Abstract
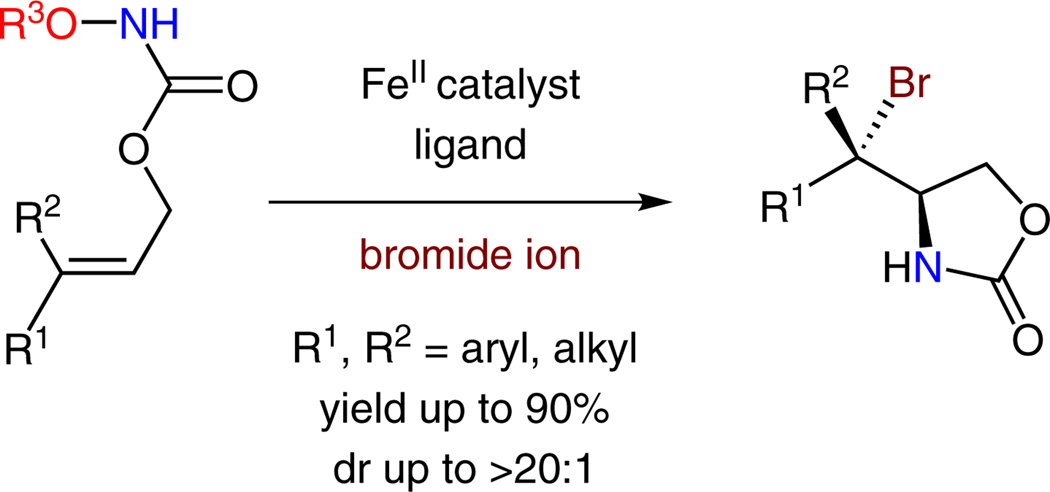
A new iron-catalyzed diastereoselective aminobromination method is reported for both internal and terminal olefins (yield up to 90% and dr up to >20:1). In this transformation, a functionalized hydroxylamine and bromide ion were used as the nitrogen and bromine source, respectively. This method is compatible with a broad range of olefins and provides a convenient approach to synthetically valuable vicinal bromo primary amines. Our studies suggest that both the diastereoselectivity and enantioselectivity for the olefin aminobromination can be controlled by iron catalysts.
Keywords: iron, nitrogen, bromine, alkenes, diastereoselectivity
Stereoselective olefin difunctionalization through nitrogen and bromine atom-transfer is an important transformation because it can readily convert hydrocarbons to vicinal bromo primary amines, a class of chiral building blocks valuable for organic synthesis.2 Although a range of excellent methods for asymmetric olefin bromo-oxygenation were reported,3 catalytic enantioselective olefin aminobromination methods are less developed.4 Additionally, most of the existing asymmetric olefin aminobromination reactions proceed through electrophilic bromonium ion intermediates. In contrast, enantioselective aminobromination of internal olefins using nucleophilic bromide ion has not been developed. In particular, stereoselective olefin aminobromination reactions via iron-nitrenoid intermediates have not been reported.5 Herein, we describe an iron-catalyzed diastereoselective intramolecular aminobromination method for a broad range of olefins (Scheme 1, yield up to 90% and dr up to >20:1). In this reaction, a functionalized hydroxylamine and bromide ion were used as nitrogen and bromine sources, respectively. Most notably, both the diastereoselectivity and enantioselectivity of this new method can be conveniently controlled by nitrogen-based ligands.
Scheme 1.
Iron-catalyzed diastereoselective olefin aminobromination with bromide ion
Prior to this research, Yoshimitsu and co-workers reported an FeBr2-catalyzed racemic intramolecular olefin aminobromination reaction with an N-tosyloxycarbamate, tetrabutylammonium bromide (TBAB), and t-BuOH under ligand-free conditions.6 In their studies, modest diastereoselectivity was observed with five substrates (1.5 to 3.8:1 dr). Our present method reported here has some unique features that complement the existing iron-catalyzed olefin aminobromination method. First, excellent anti-selectivity has been observed across a wide range of both acyclic and cyclic olefins (20:1 dr). Next, excellent asymmetric induction has been achieved with chiral iron-ligand complexes (up to 89% ee).
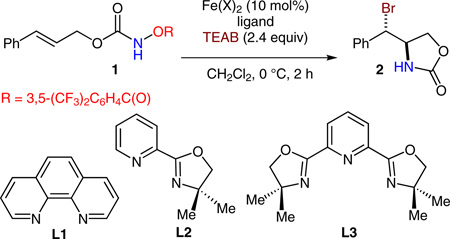
We selected an acyloxyl carbamate 1 as a model substrate for catalyst screening and observed that FeBr2 catalyzed a nondiastereoselective reaction in the presence of tetraethylammonium bromide (TEAB) under the ligand free condition (Table 1, entry 1, 84% yield, 0.86:1 dr). Interestingly, the FeBr2-phenanthroline L1 complex catalyzed the anti-aminobromination with a significantly improved dr, but in a decreased yield (entry 2, 58% yield, 18:1 dr). However, the Fe(OTf)2-L1 complex revealed essentially the same diastereoselectivity, but gave an improved yield (entry 3, 78% yield, 18:1 dr). Notably, the Fe(NTf2)2-L1 complex provided both excellent yield and dr (entry 4, 81% yield, >20:1 dr). We also observed that the Fe(NTf2)2-L2 and Fe(NTf2)2-L3 complexes are less effective for diastereocontrol with trans-olefinic substrate 1 (entries 5, 6).7
Table 1.
