Abstract
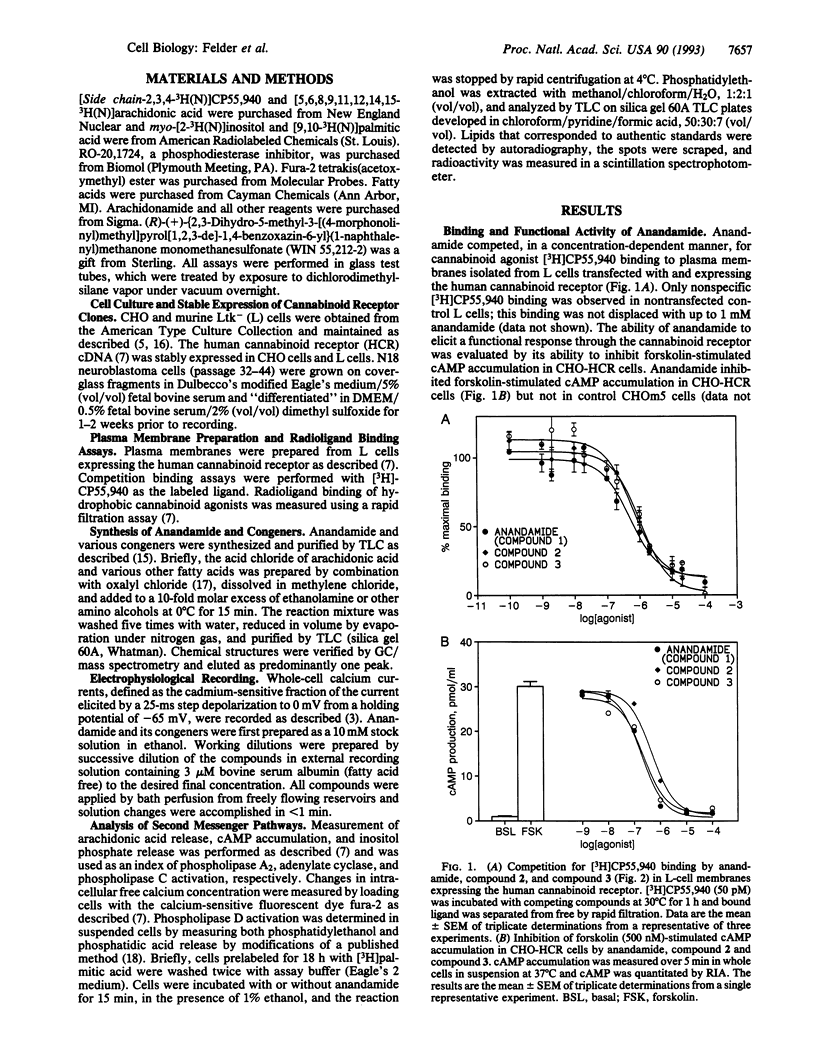
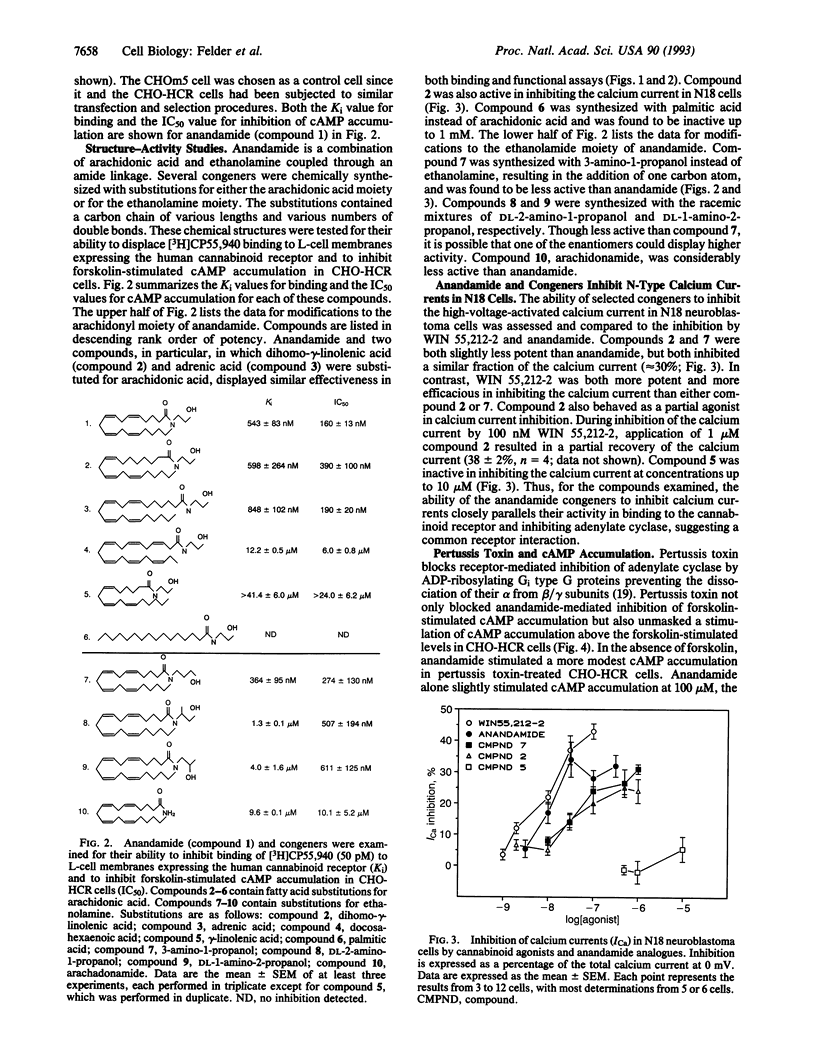
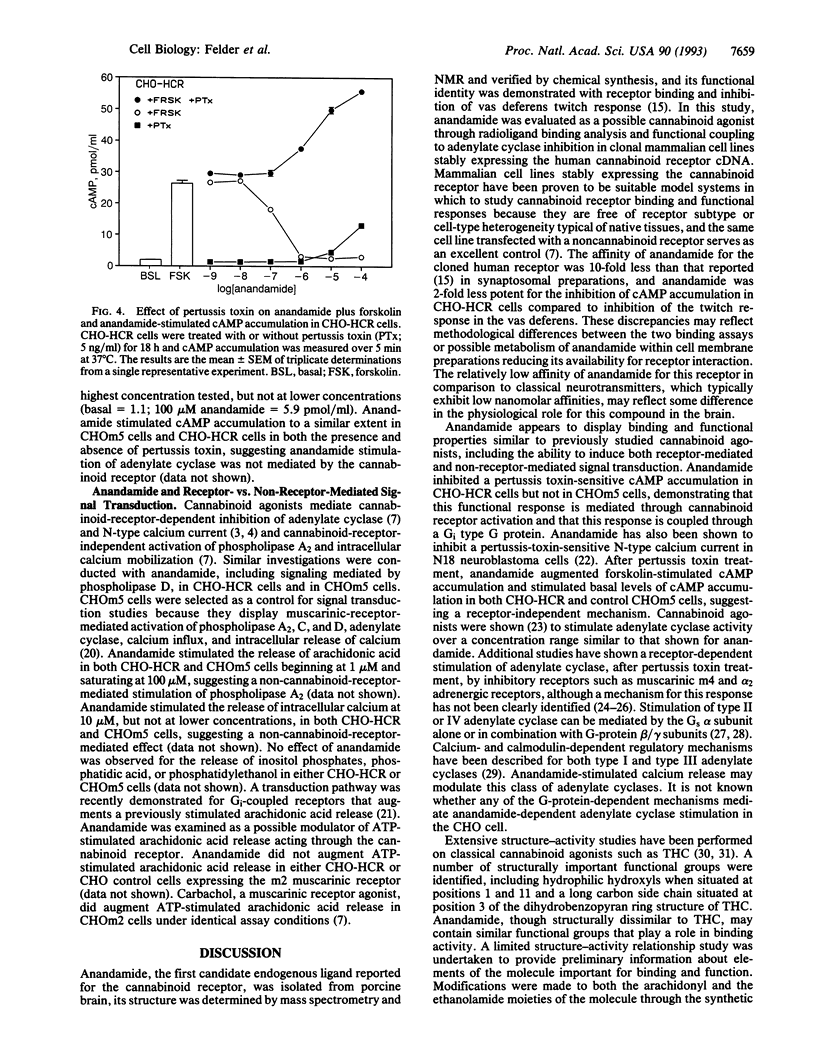
Arachidonylethanolamide (anandamide), a candidate endogenous cannabinoid ligand, has recently been isolated from porcine brain and displayed cannabinoid-like binding activity to synaptosomal membrane preparations and mimicked cannabinoid-induced inhibition of the twitch response in isolated murine vas deferens. In this study, anandamide and several congeners were evaluated as cannabinoid agonists by examining their ability to bind to the cloned cannabinoid receptor, inhibit forskolin-stimulated cAMP accumulation, inhibit N-type calcium channels, and stimulate one or more functional second messenger responses. Synthetic anandamide, and all but one congener, competed for [3H]CP55,940 binding to plasma membranes prepared from L cells expressing the rat cannabinoid receptor. The ability of anandamide to activate receptor-mediated signal transduction was evaluated in Chinese hamster ovary (CHO) cells expressing the human cannabinoid receptor (HCR, termed CHO-HCR cells) and compared to control CHO cells expressing the muscarinic m5 receptor (CHOm5 cells). Anandamide inhibited forskolin-stimulated cAMP accumulation in CHO-HCR cells, but not in CHOm5 cells, and this response was blocked with pertussis toxin. N-type calcium channels were inhibited by anandamide and several active congeners in N18 neuroblastoma cells. Anandamide stimulated arachidonic acid and intracellular calcium release in both CHOm5 and CHO-HCR cells and had no effect on the release of inositol phosphates or phosphatidylethanol, generated after activation of phospholipase C and D, respectively. Anandamide appears to exhibit the essential criteria required to be classified as a cannabinoid/anandamide receptor agonist and shares similar nonreceptor effects on arachidonic acid and intracellular calcium release as other cannabinoid agonists.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakalyar H. A., Reed R. R. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990 Dec 7;250(4986):1403–1406. doi: 10.1126/science.2255909. [DOI] [PubMed] [Google Scholar]
