Abstract
PURPOSE
The purpose of this open-label, retrospective report was to determine the safety and effectiveness of locoregional therapy with yttrium-90 (90Y) radioembolization for patients with progressing breast cancer liver metastases (BCLM) despite polychemotherapy.
MATERIALS & METHODS
Seventy-five patients with progressing BCLM and stable extrahepatic disease were treated with radioembolization at our institution. Retrospective review of a prospectively collected database was performed to evaluate clinical and biochemical toxicities, tumor response, overall survival (OS), and time to progression (TTP). Radiologic response assessments included Response Evaluation Criteria in Solid Tumors in primary index lesions and metabolic activity on positron emission tomography. Univariate and multivariate analyses were performed.
RESULTS
30-day mortality was 4% (n=3). Grade 3+ clinical toxicity and hyperbilirubinemia occurred in 7.6% (n=5) and 5.9% (n=4), respectively. The rate of partial response was 35.3% (n=24), 63.2% (n=43) had stable disease, and progressive disease occurred in 1.5% (n=1). PET imaging was available in 25 patients and 21 (84%) had a complete or partial response or stable disease. The median OS was 6.6mo (95% CI, 5.0 to 9.2mo). The hazard ratio (HR) for OS was .39 (95% CI, .23 to .66) for tumor burden <25% compared to greater tumor burden in multivariate analysis. Elevated bilirubin reduced OS. The HR for hepatic progression was .22 (95% CI, .05 to .98) for solitary compared to multifocal disease.
CONCLUSIONS
Locoregional therapy with 90Y radioembolization is safe and stops or delays the progression of targeted chemorefractory breast cancer liver metastases. Adverse prognosticators are identified.
Keywords: metastatic breast cancer, hepatic radioembolization, outcomes, radiation therapy, toxicity, survival, progression
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer in women worldwide (1) and approximately 12.4% of women (1 in 8) in the United States will be diagnosed in their lifetime (2). Metastatic breast cancer (mBC) is generally incurable, and half of mBC patients eventually develop liver metastases that worsen their prognosis(3). Hepatic failure is the cause of death in approximately 20% of patients (3) and impaired liver function necessitates modified polychemotherapy dosing, ultimately limiting systemic antitumor availability (4-6). When disease is confined to the liver prior to systemic therapy, the liver is the initial site of progression in a majority (60-97%) of patients (7). Surgical approaches for palliation of isolated BCLM have included resection (8-12) but recurrence after resection is common occurring after a median time of 13mo with hepatic recurrence in 25-35% (8,9). These aspects have sparked interest and controversy over the use of locoregional therapy targeting BCLM.
Liver-directed therapy for unresectable liver metastases may reduce tumor burden to ameliorate pain symptoms, preserve or possibly recover valuable liver function, and halt or slow disease progression in the palliative setting. The purpose of this study was to evaluate the safety and therapeutic effectiveness of yttrium-90 (90Y) radioembolization for patients with progressing BCLM after exhausting polychemotherapy options.
PATIENTS AND METHODS
Seventy-five consecutive BCLM patients were treated with 90Y radioembolization at our institution between August 2001 and August 2013. This open-label, institutional review board–approved study includes prospectively collected patient data and each patient provided consent allowing the use of their information for research. All patients had hepatic tumor progression (ie, increasing size of breast cancer liver metastases) after cytotoxic systemic chemotherapy. Patients were reviewed and discussed at multidisciplinary tumor board and radioembolization was applied as part of a continuum of care that included systemic therapy administered by the medical oncologist. The motivation in each patient was to stop hepatic progression, palliate symptoms, and preserve liver function and eligibility for future systemic treatments.
Inclusion
Inclusion criteria included (i) image- or biopsy-proven confirmation of mBC to the liver; (ii) active unresectable disease not appropriate for radiofrequency ablation, as determined by a multidisciplinary team; (iii) if present, stable extrahepatic disease allowing a break in active chemotherapy; (iv) Eastern Cooperation Oncology Group (ECOG) performance status of 0-2; (v) bilirubin <2.0mg/dL; (vi) adequate pulmonary function, and (vi) acceptable hematology including granulocyte count >1.5×109/L, platelet count >50×109/L.
Exclusion
Exclusion criteria were (i) life-expectancy <2mo; (ii) flow to the gastrointestinal tract not correctable by repositioning or coil embolization; or (iii) estimated radiation doses to the lungs greater than 30Gy in a single administration or 50Gy cumulatively.
Baseline Patient Characteristics
Table 1 summarizes baseline patient characteristics for the 75 patient cohort. The mean age was 54.4 years. Chemotherapy including taxanes, anthracyclines, and trastuzumab where appropriate failed in each patient. Table E1 includes receptor status and past exposure to systemic treatments.
Table 1.
Patient Characteristics
| Patient No. | % | |
|---|---|---|
| Demographics | ||
| Median Treatment Age & Range (years) | 53.7 | 26.7 - 82.4 |
| < 65 y | 59 | 78.7 |
| ≥ 65 y | 16 | 21.3 |
| Female | 75 | 100.0 |
| Ethnicity# | ||
| Declined | 21 | 30.4 |
| Caucasian | 41 | 59.4 |
| African American | 6 | 8.7 |
| Hispanic | 0 | 0.0 |
| Asian | 1 | 1.4 |
| Other | 0 | 0.0 |
| Risk Factors | ||
| Prior Cancer History# | ||
| First Cancer | 64 | 92.8 |
| Second Cancerγ | 4 | 5.8 |
| > Second Cancerγ | 1 | 1.4 |
| Tumor Burden | ||
| < 25% | 44 | 58.7 |
| 25%-50% | 26 | 34.7 |
| > 50% | 5 | 6.7 |
| Distribution | ||
| Unilobar | 14 | 18.7 |
| Bilobar | 61 | 81.3 |
| No. of lesions | ||
| Solitary | 11 | 14.7 |
| Multifocal | 64 | 85.3 |
| Central Lesion | ||
| No | 64 | 85.3 |
| Yes | 11 | 14.7 |
| Extrahepatic Metastases | ||
| No | 17 | 22.7 |
| Yes | 58 | 77.3 |
| Bone | 42 | 56.0 |
| OtherŦ | 38 | 50.7 |
| Brain | 10 | 13.3 |
| Lung | 19 | 25.3 |
| Lymph Nodes | 17 | 22.7 |
| Previous Liver-directed Therapy✪ | ||
| None | 66 | 88.0 |
| Resection | 5 | 6.7 |
| RF ablation | 5 | 6.7 |
| 90Y Radioembolization | 0 | 0.0 |
| TACE | 1 | 1.3 |
| ECOG (Zubrod) Performance Status | ||
| 0 | 39 | 52.0 |
| 1 | 29 | 38.7 |
| 2+ | 7 | 9.3 |
| Liver Function | ||
| Bilirubin, total serum (mg/dL)* | 0.7 | 0.6 - 1.2 |
| Albumin (g/dL)* | 3.2 | 3.1 - 3.3 |
| Portal Vein Thrombosis/Attenuation | ||
| No | 69 | 92.0 |
| Yes | 6 | 8.0 |
| Ascites | ||
| No | 69 | 92.0 |
| Yes | 6 | 8.0 |
| Cirrhotic Morphology | ||
| No | 73 | 97.3 |
| Yes | 2 | 2.7 |
| Method of Diagnosis | ||
| Biopsy | 32 | 45.7 |
| Imaging | 43 | 57.3 |
Note.
