Abstract
Background
Observational studies of older adults showed higher mortality for first-generation antipsychotics than their second-generation counterparts which led to FDA warnings, but the actual mechanisms involved remain unclear.
Methods
A cohort of 9,060 initiators of first-generation antipsychotics and 17,137 of second-generation antipsychotics enrolled in New Jersey and Pennsylvania Medicare were followed for 180 days. Medical events were assessed using diagnostic and procedure codes on inpatient billing claims. For the individual and joint set of medical events (mediators), we estimated the total, direct, and indirect effects of antipsychotic type (first versus second generation) on mortality on the risk ratio scale (RR) and the proportion mediated on the risk difference scale, obtaining 95% confidence intervals (CI) through bootstrapping. We performed bias analyses for false negative mediator misclassification in claims data, with sensitivity ranging from 0.25 to 0.75.
Results
There were 3,199 deaths (outcomes), 862 cardiovascular events, 675 infectious events, and 491 hip fractures (potential mediators). Mortality was higher for first-than second-generation antipsychotic initiators (adjusted RR 1.14; 95%CI 1.06-1.22). In naïve analyses that ignored potential misclassification, less than 4% of this difference was explained by any particular medical event. In bias analyses the proportion mediated ranged from 6% to 16% for stroke, 3% to 9% for ventricular arrhythmia, 3% to 11% for myocardial infarction, 0% venous thromboembolism, 3% to 9% for pneumonia, 0% to 1% for other bacterial infection, and 1% to 3% for hip fracture.
Conclusions
Acute cardiovascular events and pneumonia may explain part of the mortality difference between first- and second-generation antipsychotic initiators in this analysis.
INTRODUCTION
Antipsychotic medications are heavily used off-label to treat behavioral and psychiatric symptoms of dementia.1 These treatment decisions are ideally weighed against the safety evidence from randomized trials, which report 1.6 times higher mortality for second-generation antipsychotics than for placebo over a two to three month period2. First-generation antipsychotics have shown even higher mortality (1.4 fold) early after starting therapy, as when compared to second-generation antipsychotics in observational post-marketing studies.3 These findings led the FDA to issue Black Box warnings.4
The mechanisms by which first- and second-generation antipsychotics increase mortality are poorly understood. Most of the deaths in the Food and Drug Administration's meta-analysis of 17 placebo-controlled randomized trials of second-generation antipsychotics were attributed to cardiovascular events or infections.4 Similar results were reported in a large observational study of Canadian administrative healthcare data: 49% of all deaths were from cardiovascular causes, 60% of which occurred outside of a hospital; and 10% of all deaths were infection-related, of which 88% were pneumonia-related.6 While these findings point towards plausible disease pathways, the causes of death are too broad to distinguish between specific complications like myocardial infarction and Torsades De Pointes, a rare but lethal ventricular arrhythmia associated with some first-generation antipsychotics. Moreover, cause-of-death information on autopsy reports is particularly vulnerable to misclassification7,8 and may over-represent proximate causes.
Several studies have investigated differences between first- and second-generation antipsychotic risk for stroke, ventricular arrhythmia, myocardial infarction, venous thromboembolism, pneumonia, bacterial infections, and hip fracture, but report conflicting results due to differences in study design.9 A systematic review of observational post-marketing studies using mostly administrative claims data compared risk of first- and second-generation antipsychotics for these medical events and concluded that stroke, hip fracture, myocardial infarction, and ventricular arrhythmia combined could explain between 17% and 42% of the mortality difference.3 But this study was limited by its reliance on study-level data and published mortality rates.
Here, we used individual-level Medicare data and causal mediation analysis to quantify the contribution of various medical events to the mortality differences between older adults who initiate first- versus second-generation antipsychotics, while adjusting for potential confounders. We compared the separate and combined contributions of medical events previously studied in the literature: stroke, ventricular arrhythmia, myocardial infarction, venous thromboembolism, pneumonia, bacterial infection (other than pneumonia), and hip fracture. We also provide estimates for the relative risk for each medical event comparing first to second-generation antipsychotics that control for more covariates than considered previously.
METHODS
Causal Mediation Analysis Framework
Using causal mediation analysis, we sought to decompose the total effect of antipsychotic-type (exposure) on mortality (outcome) into natural direct and indirect effects through various individual medical events (mediators) on the risk ratio scale, and the proportion of the total effect mediated by each medical event on the risk difference scale. While this approach allows for possible exposure-mediator interactions, it also requires that all confounders of the exposure-outcome, exposure-mediator, and mediator-outcome relationships are measured and adjusted for, and prohibits the existence of any mediator-outcome confounder that is itself affected by exposure. These are strong assumptions but can be assessed through bias analyses.10,11
Recently, this framework was expanded to consider a mediation analysis for multiple mediators, wherein the direct and indirect effects for a set of mediators are of interest, and the above assumptions are required to hold for the set of mediators.12 If none of the mediators are causally or otherwise associated with one another except through the exposure, and none interact with each other (on the additive scale) to cause mortality, the joint natural indirect effect will equal the product of the natural indirect effect on the risk ratio scale. Likewise, under the same conditions, the proportion mediated by each mediator will sum to equal the proportion mediated by the joint set of mediators. In the online appendix, we provide empirical evidence to support these assumptions.
Data Source
Pharmacy dispensing records from individuals enrolled in statewide pharmacy assistance programs in New Jersey and Pennsylvania were linked to Medicare claims data on procedures, diagnoses, and dates of service for physician visits, hospitalizations, and stays in long-term care facilities. Mortality records were linked from the Social Security Death Master File.13 Institutional Review Board approval was granted by the Brigham and Women's Hospital and the study was covered by a signed data agreement with the Center for Medicaid and Medicare Services.
