Abstract
The vertebrate retina has specific functions and structures that give it a unique set of constraints on the way in which it can produce and use metabolic energy. The retina’s response to illumination influences its energy requirements, and the retina’s laminated structure influences the extent to which neurons and glia can access metabolic fuels. There are fundamental differences between energy metabolism in retina and that in brain. The retina relies on aerobic glycolysis much more than the brain does, and morphological differences between retina and brain limit the types of metabolic relationships that are possible between neurons and glia. This Mini-Review summarizes the unique metabolic features of the retina with a focus on the role of lactate shuttling.
Keywords: retina, Müller cell, glia, neuron, glucose, lactate shuttle, photoreceptor, astrocyte neuronal lactate shuttle
Within a vertebrate eye, light from the environment creates an image directly on the surface of the retina. The retina detects the pattern of illumination and encodes its temporal and spatial characteristics for further processing by the brain. The retina consumes energy to do this, and its structural and functional requirements introduce a unique set of constraints for the metabolism that produces that energy.
FUNCTIONS OF THE VERTEBRATE RETINA AND THEIR RELATIONSHIPS TO ENERGY METABOLISM
Light and darkness are detected initially by photoreceptors, the neurons in the outermost layer of the retina (Dowling, 2012). In darkness, cyclic guanosine monophosphate (cGMP) activates ion channels in the outer segments of photoreceptors so that Na+ and Ca2+ flow into the cell (Arshavsky et al., 2002). Photoreceptors in darkness hydrolyze substantial amounts of ATP to power very active ion pumps that maintain the membrane potential (Ames et al., 1992; Okawa et al., 2008). These pumps are located in the photoreceptor’s inner segment (Molday et al., 2007).
Light stimulates hydrolysis of cGMP (for review see Arshavsky et al., 2002), which slows influx of Na+ and Ca2+ through cGMP-gated channels. This substantially diminishes the ATP consumption required to maintain ion gradients across the plasma membrane. The metabolism of the cell shifts away from energy production to more anabolic activities (Hurley et al., 2014). The cell hyperpolarizes, which slows the rate at which it releases glutamate into the synapse. Light also stimulates specific anabolic requirements, regeneration of visual pigment (Cornwall et al., 2003), and replacement of membranes (Rajala and Anderson, 2003).
Differences in energy supply and demand between light and darkness have been detected experimentally in many studies (Kimble et al., 1980; Winkler, 1981; Linsenmeier, 1986; Ames et al., 1992; Medrano and Fox, 1995; Wang et al., 1997a; Xu et al., 2007; Okawa et al., 2008; Winkler et al., 2008). Energy consumption was inferred from measurements of the byproducts of energy production, i.e., rates of reduction of O2 or production of CO2 or lactate. Darkness increased these parameters. The increase ranged from as little as 6% in some studies to as much as 40% in others.
A study by Okawa et al. (2008) made a different kind of prediction; instead of evaluating how much energy is produced, the investigators calculated how much energy is consumed in light vs. in darkness. They predicted the amount of energy consumed by photoreceptors in light vs. in darkness based on quantification of ion currents. The authors concluded that each mouse rod photoreceptor consumes 108 ATP per second in darkness and only 2 × 107 ATP per second in light. That is a 400% increase in energy consumption in darkness vs. in light. At least half of the metabolites and metabolic activity in a retina are in the photoreceptor layer (Medrano and Fox, 1995; Du et al., 2013). In the inner retina, darkness increases the activity of some neurons, whereas it decreases the activity of others. Therefore, overall retinal energy consumption increases by at least 200% in darkness. There is a substantial discrepancy between this prediction of energy consumption vs. the measured differences in energy production (described in the previous paragraph). This remains to be resolved, but all researchers do agree that metabolic flux in the retina is greater in darkness than in light. The ways by which retinas control the distribution and pace of their metabolism to meet the different metabolic demands of light and darkness are only beginning to be understood.
STRUCTURAL FEATURES OF THE RETINA AND THEIR INFLUENCE ON ENERGY METABOLISM
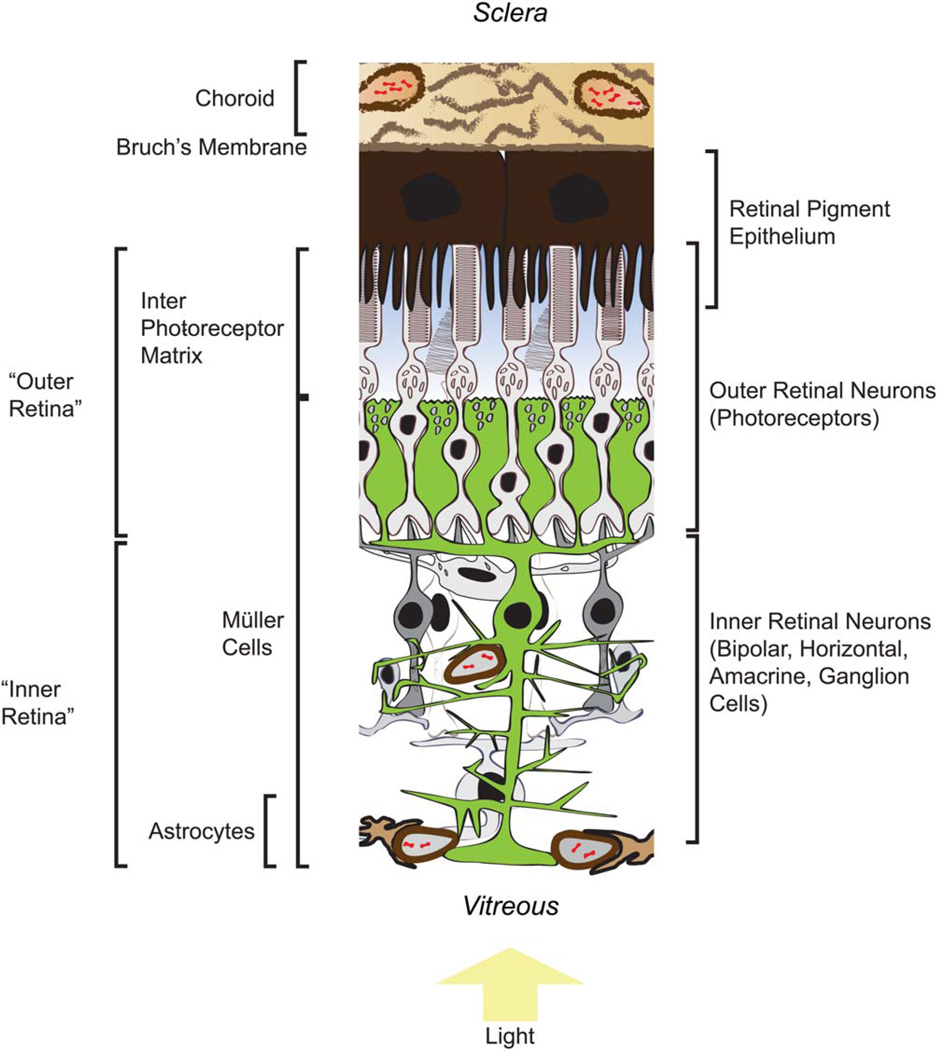
Figure 1 illustrates key structural features of the retina that influence its energy metabolism.
Fig. 1.
Key structural features that influence production and consumption of metabolic energy in a vascularized retina. See text for descriptions of the metabolic roles of these features. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Choroid
The choroid is the vascular layer of the eye between the sclera and retinal pigment epithelium (RPE; Kolb, 2005). Collagenous material, blood vessels, and fenestrated capillaries (choriocapillaris) that make up the choroid are bounded at the innermost side by basement membranes of the capillary bed. The elastic layer between this basement membrane and the basement membrane of the RPE is called Bruch’s membrane (Booij et al., 2010).
RPE
Adjacent to Bruch’s membrane is the RPE, which forms the outer blood–retina barrier. Its highly polarized cells form a basement membrane on the choroid side, whereas on the retina side apical processes intercalate between the outer segments of the photoreceptors (Bonilha, 2014). The RPE serves as a selective barrier between the blood and the retina because its cells are joined together by tight junctions that prevent diffusion.
Interphotoreceptor Matrix
Between the outer surface of the retina and the inner surface of the RPE is a proteoglycan-based material called the interphotoreceptor matrix (IPM). This is the medium through which metabolites are exchanged between the RPE and the retina (Strauss, 2005). There is evidence for a concentration gradient of metabolic fuel between the RPE and the retina. In one study, rabbit eyes were enucleated and immediately frozen before cutting the eye into serial sections and then quantifying glucose and other metabolites in each of the retinal layers (MacGregor et al., 1986). The investigators found that the amount of glucose at the photoreceptor inner segments was only ~55% of the amount of glucose at the RPE. This steepness of that glucose gradient probably was underestimated in that study because curvature of the retina in the intact eye might have caused cross-contamination of the retinal layers. No controls were available at that time to evaluate the extent of cross-contamination. More recent applications of serial section analysis used flattened retinas (Sokolov et al., 2002; Linton et al., 2010). They also used antiopsin antibodies and antibodies against other retinal proteins to screen and select only section sets that had the least amount of cross-layer contamination. These improved methods and controls have not been applied to the measurement of glucose or lactate in retinas. Nevertheless, a particularly surprising finding of the MacGregor et al. (1986) study (see Fig. 3 in that report) is that the concentration of glucose starting at the photoreceptor layer increases toward the vitreal side of the retina. This is surprising because there is no source of exogenous glucose at the vitreal side of the avascular rabbit retinas that were used in that study. This unexpected distribution of glucose cannot be explained by cross-contamination caused by curvature because the most vitreal layers (the ones with the unexpectedly high glucose) would not be contaminated by the most scleral layers.
