Abstract
Systemic sclerosis (SSc) is a disorder characterized by immune dysfunction, microvascular injury, and fibrosis. Organ involvement in patients with SSc is variable; however, pulmonary involvement occurs in up to 90% of patients with SSc. Interstitial lung disease (ILD) is a major cause of mortality and, thus, a major determinant in the prognosis of patients with SSc. This review summarizes current findings about the characteristics of ILD in patients with SSc, selection of patients with SSc-ILD who are candidates for the treatment, and current treatment options.
Keywords: systemic sclerosis, interstitial lung disease, pulmonary hypertension
Introduction
Systemic sclerosis (SSc) is a disorder characterized by immune dysfunction, microvascular injury, and fibrosis. Interstitial lung disease (ILD) is an important component of SSc and is detected by high-resolution computed tomography (HRCT) scanning in up to 90% of patients with SSc.1 It can also be complicated with pulmonary arterial hypertension (PAH) and/or pulmonary veno-occlusive disease (PVOD) and potentially precipitated with gastroesophageal reflux and dysmotility. SSc-ILD is the major cause of morbidity and mortality in SSc, which has a great impact on the mortality of SSc, with an estimated mortality from pulmonary disease of all causes to be around 30%. This article reviews the recent findings of the characteristics and treatments of ILD in patients with SSc.
The Natural History and Clinical Course of SSc-ILD
Patients with SSc-ILD are predominantly women between the ages of 30 and 55 years.2,3 The prevalence of SSc-ILD dose not increase with age, such as idiopathic pulmonary fibrosis (IPF).3 SSc-ILD is responsible for up to 30% of the mortality of patients with SSc,2 and the median survival is 5–8 years for SSc-ILD.3,4 However, the survival of patients with SSc-ILD is variable, with five-year survival of about 90% (estimated as 85% in 1994).5 The nine-year survival (including all causes of death) is 38% for those with diffuse cutaneous SSc (dcSSc)-ILD.6 Overall, 12% of patients with SSc-ILD develop severe chronic respiratory insufficiency.7 Interestingly, the prognosis of SSc-ILD is less severe than that of IPF.8 In the natural course of SSc-ILD, 45%–55% of patients show deteriorating pulmonary function tests (PFTs) in the earlier phase (during the first three years), even when they are asymptomatic.5,6 Not all these patients have progressive disease and only 16% develop severe restrictive lung disease (predicted forced vital capacity [FVC] <55%).6 However, the acute deterioration of lung function may also occur in SSc-ILD, it is not well-characterized.9 Physiological parameters such as advanced age (>60 years) at disease onset and reduced lung function such as lower diffusing capacity for carbon monoxide (predicted the diffusing capacity of the lungs for carbon monoxide [DLCO] <65%) are predictive of mortality in SSc.10 Circulating biomarkers that can accurately predict the clinical course of lung disease are still lacking for SSc-ILD.
Findings from Physical Examination and Laboratory Tests of Patients with SSc-ILD
On physical examination, patients present with dyspnea on exertion, and this may be accompanied by a nonproductive cough and inspiratory fine crackles at the bilateral basilar area on auscultation. Digital clubbing is infrequent in SSc-ILD.3 Since some patients with SSc may not develop dyspnea, HRCT must be performed periodically in patients with SSc along with PFT. ILD can be the initial disease manifestation of SSc.11 In these patients, telangiectasia, Raynaud’s phenomenon, and abnormal nailfold capillary changes are observed during the examination.12 While various environmental factors, such as exposure to drugs or to chemical substances such as vinyl chloride and silica, might be associated with SSc.11 Antinuclear antibodies, including anti-Scl-70, anti-Th/To, or other SSc-specific autoantibodies,11 are detected in almost all patients with SSc-ILD. SSc is categorized into two clinical subsets based on the extent of skin thickening: limited cutaneous SSc (lcSSc) and dcSSc. dcSSc shows a significant incidence of an ILD, the onset of Raynaud’s phenomenon within one year of onset skin changes, truncal and acral skin involvement, presence of tendon friction rubs, early involvement of organs such as the heart, lungs, and kidneys, oliguric renal failure, diffuse gastrointestinal disease, and myocardial involvement, absence of anticentromere antibodies, and positive with anti-Scl-70 antibodies (30% of patients), which is more progressive than lcSSc. In contrast, lcSSc has slower onset, usually Raynaud’s phenomenon for years (occasionally decades), skin involvement limited to hands, face, feet, and forearms (acral) or absent, a significant late incidence of pulmonary hypertension (PH), sometimes with ILD, trigeminal neuralgia, skin calcifications, and telangiectasia, and a high incidence of anticentromere antibodies.13 Although the frequency and severity of ILD are associated mainly with patients with dcSSc, ILD also affects patients with lcSSc. PFT shows restrictive characteristics, with reduced FVC and DLCO.3
Findings from Bronchoscopic Analysis in Patients with SSc
Bronchoalveolar lavage (BAL) fluid from healthy nonsmokers contains a predominance of macrophages (80%–90%), with lower percentages of lymphocytes (5%–15%) and neutrophils (≤3%).14 Inflammatory cells and other inflammation mediators were present in the BAL fluid of patients with SSc-ILD, reflecting ongoing inflammation in the lungs.15,16 An abnormal BAL cellular profile (defined as ≥15% lymphocytes and/or ≥5% neutrophils and/or ≥5% eosinophils in the BAL fluid) was present in 38% of patients with SSc who showed parenchymal involvement on HRCT,17 but 47% of patients with normal HRCT findings also had abnormal BAL cell counts (defined as ≥18% lymphocytes and/or >4% neutrophils and/or >2% eosinophils in the BAL fluid).18 One group reported an association between abnormal BAL findings and changes in PFT over time.19 This group also found an association between neutrophilia in BAL fluid and early mortality that occurred within two years of presentation of ILD (defined as percentages of lymphocytes was >14%, neutrophils >4%, eosinophils >2%, or granulocytosis [neutrophils >4%, and/or eosinophils >2%] on BAL).19 However, they observed no associations between neutrophilia, eosinophilia, or lymphocytosis in the BAL fluid with the rapidity of functional deterioration or progression-free survival.19 Active alveolitis, as defined by an increase in neutrophils or eosinophils in the BAL fluid, was demonstrated to correlate with more severely affected lung function, including reduced FVC, total lung capacity (TLC), and DLCO, and with more extensive ground-glass opacity and fibrosis.20 However, the presence of active alveolitis based on BAL fluid findings did not predict disease progression or the response after one year of the treatment with cyclophosphamide (CYC).20 Thus, the prognostic value of abnormal BAL findings in SSc-ILD is very limited, and standardization of the definition of “alveolitis” using BAL is difficult, as discussed earlier. Despite these limitations, BAL may be useful for detecting unsuspected infections that can complicate the management of SSc-related lung disease.
Histology
Nonspecific interstitial pneumonitis (NSIP) is the most common histological finding in SSc-ILD. Usual interstitial pneumonitis (UIP), organizing pneumonia, and diffuse alveolar damage in addition to NSIP represent histological findings in 90% of patients with SSc at autopsy.21 A series of 80 patients with SSc was examined using surgical lung biopsies, and most SSc-ILD was categorized as NSIP,22 based on the classification criteria for interstitial pneumonias.23 In this context, NSIP was much more prevalent (76%) than UIP (11%).22 In a larger patient subgroup, the histopathological findings consisted of a mixture of inflammation and fibrosis. However, fewer than 20% of patients with SSc-ILD showed inflammation-dominant lesions at lung biopsy.22 These findings might be one of the rationales for immunosuppressive treatment; however, recent analysis showed that the histopathological pattern did not seem to predict the progressive disease course or treatment response in patients with SSc-ILD.22
Findings of High-Resolution CT Scan and the Role of Scoring System
HRCT has been shown to be more accurate than chest radiography in detecting and characterizing diffuse lung diseases. Abnormalities on CT correlate more closely with PFT abnormalities.24–26 HRCT is now well-established as a sensitive and noninvasive means of detecting and characterizing SSc-ILD.27–30 Several scoring systems that reflect the severity and extent of ILD based on disease distribution, relative proportions of reticulations and ground-glass opacity, and the presence of fibrosis on CT scans at one or more levels throughout the chest have been reported.30–32 These are useful and important for detecting and selecting the SSc-ILD candidates who should be treated and for following-up the patients who are treated. A scoring system from the UK group revealed that it was a predictor of mortality in an observational study for SSc-ILD.31 Goldin et al showed that their scoring system could be utilized in controlled clinical trials of interventions in patients with SSc-ILD (or potentially in other interstitial pneumonitis) as a usual adjunct to conventional physiologic and patient-centered end points.33 Recently, Frauenfelder et al showed a novel screening system with limited number of slices to avoid excess radiation exposure.34 These scoring systems are very convenient compared with the examination of full set of images of HRCT; however, some of the important images might be missing because of the gaps of the images.
Complications with Pulmonary Hypertension
Although SSc is characterized by fibrotic lesions, such as ILD, vascular lesions, such as PAH, are another aspect of the disease. In terms of SSc-PAH, the prognosis is improved by the use of vasodilators such as prostanoids, endothelin receptor antagonists, and phosphodiesterase 5 inhibitors, but it is still worse than the prognosis for idiopathic PAH. Even when patients with SSc-ILD are asymptomatic and ILD, or PAH are not detected by various imaging or laboratory modalities, fibrosis, and/or vasculopathy may progress although the extent of each process may be variable in each patient. This might be one reason why it has been so difficult to treat lung involvement and why the prognosis has not improved as expected. Chang et al examined the frequencies of the coexistence of ILD and PH in 619 patients with SSc.35 ILD, defined as % TLC <80%, was present in 251 patients (41%), while PH, defined as a systolic PA pressure >35 mmHg as estimated by Doppler echocardiography, was detected in 231 patients (37%). A total of 112 patients (18%) had both ILD and PH; thus, the proportion of PH in SSc-ILD was 44%. Trad et al reported ILD in 52 (60%) of 86 patients with dcSSc; 18 (35%) of the 52 had concomitant PH based on Doppler echocardiography.36 These estimated proportions might be higher than those as they are due to the overestimation of systolic PA pressure by Doppler echocardiography.37 However, the prevalence of PH is <5% in patients with chronic obstructive pulmonary disease or IPF, so the prevalence of PH in patients with SSc with ILD appears to be much higher.38 In patients with SSc, the prevalence of PH was reported to be in the range of 7%–12%, when PH was detected by Doppler echocardiography screening followed by confirmatory right heart catheterization.39 Several cohort studies have evaluated the prevalence of “PH owing to ILD” in patients with SSc, with prevalence findings ranging from 18% to 21%.36,40,41 PAH had a significant adverse impact on survival in both SSc-ILD and IPF,35,36,42,43 and the short-term survival rates of patients with SSc with combined ILD and PH were worse than the rates of those with PAH alone. The three-year survival rates of patients with SSc with combined ILD and PH were disappointingly low, ranging from 28% to 47%. A multivariate analysis of all patients with PH found that ILD was an independent parameter associated with the worst survival.40 Notably, the risk of death was increased fivefold for patients with SSc with combined ILD and PH compared to those with PAH alone. Moreover, histological analysis of lung tissues in patients with SSc-PAH revealed that half of them had fibrosis of not only in arteries/arterioles but veins/venules, which was associated with capillary congestion as in PVOD, which was also associated with severe prognosis of SSc.44 Thus, the patients with SSc have complicated organ involvements simultaneously even in the lung, and it make us difficult to target the treatment.