Values expressed as median and 95% confidence intervals.
ECOG, Eastern Cooperative Oncology Group; RF, radiofrequency; TACE, transcatheter aterial chemoembolization.
Cancer history included metastatic colorectal, uterine, hemangioma (n=2), and renal cell.
Sites of extrahepatic metastasis also included kidney, ovary, peritoneum (n=3), mediastinum, retina, pancreas, and adrenal gland.
Observe total n ≠ 75 due to missing patient data in a small number of cases.
Note that some patients received more than one liver-directed therapy.
90Y Radioembolization
Baseline laboratory tests including liver function tests, complete blood count, coagulation profile, albumin, total bilirubin, and tumor marker (CA-27.29) were obtained on the day of treatment. Estimated lung shunting was determined using technetium-99m macroaggregated albumin (99mTc-MAA) scan during treatment planning angiography and selective visceral catheterization. Prophylactic coil embolization was performed in cases of non-target arterial flow to the GI tract. The methods for calculating the required activity for the prescribed dose with and without lung shunt fraction calculations have been previously published (13-15). Uniform Medical Internal Radiation Dose (MIRD) assumptions were used (13,16,17).
Treatment was administered in a segmental (≤2 segmental feeding arteries), lobar, or sequential bilobar fashion. According to the disease presentation, sequential bilobar patients received treatment in the dominant lobe and the contralateral lobe was targeted within 30–90d to complete the treatment cycle. Each patient was treated on an outpatient basis. At each follow-up visit (1 mo, 3 mo, and every 3 mo thereafter), patients were assessed for clinical and biochemical treatment toxicity and imaging response with computed tomography (CT) or magnetic resonance imaging (MRI). Positron emission tomography (PET) was possible in a limited number of patients depending on insurance coverage and the discretion of the ordering physician.
Data Collection and Outcome Measures
This manuscript was prepared using reporting standards of the Society of Interventional Radiology Technology Assessment Committee and Interventional Oncology Task Force (18). A retrospective cohort analysis was completed utilizing prospectively acquired medical, laboratory, clinical, and imaging data. Patients were contacted by telephone at 2-3wks and seen in clinic at 1mo follow-up to assess toxicity and response to treatment.
Tumor response on venous phase CT or delayed post-contrast MRI was determined using unidimensional Response Evaluation Criteria in Solid Tumors (RECIST) (19,20) with long-axis measurements of the primary index lesion as previously reported (21). Up to two index lesions were defined per patient if bilobar disease was present and treated. In these cases, maximum tumor responses were averaged. Pre- and post-treatment studies were compared side-by-side on a computer display by a board-certified radiologist and tumor axis measurements in the axial view were drawn in a similar anatomical plane and orientation over longitudinal studies. Metabolic tumor response on positron emission tomography (PET) was visually assessed in the dominant lesion and scored as zero (complete response), decreased (partial response), stable (stable disease), or increased (progressive disease) fluorodeoxyglucose (FDG) uptake in comparison to pre-treatment scans (22). Baseline serologic CA-27.29 values (>38U/ml considered producers) were acquired on the day of treatment and reevaluated at the above follow-ups to assess tumor marker response. CA-27.29 was utilized as a study marker for response but not for progression because false elevations may be observed 1-2mo after the initiation of a new treatment (23).
Study Endpoints
The primary endpoints were toxicity and progression with overall survival (OS) as a secondary endpoint. The Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute (version 4.0) was used to grade immediate (<24hrs), early (1-30d), or delayed (>30d) clinical and biochemical adverse events following radioembolization (24). The endpoint for tumor progression was defined by RECIST criteria as a 20% increase in long-axis measurements. Hepatic progression was defined as any appearance of new lesions or any enlargement of existing disease in treated or untreated volumes. Distant progression was defined as the appearance of new lesions or any enlargement of existing extrahepatic disease. Median OS was determined by all-cause mortality and verified with the Social Security Death Index.
Statistical Analyses
All analyses are calculated from the day of first radioembolization using the Kaplan-Meier method in this intent-to-treat cohort. In the absence of progression, patients were censored on the day of last imaging. OS was censored on the day of last follow up. The log-rank test was used to assess differences in estimates between groups and significant predictors were included in a multivariate Cox proportional hazards model with adjustment for possible confounders. 95% confidence intervals were calculated. Log-minus-log plots and Schoenfield residuals were used to assess validity of the proportional hazards assumption for univariate and multivariate analyses, respectively. All data were analyzed with STATA (12.1; StataCorp, College Station, Texas). Differences were considered statistically significant at two-tailed P-values of less than .05.
RESULTS
Treatment and Toxicity
Table 2 presents patient dosimetry. 161 infusions were delivered into 125 different hepatic treatment volumes over 122 treatment sessions. The mean infused activity was 1.52GBq (95% CI,1.38 to 1.67GBq) and residual activity in the dose vial was 4.6% (95% CI,3.0 to 6.3%). The implemented embolic 90Y device was glass microspheres (TheraSphere, BTG, Canada). There were no misadministrations. Repeat radioembolization was performed in 33 patients with retreatment of a prior target volume in 42.4% (14/33).
Table 2.
