Abstract
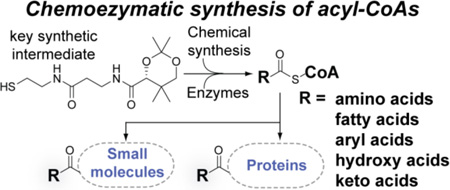
A chemoenzymatic approach to generate fully functional acyl coenzyme A molecules that are then used as substrates to drive in situ acyl transfer reactions is described. Mass spectrometry-based assays to verify the identity of acyl coenzyme A enzymatic products are also illustrated. The approach is responsive to a diverse array of carboxylic acids that can be elaborated to their corresponding coenzyme A thioesters, with potential applications in wide-ranging chemical biology studies that utilize acyl coenzyme A substrates.
Graphical abstract
In biological chemistry, coenzyme A (CoA, 1, Scheme 1) acts as a molecular shuttle for carboxylic acids linked to its terminal thiol. S-acylated derivatives of 1 (acyl-CoAs, Scheme 1) participate in numerous enzymatic reactions, including primary energy metabolism, synthesis of biomolecules, posttranslational modification of proteins, and other processes. Acyl-CoAs are also preeminent in their participation in secondary metabolism, such as in the biosynthesis of polyketide and non-ribosomal peptide antibiotics.1 In each of the above applications, the reactive thioester moiety of acyl-CoA is of primary importance, activating the attached carboxylic acid for participation in downstream enzymatic processes.
Scheme 1. Natural route for biosynthesis of acyl-CoAs.
Reactions catalyzed by CoaB and CoaC can be bypassed by synthetically appending the β-mercaptoethylamine moiety to 2.
As shown in Scheme 1, 1 is biosynthesized in bacteria via the action of the five enzymes CoaA–E.2 Pantothenic acid (2, vitamin B5) is converted into phosphopantetheine (3) by the action of three enzymes (CoaA–C). Further adenylation, followed by phosphorylation of the ribose 3’-hydroxyl, affords 1. Carboxylic acids are thioesterified to 1 by the action of ATP-dependent acyl-CoA ligases to afford acyl-CoAs. While a wide diversity of acyl-CoA ligases have been discovered, their limited substrate promiscuity has restricted their development as synthetically useful catalysts. As a result, the traditional synthesis of acyl-CoAs has relied on the chemical ligation of carboxylic acids to 1 with the aid of peptide coupling reagents to generate the thioester linkage. However, this approach suffers from several drawbacks, including the high cost of 1 as the starting material, poor yields for the coupling step, and labor-intensive HPLC-based purification. Acyl-CoAs are also known for their inherent instability by virtue of the chemically labile thioester and phosphoester linkages in the molecule. In the course of our previous studies3 requiring the preparation of acyl-CoA substrates, we encountered these problems, often representing significant challenges. These difficulties prompted us to seek an alternative and more convenient access to acyl-CoAs.
We herein report a straightforward and efficient methodology for the preparation of a wide range of acyl-CoAs. Our strategy relies on the combination of an initial chemical preparation of S-acylated pantetheine derivatives, followed by a one-pot enzymatic transformation into the desired acyl-CoA end product. We also establish a mass spectrometric assay to characterize acyl-CoA products. Furthermore, we demonstrate the applicability of chemoenzymatically generated acyl-CoAs as acyl donors in acyl-transfer reactions.
Begley and coworkers have shown that Escherichia coli CoaA, CoaD, and CoaE enzymes are promiscuous, in that they can accept substrates modified at either the carboxy terminus of 2, or at the cysteamine moiety of 3.4 Burkart and coworkers reported that this observation allows for an access route to amide and ester analogues of acyl-CoAs that relies on bypassing the first enzymatic steps (Scheme 1) by employing appropriately designed derivatives of 2 that can be extended by CoaA, CoaD, and CoaE enzymes.5 Despite these advances, it should be noted that functional acyl-CoAs with a thioester linkage that are capable of acting as physiological acyl-donors have not been previously elaborated. We have chosen a similar strategy to efficiently prepare thioester linked acyl-CoA derivatives by a chemoenzymatic route.