- Baumgold J., Paek R., Fiskum G. Calcium independence of phosphoinositide hydrolysis-induced increase in cyclic AMP accumulation in SK-N-SH human neuroblastoma cells. J Neurochem. 1992 May;58(5):1754–1759. doi: 10.1111/j.1471-4159.1992.tb10050.x. [DOI] [PubMed] [Google Scholar]
- Burstein S., Hunter S. A. Prostaglandins and cannabis--VI. Release of arachidonic acid from HeLa cells by delta1-tetrahydrocannabinol and other cannabinoids. Biochem Pharmacol. 1978;27(8):1275–1280. doi: 10.1016/0006-2952(78)90463-x. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Gilman A. G. G protein involvement in receptor-effector coupling. J Biol Chem. 1988 Feb 25;263(6):2577–2580. [PubMed] [Google Scholar]
- Caulfield M. P., Brown D. A. Cannabinoid receptor agonists inhibit Ca current in NG108-15 neuroblastoma cells via a pertussis toxin-sensitive mechanism. Br J Pharmacol. 1992 Jun;106(2):231–232. doi: 10.1111/j.1476-5381.1992.tb14321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane W. A., Dysarz F. A., 3rd, Johnson M. R., Melvin L. S., Howlett A. C. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988 Nov;34(5):605–613. [PubMed] [Google Scholar]
- Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992 Dec 18;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Federman A. D., Conklin B. R., Schrader K. A., Reed R. R., Bourne H. R. Hormonal stimulation of adenylyl cyclase through Gi-protein beta gamma subunits. Nature. 1992 Mar 12;356(6365):159–161. doi: 10.1038/356159a0. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Dieter P., Kinsella J., Tamura K., Kanterman R. Y., Axelrod J. A transfected m5 muscarinic acetylcholine receptor stimulates phospholipase A2 by inducing both calcium influx and activation of protein kinase C. J Pharmacol Exp Ther. 1990 Dec;255(3):1140–1147. [PubMed] [Google Scholar]