Catalyst Screening for the Iron-Catalyzed Diastereoselective Olefin Aminobromination
 | |||||
|---|---|---|---|---|---|
| Entrya | Fe(X)2 | Ligand (mol%) |
Conversion (%)b |
Yield (%)c | dr (anti/syn)b |
| 1 | FeBr2 | none | >95 | 84 | 0.86:1 |
| 2 | FeBr2 | L1 (20) | >95 | 58 | 18:1 |
| 3 | Fe(OTf)2 | L1 (20) | >95 | 78 | 18:1 |
| 4 | Fe(NTf2)2 | L1 (20) | >95 | 81 | >20:1 |
| 5 | Fe(NTf2)2 | L2 (20) | >95 | 78 | 12:1 |
| 6 | Fe(NTf2)2 | L3 (20) | >95 | 75 | 13:1 |
Unless stated otherwise, the reactions were carried out under N2 in the presence of 4 Å molecular sieves.
Conversion and dr were determined by 1H NMR analysis.
Isolated yield. TEAB: Tetraethylammonium bromide.
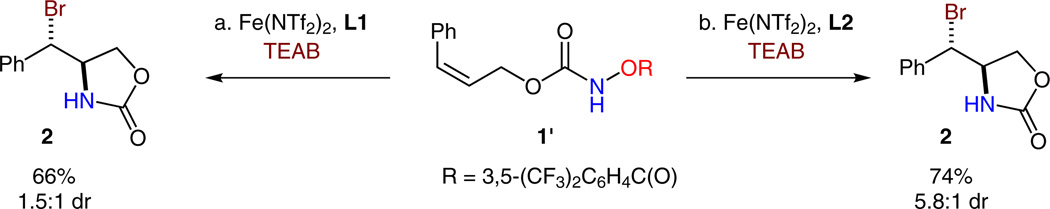
Since nonstereospecificity was observed in the iron-catalyzed olefin aminochlorination,8 cis-olefin 1′ was subsequently evaluated for the aminobromination reaction (Scheme 2). To our surprise, the Fe(NTf2)2-L1 complex catalyzed an essentially nondiastereoselective reaction (1.5:1 dr), while the Fe(NTf2)2-L2 complex catalyzed an anti-selective addition with a significant diastereomeric ratio (5.8:1 dr). The different reaction profiles for isomeric olefins 1 and 1′ revealed that the aminobromination reaction is neither stereospecific nor fully stereoconvergent. These results also suggest that the aminobromination occurs in a stepwise fashion and the C–N bond formation step may be ratedetermining.
Scheme 2.
Ligand effect in the iron-catalyzed aminobromination of a cis-olefin. a) Reaction conditions: Fe(NTf2)2 (10 mol%), L1 (20 mol%), TEAB (2.4 equiv), CH2Cl2, 0 °C, 2 h. b) Reaction conditions: Fe(NTf2)2 (10 mol%), L2 (20 mol%), TEAB (2.4 equiv), CH2Cl2, 0 °C, 2 h.
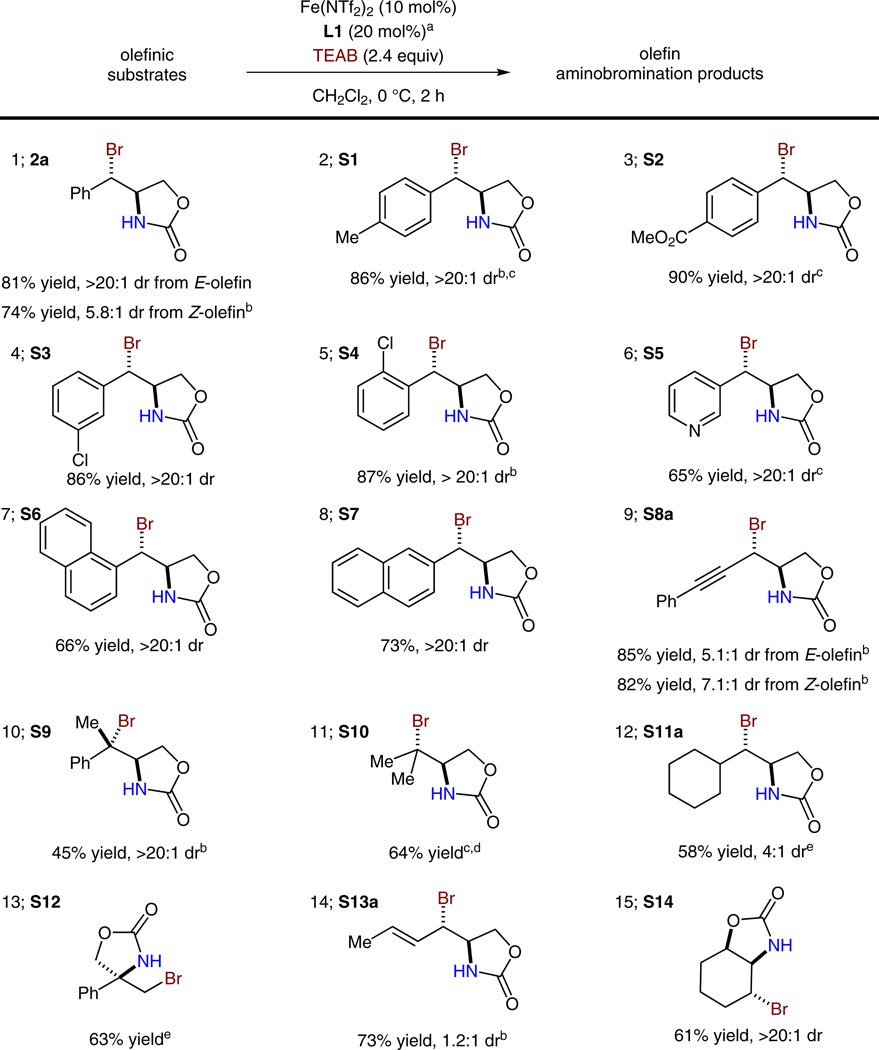
With the optimized conditions in hand, a variety of substrates were explored to evaluate the scope and limitations of this anti-aminobromination method (Scheme 3). First, disubstituted styrenyl olefins are found to be generally good substrates; both electron-donating and -withdrawing substituents can be tolerated by this method (Scheme 3, entries 1–4). Notably, this method is also compatible with ortho-substituents and pyridyl groups (entries 5, 6). Furthermore, isomeric naphthyl olefins are both excellent substrates for providing high diastereoselectivity (entries 7, 8). Cis- and trans-eneynes are also good substrates for the stereoconvergent and anti-selective method (entry 9). Additionally, both styrenyl and nonstyrenyl trisubstituted olefins underwent aminobromination smoothly with excellent diastereoselectivity (entries 10, 11). Furthermore, a cyclohexyl-substituted olefin could participate in the reaction with reasonable yield and diastereoselectivity (entry 12, 58% yield, 4:1 dr). Moreover, 1,1-disubstituted olefins and dienes are viable substrates with excellent regioselectivity (entries 13, 14). Finally, a cyclic olefin proved to be an excellent substrate for the anti-aminobromination reaction (entry 15, dr >20:1 dr).