Yttrium-90 Dosimetry
| Location | No. of Patients | Treatment Cycles | Treated Volume (cc) | Dose (Gy) | Cumulative Lung Dose (Gy) | LSF (%) |
|---|---|---|---|---|---|---|
| Segmental* | 12 | 30 | 312.6 (276.8 - 475.6) | 118.5 (114.2 - 137.4) | 3.4 (1.8 - 13.4) | 2.6 (1.3 - 9.6) |
| Right Lobe | 37 | 46 | 1,018.4 (912.4 - 1,170.8) | 113.1 (100.7 - 119.1) | 4.5 (4.2 - 6.7) | 2.8 (2.7 - 4.1) |
| Left Lobe | 5 | 9 | 800.0 (561.8 - 946.5) | 115.0 (92.2 - 131.7) | 2.5 (0.4 - 5.9) | 3.0 (2.1 - 5.3) |
| Bilobar | 21 | 23 | 1,426.0 (1,324.2 - 1,687.1) | 120.2 (104.0 - 124.1) | 5.0 (4.0 - 7.8) | 2.6 (2.1 - 3.5) |
Note.–Values expressed as medians and 95% confidence intervals.
LSF, lung shunt fraction.
Infusion at the level of ≤ 2 segmental feeding arteries.
Four patients (5.3%) were enrolled in a Phase 1 trial (NCT00858429) evaluating 90Y dose-escalation with concomitant capecitabine to capitalize on radiation-induced increases in thymidine phosphorylase and to interrogate potential radiosensitizing effects of chemoradiation (25-27). Capecitabine was administered for 14d followed by a 7d rest period x3 cycles. These patients received doses of 97-166Gy on days 1-7 of the second cycle. Two patients discontinued capecitabine during the second cycle due to Grade 3 skin toxicity plus transient cholecystitis in one patient and hand-foot syndrome in the second patient.
Table 3 summarizes Grade 3 clinical and biochemical toxicities after the first treatment. Clinical toxicity follow-up was available in sixty-six (91.7%) of seventy-two living patients at 1mo. 30-day mortality was 4% (n=3). Each of these patients had elevated total serum bilirubin at baseline, extrahepatic disease in 2+ sites, and 25-50% (n=2) or >50% (n=1) hepatic tumor burden. The cause of death was sepsis in a patient with peritoneal involvement and hepatic decompensation in the remaining two patients. Two patients (2.8%) had rectus sheath irritation due patency of the falciform artery. Two patients (2.8%) had cholecystitis and one required surgery with radiation-induced cholecystitis diagnosed on surgical pathology. Vascular complications included groin hematoma and ecchymosis (n=1) and symptomatic anemia requiring transfusion (n=1). The most common Grade 1/2 clinical toxicities were fatigue (74.2%), nausea (31.8%), and abdominal pain (30.3%); however, the incidence of any severe Grade 3 clinical toxicity was 7.6% (n=5). There were no Grade 4 clinical toxicities.
Table 3.
Toxicity According to CTCAE Version 4.0
| Adverse Event |
Grade 3 |
|
|---|---|---|
| Patient No. | % | |
| Clinical | ||
| Any Cause | 5 | 7.6 |
| Fatigue | 1 | 1.5 |
| Abdominal Pain | 4 | 6.1 |
| Nausea | 1 | 1.5 |
| Vomiting | 0 | 0.0 |
| Fever | 1 | 1.5 |
| Biochemical | ||
| Any Cause | 37 | 54.4 |
| Decreased Albumin | 3 | 4.2 |
| Increased Total Serum Bilirubin | 4 | 5.9 |
| Increased Alkaline Phosphatase | 4 | 5.9 |
| Increasted ALT | 3 | 4.4 |
| Increasted AST | 6 | 8.8 |
| Leukopenia | 3 | 4.3 |
| Lymphopenia | 29 | 42.0 |
Note.–Values expressed as incidence and percent.
ALT, alanine transaminase; AST, aspartate transaminase; CTCAE, Common Terminology Criteria for Adverse Events.
Biochemical toxicity follow-up was available for seventy-two patients (100%) and complete for sixty-eight patients (94.4%). The most common Grade 1/2 biochemical toxicities were increased alkaline phosphatase (64.7%) and AST (63.2%). Lymphocyte radiosensitivity resulted in Grade 3 lymphopenia for 29 patients (42%). Grade 3 hyperbilirubinemia occurred in four patients, of whom two had hyperbilirubinemia at baseline. There were no Grade 4 biochemical toxicities.
Eligibility for Systemic Therapy
Twenty-seven patients included in the present study saw an oncologist at an outside hospital and details regarding their systemic treatment were not available in retrospective chart review at our center. At our institution, 66.7% (32/48) of patients received additional systemic therapy after radioembolization while 16.7% (8/48) did not. One patient was lost to follow-up due to international travel. Seven patients did not have information regarding subsequent systemic therapy in the medical record.
Tumor Response
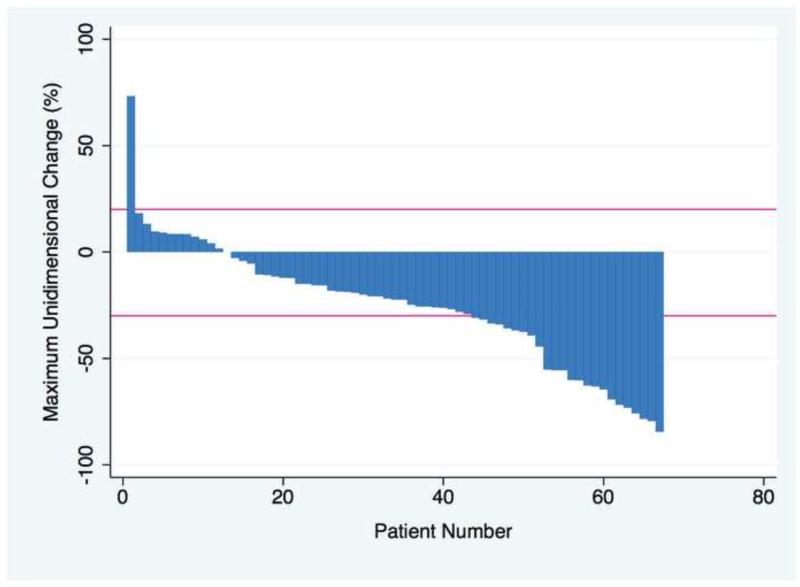
Table E2 presents imaging response by patient and by index lesion. Waterfall plot appears in Figure 1. Index lesions (n=104) were defined in 276 reviewed studies (CT and/or MRI). The average tumor size was 3.9cm (95% CI,3.4 to 4.4cm). Imaging after radioembolization was achieved in 93.2% (68 of 73 living patients). The mean and 95% CI for follow-up scans (n=201) after first treatment was 3.2mo (2.6 to 3.7mo) but was positively skewed with a median of 1.4mo. The overall response rate defined as any decrease in long-axis was 82.4% (n=59).