- Felder C. C., MacArthur L., Ma A. L., Gusovsky F., Kohn E. C. Tumor-suppressor function of muscarinic acetylcholine receptors is associated with activation of receptor-operated calcium influx. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1706–1710. doi: 10.1073/pnas.90.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Veluz J. S., Williams H. L., Briley E. M., Matsuda L. A. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol Pharmacol. 1992 Nov;42(5):838–845. [PubMed] [Google Scholar]
- Felder C. C., Williams H. L., Axelrod J. A transduction pathway associated with receptors coupled to the inhibitory guanine nucleotide binding protein Gi that amplifies ATP-mediated arachidonic acid release. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6477–6480. doi: 10.1073/pnas.88.15.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E., Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmacol. 1993 Feb 9;231(2):313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- Gao B. N., Gilman A. G. Cloning and expression of a widely distributed (type IV) adenylyl cyclase. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10178–10182. doi: 10.1073/pnas.88.22.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard C. M., Mollereau C., Vassart G., Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991 Oct 1;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Lynn A. B., Johnson M. R., Melvin L. S., de Costa B. R., Rice K. C. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991 Feb;11(2):563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C. J., Bloom A. S. Possible role of prostaglandins in the effects of the cannabinoids on adenylate cyclase activity. Eur J Pharmacol. 1983 Jul 15;91(1):21–27. doi: 10.1016/0014-2999(83)90357-6. [DOI] [PubMed] [Google Scholar]
- Holbrook P. G., Pannell L. K., Murata Y., Daly J. W. Molecular species analysis of a product of phospholipase D activation. Phosphatidylethanol is formed from phosphatidylcholine in phorbol ester- and bradykinin-stimulated PC12 cells. J Biol Chem. 1992 Aug 25;267(24):16834–16840. [PubMed] [Google Scholar]