Population & Study Design
We assembled a retrospective cohort of Medicare beneficiaries aged 65 and over from January 1 1994 to December 31 2005, who filled a new antipsychotic prescription dispensing (index date) after a 180 day period of non-use. For cohort entry, we required continuous Medicare enrollment and at least one encounter or prescription claim in the six months preceding the index date (enrollment period). Indications were not available for prescription claims. Thus, to identify our population of interest (patients with behavioral and psychosocial symptoms of dementia) and to minimize the potential for confounding by indication, we excluded patients with the following characteristics during the enrollment period (see eFigure 1 for details): two or more claims with diagnoses that indicate antipsychotic treatment for conditions besides dementia (e.g. schizophrenia), an initial dispensing consistent with pro-re-nata use or severe psychiatric illness (e.g. less than 30 days’ supply, clozapine use, etc.). We also excluded patients with very low risk for hip fracture (i.e. lower-limb paralysis) as well as those with a nursing home stay during the enrollment period where claims may poorly document some medical events. Follow-up began on the index date and lasted for 180 days or until death. Patients were not allowed to re-enter the cohort after the end of follow-up.
Antipsychotic Exposure
We defined exposure as a binary variable comparing first-generation to second-generation antipsychotic initiation (reference). Second-generation antipsychotic use included oral aripiprazole, olanzapine, quetiapine, risperidone, or ziprasidone; First-generation antipsychotic use included oral chlorpromazine, fluphenazine, haloperidol, loxapine, mesoridazine, molindone, perphenazine, pimozide, promazine, thioridazine, thiothixene, thiothixene, trifluoperazine, or triflupromazine. Clozapine use was not considered.
Medical Events (Mediators) and Mortality (Outcome)
We considered the following as potential mediators: stroke, ventricular arrhythmia, acute myocardial infarction, venous thromboembolism, pneumonia, bacterial infection (besides pneumonia), and hip fracture. These were defined as binary variables indicating their occurrence between the index prescription date (inclusive) and the end of follow up (180 days) or death, and were classified using diagnostic and procedure codes based on the International Classification of Diseases, Ninth Revision, Clinical Modification (as detailed in eTable 5). Death during follow-up was defined as a binary variable.
Covariates
We selected 70 risk factors for mortality or the medical events under study. This rich set of covariates included demographic characteristics, health service utilization and medication usage, co-existing medical and psychiatric illness, and indicators of functional impairment (the entire list is given as a footnote in Table 1). These were assessed using demographic, drug dispensing, and diagnostic information on pharmacy and Medicare insurance claims during the enrollment period.
Table 1.
Selected characteristicsa of first-generation antipsychotic (FGA) and second-generation antipsychotic (SGA) users in the 180 days prior to antipsychotic initiation among FGA and SGA initiators enrolled in Medicare and pharmacy assistance programs in New Jersey and Pennsylvania from 1994 to 2005.
| SGAa | FGAa | % Differencec | |||
|---|---|---|---|---|---|
|
|
|||||
| N=17,137 | %c | N=9,060 | %c | ||
| Demographics | |||||
| Female | 13945 | 81 | 7197 | 79 | −2 |
| Cardiovascular Disease | |||||
| Atrial fibrillation | 597 | 4 | 400 | 4 | 1 |
| Ischemic stroke | 190 | 1 | 311 | 3 | 2 |
| Cardiac arrhythmia | 4660 | 27 | 2594 | 29 | 1 |
| Coronary artery disease | 2295 | 13 | 1581 | 18 | 4 |
| Congestive heart failure | 3818 | 22 | 2386 | 26 | 4 |
| DVT/PEb | 38 | 0 | 28 | 0 | 0 |
| Hyperlipidemia | 826 | 5 | 200 | 2 | −3 |
| Hypertension | 9482 | 55 | 4388 | 48 | −7 |
| Myocardial infarction | 1083 | 6 | 670 | 7 | 1 |
| Peripheral vascular disease | 1741 | 10 | 605 | 7 | −4 |
| Psychiatric Disorder | |||||
| Anxiety | 2676 | 16 | 1082 | 12 | −4 |
| Dementia | 9283 | 54 | 3815 | 42 | −12 |
| Delirium | 1894 | 11 | 1004 | 11 | 0 |
| Depressive illness | 5347 | 31 | 2387 | 26 | −5 |
| Manic-depressive illness | 1252 | 7 | 475 | 5 | −2 |
| Other Conditions | |||||
| Chronic lung disease | 3150 | 18 | 1809 | 20 | 2 |
| Diabetes | 2776 | 16 | 1469 | 16 | 0 |
| Renal impairment | 1872 | 11 | 880 | 10 | −1 |
| Hip Fracture Risk Factors | |||||
| Fracture | 553 | 3 | 304 | 3 | 0 |
| Fecal incontinence | 1370 | 8 | 457 | 5 | −3 |
| Motor impairment | 2239 | 13 | 441 | 5 | −8 |
| Postural hypotension | 749 | 4 | 431 | 5 | 0 |
| Pneumonia/Infection Risk Factors | |||||
| Gastroesophageal reflux disease | 1864 | 11 | 550 | 6 | −5 |
| Pneumonia | 1233 | 7 | 861 | 10 | 2 |
| ACE inhibitor Rx | 4320 | 25 | 2283 | 25 | 0 |
| Immunosuppressive Rx | 2733 | 16 | 1189 | 13 | −3 |
| Anti-infectious Rx | 5924 | 35 | 2833 | 31 | −3 |
| Proton pump inhibitor Rx | 3990 | 23 | 902 | 10 | −13 |
Abbreviations: DVT/PE (venous thromboembolism); FGA (first-generation antipsychotics); SGA (second-generation antipsychotics).
See etable1 for data on all patient characteristics used in the adjusted analysis, which includes demographics: age, female, race (white / black / other); calendar quarter of antipsychotic initiation; health services / medication use: # physician visits, # hospitalizations, # total medications, # psychotropic medications, # infection-related visits, # hospitalizations ≥ 3 days; cardiovascular disease: angina, atrial fibrillation, intracranial hemorrhage, ischemic stroke, other cerebrovascular disease, cardiac arrhythmia, coronary artery disease, congestive heart failure, venous thromboembolism, hyperlipidemia, hypertension, myocardial infarction, peripheral vascular disease, valvular heart disease; cardiovascular medications: angiotensin II receptor blocker, antiarrhythmia, anticoagulant, beta blocker, calcium channel blocker, diuretic, statin; psychiatric disorders: ADHD (attention deficit hyperactivity disorder), anxiety, dementia, delirium, depressive illness, manic-depressive illness, drug abuse/dependence, alcohol abuse/dependence, other psychiatric disorder; psychiatric medications: antidepressant Rx, anticonvulsant Rx, anxiolytic/sedative/hypnotic Rx; other conditions: chronic lung disease, diabetes, hepatic impairment, renal impairment, sleep disorder; hip fracture risk factors: arthritis, epilepsy, fracture, fall history, fecal incontinence, gait disorder, hormone therapy Rx, impaired vision, hyperthyroidism, motor impairment, osteoporosis Rx, postural hypotension, recent weight change, vertigo; pneumonia/infection risk factors: dysphagia, gastroesophageal reflux disease, hernia, pneumonia, ace inhibitor Rx, immunosuppressive Rx, anti-infectious Rx, proton pump inhibitor Rx.