Figure 1.
Maximum unidimensional change over the course of follow up for 68 patients. Negative values represent % decrease in tumor size and bars represent RECIST cutoffs for partial response (−30%) and progressive disease (+20%).
Pre- and post-treatment PET imaging was available in 25 patients. In this subset, 12% (n=3) had a complete response, 72% (n=18) had a partial response or stable disease, and 16% (n=4) had progressive disease (PD). Fifty-five patients (73.3%) were CA-27.29 producers and 16 of these 55 (29.1%) had a 30% decrease in serum CA-27.29.
Survival & Progression
OS
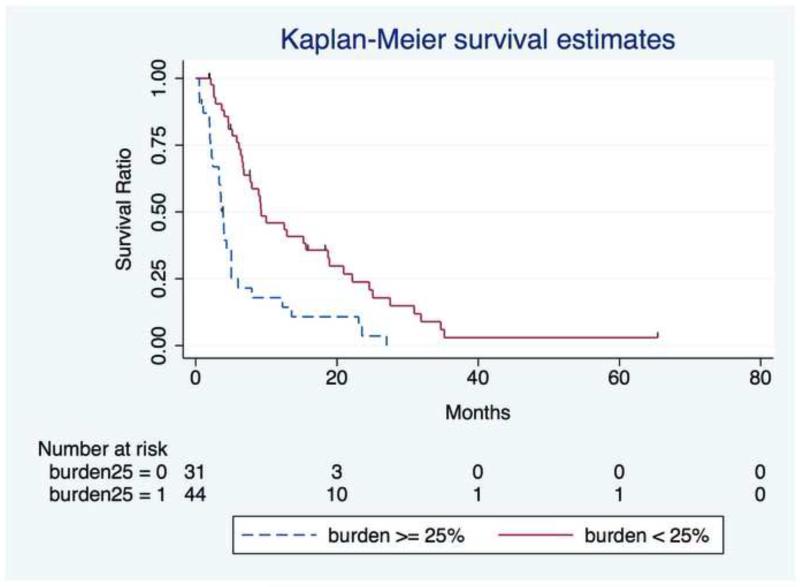
Table 4 presents stratified median survival times and 95% CIs. The median OS was 6.6mo (95% CI,5.0 to 9.2mo). Estimated OS (and 95% CI) was 96% (88.1-98.7%), 80.7% (69.6-88.1%), 53.7% (41.5-64.5%), and 34.5% (23.5-45.7%) at 1mo, 3mo, 6mo, and 12mo, respectively. Univariate analyses identified tumor burden ≥25% (P < 0.0001) and total serum bilirubin > 1.1 mg/dL (P < 0.0001) as risk factors for worsened survival. Tumor burden <25% (HR,.39;95% CI,.23 to .66;P=.001;Figure 2) and elevated bilirubin (HR,1.38;95% CI,1.10 to 1.73;P=.005) as a continuous variable remained significant in the multivariate analysis after adjusting for potential confounders.
Table 4.
Survival Analysis
| Univariate | MultivariateŦ | |||
|---|---|---|---|---|
| Variable | Time to Event (mo) | P Value | Hazard Ratio | P Value |
| Treatment Age | 0.4993 | |||
| < 65 y | 6.8 (4.6 - 10.0) | - | ||
| ≥ 65 y | 5.0 (3.6 - 12.5) | - | ||
| Systemic Treatment History | 0.0594 | 0.067 | ||
| < 6 Medications | 4.6 (3.6 - 6.1) | 1.61 (.97 - 2.68) | ||
| ≥ 6 Medications | 8.9 (6.4 - 12.5) | 1.00 | ||
| ECOG Performance Status | 0.3895 | |||
| 0 | 6.7 (5.0 - 10.0) | - | ||
| 1+ | 6.1 (3.5 - 12.3) | - | ||
| Tumor Burden | < 0.0001 | 0.001 | ||
| < 25% | 9.3 (6.7 - 18.7) | .39 (.23 - .66) | ||
| ≥ 25% | 3.9 (2.4 - 5.0) | 1.00 | ||
| Distribution | 0.0665 | 0.558 | ||
| Unilobar | 6.7 (4.0 - 34.7) | .72 (.24 - 2.18) | ||
| Bilobar | 6.4 (4.6 - 9.1) | |||
| No. of Lesions | 0.1062 | 0.705 | ||
| Solitary | 6.7 (4.0 - 34.7) | .81 (.26 - 2.47) | ||
| Multifocal | 6.6 (4.4 - 9.1) | 1.00 | ||
| Bilirubin* | < 0.0001 | 0.005 | ||
| ≤ 1.1 mg/dL | 7.9 (5.8 - 12.3) | 1.00 | ||
| > 1.1 mg/dL | 2.0 (0.5 - 2.6) | 1.38 (1.10 - 1.73) | ||
| Extrahepatic Metastases | 0.095 | 0.164 | ||
| No | 9.3 (5.2 - 23.1) | 1.00 | ||
| Yes | 5.8 (4.0 - 8.9) | 1.59 (0.83 - 3.03) | ||
| Brain Metastases | 0.2906 | |||
| No | 6.4 (4.6 - 12.3) | - | ||
| Yes | 6.8 (2.4 - 9.1) | - | ||
| CA-27.29 Producer* | 0.3061 | |||
| No | 5.0 (3.7 - 7.9) | - | ||
| Yes | 7.9 (4.6 - 12.3) | - | ||
Note.–Values expressed as median (95% confidence interval) where appropriate.
ECOG, Eastern Cooperative Oncology Group.
Multivariate analyses adjusted for continuous pre-treatment bilirubin and CA-27.29 values.
Variables included in multivariate Cox proportional hazards model if the proportional hazards assumption was not violated and P was < 0.25 on univariate log-rank testing.
Figure 2.
Overall Survival from the day of first treatment (n=9 patients are censored) stratified by tumor burden < 25% or ≥ 25%.
