Abstract
Individuals with obsessive-compulsive disorder (OCD) often complain of doubt related to memory. As neuropsychological research has demonstrated that individuals with OCD tend to focus on details and miss the larger context, the construct of source (contextual) memory may be particularly relevant to memory complaints in OCD. Memory for object versus contextual information relies on partially distinct regions within the prefrontal cortex, parietal and medial temporal lobe, and may be differentially impacted by OCD. In the present study we sought to test the hypothesis that individuals with OCD exhibit impaired source memory retrieval using a novel memory paradigm-The Memory for Rooms Test (MFRT)- a four-room memory task in which participants walk through four rooms and attempt to encode and remember objects. Demographically matched individuals with OCD and healthy controls studied objects in the context of four rooms, and then completed a memory retrieval test while undergoing functional magnetic resonance imaging (fMRI). While no differences were observed in source memory accuracy, individuals with OCD exhibited greater task related activation in the posterior cingulate cortex (PCC) relative to healthy controls during correct source memory retrieval. During correct object recognition, individuals with OCD failed to recruit the dorsolateral prefrontal(DLPFC)/premotor, left mPFC, and right parietal regions to the same extent as healthy controls. Our results suggest abnormal recruitment of frontal-parietal and PCC regions during source verses object memory retrieval in OCD. Within the OCD group, activation in the PCC and the premotor/DLPFC was associated with greater pathological doubt. This finding is consistent with the observation that OCD patients often experience extreme doubt, even when memory performance is intact.
1. Introduction
Individuals with obsessive-compulsive disorder (OCD) often complain of poor memory and neuropsychological research has demonstrated impairments, particularly on tasks involving strategic processing (Deckersbach et al. , 2000, Savage et al. , 1999, Savage et al. , 2000, Vandborg et al. , 2014). Meaningful organization of information is known to enhance encoding and retrieval of new memories, and previous research has shown that individuals with OCD over-focus on details and miss the larger context (Savage, Deckersbach, 2000). Given findings that individuals with OCD tend to miss the “big picture,” source memory may be particularly relevant to OCD. Source memory refers to the ability to remember the specific context of a learning episode. Poor source memory may contribute to doubt, which in OCD is often characterized by a feeling of uncertainty that an action aimed at preventing harm has been completed (Radomsky et al. , 2014). Compulsive checking may further contribute to doubt related to memory as checking leads to the creation of multiple episodes under very similar contexts (e.g., “How do I know I am remembering the most recent time I turned off the stove and not some other time?”). Indeed van den Hout and Kindt (2003) showed that repeated checking reduced memory confidence and vividness of the last checking episode in a non-clinical sample(van den Hout and Kindt, 2003). The goal of the present study was to test the hypothesis that OCD is associated with impairment in source memory retrieval, and abnormal recruitment of frontal-parietal and cingulate cortices regions.
Source memory involves the binding of content with context. One example of source memory is the need to remember where objects are located. The ability to recall where an object was seen involves linking the item (object) with source (location). Support for a distinction between source and item memory comes from results of behavioral and neuroimaging studies in healthy and neurologically impaired groups. For example, a study by Koriat et al (1991) found that performing an action increases memory for that action (item specific memory) while decreasing memory for the context of the action (source memory). Therefore, it is possible that the act of compulsive checking may increase item specific memory while decreasing source memory. While memory for the action may be intact, low memory confidence likely arises from poor memory for the context of the action (e.g., “I remember checking the stove. Am I remembering the last time or some other time?”). This may partially explain the paradoxical observation that repeated checking diminishes memory confidence in OCD (Radomsky et al. , 2006, van den Hout and Kindt, 2003).
In healthy subjects, the prefrontal cortex, medial temporal lobe and parietal regions support source memory retrieval (Mitchell and Johnson, 2009, Slotnick et al. , 2003). Greater activation in prefrontal cortex has been consistently associated with source versus item memory (Dennis et al. , 2008, Mitchell and Johnson, 2009). Within the medial temporal lobe, the hippocampus is thought to bind different aspects of experience, and therefore contributes to source memory (DeVito and Eichenbaum, 2010, Eldridge et al. , 2000). Activity in the posterior cingulate and parietal regions are also consistently observed during episodic and source retrieval, the later may be dependent on top-down attention driven processes (Cabeza et al. , 2008, Maddock et al. , 2001). Memory for different types of information (contextual verses object) may, therefore, rely on distinct regions within the PFC, parietal, posterior cingulate cortex and medial temporal lobe, and may be differentially impacted by obsessive-compulsive symptoms. While no studies that we are aware of have examined neural correlates of source memory in OCD, functional neuroimaging studies have found abnormal recruitment of frontal-parietal regions during tasks that tap episodic memory. Based on this past research, we predicted that individuals with OCD would exhibit impaired source memory retrival coupled with abnormal recruitment of frontal-parietal and posterior cingulate cortex regions during source versus item memory retrieval.
The type of source information may also be important (Baddeley, 1982, Moscovitch, 1992). Associative source information is more closely associated with the stimulus itself (e.g., color of the word or mode of presentation), while extrinsic or organizational source information is independent of the stimulus (e.g., where an object appeared or in what order). Because associative source information is more closely tied with the stimulus it may be less dependent on organization (Spencer and Raz, 1995). While some studies support memory deficits in OCD (Kim et al. , 2009, Rubenstein et al. , 1993), other studies have failed to find differences in behavioral performance between individuals with OCD and healthy controls (Constans et al. , 1995, McNally and Kohlbeck, 1993, Moritz et al. , 2006). Given findings that individuals with OCD neglect the context of the learning episode and focus on irrelevant details rather than structural elements (Savage, Baer, 1999), impairments are likely to be greater on organizational source memory tasks. The present study focused on organizational source memory in order to maximally stress effortful linkages in source memory. It is also possible that individuals with OCD demonstrate similar behavioral performance, but still engage alternate brain networks, possibly in a compensatory fashion. For example, a previous study demonstrated altered frontoparietal activation during a working memory task in OCD, even while behavioral performance was unaffected (de Vries et al. , 2013).
