant metastasis, d)T1 < 2 cm; T2 ≥ 2 cm, < 5 cm; T3 ≥ 5 cm; T4, invades other organ, e)N1, metastasis in perirectal lymph node; N2, metastasis in unilateral internal iliac and/or inguinal lymph node; N3, metastasis in perirectal and inguinal lymph node and/or bilateral internal iliac and/or inguinal lymph nodes.
2. Survival
The 5-year OS in patients grouped by MM stage was 88±12% (mean±standard error of mean) for stage I and 43±12% for stage II, and the 5-year DFS was 64±17% and 39±12%, respectively. The figures for 5-year OS and DFS for patients at rectal TNM stage I or III at diagnosis were the same as the respective figures for patients at MM stage I or II at diagnosis. The 5-year OS was 67±27% for anal TNM stage I, 100% for anal TNM stage II, and 43±12% for anal TNM stage III at diagnosis, and the 5-year DFS was 75±21.7% for anal TNM stage I, 60±21.9% for anal TNM stage II, and 38.8±11.8% for anal TNM stage III at diagnosis.
Patients with MM stage I disease (n=9) were divided into two stages by anal TNM, and the 5-year OS and DFS were compared between the two stages: there was no significant difference in survival between the two stages (Fig. 3). Patients with MM stage II (n=19) were reclassified by rectal TNM into three subgroups and were reclassified by anal TNM into two subgroups (Fig. 3). There was no significant difference in either 5-year OS or DFS between patients classified as anal TNM stage IIIA and stage IIIB (OS: stage IIIA, 38±13% vs. stage IIIB, 50±25%, p=0.7; DFS: stage IIIA, 44±13% vs. stage IIIB, 38±29%, p=0.88). By contrast, the 5-year OS for rectal TNM stage III patients varied between subgroups IIIC and either IIIA (p=0.002) or IIIB (p=0.07), but not between subgroups IIIA and IIIB (p=0.21) (67±19% for stage IIIA, 35±16% for stage IIIB, and 0% stage IIIC). Similarly, the 5-year DFS for rectal TNM stage III patients varied between the three subgroups, with significant differences between stage IIIC and either IIIA (p < 0.001) or IIIB (p < 0.001), but not between IIIA and IIIB (p=0.41) (51±20.4% for stage IIIA, 38±16% for stage IIIB, and 0% for stage IIIC) (Fig. 3).
Fig. 3.
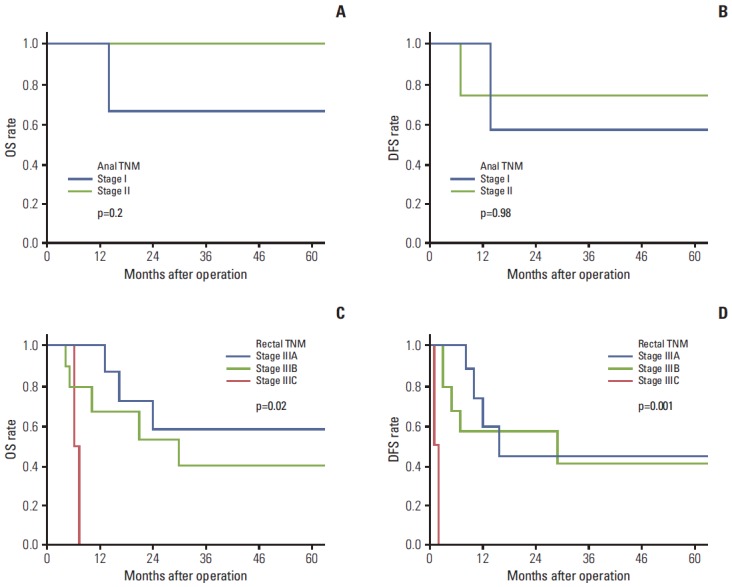
(A-D) Overall survival (OS) (A) and disease-free survival (DFS) (B) of patients with local disease (malignant melanoma [MM] stage I) grouped by anal TNM after converting MM stage to anal TNM, OS (C) and DFS (D) of patients with regional disease (MM stage II) grouped by rectal TNM after converting MM stage to rectal TNM. Anal TNM, tumor node metastasis (TNM) staging of anal cancer based on the 7th American Joint Committee on Cancer (AJCC) staging system; rectal TNM, TNM staging of rectal cancer based on the 7th AJCC staging system.