Scheme 3.
Substrate scope for the iron-catalyzed diastereoselective olefin aminobromination reaction. a Unless stated otherwise, the reactions were carried out under N2 in the presence of 4 Å molecular sieves. b L2 (20 mol%) was used as the ligand. c Reaction conditions: –15 °C, 6 h. d TOAB was used as the bromide source. e Reaction conditions: Fe(NTf2)2·(L2)2 (15 mol%). TOAB: Tetraoctylammonium bromide.
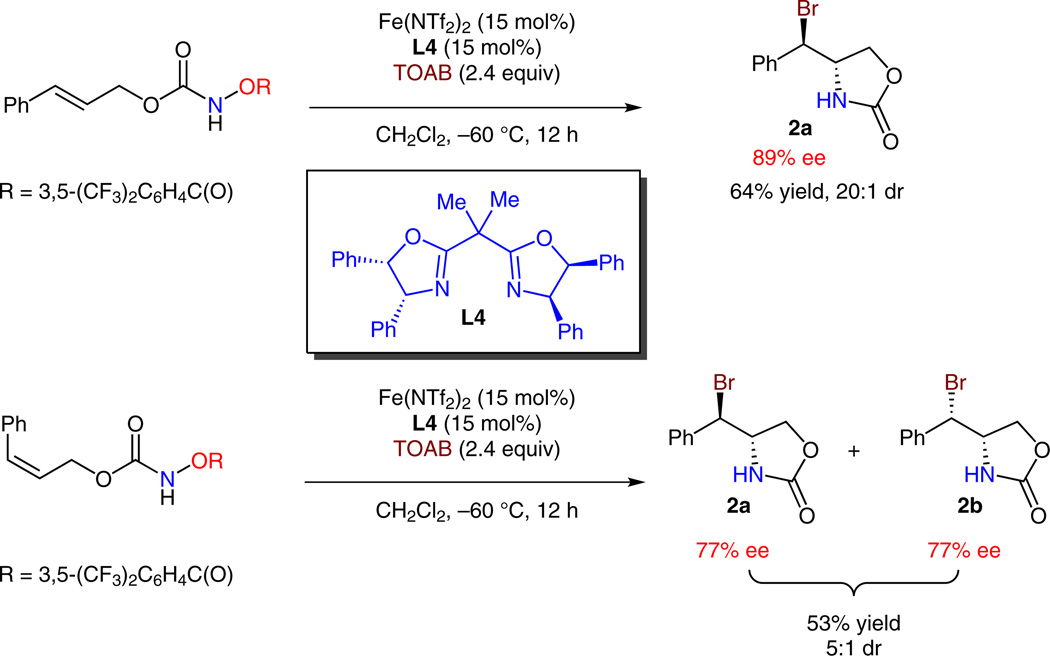
Since the iron–ligand complexes can control diastereoselectivity and they are involved in the stereo-determining transition state, the asymmetric aminobromination was further explored with chiral iron–ligand complexes. We discovered that the Fe(NTf2)2-chiral ligand L4 complex catalyzed both diastereoselective and enantioselective aminobromination of trans-olefin 1 (Scheme 4, >20:1 dr, 89% ee for 2a). Interestingly, the Fe(NTf2)2-L4 complex also catalyzed a less-stereoselective reaction with cis-olefin 1′ (5.0:1 dr, 77% ee for both 2a and 2b), a phenomenon that is very different from iron-catalyzed intramolecular olefin aminochlorination reaction.8 This result suggests that there may be mechanistic subtleties between the chlorine- and bromine-atom-transfer step.
Scheme 4.
Iron-catalyzed asymmetric aminobromination with isomeric olefins
In conclusion, we have described an iron-catalyzed diastereoselective aminobromination method for both internal and terminal olefins. In this reaction, a functionalized hydroxylamine and a bromide ion were used as the nitrogen and bromine source, respectively. This method is compatible with a broad range of olefins and provides a convenient approach to vicinal bromo primary amines, a class of valuable building blocks in synthetic chemistry. Our studies suggest that both the diastereoselectivity and enantioselectivity for this reaction can be controlled by the iron ligand complexes and our efforts were focused on the method of development for the stereoselective intermolecular olefin aminobromination.