Past research has also suffered from a lack of ecological validity. Although memory deficits have been reported on some neuropsychological tests (Anderson and Savage, 2004, Savage, Deckersbach, 2000), it is not clear how differences in performance observed in OCD on these abstract tests translate to real life settings. Accordingly, the present study examined performance in the context of a memory test with high ecological validity. The task involved objects presented in the context of four different household rooms that participants walked through prior to the scanning session. This allowed us to test memory retrieval for objects as well as memory for source (which room the object was viewed in) during scanning. Given previous work supporting the role of the PFC, PCC and parietal regions in source memory in healthy adults (Glisky et al. , 1995, Johnson et al. , 1997) and evidence of frontal-parietal dysfunction in OCD during working memory (de Vries, de Wit, 2013), we hypothesized that individuals with OCD would exhibit source memory impairments and that such impairments would be coupled with abnormal recruitment of these regions during correct source versus object retrieval. As individuals with OCD often report doubt and decreased vividness for memory episodes, even when no obvious differences in behavioral performance are observed (Boschen and Vuksanovic, 2007, Radomsky, Dugas, 2014), we expected to observe relationships between severity of doubting symptoms and abnormal activation of these regions during source versus object retrieval.
2. Methods and Materials
2.1 Participants
Participants were sixteen females with OCD and seventeen healthy control females. Because past studies suggest that males and females process context information differently (Piefke and Fink, 2005), for this initial study we selected an all female sample. Participants were recruited from an outpatient treatment program, advertisements, and an undergraduate research subject pool. All participants were between 18 and 50 years of age. Groups were matched for age, gender (all female), handedness (all R), education, and general cognitive ability (estimated IQ). Each participant was administered the Mini International Neuropsychiatric Interview (MINI;Sheehan et al. , 1998) and the Wechsler Abbreviated Scale of Intelligence Vocabulary and Matrix Reasoning subtests (WASI;Corporation, 1999) by a trained clinician. Participants in the OCD group met criteria for OCD as assessed by the MINI.
Study exclusion criteria included the presence of any other Axis I disorder, neurological illness or injury, MRI contradiction, or current use of antipsychotic, stimulant, or anxiolytic (e.g., benzodiazepine) medication. As previous research suggests that compulsive hoarding may be distinct from OCD in terms of neurobiology and clinical course (Saxena, 2007), individuals with clinically significant compulsive hoarding symptoms (Steketee and Frost, 2003) were also excluded. Individuals who were taking antidepressant medication (SSRI only) were on a stable dose for at least two months prior to scanning. The University of Kansas Medical Center Human Subjects Committee approved all study procedures, and informed written consent was obtained from all study participants.
2.2 Memory Encoding Task
The current study aimed to build on previous memory paradigms (Dennis, Hayes, 2008, Mitchell et al. , 2006), by employing a recently developed ecologically valid task to tap into object and source memory. Traditionally, item and source memory tasks have be limited to photographs of objects (e.g., abstract shape) and sources (e.g., red or blue background color or left or right side of the screen). Unlike previous source memory paradigms, we used a physical space (objects within rooms), which were encoded outside the scanner. Participants actually walked through these four rooms. This allowed us to examine source memory in a more naturalistic manner, and tap into the real world experience of remembering where an object was seen. Based on our prior work, the MFRT was found to have good internal reliability (split half reliability, r(58) =.7), and a high level of internal consistency (Cronbach alpha=.8).
2.3 Study Stimuli
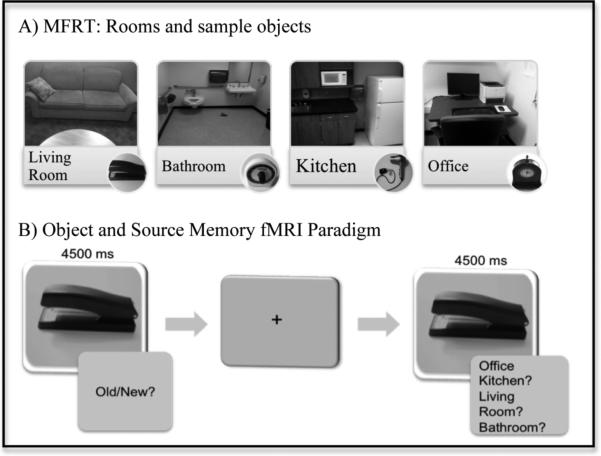
The study stimuli consisted of 96 objects and four rooms. Objects were exemplifiers of each context (e.g., bathroom-toothbrush, living room-coasters, office-stapler). Each room contained six objects that were context-congruent and six context-incongruent objects. Incongruent objects were selected from the other categories, for example the bathroom always contained six bathroom objects, two living room objects, two kitchen objects, and two office objects. Both context-congruent and incongruent items were used to make source memory judgments more difficult. Objects were rotated and counterbalanced across conditions. The order of room presentation was rotated across conditions. Objects were photographed on a grey background for use during the fMRI memory retrieval test. For room pictures and sample objects see figure 1.
Figure 1.