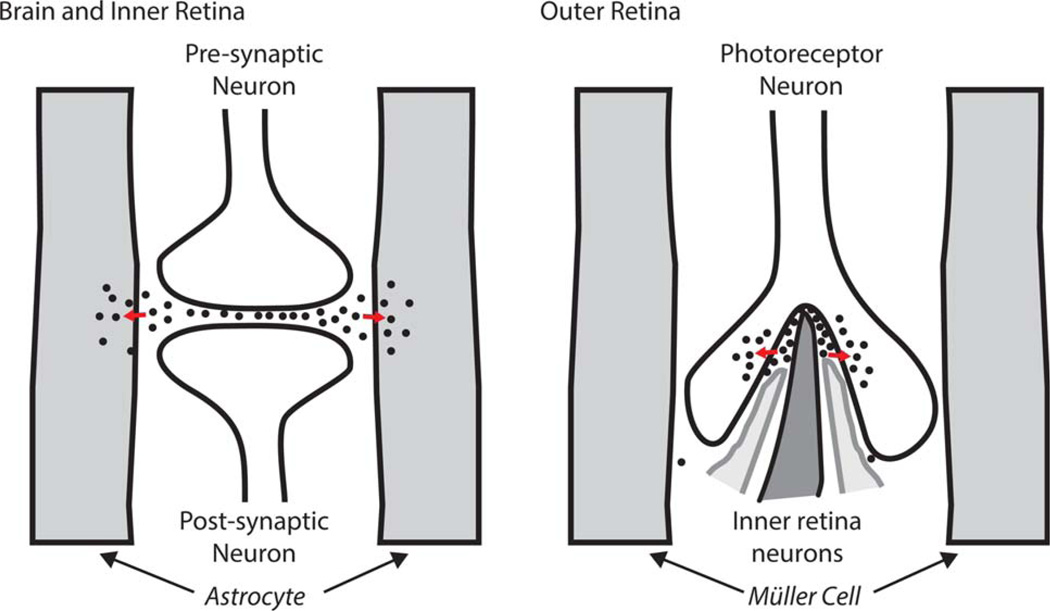
Fig. 3.
Differences in glutamate distribution in conventional synapses compared with photoreceptor synapses. In brain and in inner retinal neurons (left), glutamate (black dots) released by a presynaptic neuron can be taken up by glia and used to synthesize glutamine. At the photoreceptor synapse (right), glutamate is rapidly sequestered back into the photoreceptor before it can escape from the synapse. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Another approach has been used to evaluate the distribution of fuels in the space between the RPE and the retina isolated from bovine eyes. The surfaces of the RPE and the retina were extracted separately (Adler and Southwick, 1992). Glucose was ~50 times more abundant on the RPE surface than on the retina surface. In contrast, lactate was ~7 times more abundant at the retina surface than at the RPE surface. Of concern for these experiments is that some of the fuels might have been consumed postmortem while the tissue surfaces were being rinsed. However, the implication of the opposite directions of the apparent glucose vs. lactate gradients is important. Even if the values for glucose and lactate in that study do not reflect in vivo levels, the results still show that RPE releases more glucose and consumes more lactate than the retina, whereas the retina releases more lactate and consumes more glucose than the RPE. A more reliable evaluation of these gradients will require more sophisticated experimental strategies that allow measurements of metabolites under in vivo conditions, perhaps with fluorescent sensors.
Outer Retinal Neurons (Photoreceptors)
Photoreceptors have a uniquely polarized, elongated structure. At one end is the outer segment, highly enriched with phospholipid membranes and specialized to detect light. The other end is a synaptic terminal. The unique morphology of the photoreceptor synaptic terminal physically encapsulates dendritic tips of downstream bipolar and horizontal cells. The central portion of the photoreceptor, adjacent to the base of the outer segment, is sometimes referred to as an ellipsoid. This is where most of the transporters, glycolytic enzymes, and mitochondria are located. Most of the Na+ /K+ -ATPase that maintains ion gradients is located there (MacGregor and Matschinsky, 1986; Molday et al., 2007). This region of the retina consumes nearly all of the O2 available to it (Yu and Cringle, 2001; Wangsa-Wirawan and Linsenmeier, 2003).
Hexokinase, which catalyzes the initial phosphorylation of glucose, also is mostly localized to this region of the retina (Lowry et al., 1961; Rajala et al., 2013). Hexokinase appears to be segregated from enzymes that catalyze subsequent reactions in glycolysis and the pentose phosphate pathway. Hexokinase activity is tightly localized to just below the outer segment, whereas phosphofructokinase and glucose-6-phosphate dehydrogenase activities are displaced toward the synaptic terminal (Lowry et al., 1961). Pyruvate kinase, which catalyzes the final step in glycolysis, is unusually abundant in photoreceptors (Lindsay et al., 2014). The specific isoform pyruvate kinase M2 (PKM2) is expressed exclusively in mouse rod photoreceptors (Lindsay et al., 2014).
Mitochondria in the ellipsoid influence the refractive index of this portion of the photoreceptor and may enhance the wave-guide properties of the photoreceptor (Hoang et al., 2002). There are more mitochondria in cones than in rods, and it has been suggested that mitochondrial distribution in cones may influence the optical properties of cones to optimize visual resolution in the central fovea (Hoang et al., 2002).
Müller Cells
These specialized glial cells extend across the thickness of the retina. On the photoreceptor side they form adherens junctions with photoreceptors just below the level of the photoreceptor cell bodies. Under a microscope this layer looks like a membrane, so it was named the external-limiting membrane (ELM). Mitochondria in Müller cells are concentrated mostly in this apical part of the cell. Both the photoreceptors and the apical surfaces of the Müller cells have direct access to the IPM.
Müller cells have transporters for monocarboxylates, glucose, and amino acids (Reichenbach and Bringmann, 2013), and they synthesize glutamine (Bringmann et al., 2009). Glutamine synthesis is required to sustain neurotransmission. Injection of a glutamine synthetase inhibitor into rat eyes blocks the electroretinogram b-wave, a field potential response that depends on neurotransmission in the retina (Barnett et al., 2000).
Müller cells also synthesize, store, and degrade glycogen. The enzymes that synthesize and degrade glycogen are present in Müller cells (Pfeiffer-Guglielmi et al., 2005; Rothermel et al., 2008; Perezleon et al., 2013). The amount of glycogen stored in the retina varies across species. In general the amount of glycogen stored correlates inversely with the extent of vascularization (Kuwabara and Cogan, 1961). Guinea pig and rabbit retinas, which are avascular, store the most glycogen, whereas mouse and rat retinas, which are vascularized, have the least. In a study of guinea pig retinas, most of the glycogen was stored at the Müller cell endfeet (see Fig. 3 of Poitry-Yamate and Tsacopoulos, 1991). In another study, the concentration of glycogen in rabbit retinas was found to be ~100 times higher at the side of the retina with the Müller cell endfeet compared with the photoreceptor layer (Matschinsky et al., 1968). Dark adaptation decreases the amount of glycogen stored in rat retinas by ~60% (Coffe et al., 2004), and glycogen metabolism in retinas appears to be regulated by the insulin signaling pathway (Perezleon et al., 2013).
Enzymes associated with gluconeogenesis have been found in amphibian retinas (Goldman, 1988), and there is evidence that Müller cells synthesize their glycogen primarily from glucose that they make by gluconeogenesis (Goldman, 1988, 1990; Rodriguez and Fliesler, 1990). Glucose-6-phosphatase, required for the release of glucose from a cell, also appears to be present primarily at the Müller cell endfeet (Lessell and Kuwabara, 1964). In amphibian retinas, lactate is more effective than glucose or glutamine as a source of carbons for gluconeogenesis (Goldman, 1988). From these findings, Goldman (1990) suggested that there is a “Cori-like” cycle in the retina in which Müller cells are the site of gluconeogenesis, and the carbons for glucose synthesis come from lactate, produced by other cells in the retina. Müller cells express very little of any isoform of pyruvate kinase (Lindsay et al., 2014). Very low pyruvate kinase activity enhances the efficiency of gluconeogenesis as the dominant metabolic pathway for glucose metabolism in Müller cells.
The endfeet of Müller cells form a regular mosaic across the vitreal surface of a retina. The distribution and morphology of Müller cells are not influenced directly by the proximity of blood vessels in the way that astrocyte morphology and distribution are (Stone and Dreher, 1987). However, vascularization has several indirect effects on Müller cells (see Fig. 12 of Dreher et al., 1992). Müller cells in avascular retinas are shorter and have thicker trunks than in vascularized retinas. They have more mitochondria adjacent to their apical processes (Uga and Smelser, 1973), and they store more glycogen than Müller cells in vascularized retinas (Kuwabara and Cogan, 1961). Finally, the branching pattern and the ratio of Müller cells to neurons also are different in avascular and vascularized retinas (Dreher et al., 1992).
Inner Retinal Neurons
The inner retina consists of a diverse collection of relatively conventional neurons, including bipolar cells, horizontal cells, amacrine cells, and ganglion cells. These neurons are morphologically diverse, and their synaptic terminals are generally less encapsulated than the photoreceptor terminals. The Müller cells in this part of the retina have a morphology distinct from their morphology in the outer retina, and they do not form adherens junctions with the inner retinal neurons (Kolb, 2005). In vascularized retinas, blood vessels in the inner retina are similar to cerebral blood vessels. The inner blood–retina barrier is a layer of nonfenestrated endothelial cells with tight junctions that limit access of biomolecules to the retina.