All reactions were performed in flame-dried round-bottomed flasks and vials. Stainless steel syringes and cannula were used to transfer air- and moisture-sensitive liquids. Flash chromatography was performed using silica gel 60 (230–400 mesh) from Sigma-Aldrich. Et4NBr (TEAB) and tetraoctylammonium bromide (TOAB) were purchased from Sigma-Aldrich. They were further purified by recrystallization from Et2O–acetone mixture and stored in a glove box under N2 atmosphere. Other reagents were purchased from Sigma-Aldrich, Fluka, EM Science, and Lancaster, and used directly as received. All solvents were used after being freshly distilled. 1H NMR and 13C NMR spectra were recorded on Bruker UltraShield-400 (400 MHz). The mass spectroscopic data were obtained at the Georgia State University mass spectrometry facility using a Micromass Platform II single quadruple instrument. IR spectra were obtained using a PerkinElmer Spectrum 100 FT-IR spectrometer.
Racemic Diastereoselective Olefin Aminobromination; General Procedure
To a flame-dried sealable 2-dram vial (vial A) equipped with a magnetic stir bar were added Fe(NTf2)2 (12.3 mg, 0.02 mmol, 10 mol%) and 1,10-phenanthroline (7.2 mg, 0.04 mmol, 20 mol%). After the vial was evacuated and backfilled three times with N2, anhydrous CH2Cl2 (1.0 mL) was added and the mixture was stirred at r.t. for 20 min. During this time, the olefinic substrate (0.2 mmol) and anhydrous TEAB (51 mg, 0.24 mmol) were dissolved in CH2Cl2 (4.0 mL) in a second flame-dried 3-dram vial (vial B) with a magnetic stir bar and freshly activated 4 Å molecular sieves under N2 atmosphere. Both vials were degassed twice by brief evacuation and backfilling with N2. The vial B was cooled down to 0 °C, and the solution in vial A was added to vial B dropwise via a syringe. The resulting solution was stirred at the same temperature until all the starting material was fully consumed monitored by TLC (eluent: hexanes–acetone, 10:1). The reaction was quenched by sat. aq NaHCO3 (1 mL). After extraction with CH2Cl2 (3 × 1.5 mL), the combined organic phases were concentrated and the residue was purified through a gradient silica gel flash column chromatography (hexanes–acetone, 15:1 to 4:1) to afford the product. The dr was determined by 1H NMR analysis.
4-[Bromo(phenyl)methyl]oxazolidin-2-one (2a)
By following the General Procedure, 2a was obtained as a white solid; yield: 41 mg (81%); dr >20:1; mp 113–115 °C.
IR (ATR, neat): 3232 (m), 3133 (w), 2957 (w), 2853 (w), 1730 (s), 1236 (s), 1094 (s), 1022 (s), 650 cm−1 (s).
1H NMR (400 MHz, CDCl3): δ = 7.49–7.32 (m, 5 H), 5.22 (s, 1 H), 4.76 (d, J = 9.4 Hz, 1 H), 4.69–4.58 (m, 1 H), 4.52–4.36 (m, 2 H).
13C NMR (100 MHz, CDCl3): δ = 158.0, 137.0, 129.7, 129.4, 128.0, 69.5, 58.0, 54.5.
HRMS (ESI): m/z calcd for C10H10BrNO2Na+ (M + Na+): 277.9793; found: 277.9801.
4-[Bromo(phenyl)methyl]oxazolidin-2-one (2b)
By following the General Procedure under the conditions described in Table 1 entry 1, 2a and 2b were obtained as a mixture; yield: 38 mg (84%); dr 0.86:1; mp 111–118 °C.
1H NMR (400 MHz, CDCl3): δ = 7.48–7.31 (m, 5 H), 5.68 (s, 1 H), 4.87 (d, J = 9.6 Hz, 1 H), 4.50–4.43 (m, 1 H), 4.23 (t, J = 9.4 Hz, 1 H), 3.92 (dd, J = 9.5, 5.2 Hz, 1 H).
13C NMR (100 MHz, CDCl3): δ = 157.9, 136.4, 129.7, 129.4, 127.9, 67.3, 58.7, 56.7.
HRMS (ESI): m/z calcd for C10H10BrNO2Na+ (M + Na+): 277.9793; found: 277.9799.
4-[Bromo(p-tolyl)methyl]oxazolidin-2-one (S1)
By following the General Procedure under the conditions described in Scheme 3, S1 was obtained as a white solid; yield: 46 mg (86%); dr >20:1; mp 121–123 °C.
IR (ATR, neat): 3239 (m), 3099 (w), 2921 (w), 1748 (s), 1239 (m), 1028 (m), 650 cm−1 (s).
1H NMR (400 MHz, CDCl3): δ = 7.39–7.17 (m, 4 H), 5.09 (s, 1 H), 4.77 (d, J = 9.1 Hz, 1 H), 4.72–4.61 (m, 1 H), 4.55–4.39 (m, 2 H), 2.38 (s, 3 H).
13C NMR (100 MHz, CDCl3): δ = 158.1, 139.8, 134.0, 130.0, 127.9, 69.5, 57.9, 54.7, 21.2.
HRMS (ESI): m/z calcd for C11H13BrNO2+ (M + H+): 270.0130; found: 270.0127.
Methyl 4-[Bromo(2-oxooxazolidin-4-yl)methyl]benzoate (S2)
By following the General Procedure under the conditions described in Scheme 3, S2 was obtained as a white solid; yield: 56 mg (90%); dr >20:1; mp 101–103 °C.
IR (ATR, neat): 3323 (m), 2946 (w), 2834 (w), 1656 (m), 1449 (m), 1019 cm−1 (s).
1H NMR (400 MHz, CDCl3): δ = 8.06 (d, J = 7.9 Hz, 2 H), 7.48 (d, J = 7.9 Hz, 2 H), 5.25 (s, 1 H), 4.79 (d, J = 9.2 Hz, 1 H), 4.71–4.63 (m, 1 H), 4.51–4.41 (m, 2 H), 3.93 (s, 3 H).