Role of Gastroesophageal Reflux in the Pathogenesis of SSc-ILD
Esophageal disease and gastroesophageal regurgitation have been reported in 50%–90% of patients with SSc45–47 and are risk factors for lung injury.48 There was a correlation between the degree of DLCO impairment and the degree of gastroesophageal reflux and esophageal motor impairment.46,49 Followed over time, the patients with severe esophageal motor disturbances had a faster deterioration in their DLCO and a higher frequency of ILD on HRCT.49 Recently, the hypothesis that reflux of gastric content into the airway may cause interstitial fibrosis has been proposed by several investigators in experimental models and in other diseases, which is called centrilobular fibrosis. Isolated centrilobular fibrosis was found in 21% of the biopsies and also found associated to nonspecific interstitial pneumonia in 84% of these patients. The constant clinical finding in all isolated centrilobular fibrosis cases was dyspnea, esophageal abnormalities, and moderate lung impairment. Lung function parameters in isolated centrilobular fibrosis patients remained stable after one year of exclusively intensive antireflux treatment.50 These data suggest that gastroesophogeal reflux may contribute to the onset or progression of the disease.
Patients with SSc-ILD who are Candidates for Treatment
Patients with advanced SSc-ILD need to be treated, and patients with earlier disease should have therapeutic intervention as well, especially when they are at high risk of progression. However, it is difficult to identify the latter group. A literature search identified the following key findings that are relevant to the selection and management of patients with SSc-ILD51:
The risk of progression of SSc-ILD is highest in the first four years of the disease, especially in the first two years, and in a small subset of patients with lung disease that precedes the cutaneous manifestations of SSc.52 Therefore, regular monitoring using PFT is important.12,22,53
It is generally accepted that treatment should be introduced if there is evidence of an ongoing progression based on pulmonary function decline or radiographic deterioration, with or without declining exercise tolerance. However, there is limited supporting evidence linking pulmonary function decline to mortality in SSc-ILD.12,53
The link between disease severity at presentation and patient outcome has been widely explored, and more severe pulmonary functional impairment at presentation is indicative of higher risk of mortality.
The histopathological pattern22 and the presence of active alveolitis as detected by BAL54 do not seem to predict the clinical course in patients with SSc-ILD.
Therefore, based on the findings, the clinician must decide which is better for the patient: watchful waiting or treatment. In a recent study of 215 patients with SSc-ILD as judged by CT, the extent of disease on CT was predictive of mortality and decline in FVC.31 The authors proposed a sub-classification of SSc-ILD as mild or extensive based on rapid semiquantitative evaluation using CT and FVC levels. Basically, ILD can be categorized as mild or extensive based on the finding that the total disease extent is greater than or less than 20% of the lung fields. If the CT evaluation is inconclusive, a categorization of mild or extensive disease is made using a threshold of 70% for predicted FVC. The distinction between mild and extensive disease using a combination of CT and PFT data provides a more accurate prognostic separation for SSc-ILD than that has been achieved with any single modality. A recent systematic review found that the extent of fibrosis on lung HRCT scan was the only variable that independently predicted both ILD progression and mortality.55
These data suggest that two types of patients should be treated. First, the patients who initially present with either lung disease with an extent >20% on HRCT or with indeterminate disease extent plus an FVC <70%, second, the patients experiencing a significant decrease in pulmonary functional assessment during the follow-up. The latter is based on a recently proposed consensus for monitoring clinically meaningful progression of ILD in connective tissue disease (even though no factors are associated with disease progression per se).56 The Outcome Measures in RA Clinical Trials (OMERACT) consensus may be one criterion for treatment,56 specifically >10% relative decline in FVC or >5% to <10% relative decline in FVC plus >15% relative decline in DLCO, regardless of the extent of lung involvement.
With increased awareness of the morbidity and mortality associated with SSc-ILD, there is an increasing trend toward performing HRCT and PFT even in patients with asymptomatic SSc, since some patients with moderate-to-severe loss of lung function or advanced fibrosis do not show dyspnea. Limited interstitial abnormalities of questionable clinical significance are increasingly being detected. In a recent series, some patients with SSc-ILD exhibited pulmonary abnormalities on HRCT that made up less than 10% of the total lung volume. When the extent of disease is trivial, a “watchful waiting” approach without immediate intervention is appropriate.
Treatment Approaches for the Treatment of SSc-ILD
Cyclophosphamide
The results of two prospective, randomized, placebo-controlled trials suggest that oral (2 mg/kg/day × 12 months)42 and intravenous (600 mg/m2/month × 6 + 20 mg oral prednisolone (PSL) on alternate day followed by azathioprine [2.5 mg/kg/day])57 CYC may slow or prevent the progression of SSc-ILD. In the Scleroderma Lung Study (SLS), daily oral CYC was administered for one year in patients considered to have mild-to-moderate SSc-ILD (active alveolitis on examination of BAL fluid or any ground-glass opacity based on CT findings; the onset of the first symptom of SSc other than Raynaud’s phenomenon within the previous seven years; an FVC between 45% and 85% of the predicted value; and grade 2 exertional dyspnea according to the baseline instrument of the Mahler Dyspnea Index).42 A significant effect was observed for the primary end point, FVC. Interestingly, a greater effect was seen in patients with more severe lung fibrosis as evaluated by HRCT scoring. Among the secondary outcome measures, significant treatment effects were observed for skin thickness and dyspnea scores. In the UK study, the use of monthly intravenous CYC was associated with a similar treatment effect for FVC,57 but this effect was not statistically significant. Most importantly, both studies showed that the CYC treatment prevented the progression of fibrotic disease in SSc-ILD. In the SLS study, the main effect of the treatment was observed because of the decline of FVC in the placebo group in patients with more extensive disease on HRCT.42 However, the therapeutic effect was lost at one year of follow-up because patients with more extensive disease in the active treatment arm progressed selectively after CYC cessation.58 These findings strongly indicate that the beneficial effect of suppression of alveolitis is largely confined to patients with extensive fibrotic disease. It also illustrates that even treatment that stabilizes extensive disease can be considered as therapeutic success in SSc-ILD.54 A summary of drugs associated with SSc-ILD treatment is shown in Table 1.
Table 1.
Selected evidence of SSc-ILD treatment with cyclophosphamide.
| STUDY [REF] | STUDY DESIGN | TREATMENT | N | INCLUSION | F/U | EPs | OUTCOME |
|---|---|---|---|---|---|---|---|
| Tashkin et al, 2006 [42] | Prospective, randomized, double-blind, placebo-controlled | Oral CY (2 mg/kg/day) vs placebo | 158 | DD less than 7 years Active alveolitis by BAL or HRCT or PFT | 24 Mo | FVC | – Difference in FVC (Favor to CY) |
| Hoyles et al, 2009 [57] | Prospective, randomized, double-blind, placebo-controlled | IV CY (600 mg/m2/month) x6 + 20 mg oral PSL on alternate day vs placebo | 45 | SSc-ILD on HRCT or lung biopsy | 12 Mo | FVC and DLCO | – T rend of difference in FVC (Favor to CY) – No difference in DLCO |
Abbreviations: EP, endpoint; DD, disease duration; BAL, broncoalveolar lavage; HRCT, high-resolution computed tomography; FVC, forced vital capacity; ILD, interstitial lung disease; IV, intravenous; PSL, prednisolone; Mo, months; CY, cyclophosphamide.
Glucocorticoids
As in IPF, the therapeutic response to immunosuppressive therapy is limited in SSc-ILD. However, there are concerns of an increased risk of scleroderma renal crisis, especially in patients treated with corticosteroids.59
On the basis of the evidence suggesting that inflammation precedes fibrosis in SSc, glucocorticoid, and immunosuppressive therapy have been proposed in SSc-ILD. Historically, glucocorticoid represented the treatment of choice of SSc-ILD. The efficacy of glucocorticoid is still controversial and still lacks a strong evidence. However, in most clinical trials, the use of glucocorticoid was permitted with the drug under investigation. As for combination therapy, there were both reports showing efficacy of CYC (500 mg/m2 initially, followed by 750 mg/m2 one month later, monthly for 6 months then bimonthly up to 12 months) combination with a high dose of glucocorticoid (1 mg/kg/day for four weeks, then reducing the PSL by 5 mg/day on alternating days each two weeks) versus with low dose (less than 10 mg/day PSL),60 and showing comparable effect of both higher (1 mg/kg/day PSL for four weeks and then lowered to 5 mg every two weeks up to 10 mg/day PSL) and lower doses (10 mg/day PSL).61 Comparison of the effect of combination with CYC alone (1 g/m2/month × 12) versus CYC plus glucocorticoid (1 g/m2/month × 12 plus 60 mg/day PSL for four weeks and then lowered to 10 mg/day on the end of second month and maintain) showed similar effect,62 suggesting that low dose or CYC alone is enough for the treatment of ILD. Also, comparable effect of monotherapy with glucocorticoid (0.5–1.0 mg/kg/day PSL) compared with CYC (six cycles of intravenous CYC 500 mg/m2 monthly and 0.3–0.5 mg/kg/day PSL) was also shown by Ando et al.63 In addition, some of case reports suggest that glucocorticoids may be effective in SSc-ILD in certain situations.64–66 From the point of view of side effect of glucocorticoid, a number of studies reported an increased risk of developing scleroderma renal crisis in patients receiving high-dose corticosteroids;59 in general, we recommend to use corticosteroids at low dose only in patients with worsening ILD and high dose for rescue of respiratory failure. A large, well-controlled clinical trial is needed to develop clinical recommendation or guidelines for glucocorticoid treatment. A summary of drugs associated with SSc-ILD treatment is shown in Table 2.