Memory for Rooms Test: A) Rooms and sample objects: Each room contained 12 target objects. Target and distracter objects were counter balanced across rooms and across participants. B) Object and source memory fMRI paradigm: Participants viewed pictures of objects, which were present in the rooms during encoding (targets) and some that were not (distracters). They were asked to indicate (using a response button) whether each object was old or new, and also for objects that were identified as “old” to indicate the source (which room the object seen in). The order of these responses choices was counterbalanced between participants. A variable length of fixation was viewed between trials.
2.4 Procedures
Following the consent procedure, a trained clinician administered clinical assessments. Anxiety and depressive symptoms were measured by the Beck Anxiety Inventory (BAI;Beck, 1993) and Beck Depression Inventory-II (BDI-II;Beck, 1996). Obsessive-compulsive symptoms were measured by the Dimensional Obsessive Compulsive Scale (DOCS;Abramowitz et al. , 2010), which includes four OCD symptom dimensions (Contamination, Symmetry, Unacceptable Thoughts, and Responsibility for Harm/Mistakes), and the Yale-Brown Obsessive Compulsive Scale (YBOCS;Goodman et al. , 1989). During a second visit, participants were asked to walk through four different rooms (office, living room, bathroom, and kitchen). Individuals were given the following instructions: “I will be showing you a number of rooms, try to remember as much as you can about the things you see. You may walk around inside the room, but do not touch anything. You will have 30 seconds per room.”
2.5 fMRI Paradigm
Following the encoding of rooms outside the scanner, participants completed a recognition test in the scanner during which they made both item and source memory judgments. Participants were presented with ninety-six pictures (48 target items and 48 distracters) and were asked to identify whether the object was old or new. Recognition stimuli (item and source) were presented for 4.5 seconds each and followed by fixation ranging from 0.5 - 23 seconds. The intertrial interval was “jittered” by a variable duration of fixation in order to allow separation of the brain response from one trial to the next. Following the fixation, if an object was identified as old, a second screen appeared and participants were instructed to indicate the location (room) in which the item was learned. If an object was identified as new, the next object appeared on the screen (see figure 1). Participants indicated their choice by pressing the appropriate button on the response box. The order of response options was counterbalanced across participants. Old and new items were presented randomly, the order of the old and new items as well as the duration of the intertrial intervals were optimized by AFNI's the RFSgen program (Cox, 1996).
2.5.1 Scanning Parameters
fMRI scanning was performed using a 3-Tesla head-only Siemens Allegra Scanner (Siemens, Erlangen, Germany) fitted with a quadrature head coil. Structural scanning included T1-weighted anatomical images with 3D MPRAGE sequence (TR/TE= 23/4ms, flip angel =8°, FOV=256 mm, matrix=256×192, slice thickness=1 mm). This scan was used for slice localization for the functional scans, Talairach transformation, and coregistration with fMRI data. Gradient echo blood oxygen level dependent (BOLD) scans were acquired in 42 ascending interleaved oblique axial 3 mm slices at a 40° angle (TR= 2500 ms, TE= 30ms, flip angle = 90°, in-plane resolution=3mm). Visual stimuli were back-projected to a screen from a shielded LCD projector.
2.6 fMRI Analysis
fMRI data were analyzed using the BrainVoyager QX (version 2.4.2) statistical package (Brain Innovation, Maastricht, Netherlands, 2012). Following standard preprocessing steps (motion correction, temporal filtering, and spatial smoothing), functional images were aligned to the anatomic images obtained within each session and normalized to Talairach space (Talairach, 1988). Functional scans were discarded if participants moved greater than 3mm. No subjects were left out of the analysis due to motion. A general linear model was carried out for each subject individually. This GLM incorporating task effects, and six motion parameters derived from realignment corrections, was convolved with the hemodynamic response and used to compute parameter estimates and contrast images at each voxel. Contrast images for each subject were then submitted to a second level analysis in which subjects were treated as a random effect. Between group comparisons were conducted using independent sample t-tests across the whole brain. Contrasts were restricted to those of theoretical interest. To assess the neural regions associated with object memory, item memory hits were contrasted with correct rejection of distracters. As source memory involves both object recognition and memory for spatial and temporal context, source memory related activity was obtained by contrasting source memory hits vs. item memory hits. Voxel values were considered significant if the activation survived a statistical threshold of p < .01. A family-wise approach was used to correct for multiple comparisons at p < .05 corrected, determined by Monte Carlo simulation in Brain Voyager. Secondary analyses of extracted cluster data were performed in SPSS to examine the relationship to OCD symptom dimensions.
3. Results
3.1 Demographic Data
There were no significant differences between the OCD and HC group in terms of age, education, or general cognitive ability (Table 1). Regarding medication use, 27% (n = 9) of participants (in the OCD group) were on a stable dose of a selective serotonin reuptake inhibitor (SSRI). Participants with OCD reported a moderate level of OC symptoms compared to the healthy participants, who reported minimal symptoms.
Table 1.