13C NMR (100 MHz, CDCl3): δ = 166.1, 158.0, 141.7, 131.3, 130.6, 128.2, 69.4, 57.8, 53.4, 52.4.
HRMS (ESI): m/z calcd for C12H13BrNO4+ (M + H+): 314.0028; found: 314.0027.
4-[Bromo(3-chlorophenyl)methyl]oxazolidin-2-one (S3)
By following the General Procedure under the conditions described in Scheme 3, S3 was obtained as a white solid; yield: 50 mg (86%); dr >20:1; mp 107–109 °C.
IR (ATR, neat): 3240 (w), 2985 (w), 2863 (w), 1737 (s), 1235(s), 1044 (s), 732 cm−1 (s).
1H NMR (400 MHz, CDCl3): δ = 7.42–7.27 (m, 4 H), 5.64 (s, 1 H), 4.71 (d, J = 8.8 Hz, 1 H), 4.67–4.57 (m, 1 H), 4.46–4.36 (m, 2 H).
13C NMR (100 MHz, CDCl3): δ = 158.4, 139.0, 135.2, 130.6, 129.82, 128.3, 126.3, 69.4, 57.9, 53.5.
HRMS (ESI): m/z calcd for C10H10BrClNO2+ (M + H+): 289.9583; found: 289.9584.
4-[Bromo(2-chlorophenyl)methyl]oxazolidin-2-one (S4)
By following the General Procedure under the conditions described in Scheme 3, S4 was obtained as a white solid; yield: 51 mg (87%); dr >20:1; mp 123–125 °C.
IR (ATR, neat): 3260 (m), 3102 (w), 2923 (w), 1752 (s), 1475 (m), 1233 (m), 1030 (m), 734 (m), 509 cm−1 (s).
1H NMR (400 MHz, CDCl3): δ = 7.53 (dd, J = 7.7, 1.3 Hz, 1 H), 7.43 (dd, J = 7.7, 1.2 Hz, 1 H), 7.39–7.29 (m, 2 H), 5.40 (s, 1 H), 5.39 (d, J = 8.8 Hz, 1 H), 4.66–4.54 (m, 2 H), 4.45 (dd, J = 8.8, 4.3 Hz, 1 H).
13C NMR (100 MHz, CDCl3): δ = 158.3, 134.5, 133.9, 130.6, 130.4, 129.1, 128.0, 69.1, 57.0, 49.5.
HRMS (ESI): m/z calcd for C10H10BrClNO2+ (M + H+): 289.9583; found: 289.9580.
4-[Bromo(pyridin-3-yl)methyl]oxazolidin-2-one (S5)
By following the General Procedure under the conditions described in Scheme 3, S5 was obtained as a white solid, yield: 33 mg (65%); dr >20:1; mp >200 °C.
IR (ATR, neat): 3300 (m), 2985 (w), 1737 (s), 1372 (m), 1234 (s), 1044 (s), 504 cm−1 (s).
1H NMR (400 MHz, CDCl3): δ = 8.62 (s, 1 H), 8.56 (d, J = 4.0 Hz, 1 H), 7.77 (d, J = 8.0 Hz, 1 H), 7.35 (dd, J = 7.9, 4.8 Hz, 1 H), 5.60 (s, 1 H), 4.78 (d, J = 9.0 Hz, 1 H), 4.66 (t, J = 8.2 Hz, 1 H), 4.50–4.41 (m, 2 H).
13C NMR (100 MHz, CDCl3): δ = 158.7, 150.1, 148.8, 136.1, 133.5, 124.1, 69.2, 58.0, 51.4.
HRMS (ESI): m/z calcd for C9H10BrN2O2+ (M + H+): 256.9926; found: 256.9935.
4-[Bromo(naphthalen-1-yl)methyl]oxazolidin-2-one (S6)
By following the General Procedure under the conditions described in Scheme 3, S6 was obtained as a white solid; yield: 40 mg (66%); dr >20:1; mp 119–121 °C.
IR (ATR, neat): 3242 (m), 2913 (w), 1766 (s), 1702 (s), 1480 (m), 1410 (m), 1212 (s), 1028 (s), 763 cm−1 (s).
1H NMR (400 MHz, CDCl3): δ = 8.11 (s, 1 H), 7.92 (t, J = 7.7 Hz, 2 H), 7.66–7.62 (m, 2 H), 7.57 (t, J = 7.2 Hz, 1 H), 7.51 (t, J = 7.7 Hz, 1 H), 5.63 (s, 1 H), 5.09 (s, 1 H), 4.90–4.80 (m, 1 H), 4.76 (t, J = 9.2 Hz, 1 H), 4.61 (dd, J = 9.3, 5.0 Hz, 1 H).
13C NMR (100 MHz, CDCl3): δ = 158.0, 134.1, 130.7, 130.5, 129.3, 127.3, 126.6, 125.4, 122.5, 77.2, 69.9, 56.7.
HRMS (ESI): m/z calcd for C14H13BrNO2+ (M + H+): 306.0105; found: 306.0110.
4-[Bromo(naphthalen-2-yl)methyl]oxazolidin-2-one (S7)
By following the General Procedure under the conditions described in Scheme 3, S7 was obtained as a white solid; yield: 45 mg (73%); dr >20:1; mp 116–118 °C.
IR (ATR, neat): 3250 (m), 3134 (w), 2920 (w), 1756 (s), 1712 (s), 1434 (m), 1409 (m), 1248 (s), 1019 (s), 766 cm−1 (s).