The 5-year OS and DFS for MM stage I were 75±22% and 27±23%, respectively, in patients who were treated with LE and 100% for both in patients treated with APR (p=0.31 and p=0.05 for 5-year OS and DFS, respectively, following LE group vs. APR group) (Fig. 4). One patient in the LE group had local recurrence and underwent subsequent APR; at the study period, the patient had been free of recurrence for 14 months. Two patients in the LE group developed distant metastases. Of 19 MM stage II patients, seven patients (37%) underwent adjuvant chemotherapy (cisplatin-based in 2 patients or dacarbazine-based chemotherapy in 5 patients) and two patients (10.5%) received immunotherapy (Table 3). There was no significant difference in 5-year OS and DFS between the nine patients that received adjuvant chemo- or immunotherapy and the 10 patients without therapy (p=0.82 and p=0.43, respectively).
Fig. 4.
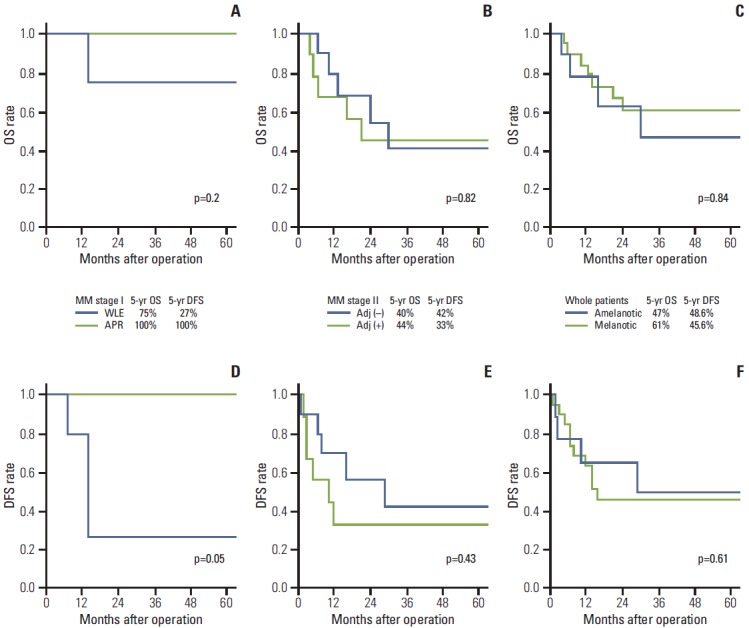
(A-F) Overall survival (OS) and disease-free survival (DFS) according to the operative type, whether received adjuvant treatment or not, and type of melanosis. The 5-year OS (A) and the 5-year DFS (D) of patients with local disease (malignant melanoma [MM] stage I) grouped by operative type (wide local excision [WLE] vs. abdominoperineal resection [APR]), the 5-year OS (B) and the 5-year DFS (E) of patients with regional disease (MM stage II) grouped by adjuvant therapy (received adjuvant chemotherapy or immunotherapy, adj [+] vs. not received adjuvant therapy, adj [–]), the 5-year OS (C) and the 5-year DFS (F) of patients with malignant anorectal melanoma grouped by melanosis (amelanotic melanoma vs. melanotic melanoma).
Table 3.