Demographics
| OCD Mean (SD) | HC Mean (SD) | t | df | p | |
|---|---|---|---|---|---|
| Age | 25.1 (8.6) | 26.7 (9.7) | .47 | 31 | .64 |
| Education | 15.1 (1.6) | 15.3 (1.9) | .28 | 31 | .78 |
| WASI | 119.3 (10.1) | 120.2 (5.0) | .33 | 21.6 | .75 |
| Y-BOCS | 18.9 (3.6) | .89 (1.4) | −18.9 | 19.3 | .0000001 |
| DOCS | 24.5 (9.8) | 2.7 (2.9) | −8.5 | 17.4 | .0000001 |
| BAI | 14.2 (7.1) | 2.6 (3.0) | −5.8 | 19.1 | .00001 |
| BDI | 16.6 (10.5) | 2.7 (3.9) | −5.0 | 18.8 | .00009 |
Note. WASI= Wechsler Abbreviated Scale of Intelligence; Y-BOCS= Yale-Brown Obsessive Compulsive Scale- Self Report Version; DOCS= Dimensional Obsessive Compulsive Scale; BAI= Beck Anxiety Inventory; BDI-II= Beck Depression Inventory-Second Edition
3.2 Behavioral Performance
Accuracy and reaction time results are shown in table 2. There were no significant differences in behavioral performance between the OCD and HC groups on target (t= 1.2, p=0.26), distracter (t= −0.239, p = 0.81), or source memory accuracy (t= −0.79, p= 0.43). In addition, no significant differences were observed in terms of reaction time (all p's > .05). Furthermore, there were no significant differences in accuracy or reaction time between medicated (SSRI) and unmedicated participants within the OCD group (all p's >.10).
Table 2.
Behavioral Performance
| OCD Mean (SD) | HC Mean (SD) | t | df | p | |
|---|---|---|---|---|---|
| Target % Accuracy | 85.9 (4.5) | 82.3 (11.9) | 1.2 | 20.7 | .26 |
| Target % Accuracy for Incongruent Objects | 89.58(1.57) | 83.82(13.3) | −1.60 | 23.05 | .12 |
| Target % Accuracy for Congruent Objects | 82.55(8.50) | 80.88(13.4) | −.42 | 31 | .68 |
| Distracter % Accuracy | 86.1 (11.2) | 86.9 (8.4) | −.239 | 31 | .81 |
| Object Discriminability | 86.0 (6.0) | 84.6 (7.8) | −.57 | 31 | .57 |
| Target Response Time (ms) | 1851 (232) | 1778 (248) | −.86 | 31 | .40 |
| Distracter Response Time (ms) | 2265 (434) | 2114 (273) | −1.18 | 31 | .25 |
| Source % Accuracy | 76.3 (22.1) | 81.5 (14.4) | −.79 | 31 | .43 |
| Source Reaction Time (ms) | 1560 (355) | 1377 (244) | −1.7 | 31 | .10 |
| Source % Accuracy for Incongruent Objects | 74.7(19.2) | 76.32(17.1) | 2.62 | 22.8 | .80 |
| Source % Accuracy for Congruent Objects | 78.15(25.9) | 86.35(14.1) | 1.12 | 31 | .28 |
3.3 fMRI Results
3.3.1 Contrast 1: Target-Hits versus Correct Rejections
An analysis was performed examining group differences for the contrast Target Hits versus Correct Rejections. The regions resulting from this analysis identified brain regions in which the OCD group showed greater activation compared to healthy controls during correct recognition of target objects compared to correct rejection of distracters. There were no regions of significant difference for this contrast.
3.3.2 Contrast 2: Source Correct versus Target Hits
Second, we performed an analysis examining group differences on source versus object memory, this time focusing Source Hits versus Target Hits. Statistically significant differences were observed between the OCD and HC groups (see Table 3). Specifically, the OCD group exhibited greater source versus item memory effects in the left mPFC, right premotor cortex/dorsolateral PFC, bilateral posterior cingulate cortex (PCC), and right parietal cortex, when compared to the control group. Conversely, the HC group did not show significantly greater brain activation during source memory performance compared to item memory, in any brain region, compared to individuals with OCD. These relationships are depicted in Figure 2.
Table 3.
Regions of Significant activation for Random Effects GLM Contrast of Source Hit versus Target Hit, OCD versus HC
| Regions Reaching Significance During GLM Contrasts of OCD > Healthy Control | |||||||
|---|---|---|---|---|---|---|---|
| Talairach Coordinates |
|||||||
| Region and Contrast | L/R | X | Y | Z | Voxels | t | P value |
| Source Hit vs. Target Hit | |||||||
| Left Medial Prefrontal Cortex | L | −10 | 46 | 15 | 7 | 3.63 | .0010 |
| Premotor cortex/Dorsolateral prefrontal cortex | R | 32 | 4 | 36 | 12 | 4.04 | .00034 |
| Right Posterior Cingulate Cortex | R | 5 | −47 | 24 | 11 | 4.02 | .00035 |
| Left Posterior Cingulate Cortex | L | −13 | −44 | 27 | 13 | 4.26 | .00018 |
| Right Parietal Lobe | R | 29 | −65 | 39 | 7 | 3.45 | .0016 |
Figure 2.
Comparison of Source Correct versus Target Hits by OCD versus HC groups. Mean percent signal change in the maximally activated voxel for each cluster was extracted and calculated for each condition. Areas of activation were found in the left medial PFC (A), right premotor cortex/DLPFC (B), the right parietal lobe (C), left cingulate cortex (D), and right cingulate cortex (E).
In the left mPFC, group differences were driven by a greater decrease in activation in the OCD group during correct recognition of targets compared to the HC group. In the right premotor cortex/DLPFC, group differences were driven by the HC group exhibiting increased activation during correct recognition of targets compared to the OCD group (See Figure 2). The HC also exhibited greater activation during target correct versus source correct trials, while no differences between target correct and source correct trials were observed for the OCD group.
In the bilateral PCC, group differences were driven by increased activation in the bilateral PCC in the OCD group compared to the HC during source correct. The OCD group exhibited deactivation in the right PCC during correct object recognition, while the HC group exhibited deactivation during both correct target and source recall. In the left PCC, the OCD group exhibited increased activation for source recognition, and deactivation during object recognition. The HC group exhibited the opposite pattern, with increased activation for object recognition, and decreased activation during source recall. Thus, the OCD group exhibited greater source memory-related activation in the bilateral PCC, while the HC group exhibited greater object memory activation in left PCC region. Finally, in the right parietal lobe, group differences were driven by increased activation during object recognition in the HC compared to the OCD group.