1 H NMR (400 MHz, CDCl3): δ = 7.90 (d, J = 8.6 Hz, 1 H), 7.88–7.79 (m, 3 H), 7.59–7.52 (m, 2 H), 7.50 (d, J = 8.5 Hz, 1 H), 5.02 (s, 1 H), 4.92 (d, J = 9.6 Hz, 1 H), 4.66 (t, J = 8.4 Hz, 1 H), 4.60–4.51 (m, 1 H), 4.48 (dd, J = 8.7, 4.8 Hz, 1 H).
13C NMR (100 MHz, CDCl3): δ = 158.0, 134.1, 133.6, 132.9, 129.7, 128.1, 127.8, 127.8, 127.4, 127.2, 124.5, 69.6, 57.8, 55.1.
HRMS (ESI): m/z calcd for C14H13BrNO2+ (M + H+): 306.0105; found: 306.0110.
4-(1-Bromo-3-phenylprop-2-yn-1-yl)oxazolidin-2-one (S8a)
By following the General Procedure under the conditions described in Scheme 3, S8a and its syn-diastereomer S8b were obtained as a white foam; yield: 47 mg (85%); dr 7.1:1.
S8a
1H NMR (400 MHz, CDCl3): δ = 7.46–7.44 (m, 2 H), 7.37–7.32 (m, 3 H), 6.71 (s, 1 H), 4.73 (d, J = 5.7 Hz, 1H), 4.59–4.51 (m, 2 H), 4.29–4.19 (m, 1 H).
13C NMR (100 MHz, CDCl3): δ = 159.1, 132.0, 129.4, 128.4, 121.0, 89.3, 82.5, 67.6, 57.4, 39.0.
S8b
IR (ATR, neat): 3269 (m), 2987 (w), 2224 (m), 1754 (s), 1228 (w), 1037 (m), 933 (m), 758 cm−1 (m).
1H NMR (400 MHz, CDCl3): δ = 7.46–7.44 (m, 2 H), 7.37–7.32 (m, 3 H), 6.61 (s, 1 H), 4.70 (d, J = 6.2 Hz, 1 H), 4.59–4.51 (m, 2 H), 4.40 (dd, J = 9.5, 4.1 Hz, 1 H).
13C NMR (100 MHz, CDCl3): δ = 159.1, 132.1, 129.6, 128.5, 120.8, 88.9, 81.8, 67.1, 57.6, 39.2.
HRMS (ESI): m/z calcd for C12H11BrNO2+ (M + H+): 279.9973; found: 279.9976.
4-(1-Bromo-1-phenylethyl)oxazolidin-2-one (S9)
By following the General Procedure under the conditions described in Scheme 3, S9 was obtained as a white solid; yield: 24 mg (45%); dr >20:1; mp 121–123 °C.
IR (ATR, neat): 3270 (m), 3101 (w), 2995 (w), 1751 (s), 1407 (w), 1239 (m), 1052 cm−1 (m).
1H NMR (400 MHz, CDCl3): δ = 7.55 (d, J = 7.3 Hz, 2 H), 7.43–7.32 (m, 3 H), 5.57 (s, 1 H), 4.56–4.50 (m, 3 H), 2.15 (s, 3 H).
13C NMR (100 MHz, CDCl3): δ = 158.7, 140.9, 128.9, 127.1, 67.6, 67.4, 62.3, 24.9.
HRMS (ESI): m/z calcd for C11H13BrNO2+ (M + H+): 270.0130; found: 270.0138.
4-(2-Bromopropan-2-yl)oxazolidin-2-one (S10)
By following the General Procedure under the conditions described in Scheme 3, S10 was obtained as a white solid; yield: 27 mg (64%); mp 65–68 °C.
IR (ATR, neat): 2920 (s), 2870 (s), 1767 (s), 1483 (w), 1342 (m), 1215 (s), 1040 (s), 859 cm−1 (s).
1H NMR (400 MHz, CDCl3): δ = 7.20 (s, 1 H), 4.48 (t, J = 9.2 Hz, 1 H), 4.37 (dd, J = 9.5, 4.7 Hz, 1 H), 4.01 (dd, J = 8.3, 4.7 Hz, 1 H), 1.73 (s, 3 H), 1.72 (s, 3 H).
13C NMR (100 MHz, CDCl3): δ = 160.0, 67.6, 65.4, 62.4, 29.7, 27.9.
HRMS (ESI): m/z calcd for C6H11BrNO2+ (M + H+): 207.9922; found: 207.9926.
(±)-4-[Bromo(cyclohexyl)methyl]oxazolidin-2-one (S11a)
By following the General Procedure under the conditions described in Scheme 3, S11a and its diastereomer S11b were obtained as a white solid, which can be separated through a gradient silica gel flash column chromatography (hexanes–acetone, from 15:1 to 4:1); combined yield: 30 mg (58%); dr 4:1.
S11a
Mp 72–76 °C.
1H NMR (400 MHz, CDCl3): δ = 6.88 (s, 1 H), 4.54 (t, J = 8.7 Hz, 1 H), 4.30 (dd, J = 9.1, 5.7 Hz, 1 H), 4.20 (td, J = 8.6, 5.6 Hz, 1 H), 3.87 (dd, J = 8.9, 3.0 Hz, 1 H), 1.87–1.74 (m, 2 H), 1.73–1.51 (m, 4 H), 1.47–1.07 (m, 5 H).
13C NMR (100 MHz, CDCl3): δ = 159.8, 70.1, 65.0, 54.6, 39.7, 31.9, 27.2, 25.9, 25.9, 25.4.
S11b
Mp 71–74 °C.
IR (ATR, neat): 2925 (s), 2851 (s), 1759 (s), 1482 (w), 1375 (m), 1227(s), 1149 (s), 1035 (s), 820 cm−1 (s).