Clinico-pathologic features of each patient participating in the study
| No. | Age (yr) | Sex | Chief complaint | Op | F/U | Amelanosis | MM stage | Rectal TNM | Anal TNM | Adjuvant Tx | Last status | 1st Rec site |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | F | Routine exam | WLE | 78 | No | 1 | I (T1N0M0) | I (T1N0M0) | No | NED | - |
| 2 | 39 | F | Hematochezia | APR | 9 | No | 2 | IIIB (T3N2aM0) | IIIB (T3N3M0) | No | Death | Hepatic |
| 3 | 58 | M | Hematochezia | APR | 13 | No | 2 | IIIA (T2N1bM0) | IIIA (T3N1M0) | No | Death | Brain |
| 4 | 50 | M | Hematochezia | APR | 4 | Yes | 2 | IIIB (T1N2bM0) | IIIA (T2N1M0) | CTx | Death | Bone |
| 5 | 63 | F | Difficult defecation | APR | 128 | Yes | 2 | IIIA (T1N1aM0) | IIIA (T3N1M0) | CTx | NED | - |
| 6 | 69 | F | Difficult defecation | APR | 6 | No | 1 | I (T1N0M0) | II (T3N0M0) | CTx | Death s Rec | - |
| 7 | 66 | F | Hematochezia | APR | 6 | No | 2 | IIIC (T3N2bM0) | IIIA (T3N1M0) | No | Death | Local |
| 8 | 54 | F | Anal mass | APR | 31 | Yes | 2 | IIIIB (T3N2aM0) | IIIA (T3N1M0) | No | Death | Hepatic |
| 9 | 58 | F | Hematochezia | APR | 8 | Yes | 2 | IIIC (T4N2aM0) | IIIB (T4N1M0) | CTx | Death | Multiple |
| 10 | 60 | F | Difficult defecation | APR | 16 | Yes | 2 | IIIA (T3N1aM0) | IIIA (T2N1M0) | CTx | Death | Multiple |
| 11 | 38 | M | Difficult defecation | APR | 146 | No | 2 | IIIA (T1N1bM0) | IIIA (T2N1M0) | CTx | NED | - |
| 12 | 66 | F | Difficult defecation | APR | 18 | No | 2 | IIIB (T3N2aM0) | IIIA (T3N1M0) | CTx | Death | Local |
| 13 | 42 | M | Hematochezia | WLE | 9 | No | 1 | I (T1N0M0) | I (T1N0M0) | No | Death | Hepatic |
| 14 | 41 | M | Anal pain | APR | 118 | No | 2 | IIIB (T2N1bM0) | IIIA (T2N1M0) | CTx | NED | - |
| 15 | 63 | F | Hematochezia | APR | 23 | No | 2 | IIIA (T1N1bM0) | IIIA (T2N1M0) | No | Death | Seeding |
| 16 | 55 | F | Hematochezia | APR | 44 | No | 2 | IIIA (T1N1bM0) | IIIA (T1N1M0) | IFN-α | Alive c Rec | distant LN |
| 17 | 69 | F | Hematochezia | APR | 116 | Yes | 1 | I (T1N0M0) | I (T1N0M0) | IFN-α | NED | |
| 18 | 69 | M | Incontinence | APR | 4 | No | 2 | IIIB (T3N1bM0) | IIIA (T2N1M0) | IFN-α | Death s Rec | - |
| 19 | 53 | F | Hematochezia | APR | 106 | Yes | 2 | IIIA (T2N1bM0) | IIIA (T1N1M0) | No | NED | - |
| 20 | 62 | F | Bowel habit change | WLE | 90 | No | 1 | I (T1N0M0) | II (T2N0M0) | No | Alive c Rec | Local |
| 21 | 50 | F | Bowel habit change | APR | 83 | No | 1 | I (T2N0M0) | II (T2N0M0) | No | NED | - |
| 22 | 61 | F | Anal pain | WLE | 38 | No | 1 | I (T2N0M0) | II (T2N0M0) | No | Alive c Rec | Multiple |
| 23 | 51 | F | Anal pain | WLE | 4 | Yes | 1 | I (T1N0M0) | I (T1N0M0) | No | NED | - |
| 24 | 71 | F | Hematochezia | APR | 29 | No | 2 | IIIB (T2N2bM0) | IIIB (T2N2M0) | RTx | NED | - |
| 25 | 50 | M | Hematochezia | APR | 28 | No | 2 | IIIB (T3N1aM0) | IIIA (T3N1M0) | No | NED | - |
| 26 | 55 | M | Bowel habit change | APR | 14 | No | 1 | I (T1N0M0) | II (T2N0M0) | No | NED | - |
| 27 | 61 | F | Hematochezia | APR | 1 | Yes | 2 | IIIB (T2N2aM0) | IIIA (T2N1M0) | No | NED | - |
| 28 | 71 | M | Hematochezia | APR | 7 | No | 2 | IIIA (T2N1bM0) | IIIB (T3N2M0) | No | NED | - |
Op, operation; F/U, follow-up, months; MM stage, clinical stage of mucosal melanoma; rectal TNM, tumor node metastasis (TNM) staging of rectal cancer based on the 7th American Joint Committee on Cancer (AJCC) staging system; anal TNM, TNM staging of anal cancer based on the 7th AJCC staging system; Tx, treatment; Rec, recurrence; WLE, wide local excision; NED, no evidence of disease; APR, abdomino-perineal resection; CTx, chemotherapy; Death s Rec, death without recurrence; LN, distant lymph node involvement; IFN-α, interferon-α; Alive c Rec, alive with recurrence; RTx, radiotherapy.