Tumor Progression
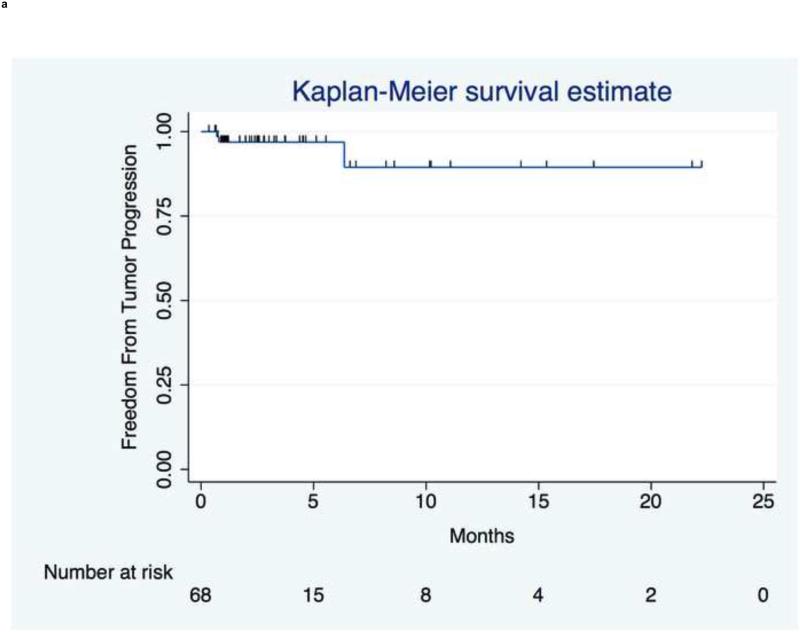
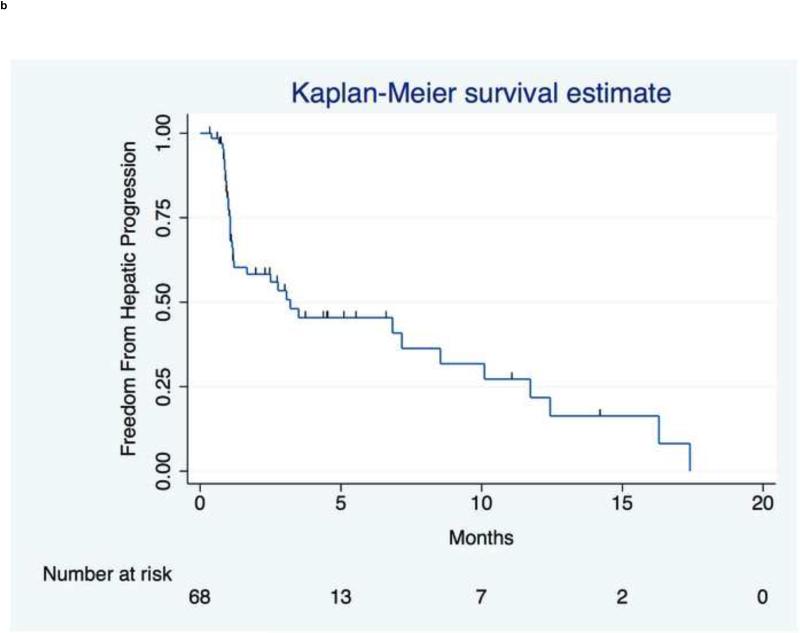
Figure 3 presents tumor, hepatic, and distant progression and Table E3 summarizes disease progression at 1, 3, 6, and 12mo. Three patients had index lesion progression over the course of follow-up by RECIST criteria.
Figure 3.
Time to Progression. (a) TTP in index lesions by RECIST criteria from the day of first treatment (n=3 patients had progressive disease). (b) Hepatic TTP in treated or untreated liver. Median hepatic TTP was 3.2mo (95% CI,1.2 to 8.5mo). (c) Extrahepatic TTP in distant sites. Median extrahepatic TTP was 4.1mo (95% CI,2.4 to 7.0mo).
Hepatic Progression
The median time to hepatic progression was 3.2mo (95% CI,1.2 to 8.5mo). Solitary versus multifocal disease was the only significant univariate prognosticator (12.4 v 2.5mo;P=.0143) and was significant in the multivariate analysis (HR,.22,95% CI,.05 to .98;P=.046) after adjusting for unilobar versus bilobar disease.
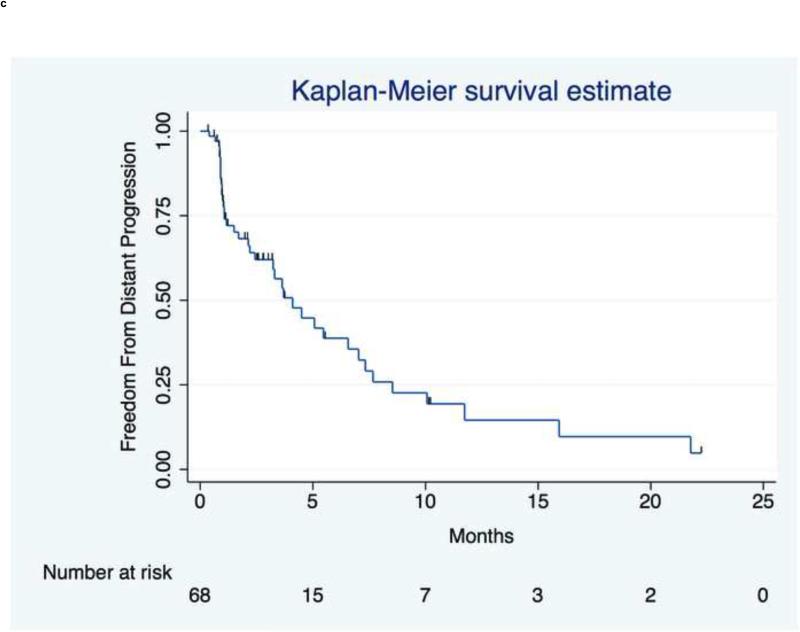
Distant Progression
The median time to distant progression was 4.1mo (95% CI,2.4 to 7.0mo). Distant progression of existing disease occurred in the brain for five patients (50%). New brain tumors were detected on MRI in seven patients (11.3%) without a prior history. Other initial sites of extrahepatic progression were lung (n=15), abdominal lymph nodes (n=13), bone (n=7), spleen (n=3), peritoneum (n=2), pancreas (n=2), pericardial lymph nodes (n=2), and adrenal (n=1).
DISCUSSION
This series expands on a 27 patient pilot study (28), including these data to report toxicity, therapeutic effectiveness, and important prognosticators in a small but growing literature (29) on radioembolization in BCLM patients. Locoregional therapy with 90Y radioembolization had an acceptable safety profile with fewer severe adverse events (Grade 3+) in comparison to systemic toxicities from several chemotherapy approaches applied in breast cancer patients with visceral metastases (30-34). The incidence of Grade 3 clinical toxicity was low and agreed with the resin microsphere literature with a decreased incidence of abdominal pain in our cohort (35-38). Most patients received additional systemic therapy after radioembolization suggesting preserved eligibility for nth-line chemotherapy despite advanced disease but timing, dosing, safety, and efficacy must be evaluated prospectively.