Table 2.
Selected evidence of SSc-ILD treatment with glucocorticoids.
| STUDY [REF] | STUDY DESIGN | TREATMENT | N | INCLUSION | F/U | EPs | OUTCOME |
|---|---|---|---|---|---|---|---|
| Pakas et al, 2002 [60] | Open-label | IV CY (500 mg/m2 initially, followed by 750 mg/m2/month × 5 and bimonthly × 3) in combination with PSL (low dose (<10 mg/day) or high dose (1 mg/kg/day and decrease to 5 mg/day on alternate day)) | 12 and 16 | SSc-ILD with FVC <70% | 12 Mo | HRCT, PFT, dyspnea score | (Low dose group) – No improvement (High dose group) – I mprovement of %GGO, %FVC, %DLCO, and severity of dyspnea at 12 months |
| Campos et al, 2009 [61] | Randomized, single-blinded | IV CY (750–1000 mg/m2/monthly × 6 and bimonthly × 3) in combination with PSL (low dose (<10 mg/day) or high dose (1 mg/kg/day and decrease to 5 mg/day on alternate day)) | 10 and 13 | SSc-ILD with GGO or honeycombing and/or the presence of active alveolitis in BALF with DOE, and decrease of FVC | 12 Mo | %FVC and %DLCO and HRCT | (Low dose group) – R adiographic improvement |
| Domiciano et al, 2009 [62] | Prospective, open label, controlled | IV CY (1000 mg/m2 × 12 with or without PSL (60 mg/day for a month and decrease to 10 mg/day) | 9 and 9 | SSc-ILD with NISP pattern by lung biopsy | 12 and 36 Mo | %FVC, %DLCO, %FEV1, %TLC | – %FVC, %DLCO comparable |
| Ando et al, 2009 [62] | Retrospective | IV CY (500 mg/m2 × 6) with 0.3–0.5 mg/kg/day of PSL), PSL alone (0.5–1.0 mg/kg/day), without treatment | 7 and 14 and 50 | SSc-ILD based on PFT and CT findings | Median 9.8 + 3.3 y | Change of FVC, | – Change of FVC: better in IVCY combination or PSL alone compared to without treatment |
Abbreviations: EP, endopoint; CY, cyclophosphamide; DD, disease duration; BAL, broncoalveolar lavage; HRCT, high-resolution computed tomography; PFT, pulmonary function test; GGO, ground-glass opacity; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; ILD, interstitial lung disease; IV, intravenous; NSIP, nonspecific interstitial pneumonia; PSL, prednisolone; Mo, months; y, years.
Mycophenolate mofetil
Mycophenolate mofetil (MMF) suppresses lymphocyte proliferation. In patients with rheumatoid arthritis (RA), MMF has been reported to have anti-fibrotic effects.67 In SSc-ILD, MMF has primarily been the subject of retrospective studies and observational studies. In a prospective observational one-year study of 14 patients who took MMF (720 mg bid for 12 months, except for the first week of treatment [360 mg bid]) plus 5 mg/day PSL), six patients experienced at least a 10% improvement in their FVC and five patients’ pulmonary function remained stable.68 Some other small studies have had mixed results that showed documented improvements in FVC and DLCO.68–70 A retrospective study enrolled 109 patients treated with MMF and 63 control subjects receiving other drugs were reviewed. A lower frequency of clinically significant pulmonary fibrosis in the MMF-treated cohort and better five-year survival from disease onset and from commencement of treatment were observed. There was no significant difference between the two groups in terms of modified Rodnan skin score and FVC change.71 Larger randomized trials are needed since this drug represents an attractive, less toxic possible alternative to CYC. Importantly, an MMF is currently being studied in the SLS II (MMF titrated to 3 g/day over two years versus oral CY titrated to 2 mg/kg/day for one year followed by placebo for another year) in direct comparison to CYC.72 A summary of drugs associated with SSc-ILD treatment is shown in Table 3.
Table 3.
Selected evidence of SSc-ILD treatment with mycofenolate mofetil.
| STUDY [REF] | STUDY DESIGN | TREATMENT | N | INCLUSION | F/U | EPs | OUTCOME |
|---|---|---|---|---|---|---|---|
| Simeon-Aznar et al, 2002 [68] | Prospective, observational | MMF 720 mg bid for 12 months + 5 mg/day PSL | 14 | HRCT findings compatible with ILD and/or FVC ≤80% | 12 Mo | FVC, FEV1, DLCO | 6/14 increase ≥10% in FVC |
| Koutroumpas et al, 2010 [69] | Retrospective | MMF 2 g/day + 5–7.5 mg/day PSL | 10 | dcSSc-ILD | 12 Mo | PFT | – Increase of FVC and DLCO |
| Swigris et al, 2006 [70] | Retrospective | MMF 2–2.5 g/day + 4–15 mg/day PSL | 28 | CTD-ILD (9 SSc) | NA | %FVC, %TLC, %DLCO | – Increase of %FVC, %TLC, %DLCO |
| Nihtyanova et al, 2007 [71] | Retrospective | MMF (2 g/day (73%), ≤1.5 mg/day (24%)) and controls | 109 and 63 | SSc (ILD patients were detected by HRCT, 61%) | NA | 5 y-survival, development of PF, PFT | – Better 5 y-survival from commencement of treatment and from disease onset – Lower % of PF development |
Abbreviations: EP, endopoint; MMF, mycofenolate mofetil; HRCT, high-resolution computed tomography; PFT, pulmonary function test; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; TLC, total lung capacity; DLCO, diffusing capacity of the lung for carbon monoxide; ILD, interstitial lung disease; PSL, prednisolone; Mo, months; y, years; PF, Pulmonary fibrosis.
Pirfenidone
Pirfenidone is a pyridone with both anti-inflammatory and antifibrotic effects. In clinical studies, pirfenidone slowed a decline in the lung function and exercise capacity.73 A randomized, double-blind, placebo-controlled Phase III trial in IPF (assigned to 1,800 mg/day or 1,200 mg/day) in Japan demonstrated a decrease in the rate of decline of vital capacity and an increase in progression-free survival time over 52 weeks; however, the primary endpoint of change in the percentage predicted FVC at week 72 was not met.74 The CAPACITY trial, using a lower dose of pirfenidone, reached its primary endpoint (2,403 mg/day of pirfenidone was the most effective although a smaller dose of 1,197 mg/day also slowed the decline in FVC).75 Miura et al reported improvement of vital capacity after pirfenidone treatment in five Japanese patients with SSc-ILD.76 Nagai et al also showed that 40 mg/kg/day of pirfenidone had a stabilizing effect on the course of 10 patients with chronic progressive pulmonary fibrosis based on chest radiographic scores and arterial oxygen pressure, two of which were patients with SSc-ILD.77 In another case report, even 600 mg/day of pirfenidone was used and the patient had stable clinical course.78 The LOTUSS study, which was an open-label, 16-week study, was designed to assess the safety and tolerability of pirfenidone in patients with SSc-ILD. Patients were randomized to a two- or four-week titration of pirfenidone to the target dose of 2,403 mg/day. Eligibility required a diagnosis of SSc ≤7 years from first non-Raynaud’s symptom, HRCT-confirmed ILD, FVC ≥50% and DLCO ≥40%. Of the 63 patients enrolled, 61 patients showed Treatment emergent adverse events (TEAE). From the 61 patients (96.8%) who experienced TEAEs, 19 patients (30.2%) experienced mild TEAEs, 30 (47.6%) experienced moderate TEAEs, and 12 patients (19%) experienced severe TEAEs. Three patients experienced serious adverse events such as bronchitis, PH, small intestinal obstruction, and worsening ILD. The study did not assess any efficacy endpoint; however, the median change between the baseline and at week 16 showed that the FVC was decreased to 0.5% and that 16.7% of the patients had an increase of 5% or more, whereas there was a decrease of more than 5% in 8.3% of the patients. From baseline, there was a median increase of 1.5% in the levels of DLCO. In 31.7% of patients, there was an increase of 5% or more, whereas 16.7% had reduced DLCO by more than 5%,79 which seems promising. A summary of drugs associated with SSc-ILD treatment is shown in Table 4.
Table 4.
Selected evidence of IPF and SSc-ILD treatment with pirfenidone.
| STUDY [REF] | STUDY DESIGN | TREATMENT | N | INCLUSION | F/U | EPs | OUTCOME |
|---|---|---|---|---|---|---|---|
| Azuma et al, 2005 [73] | Prospective, randomized, double-blind, placebo-controlled | Pirfenidone 720 mg bid for 12 months + 5 mg/day PSL | 72 and 35 | IPF | 12 Mo | Lowest SpO2 during the 6MWT Change in VC, episode of exacerbation | – Better in change in VC measurement, episode of acute exacerbation |
| Taniguchi et al, 2010 [74] | Prospective, randomized, double-blind, placebo-controlled | Pirfenidone 1.8 g/day, 1.2 mg/day, or placebo | 108 and 55 and 104 | IPF | 12 Mo | The change in VC, progression-free survival | – Better in VC decline, progression free survival |
| Noble et al, 2006 [75] | Prospective, randomized, double-blind, placebo-controlled | Pirfenidone 2403 mg/day, 1197 mg/day, or placebo | 174 and 87 and 174 | IPF | 72 Wk | The change in %FVC | – Better in change in %FVC |
| Miura et al, 2014 [76] | Case series | Pirfenidone | 5 | SSc-ILD | N/A | N/A | – Increase in VC |
| Nagai et al, 2002 [77] | Open-label | Pirfenidone 40 mg/kg/day | 10 | 8 IPF 2 SSc-ILD | 12 Mo | Overall survival, chest radiographic score, arterial oxygen pressure | – No deterioration of chest radiographic score, arterial oxygen pressure |
| Udiwadia ZF et al, 2015 [78] | Case report | Pirfenidone 600 mg/day | 1 | SSc-ILD | 20 Mo | The change of %FVC, %DLCO, 6MWD | – PFT stabilized, 6MWD improved |
Abbreviations: EP, endpoint; IPF, idiopathic pulmonary fibrosis; VC, vital capacity of lungs; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; TLC, total lung capacity; DLCO, diffusing capacity of the lung for carbon monoxide; ILD, interstitial lung disease; Mo, months; Wk, weeks; 6MWT, 6-minute walk test; 6MWD, 6-minute walk distance; N/A, not available.