3.4 Relationship to OC Symptom Dimensions and Behavioral Performance
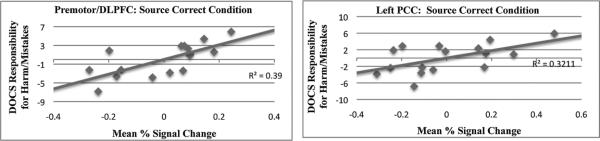
Partial correlation analyses (controlling for age) were conducted within the OCD group only to examine the relationship between depression, anxiety, overall OCD symptom severity (measured by the Y-BOCS), and four OCD symptom dimensions (Contamination, Symmetry, Unacceptable Thoughts, and Responsibility for Harm/Mistakes) with each cluster. During correct source memory retrieval, obsessional doubt related to responsibility for harm/mistakes positively correlated with activation in the premotor/DLPFC (r(13)= .71, p= .003) and left PCC (r(13)=.57, p=.028) regions (See Figure 3). With regard to anxiety, depressive symptoms, and overall OCD symptom severity, no significant relationships were observed between any cluster and BDI-II, BAI, or Y-BOCS score (all p's >.05).
Figure 3.
Greater activation in premotor/DLPFC and left PCC during source correct retrieval was positively associated with OCD participant's severity scores on the DOCS Responsibility for Harm/Mistakes scale.
Partial correlation analyses (controlling for age) were conducted within each group to examine the relationship between behavioral performance and cluster activation. Within the HC group, greater differential activation between source and target in the premotor/DLPFC was associated with increased source memory accuracy, (r(14)=.52, p=.038). No significant relationship was observed within the OCD group between premotor/DLPFC activation and memory accuracy. Within the OCD group, greater activation during target correct in the parietal region was associated with lower distracter accuracy (r(13)= −.69, p=.004). In contrast, for the HC group, there was a trend in the opposite direction, such that activation during target correct in the parietal region was associated with greater accuracy for novel (distracter) objects (r(14)=.46, p=.06).
The relationship between extracted cluster data and SSRI medication use was examined using independent t-tests. Within the OCD group, no significant differences in cluster activation for source-target were observed between medicated and unmedicated participants, all p's>.10. Group contrast results also did not change markedly when covarying for age, education, IQ, and medication use. See supplementary information for more detailed information on these analyses.
4. Discussion
In this study we examined object and source memory in individuals with OCD using an ecologically valid paradigm. No differences were observed between the OCD and HC group in terms of behavioral performance. Our findings are consistent with some past studies (Constans, Foa, 1995, McNally and Kohlbeck, 1993, Moritz, Jacobsen, 2006), which failed to find behavioral differences in memory performance between individuals with OCD and healthy controls. One possible explanation for inconsistent findings related to OCD and memory may be that individuals with OCD have latent deficits that are not always apparent at the behavioral level. In addition, it has been proposed that memory problems in OCD my not so much affect “what” is remembered but rather “how” information is remembered (Savage, 2002). Consistent with this theory, we found that individuals with OCD engage alternative brain networks to support memory retrieval, even in the context of normal behavioral performance. Despite similarities in performance, individuals with OCD also exhibited different patterns of task-related activation in the left mPFC, right premotor cortex/DLPFC, parietal lobe, and PCC relative to healthy controls during correct source versus object recognition. The absence of performance differences is advantageous in interpreting the neuroimaging results, because differences in the observed neural correlates of memory between the two groups cannot be attributed to differences in overall performance (Morcom et al. , 2007). The mPFC, DLPFC, parietal lobe, and PCC have been implicated in memory (Fan et al. , 2003, Suzuki and Amaral, 1994), as well as neurobiological processes underlying OCD (Busatto et al. , 2000, Friedlander and Desrocher, 2006, Rauch et al. , 2001a, Rauch et al. , 2001b). Within the OCD group, activation in the premotor/DLPFC and left PCC regions were associated with OC symptoms. These regions were differentially activated during source versus object retrieval, and positively correlated with pathological doubt. This finding is consistent with the observation that OCD patients often experience extreme doubt, even when memory performance is intact (Tolin et al. , 2001, van den Hout and Kindt, 2003).
4.1 Medial PFC
The OCD and HC groups exhibited differential activation in the mPFC during correct source versus object recognition. Specifically, the OCD group had a greater reduction in left mPFC activation during correct object recognition (vs. source judgments) compared to the HC group. The mPFC serves executive functions that are essential for behavioral flexibility, action monitoring, and behavioral control (Passetti et al. , 2002). PFC dysfunction has been identified as a neurobiological marker of cognitive inflexibility and behavioral disinhibition associated with neuropsychiatric disorders such as OCD. The important role of the mPFC in top-down cognitive control mechanisms is particularly well documented (Narayanan and Laubach, 2006, Passetti, Chudasama, 2002). A greater reduction in activation in this region, may suggest diminished flexibility and action monitoring. This finding is consistent with previous work that found decreased mPFC activation in individuals with OCD, and their unaffected relatives compared to HC subjects during a working memory task, despite no differences in behavioral performance (Chamberlain et al. , 2008).