rounded to the nearest whole number
Descriptive Analysis
For first- and second-generation antipsychotic users, antipsychotic use was described according to the frequency of specific drugs, average chlorpromazine-equivalent dose,14 and treatment discontinuation (with a 14-day no-refill grace period). Exposure covariate imbalance comparing first- to second-generation antipsychotic initiators was evaluated using prevalence and mean differences. Separately for first- and second-generation antipsychotic initiators, mediator covariate imbalance was evaluated using prevalence differences for binary covariates and standardized mean differences for continuous and count variables, comparing those who developed the medical event during follow-up to those who did not.15
For each mediator, we evaluated its frequency and the strength of its relationships with antipsychotic-type and mortality. Specifically, using logistic regression, we estimated the crude risk at 180 days and the covariate-adjusted relative risk comparing first- and second-generation antipsychotic use. Within groups defined by antipsychotic type, we used g-computation (i.e. model-based standardization with 95% confidence intervals (CI) obtained from 1,000 bootstrap samples) to estimate the covariate-adjusted percent difference16 in mortality by 180 days of follow-up, comparing those who developed the medical event to those who did not. This stratified measure provides qualitative evidence for whether the association between a medical event and mortality is modified by antipsychotic-type on the additive scale.
Mediation Analyses
We used a regression-based approach17 for causal mediation analysis involving a binary exposure, mediator, and outcome to estimate crude and adjusted direct and indirect effects of antipsychotic-type on 180 day mortality through each medical event on the risk-ratio scale. For each medical event, we estimated two models: (1) a logistic regression model for the mediator’s occurrence conditional on antipsychotic type and main effects for all baseline covariates listed in the footnote of Table 1, and (2) a Poisson regression model for mortality conditional on antipsychotic type, the mediator’s occurrence, a product term for their interaction, and the same baseline covariates. Crude results were obtained from analogous models omitting the covariates. The parameter estimates from these models were combined to estimate the direct and indirect effect risk ratios using closed form estimators, which were then used to compute the proportion mediated on the risk difference scale.18 The non-parametric bootstrap (n=1,000 samples) was used to obtain standard errors through the percentile method. Relying on the assumptions of no mediator-mediator interactions and that no mediators affected one another, we summed over the proportion mediated by individual mediators to obtain the proportion mediated by the set of mediators.
Bias Analysis for False-Negative Medical Event Misclassification
Medicare claims data are created to facilitate the payment and reimbursement of clinical services performed by healthcare providers. To avoid false positive medical events during follow-up, we used restrictive classification algorithms with high positive-predictive values19-26 as described in eTable 5. These algorithms are very specific for rare events such as these,27 but the sensitivity will be moderate or low.28 False negatives may occur more often among those who die, especially with events where pre-hospital mortality is common (e.g. ventricular arrhythmia) or do-not-resuscitate orders dictate whether aggressive treatment is pursued (e.g. pneumonia).
Such misclassification can substantially bias the indirect effect.29,30 We thus performed a bias analysis to explore how results would change under various scenarios of non-differential and differential misclassification. In each case we assumed perfect specificity for observing the medical event, but varied the sensitivity from 0.25 to 0.75 separately for those who survived and for those who died. From these, we restricted our attention (a priori) to scenarios where sensitivity was equal or greater among survivors than non-survivors as we expected more false-negative coding among the deceased. To be clear, each scenario assumed that mediator misclassification was non-differential with respect to antipsychotic type, covariates, and other mediators but some scenarios allowed for differential misclassification with respect to death. We address the plausibility of these assumptions in the Discussion. A hybrid approach28 was used to obtain adjusted mediator and outcome model parameters for each scenario, which were then used to estimate the corresponding direct and indirect effects with 95% CI obtained through bootstrapping the entire process with 1,000 samples.
We used maximum likelihood30,31 to obtain adjusted logistic regression parameters for the mediator model under the assumed values for sensitivity and specificity. In the main analysis, the model for the misclassified medical event M* was conditional on antipsychotic-type A and baseline covariates C. However, in the bias analysis we instead aimed to adjust a model for the misclassified mediator M* conditional on antipsychotic-type and propensity score decile X formed from the predicted values of a logistic regression model for antipsychotic-type A conditional on baseline covariates C. This choice was made to overcome convergence problems in the setting of many covariates, and we checked that both models yielded similar results (for the mediation analysis) before proceeding. To adjust the model, we maximized a likelihood for the true value of the mediator M where sensitivity SN and specificity SP were (in some scenarios) allowed to depend on 180 day mortality Y:
We used predictive value weighting30,32 to adjust the parameters of the Poisson regression model for the outcome under the assumed values for sensitivity and specificity. For each observed medical event M* a generalized additive logistic model was fit (conditional on baseline covariates C with cubic splines for age and its interactions with antipsychotic-type A and mortality Y) to obtain P[M*|Y, A, C] for each person. With Bayes Rule, these predictions and the assumed values for sensitivity and specificity were used to estimate predictive value weights of the form P[M|M*, Y, A, C] in a dataset with two copies of each person, where a hypothetically true medical event M was set to “occurred” for one copy and “not occurred” for the other. A weighted outcome model was fit in the stacked dataset to yield the adjusted parameters. All analyses were carried out with SAS version 9.3 (Cary, NC) and R version 2.15 (Vienna, Austria).
RESULTS
Cohort Description
We identified a cohort of 9,060 FGA and 17,137 SGA initiators. Haloperidol (66%) and thioridazine (13%) were the most frequently prescribed first-generation antipsychotics, whereas risperidone (55%), olanzapine (32%), and quetiapine (12%) were the most widely prescribed second-generation antipsychotics. Ninety-eight percent were dispensed a tablet or capsule. Between first- and second-generation antipsychotics, the chlorpromazine-equivalent dose was similar (45 versus 49 mg) but discontinuation was higher for first-generation antipsychotics (80% versus 71%).