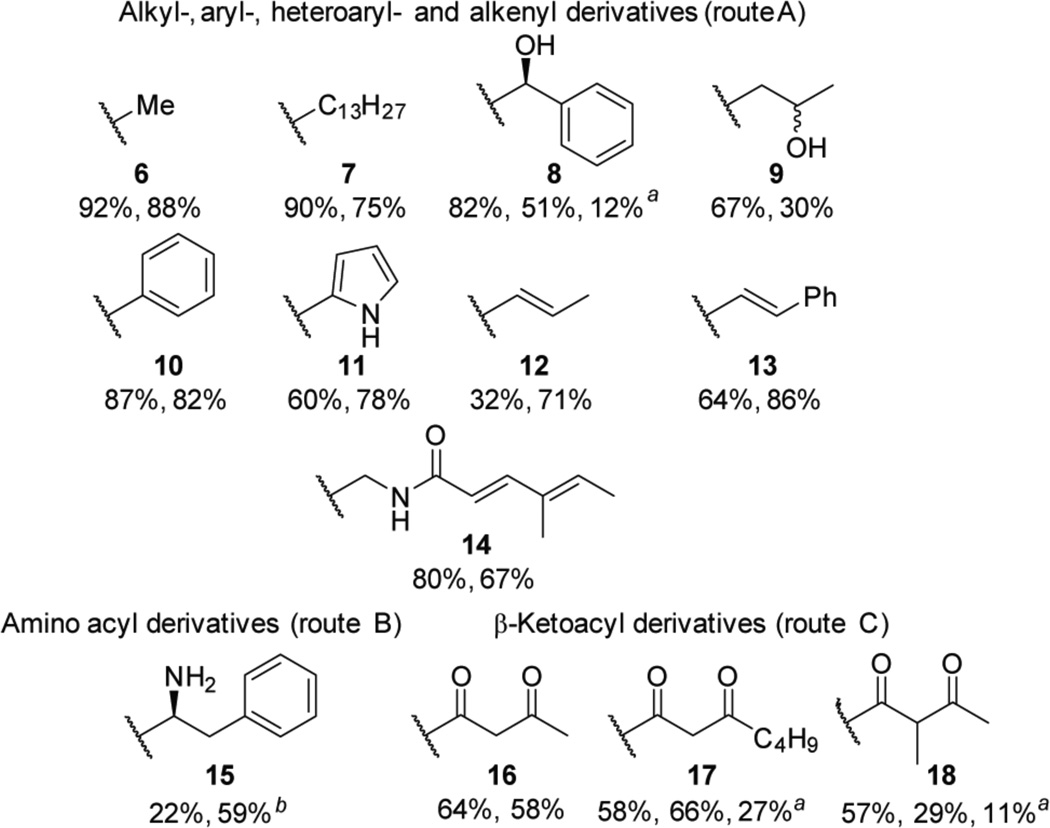
We first sought to identify an efficient synthetic strategy for the preparation of functionally diverse S-acyl pantetheines. As shown in Scheme 2, we chose acetonide 4 as a starting point.6 Peptide coupling of cysteamine to 4 delivered thiol 5 in 96% yield.7 Intermediate 5 can now be used to attach a variety of carboxylic acids (Figure 1) to the thiol head group elaborating the reactive thioester linkage. For alkyl-, aryl-, heteroaryl-, and alkenyl carboxylates, we identified an EDC-based protocol as most efficient and general.8 In particular, some challenging substrates were successfully coupled to 5 in synthetically useful yields. These include crotonic acid (12),9 pyrrole carboxylic acid (11), as well as α- and β-hydroxyacids (8, 9) (Figure 1). Subsequent removal of the acetonide protecting group proceeded smoothly using AcOH/H2O (route A, Scheme 2).10 This mild deprotection protocol did not affect sensitive functional groups, such as β-hydroxy carbonyls (9) or alkenes (12–14).11 Furthermore, no HPLC purification step was needed for most products. For the synthesis of an aminoacyl-S-pantetheine derivative, we found that a trityl-protected precursor was most convenient among several tested.12 Despite the lower coupling yield, mild conditions were sufficient for the additional deprotection step required to access this compound class (15, 1% TFA in CH2Cl2, route B, Scheme 2). Initial attempts to couple β-ketoacids to 5 remained unsuccessful. Gratifyingly, we were able to prepare derivatives 16–18 by reaction of 5 with masked ketoacids (route C, Scheme 2) in refluxing toluene.13 Using this protocol, the corresponding β-keto thioesters were obtained in acceptable yields (Figure 1).