4.2 Premotor/Dorsolateral PFC
The OCD group exhibited reduced activation across a brain region comprising both premotor cortex and DLPFC during correct object recognition compared to the HC group. Within the HC group, greater differential activation between source and target in the premotor/DLPFC was also associated with increased source memory accuracy. The DLPFC is involved in motor planning, working memory, and anticipating actions. Reduced activation in the DLPFC during correct object recognition compared to the HC group may suggest impaired motor planning in OCD (den Braber et al. , 2010, Schlosser et al. , 2010, van den Heuvel et al. , 2005). During source retrieval, both groups engaged the premotor/DLPFC to the same extent; however within the OCD group, activation was positively associated with obsessional doubt regarding past mistakes and responsibility for harm.
4.3 Posterior Cingulate Cortex
The OCD group exhibited greater source memory activation in the bilateral PCC compared to the HC group. The PCC plays a key role in memory, as it has strong reciprocal connections with memory structures in the medial temporal lobe (Suzuki and Amaral, 1994). Imaging studies have found the PCC to be activated during naturally acquired autobiographical memory retrieval (Andreasen et al., 1995, Maddock, Garrett, 2001). The findings of increased activation in this region, observed in the present study, may represent compensatory activation in individuals with OCD since no performance differences between the HC and OCD group were observed. Although neurobiological models of OCD have emphasized frontal-stratial dysfunction, past studies also suggest a role for the PCC in OCD (Busatto, Zamignani, 2000, Rauch, Dougherty, 2001a, Rauch, Makris, 2001b). For example, a study examining preoperative predictors of treatment for OCD patients undergoing an anterior cingulotomy found that preoperative glucose metabolic rates in the right PCC significantly correlated with subsequent reduction in OCD symptom severity following anterior cingulotomy (Rauch, Dougherty, 2001a). A positive correlation between regional cerebral blood flow in the PCC and subsequent symptomatic improvement following treatment with medication has also been reported (Rauch et al. , 2002). The PCC is also a part of the default mode network (DMN), and several studies demonstrate abnormal DMN network connectivity in OCD (Stern et al. , 2012). The PCC functionally plays a role in attention to internal mental processes. Individuals with OCD may have difficulty disengaging from irrelevant internal mental processes during memory retrieval and this may contribute to doubt. If OCD impairs inhibition, as proposed, lack of inhibition may reflect lack of suppression of irrelevant details of the learning episode or failure to inhibit internal mental processes in individuals with OCD. Consistent with the theory of failed inhibition, we found that in individuals with OCD, activation in the left PCC during source memory retrieval was associated with greater obsessional doubt related to responsibility for harm and past mistakes.
4.4 Parietal Cortex
Differences between the OCD group and HC were also observed in the parietal cortex. Specifically, the HC group exhibited greater activation for target correct compared to the OCD group. The parietal cortex is frequently activated during episodic retrieval (Cabeza and Nyberg, 2000). Several studies found that certain parietal regions, specifically the superior parietal region, show greater activity for recollection than for familiarity (Eldridge, Knowlton, 2000, Henson et al. , 1999). This region is thought to be involved in the voluntary allocation of attention, and is associated with top-down (attention guided by goals) processes supporting retrieval search, monitoring and verification (Cabeza, Ciaramelli, 2008). As such, dysfunction related to this region does not always translate to deficits in behavioral performance. Past studies examining object and source performance in individuals with parietal lesions, revealed intact object and source memory. However, subjects with parietal lesions reported decreased vividness and lack of confidence in their memory, despite intact behavioral performance (Davidson et al. , 2008). The present study found reduced activation in this region during correct object recognition in individuals with OCD compared to controls, which may reflect greater attention-driven recollection in the HC group compared to the OCD group.
4.5 Strengths/Limitations and Future Directions
To date, no studies have examined neural correlates of source memory in individuals with OCD and the current study did so with a novel and ecologically valid task. An additional strength was the exclusion of individuals with OCD who met criteria for other axis I disorders. Groups were also carefully matched for gender, age, education, handedness (all right), and general cognitive ability.
The present study revealed novel findings regarding altered source memory processing in OCD. However, some limitations of the study should be noted. These include the modest sample size, and exclusion of males. Because past studies suggest that males and females process context information differently (De Goede and Postma, 2008, Lejbak et al. , 2009, Wang and Fu, 2009), collapsing males and females into statistical comparison may bias fMRI results. Future studies may explore potential sex differences in functional activation in OCD compared to healthy adults using this paradigm.
A further limitation includes the inclusion of individuals with OCD who were taking an SSRI medication. While it is possible that antidepressant medication may impact the findings of this study, a study comparing neuropsychological performance between SSRI medicated and unmedicated individuals with OCD found no differences between the two groups on a comprehensive battery of neuropsychological tests (Mataix-Cols et al. , 2002). Additionally, a study examining the effects of an SSRI (escitalopram) found no significant effects on performance or the hemodynamic response during working memory tasks (Rose et al. , 2006). In this study, no significant performance or cluster activation differences were observed between medicated and unmedicated OCD participants. Therefore, we do not believe that the observed differences between the OCD and HC group were due to medication effects.
5. Conclusions
Results of this study suggest that, despite similarity on behavioral performance measures, individuals with OCD exhibited different patterns of task-related brain activation. While the level of performance was matched between individuals with OCD and healthy controls, the groups differentially recruited several brain regions to support memory retrieval. Differences between the OCD and HC groups emerged in brain regions known to play a role in planning, anticipating actions, and cognitive flexibility, as well as brain regions implicated in episodic retrieval. Our results also suggest that for individuals with OCD, activation in the premotor/DLPFC and PCC during source retrieval is associated with greater obsessional doubt regarding responsibility for harm and concerns about past mistakes. Future longitudinal studies should examine how cognitive processes such as cognitive flexibility and memory contribute to OCD symptoms, such as doubt. Increased understanding of the role of neural processes related to memory and cognitive processing in OCD may lead to increased understanding of this debilitating disorder, and more targeted interventions.