This community-dwelling cohort of 81% female and 92% Caucasian antipsychotic initiators was, on average, 82 years old and in regular contact with the healthcare system (data for selected covariates are shown in Table 1; for all covariates see eTable 1). There was substantial cardiovascular, neurological, and psychological comorbidity. On average, patients received seven distinct medications; 24% received a diagnosis of congestive heart failure; 16% diabetes; 50% treated dementia; 30% depressive illness; and 33% filled a prescription for an antibiotic or antiviral drug. Neither initiator group appeared healthier across all risk factors. First-generation antipsychotic users had higher prevalence of cardiovascular disease, and fewer use of cardiovascular medications, but lower prevalence of treated dementia, peripheral vascular disease, and motor impairment. The average number of physician visits, hospitalizations, and psychotropic medications were similar for first- and second-generation antipsychotic users.
Medical events and mortality
There were 3,199 deaths (12%) during follow-up. Table 2 presents the medical event occurrence and the adjusted effect of antipsychotic type (exposure) on each medical event (the mediators). A total of 1,959 antipsychotic initiators experienced a medical event, of whom 84 experienced two events (of the 1,959 events, 862 involved cardiovascular events, 675 involved infectious events, and 491 involved hip fractures). The most frequent events were bacterial infection (91% bacteremia or cellulitis), hip fracture, stroke, and myocardial infarction. Antipsychotic type was most associated with pneumonia (OR=1.57), stroke (OR=1.31), and hip fracture (OR=1.10). Table 3 presents the adjusted effect of each medical event on mortality on the difference scale by antipsychotic type. Excess mortality was greatest for ventricular arrhythmia (60.7% first-generation, 76.3% second-generation antipsychotic), lowest for venous thromboembolism (7.8% first-generation, 7.2% second-generation antipsychotic) and hip fracture (10.5% first-generation, 3.7% second-generation antipsychotic). Among first-generation antipsychotic users excess mortality ranged from 15.8% to 20.2% for stroke, myocardial infarction, pneumonia, and bacterial infection (16.3% to 20.4% among second-generation antipsychotic users).
Table 2.
180-day medical event (mediator) risk and association with antipsychotic type among first-generation antipsychotic (FGA) and second-generation antipsychotic (SGA) initiators enrolled in Medicare and pharmacy assistance programs in New Jersey and Pennsylvania from 1994 to 2005.
| Number of cases |
Crude Risk (%) |
Adjusted odds ratio (95%CI) for
the medical event given antipsychotic type (FGAa vs. SGAa) |
|
|---|---|---|---|
| Cardiovascular Mediators | 862 | 3.29 | |
| Stroke | 361 | 1.38 | 1.31 (1.04-1.65) |
| Ventricular Arrhythmia | 114 | 0.44 | 1.09 (0.72-1.65) |
| Myocardial Infarction | 252 | 0.96 | 1.08 (0.81-1.42) |
| DVT/PEa | 150 | 0.57 | 0.81 (0.55-1.19) |
| Infection Mediatorsa | 675 | 2.58 | |
| Pneumonia | 118 | 0.45 | 1.57 (1.05-2.34) |
| Bacterial Infectionb | 569 | 2.17 | 0.90 (0.75-1.10) |
| Injury Mediator | |||
| Hip Fracture | 491 | 1.87 | 1.10 (0.90-1.35) |
Abbreviations: DVT/PE (venous thromboembolism); FGA (first-generation antipsychotics); SGA (second-generation antipsychotics).
Excluding pneumonia
Table 3.
180-day mortality difference, comparing those who experienced the medical event to those who did not (i.e. mediator vs. no mediator) among first-generation antipsychotic (FGA) and second-generation antipsychotic (SGA) initiators enrolled in Medicare and pharmacy assistance programs in New Jersey and Pennsylvania from 1994 to 2005.
| Among SGAa RD (95% CI) |
Among FGAa RD (95% CI) |
|
|---|---|---|
|
Cardiovascular
Mediators |
||
| Stroke | 20.4% (14.9-26.9) | 20.2% (12.9-28.0) |
| Ventricular Arrhythmia | 60.7% (48.5-71.9) | 76.3% (64.5-86.6) |
| Myocardial Infarction | 22.5% (15.7-29.4) | 19.5% (10.6-28.7) |
| DVT/PEa | 7.8% (0.0-15.4) | 7.2% (−4.1-20.7) |
| Infection Mediators | ||
| Pneumonia | 19.9% (7.8-34.4) | 15.8% (5.6-27.3) |
| Bacterial Infectionb | 16.3% (12.1-20.7) | 15.8% (9.9-21.6) |
| Injury Mediator | ||
| Hip Fracture | 10.5% (6.1-15.0) | 3.7% (−1.2-9.3) |
Abbreviations: DVT/PE (venous thromboembolism); FGA (first-generation antipsychotics); SGA (second-generation antipsychotics).
Excluding pneumonia
A covariate balance plot designed to portray measured mediator-outcome confounding for each medical event is presented in eFigure 2. If there were no confounding between any of the medical event and mortality relationships, the prevalence of pre-existing risk factors would be equal for those who experienced a medical event during follow-up and those who did not, after stratifying by the type of antipsychotic used at baseline (i.e. the solid and open circles would line up at zero). In most cases, first- and second-generation antipsychotic initiators who experienced a medical event during follow-up were more likely to have been diagnosed with a pre-existing risk factor or use a related medication during the enrollment period, but these sometimes differ by type of medical event. These imbalances underscore our decision to include many risk factors to adjust for mediator-outcome confounding.
Mediation Analysis
The crude and covariate-adjusted analyses accounting for exposure-mediator interaction are presented in Table 4, which were similar to those ignoring such interaction (eTable 2). Crude indirect effects were close to the null and were lower after covariate adjustment. Although covariate adjustment attenuated the total effect from RR=1.23 (95%CI 1.16 to 1.32) to RR=1.14 (95%CI 1.06 to 1.22), the direct effects in crude and adjusted analyses were similar to the total effect in both cases. The proportion mediated was highest for stroke (5%) and we hypothesized that these low values might be explained by miscoding or under-ascertainment of medical events.