Intravenous immunoglobulin
Effects of intravenous immunoglobulin (IVIg) on patients with SSc remain unclear. Some reports suggested the benefit of IVIg therapy in individual patients, but only few of these documented of clinical course of SSc-ILD. For example, Levy et al showed three cases and one of three patients had a mild restrictive lung disease and improved pulmonary diffusion indices after the treatment with a total dose of 56 g of IVIg.80 This group also reported the efficacy of IVIg (monthly infusion of 2 g/kg over a five-day period for each course) for cutaneous involvement in 15 patients with SSc. Of these patients, nine patients had pulmonary involvement; however, the clinical courses of these patients were not documented.81 Two placebo-controlled studies have been conducted. Kudo et al reported the clinical trial of six patients with SSc (treated with five-day administration of IVIg 400 mg/kg/day once or twice), showing changes in cytokine levels with no report of clinical outcomes.82 Another study by Takehara et al reported the use of single cycle of IVIg (five-day administration of IVIg 400 mg/kg/day) in a randomized, double-blind, placebo-controlled trial of 63 patients with dcSSc. At 12 weeks, there were no differences in change in modified Rodnan’s skin score (mRSS) between the two groups. The participants whose skin score did not improve by at least five points at 12 weeks were given an additional cycle of IVIg. However, the patients who received two IVIg treatments had greater improvements in skin score over time than those who initially received placebo followed by a later IVIg infusion.83 These data suggest that repeated IVIg cycles may influence long-term cutaneous outcomes. Poelman et al retrospectively reviewed patients treated with six monthly cycles dosed at 2 g/kg/month of IVIg. Pulmonary function remained stable from baseline to 12 months. The mean FVC at baseline and 12 months were 83.0% and 83.8% predicted, respectively, and the mean DLCO at baseline and 12 months were 76.3% and 79.9% predicted, respectively. There was no difference in pulmonary severity score from baseline to 12 months.84 Thus, we still need more evidence for IVIg treatment for SSc-ILD. A summary of drugs associated with SSc-ILD treatment is shown in Table 5.
Table 5.
Selected evidence of SSc-ILD treatment with intravenous immunoglobulin.
| STUDY [REF] | STUDY DESIGN | TREATMENT | N | INCLUSION | F/U | EPs | OUTCOME |
|---|---|---|---|---|---|---|---|
| Levy et al, 2000 [80] | Case series | IVIg 2 g/kg/month × 6 Mo | 3 | N/A | N/A | N/A | – One had ILD and improved diffusion indices |
| Poelman et al, 2015 [84] | Randomized, double-blinded, placebo-controlled | IVIg 2 g/kg/month × 6 Mo | 30 | dcSSc | 12 Mo | %FVC, %DLCO | – N o difference between base-line and 12 Mo |
Abbreviations: EP, endpoint; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; ILD, interstitial lung disease; Mo, months; N/A, not available; IVIg, intravenous immunoglobulin.
Hematopoietic stem cell transplantation
Since treatment with CYC does not show any survival benefit,85 high-dose immunosuppression is an alternative to conventional-dose immunosuppression. SSc is a progressive autoimmune disorder and is associated with systemic chronic inflammation,86 which is a rationale for high-dose immunosuppression despite the increased risk of toxicity. Studies of high-dose immunosuppression with autologous hematopoietic stem cell transplantation (HSCT) in patients with SSc showed prevention of disease progression,87,88 and a single-center trial demonstrated the superiority of autologous HSCT over conventional therapy.88 The European Group for Blood and Marrow Transplantation and the European League Against Rheumatism has reported the results from the Autologous Stem Cell Transplantation International Scleroderma (ASTIS) trial. This was the first international, randomized controlled trial of autologous HSCT versus pulse monthly CYC in 156 patients with dcSSc with a maximum disease duration of four years; the patients had a mRSS >15, and heart, lungs, or kidney involvement.89 Patients with severe ILD or PAH were excluded (mean predicted %FVC: 81.4%, mean predicted %DLCO: 58.5% in patients included). The study showed significantly better long-term event-free survival and overall survival at a median follow-up of approximately six years plus clinically meaningful improvements in objective and patient-reported outcome measures at two years ASTIS and other studies show that patient selection is critical in determining successful outcomes for HSCT,88–90 especially because cardiopulmonary involvement is associated with high mortality in HSCT. Also, disease relapse occurred up to 37% of patients at later points after HSCT.87,91 In the ASTIS trial, there was disease relapse in 22% of the patients assigned to HSCT after 12–24 months, as defined by the requirement for additional immunosuppressive therapy. Thus, it is better to limit the consideration of HSCT to patients with SSc who have failed to improve or have worsened on conventional immunosuppressive agents.
Tumor necrosis factor-α inhibitors
Since TNF-α inhibitors have significant effects for the treatment of RA, etanercept was administered to 18 patients with SSc, 6 with dcSSc, and 12 with lcSSc, whose disease was complicated with joint involvement for a 2–66-month period. Of these, 15 patients showed clinical benefits. In these patients, the FVC and DLCO values declined slightly during treatment but were comparable to those in patients with SSc who were not treated with etanercept (25 mg twice weekly or 50 mg once weekly).92 No data on lung function were reported in two studies of infliximab treatment,93,94 and the reports were contradictory in terms of the effect of anti-TNF-α inhibitors on skin involvement (infliximab 3 mg/kg/injection × 4 and switched to etanercept93 and infliximab 5 mg/kg/injection × 5),94 Recently expert consensus of the European Scleroderma Trials and Research (EUSTAR) group did not recommend the routine use of anti-TNF-α inhibitors for SSc because there is no evidence that these drugs successfully counteract the fibrotic process.95 A summary of drugs associated with SSc-ILD treatment is shown in Table 6.
Table 6.
Selected evidence of SSc-ILD treatment with biologics.
| STUDY [REF] | STUDY DESIGN | TREATMENT | N | INCLUSION | F/U | EPs | OUTCOME |
|---|---|---|---|---|---|---|---|
| Lam et al, 2002 [92] | Retrospective | Etanercept 25 mg twice/wk or 50 mg once/wk | 18 | Met 1980 ACR criteria or 3 of 5 features of CREST | N/A | %FVC,%DLCO | – %FVC and %DLCO declined |
| Daoussis et al, 2005 [96] | Open-label, randomized, controlled | Rituximab (four weekly 375 mg/m2 rituximab infusions/cycle × 2) vs placebo | 8 and 6 | SSc-ILD by HRCT and/or PFT | 12 Mo | PFT, HRCT | – Increase of %FVC and %DLCO in Rituximab compared to controls |
| Daoussis et al, 2006 [97] | Retrospective | Rituximab (four weekly 375 mg/m2 rituximab infusions/cycle × 4) vs placebo | 8 | SSc-ILD by HRCT and/or PFT | 24 Mo | PFT, HRCT | – I ncrease of %FVC and %DLCO in Rituximab compared to baseline |
| McGonagle et al, 2007 [99] | Case report | Rituximab 1000 mg together with 100 mg mPSL at baseline and day 15 | 1 | An SSc-ILD patient failed to PSL + IVCY | 3 wks | 6MWD, FVC, DLCO, HRCT | – All parameters improved |
| Daoussis et al, 2010 [100] | Case report | Rituximab (four weekly 375 mg/m2 rituximab infusions/cycle × 4) | 1 | An SSc-ILD patient failed to PSL + IVCY | 6, 12, and 18 Mo | 6MWD, SpO2, FVC, DLCO, NYHA class | – All parameters improved |
| Elhai et al, 2013 [103] | Case report | Abatacept (10 mg/kg/month) | 7 | SSc with refractory myopathy | Mean 18 Mo | % of the number patients who were TLCO <70% or FVC <75% | – N o change compared with baseline and last visit |
| Becker et al, 2006 [118] | Case report | Basiliximab (20 mg/month 6×) | 10 | dcSSc with rapidly progressive disease and organ involvement (8 with ILD) | 44 wk | %FVC, %DLCO | – %FVC, %DLCO improved – 4 patients improved ≥10% of FVC – 2 patients improved ≥10% of DLCO |
Abbreviations: EP, endpoint; HRCT, high-resolution computed tomography; PFT, pulmonary function test; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; TLC, total lung capacity; DLCO, diffusing capacity of the lung for carbon monoxide; ILD, interstitial lung disease; PSL, prednisolone; Mo, months; y, years.
B-cell depletion therapies
An open-label, randomized, controlled study was conducted to evaluate the efficacy of rituximab in patients with SSc-ILD. All of the enrolled patients with SSc-ILD were positive for the anti-Scl-70 antibody and on stable therapy during the previous 12 months. The dose of rituximab was 375 mg/m2 once a week for four consecutive weeks at baseline, and the treatment was repeated after 24 weeks. Of the 12 patients, eight were randomized to rituximab and six to placebo. After one year, PFT was improved in the rituximab group: the predicted FVC increased from 68.13% ± 19.69% to 75.63% ± 19.73% (P = 0.0018), and the predicted DLCO increased from 52.25% ± 20.71% to 62% ± 23.21% (P = 0.017). Both parameters declined slightly in the placebo control group. Thus, the comparison of changes in FVC and DLCO after one year revealed that patients receiving rituximab improved significantly compared to controls. None of the patients receiving rituximab exhibited deterioration in the lung function, whereas five of six control cases showed worsening of FVC and/or DLCO values. In addition, skin thickening (as evaluated by mRSS), collagen deposition in skin specimens, and health quality improved in rituximab-treated patients (two cycles at baseline and week 24 [four weekly 375 mg/m2 rituximab infusions/each cycle]) but not in those treated with placebo.96 Interestingly, the eight cases who were given rituximab continued to improve both in terms of PFT and skin thickening after four cycles of rituximab (two cycles at baseline and week 24),97 suggesting that repeated cycles confer increasing benefit, as has been shown in RA.98 Improvement in PFT after rituximab therapy was also reported in three single SSc cases (1,000 mg rituximab together with 100 mg methylprednisolone administration at baseline and day 15)99,100 These results are noteworthy considering that patients with early diffuse disease have a higher risk of developing severe visceral complications. Skin involvement, as evaluated by mRSS, also improved in two studies (1,000 mg rituximab together with 100 mg methylprednisolone administration at baseline and day 15),101 (four cycles [four weekly 375 mg/m2 rituximab infusions]).102 Large controlled randomized trials comparing rituximab to placebo or to CYC are required to better understand the role of B-cell depletion therapy in the context of a disease. A summary of drugs associated with SSc-ILD treatment is shown in Table 6.