Inner Retinal Vascularization
Nutrients reach the eye via two sources (Saint-Geniez and D’Amore, 2004). The outer retina in all species and at all developmental stages receives nutrients and O2 from the choriocapillaris. There are no blood vessels in the outer part of the retina. The other source of nutrients for some retinas is a central artery that, during early development of the eye, supplies blood to the lens. Later in development these vessels regress and attach to the vitreal surface of the retina (Saint-Geniez and D’Amore, 2004; Alvarez et al., 2007). In species with the thickest retinas, these vessels penetrate into the inner layers of the retina (Provis, 2001). In species with thinner retinas, the vessels simply remain attached to the vitreal surface. In species with the thinnest retinas, the blood vessels from the central artery withdraw altogether. Mouse, rat, cat, bovine, monkey, and human retinas are examples of thick and thoroughly vascularized retinas, whereas rabbit, guinea pig, and brush-tailed possum retinas are examples of thin, mostly avascular retinas (Freeman and Tancred, 1978; Chase, 1982; Buttery et al., 1991). Vascularization also depends on developmental stage. Inner retinas of zebrafish at early developmental stages are avascular, but at later stages they can receive nutrients from blood vessels attached to the vitreal surface of the retina (Alvarez et al., 2007).
As noted above, vascularization of a retina correlates inversely with the amount of glycogen stored in the retina (Kuwabara and Cogan, 1961). Vascularization also influences the distribution of mitochondria. When there is no inner retinal vascularization, mitochondria are confined to the ellipsoid region of the photoreceptor (Hughes et al., 1972; Bentmann et al., 2005; Stone et al., 2008). When retinas are vascularized, mitochondria also are present at the photoreceptor synaptic terminals and in neurons of the inner retina (Bentmann et al., 2005; Stone et al., 2008; Linton et al., 2010). The morphological features of ellipsoid mitochondria also are strikingly different in vascularized vs. nonvascularized retinas. Examples of this diversity are apparent in a recent report from our laboratory (see Fig. S1 of Linton et al., 2010).
Astrocytes
Astrocytes are mostly absent from avascular retinas, but they are present near the inner surface of vascularized retinas. Their distribution in those retinas is centered at the optic nerve head and correlates with regions of vascularization (Stone and Dreher, 1987). A striking difference between astrocytes and Müller cells is that the latter are distributed throughout the retina independently of vascularization (Dreher et al., 1992), whereas astrocytes are present only in vascularized regions.
The morphology of an astrocyte appears to be influenced by its affinity for the walls of blood vessels. At the inner surface of the retina, astrocytes wrap around both blood vessels and ganglion cell axon bundles (see, e.g., Fig. 1 of Stone and Dreher, 1987). Capillaries in the middle layers of a retina are not wrapped by astrocytes but instead are wrapped by processes extending from Müller cells (Kondo et al., 1984). Whereas Müller cells are born within the developing retina, astrocytes in a retina probably are not derived from the retina itself. It has been suggested that they originate in the brain and reach the retina by following blood vessels into the eye (Stone and Dreher, 1987).
UNIQUE METABOLIC FEATURES OF VERTEBRATE RETINAS
Aerobic Glycolysis
Energy metabolism of the vertebrate retina is dominated by aerobic glycolysis, i.e., production of lactate from glucose even when O2 is abundant. Warburg (1927) noted this as early as the 1920s when comparing metabolism of cancer cells with metabolism in a variety of normal tissues. He reported that the retina uses aerobic glycolysis as much as cancer cells do, an observation that has been confirmed in many subsequent investigations (Cohen and Noell, 1960; Winkler, 1981; Du et al., 2013).
Between 80% and 96% of glucose consumed by an isolated retina is made into lactate and released into the medium. In an intact eye, the outer and inner parts of the retina are served by distinct blood supplies. A comparison of arterial vs. venous glucose, lactate, and O2 in these blood supplies revealed that the majority of aerobic glycolysis occurs in the outer retina, i.e., the photoreceptor layer (Wang et al., 1997b,c).
Aerobic glycolysis dominates energy production in the outer retina, but the retina requires both glycolysis and oxidative phosphorylation to initiate vision (Ames et al., 1992). Responses of neurons downstream of photo-receptors are abolished either by inhibiting glycolysis or by removing O2 (Noell, 1951; Wilkus et al., 1971; Winkler, 1981).
Phosphocreatine Shuttle
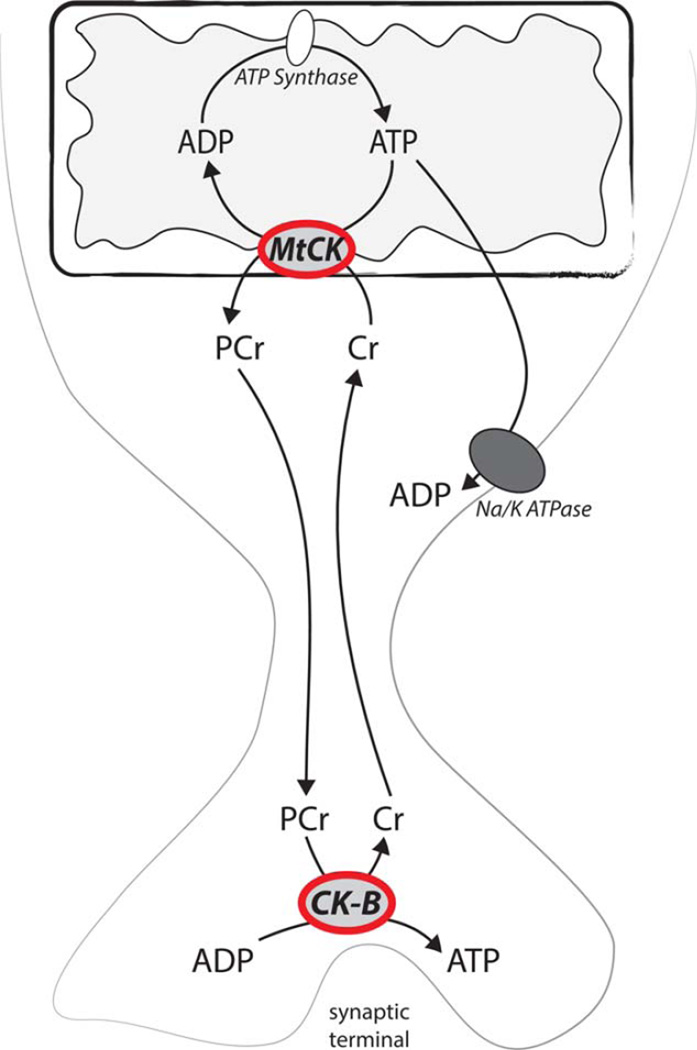
Most of the mitochondria in vertebrate photoreceptor neurons are located centrally, adjacent to the outer segment, in a region of the cell sometimes called the ellipsoid. In species in which the inner retina is vascularized, mitochondria in photoreceptors also are present at the synaptic terminal (Bentmann et al., 2005; Stone et al., 2008; Linton et al., 2010). Photoreceptors in avascular retinas have all their mitochondria only in the cell body. In these retinas there is a problem for ATP-dependent reactions that occur at the synaptic terminal, far from the ellipsoid (Linton et al., 2010). ATP is required to load neurotransmitter into synaptic vesicles. ATP made in the ellipsoid region by glycolytic enzymes (Lindsay et al., 2014) or by mitochondria may not reach the synapse because, during its transit, the ATP is exposed to highly active Na+/K+-ATPases in the plasma membranes between the mitochondria and the synaptic terminal (Molday et al., 2007). Photoreceptors in avascular retinas use a unique type of energy shuttle to solve this problem (Linton et al., 2010). They use phosphocreatine (PCr) rather than ATP to deliver high-energy phosphate from the centrally located mitochondria to the synaptic terminal. Mitochondrial creatine kinase uses ATP to phosphorylate creatine to generate PCr. After the PCr reaches the synapse, CK-B, a different isoform of creatine kinase that is localized mostly at the synapse, transfers the stored energy from PCr back to ATP to fuel neurotransmission. We demonstrated the importance of this shuttle in larval zebrafish retinas, which are not vascularized (Linton et al., 2010). The operation of this shuttle is summarized in Figure 2. Although the shuttle does not appear to have an essential role in vascularized mouse retinas (Linton et al., 2010), we found that the remarkable localization of CK-B to the synaptic terminal is retained (Linton et al., 2010).
Fig. 2.
Phosphocreatine shuttle in photoreceptors in nonvascularized retinas. When mitochondria produce ATP, mtCK converts it to PCr, which shuttles the high-energy phosphate past ion pumps in the cell body of the photoreceptor. After it has reached the synaptic terminal, the PCr is used to make ATP. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Lactate Shuttles
Lactate, the reduced form of pyruvate, can transport reducing power and carbons between cell types and between tissues (Brooks, 2009). Three well-documented examples of lactate shuttles are relevant to metabolism in the retina:
The Cori cycle
Lactate is made in muscle when the rate of glycolysis exceeds the rate at which mitochondria can oxidize pyruvate. This reaction generates the NAD+ required to sustain glycolysis in the muscle. The reducing power stored in lactate, together with its three carbons, can be used effectively by transporting it from the muscle through the blood to the liver, where it provides carbon for the synthesis of glucose by gluconeogenesis. Glucose made in this way can be transported by the blood back to the muscles to fuel glycolysis (Berg et al., 2012).