Table 4.
Crude and adjusted direct, indirect, and total effects for potential mediators (allowing for exposure-mediator interaction) of the 180-day mortality difference between first-generation antipsychotic and second-generation antipsychotic initiators enrolled in Medicare and pharmacy assistance programs in New Jersey and Pennsylvania from 1994 to 2005.
| Unadjusted | Baseline covariate-adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Direct RR (95% CI) |
Indirect RR (95% CI) |
Total RR (95%CI) |
PMa (%) |
Direct RR (95% CI) |
Indirect RR (95% CI) |
Total RR (95% CI) |
PMa (%) |
|
|
|
|
|||||||
| Cardiovascular | ||||||||
| Stroke | 1.23 (1.15, 1.31) | 1.007 (1.001, 1.013) | 1.23 (1.16, 1.32) | 4 | 1.13 (1.06-1.22) | 1.005 (1.001-1.011) | 1.14 (1.06-1.22) | 5 |
| Arrhythmia | 1.23 (1.15, 1.31) | 1.004 (0.995, 1.014) | 1.23 (1.15, 1.32) | 2 | 1.13 (1.06-1.21) | 1.002 (0.996-1.009) | 1.14 (1.06-1.22) | 2 |
| Myocardial Infarction |
1.23 (1.15, 1.31) | 1.005 (0.999, 1.011) | 1.23 (1.15, 1.32) | 3 | 1.14 (1.06-1.22) | 1.002 (0.999-1.006) | 1.14 (1.06-1.22) | 2 |
| DVT/PEa | 1.23 (1.16, 1.32) | 0.999 (0.997, 1.000) | 1.23 (1.16, 1.32) | −1 | 1.14 (1.06-1.22) | 0.999 (0.997-1.000) | 1.14 (1.06-1.22) | −1 |
| Infection | ||||||||
| Pneumonia | 1.22 (1.15, 1.31) | 1.007 (1.003, 1.012) | 1.23 (1.15, 1.32) | 4 | 1.14 (1.06-1.22) | 1.002 (1.000-1.004) | 1.14 (1.06-1.22) | 2 |
| Bacterial Infectionb |
1.23 (1.15, 1.31) | 1.000 (0.993, 1.007) | 1.23 (1.15, 1.32) | 0 | 1.14 (1.07-1.22) | 0.999 (0.995-1.003) | 1.14 (1.07-1.22) | −1 |
| Injury | ||||||||
| Hip Fracture | 1.23 (1.15, 1.31) | 1.002 (0.999, 1.005) | 1.23 (1.16, 1.31) | 1 | 1.14 (1.06-1.23) | 1.001 (1.000-1.002) | 1.14 (1.06-1.23) | 1 |
Abbreviations: DVT/PE (venous thromboembolism); PM (proportion mediated).
Excluding pneumonia.
In Figure 1 we present results from the bias analyses for false-negative mediator misclassification. Corresponding risk ratios and 95%CI for the direct and indirect effects for each scenario are presented in eTable 4. The proportion mediated was higher than the naïve estimators for some medical events and grew as sensitivity decreased from 0.75 to 0.25, particularly for stroke (6% to 16%), ventricular arrhythmia (3% to 9%), myocardial infarction (3% to 11%), and pneumonia (3% to 9%). The sensitivity among those who survived, rather than those who died, appeared to have more influence on these results. Conversely, the proportion mediated was low and did not appreciably change across scenarios for venous thromboembolism (0%), other bacterial infection (0% to 1%), or hip fracture (1% to 3%). Of the scenarios considered, we suspect that those with sensitivity equal to 0.5 (possibly lowered to 0.25 among those who died) were the most realistic and present these results allowing for exposure-mediator interaction in Table 5 and corresponding results without exposure-mediator interaction in eTable 3. Note that the bias analyses shifted results farther than adjustments for confounding even in mild misclassification scenarios.
Figure 1.
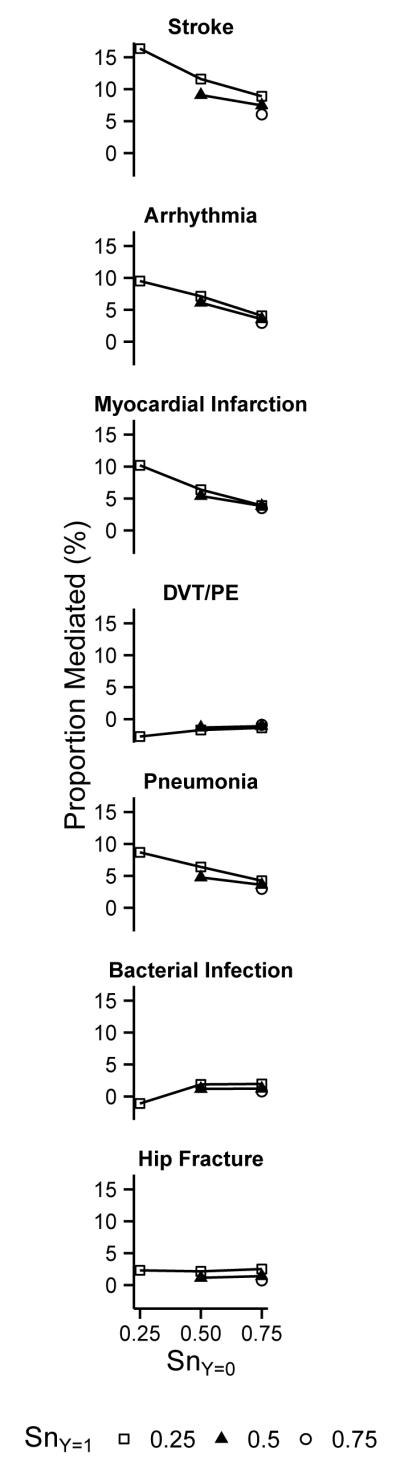
Bias analysis for false-negative medical event misclassification. Each panel presents the results for a specific medical event: the adjusted proportion-mediated (y-axis) under various bias scenarios characterized by sensitivity among those survived (SnY=0; x-axis) and among those who died (SnY=1; symbols), assuming perfect specificity. The nature of bias due to misclassification can be observed from the graph: if it were driven by non-differential misclassification, the lines for all symbols would be identical; the influence of misclassification among those who died is reflected by a line’s slope, and the influence of misclassification among those who survived is reflected in the degree of vertical separation between the lines.