Tocilizumab and abatacept
One study examined the safety and effectiveness of tocilizumab (8 mg/kg/month) and abatacept (10 mg/kg/month) for SSc-polyarthritis and SSc-myopathy.103 The study included 20 patients with SSc with refractory polyarthritis and seven with refractory myopathy from the EUSTAR network; 15 patients received tocilizumab and 12 patients received abatacept. All patients with SSc-myopathy received abatacept. After five months of tocilizumab treatment, there was a significant improvement in joint involvement, and 10/15 patients achieved a good response according to the EULAR response criteria for RA.104 After 11 months of abatacept treatment, joint parameters showed significant improvement, with 6/11 patients achieving a good response according to the EULAR criteria. Abatacept did not improve muscle outcome measures in SSc-myopathy, and no significant changes were seen for skin or lung fibrosis in the different groups. Both treatments were well tolerated.103 Another case report showed that skin involvement, as evaluated by mRSS, histology, and Vesmeter were also improved by tocilizumab treatment (8 mg/kg/month × six months) in two cases with SSc.105 A summary of drugs associated with SSc-ILD treatment is shown in Table 6.
Anti-transforming growth factor β (TGF-β) therapies
Excessive TGF-β activity is a common feature of fibrotic conditions. Thus, fibrotic disorders, including SSc, are candidates for TGF-β therapy.106 In SSc, there was a subset of patients that showed a “TGF-β-responsive gene signature” in skin samples.107,108 These patients had higher mRSS and more severe lung involvement than those without this gene signature.108 In the first clinical trial of neutralizing antibodies to TGF-β, the human monoclonal antibody metelimumab (CAT-192; three different doses: 0.5, 5, and 10 mg/kg on baseline and weeks 6, 12, and 18), was compared with placebo in 45 patients with SSc (disease duration <18 months).109 In this randomized, placebo-controlled Phase I/II trial, the antibody was given by intravenous infusion at baseline and at weeks 6, 12, and 18. The patients were evaluated at 24 weeks. The trial showed no evidence of efficacy for improving skin scores or other manifestations. Another study evaluated the efficacy of a monoclonal antibody, fresolismab (GC-1008, 1 mg/kg or 5 mg/kg infusion), that targets all three isoforms of TGF-β. Enrolled patients had IPF or SSc with mRSS ≥15. The study excluded patients with moderate to severe lung involvement (patients with FVC <80%, DLCO <70%, or ground glass opacity and/or fibrosis >20% of lung fields by HRCT). These studies were already completed recently.
Type I interferon (IFN)-receptor-targeted therapies
A recent report showed an association between a type I IFN responsive gene expression profile and SSc pathogenesis. Studies also show an increase in plasmacytoid dendritic cells, which are a source of type I IFN, in the circulation and the skin of patients with SSc.110–112 Based on these findings, a Phase I clinical trial was conducted for anifrolumab (MEDI-546), an IgG-κ type fully human monoclonal antibody against subunit 1 of the type I IFN-α receptor.113 The study enrolled 34 patients with dcSSc, and the drug was well-tolerated. The expression of upregulated type I IFN-related genes in skin and blood cells was normalized by the administration of MEDI−546.
Other biologics
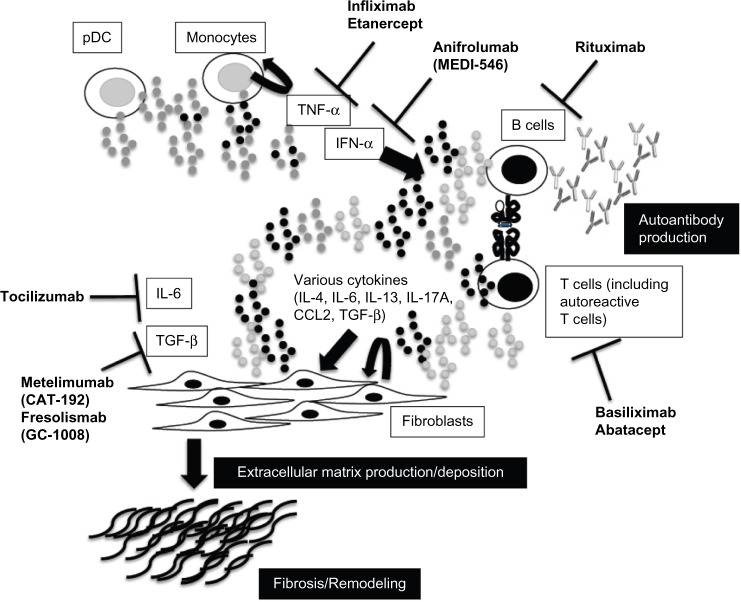
In SSc, autoreactive T cells are involved in autoantibody production.114 Thus, the activation of such kind of T cells plays an important role in the pathogenesis of SSc. There is a correlation between fibrosis progression in the skin and lungs, patient prognosis, and plasma levels of the soluble interleukin-2 (IL-2) receptor.115,116 Basiliximab is a chimeric monoclonal antibody that selectively inhibits the α-chain of the IL-2 receptor that is expressed on activated T- and B-cells. This drug is approved for the treatment of kidney allograft rejection; moreover, it has been used successfully to treat steroid-resistant graft versus host disease,117 a disorder with some similarities to SSc. Becker et al recently published a small open-label trial on the use of basiliximab in SSc118; this followed an earlier case report from the same group.119 The study enrolled 10 rapidly progressive patients with dcSSc (mean disease duration 2.1 years), and eight were with SSc-ILD. Basiliximab was administrated for six months (every four weeks injection [20 mg/injection]). Both FVC and DLCO were slightly improved; the authors noted that four cases showed an increase of predicted FVC >10% and two cases showed an increase in DLCO >10% (Table 6). Moreover, skin thickening also improved, and the drug was quite well-tolerated. However, these reports are case series, so larger trials are needed. Figure 1 summarizes the targets of biologics for SSc.
Figure 1.
Novel targets and treatment approaches for systemic sclerosis. Activation of fibroblasts and the production and deposition of extracellular matrix components are important in the pathogenesis of systemic sclerosis. The release of various cytokines from activated lymphocytes plays a key role in fibroblast activation, and activated lymphocytes contribute to the production of autoantibodies. Plasmacytoid dendritic cells (pDCs) and/or monocytes activate lymphocytes through cytokines such as interferon (IFN) alpha. It might be possible to modify disease activity in patients with systemic sclerosis by regulating these targets.
Tyrosine kinase inhibitors
Tyrosine kinase inhibitors (TKIs) such as imatinib or nilotinib are other options not only for treating ILD but also for PH, since this class of drugs is potentially effective for both. TKIs suppress the intracellular signals involved in vascular remodeling and excessive fibrosis, such as signals involving TGF-β, Platelet-derived growth factor (PDGF), and c-kit.120 A clinical trial found that imatinib treatment (600 mg/day) of patients with IPF did not significantly improve survival or lung function compared with placebo; however, imatinib-treated patients showed significantly improved oxygenation at 48 weeks, and these positive effects were sustained for 96 weeks.121 Current evidence does not support imatinib as an effective treatment for SSc-ILD, but TKIs may be a potential treatment strategy for this serious condition. At least three clinical trials of imatinib have been conducted in patients with early dcSSc. Two pilot open-label studies showed a trend toward the improvement of skin thickening and FVC (400 mg/day)122 (up to 600 mg/day),123 but no efficacy was found in a randomized, double-blind, placebo-controlled trial (400 mg/day).124 Patients with advanced ILD were excluded from these trials, but it should be noted that many patients dropped out because of adverse events, including gastrointestinal symptoms and peripheral edema, and one trial was terminated because of safety concerns. The dosage of imatinib is one of the factors associated with the occurrence of adverse events, but in general, the safety precludes clinicians from using imatinib for patients with SSc who have multiple organ involvement. A recent report including 30 patients with SSc showed that low-dose oral imatinib (200 mg/day) for six months followed by a six-month follow-up stabilized ILD in a large proportion of patients (73%) unresponsive to CYC therapy, without serious side effects associated with imatinib.125 Also, a TKI for multitargets, nintedanib (BIBF1120; 150 mg twice a day), was also recently introduced to patients with IPF as a phase III trial followed by phase II trial.126 A total of 1,066 patients were randomly assigned to receive nintedanib or placebo. The administration of 300 mg/day of nintedanib significantly reduced the deterioration of pulmonary function, especially the suppression of the reduction of FVC.127 Thus, these approaches may shed the light on the novel paths for the treatment of SSc-ILD in the future. A summary of drugs associated with SSc-ILD treatment is shown in Table 7.
Table 7.
Selected evidence of IPF or SSc-ILD treatment with tyrosine kinase inhibitors.
| STUDY [REF] | STUDY DESIGN | TREATMENT | N | INCLUSION | F/U | EPs | OUTCOME |
|---|---|---|---|---|---|---|---|
| Daniels et al, 2010 [121] | Randomized, double-blinded, placebo-controlled | Imatinib 600 mg/day vs placebo | 119 | IPF | 96 w | TTDP Change in %FVC, %DLCO | – N o change of TTDP, change in %FVC, %DLCO |
| Spiera et al, 2011 [122] | Open-label | Imatinib 400 mg/day | 30 | dcSSc (16 with ILD) | 12 Mo | Change in %FVC, %DLCO | – Increase in %FVC and–Trend toward increase in %DLCO |
| Khanna et al, 2011 [123] | Open-label | Imatinib 600 mg/day | 20 | SSc-ILD FVC <85%, DOE, GGO on HRCT | 12 Mo | %FVC, %DLCO, %TLC | – 7 patients dropped – T rends toward improvement of %FVC, %DLCO, %TLC |
| Pope et al, 2011 [124] | Randomized, double-blinded, placebo-controlled | Imatinib 400 mg/day vs placebo | 9 and 1 | dcSSc | 6 Mo | FVC, TLC, DLCO | – 5 of 9 patients dropped |
| Fraticelli et al, 2014 [125] | Open-label | Imatinib 200 mg/day | 30 | SSc-ILD patients with grade 2 by MBDI plus HRCT findings or BAL findings | 6 Mo | FVC, DLCO, PaO2, FVC, HRCT | – 4 patients dropped – 4 good response, 15 stabilized, 7 worsened |
| Richeldi et al, 2014 [126] | Randomized, double-blinded, placebo-controlled | Nintedanib(300 mg/day) vs placebo | 7 | IPF | 52 wk | FVC | – Reduced the decline in FVC |
Abbreviations: EP, endpoint; HRCT, high-resolution computed tomography; TTDP, time to disease progression (10% decline in%FVC from baseline); PFT, pulmonary function test; FVC, forced vital capacity; TLC, total lung capacity; DLCO, diffusing capacity of the lung for carbon monoxide; ILD, interstitial lung disease; MBDI, Mahler Baseline Dyspnea Index; Mo, months; wk, weeks; DOE, dyspnea on exertion.