Scheme 2. Synthetic strategy for the preparation of S-acyl pantetheine derivatives.
Figure 1.
Substrate scope for the synthesis of S-acyl pantetheine derivatives (R groups shown). Yields given correspond to the coupling reaction (first yield) followed by the deprotection (second yield) as outlined in Scheme 2. aYields following HPLC purification; bcombined yield for both deprotection steps.
In order to elaborate pantetheine thioesters 6–18 into the corresponding acyl-CoAs, we next cloned, expressed, and purified recombinant E. coli CoaA, CoaD, and CoaE enzymes. Substrates 6–18 were assayed using a premixed enzyme-cocktail of purified CoaA, CoaD, and CoaE as catalyst. In addition to the substrate and the enzyme-cocktail, the only other component that needed to be provided was freshly prepared ATP (see Supplementary Information for detailed assay procedures). Using a three-fold molar excess of ATP, the enzyme-cocktail catalyzed stoichiometric conversion of 1000-fold molar excess substrates to their corresponding acyl-CoA products in three hours at 30 °C, while no conversion was observed in the absence of either ATP, or the enzymes (Figure 2a). Identity of the enzymatically synthesized benzoyl-CoA (Figure 2a), generated using 10 as the substrate was verified by NMR (see Supplementary Information).
Figure 2.
Enzymatic synthesis and characterization of acyl-CoAs starting from S-acyl pantetheine substrates. (a) Illustratively, HPLC analysis of reaction substrate 10 and the product benzoyl-CoA at 280 nm demonstrates that product formation is dependent on the presence of ATP and CoaA/D/E enzyme catalysts. (b) Mass spectrometric characterization of the enzymatic product benzoyl-CoA. In the MS1 spectra, dominant [M+H]1+ and [M+2H]2+ ions (labeled with red diamonds) are observed that correspond to the molecular formula of benzoyl-CoA (C28H40N7O17P3S). Upon fragmentation of the [M+H]1+ ion, characteristic benzoyl-(cyclo)pantetheine and (cyclo)pantetheine MS2 product ions are observed. A proposed chemical route for the generation of the observed (cyclo)pantetheine MS2 product ions is shown in Figure S29.
Next, we developed a mass spectrometric assay to query the identity of the acyl-CoA products in order to circumvent their preparative isolation and NMR characterization. Guided by our previous reports describing ‘phosphopantetheine ejection’ MS2 product ions,14 we observed characteristic acyl-(cyclo)pantetheine and (cyclo)pantetheine MS2 product ions upon fragmentation of the acyl-CoA [M+H]1+ parent MS1 ion (Figure 2b and Figures S1–S13). Note that the observation of the (cyclo)pantetheine MS2 ion is indicative of the thioester linkage present in the acyl-CoA enzymatic product. Modulation of MS/MS parameters demonstrated that with increasing fragmentation energy, the abundance of the (cyclo)pantetheine MS2 product ion increased relative to that of the acyl-(cyclo)pantetheine ion (Figure S6).
Having verified the chemoenzymatic production of acyl-CoAs, we next verified their viability to perform their physiological roles- that is, to act as donors in acyl transfer reactions. To illustrate, we employed chloramphenicol acetyltransferase (CAT), an enzyme that catalyzes the acetylation of chloramphenicol (19) using acetyl-CoA as the acetyl donor (Figure 3a).14 Starting from 6, in a single-pot assay, we produced acetyl-CoA that was then used as a substrate by CAT to generate acetylated-19. Two monoacetylated-19 products were observed (Figure 3b, trace iii), consistent with the slow noncatalytic transfer of the acetyl group from 3-acetyl-19 to the 1-hydroxyl of 19.15 This then facilitated a second acetylation event at the 3-hydroxyl position, leading to production of diacetylated-19 (Figure S14).