Radiologic tumor response following radioembolization with resin microspheres has shown progressive disease (PD) in only 12.5% (17/136) by RECIST criteria (35,37-39) and 6% (2/36) by World Health Organization (WHO) criteria (36) at 2-4mo. Disease control (98.5% in this study) may capture a bias towards positive treatment effects in slow growing neoplasms. Therefore, we also report objective decreases in tumor size in 82.4% of patients. Metabolic disease control was 84% (21/25) on PET and imaging modalities like PET/CT (35) and diffusion-weighted MRI (40) may allow early response assessments. Interestingly, all four patients with PD on PET had SD (2/4) or PR (2/4) by RECIST with potential overestimation of PD due to treatment-related inflammation (41).
Radioembolization is mechanistically distinct in comparison to transarterial chemoembolization (TACE). Though TACE may theoretically offer escalated local dosing or combinations of chemotherapeutics, therapeutic failure rates are high with PD in 51-60% of patients (42,43) even when multiple cytotoxic agents and dose selection are tailored to individual mBC patients based on their history of systemic treatment (43). Buijs et al. reported 26% of treated lesions had partial RECIST response after multi-agent TACE yet 0 of 14 patients had a net partial response based on total tumor burden and the number of patients with stable or PD and toxicity were not specified (40). Differences in reporting, variations in treatment protocols, and heterogeneity of baseline patient characteristics limit comparisons of OS following TACE. Infiltrative patterns often observed in BCLM may not be amenable to embolization and these tumors are often chemorefractory at the time of intraarterial therapy, reducing enthusiasm for TACE mechanisms of action.
Substratification analyses were performed to identify baseline variables that have been associated with outcomes in unresectable liver metastases treated with 90Y (37,38,44-46). Increased hepatic tumor burden and bilirubin were significant multivariate prognosticators of OS suggesting that the presence of hepatic metastases not only portends a worsened prognosis (47) but that the degree of liver involvement and dysfunction adversely influences patient survival. When polychemotherapy options are exhausted, BCLM patients often have substantial extrahepatic disease burden or micrometastases that are chemorefractory and this likely limits survival even when liver-directed therapy is applied successfully. Earlier locoregional therapy at the time of hepatic metastases, especially for niche subgroups with known chemoresistance (ie, triple negative patients), may be warranted. It should be noted that analyses of OS stratified by endpoint measures of therapeutic success/failure over follow-up such as imaging response (35-38,43) are prone to guarantee-time bias (48). Moving forward, propensity score matched analyses may provide statistical post hoc randomization for informative retrospective analyses in settings where patient recruitment for prospective randomized studies is not feasible.
The localized radiation effect of 90Y had the intended therapeutic effect and benefited BCLM patients by consistently delaying or stopping tumor progression in targeted lesions. Differences between hepatic progression and tumor progression suggest the 3.2mo median time to hepatic progression primarily represents progression in untreated volumes or new hepatic tumors. The identification of multifocal disease as an independent prognosticator for reduced hepatic TTP may be indicative of bad biology serving as a “canary in the coal mine” reflective of global metastatic potential. This prognosticator could also conceivably reflect an increased number of tumors that can progress or more challenging tumor targeting but these explanations were not supported given the rare occurrence of progression in index lesions.
Radiofrequency ablation (RFA) offers a median survival after ablation of 29.9 to 60mo with 20-32% 5-year survival (49-53). Alternative techniques include MRI-guided laser-induced interstitial thermotherapy and high-dose-rate brachytherapy with iridium-192 with median OS of 37.6mo (n=276) and 18mo (n=37), respectively (54-56). 90Y allows treatment of unresectable hepatic tumors when the number, size, shape, or location of lesions is not appropriate for RFA or alternative ablative techniques.
Our reported outcomes primarily reflect safety and antitumor efficacy for 90Y radioembolization in patients with advanced disease after exhausting chemotherapy options but future comparative studies must determine benefits relative to active nth-line systemic agents. These studies should not only consider survival for BCLM patients but also benefits in quality of life metrics as has been done for hepatocellular carcinoma (57). The maximum tolerated dose for radioembolization with glass microspheres in combination with capecitabine (27) exceeds the target dose of 120Gy reported herein where 82.4% of patients had an objective index lesion response. Therefore, chemoradiation combining capecitabine and radioembolization with the glass 90Y microsphere delivery device may potentially serve as a safe and effective arm for a randomized trial.
This study has important limitations. These analyses were retrospective and there was no comparison arm as patients had exhausted alternative treatment options. Intent-to-treat study designs obscure the effects of subsequent systemic or locoregional therapies creating potential for type II error in OS analyses. The presence of concomitant capecitabine administration in a small number of patients (5.3%) is a confounder that may have increased measures of toxicity and anti-tumor activity. Follow-up imaging was not possible for all patients and many patients did not have PET imaging. Radiologic antitumor response was evaluated by defining primary index lesions; a method validated for hepatocellular carcinoma (21) but not for BCLM and the use of RECIST is limited by discretization of response, assumed tumor geometry, and it may not capture tumor growth kinetics.
Chemoresistance mechanisms are often varied and there is a need for molecular diagnostics to identify patient-specific chemoresistance mechanisms. 90Y radioembolization is safe and stabilizes hepatic disease in a heterogeneous population of BCLM patients with a low therapeutic failure rate. Liver-directed therapy with 90Y may supplement active systemic therapies in the chemorefractory setting and rationally designed combinations with systemic therapy are under investigation. Our outcomes data should allow sample size estimation for future trials (58) and guide patient selection given confirmation of independent risk factors for reduced survival.
Supplementary Material
Acknowledgments
The authors thank advanced clinical research nurses Krystina Salzig RN, and Karen Marshall RN, for their help and support.
Role of Funding: There was no funding provided for this study. RS is supported in part by NIH grant CA126809. ACG is supported in part by an Allied Scientist grant from the SIR Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: RJL and RS served as advisors to BTG. None of the other authors have any conflict of interest.