Table 5.
Covariate-adjusted direct, indirect, and total effects for potential mediators (allowing for exposure-mediator interaction) of the 180-day mortality difference between first-generation antipsychotic and second-generation antipsychotic initiators enrolled in Medicare and pharmacy assistance programs in New Jersey and Pennsylvania from 1994 to 2005, assuming particular non-differential and differential mediator misclassification scenarios.
| Naïveb | Non-differential Mediator Misclassificationc | Differential Mediator Misclassificationd | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| PMa (%) |
Direct RR (95% CI) |
Indirect RR (95% CI) |
Total RR (95% CI) |
PMa (%) |
Direct RR (95% CI) |
Indirect RR (95% CI) |
Total RR (95% CI) |
PMa (%) |
|
|
|
|
|
|||||||
| Cardiovascular | |||||||||
| Stroke | 5 | 1.13 (1.05-1.2) | 1.010 (1.001-1.02) | 1.14 (1.06-1.22) | 9 | 1.12 (1.04-1.2) | 1.013 (1.002-1.025) | 1.14 (1.06-1.22) | 12 |
| Arrhythmia | 2 | 1.14 (1.06-1.22) | 1.008 (0.992-1.023) | 1.14 (1.07-1.23) | 6 | 1.14 (1.06-1.22) | 1.009 (0.994-1.024) | 1.15 (1.06-1.23) | 7 |
| Myocardial Infarction |
2 | 1.13 (1.06-1.21) | 1.006 (0.999-1.015) | 1.14 (1.07-1.22) | 5 | 1.13 (1.06-1.21) | 1.007 (0.998-1.018) | 1.14 (1.07-1.22) | 6 |
| DVT/PEa | −1 | 1.14 (1.06-1.22) | 0.999 (0.995-1.001) | 1.14 (1.06-1.22) | −1 | 1.13 (1.06-1.22) | 0.998 (0.993-1.001) | 1.13 (1.06-1.21) | −2 |
| Infection | |||||||||
| Pneumonia | 2 | 1.13 (1.06-1.21) | 1.005 (1.001-1.011) | 1.14 (1.07-1.22) | 5 | 1.13 (1.05-1.21) | 1.007 (1.002-1.014) | 1.14 (1.06-1.22) | 6 |
| Bacterial Infectione |
−1 | 1.14 (1.07-1.22) | 1.002 (0.992-1.011) | 1.15 (1.07-1.23) | 1 | 1.15 (1.07-1.22) | 1.002 (0.991-1.015) | 1.15 (1.07-1.23) | 2 |
| Injury | |||||||||
| Hip Fracture | 1 | 1.13 (1.06-1.21) | 1.001 (0.999-1.005) | 1.13 (1.06-1.22) | 1 | 1.13 (1.06-1.21) | 1.002 (1-1.008) | 1.13 (1.06-1.21) | 2 |
Abbreviations: DVT/PE (venous thromboembolism; PM (proportion mediated on risk-difference scale).
Covariate-adjusted proportion mediated without adjustment for medical event misclassification, reproduced from Table 4.
Non-differential misclassification model for all medical events: sensitivity=0.5 and specificity=1.0.
Differential misclassification model for all medical events: sensitivity=0.5 given survival at end of follow-up and 0.25 given death by end of follow-up; specificity=1.0.
Excluding pneumonia.
Under the assumptions of no mediator-mediator interactions and that the mediators do not affect one another, the naïve covariate-adjusted results suggested that 9% of the mortality difference might be explained by stroke, ventricular arrhythmia, myocardial infarction, and pneumonia. In the bias analyses that accounted for suspected mediator misclassification, this value ranged from 15% to 45% over a wide array of scenarios.
DISCUSSION
This study found a 1.14 fold increase in 180-day mortality for first-generation antipsychotic initiators compared to second-generation antipsychotic initiators. In this setting of claims data where medical event misclassification is expected, the bias analyses for mediator misclassification suggested that stroke, ventricular arrhythmia, myocardial infarction, and pneumonia might explain 15% to 45% of this mortality difference. The contributions of some medical events were due to their stronger associations with antipsychotic-type (e.g. stroke and pneumonia), whereas other medical events' contributions were driven by their frequency (e.g. myocardial infarction) or lethality (e.g. ventricular arrhythmia), or were diminished by a multiplicative statistical interaction with antipsychotic-type (e.g. hip-fracture). We note that these summary estimates across mediators may be overestimates to the extent that mediators affect one another and mediator-mediator interactions exist.
Overall, our findings are consistent with the published literature on mortality and medical event risk in antipsychotic initiators, particularly those that reported higher cardiovascular, infectious, and respiratory-related mortality among first- versus second-generation antipsychotic initiators. Elsewhere we provide a detailed review of the epidemiologic evidence and biological plausibility of these differences in mortality and risk for many of these medical events.3 Summary data from these studies suggest that between 17% and 42% of the mortality difference could be mediated by stroke, ventricular arrhythmia, myocardial infarction, and hip fracture. Over 60 studies compared mortality or medical event risk between first- and second-generation antipsychotic initiators but many study designs were ill-suited to detect short-term effects (e.g. prevalent users, poor temporality, immortal person-time). The few studies that avoided these potential biases reported higher first-generation antipsychotic risk for ventricular arrhythmia33, myocardial infarction33,34 and hip-fracture,34,35 but lower risk for venous thromboembolism. But the evidence for stroke was mixed33,34,36-39 and none reported a difference in risk for pneumonia or other bacterial infections.33-35 The divergent evidence for hip fracture and pneumonia may be partly explained by our focus on community-dwelling older adults to improve internal validity for comparisons across events, whereas many other studies focused on or included nursing home residents. Differences in medical event coding may also be a contributing factor.