Lactate shuttle in tumors
Cancer cells in the hypoxic core of a tumor can release lactate, which diffuses to the more aerobic cortex of the tumor for oxidation (Draoui and Feron, 2011; Doherty and Cleveland, 2013).
Astrocyte neuronal lactate shuttle
Astrocytes may have better access to glucose and O2 than some neurons in brain tissue. The astrocyte neuronal lactate shuttle (ANLS) model describes how lactate can be used to shuttle reducing power from the astrocytes to those neurons (Belanger et al., 2011; Pellerin and Magistretti, 2012). According to this model, astrocytes convert glucose to lactate and then release the lactate to be taken up and oxidized by neurons. The model suggests that ANLS activity is stimulated by energy-consuming uptake of glutamate into astrocytes and energy-dependent conversion of glutamate to glutamine (Pellerin and Magistretti, 1994). However, evidence against this sort of role for glutamate metabolism also has been reported (Bauer et al., 2012; McKenna, 2013; Whitelaw and Robinson, 2013; Jackson et al., 2014; Olsen and Sonnewald, 2014). Furthermore, blockade of astrocytic glutamate transporters does not affect lactate production (Caesar et al., 2008). Overall, the ANLS model does not adequately explain many key features of the metabolic relationships between neurons and glia (Dienel, 2012a,b, 2014).
Evidence for ANLS Activity in the Vertebrate Retina
ANLS activity has been implicated in the metabolism of mammalian retinas. In one study, investigators compared metabolic characteristics of isolated Müller cells vs. Müller cells that are attached to photoreceptors. The preparations were made from guinea pig retinas, which are avascular. The investigators found that the Müller cells with attached photoreceptors released less lactate into the medium than Müller cells without attached photoreceptors (Poitry-Yamate and Tsacopoulos, 1992; Poitry-Yamate et al., 1995). The investigators concluded that this finding was consistent with the ANLS model. They reasoned that less lactate was released from the clusters because lactate made by Müller cells in the clusters was imported into photoreceptors before it could be released. The presence of monocarboxylate transporters on Müller cells was thought to be consistent with the ANLS model (Gerhart et al., 1999).
Indicators That the ANLS Might Not Accurately Represent Metabolic Relationships Between Photoreceptor Neurons and Müller Glia in the Outer Retina
Arguments have been presented both for and against a role for ANLS in brain, but there are key structural and metabolic differences between brain and retina that must limit the role of the ANLS in retina. 1) Brain astrocytes wrap around blood vessels, so they must be able to access nutrients and O2 directly from the vasculature. Many brain neurons also take up glucose directly (Patel et al., 2014), but it is possible that some brain neurons are not in locations with such direct access. It has been suggested that those types of neurons use astrocytes as a source of fuel (Belanger et al., 2011; Pellerin and Magistretti, 2012). From the morphology of the vertebrate outer retina, fewer types of metabolic relationships are possible. All photoreceptor neurons have very direct access to nutrients and O2 from the IPM (Adler and Southwick, 1992; Mieziewska, 1996). In fact, the cell bodies of the photoreceptors are closer than Müller cells are to the RPE, which is the source of glucose. This can be seen in the electron micrograph of the outer retina shown in a recent article on the zebrafish retina (see Fig. 4 of Tarboush et al., 2012). Because photoreceptors are very metabolically active, it is likely that they use up most of the glucose from the IPM before it has a chance to reach the Müller cells (Fig. 1). 2) The open structure of neuronal synapses in brain allows glutamate released at the synapse to diffuse away and be sequestered into astrocytes (Fig. 3, left). In striking contrast, photoreceptor synapses are encapsulated structures with abundant EAAT5 transporters that sequester glutamate back into the photoreceptor before it can diffuse out of the synapse (Hasegawa et al., 2006; Fig. 3, right). Experimentally, we have found that brain slices release abundant amounts of glutamate into the culture medium, whereas retinas do not (Du et al., 2013). 3) Aerobic glycolysis accounts for 2–25% of Glc metabolism in brain (Goyal et al., 2014), but it accounts for 80–96% of glucose consumption in retina (Winkler 1981; Ames et al., 1992; Wang et al., 1997a). PKM2, an isoform of pyruvate kinase associated with aerobic glycolysis in tumors, is barely detectable in brain, but it is abundant in retina, where it is expressed specifically in photoreceptor neurons (Lindsay et al., 2014). In fact, Müller cells in retina express very little of any isoform of pyruvate kinase, so it is unlikely that they can synthesize much pyruvate or much lactate from glucose. 4) Brain neurons release glutamate primarily when stimulated. In contrast, photoreceptors continuously release glutamate at their terminals in darkness because they are in a state of constant partial depolarization (Dowling, 2012).
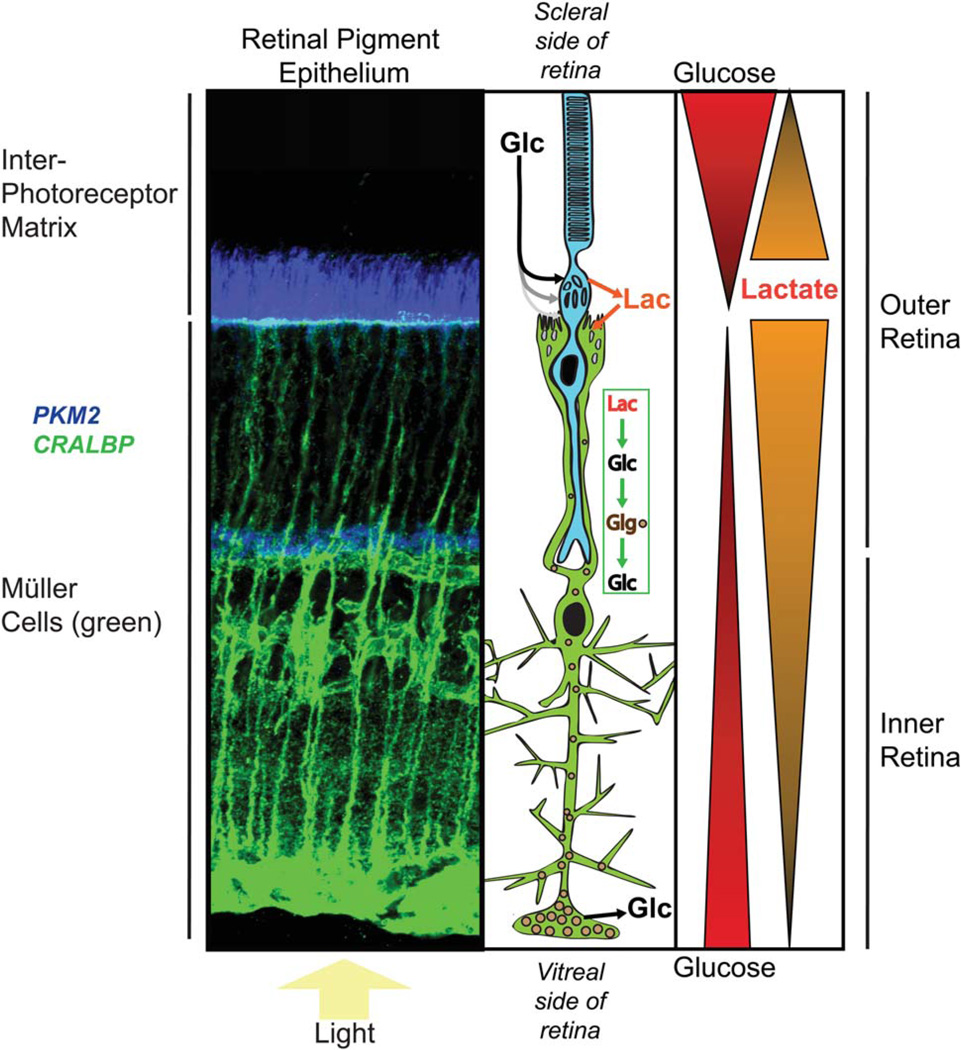
Fig. 4.
Model for distribution of metabolic fuels in an avascular retina. A z-stack of confocal images of a fixed mouse retina stained with antibodies to CRALBP (green) and PKM2 (blue) is shown for reference next to a schematic diagram showing a rod photoreceptor (blue) and a Müller glial cell (green). Evidence has been reported (MacGregor et al., 1986; Adler and Southwick, 1992) that there are gradients of glucose and lactate in the IPM, as shown at right. With this model we are hypothesizing that there also is a gradient of lactate in the retina, highest at the photoreceptor side and decreasing toward the ganglion cell side of the retina (consistent with Fig. 3 of Du et al., 2013). Evidence cited in the text shows that Müller cells can convert lactate to glucose and glycogen. The model includes this as a source of glucose in the inner retina. This model would generate a glucose gradient similar to that detected in nonvascularized rabbit retinas by MacGregor et al. (1986). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Each of these observations makes it unlikely that ANLS could have a prominent role in the outer part of the vertebrate retina. Nevertheless, previously published data have been cited as evidence for a role of ANLS in retina. The following section shows why those data do not convincingly show that ANLS influences metabolic relationships between glia and neurons in the outer retina.