1H NMR (400 MHz, CDCl3): δ = 5.58 (s, 1 H), 4.49 (t, J = 8.2 Hz, 1 H), 4.28–4.17 (m, 2 H), 3.90 (dd, J = 7.7, 3.9 Hz, 1 H), 1.84–1.74 (m, 2 H), 1.74–1.61 (m, 3 H), 1.53–1.13 (m, 7 H).
13C NMR (100 MHz, CDCl3): δ = 158.1, 68.0, 65.8, 55.4, 40.6, 31.8, 28.7, 25.9, 25.9, 25.7.
HRMS (ESI): m/z calcd for C10H17ClNO2+ (M + H+): 218.0942; found: 218.0937.
4-(Bromomethyl)-4-phenyloxazolidin-2-one (S12)
By following the General Procedure under the conditions described in Scheme 3, S12 was obtained as a white solid; yield: 32 mg (63%); mp 94–96 °C.
IR (ATR, neat): 3252 (m), 2932 (w), 2853 (w), 1733 (s), 1376 (w), 1089 (s), 1061 cm−1 (w).
1H NMR (400 MHz, CDCl3): δ = 7.46–7.28 (m, 5 H), 6.90 (s, 1 H), 4.68 (d, J = 8.8 Hz, 1 H), 4.49 (d, J = 8.8 Hz, 1 H), 3.80 (q, J = 11.0 Hz, 2 H).
13C NMR (100 MHz, CDCl3): δ = 158.7, 139.9, 129.3, 128.7, 124.8, 74.7, 63.5, 40.6.
HRMS (ESI): m/z calcd for C10H11BrNO2+ (M + H+): 255.9973; found: 255.9965.
4-[(E)-1-Bromobut-2-en-1-yl]oxazolidin-2-one (S13a)
By following the General Procedure under the conditions described in Scheme 3, S13a and its syn-diastereomer S13b were obtained as a white foam; yield: 33 mg (73%); dr 1.2:1.
S13a
1H NMR (400 MHz, CDCl3): δ = 6.23 (s, 1 H), 5.93–5.83 (m, 1 H), 5.59– 5.50 (m, 1 H),4.50 (t, J = 8.8 Hz, 1 H), 4.34 (dd, J = 9.8, 7.9 Hz, 1 H), 4.27 (dd, J = 9.3, 5.0 Hz, 1 H), 4.13–4.05 (m, 1 H), 1.78–1.76 (m, 3 H).
13C NMR (100 MHz, CDCl3): δ = 159.0, 133.8, 127.1, 68.7, 57.5, 56.6, 17.9.
S13b
IR (ATR, neat): 3276 (m), 2919 (w), 1751 (s), 1407 (w), 1233 (s), 1023 (m), 532 cm−1 (m).
1H NMR (400 MHz, CDCl3): δ = 6.29 (s, 1 H), 5.93–5.83 (m, 1 H), 5.59– 5.50 (m, 1 H),4.45–4.41 (m, 2 H), 4.18 (dd, J = 9.4, 4.7 Hz, 1 H), 4.11– 4.06 (m, 1 H), 1.76–1.74 (m, 3 H).
13C NMR (100 MHz, CDCl3): δ = 158.8, 133.7, 126.3, 67.3, 57.2, 55.6, 17.8.
HRMS (ESI): m/z calcd for C7H11BrNO2+ (M + H+): 219.9973; found: 219.9976.
4-Bromohexahydrobenzo[d]oxazol-2(3H)-one (S14)
By following the General Procedure under the conditions described in Scheme 3, S14 was obtained as a white solid; yield: 27 mg (61%); dr >20:1; mp 115–117 °C.
IR (ATR, neat): 3272 (m), 2948 (w), 2885 (w), 1751 (s), 1201 cm−1 (w).
1H NMR (400 MHz, CDCl3): δ = 5.71 (s, 1 H), 4.67–4.65 (m, 1 H), 3.90– 3.84 (m, 1 H), 3.78 (dd, J = 8.8, 5.9 Hz, 1 H), 2.27–2.23 (m, 2 H), 1.78– 1.58 (m, 4 H).
13C NMR (100 MHz, CDCl3): δ = 159.0, 77.0, 60.9, 53.8, 32.7, 25.9, 20.7.
HRMS (ESI): m/z calcd for C7H11BrNO2+ (M + H+): 219.9973; found: 219.9972.
Asymmetric Olefinic Aminobromination; (S)-4-[(R)-Bromo(phenyl) methyl]oxazolidin-2-one (2a); Typical Procedure
To a flame-dried sealable 2-dram vial (vial A) equipped with a magnetic stir bar were added Fe(NTf2)2 (9.2 mg, 0.015 mmol, 15 mol%) and chiral ligand L4 (7.3 mg, 0.015 mmol, 15 mol%). After the vial was evacuated and backfilled three times with N2, anhydrous CH2Cl2 (1.0 mL) was added and the mixture was stirred at r.t. for 20 min. Meanwhile, a second flame-dried and N2-protected 2-dram vial (vial B) with a magnetic stir bar was charged with 1 (0.1 mmol), anhydrous TOAB (137 mg, 0.24 mmol), freshly activated 4 Å molecular sieves, and anhydrous CH2Cl2 (3.0 mL). Both vials were degassed twice by brief evacuation and backfilling with N2. Vial B was cooled down to −60 °C, and the catalyst solution in vial A was added to vial B dropwise via a syringe. The resulting solution was stirred at this temperature for 12 h and then gradually warmed up to r.t. The reaction was quenched with sat. aq NaHCO3 (1 mL). The reaction mixture was extracted with CH2Cl2 (3 × 1.5 mL), and the combined organic phases were concentrated in vacuo. The residue was purified by a gradient silica gel flash column chromatography (hexanes–acetone, from 15:1 to 4:1) to afford 2a as a white solid; yield: 17 mg (64%); dr >20:1; 89% ee. The dr was determined by crude 1H NMR analysis and the ee was determined by Chiral HPLC analysis [Chiral AD-H column, 10% i-PrOH in hexanes, flow rate = 1.0 mL/min, UV detection at 205 nm; tR (minor) = 17.45 min, tR (major) = 21.20 min]; [α]D20 + 77 (c = 0.65, CH2Cl2).