Lung transplantation
Lung transplantation is a viable and potentially life-saving approach for managing with patients with SSc with end-stage ILD. However, SSc is considered as a poor candidate for transplantation, since multiple comorbidities (including gastroesophageal reflux, dysmotility, renal impairment (a creatinine clearance below 50 mL/min), skin breakdown because of ulceration, and significant arrhythmia) increase the risk of procedure-related death.128 Despite strict inclusion and exclusion criteria being used to select patients with SSc without those risk factors in one study, the cumulative survival rate at six months posttransplantation was still 69% in the SSc group compared with 80% in the IPF group.129 On the other hand, another study showed that outcomes, such as survival rate at four years, annual incidence rate for acute rejection, and infection or serum creatinine elevation, were comparable between a similar group of transplant patients with scleroderma and with IPF.130 In a recent single-center study that evaluated the prognosis of bilateral lung transplantation, the one-year all-cause mortality rate was 6.6% in patients with SSc and 13.6% in those with IPF.131 In patients with SSc-PAH, transplantation remains an option in those who fail therapy.
Conclusion
Among patients with SSc, some patients with progressive SSc-ILD have a poor prognosis despite recent therapeutic advances. Furthermore, the underlying pathophysiology is complicated, such as combined ILD and PH in some patients with SSc. The limited effectiveness of current treatments and the devastating nature of this condition continue to drive the search for novel therapeutic targets.
Footnotes
ACADEMIC EDITOR: Hussein D. Foda, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 652 words, excluding any confidential comments to the academic editor.
FUNDING: Author discloses no external funding sources.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived the concepts: HY Analyzed the data: HY. Wrote the first draft of the manuscript: HY. Contributed to the writing of the manuscript: HY. Agree with manuscript results and conclusions: HY. Jointly developed the structure and arguments for the paper: HY. Made critical revisions and approved final version: HY. The author reviewed and approved of the final manuscript.
REFERENCES
- 1.Schurawitzki H, Stiglbauer R, Graninger W, et al. Interstitial lung disease in progressive systemic sclerosis: high-resolution CT versus radiography. Radiology. 1990;176:755–9. doi: 10.1148/radiology.176.3.2389033. [DOI] [PubMed] [Google Scholar]
- 2.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altman RD, Medsger TA, Jr, Bloch DA, Michel BA. Predictors of survival in systemic sclerosis (scleroderma) Arthritis Rheum. 1991;34:403–13. doi: 10.1002/art.1780340405. [DOI] [PubMed] [Google Scholar]
- 5.Wells AU, Cullinan P, Hansell DM, et al. Fibrosing alveolitis associated with systemic sclerosis has a better prognosis than lone cryptogenetic fibrosing alveolitis. Am J Respir Crit Care Med. 1994;149:1583–90. doi: 10.1164/ajrccm.149.6.8004317. [DOI] [PubMed] [Google Scholar]
- 6.Steen VD, Medsger TA., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–44. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Steen VD, Conte C, Owens GR, Medsger TA. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37:1283–9. doi: 10.1002/art.1780370903. [DOI] [PubMed] [Google Scholar]
- 8.Herzog EL, Mathur A, Tager AM, Feghali-Bostwick C, Schneider FR, Varga J. Interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis. How similar and distinct? Arthritis Rheumatol. 2014;66:1967–78. doi: 10.1002/art.38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132:214–20. doi: 10.1378/chest.07-0323. [DOI] [PubMed] [Google Scholar]
- 10.Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long term survival in systemic sclerosis. Arthritis Rheumatol. 2014;66:1625–35. doi: 10.1002/art.38390. [DOI] [PubMed] [Google Scholar]
- 11.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 12.Solomon JJ, Olson AL, Fischer A, Bull T, Brown KK, Raghu G. Scleroderma lung disease. Eur Respir Rev. 2013;22:6–19. doi: 10.1183/09059180.00005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 14.Meyer KC, Raghu G. Bronchoalveolar lavage for the evaluation of interstitial lung disease: is it clinically useful? Eur Respir J. 2011;38:761–9. doi: 10.1183/09031936.00069509. [DOI] [PubMed] [Google Scholar]
- 15.Behr J, Vogelmeier C, Beinert T, et al. Bronchoalveolar lavage for evaluation and management of scleroderma disease of the lung. Am J Respir Crit Care Med. 1996;154(2 pt 1):400–6. doi: 10.1164/ajrccm.154.2.8756813. [DOI] [PubMed] [Google Scholar]
- 16.Bolster MB, Ludwicka A, Sutherland SE, Strange C, Silver RM. Cytokine concentrations in bronchoalveolar lavage fluid of patients with systemic sclerosis. Arthritis Rheum. 1997;40:743–51. doi: 10.1002/art.1780400422. [DOI] [PubMed] [Google Scholar]
- 17.De Santis M, Bosello S, La Torre G, et al. Functional, radiological and biological markers of alveolitis and infections of the lower respiratory tract in patients with systemic sclerosis. Respir Res. 2005;6:96. doi: 10.1186/1465-9921-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remy-Jardin M, Remy J, Wallaert B, Bataille D, Hatron PY. Pulmonary involvement in progressive systemic sclerosis: sequential evaluation with CT, pulmonary function tests, and bronchoalveolar lavage. Radiology. 1993;188:499–506. doi: 10.1148/radiology.188.2.8327704. [DOI] [PubMed] [Google Scholar]
- 19.Goh NS, Veeraraghavan S, Desai SR, et al. Bronchoalveolar lavage cellular profiles in patients with systemic sclerosis-associated interstitial lung disease are not predictive of disease progression. Arthritis Rheum. 2007;56:2005–12. doi: 10.1002/art.22696. [DOI] [PubMed] [Google Scholar]
- 20.Strange C, Bolster MB, Roth MD, et al. Scleroderma Lung Study Research Group Bronchoalveolar lavage and response to cyclophosphamide in scleroderma interstitial lung disease. Am J Respir Crit Care Med. 2008;177:91–8. doi: 10.1164/rccm.200705-655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferri C, Valentini G, Cozzi F, et al. Systemic Sclerosis Study Group of the Italian Society of Rheumatology (SIR-GSSSc) Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002;81:139–53. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Bouros D, Wells AU, Nicholson AG, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165:1581–6. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society, European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 24.Kim DS, Yoo B, Lee JS, et al. The major histopathologic pattern of pulmonary fibrosis in scleroderma is nonspecific interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:121–7. [PubMed] [Google Scholar]
- 25.Minai OA, Dweik RA, Arroliga AC. Manifestations of scleroderma pulmonary disease. Clin Chest Med. 1998;19:713–31. doi: 10.1016/s0272-5231(05)70112-x. [DOI] [PubMed] [Google Scholar]
- 26.Hunninghake GW, Lynch DA, Galvin JR, et al. Radiologic findings are strongly associated with a pathologic diagnosis of usual interstitial pneumonia. Chest. 2003;124:1215–23. doi: 10.1378/chest.124.4.1215. [DOI] [PubMed] [Google Scholar]
- 27.Piper WN, Helwig EB. Progressive systemic sclerosis: visceral manifestations in generalized scleroderma. AMA Arch Derm. 1955;72:535–46. doi: 10.1001/archderm.1955.03730360041004. [DOI] [PubMed] [Google Scholar]
- 28.Fischer A, Swigris JJ, Groshong SD, et al. Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest. 2008;134:601–5. doi: 10.1378/chest.08-0053. [DOI] [PubMed] [Google Scholar]
- 29.Clements PJ, Roth MD, Elashoff R, et al. Scleroderma Lung Study Group Scleroderma Lung Study (SLS): differences in the presentation and course of patients with limited versus diffuse systemic sclerosis. Ann Rheum Dis. 2007;66:1641–7. doi: 10.1136/ard.2007.069518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol. 1997;169:977–83. doi: 10.2214/ajr.169.4.9308447. [DOI] [PubMed] [Google Scholar]
- 31.Goh NS, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248–54. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Li G, Gjertson D, et al. Classification of parenchymal abnormality in scleroderma lung using a novel approach to denoise images collected via a multicenter study. Acad Radiol. 2008;15:1004–16. doi: 10.1016/j.acra.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldin J, Elashoff R, Kim HJ, et al. Treatment of Scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo. Chest. 2009;136:1333–40. doi: 10.1378/chest.09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frauenfelder T, Winklehner A, Nguyen TD, et al. Screening for interstitial lung disease in systemic sclerosis: performance of high-resolution CT with limited number of slices: a prospective study. Ann Rheum Dis. 2014;73:2069–73. doi: 10.1136/annrheumdis-2014-205637. [DOI] [PubMed] [Google Scholar]
- 35.Chang B, Wigley FM, White B, Wise RA. Scleroderma patients with combined pulmonary hypertension and interstitial lung disease. J Rheumatol. 2003;30:2398–405. [PubMed] [Google Scholar]
- 36.Trad S, Amoura Z, Beigelman C, et al. Pulmonary arterial hypertension is a major mortality factor in diffuse systemic sclerosis, independent of interstitial lung disease. Arthritis Rheum. 2006;54:184–91. doi: 10.1002/art.21538. [DOI] [PubMed] [Google Scholar]
- 37.Fischer A, Bull TM, Steen VD. Practical approach to screening for scleroderma-associated pulmonary arterial hypertension. Arthritis Care Res. 2012;64:303–10. doi: 10.1002/acr.20693. [DOI] [PubMed] [Google Scholar]
- 38.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–94. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 39.Avouac J, Airò P, Meune C, et al. Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J Rheumatol. 2010;37:2290–8. doi: 10.3899/jrheum.100245. [DOI] [PubMed] [Google Scholar]
- 40.Mathai SC, Hammers LK, Champion HC, et al. Survival in pulmonary hypertension associated with the scleroderma spectrum of diseases. Arthritis Rheum. 2009;60:569–77. doi: 10.1002/art.24267. [DOI] [PubMed] [Google Scholar]
- 41.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med. 2009;179:151–7. doi: 10.1164/rccm.200806-953OC. [DOI] [PubMed] [Google Scholar]