Critical Evaluation of Evidence That the ANLS Governs Metabolic Relationships Between Photoreceptors and Müller Cells
The cell preparation
Poitry-Yamate et al. (1995) and Poitry et al. (2000) reported several experiments designed to address the hypothesis that Müller glia produce lactate, which then fuels photoreceptor metabolism. Their studies were based on careful analysis of three well-defined preparations: 1) acutely isolated Müller cells, 2) acutely isolated Müller cells in complexes with photo-receptors, and 3) acutely isolated photoreceptor inner segment/outer segment cell fragments. These preparations were made by using specific protocols in which guinea pig retinas were treated with collagenase, hyaluronidase, and papain; dissociated by gentle trituration; and then fractionated by density gradient centrifugation. Müller cells isolated by this type of method have a morphology that generally resembles their morphology in an intact retina. The method appears to be the best type of preparation reported to date for isolating Müller cells in a metabolic state that may be close to their state in intact retinas. Nevertheless, experiments, even with these types of preparations, must be interpreted cautiously. Müller cells undergo rapid dedifferentiation in response to stress (Goldman, 2014). The enzymes used for isolation and the physical disruption of the tissue could induce stress, alter cell surface receptors, and induce metabolic changes in the isolated cells. Consistent with this, the Tsacopoulos group (Poitry et al., 2000), in their detailed description of the method that they used to isolate Müller cells and cell clusters, noted considerable instability of the metabolic responses of these cells during the first hours after isolation.
The conclusion that only Müller cells in guinea pig retinas take up radioactively labeled 2-deoxy glucose (2-DG)
A 1990 article described auto-radiography of guinea pig retinas that had been isolated, quartered, and incubated with [3H]2-DG (Poitry-Yamate and Tsacopoulos, 1991). The authors reported that silver grains were detected only over Müller cell bodies and endfeet. However, there was no specific labeling to identify specific locations of Müller cells reliably and no statistical analysis of overlap between silver grains and Müller cells vs. silver grains and neurons. Another concern with this study is that the label 2-DG was delivered to the dissected retina while it was surrounded on all sides by the radioactive solution. It is important to note that the guinea pig retina is avascular. In an intact guinea pig eye, glucose is delivered to the retina vectorially, i.e., only from the photoreceptor side of the retina. It is important to repeat this analysis with improved methods, such as genetically encoded markers to label Müller cells, and with fluorescently labeled 2-DG.
The conclusion that Müller cell/photoreceptor complexes release less lactate than is released by Müller cells alone
Poitry-Yamate et al. (1995) used preparations of acutely isolated Müller cells and Müller cell/photoreceptor complexes to test the hypothesis that Müller cells use glucose to make and release lactate, which then fuels respiration in photoreceptor cells. Their analysis showed that isolated Müller cells convert [14C]glucose into [14C]lactate, which then was released into the culture medium. The study also found that isolated photoreceptors readily metabolize glucose. The photoreceptors had a slight preference for lactate over glucose as a fuel for respiration. The investigators found that complexes of Müller cells and photoreceptors release less lactate (per microgram protein) into the culture medium than Müller cells alone. Altogether, these experiments were interpreted to indicate that Müller cells produce lactate and photoreceptors oxidize it, consistent with the ANLS model.
However, there are straightforward and reasonable alternative explanations for those findings. Chih, Roberts, and colleagues (Chih et al., 2001a,b) described how the lactate levels were normalized incorrectly in the analysis of Poitry-Yamate and colleagues (1995). Moreover, the preference of photoreceptors for lactate as a fuel probably is not a feature that is specific for photoreceptors. It reflects a general metabolic feature of most cells; the path to oxidation from lactate is more direct than the path from glucose. The rate of glucose oxidation is limited by the rate-limiting step in glycolysis, whereas that ratelimiting step is bypassed when lactate is used as a fuel. It also is important to consider that Müller cell metabolism and homeostasis are altered by proteolysis-induced loss of the normal physical attachment to a photoreceptor cell (via adherens junctions) that occurs in an intact retina. This is especially important because Müller cells dedifferentiate very rapidly in response to stress (Goldman, 2014).
The observation that the specific activity of lactate from Müller cell/photoreceptor complexes is lower than the specific activity of lactate made by isolated Müller cells
Poitry-Yamate et al. (1995) incubated cell preparations with [14C]glucose and found that [14C]lactate released from dark-adapted Müller cell/ photoreceptor complexes has a lower specific activity than [14C]lactate released from isolated Müller cells. The authors suggested that this reflects the use of endogenous pools of unlabeled glycogen by glycolysis in Müller cells when stimulated by the presence of dark-adapted photo-receptors. However, an equally plausible explanation for this result is that the glucose released from the glycogen in Müller cells is used by photoreceptors to make lactate. Because the decrease in specific activity did not occur when the complexes were incubated in light instead of in darkness, this alternative explanation is consistent with the higher energy demands of photoreceptors in darkness compared with in light.
The observation that glutamate and ammonia stimulate production of lactate by isolated Müller cells
Poitry et al. (2000) used the preparation of acutely isolated Müller cells to investigate metabolic effects of glutamate and ammonia. Glutamate and ammonia together stimulated production and release of lactate from the isolated Müller cells. The most likely explanation for this is that ATP consumption by glutamine synthetase stimulated glycolysis and production and release of lactate, consistent with the effect of glutamine synthesis on other glial cells. This biochemical outcome would occur regardless of whether the ANLS is the dominant metabolic pathway in the retina.
Constraints on Metabolic Relationships Within the Retina Are Imposed by the Uniquely Laminated Nature of Retinal Morphology
Fuels that reach isolated Müller cells and Müller cell/photoreceptor complexes in culture dishes must be very different from fuels that reach these cells in an intact retina in an intact eye. Immunocytochemical analyses indicate that the glucose transporter Glut-1 is located on photoreceptor cell bodies that extend into the IPM beyond the ELM in rat retinas (Bergersen et al., 1999; Philp et al., 2003; Gospe et al., 2010). The ELM marks the ends of the distal processes of the Müller cells.
In vitro evidence indicates that there is a gradient of glucose in the IPM from higher at the RPE surface to lower at the retina surface (MacGregor et al., 1986; Adler and Southwick, 1992). The closer proximity of the photoreceptor cell bodies to the main source of glucose could give them a substantial advantage over Müller cells for access to glucose (Fig. 4; see also the EM image in Fig. 4 of Tarboush et al., 2012).
The apical processes of Müller cells in rat retina contain Glut-2, the glucose transporter isoform on liver and pancreatic β-cells (Watanabe et al., 1994). Glut-2 is a low-affinity, high-capacity transporter that enhances the ability of cells to sense and regulate extracellular glucose levels. Its presence on Müller cell apical processes suggests that Müller cells could have a similar role in retina.
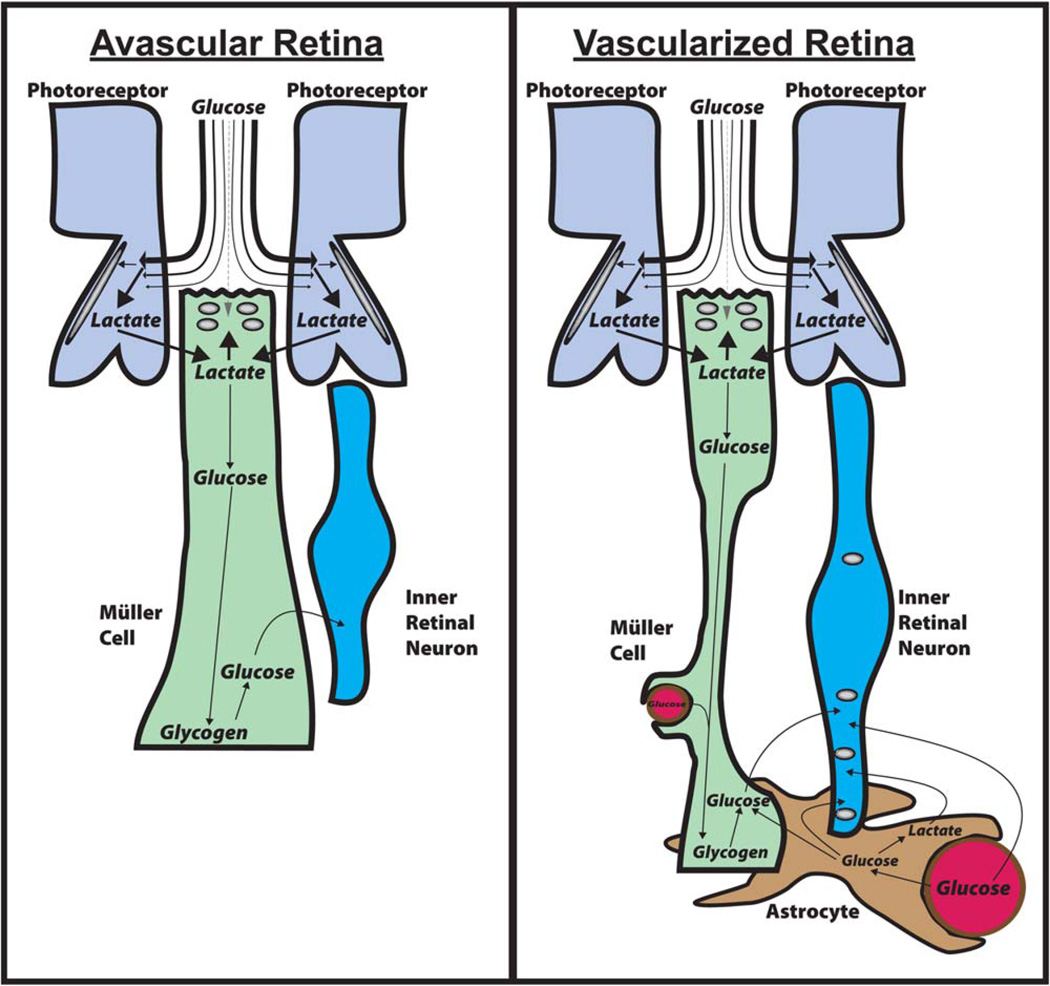
These observations are important because they suggest that photoreceptors have the opportunity to deplete most of the glucose from the IPM before it reaches Müller cells. Photoreceptors use most of this glucose to make lactate, which they release through monocarboxylate transporters. Müller cells can take up this lactate and use it for gluconeogenesis, they can store the glucose they make as glycogen and they can use glucose-6-phosphatase (Lessell and Kuwabara, 1964) to distribute glucose to other cells in the retina. These observations suggest that Müller cells control the distribution of glucose in the retina. They can make glucose, they can store it as glycogen and they can supply glucose to other cells within the retina when it is needed. This model is described and illustrated in figures 4 and 5.