IR (ATR, neat): 3232 (m), 3133 (w), 2957 (w), 2853 (w), 1730 (s), 1236 (s), 1094 (s), 1022 (s), 650 cm−1 (s).
1H NMR (400 MHz, CDCl3): δ = 7.49–7.32 (m, 5 H), 5.22 (s, 1 H), 4.76 (d, J = 9.4 Hz, 1 H), 4.69–4.58 (m, 1 H), 4.52–4.36 (m, 2 H).
13C NMR (100 MHz, CDCl3): δ = 158.0, 137.0, 129.7, 129.4, 128.0, 69.5, 58.0, 54.5.
HRMS (ESI): m/z calcd for C10H10BrNO2Na+ [M + Na+]: 277.9793; found: 277.9801.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM110382) and Georgia State University.
Footnotes
Supporting information for this article is available online at http://dx.doi.org/10.1055/s-0034-1378719.
References
- 2.For selected reviews of enantioselective olefin halofunctionalization, see: Denmark SE, Kuester WE, Burk MT. Angew. Chem. Int. Ed. 2012;51:10938. doi: 10.1002/anie.201204347. Zheng S, Schienebeck CM, Zhang W, Wang H-Y, Tang W. Asian J. Org. Chem. 2014;3:366.
- 3.For selected references of catalytic asymmetric olefin bromooxygenation, see: Zhang W, Zheng S, Liu N, Werness JB, Guzei IA, Tang W. J. Am. Chem. Soc. 2010;132:3664. doi: 10.1021/ja100173w. Zhou L, Tan CK, Jiang X, Chen F, Yeung Y-Y. J. Am. Chem. Soc. 2010;132:15474. doi: 10.1021/ja1048972. Huang D, Wang H, Xue F, Guan H, Li L, Peng X, Shi Y. Org. Lett. 2011;13:6350. doi: 10.1021/ol202527g. Denmark SE, Burk MT. Org. Lett. 2012;14:256. doi: 10.1021/ol203033k. Paull DH, Fang C, Donald JR, Pansick AD, Martin SF. J. Am. Chem. Soc. 2012;134:11128. doi: 10.1021/ja305117m. Murai K, Matsushita T, Nakamura A, Fukushima S, Shimura M, Fujioka H. Angew. Chem. Int. Ed. 2010;49:9174. doi: 10.1002/anie.201005409. Wang Y-M, Wu J, Hoong C, Rauniyar V, Toste FD. J. Am. Chem. Soc. 2012;134:12928. doi: 10.1021/ja305795x. Zhang W, Liu N, Schienebeck CM, Zhou X, Izhar II, Guzei IA, Tang W. Chem. Sci. 2013;4:2652.
- 4.For selected reports on catalytic asymmetric olefin aminobromination via an electrophilic bromonium ion, see: Zhou L, Chen J, Tan CK, Yeung Y-Y. J. Am. Chem. Soc. 2011;133:9164. doi: 10.1021/ja201627h. Alix A, Lalli C, Retailleau P, Masson G. J. Am. Chem. Soc. 2012;134:10389. doi: 10.1021/ja304095z. Huang D, Liu X, Li L, Cai Y, Liu W, Shi Y. J. Am. Chem. Soc. 2013;135:8101. doi: 10.1021/ja4010877. Cai Y, Liu X, Hui Y, Jiang J, Wang W, Chen W, Lin L, Feng X. Angew. Chem. Int. Ed. 2010;49:6160. doi: 10.1002/anie.201002355. For a reference of asymmetric aminobromination of terminal olefins with bromine radical donors, see: Bovino MT, Chemler SR. Angew. Chem. Int. Ed. 2012;51:3923. doi: 10.1002/anie.201109044.
- 5.For iron-catalyzed stereoselective olefin aminohydroxylation and aminofluorination that proceed through an iron-nitrenoid intermediate, see: Liu G-S, Zhang Y-Q, Yuan Y-A, Xu H. J. Am. Chem. Soc. 2013;135:3343. doi: 10.1021/ja311923z. Zhang Y-Q, Yuan Y-A, Liu G-S, Xu H. Org. Lett. 2013;15:3910. doi: 10.1021/ol401666e. Lu D-F, Zhu C-L, Jia Z-X, Xu H. J. Am. Chem. Soc. 2014;136:13186. doi: 10.1021/ja508057u. Lu D-F, Liu G-S, Zhu C-L, Yuan B, Xu H. Org. Lett. 2014;16:2912. doi: 10.1021/ol501051p.
- 6.(a) Kamon T, Shigeoka D, Tanaka T, Yoshimitsu T. Org. Biomol. Chem. 2012;10:2363. doi: 10.1039/c2ob07190h. [DOI] [PubMed] [Google Scholar]; (b) Shigeoka D, Kamon T, Yoshimitsu T. Beilstein J. Org. Chem. 2013;9:860. doi: 10.3762/bjoc.9.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For the synthesis of ligands see references 4c and 4d.
- 8.For the iron-catalyzed asymmetric aminochlorination method for internal olefins, see: Zhu C-L, Tian J-S, Gu Z-Y, Xing G-W, Xu H. Chem. Sci. 2015;6:3044. doi: 10.1039/c5sc00221d. For early work on ironcatalyzed racemic intramolecular olefin aminochlorination, see: Bach T, Schlummer B, Harms K. Chem. Commun. 2000:287.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.