- 42.Tashkin DP, Elashoff R, Clements PJ, et al. Scleroderma Lung Study Research Group Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 43.Hinchcliff M, Fisher A, Schiopu E, Steen VD, PHAROS Investigators Pulmonary hypertension and recognition of outcomes in scleroderma (PHAROS): Baseline characteristics and description of study population. J Rheumatol. 2011;38:2172–9. doi: 10.3899/jrheum.101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overbeek MJ, Vonk MC, Boonstra A, et al. Pulmonary arterial hypertension in limited cutaneous systemic sclerosis: a distinctive vasculopathy. Eur Respir J. 2009;34:371–9. doi: 10.1183/09031936.00106008. [DOI] [PubMed] [Google Scholar]
- 45.Ntoumazios SK, Voulgari PV, Potsis K, Koutis E, Tsifetaki N, Assimakopoulos DA. Esophageal involvement in scleroderma: gastroesophageal reflux, the common problem. Semin Arthritis Rheum. 2006;36:173–81. doi: 10.1016/j.semarthrit.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Johnson DA, Drane WE, Curran J, et al. Pulmonary disease in progressive systemic sclerosis. A complication of gastroesophageal reflux and occult aspiration? Arch Intern Med. 1989;149:589–93. [PubMed] [Google Scholar]
- 47.Maddern GJ, Horowitz M, Jamieson GG, Chatterton BE, Collins PJ, Roberts-Thomson P. Abnormalities of esophageal and gastric emptying in progressive systemic sclerosis. Gastroenterology. 1984;87:922–6. [PubMed] [Google Scholar]
- 48.Christmann RB, Wells AU, Capelozzi VL, Silver RM. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin Arthritis Rheum. 2010;40:241–9. doi: 10.1016/j.semarthrit.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Marie I, Dominique S, Levesque H, et al. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis Rheum. 2001;45:346–54. doi: 10.1002/1529-0131(200108)45:4<346::AID-ART347>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 50.de Souza RB, Borges CT, Capelozzi VL, et al. Centrilobular fibrosis: an under-recognized pattern in systemic sclerosis. Respiration. 2009;77:389–97. doi: 10.1159/000156958. [DOI] [PubMed] [Google Scholar]
- 51.Antoniou KM, Wells AU. Scleroderma lung disease: evolving understanding in light of newer studies. Curr Opin Rheumatol. 2008;20:686–91. doi: 10.1097/BOR.0b013e3283126985. [DOI] [PubMed] [Google Scholar]
- 52.Steen V. Predictors of end stage lung disease in systemic sclerosis (editorial) Ann Rheum Dis. 2003;62:97–9. doi: 10.1136/ard.62.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenwald R, Tashkin DP, Gong H, et al. Longitudinal changes in lung function and respiratory symptoms in progressive systemic sclerosis. Am J Med. 1987;83:83–92. doi: 10.1016/0002-9343(87)90501-8. [DOI] [PubMed] [Google Scholar]
- 54.Wells AU, Latsi P, McCune WJ. Daily cyclophosphamide for scleroderma: are patients with the most to gain underrepresented in this trial? Am J Respir Crit Care Med. 2007;176:952–3. doi: 10.1164/rccm.200708-1185ED. [DOI] [PubMed] [Google Scholar]
- 55.Winstone TA, Assayag D, Wilcox PG, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 2014;146:422–36. doi: 10.1378/chest.13-2626. [DOI] [PubMed] [Google Scholar]
- 56.Khanna D, Mittoo S, Aggarwal R, et al. Connective Tissue Disease-associated Interstitial Lung Diseases (CTD-ILD) – Report from OMERACT CTD-ILD Working Group. J Rheumatol. 2015;42(11):2168–71. doi: 10.3899/jrheum.141182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoyles RK, Ellis RW, Wellsbury J, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 58.Tashkin DP, Elashoff R, Clements PJ, et al. Scleroderma Lung Study Research Group Effects of 1- year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–34. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steen VD, Medsger TA., Jr Case–control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum. 1998;41:1613–9. doi: 10.1002/1529-0131(199809)41:9<1613::AID-ART11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 60.Pakas I, Ioannidis JP, Malagari K, Skopouli FN, Moutsopoulos HM, Vlachoyiannopoulos PG. Cyclophosphamide with low or high dose prednisolone for systemic sclerosis lung disease. J Rheumatol. 2002;29:298–304. [PubMed] [Google Scholar]
- 61.Campos DP, Del Toro ME, Casanovas AP, et al. Are high dose of prednisone necessary for treatment of interstitial lung disease in systemic sclerosis? Reumatol Clin. 2012;8:58–62. doi: 10.1016/j.reuma.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Domiciano DS, Bonfá E, Borges CT, et al. A long-term prospective randomized controlled study of non-specific interstitial pneumonia (NSIP) treatment in scleroderma. Clin Rheumatol. 2011;30:223–9. doi: 10.1007/s10067-010-1493-4. [DOI] [PubMed] [Google Scholar]
- 63.Ando K, Motojima S, Doi T, et al. Effect of glucocorticoid monotherapy on pulmonary function and survival in Japanese patients with scleroderma-related interstitial lung disease. Respir Investig. 2013;51:69–75. doi: 10.1016/j.resinv.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 64.White B. Interstitial lung disease in scleroderma. Rheum Dis Clin North Am. 2003;29:371–90. doi: 10.1016/s0889-857x(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 65.Griffiths B, Miles S, Moss H, Robertson R, Veale D, Emery P. Systemic sclerosis and interstitial lung disease: a pilot study using pulse intravenous methylprednisolone and cyclophosphamide to assess the effect on high resolution computed tomography scan and lung function. J Rheumatol. 2002;29:2371–8. [PubMed] [Google Scholar]
- 66.Pai BS, Srinivas CR, Sabitha L, Shenoi SD, Balachandran CN, Acharya S. Efficacy of dexamethasone pulse therapy in progressive systemic sclerosis. Int J Dermatol. 1995;34:726–8. doi: 10.1111/j.1365-4362.1995.tb04664.x. [DOI] [PubMed] [Google Scholar]
- 67.Saketkoo LA, Espinoza LR. Rheumatoid arthritis interstitial lung disease: mycophenolate mofetil as an antifibrotic and disease-modifying antirheumatic drug. Arch Intern Med. 2008;168:1718–9. doi: 10.1001/archinte.168.15.1718. [DOI] [PubMed] [Google Scholar]
- 68.Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, Selva-O’Callaghan A, Solans-Laqué R, Vilardell-Tarrés M. Effect of mycophenolate sodium in scleroderma- related interstitial lung disease. Clin Rheumatol. 2011;30:1393–8. doi: 10.1007/s10067-011-1823-1. [DOI] [PubMed] [Google Scholar]
- 69.Koutroumpas A, Ziogas A, Alexiou I, Barouta G, Sakkas LI. Mycophenolate mofetil in systemic sclerosis-associated interstitial lung disease. Clin Rheumatol. 2010;29:1167–8. doi: 10.1007/s10067-010-1498-z. [DOI] [PubMed] [Google Scholar]
- 70.Swigris JJ, Olson AL, Fischer A, et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest. 2006;130:30–6. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 71.Nihtyanova SI, Brough GM, Black CM, et al. Mycophenolate mofetil in diffuse cutaneous systemic sclerosis – a retrospective analysis. Rheumatology. 2007;46:442–5. doi: 10.1093/rheumatology/kel244. [DOI] [PubMed] [Google Scholar]
- 72.Tashkin DP, Roth MD, Furst DE, et al. Double-blind comparison of mycophenolate mofetil and oral cyclophosphamide for treatment of scleroderma-related interstitial lung disease (Scleroderma Lung Study (SLS)II): Rationale, design, methods, baseline characteristics/intercorrelations and patients disposition. Am J Respir Crit Care Med. 2013;187:A2921. [Google Scholar]
- 73.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 74.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone Clinical Study Group in Japan Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2009;35:821–9. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 75.Noble PW, Albera C, Bradford WZ, et al. CAPACITY Study Group Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomized trials. Lancet. 2011;377:1760–9. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 76.Miura Y, Saito T, Fujita K, et al. Clinical experience with pirfenidone in five patients with scleroderma-related interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:235–8. [PubMed] [Google Scholar]
- 77.Nagai S, Hamada K, Shigematsu M, et al. Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med. 2002;41:1118–23. doi: 10.2169/internalmedicine.41.1118. [DOI] [PubMed] [Google Scholar]
- 78.Udwadia ZF, Mullerpattan JB, Balakrishnan C, Richeldi L. Improved pulmonary function following pirfenidone treatment in a patient with progressive interstitial lung disease associated with systemic sclerosis. Lung India. 2015;32:50–2. doi: 10.4103/0970-2113.148451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khanna D, Albera C, Fischer A, et al. Safety and tolerability of pirfenidone in patients with systemic sclerosis-associated interstitial lung disease – The LOTUSS study. Ann Rheum Dis. 2015;74:S816. [Google Scholar]
- 80.Levy Y, Sherer Y, Langevitz P, et al. Skin score decrease in systemic sclerosis patients treated with intravenous immunoglobulin – a preliminary report. Clin Rheumatol. 2000;19:207–11. doi: 10.1007/s100670050158. [DOI] [PubMed] [Google Scholar]
- 81.Levy Y, Amital H, Langevitz P, et al. Intravenous immunoglobulin modulates cutaneous involvement and reduces skin fibrosis in systemic sclerosis: an open-label study. Arthritis Rheum. 2004;50:1005–7. doi: 10.1002/art.20195. [DOI] [PubMed] [Google Scholar]
- 82.Kudo H, Jinnin M, Yamane K, et al. Intravenous immunoglobulin treatment recovers the down-regulated levels of Th1 cytokines in the sera and skin of scleroderma patients. J Dermatol Sci. 2013;69:77–80. doi: 10.1016/j.jdermsci.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 83.Takehara K, Ihn H, Sato S. A randomized, double-blind, placebo-controlled trial: intravenous immunoglobulin treatment in patients with diffuse cutaneous systemic sclerosis. Clin Exp Rheumatol. 2013;31:S151–6. [PubMed] [Google Scholar]
- 84.Poelman CL, Hummers LK, Wigley FM, Anderson C, Boin F, Shah AA. Intravenous immunoglobulin may be an effective therapy for refractory active diffuse cutaneous systemic sclerosis. J Rheumatol. 2015;42:236–42. doi: 10.3899/jrheum.140833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khanna D, Denton CP. Evidence-based management of rapidly progressing systemic sclerosis. Best Pract Res Clin Rheumatol. 2010;24:387–400. doi: 10.1016/j.berh.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nash RA, McSweeney PA, Crofford LJ, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood. 2007;110:1388–96. doi: 10.1182/blood-2007-02-072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burt RK, Shah SJ, Dill K, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomized phase 2 trial. Lancet. 2011;378:498–506. doi: 10.1016/S0140-6736(11)60982-3. [DOI] [PubMed] [Google Scholar]