Supplementary Material
Highlights.
This study examined object and source memory in OCD using a novel task.
Group differences emerged in neural correlates of object and source retrieval.
Altered source retrieval was associated with doubting symptoms in OCD.
Acknowledgements
This research was supported by the National Institute of Mental Health National Research Service Award (F31 MH090690), and the Hoglund Brain Imaging Center Pilot Fund. The Hoglund Brain Imaging Center at the University of Kansas Medical Center is supported by a generous gift from Forrest and Sally Hoglund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors have no biomedical financial conflicts of interest or potential conflicts to report.
References
- Abramowitz JS, Deacon BJ, Olatunji BO, Wheaton MG, Berman NC, Losardo D, et al. Assessment of obsessive-compulsive symptom dimensions: development and evaluation of the Dimensional Obsessive-Compulsive Scale. Psychological assessment. 2010;22:180–98. doi: 10.1037/a0018260. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Savage CR. Cognitive and neurobiological findings in obsessive-compulsive disorder. The Psychiatric clinics of North America. 2004;27:37–47, viii. doi: 10.1016/S0193-953X(03)00107-2. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. The American journal of psychiatry. 1995;152:1576–85. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Implications of neuropsychological evidence for theories of normal memory. Philosophical transactions of the Royal Society of London. 1982;298:59–72. doi: 10.1098/rstb.1982.0072. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Boschen MJ, Vuksanovic D. Deteriorating memory confidence, responsibility perceptions and repeated checking: comparisons in OCD and control samples. Behav Res Ther. 2007;45:2098–109. doi: 10.1016/j.brat.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Zamignani DR, Buchpiguel CA, Garrido GE, Glabus MF, Rocha ET, et al. A voxel-based investigation of regional cerebral blood flow abnormalities in obsessive-compulsive disorder using single photon emission computed tomography (SPECT). Psychiatry research. 2000;99:15–27. doi: 10.1016/s0925-4927(00)00050-0. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–25. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–2. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- Constans JI, Foa EB, Franklin ME, Mathews A. Memory for actual and imagined events in OC checkers. Behav Res Ther. 1995;33:665–71. doi: 10.1016/0005-7967(94)00095-2. [DOI] [PubMed] [Google Scholar]
- Corporation TP. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. The Psychological Corporation; San Antonia, TX: 1999. [Google Scholar]
- Davidson PS, Anaki D, Ciaramelli E, Cohn M, Kim AS, Murphy KJ, et al. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–55. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Goede M, Postma A. Gender differences in memory for objects and their locations: a study on automatic versus controlled encoding and retrieval contexts. Brain Cogn. 2008;66:232–42. doi: 10.1016/j.bandc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- de Vries FE, de Wit SJ, Cath DC, van der Werf YD, van der Borden V, van Rossum TB, et al. Compensatory Frontoparietal Activity During Working Memory: An Endophenotype of Obsessive-Compulsive Disorder. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Otto MW, Savage CR, Baer L, Jenike MA. The relationship between semantic organization and memory in obsessive-compulsive disorder. Psychotherapy and Psychosomatics. 2000;69:101–7. doi: 10.1159/000012373. [DOI] [PubMed] [Google Scholar]
- den Braber A, van 't Ent D, Cath DC, Wagner J, Boomsma DI, de Geus EJ. Brain activation during cognitive planning in twins discordant or concordant for obsessive-compulsive symptoms. Brain : a journal of neurology. 2010;133:3123–40. doi: 10.1093/brain/awq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Eichenbaum H. Distinct contributions of the hippocampus and medial prefrontal cortex to the “what-where-when” components of episodic-like memory in mice. Behav Brain Res. 2010;215:318–25. doi: 10.1016/j.bbr.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Knight RT. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. The Journal of neuroscience : the official journal of the Society for Neuroscience.;2005;25:8333–7. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–52. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fan J, Gay Snodgrass J, Bilder RM. Functional magnetic resonance imaging of source versus item memory. Neuroreport. 2003;14:2275–81. doi: 10.1097/00001756-200312020-00028. [DOI] [PubMed] [Google Scholar]
- Friedlander L, Desrocher M. Neuroimaging studies of obsessive-compulsive disorder in adults and children. Clinical psychology review. 2006;26:32–49. doi: 10.1016/j.cpr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhuieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–35. [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:3962–72. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Kounios J, Nolde SF. Electrophysiological brain activity and memory source monitoring. Neuroreport. 1997;8:1317–20. doi: 10.1097/00001756-199703240-00051. [DOI] [PubMed] [Google Scholar]
- Kim YY, Roh AY, Yoo SY, Kang DH, Kwon JS. Impairment of source memory in patients with obsessive-compulsive disorder: equivalent current dipole analysis. Psychiatry research. 2009;165:47–59. doi: 10.1016/j.psychres.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Koriat A, Ben-Zur H, Druch A. The contextualization of input and output events in memory. Psychological Resarch. 1991;53:260–70. doi: 10.1007/s004260050034. [DOI] [PubMed] [Google Scholar]