Figure 3.
Chemoenzymatically synthesized acyl-CoAs are acyl donors for in situ labeling of small molecules. (a) Scheme for the conversion of 6 to acetyl-CoA, followed by the transfer of the acetyl group to 19. (b) HPLC characterization at 280 nm of standards of acetyl-CoA (i) and 19 (ii) and the enzymatic reaction (iii) demonstrating the formation of two acetyl-19 products (also see Figure S14). Absence of ATP (iv) or CoaADE enzymes (v) abolished acetylation of 19, while absence of CAT (vi) or 19 (vii) led to the production of acetyl-CoA but no acetylated-19 products.
A second physiological role of 1 is to donate its phosphopantetheine moiety, such as in the conversion of apo-acyl carrier proteins (-ACPs) to their holo forms. Substrate promiscuity of the phosphopantetheinyl transferase enzyme Sfp, which allows for the transfer of the acyl-phosphopantetheine moiety from acyl-CoAs to generate acyl-ACPs, has been widely used to study assembly line biosynthesis of natural products, among several other biochemical transformations.16 We next queried whether the chemoenzymatic acyl-CoA synthetic scheme described above can be used to drive in situ production of acyl-ACPs using Sfp.
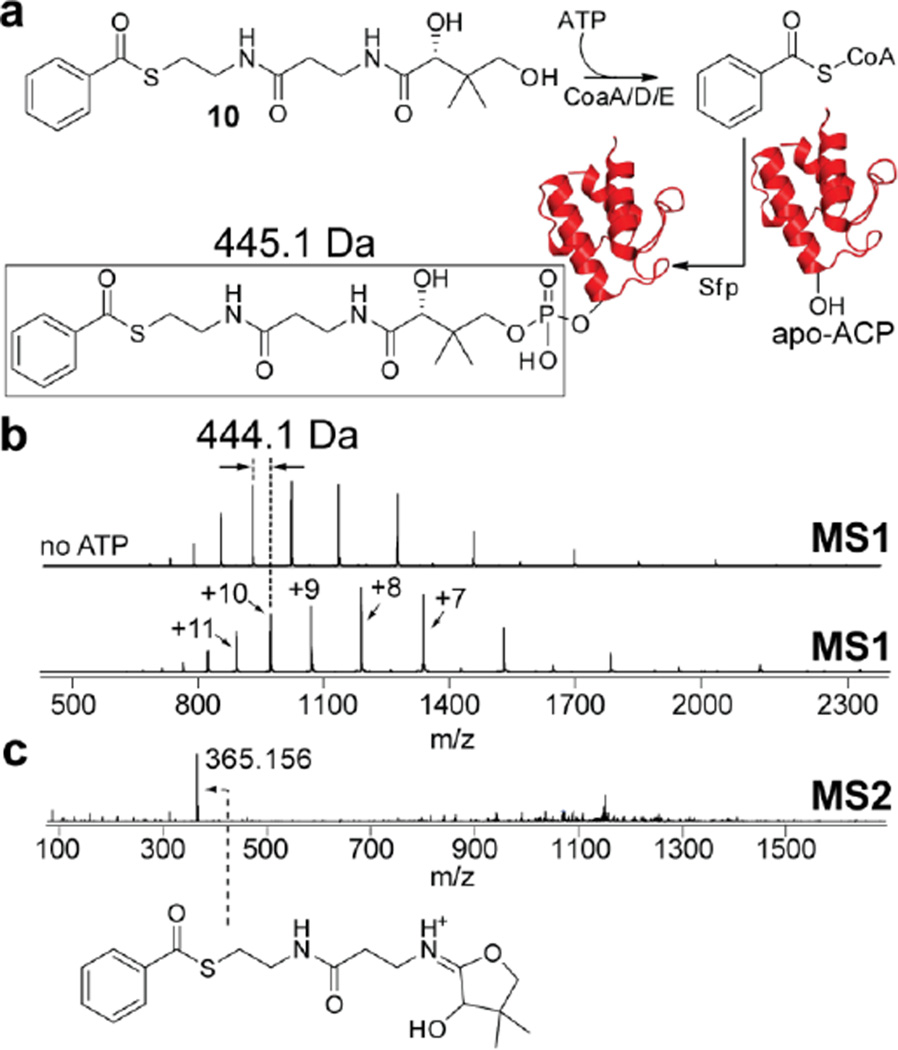
Illustratively, in a single-pot reaction starting from 10 and apo-ACP as substrates, and CoaA/D/E and Sfp as catalysts (Figure 4a), we observed the ATP-dependent stoichiometric formation of benzoyl-S-ACP as the product. The mass difference between the MS1 ions observed in the absence and presence of ATP in the reaction correspond to the benzoyl-phosphopantetheine moiety that is transferred by Sfp (Figure 4b). Furthermore, fragmentation of the peptidyl MS1 ions demonstrated identical benzoyl-(cyclo)pantetheine MS2 product ions that we previously observed for benzoyl-CoA enzymatic product (Figure 4c). Hence, without intermediary purification of the acyl-CoAs, we could achieve in situ labeling of protein substrates by acyl-CoAs. Additionally, each of the S-acyl pantetheine substrates 6–18 were responsive to CoaA/D/E-Sfp loading onto apo-ACP, thus underscoring the vast chemical space that can be explored by the above methodology (Figure S15–S27). Furthermore, a preparative scale synthesis of acyl-ACP using the scheme described above, followed by purification by size exclusion chromatography yielded several milligrams of purified and fully acylated ACP product (Figure S28).