- Howlett A. C. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985 Apr;27(4):429–436. [PubMed] [Google Scholar]
- Jones S. V., Heilman C. J., Brann M. R. Functional responses of cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1991 Aug;40(2):242–247. [PubMed] [Google Scholar]
- Kaminski N. E., Abood M. E., Kessler F. K., Martin B. R., Schatz A. R. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Mol Pharmacol. 1992 Nov;42(5):736–742. [PMC free article] [PubMed] [Google Scholar]
- Laychock S. G., Hoffman J. M., Meisel E., Bilgin S. Pancreatic islet arachidonic acid turnover and metabolism and insulin release in response to delta-9-tetrahydrocannabinol. Biochem Pharmacol. 1986 Jun 15;35(12):2003–2008. doi: 10.1016/0006-2952(86)90733-1. [DOI] [PubMed] [Google Scholar]
- Mackie K., Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda L. A., Bonner T. I., Lolait S. J. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993 Jan 22;327(4):535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R., Lander N., Srebnik M., Breuer A., Segal M., Feigenbaum J. J., Jarbe T. U., Consroe P. Stereochemical requirements for cannabimimetic activity. NIDA Res Monogr. 1987;79:15–30. [PubMed] [Google Scholar]
- Razdan R. K. Structure-activity relationships in cannabinoids. Pharmacol Rev. 1986 Jun;38(2):75–149. [PubMed] [Google Scholar]
- Reichman M., Nen W., Hokin L. E. Delta 9-tetrahydrocannabinol increases arachidonic acid levels in guinea pig cerebral cortex slices. Mol Pharmacol. 1988 Dec;34(6):823–828. [PubMed] [Google Scholar]
- Reichman M., Nen W., Hokin L. E. Delta 9-tetrahydrocannabinol inhibits arachidonic acid acylation of phospholipids and triacylglycerols in guinea pig cerebral cortex slices. Mol Pharmacol. 1991 Oct;40(4):547–555. [PubMed] [Google Scholar]
- Sinclair A. J. Long-chain polyunsaturated fatty acids in the mammalian brain. Proc Nutr Soc. 1975 Dec;34(3):287–291. doi: 10.1079/pns19750051. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991 Dec 6;254(5037):1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]