This work was presented at the 2014 SIR Annual Scientific Meeting in San Diego, California. It was selected (1/4 abstracts) for an SIR Press Release.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute; Bethesda, MD: 2013. [October 2013]. Available at http://seer.cancer.gov/csr/1975_2010/. [Google Scholar]
- 3.Zinser JW, Hortobagyi GN, Buzdar AU, Smith TL, Fraschini G. Clinical course of breast cancer patients with liver metastases. J Clin Oncol. 1987;5:773–82. doi: 10.1200/JCO.1987.5.5.773. [DOI] [PubMed] [Google Scholar]
- 4.Eklund JW, Trifilio S, Mulcahy MF. Chemotherapy dosing in the setting of liver dysfunction. Oncology (Williston Park, NY) 2005;19:1057–69. [PubMed] [Google Scholar]
- 5.Field KM, Dow C, Michael M. Part I: Liver function in oncology: biochemistry and beyond. Lancet Oncol. 2008;9:1092–101. doi: 10.1016/S1470-2045(08)70279-1. [DOI] [PubMed] [Google Scholar]
- 6.Field KM, Michael M. Part II: Liver function in oncology: towards safer chemotherapy use. Lancet Oncol. 2008;9:1181–90. doi: 10.1016/S1470-2045(08)70307-3. [DOI] [PubMed] [Google Scholar]
- 7.Atalay G, Biganzoli L, Renard F, Paridaens R, Cufer T, Coleman R, et al. Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Cancer. 2003;39:2439–49. doi: 10.1016/s0959-8049(03)00601-4. [DOI] [PubMed] [Google Scholar]
- 8.Thelen A, Benckert C, Jonas S, Lopez-Hänninen E, Sehouli J, Neumann U, et al. Liver resection for metastases from breast cancer. J Surg Oncol. 2008;97:25–9. doi: 10.1002/jso.20911. [DOI] [PubMed] [Google Scholar]
- 9.Vlastos G, Smith DL, Singletary SE, Mirza NQ, Tuttle TM, Popat RJ, et al. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol. 2004;11:869–74. doi: 10.1245/ASO.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Díaz R, Santaballa A, Munárriz B, Calderero V. Hepatic resection in breast cancer metastases: should it be considered standard treatment? Breast. 2004;13:254–8. doi: 10.1016/j.breast.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Ercolani G, Grazi GL, Ravaioli M, Ramacciato G, Cescon M, Varotti G, et al. The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol. 2005;12:459–66. doi: 10.1245/ASO.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Metcalfe MS, Mullin EJ, Maddern GJ. Hepatectomy for metastatic noncolorectal gastrointestinal, breast and testicular tumours. ANZ J Surg. 2006;76:246–50. doi: 10.1111/j.1445-2197.2006.03689.x. [DOI] [PubMed] [Google Scholar]
- 13.Salem R, Lewandowski RJ, Atassi B, Gordon SC, Gates VL, Barakat O, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627–39. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- 14.Murthy R, Nunez R, Szklaruk J, Erwin W, Madoff DC, Gupta S, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics. 2005;25:S41–55. doi: 10.1148/rg.25si055515. [DOI] [PubMed] [Google Scholar]
- 15.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–78. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 16.Campbell AM, Bailey IH, Burton MA. Tumour dosimetry in human liver following hepatic yttrium-90 microsphere therapy. Phys Med Biol. 2001;46:487–98. doi: 10.1088/0031-9155/46/2/315. [DOI] [PubMed] [Google Scholar]
- 17.Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163–71. doi: 10.1016/j.ijrobp.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 18.Salem R, Lewandowski RJ, Gates VL, Nutting CW, Murthy R, Rose SC, et al. Research reporting standards for radioembolization of hepatic malignancies. Journal Of Vascular And Interventional Radiology. 2011;22:265–78. doi: 10.1016/j.jvir.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padhani AR, Ollivier L. The RECIST criteria: implications for diagnostic radiologists. British Journal of Radiology. 2001;72:983–986. doi: 10.1259/bjr.74.887.740983. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Riaz A, Miller FH, Kulik LM, Nikolaidis P, Yaghmai V, Lewandowski RJ, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA. 2010;303:1062–9. doi: 10.1001/jama.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewandowski RJ, Thurston KG, Goin JE, Wong C-YO, Gates VL, Van Buskirk M, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135-150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J Vasc Interv Radiol. 2005;16:1641–51. doi: 10.1097/01.RVI.0000179815.44868.66. [DOI] [PubMed] [Google Scholar]
- 23.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute [September 2013];Cancer Therapy Evaluation Program (CTEP) Available at: http://evs.nci.nih,gov/ftp1/CTCAE/About.html.
- 25.Sawada N, Ishikawa T, Sekiguchi F, Tanaka Y, Ishitsuka H. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin Cancer Res. 1999;5:2948–53. [PubMed] [Google Scholar]
- 26.Unger KR, Romney DA, Koc M, Moskaluk CA, Friel CM, Foley EF, et al. Preoperative chemoradiation for rectal cancer using capecitabine and celecoxib correlated with posttreatment assessment of thymidylate synthase and thymidine phosphorylase expression. Int J Radiat Oncol Biol Phys. 2011;80:1377–82. doi: 10.1016/j.ijrobp.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Hickey R, Mulcahy MF, Lewandowski RJ, Gates VL, Vouche M, Habib A, et al. Chemoradiation of hepatic malignancies: prospective, phase 1 study of full-dose capecitabine with escalating doses of yttrium-90 radioembolization. Int J Radiat Oncol Biol Phys. 2014;88:1025–31. doi: 10.1016/j.ijrobp.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 28.Bangash AK, Atassi B, Kaklamani V, Rhee TK, Yu M, Lewandowski RJ, et al. 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol. 2007;18:621–8. doi: 10.1016/j.jvir.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Smits MLJ, Prince JF, Rosenbaum CENM, van den Hoven AF, Nijsen JFW, Zonnenberg BA, et al. Intra-arterial radioembolization of breast cancer liver metastases: a structured review. Eur J Pharmacol. 2013;709:37–42. doi: 10.1016/j.ejphar.2012.11.067. [DOI] [PubMed] [Google Scholar]
- 30.Zelek L, Barthier S, Riofrio M, Fizazi K, Rixe O, Delord JP, et al. Weekly vinorelbine is an effective palliative regimen after failure with anthracyclines and taxanes in metastatic breast carcinoma. Cancer. 2001;92:2267–72. doi: 10.1002/1097-0142(20011101)92:9<2267::aid-cncr1572>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 31.Chan S, Friedrichs K, Noel D, Pintér T, Van Belle S, Vorobiof D, et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol. 1999;17:2341–54. doi: 10.1200/JCO.1999.17.8.2341. [DOI] [PubMed] [Google Scholar]