There was no significant difference in 5-year OS and DFS between the melanotic melanoma group and amelanotic melanoma group (OS: 61±12% vs. 47±19%, p=0.84; DFS: 45.6±11.7% vs. 48.6±18.7%, p=0.61).
Discussion
The current study showed that the survival of patients, identified by MM staging as having AMM with LN involvement, differed according to their respective rectal TNM staging (Fig. 3). The MM and rectal TNM staging systems differ in their consideration of regional LN involvement (N category); in other words, rectal TNM stage III is subdivided in accordance to the number of LN metastases (Fig. 2). The Surveillance, Epidemiology, and End Results (SEER) database analyzed 126 patients from 1973 to 2001 with anal melanoma and concluded that the extent of disease correlated with OS [13]. A recent study showed that lymphatic metastasis had prognostic significance and that selective lymphadenectomy was reasonable [7]. Taken together with the current study, it can be inferred that the prognosis of AMM presenting at an advanced stage is better predicted by including the information regarding the number of regional LN involvement.
The current study found that the incidence of AMM was as low as 0.15% of all colorectal malignancies treated at the tertiary referral hospital in the same period, similarly reported in the other studies [14-16]. The female predominance of approximately 2:1 in the current study was similar to that reported elsewhere [16-18], with the exception of an earlier study that indicates no gender preference through a report of 12 patients and an analysis of 255 additional cases from literature review [19]. The median age at diagnosis in the current study (57 years) was similar to that reported by some studies [17,19], but lower than the median age of 72 years reported by the other study [16]. Hematochezia was the most frequent symptom in the current study, similar to other reports in the literatures [16-18]; indeed this symptom is sometimes mistaken for hemorrhoidal disease. The poor survival associated with anorectal melanoma may be explained by its tendency for late diagnosis. The median duration of symptoms before diagnosis was 5 months in the current study, similar to previously published reports (4-5 months) [20], indicating that early diagnosis might be improved if more attention was paid to this non-specific symptom.
The incidence of amelanotic melanoma in the current study (32%) was somewhat higher than that in the previously published reports (12%-28%) [16,17,21]. The amelanotic melanoma was reported to have a worse prognosis than the melanotic melanoma, because it is either more difficult to detect or possibly more invasive in nature [21]. The histological distinction between amelanotic melanoma and anaplastic squamous cell carcinoma is subtle [20]. In the current study, there was no significant difference in 5-year OS and DFS between the amelanotic melanoma group and melanotic melanoma group. By contrast, one study concluded that amelanotic melanomas carried a worse prognosis than melanotic melanomas [20].
In the current study, MM stage I (local disease) was reclassified into anal TNM stage I and II, and the 5-year OS and DFS between anal TNM stage I and II were not significantly different. The current study could not provide sufficient evidence that tumor size was a prognostic indicator for AMM, as the two staging system differed in tumor size with respect to T category. However, a previous study that included 33 patients suggested that there was a correlation between OS and tumor size: of 33 patients, two patients were characterized by a tumor size of less than 2 cm in diameter and were long-term survivors [18]. Therefore, tumor size as a prognostic parameter for AMM remains to be determined, requiring accumulation of more data on this rare malignancy.
This study indicated that 5-year OS in patients diagnosed with local disease (stage I) was not significantly different whether patients were treated LE or APR (p=0.32); however, 5-year DFS tended to be different (p=0.05). This contrasts with previously published case-series, which reported no difference between wide LE and APR, although APR has been considered more effective to control the local disease [1,22]. In addition, a recent study from an analysis of SEER did not reveal a survival difference in patients treated with LE compared with APR [7]. However, the survival outcomes of this study would suggest that selection of surgical treatment in patients with localized AMM was important in terms of recurrence. The optimal treatment approach for AMM remains the subject for debate.