Fig. 5.
Shuttling mechanisms that distribute glucose and lactate in retinas. Left: Shuttling in avascular retinas. Most of the glucose from the IPM is taken up by photoreceptors and converted to lactate. The lactate is released from photoreceptors, taken up by Müller glia, and converted to glucose by gluconeogenesis. Müller cells store the glucose as glycogen primarily at their endfeet, where it can be broken down to glucose when required to fuel inner retinal neurons. Right: In vascularized retinas the retinas are thicker and the Müller cells are thinner. Astrocytes and Müller cells wrap around blood vessels in the inner retina. In addition to the Müller cells synthesizing glucose and storing glycogen, blood vessels supply glucose to astrocytes and neurons. The astrocytes can redistribute glucose and lactate to other retinal glia and neurons. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
This hypothesis is based on published data, but more extensive and direct confirmations of these findings are required. Many of the experiments cited here were performed with the most advanced technologies available at the time, but we now have access to tools with greater sensitivity, reliability, and chemical and spatial resolution. Reliable measurements of distributions of glucose, lactate, glucose transporters, and other key players under in vivo conditions are required both to confirm the models shown in Figures 4 and 5 and to develop more accurate explanations for nutrient distribution in the retina.
Metabolic Roles of Müller Cells in Outer vs. Inner Retina Could Be Different
Metabolite shuttling is an adaptation that enhances the ability of cells to function and survive within an organism or within a tissue. Diverse shuttling mechanisms (Brooks, 2009) have evolved to meet many specific types of structures and functions. The laminated structure of the retina is required so that it can detect and process images focused on it by a lens. This unique morphological feature imposes constraints on the types of shuttling mechanisms that can be used successfully to distribute energy to the appropriate location when and where it is required. Even within the retina, the functional and structural constraints of the outer retina, which detects light, are different from those of the inner retina, which processes complex neuronal signals.
Our review of factors that influence energy metabolism in the vertebrate retina has led us to conclude that ANLS does not have a significant metabolic role in the outer retina. However, relationships between glia and neurons in the inner retina could be different from those in the outer retina. Consideration of glutamate/glutamine cycling and lactate shuttling suggests that other shuttling mechanisms, possibly including ANLS activity, could contribute more to metabolism in the inner retina than in the outer retina.
The role of Müller Cells in the Glutamate/Glutamine Cycle in the Outer and Inner retina
Müller cells in an intact eye span the retina from just below the photoreceptor cell bodies on the scleral side of the retina down to the ganglion cell fibers on the vitreal side of the retina. Glutamine synthetase is expressed throughout the length of the Müller cells. An important role of glutamine synthetase in glial cells is to supply glutamine to neurons to replenish their stores of glutamate. In a recent report, we showed that lactate and aspartate, most likely provided by photoreceptors, are the most effective fuels for synthesis of glutamine in the retina (Lindsay et al., 2014). We found evidence for pyruvate carboxylase activity in mouse retinas and in Müller cells isolated from mouse retinas, but glutamine synthesis was greatest when lactate and aspartate were present. Results of pulse-chase analyses in that study were consistent with conversion of glutamine to aspartate in photoreceptors, which then is used to make glutamine in Müller cells.
The importance of glutamine synthesis in maintaining glutamate pools in the inner vs. the outer retina has been investigated (Pow and Robinson, 1994). Glutamate pools in retinas were evaluated by immunocytochemical staining of sections of fixed rabbit retinas. Inhibiting glutamine synthesis substantially depleted the inner retinal pools of glutamate, whereas glutamate in the photoreceptor layer was insensitive to inhibition of glutamine synthetase.
Outer retina
Photoreceptors rely on Müller cells for glutamine less than conventional neurons do. The enclosed structure of the photoreceptor synapse and the high density of EAAT5 on the photoreceptor terminal reduce the amount of glutamate that escapes the synapse (Fig. 3, right).
Inner retina
Like many neurons in brain, inner retina neurons have synapses with an open structure that allows glutamate to diffuse to Müller cells (Fig. 3, at left). This depletion of glutamate makes inner retinal neurons dependent on recycling of glutamate in a glutamate/glutamine cycle.
The Roles of Müller Cells in Lactate Shuttling in the Outer and Inner Retina
Recognition that Müller cells can have distinct roles in the outer vs. the inner retina could help reconcile discrepancies between what is known about retinal structure, function, and metabolism and the idea that ANLS could influence retinal metabolism. There are several reasons why the role of lactate in the outer retina could be different from its role in the inner retina.
Outer retina
In an intact eye, photoreceptors are unlikely to be the cells with the greatest requirement for lactate as a fuel. First, among all the structures in the retina, photoreceptor cell bodies have the most direct access to glucose. In contrast, Müller cell apical surfaces are recessed below the photoreceptor cell bodies and essentially covered by a carpet of photoreceptors (Fig. 4; see also Fig. 4 of Tarboush et al., 2012). Based on the physical appearance of this part of the retina, Müller cells’ access to glucose from the IPM would be limited to what is left over after photoreceptors take what they require. Consistent with this idea, there is a steep gradient of glucose concentration that declines from the RPE to the cell bodies of photoreceptors (MacGregor et al., 1986). Second, Müller cells in the outer retina are deficient for pyruvate kinase, so they must have only a limited capacity to synthesize lactate (Lindsay et al., 2014). In contrast, photoreceptors express very high levels of the M2 isoform of pyruvate kinase (Lindsay et al., 2014). Therefore, in the outer retina, photoreceptors are most likely to be the primary consumers of glucose and producers of lactate.
Lactate released by photoreceptors can be used by Müller cells to make glucose for the inner retina (as illustrated schematically in Fig. 4). From the finding that Müller cells are capable of gluconeogenesis, glycogen synthesis, and breakdown and release of glucose (Goldman, 1990), we propose that Müller cells can use lactate from photoreceptors to synthesize glucose, store it as glycogen, and provide it to neurons of the inner retina when required. The very low expression of pyruvate kinase in Müller cells is consistent with gluconeogenesis being the predominant pathway for glucose metabolism in these cells. An important caveat for this model is that the evidence for Müller cell gluconeogenesis, thus far, comes only from amphibian retinas (Goldman, 1990). Future studies should test whether gluconeogenesis also occurs in retinas from other animals.
Inner retina
The inner retinal neurons require a source of glucose or lactate, which may be supplied through different pathways in avascular vs. vascularized retinas.
Avascular retinas
The model shown in Figures 4 and 5 (at left) make up our proposal for the network of metabolic relationships in an avascular retina. It is consistent with the findings described in this Mini-Review, including the distribution of glucose in an avascular retina described by MacGregor et al. (1986).
Vascularized retinas
Whereas photoreceptor neurons in the outer retina have more direct access than Müller cells to glucose, the relationships between neurons and glia in the vascularized inner retina may be quite different (Fig. 5, right). Processes from Müller cells contact capillaries within the inner retina (Kondo et al., 1984), and astrocytes, which wrap around blood vessels, are abundant in retinas that are vascularized (Stone and Dreher, 1987). Some glucose may reach the neurons directly, and some glucose may reach the neurons after passing either through astrocytes (Gandhi et al., 2009) or through Müller cells. Astrocytes also may convert glucose from blood into lactate and then export the lactate as fuel for inner retinal neurons. Export of lactate from astrocytes and reuptake into neurons is an inefficient process in brain (Gandhi et al., 2009), but currently there is no direct evidence that this type of relationship between astrocytes and neurons cannot occur in specific locations in vascularized inner retinas.
The studies cited in this Mini-Review are consistent with these models. However, additional studies are required to test them further. In some cases it will be helpful to use more powerful techniques that were not available when the pioneering studies were performed. For example, it will be important to determine the gradients of metabolites in live retinas. New advances in fluorescent metabolite sensors may facilitate this (Tantama et al., 2012). It also is possible to use serial sectioning to perform isotopomer analyses of metabolites to measure metabolic flux in each of the layers of an intact retina (Du et al., 2013). Such experimental approaches will more directly and precisely quantify and localize the metabolic contributions of each cell type and their relationships in functioning retinas.