- 32.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 33.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 34.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 35.Haug AR, Tiega Donfack BP, Trumm C, Zech CJ, Michl M, Laubender RP, et al. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53:371–7. doi: 10.2967/jnumed.111.096230. [DOI] [PubMed] [Google Scholar]
- 36.Coldwell DM, Kennedy AS, Nutting CW. Use of yttrium-90 microspheres in the treatment of unresectable hepatic metastases from breast cancer. Int J Radiat Oncol Biol Phys. 2007;69:800–4. doi: 10.1016/j.ijrobp.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 37.Jakobs TF, Hoffmann R-T, Fischer T, Stemmler H-J, Tatsch K, La Fougere C, et al. Radioembolization in patients with hepatic metastases from breast cancer. J Vasc Interv Radiol. 2008;19:683–90. doi: 10.1016/j.jvir.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Saxena A, Kapoor J, Meteling B, Morris DL, Bester L. Yttrium-90 Radioembolization for Unresectable, Chemoresistant Breast Cancer Liver Metastases: A Large Single-Center Experience of 40 Patients. Ann Surg Oncol. 2014;21:1296–1303. doi: 10.1245/s10434-013-3436-1. [DOI] [PubMed] [Google Scholar]
- 39.Cianni R, Urigo C, Notarianni E, Saltarelli A, D'Agostini A, Iozzino M, et al. Radioembolisation using yttrium 90 (Y-90) in patients affected by unresectable hepatic metastases. Radiol Med. 2010;115:619–33. doi: 10.1007/s11547-010-0496-1. [DOI] [PubMed] [Google Scholar]
- 40.Buijs M, Kamel IR, Vossen JA, Georgiades CS, Hong K, Geschwind J-FH. Assessment of metastatic breast cancer response to chemoembolization with contrast agent enhanced and diffusion-weighted MR imaging. J Vasc Interv Radiol. 2007;18:957–63. doi: 10.1016/j.jvir.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 41.Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–80. [PubMed] [Google Scholar]
- 42.Eichler K, Jakobi S, Gruber-Rouh T, Hammerstingl R, Vogl TJ, Zangos S. Transarterial chemoembolisation (TACE) with gemcitabine: Phase II study in patients with liver metastases of breast cancer. Eur J Radiol. 2013;82:1816–22. doi: 10.1016/j.ejrad.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 43.Cho SW, Kitisin K, Buck D, Steel J, Brufsky A, Gillespie R, et al. Transcatheter arterial chemoembolization is a feasible palliative locoregional therapy for breast cancer liver metastases. Int J Surg Oncol. 2010;2010:1–8. doi: 10.1155/2010/251621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulcahy MF, Lewandowski RJ, Ibrahim SM, Sato KT, Ryu RK, Atassi B, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer. 2009;115:1849–58. doi: 10.1002/cncr.24224. [DOI] [PubMed] [Google Scholar]
- 45.Dunfee BL, Riaz A, Lewandowski RJ, Ibrahim S, Mulcahy MF, Ryu RK, et al. Yttrium-90 radioembolization for liver malignancies: prognostic factors associated with survival. J Vasc Interv Radiol. 2010;21:90–5. doi: 10.1016/j.jvir.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Sato KT, Lewandowski RJ, Mulcahy MF, Atassi B, Ryu RK, Gates VL, et al. Unresectable Chemorefractory Liver Metastases: Radioembolization with 90Y Microspheres--Safety, Efficacy, and Survival. Radiology. 2008;247:507–15. doi: 10.1148/radiol.2472062029. [DOI] [PubMed] [Google Scholar]
- 47.Largillier R, Ferrero J-M, Doyen J, Barriere J, Namer M, Mari V, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19:2012–9. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of Guarantee-Time Bias. J Clin Oncol. 2013;31:2963–9. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sofocleous CT, Nascimento RG, Gonen M, Theodoulou M, Covey AM, Brody LA, et al. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol. 2007;189:883–9. doi: 10.2214/AJR.07.2198. [DOI] [PubMed] [Google Scholar]
- 50.Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation--intermediate and long-term survival rates. Radiology. 2009;253:861–9. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakobs TF, Hoffmann R-T, Schrader A, Stemmler H-J, Trumm C, Lubienski A, et al. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Inter Rad. 2009;32:38–46. doi: 10.1007/s00270-008-9384-7. [DOI] [PubMed] [Google Scholar]
- 52.Carrafiello G, Fontana F, Cotta E, Petullà M, Brunese L, Mangini M, et al. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med. 2011;116:1059–66. doi: 10.1007/s11547-011-0697-2. [DOI] [PubMed] [Google Scholar]
- 53.Gunabushanam G, Sharma S, Thulkar S, Srivastava DN, Rath GK, Julka PK, et al. Radiofrequency ablation of liver metastases from breast cancer: results in 14 patients. J Vasc Interv Radiol. 2007;18:67–72. doi: 10.1016/j.jvir.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 54.Vogl TJ, Freier V, Nour-Eldin N-EA, Eichler K, Zangos S, Naguib NNN. Magnetic resonance-guided laser-induced interstitial thermotherapy of breast cancer liver metastases and other noncolorectal cancer liver metastases: an analysis of prognostic factors for long-term survival and progression-free survival. Invest Radiol. 2013;48:406–12. doi: 10.1097/RLI.0b013e31828328d7. [DOI] [PubMed] [Google Scholar]
- 55.Mack MG, Straub R, Eichler K, Söllner O, Lehnert T, Vogl TJ. Breast cancer metastases in liver: laser-induced interstitial thermotherapy--local tumor control rate and survival data. Radiology. 2004;233:400–9. doi: 10.1148/radiol.2332030454. [DOI] [PubMed] [Google Scholar]
- 56.Collettini F, Golenia M, Schnapauff D, Poellinger A, Denecke T, Wust P, et al. Percutaneous computed tomography-guided high-dose-rate brachytherapy ablation of breast cancer liver metastases: initial experience with 80 lesions. J Vasc Interv Radiol. 2012;23:618–26. doi: 10.1016/j.jvir.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 57.Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, et al. Increased Quality of Life Among Hepatocellular Carcinoma Patients Treated With Radioembolization, Compared With Chemoembolization. Clin Gastroenterol Hepatol. 2013;11:1358–65. doi: 10.1016/j.cgh.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 58.Eng J. Sample size estimation: how many individuals should be studied? Radiology. 2003;227:309–13. doi: 10.1148/radiol.2272012051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.