A total of 12 patients in the current study received adjuvant treatment using chemotherapy, immunotherapy, and radiotherapy. The current study showed that adjuvant treatment did not improve survival. The role of chemotherapy, immunotherapy, and radiotherapy in the treatment of AMM remains controversial [23]. Several regimens have been recommended, including dacarbazine, levamisole, and interferon α, but none have shown survival benefit for cutaneous melanoma [23]. A previous study showed that radiotherapy for AMM improved local control in three patients [24]. Most series for adjuvant treatment have been related to the outcome of cutaneous melanoma; therefore, the efficacy of adjuvant treatment for AMM requires further study.
The current study has a number of limitations that should be considered when interpreting the results. As is commonly pointed out, retrospective observational studies conducted at a single-center are potentially liable to referral and selection bias. Additionally, the cohort was small and confined to patients without distant metastases. This may explain why the current study demonstrated a favorable survival outcome compared with previous studies [1,7,10,12]. Finally, patients received various regimens of adjuvant treatment, meaning that comparison between individual regimens was not possible.
Conclusion
The accuracy of prognosis in patients diagnosed with stage III AMM would be improved by using the rectal TNM staging system, which includes information about the number of LN metastases. The optimal surgical treatment between APR and LE remains unclear, as does the choice of adjuvant treatment. Considering the rarity of AMM, a multicenter study is urgently needed to validate the prognostic markers and efficient staging system to optimize adequate treatment.
Acknowledgments
This study was supported by grants (to J.C. Kim) from the Asan Institute for Life Sciences (2013-069 and 9-490), the Korea Research Foundation (2013R1A2A1A03070986), Ministry of Science, ICT, and Future Planning, and the Korea Health 21 R&D Project (HI06C0868 and HI13C1750), Ministry of Health, Welfare, and Family Affairs, Republic of Korea. We gratefully appreciate for supervising the review of pathologic slide by Prof. Chan-Sik Park.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Belbaraka R, Elharroudi T, Ismaili N, Fetohi M, Tijami F, Jalil A, et al. Management of anorectal melanoma:report of 17 cases and literature review. J Gastrointest Cancer. 2012;43:31–5. doi: 10.1007/s12029-010-9216-2. [DOI] [PubMed] [Google Scholar]
- 2.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–78. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Falch C, Stojadinovic A, Hann-von-Weyhern C, Protic M, Nissan A, Faries MB, et al. Anorectal malignant melanoma: extensive 45-year review and proposal for a novel staging classification. J Am Coll Surg. 2013;217:324–35. doi: 10.1016/j.jamcollsurg.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387–402. doi: 10.1046/j.1365-2559.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- 5.Roumen RM. Anorectal melanoma in The Netherlands: a report of 63 patients. Eur J Surg Oncol. 1996;22:598–601. doi: 10.1016/s0748-7983(96)92346-x. [DOI] [PubMed] [Google Scholar]