REFERENCES
- Adler AJ, Southwick RE. Distribution of glucose and lactate in the interphotoreceptor matrix. Ophthalmic Res. 1992;24:243–252. doi: 10.1159/000267174. [DOI] [PubMed] [Google Scholar]
- Alvarez Y, Cederlund ML, Cottell DC, Bill BR, Ekker SC, Torres-Vazquez J, Weinstein BM, Hyde DR, Vihtelic TS, Kennedy BN. Genetic determinants of hyaloid and retinal vasculature in zebrafish. BMC Dev Biol. 2007;7:114. doi: 10.1186/1471-213X-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A, 3rd, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci. 1992;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototrans-duction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- Barnett NL, Pow DV, Robinson SR. Inhibition of Muller cell glutamine synthetase rapidly impairs the retinal response to light. Glia. 2000;30:64–73. doi: 10.1002/(sici)1098-1136(200003)30:1<64::aid-glia7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Bauer DE, Jackson JG, Genda EN, Montoya MM, Yudkoff M, Robinson MB. The glutamate transporter, GLAST, participates in a macromolecular complex that supports glutamate metabolism. Neurochem Int. 2012;61:566–574. doi: 10.1016/j.neuint.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Bentmann A, Schmidt M, Reuss S, Wolfrum U, Hankeln T, Burmester T. Divergent distribution in vascular and avascular mammalian retinae links neuroglobin to cellular respiration. J Biol Chem. 2005;280:20660–20665. doi: 10.1074/jbc.M501338200. [DOI] [PubMed] [Google Scholar]
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. New York: W.H. Freeman and Company; 2012. [Google Scholar]
- Bergersen L, Johannsson E, Veruki ML, Nagelhus EA, Halestrap A, Sejersted OM, Ottersen OP. Cellular and subcellular expression of monocarboxylate transporters in the pigment epithelium and retina of the rat. Neuroscience. 1999;90:319–331. doi: 10.1016/s0306-4522(98)00427-8. [DOI] [PubMed] [Google Scholar]
- Bonilha VL. Retinal pigment epithelium (RPE) cytoskeleton in vivo and in vitro. Exp Eye Res. 2014;126:38–45. doi: 10.1016/j.exer.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res. 2010;29:1–18. doi: 10.1016/j.preteyeres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Biedermann B, Francke M, Iandiev I, Grosche J, Wiedemann P, Albrecht J, Reichenbach A. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009;54:143–160. doi: 10.1016/j.neuint.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol. 2009;587:5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery RG, Hinrichsen CF, Weller WL, Haight JR. How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vis Res. 1991;31:169–187. doi: 10.1016/0042-6989(91)90110-q. [DOI] [PubMed] [Google Scholar]
- Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M. Glutamate receptor-dependent increments in lactate, glucose, and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. The evolution of retinal vascularization in mammals. A comparison of vascular and avascular retinae. Ophthalmology. 1982;89:1518–1525. doi: 10.1016/s0161-6420(82)34608-4. [DOI] [PubMed] [Google Scholar]
- Chih CP, He J, Sly TS, Roberts EL., Jr Comparison of glucose and lactate as substrates during NMDA-induced activation of hippocampal slices. Brain Res. 2001a;893:143–154. doi: 10.1016/s0006-8993(00)03306-0. [DOI] [PubMed] [Google Scholar]
- Chih CP, Lipton P, Roberts EL., Jr Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001b;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Coffe V, Carbajal RC, Salceda R. Glycogen metabolism in the rat retina. J Neurochem. 2004;88:885–890. doi: 10.1046/j.1471-4159.2003.02207.x. [DOI] [PubMed] [Google Scholar]
- Cohen LH, Noell WK. Glucose catabolism of rabbit retina before and after development of visual function. J Neurochem. 1960;5:253–276. doi: 10.1111/j.1471-4159.1960.tb13363.x. [DOI] [PubMed] [Google Scholar]
- Cornwall MC, Tsina E, Crouch RK, Wiggert B, Chen C, Koutalos Y. Regulation of the visual cycle: retinol dehydrogenase and retinol fluorescence measurements in vertebrate retina. Adv Exp Med Biol. 2003;533:353–360. doi: 10.1007/978-1-4615-0067-4_45. [DOI] [PubMed] [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012a;32:1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Fueling and imaging brain activation. ASN Neuro 4. 2012b doi: 10.1042/AN20120021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Lactate shuttling and lactate use as fuel after traumatic brain injury: metabolic considerations. J Cereb Blood Flow Metab. 2014;34:1736–1748. doi: 10.1038/jcbfm.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. The retina: an approachable part of the brain. Cambridge, MA: Belknap Press; 2012. [Google Scholar]
- Draoui N, Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011;4:727–732. doi: 10.1242/dmm.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher Z, Robinson SR, Distler C. Muller cells in vascular and avascular retinae: a survey of seven mammals. J Comp Neurol. 1992;323:59–80. doi: 10.1002/cne.903230106. [DOI] [PubMed] [Google Scholar]
- Du J, Cleghorn W, Contreras L, Linton JD, Chan GC, Chertov AO, Saheki T, Govindaraju V, Sadilek M, Satrustegui J, Hurley JB. Cytosolic reducing power preserves glutamate in retina. Proc Natl Acad Sci U S A. 2013;110:18501–18506. doi: 10.1073/pnas.1311193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, Tancred E. The number and distribution of ganglion cells in the retina of the brush-tailed possum, Trichosurus vulpecula. J Comp Neurol. 1978;177:557–567. doi: 10.1002/cne.901770403. [DOI] [PubMed] [Google Scholar]
- Gandhi GK, Cruz NF, Ball KK, Dienel GA. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem. 2009;111:522–536. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart DZ, Leino RL, Drewes LR. Distribution of monocarboxylate transporters MCT1 and MCT2 in rat retina. Neuroscience. 1999;92:367–375. doi: 10.1016/s0306-4522(98)00699-x. [DOI] [PubMed] [Google Scholar]
- Goldman D. Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–442. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SS. Gluconeogenesis in the amphibian retina. Lactate is preferred to glutamate as the gluconeogenic precursor. Biochem J. 1988;254:359–365. doi: 10.1042/bj2540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SS. Evidence that the gluconeogenic pathway is confined to an enriched Muller cell fraction derived from the amphibian retina. Exp Eye Res. 1990;50:213–218. doi: 10.1016/0014-4835(90)90233-k. [DOI] [PubMed] [Google Scholar]
- Gospe SM3rd, Baker SA, Arshavsky VY. Facilitative glucose transporter Glut1 is actively excluded from rod outer segments. J Cell Sci. 2010;123:3639–3644. doi: 10.1242/jcs.072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa J, Obara T, Tanaka K, Tachibana M. High-density pre-synaptic transporters are required for glutamate removal from the first visual synapse. Neuron. 2006;50:63–74. doi: 10.1016/j.neuron.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Hoang QV, Linsenmeier RA, Chung CK, Curcio CA. Photoreceptor inner segments in monkey and human retina: mitochondrial density, optics, and regional variation. Vis Neurosci. 2002;19:395–407. doi: 10.1017/s0952523802194028. [DOI] [PubMed] [Google Scholar]
- Hughes JT, Jerrome D, Krebs HA. Ultrastructure of the avian retina. An anatomical study of the retina of the domestic pigeon (Columba liva) with particular reference to the distribution of mitochondria. Exp Eye Res. 1972;14:189–195. doi: 10.1016/0014-4835(72)90002-4. [DOI] [PubMed] [Google Scholar]
- Hurley JB, Chertov AO, Lindsay K, Giamarco M, Cleghorn W, Du J, Brockerhoff S. Energy metabolism in the vertebrate retina. In: Furakawa T, Hurley JB, Kawamura S, editors. Vertebrate photoreceptor functional molecular bases. New York: Springer; 2014. pp. 91–138. [Google Scholar]
- Jackson JG, O’Donnell JC, Takano H, Coulter DA, Robinson MB. Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J Neurosci. 2014;34:1613–1624. doi: 10.1523/JNEUROSCI.3510-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble EA, Svoboda RA, Ostroy SE. Oxygen consumption and ATP changes of the vertebrate photoreceptor. Exp Eye Res. 1980;31:271–288. doi: 10.1016/s0014-4835(80)80037-6. [DOI] [PubMed] [Google Scholar]
- Kolb H. Kolb H, Nelson R, Fernandez E, Jones B, editors. Glial cells of the retina. Webvision: the organization of the retina and visual system. 2005 http://webvision.med.utah.edu.