- Lejbak L, Vrbancic M, Crossley M. The female advantage in object location memory is robust to verbalizability and mode of presentation of test stimuli. Brain Cogn. 2009;69:148–53. doi: 10.1016/j.bandc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–76. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Alonso P, Pifarre J, Menchon JM, Vallejo J. Neuropsychological performance in medicated vs. unmedicated patients with obsessive-compulsive disorder. Psychiatry research. 2002;109:255–64. doi: 10.1016/s0165-1781(02)00024-0. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Kohlbeck PA. Reality monitoring in obsessive-compulsive disorder. Behav Res Ther. 1993;31:249–53. doi: 10.1016/0005-7967(93)90023-n. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull. 2009;135:638–77. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Johnson MK, Greene EJ. An fMRI investigation of short-term source memory in young and older adults. Neuroimage. 2006;30:627–33. doi: 10.1016/j.neuroimage.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb Cortex. 2007;17:2491–506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Moritz S, Jacobsen D, Willenborg B, Jelinek L, Fricke S. A check on the memory deficit hypothesis of obsessive-compulsive checking. Eur Arch Psychiatry Clin Neurosci. 2006;256:82–6. doi: 10.1007/s00406-005-0605-7. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–67. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–31. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex. 2002;12:1254–68. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Piefke M, Fink GR. Recollections of one's own past: the effects of aging and gender on the neural mechanisms of episodic autobiographical memory. Anat Embryol (Berl) 2005;210:497–512. doi: 10.1007/s00429-005-0038-0. [DOI] [PubMed] [Google Scholar]
- Radomsky AS, Dugas MJ, Alcolado GM, Lavoie SL. When more is less: doubt, repetition, memory, metamemory, and compulsive checking in OCD. Behaviour research and therapy. 2014;59:30–9. doi: 10.1016/j.brat.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Radomsky AS, Gilchrist PT, Dussault D. Repeated checking really does cause memory distrust. Behav Res Ther. 2006;44:305–16. doi: 10.1016/j.brat.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Dougherty DD, Cosgrove GR, Cassem EH, Alpert NM, Price BH, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biological psychiatry. 2001a;50:659–67. doi: 10.1016/s0006-3223(01)01188-x. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Makris N, Cosgrove GR, Kim H, Cassem EH, Price BH, et al. A magnetic resonance imaging study of regional cortical volumes following stereotactic anterior cingulotomy. CNS Spectr. 2001b;6:214–22. doi: 10.1017/s1092852900008592. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Dougherty DD, Alpert NM, Fischman AJ, Jenike MA. Predictors of fluvoxamine response in contamination-related obsessive compulsive disorder: a PET symptom provocation study. Neuropsychopharmacology. 2002;27:782–91. doi: 10.1016/S0893-133X(02)00351-2. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Simonotto E, Spencer EP, Ebmeier KP. The effects of escitalopram on working memory and brain activity in healthy adults during performance of the n-back task. Psychopharmacology (Berl) 2006;185:339–47. doi: 10.1007/s00213-006-0334-2. [DOI] [PubMed] [Google Scholar]
- Rubenstein CS, Peynircioglu ZF, Chambless DL, Pigott TA. Memory in sub-clinical obsessive-compulsive checkers. Behav Res Ther. 1993;31:759–65. doi: 10.1016/0005-7967(93)90006-g. [DOI] [PubMed] [Google Scholar]
- Savage CR. The role of emotion in strategic behavior: Insights from Psychopathology. In: Feldman BL, Solovey P, editors. The Wisdom in Feeling: Psychological Processes in Emotional Intelligence. Guilford Press; New York: 2002. pp. 211–36. [Google Scholar]
- Savage CR, Baer L, Keuthen NJ, Brown HD, Rauch SL, Jenike MA. Organizational strategies mediate nonverbal memory impairment in obsessive-compulsive disorder. Biol Psychiatry. 1999;45:905–16. doi: 10.1016/s0006-3223(98)00278-9. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Wilhelm S, Rauch SL, Baer L, Reid T, et al. Strategic processing and episodic memory impairment in obsessive compulsive disorder. Neuropsychology. 2000;14:141–51. doi: 10.1037//0894-4105.14.1.141. [DOI] [PubMed] [Google Scholar]
- Saxena S. Is compulsive hoarding a genetically and neurobiologically discrete syndrome? Implications for diagnostic classification. The American journal of psychiatry. 2007;164:380–4. doi: 10.1176/ajp.2007.164.3.380. [DOI] [PubMed] [Google Scholar]
- Schlosser RG, Wagner G, Schachtzabel C, Peikert G, Koch K, Reichenbach JR, et al. Frontocingulate effective connectivity in obsessive compulsive disorder: a study with fMRI and dynamic causal modeling. Human Brain Mapping. 2010;31:1834–50. doi: 10.1002/hbm.20980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4-57. [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10:527–39. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Steketee G, Frost R. Compulsive hoarding: current status of the research. Clin Psychol Rev. 2003;23:905–27. doi: 10.1016/j.cpr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One. 2012;7:e36356. doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:1856–77. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Steriotaxic Atlas of the Human Brain. Thieme Meical Publishers, Inc.; New York: 1988. [Google Scholar]
- Tolin DF, Abramowitz JS, Brigidi BD, Amir N, Street GP, Foa EB. Memory and memory confidence in obsessive-compulsive disorder. Behav Res Ther. 2001;39:913–27. doi: 10.1016/s0005-7967(00)00064-4. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Archives of General Psychiatry. 2005;62:301–9. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- van den Hout M, Kindt M. Repeated checking causes memory distrust. Behav Res Ther. 2003;41:301–16. doi: 10.1016/s0005-7967(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Vandborg SK, Hartmann TB, Bennedsen BE, Pedersen AD, Thomsen PH. Memory and executive functions in patients with obsessive-compulsive disorder. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2014;27:8–16. doi: 10.1097/WNN.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Wang B, Fu XL. Gender difference in the effect of daytime sleep on declarative memory for pictures. J Zhejiang Univ Sci B. 2009;10:536–46. doi: 10.1631/jzus.B0820384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.