Figure 4.
In situ labeling of ACPs with enzymatically synthesized acyl-CoAs. (a) Reaction scheme for the conversion of 10 to benzoyl-CoA and concomitant transfer to an ACP molecule (in cartoon representation colored red). (b) LC/MS ESI-ToF characterization of benzoyl-ACP. As shown for the [M+10H]10+ ion, the deconvoluted mass difference between apo-ACP (observed in the negative control reaction with no ATP added) and acylated-ACP corresponds to the benzoyl-phosphopantetheine moiety that is transferred to a conserved serine residue of the ACP (note that the difference of 1 Da between the masses is due to the serine side chain hydroxyl proton that is lost upon transfer). (c) Further conformation for ACP acylation is provided by the observation of the characteristic benzoyl-(cyclo)pantetheine MS2 product ion, identical to the MS2 product ion shown in Figure 2b.
In conclusion, we have developed a workflow to generate a multitude of S-acyl pantetheine molecules from a key synthetic intermediate, and demonstrated the enzymatic elaboration of structurally diverse acyl-pantetheines to fully functional acyl-CoA molecules that can participate in acyl- and acyl-phosphopantetheine transfer reactions to small molecules and proteins. The chemoenzymatic schemes, together with the mass spectrometry based assays described in this study, are expected to be broadly applicable in biochemical investigations involving acyl-CoA substrates and intermediates. In particular, biochemical and structural studies requiring large amounts of highly pure acyl-CoAs and acyl-ACPs will benefit from the methodology described herein.
Supplementary Material
Acknowledgments
We thank our UCSD colleagues B. M. Duggan for assistance with NMR data acquisition and analysis, and P. Jordan for helpful discussions. Funding and instrumentation support was generously provided by the NIH (P01-ES021921, R01-AI47818, and GMS10RR029121) and NSF (OCE-1313747), Bruker, and postdoctoral fellowships from the Helen Hay Whitney Foundation to V.A., the Swiss National Science Foundation to S.D., and Uehara Memorial Foundation to T.A. Mass spectrometric data is made available on http://gnps.ucsd.edu.