- Kondo H, Takahashi H, Takahashi Y. Immunohistochemical study of S-100 protein in the postnatal development of Muller cells and astrocytes in the rat retina. Cell Tissue Res. 1984;238:503–508. doi: 10.1007/BF00219865. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Cogan DG. Retinal glycogen. Arch Ophthalmol. 1961;66:680–688. doi: 10.1001/archopht.1961.00960010682013. [DOI] [PubMed] [Google Scholar]
- Lessell S, Kuwabara T. Phosphatase histochemistry of the eye. Arch Ophthalmol. 1964;71:851–860. doi: 10.1001/archopht.1964.00970010867015. [DOI] [PubMed] [Google Scholar]
- Lindsay KJ, Du J, Sloat SR, Contreras L, Linton JD, Turner SJ, Sadilek M, Satrustegui J, Hurley JB. Pyruvate kinase and aspartate–glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc Natl Acad Sci U S A. 2014;111:15579–15584. doi: 10.1073/pnas.1412441111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986;88:521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton JD, Holzhausen LC, Babai N, Song H, Miyagishima KJ, Stearns GW, Lindsay K, Wei J, Chertov AO, Peters TA, Caffe R, Pluk H, Seeliger MW, Tanimoto N, Fong K, Bolton L, Kuok DL, Sweet IR, Bartoletti TM, Radu RA, Travis GH, Zagotta WN, Townes-Anderson E, Parker E, Van der Zee CE, Sampath AP, Sokolov M, Thoreson WB, Hurley JB. Flow of energy in the outer retina in darkness and in light. Proc Natl Acad Sci U S A. 2010;107:8599–8604. doi: 10.1073/pnas.1002471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Roberts NR, Schulz DW, Clow JE, Clark JR. Quantitative histochemistry of retina. II. Enzymes of glucose metabolism. J Biol Chem. 1961;236:2813–2820. [PubMed] [Google Scholar]
- MacGregor LC, Matschinsky FM. Altered retinal metabolism in diabetes. II. Measurement of sodium–potassium ATPase and total sodium and potassium in individual retinal layers. J Biol Chem. 1986;261:4052–4058. [PubMed] [Google Scholar]
- MacGregor LC, Rosecan LR, Laties AM, Matschinsky FM. Altered retinal metabolism in diabetes. I. Microanalysis of lipid, glucose, sorbitol, and myo-inositol in the choroid and in the individual layers of the rabbit retina. J Biol Chem. 1986;261:4046–4051. [PubMed] [Google Scholar]
- Matschinsky FM, Passonneau JV, Lowry OH. Quantitative histochemical analysis of glycolytic intermediates and cofactors with an oil well technique. J Histochem Cytochem. 1968;16:29–39. doi: 10.1177/16.1.29. [DOI] [PubMed] [Google Scholar]
- McKenna MC. Glutamate pays its own way in astrocytes. Front Endocrinol. 2013;4:191. doi: 10.3389/fendo.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano CJ, Fox DA. Oxygen consumption in the rat outer and inner retina: light- and pharmacologically-induced inhibition. Exp Eye Res. 1995;61:273–284. doi: 10.1016/s0014-4835(05)80122-8. [DOI] [PubMed] [Google Scholar]
- Mieziewska K. The interphotoreceptor matrix, a space in sight. Microsc Res Techniq. 1996;35:463–471. doi: 10.1002/(SICI)1097-0029(19961215)35:6<463::AID-JEMT5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Molday LL, Wu WW, Molday RS. Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex. J Biol Chem. 2007;282:32792–32801. doi: 10.1074/jbc.M706321200. [DOI] [PubMed] [Google Scholar]
- Noell WK. The effect of iodoacetate on the vertebrate retina. J Cell Physiol. 1951;37:283–307. doi: 10.1002/jcp.1030370209. [DOI] [PubMed] [Google Scholar]
- Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GM, Sonnewald U. Glutamate: where does it come from and where does it go? Neurochem Int [E-pub ahead of print] 2014 doi: 10.1016/j.neuint.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Patel AB, Lai JC, Chowdhury GM, Hyder F, Rothman DL, Shulman RG, Behar KL. Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-toneuron lactate shuttle. Proc Natl Acad Sci U S A. 2014;111:5385–5390. doi: 10.1073/pnas.1403576111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perezleon JA, Osorio-Paz I, Francois L, Salceda R. Immunohistochemical localization of glycogen synthase and GSK3beta: control of glycogen content in retina. Neurochem Res. 2013;38:1063–1069. doi: 10.1007/s11064-013-1017-0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Guglielmi B, Francke M, Reichenbach A, Fleckenstein B, Jung G, Hamprecht B. Glycogen phosphorylase isozyme pattern in mammalian retinal Muller (glial) cells and in astrocytes of retina and optic nerve. Glia. 2005;49:84–95. doi: 10.1002/glia.20102. [DOI] [PubMed] [Google Scholar]
- Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci. 2003;44:1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- Poitry S, Poitry-Yamate C, Ueberfeld J, MacLeish PR, Tsacopoulos M. Mechanisms of glutamate metabolic signaling in retinal glial (Muller) cells. J Neurosci. 2000;20:1809–1821. doi: 10.1523/JNEUROSCI.20-05-01809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitry-Yamate C, Tsacopoulos M. Glial (Muller) cells take up and phosphorylate [3H]2-deoxy-D-glucose in mammalian retina. Neurosci Lett. 1991;122:241–244. doi: 10.1016/0304-3940(91)90868-t. [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Tsacopoulos M. Glucose metabolism in freshly isolated Muller glial cells from a mammalian retina. J Comp Neurol. 1992;320:257–266. doi: 10.1002/cne.903200209. [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Muller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci. 1995;15:5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV, Robinson SR. Glutamate in some retinal neurons is derived solely from glia. Neuroscience. 1994;60:355–366. doi: 10.1016/0306-4522(94)90249-6. [DOI] [PubMed] [Google Scholar]
- Provis JM. Development of the primate retinal vasculature. Prog Retin Eye Res. 2001;20:799–821. doi: 10.1016/s1350-9462(01)00012-x. [DOI] [PubMed] [Google Scholar]
- Rajala A, Gupta VK, Anderson RE, Rajala RV. Light activation of the insulin receptor regulates mitochondrial hexokinase. A possible mechanism of retinal neuroprotection. Mitochondrion. 2013;13:566–576. doi: 10.1016/j.mito.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RV, Anderson RE. Light regulation of the insulin receptor in the retina. Mol Neurobiol. 2003;28:123–138. doi: 10.1385/MN:28:2:123. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A. New functions of Muller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- Rodriguez IR, Fliesler SJ. Glycogenesis in the amphibian retina: in vitro conversion of [2-3H]mannose to [3H]glucose and subsequent incorporation into glycogen. Exp Eye Res. 1990;51:71–77. doi: 10.1016/0014-4835(90)90172-q. [DOI] [PubMed] [Google Scholar]
- Rothermel A, Weigel W, Pfeiffer-Guglielmi B, Hamprecht B, Robitzki AA. Immunocytochemical analysis of glycogen phosphorylase isozymes in the developing and adult retina of the domestic chicken (Gallus domesticus) Neurochem Res. 2008;33:336–347. doi: 10.1007/s11064-007-9477-8. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal, and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Stone J, Dreher Z. Relationship between astrocytes, ganglion cells, and vasculature of the retina. J Comp Neurol. 1987;255:35–49. doi: 10.1002/cne.902550104. [DOI] [PubMed] [Google Scholar]
- Stone J, van Driel D, Valter K, Rees S, Provis J. The locations of mitochondria in mammalian photoreceptors: relation to retinal vasculature. Brain Res. 2008;1189:58–69. doi: 10.1016/j.brainres.2007.10.083. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Tantama M, Hung YP, Yellen G. Optogenetic reporters: fluorescent protein-based genetically encoded indicators of signaling and metabolism in the brain. Prog Brain Res. 2012;196:235–263. doi: 10.1016/B978-0-444-59426-6.00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarboush R, Chapman GB, Connaughton VP. Ultrastructure of the distal retina of the adult zebrafish, Danio rerio. Tissue Cell. 2012;44:264–279. doi: 10.1016/j.tice.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Uga S, Smelser Comparative study of the fine structure of retinal Muller cells in various vertebrates. Invest Ophthalmol. 1973;12:434–448. [PubMed] [Google Scholar]
- Wang L, Kondo M, Bill A. Glucose metabolism in cat outer retina. Effects of light and hyperoxia. Invest Ophthalmol Vis Sci. 1997a;38:48–55. [PubMed] [Google Scholar]
- Wang L, Tornquist P, Bill A. Glucose metabolism in pig outer retina in light and darkness. Acta Physiol Scand. 1997b;160:75–81. doi: 10.1046/j.1365-201X.1997.00030.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Tornquist P, Bill A. Glucose metabolism of the inner retina in pigs in darkness and light. Acta Physiol Scand. 1997c;160:71–74. doi: 10.1046/j.1365-201X.1997.00131.x. [DOI] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- Warbug O. Über die Klassifizierung tierischer Gewebe nach ihrem Stoffwechsel. Biochem Z. 1927;184:484–488. [Google Scholar]
- Watanabe T, Mio Y, Hoshino FB, Nagamatsu S, Hirosawa K, Nakahara K. GLUT-2 Expression in the rat retina; Localization at the Apical Ends of Müller cells. 1994 doi: 10.1016/0006-8993(94)91606-3. [DOI] [PubMed] [Google Scholar]
- Whitelaw BS, Robinson MB. Inhibitors of glutamate dehydrogenase block sodium-dependent glutamate uptake in rat brain membranes. Front Endocrinol. 2013;4:123. doi: 10.3389/fendo.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkus RJ, Chatrian GE, Lettich E. The electroretinogram during terminal anoxia in humans. Electroencephalogr Clin Neurophysiol. 1971;31:537–546. doi: 10.1016/0013-4694(71)90070-8. [DOI] [PubMed] [Google Scholar]
- Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77:667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Starnes CA, Twardy BS, Brault D, Taylor RC. Nuclear magnetic resonance and biochemical measurements of glucose utilization in the cone-dominant ground squirrel retina. Invest Ophthalmol Vis Sci. 2008;49:4613–4619. doi: 10.1167/iovs.08-2004. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ola MS, Berkich DA, Gardner TW, Barber AJ, Palmieri F, Hutson SM, LaNoue KF. Energy sources for glutamate neuro-transmission in the retina: absence of the aspartate/glutamate carrier produces reliance on glycolysis in glia. J Neurochem. 2007;101:120–131. doi: 10.1111/j.1471-4159.2006.04349.x. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]