Previously we examined natural effects for stroke as a mediator of the first-generation vs. second-generation antipsychotic mortality difference in community-dwelling and nursing home residents where the proportion mediated was small (2.7%) even after a host of bias analyses for study design and unmeasured confounding were undertaken.40 In that study, using more sensitive but less specific algorithms for detecting stroke increased the proportion mediated up to 6.7%, which was similar to the 6.2% estimate under the least extreme scenario we considered (sensitivity of 0.75 and perfect specificity). However, our results for more extreme scenarios imply that coding-based strategies for handling misclassification (which always involve tradeoffs between sensitivity and specificity) may be insufficient for recovering estimates when medical events are evaluated as mediators in claims data. In the absence of sub-study validation data, it may be best to pursue highly specific diagnostic algorithms for rare events so that bias analyses for misclassification can be applied. This would be consistent with standard pharmacoepidemiology practice where highly predictive and specific coding algorithms are sought to avoid classifying health encounters for diagnosis or management of prevalent disease as drug-induced events.
In the moderate bias analysis scenarios presented in Table 5, we explained 25% to 31% of the mortality difference (Table 5). The high mortality (12%) and comorbidity may explain why a large part of the difference was unaccounted for. Patients with extant physical and psychological illness may have higher risk for causes of death beyond the medical events we considered. Other lethal medical events may lie along the causal pathway, such as renal or respiratory failure, and rare complications such as neuroleptic malignant syndrome.
Residual confounding may have influenced our results. Delirium is a strong predictor of mortality in older adults and when it is detected—often it is not—it is frequently treated with haloperidol (a widely used first-generation antipsychotic) which could lead to exposure-mediator or exposure-outcome confounding. Delirium is poorly captured in claims data, so residual confounding at baseline could bias the total and indirect effects upwards; although it is unlikely to act as a mediator-outcome confounder during follow-up as it is often a consequence of medical events. While unmeasured behavioral risk factors (e.g. smoking, physical activity) could bias the indirect effects for several mediators through mediator-outcome confounding, it is unlikely that such bias would impact each of them to the same degree. Moreover, the medical events had strong and plausible associations with mortality. Although we adjusted for 70 risk factors, we cannot exclude residual confounding as an alternate explanation. Future studies could improve upon confounder assessment and further explore the impact of unmeasured and residual confounding through bias analyses.10
The bias analyses’ results are only valid to the extent that models are correctly specified and are based on plausible assumptions. We assumed that differences in event misclassification would be most severe by vital status, and in the case of acute cardiovascular events this is quite reasonable. But for some medical events, coding accuracy might also differ by comorbid illnesses, particularly ones that are the primary reason for admission or ones that attract greater clinical attention. Health service use is another candidate, especially for pneumonia where do-not-resuscitate orders may affect treatment decisions for very ill patients. We note that misclassification of hip fracture is probably minor as hospitalization is often necessary, pre-hospital mortality is low, and it is likely to appear as the admitting diagnosis. Interestingly, even for moderate scenarios, adjusting for mediator misclassification shifted results farther than adjusting for confounding. These results underscore the need to seriously address mediator misclassification when it is suspected, preferably through validation sub-studies or bias analyses at minimum.
Our results may be subject to a subtle form of length bias, where associations between antipsychotic-type and medical events are under-estimated. Survival differences across antipsychotic-type could lead to a higher apparent medical event rate among second-generation antipsychotic users and thus yield an attenuated indirect effect estimate. Methods for mediation analysis involving time-to-event mediators are still under development, and accounting for competing risks would demand cautious interpretation in this setting where mortality is the outcome. It may be worthwhile to investigate these types of biases through bias analyses for truncation.41
Our study represents a valuable step at synthesizing how various medical events contribute to the mortality difference between first- and second-generation antipsychotic initiators. We considered several mediators and adjusted for a host of mediator and mortality risk factors, with thorough bias analyses for mediator misclassification. While future studies could certainly improve upon this effort, the bias analyses suggest that acute cardiovascular events and pneumonia might explain between 15% and 45% of the mortality difference.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge Linda Valeri for providing statistical advice on implementing sensitivity analyses for mediator misclassification
SOURCES OF FINANCIAL SUPPORT
JWJ was supported by a NIMH Training Grant in Training in Psychiatric Genetics and Translational Research (T32 MH017119) and the Horace W. Goldsmith Fellowship at Harvard University; TJV was supported by a NIH grant (R01 ES 017876); DB was supported by a grant from the Alzheimer’s Association; SS is the primary investigator of unrestricted research grants from Pfizer, Novartis, and Boehringer Ingelheim. None of these funding sources had any bearing on the conception, design, conduct, reporting, or interpretation of this study.
REFERENCES
- 1.Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177–84. doi: 10.1002/pds.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–43. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 3.Jackson JW, Schneeweiss S, VanderWeele TJ, Blacker D. Quantifying the role of adverse events in the mortality difference between first and second-generation antipsychotics in older adults: systematic review and meta-synthesis. PLOS ONE. 2014;9(8):e105376. doi: 10.1371/journal.pone.0105376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA Issues Public Health Advisory for Antipsychotic Drugs used for Treatment of Behavioral Disorders in Elderly Patients. FDA Talk Paper; 2005. [Google Scholar]
- 5.US Food and Drug Administration [Accessed August 6, 2013];Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioral Disturbances. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm.
- 6.Setoguchi S, Wang PS, Alan Brookhart M, Canning CF, Kaci L, Schneeweiss S. Potential causes of higher mortality in elderly users of conventional and atypical antipsychotic medications. J Am Geriatr Soc. 2008;56(9):1644–50. doi: 10.1111/j.1532-5415.2008.01839.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Archives of internal medicine. 2001;161(2):277–84. doi: 10.1001/archinte.161.2.277. [DOI] [PubMed] [Google Scholar]
- 8.Roulson J, Benbow EW, Hasleton PS. Discrepancies between clinical and autopsy diagnosis and the value of post mortem histology; a meta-analysis and review. Histopathology. 2005;47(6):551–9. doi: 10.1111/j.1365-2559.2005.02243.x. [DOI] [PubMed] [Google Scholar]
- 9.Pratt N, Roughead EE, Salter A, Ryan P. Choice of observational study design impacts on measurement of antipsychotic risks in the elderly: a systematic review. BMC Med Res Methodol. 2012;12(1):72. doi: 10.1186/1471-2288-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanderWeele TJ. Bias formulas for sensitivity analysis for direct and indirect effects. Epidemiology. 2010;21(4):540–51. doi: 10.1097/EDE.0b013e3181df191c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VanderWeele TJ, Chiba Y. Sensitivity analysis for direct and indirect effects in the presence of exposure-induced mediator-outcome confounders. Epidemiology, Biostatistics, and Public Health. 2014;11(2):e9027, 1–12. doi: 10.2427/9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderweele TJ, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiologic Methods. 2013;2(1):95–115. doi: 10.1515/em-2012-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Accessed December 1, 2012];Social Security Death Master File. http://www.ssdmf.com.