Footnotes
ASSOCIATED CONTENT
Experimental details, synthetic schemes, and NMR spectroscopy and mass spectrometry data are provided as Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
V.A. and B.S.M. conceived the study, S.D., L.R. and T.A. synthesized and characterized compounds, V.A. performed enzymatic experiments, V.A. and N.G. performed mass spectrometry measurements, all authors analyzed data and wrote the manuscript.
The authors declare no competing financial interests.
REFERENCES
- 1.Walsh CT. Science. 2004;303:1805. doi: 10.1126/science.1094318. [DOI] [PubMed] [Google Scholar]
- 2.Leonardi R, Zhang YM, Rock CO, Jackowski S. Prog Lipid Res. 2005;44:125. doi: 10.1016/j.plipres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 3.(a) Agarwal V, El Gamal AA, Yamanaka K, Poth D, Kersten RD, Schorn M, Allen EE, Moore BS. Nat Chem Biol. 2014;10:640. doi: 10.1038/nchembio.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Eustaquio AS, McGlinchey RP, Liu Y, Hazzard C, Beer LL, Florova G, Alhamadsheh MM, Lechner A, Kale AJ, Kobayashi Y, Reynolds KA, Moore BS. Proc Natl Acad Sci U S A. 2009;106:12295. doi: 10.1073/pnas.0901237106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strauss E, Begley TP. J Biol Chem. 2002;277:48205. doi: 10.1074/jbc.M204560200. [DOI] [PubMed] [Google Scholar]
- 5.Worthington AS, Burkart MD. Org Biomol Chem. 2006;4:44. doi: 10.1039/b512735a. [DOI] [PubMed] [Google Scholar]
- 6.Prepared as: Zhang W, Shrestha R, Buckley RM, Jewell J, Bossmann SH, Stubbe J, Li P. ACS Chem. Biol. 2014;9:1773. doi: 10.1021/cb5002735.
- 7.5 was prepared previously: Gaudelli NM, Townsend CA. J. Org. Chem. 2013;78:6412. doi: 10.1021/jo4007893. (see Supporting Information for details).
- 8.Purification of PyBOP-mediated reactions was difficult.
- 9.Coupling of unsaturated acids to thiols has previously been reported to be problematic due to competing 1,4-addition (see ref. 13b)
- 10.Deprotection of the diol was best achieved using AcOH/H2O: Jansen PAM, van Diepen JA, Ritzen B, Zeeuwen PLJM, Cacciatore I, Cornacchia C, van Vlijmen-Willems IMJJ, de Heuvel E, Botman PNM, Blaauw RH, Hermkens PHH, Rutjes FPJT, Schalkwijk J. ACS Chem. Biol. 2013;8:530. doi: 10.1021/cb3006424.
- 11.In particular β-hydroxyester 9 and diene 14 proved highly sensitive to more acidic conditions.
- 12.(a) Despite the greater coupling yields with Fmoc and Boc protecting groups, 15 proved to be highly susceptible to the basic conditions required for Fmoc-group removal. Boc deprotection was not selective, resulting in the removal of the acetonide protecting group alongside the Boc group Isidro-Llobet A, Álvarez M, Albericio F. Chem. Rev. 2009;109:2455. doi: 10.1021/cr800323s.
- 13.(a) Clemens RJ, Hyatt JA. J. Org. Chem. 1985;50:2431. [Google Scholar]; (b) Gilbert IH, Ginty M, O’Niell JA, Simpson TJ, Staunton J, Willis CL. Bioorg. Med. Chem. Lett. 1995;5:1587. [Google Scholar]
- 14.Dorrestein PC, Kelleher NL. Nat. Prod. Rep. 2006;23:893. doi: 10.1039/b511400b. [DOI] [PubMed] [Google Scholar]
- 15.Thibault G, Guitard M, Daigneault R. Biochim. Biophys. Acta. 1980;614:339. doi: 10.1016/0005-2744(80)90223-5. [DOI] [PubMed] [Google Scholar]
- 16.Beld J, Sonnenschein EC, Vickery CR, Noel JP, Burkart MD. Nat. Prod. Rep. 2014;31:61. doi: 10.1039/c3np70054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.