- 89.van Laar JM, Farge D, Sont JK, et al. EBMT/EULAR Scleroderma Study Group transplantation in the treatment of systemic. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311:2490–8. doi: 10.1001/jama.2014.6368. [DOI] [PubMed] [Google Scholar]
- 90.Burt RK, Oliveira MC, Shah SJ, et al. Cardiac involvement and treatment-related mortality after non-myeloablative haemopoietic stem-cell transplantation with unselected autologous peripheral blood for patients with systemic sclerosis: a retrospective analysis. Lancet. 2013;381:1116–24. doi: 10.1016/S0140-6736(12)62114-X. [DOI] [PubMed] [Google Scholar]
- 91.Farge D, Passweg J, van Laar JM, et al. EBMT/EULAR Registry Autologous stem cell transplantation in the treatment of systemic sclerosis: report from the EBMT/EULAR Registry. Ann Rheum Dis. 2004;63:974–81. doi: 10.1136/ard.2003.011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lam GK, Hummers LK, Woods A, Wigley FM. Efficacy and safety of etanercept in the treatment of scleroderma-associated joint disease. J Rheumatol. 2007;34:1636–7. [PubMed] [Google Scholar]
- 93.Bosello S, De Santis M, Tolusso B, Zoli A, Ferraccioli G. Tumor necrosis factor-alpha inhibitor therapy in erosive polyarthritis secondary to systemic sclerosis. Ann Intern Med. 2005;143:918–20. doi: 10.7326/0003-4819-143-12-200512200-00019. [DOI] [PubMed] [Google Scholar]
- 94.Denton CP, Engelhart M, Tvede N, et al. An open label pilot study of infliximab therapy in diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2009;68:1433–9. doi: 10.1136/ard.2008.096123. [DOI] [PubMed] [Google Scholar]
- 95.Distler JH, Jordan S, Airo P, et al. Is there a role for TNFα antagonists in the treatment of SSc? EUSTAR expert consensus development using the Delphi technique. Clin Exp Rheumatol. 2011;29:S40. [PubMed] [Google Scholar]
- 96.Daoussis D, Liossis SN, Tsamandas AC, et al. Experience with rituximab in scleroderma: results from 1-year, proof-of principle study. Rheumatology. 2010;49:271–80. doi: 10.1093/rheumatology/kep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Daoussis D, Liossis SN, Tsamandas AC, et al. Effect of long-term treatment with rituximab on pulmonary function and skin fibrosis in patients with diffuse systemic sclerosis. Clin Exp Rheumatol. 2012;30:S17–22. [PubMed] [Google Scholar]
- 98.Emery P, Deodhar A, Rigby WF, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebocontrolled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)) Ann Rheum Dis. 2010;69:1629–35. doi: 10.1136/ard.2009.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McGonagle D, Tan AL, Madden J, et al. Successful treatment of resistant scleroderma- associated interstitial lung disease with rituximab. Rheumatology. 2008;47:552–3. doi: 10.1093/rheumatology/kem357. [DOI] [PubMed] [Google Scholar]
- 100.Daoussis D, Liossis SN, Tsamandas AC, et al. Is there a role for B-cell depletion as therapy for scleroderma? A case report and review of the literature. Semin Arthritis Rheum. 2010;40:127–36. doi: 10.1016/j.semarthrit.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Smith V, Van Praet JT, Vandooren B, et al. Rituximab in diffuse systemic sclerosis: an open-label clinical and histopathological study. Ann Rheum Dis. 2010;69:193–7. doi: 10.1136/ard.2008.095463. [DOI] [PubMed] [Google Scholar]
- 102.Bosello S, De Santis M, Lama G, et al. B cell depletion in diffuse progressive systemic sclerosis: safety, skin score modification and IL-6 modulation in an up to thirty-six months follow-up open label trial. Arthritis Res Ther. 2010;12:R54. doi: 10.1186/ar2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elhai M, Meunier M, Matucci-Cerinic M, et al. EUSTAR (EULAR Scleroderma Trials and Research group) Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: a EUSTAR observational study. Ann Rheum Dis. 2013;72:1217–20. doi: 10.1136/annrheumdis-2012-202657. [DOI] [PubMed] [Google Scholar]
- 104.Fransen J, van Riel PLCM. The disease activity score and the EULAR response criteria. Clin Exp Rheumatol. 2005;23:S93–9. [PubMed] [Google Scholar]
- 105.Shima Y, Kuwahara Y, Murota H, et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology (Oxford) 2010;49:2408–12. doi: 10.1093/rheumatology/keq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Milano A, Pendergrass SA, Sargent JL, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS One. 2008;3:e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sargent J, Milano A, Bhattacharyya S, et al. The TGF-ß responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J Invest Dermatol. 2010;130:694–705. doi: 10.1038/jid.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Denton CP, Merkel PA, Furst DE, et al. Cat-192 Study Group; Scleroderma Clinical Trials Consortium. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT−192. Arthritis Rheum. 2007;56:323–33. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 110.Duan H, Fleming J, Pritchard DK, et al. Combined analysis of monocyte and lymphocyte messenger RNA expression with serum protein profiles in patients with scleroderma. Arthritis Rheum. 2008;58:1465–74. doi: 10.1002/art.23451. [DOI] [PubMed] [Google Scholar]
- 111.Coelho LF, de Oliveira JG, de Oliveira DB, et al. Increased expression of 2′5′oligoadenylate synthetase and double-stranded RNA dependent protein kinase messenger RNAs on affected skin of systemic sclerosis patients. Arch Dermatol Res. 2007;299:259–62. doi: 10.1007/s00403-007-0737-x. [DOI] [PubMed] [Google Scholar]
- 112.Tan FK, Zhou X, Mayes MD, et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford) 2006;45:694–702. doi: 10.1093/rheumatology/kei244. [DOI] [PubMed] [Google Scholar]
- 113.Wang B, Higgs BW, Chang L, et al. Pharmacogenomics and translational stimulations to bridge indications for an anti-interferon-α receptor antibody. Clin Pharmacol Ther. 2013;93:483–92. doi: 10.1038/clpt.2013.35. [DOI] [PubMed] [Google Scholar]
- 114.Kuwana M, Medsger TA, Jr, Wright TM. Highly restricted TCR-alpha beta usage by autoreactive human T cell clones specific for DNA topoisomerase I: recognition of an immunodominant epitope. J Immunol. 1997;158:485–91. [PubMed] [Google Scholar]
- 115.Schmidt K, Martinez-Gamboa L, Meier S, et al. Bronchoalveoloar lavage fluid cytokines and chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients. Arthritis Res Ther. 2009;11:R111. doi: 10.1186/ar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Degiannis D, Seibold JR, Czarnecki M, Rasknova J, Raska K., Jr Soluble interleukin-2 receptors in patients with systemic sclerosis: clinical and laboratory correlations. Arthritis Rheum. 1990;33:375–80. doi: 10.1002/art.1780330310. [DOI] [PubMed] [Google Scholar]
- 117.Schmidt-Hieber M, Fietz T, Knauf W, et al. Efficacy of the interleukin-2 receptor antagonist basiliximab in steroid-refractory acute graft-versus-host disease. Br J Haematol. 2005;130:568–74. doi: 10.1111/j.1365-2141.2005.05631.x. [DOI] [PubMed] [Google Scholar]
- 118.Becker MO, Brückner C, Scherer HU, et al. The monoclonal anti-CD25 antibody basiliximab for the treatment of progressive systemic sclerosis: an open-label study. Ann Rheum Dis. 2011;70:1340–1. doi: 10.1136/ard.2010.137935. [DOI] [PubMed] [Google Scholar]
- 119.Scherer HU, Burmester GR, Riemekasten G. Targeting activated T cells: successful use of anti-CD25 monoclonal antibody basiliximab in a patient with systemic sclerosis. Ann Rheum Dis. 2006;65:1245–7. doi: 10.1136/ard.2005.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Distler JH, Distler O. Imatinib as a novel therapeutic approach for fibrotic disorders. Rheumatology (Oxford) 2009;48:2–4. doi: 10.1093/rheumatology/ken431. [DOI] [PubMed] [Google Scholar]
- 121.Daniels CE, Lasky JA, Limper AH, et al. Imatinib-IPF Study Investigators Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–10. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 122.Spiera RF, Gordon JK, Mersten JN, et al. Imatinib mesylate (Gleevec) in the treatment of diffuse cutaneous systemic sclerosis: results of a 1-year, Phase IIa, single-arm, open-label clinical trial. Ann Rheum Dis. 2011;70:1003–9. doi: 10.1136/ard.2010.143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khanna D, Saggar R, Mayes MD, et al. A one-year, Phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung disease. Arthritis Rheum. 2011;63:3540–6. doi: 10.1002/art.30548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pope J, McBain D, Petrlich L, et al. Imatinib in active diffuse cutaneous systemic sclerosis: results of a six-month, randomized, double-blind, placebo-controlled, proof-of-concept pilot study at a single center. Arthritis Rheum. 2011;63:3547–51. doi: 10.1002/art.30549. [DOI] [PubMed] [Google Scholar]
- 125.Fraticelli P, Gabrielli B, Pomponio G, et al. Imatinib in Scleroderma Italian Study Group Low-dose oral imatinib in the treatment of systemic sclerosis interstitial lung disease unresponsive to cyclophosphamide: a phase II pilot study. Arthritis Res Ther. 2014;16:R144. doi: 10.1186/ar4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–87. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 127.Richeldi L, du Bois RM, Raghu G, et al. INPULSIS Trial Investigators Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 128.Shitrit D, Amital A, Peled N, et al. Lung transplantation in patients with scleroderma: case series, review of the literature, and criteria for transplantation. Clin Transplant. 2009;23:178–83. doi: 10.1111/j.1399-0012.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 129.Schachna L, Medsger TA, Jr, Dauber JH, et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 2006;54:3954–61. doi: 10.1002/art.22264. [DOI] [PubMed] [Google Scholar]
- 130.Rosas V, Conte JV, Yang SC, et al. Lung transplantation and systemic sclerosis. Ann Transplant. 2000;5:38–43. [PubMed] [Google Scholar]
- 131.Saggar R, Khanna D, Furst DE, et al. Systemic sclerosis and bilateral lung transplantation: a single centre experience. Eur Respir J. 2010;36:893–900. doi: 10.1183/09031936.00139809. [DOI] [PMC free article] [PubMed] [Google Scholar]