- 14.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 15.Jackson JW. Does it look like a sequentially randomized trial? Covariate balance in studies of time-varying and other joint-exposures. Presented at: Society for Epidemiologic Research SERdigital 2014 Conference; November 6, 2014; [Accessed 1/21/2015]. https://epiresearch.org/ser50/serdigital/ [Google Scholar]
- 16.Vansteelandt S, Keiding N. Invited commentary: G-computation--lost in translation? Am J Epidemiol. 2011;173(7):739–42. doi: 10.1093/aje/kwq474. [DOI] [PubMed] [Google Scholar]
- 17.Valeri L, Vanderweele TJ. Mediation Analysis Allowing for Exposure-Mediator Interactions and Causal Interpretation: Theoretical Assumptions and Implementation With SAS and SPSS Macros. Psychol Methods. 2013;18(2):137–50. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339–48. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baron JA, Lu-Yao G, Barrett J, McLerran D, Fisher ES. Internal validation of Medicare claims data. Epidemiology. 1994;5(5):541–4. [PubMed] [Google Scholar]
- 20.McDonald KM, Hlatky MA, Saynina O, Geppert J, Garber AM, McClellan MB. Trends in hospital treatment of ventricular arrhythmias among Medicare beneficiaries, 1985 to 1995. Am Heart J. 2002;144(3):413–21. doi: 10.1067/mhj.2002.125498. [DOI] [PubMed] [Google Scholar]
- 21.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43(2):182–8. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Aronsky D, Haug PJ, Lagor C, Dean NC. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–28. doi: 10.1177/1062860605280358. [DOI] [PubMed] [Google Scholar]
- 24.White RH, Garcia M, Sadeghi B, Tancredi DJ, Zrelak P, Cuny J, Sama P, Gammon H, Schmaltz S, Romano PS. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res. 2010;126(1):61–7. doi: 10.1016/j.thromres.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):100–28. doi: 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60(4):397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Strom BL. Data validity issues in using claims data. Pharmacoepidemiol Drug Saf. 2001;10(5):389–92. doi: 10.1002/pds.610. [DOI] [PubMed] [Google Scholar]
- 28.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–37. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Ogburn EL, VanderWeele TJ. Analytic results on the bias due to nondifferential misclassification of a binary mediator. Am J Epidemiol. 2012;176(6):555–61. doi: 10.1093/aje/kws131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valeri L, VanderWeele TJ. The estimation of direct and indirect causal effects in the presence of a misclassified binary mediator. Biostatistics. 2014;15(3):498–512. doi: 10.1093/biostatistics/kxu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement Error in Nonlinear Models: A Modern Perspective. 2nd ed Chapman and Hall/CRC; 2006. [Google Scholar]
- 32.Lyles RH, Lin J. Sensitivity analysis for misclassification in logistic regression via likelihood methods and predictive value weighting. Stat Med. 2010;29(22):2297–309. doi: 10.1002/sim.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang PS, Schneeweiss S, Setoguchi S, Patrick A, Avorn J, Mogun H, Choudhry NK, Brookhart MA. Ventricular arrhythmias and cerebrovascular events in the elderly using conventional and atypical antipsychotic medications. J Clin Psychopharmacol. 2007;27(6):707–10. doi: 10.1097/JCP.0b013e31815a882b. [DOI] [PubMed] [Google Scholar]
- 34.Huybrechts KF, Schneeweiss S, Gerhard T, Olfson M, Avorn J, Levin R, Lucas JA, Crystal S. Comparative safety of antipsychotic medications in nursing home residents. J Am Geriatr Soc. 2012;60(3):420–9. doi: 10.1111/j.1532-5415.2011.03853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huybrechts KF, Rothman KJ, Silliman RA, Brookhart MA, Schneeweiss S. Risk of death and hospital admission for major medical events after initiation of psychotropic medications in older adults admitted to nursing homes. CMAJ. 2011;183(7):E411–9. doi: 10.1503/cmaj.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finkel S, Kozma C, Long S, Greenspan A, Mahmoud R, Baser O, Engelhart L. Risperidone treatment in elderly patients with dementia: relative risk of cerebrovascular events versus other antipsychotics. Int Psychogeriatr. 2005;17(4):617–29. doi: 10.1017/S1041610205002280. [DOI] [PubMed] [Google Scholar]
- 37.Salzman C. Clinical geriatric psychopharmacology. 4th ed Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 38.Sacchetti E, Trifiro G, Caputi A, Turrina C, Spina E, Cricelli C, Brignoli O, Sessa E, Mazzaglia G. Risk of stroke with typical and atypical anti-psychotics: a retrospective cohort study including unexposed subjects. J Psychopharmacol. 2008;22(1):39–46. doi: 10.1177/0269881107080792. [DOI] [PubMed] [Google Scholar]
- 39.Chan MC, Chong CS, Wu AY, Wong KC, Dunn EL, Tang OW, Chan WF. Antipsychotics and risk of cerebrovascular events in treatment of behavioural and psychological symptoms of dementia in Hong Kong: a hospital-based, retrospective, cohort study. Int J Geriatr Psychiatry. 2010;25(4):362–70. doi: 10.1002/gps.2347. [DOI] [PubMed] [Google Scholar]
- 40.Jackson JW, VanderWeele TJ, Viswanathan A, Blacker D, Schneeweiss S. The explanatory role of stroke as a mediator of the mortality difference between older adults who initiate first vs. second generation antipsychotics. Am J Epidemiol. 2014;180(8):847–852. doi: 10.1093/aje/kwu210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell E, Schliep K, Schisterman E. Trucation Investigations - Using Sensitivity Analyses to Uncover Potential Sources of Bias Due to Truncation in Time to Event Studies. Presented at: Society for Epidemiologic Research SERdigital 2014 Conference; November 6, 2014; [Accessed 1/21/2015]. https://epiresearch.org/ser50/serdigital/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