- 6.Kubo K, Fujiyoshi T, Yokoyama MM, Kamei K, Richt JA, Kitze B, et al. Lack of association of Borna disease virus and human T-cell leukemia virus type 1 infections with psychiatric disorders among Japanese patients. Clin Diagn Lab Immunol. 1997;4:189–94. doi: 10.1128/cdli.4.2.189-194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iddings DM, Fleisig AJ, Chen SL, Faries MB, Morton DL. Practice patterns and outcomes for anorectal melanoma in the USA, reviewing three decades of treatment: is more extensive surgical resection beneficial in all patients? Ann Surg Oncol. 2010;17:40–4. doi: 10.1245/s10434-009-0705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talsma K, van Hagen P, Grotenhuis BA, Steyerberg EW, Tilanus HW, van Lanschot JJ, et al. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM Classification for Esophageal Cancer. Ann Surg Oncol. 2012;19:2142–8. doi: 10.1245/s10434-012-2218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward MW, Romano G, Nicholls RJ. The surgical treatment of anorectal malignant melanoma. Br J Surg. 1986;73:68–9. doi: 10.1002/bjs.1800730127. [DOI] [PubMed] [Google Scholar]
- 10.Antoniuk PM, Tjandra JJ, Webb BW, Petras RE, Milsom JW, Fazio VW. Anorectal malignant melanoma has a poor prognosis. Int J Colorectal Dis. 1993;8:81–6. doi: 10.1007/BF00299333. [DOI] [PubMed] [Google Scholar]
- 11.Ishizone S, Koide N, Karasawa F, Akita N, Muranaka F, Uhara H, et al. Surgical treatment for anorectal malignant melanoma: report of five cases and review of 79 Japanese cases. Int J Colorectal Dis. 2008;23:1257–62. doi: 10.1007/s00384-008-0529-6. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson PJ, Ragnarsson-Olding BK. Importance of clear resection margins in anorectal malignant melanoma. Br J Surg. 2010;97:98–103. doi: 10.1002/bjs.6784. [DOI] [PubMed] [Google Scholar]
- 13.Podnos YD, Tsai NC, Smith D, Ellenhorn JD. Factors affecting survival in patients with anal melanoma. Am Surg. 2006;72:917–20. [PubMed] [Google Scholar]
- 14.Cagir B, Whiteford MH, Topham A, Rakinic J, Fry RD. Changing epidemiology of anorectal melanoma. Dis Colon Rectum. 1999;42:1203–8. doi: 10.1007/BF02238576. [DOI] [PubMed] [Google Scholar]
- 15.Luna-Perez P, Rodriguez DF, Macouzet JG, Labastida S. Anorectal malignant melanoma. Surg Oncol. 1996;5:165–8. doi: 10.1016/s0960-7404(96)80039-9. [DOI] [PubMed] [Google Scholar]
- 16.Weinstock MA. Epidemiology and prognosis of anorectal melanoma. Gastroenterology. 1993;104:174–8. doi: 10.1016/0016-5085(93)90849-8. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Izhak O, Levy R, Weill S, Groisman G, Cohen H, Stajerman S, et al. Anorectal malignant melanoma. A clinicopathologic study, including immunohistochemistry and DNA flow cytometry. Cancer. 1997;79:18–25. doi: 10.1002/(sici)1097-0142(19970101)79:1<18::aid-cncr4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 18.Goldman S, Glimelius B, Pahlman L. Anorectal malignant melanoma in Sweden. Report of 49 patients. Dis Colon Rectum. 1990;33:874–7. doi: 10.1007/BF02051925. [DOI] [PubMed] [Google Scholar]
- 19.Cooper PH, Mills SE, Allen MS., Jr Malignant melanoma of the anus: report of 12 patients and analysis of 255 additional cases. Dis Colon Rectum. 1982;25:693–703. doi: 10.1007/BF02629543. [DOI] [PubMed] [Google Scholar]
- 20.Pessaux P, Pocard M, Elias D, Duvillard P, Avril MF, Zimmerman P, et al. Surgical management of primary anorectal melanoma. Br J Surg. 2004;91:1183–7. doi: 10.1002/bjs.4592. [DOI] [PubMed] [Google Scholar]
- 21.Brady MS, Kavolius JP, Quan SH. Anorectal melanoma. A 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum. 1995;38:146–51. doi: 10.1007/BF02052442. [DOI] [PubMed] [Google Scholar]
- 22.Yap LB, Neary P. A comparison of wide local excision with abdominoperineal resection in anorectal melanoma. Melanoma Res. 2004;14:147–50. doi: 10.1097/00008390-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Bullard KM, Tuttle TM, Rothenberger DA, Madoff RD, Baxter NN, Finne CO, et al. Surgical therapy for anorectal melanoma. J Am Coll Surg. 2003;196:206–11. doi: 10.1016/S1072-7515(02)01538-7. [DOI] [PubMed] [Google Scholar]
- 24.Bujko K, Nowacki MP, Liszka-Dalecki P. Radiation therapy for anorectal melanoma: a report of three cases. Acta Oncol. 1998;37:497–9. doi: 10.1080/028418698430485. [DOI] [PubMed] [Google Scholar]


