Abstract
Background
The association between elevated admission serum uric acid (SUA) and risk of in-hospital acute kidney injury (AKI) is limited. The aim of this study was to assess the risk of developing AKI in all hospitalized patients with various admission SUA levels.
Methods
This is a single-center retrospective study conducted at a tertiary referral hospital. All hospitalized adult patients who had admission SUA available from January 2011 through December 2013 were analyzed in this study. Admission SUA was categorized based on its distribution into six groups (<3.4, 3.4–4.5, 4.5–5.8, 5.8–7.6, 7.6–9.4 and >9.4 mg/dL). The primary outcome was in-hospital AKI occurring after hospital admission. Logistic regression analysis was performed to obtain the odds ratio (OR) of AKI of various admission SUA levels using the most common SUA level range (5.8–7.6 mg/dL) as the reference group.
Results
Of 1435 patients enrolled, AKI occurred in 263 patients (18%). The incidence of AKI and need for dialysis was increased in patients with higher admission SUA levels. After adjusting for potential confounders, SUA >9.4 mg/dL was associated with an increased risk of developing AKI, with ORs of 1.79 [95% confidence interval (CI) 1.13–2.82]. Conversely, admission SUA <3.4 and 3.4–4.5 mg/dL were associated with a decreased risk of developing AKI, with ORs of 0.38 (95% CI 0.17–0.75) and 0.50 (95% CI 0.28–0.87), respectively.
Conclusions
Elevated admission SUA was associated with an increased risk for in-hospital AKI.
Keywords: acute kidney injury, hyperuricemia, length of hospital stay, mortality, uric acid
Introduction
Acute kidney injury (AKI) is a common clinical syndrome among hospitalized patients, independently associated with both short- and long-term mortality [1]. AKI-associated mortality has been reported to be as high as 23% [1]. Previous studies have attempted to identify effective interventions to prevent AKI events [2–4]. However, most were unsuccessful. Therefore further studies are needed to identify individuals who are at high risk of developing AKI.
Uric acid has been linked to AKI via crystal-independent mechanisms, including reduced renal blood flow and glomerular filtration rate (GFR), as well as crystal-dependent pathways [5]. Serum uric acid (SUA) measurement has been examined as a novel marker for early detection of AKI [6, 7]. Recent studies have demonstrated that an elevated SUA level is a risk factor for developing postoperative AKI in cardiovascular surgery patients [8–12]. However, the effect of admission SUA on the risk of in-hospital AKI in the general hospital population has not been examined. The objective of this study was to evaluate the risk of developing AKI in all hospitalized patients across a spectrum of SUA levels.
Materials and methods
Study population
The study included all adult (ages ≥18 years) patients admitted to the Mayo Clinic Rochester—a tertiary referral hospital—from 1 January 2011 through 31 December 2013. Exclusion criteria were patients without SUA measurement within 24 h of admission, patients with a history of end-stage renal disease (ESRD), patients who presented with AKI at the time of admission and patients who did not provide research authorization. Patients admitted after trauma were also not analyzed due to a higher incidence of bleeding and AKI at the time of admission [13]. For patients with multiple admissions during this period, only the first hospital admission was analyzed. ESRD was identified based on International Classification of Diseases, 9th revision (ICD-9 code assignment (Supplementary data, Table S1) or an estimated GFR (eGFR) <15 mL/min/1.73 m2.
Data collection
Clinical characteristics, demographic information and laboratory data were collected using manual and automated retrieval from the institutional electronic medical record system. The admission SUA level, defined as the first SUA level within 24 h of hospital admission, was collected. eGFR was derived using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [14]. Chronic kidney disease (CKD) was defined as a calculated eGFR <60 mL/min/1.73 m2. The Charlson comorbidity score [15] was computed for comorbidities at the time of admission. Principal diagnoses were grouped based on ICD-9 codes at admission (Supplementary data, Table S2).
Clinical outcomes
The primary outcome was AKI, based on the serum creatinine (SCr) criterion of the Kidney Disease Improving Global Outcomes (KDIGO) definition (Supplementary data, Table S3) [16]. AKI was defined as an increase in SCr ≥0.3 mg/dL within 48 h or ≥1.5 times baseline within 7 days after admission date. The baseline SCr was defined as the minimum SCr measured within 1 year before admission. If outpatient SCr was not available, the Modification of Diet in Renal Disease equation [17] was used to estimate the baseline SCr level, assuming normal baseline GFR of 75 mL/min/1.73 m2, in accordance with this guideline [16]. We performed sensitivity analysis using any in-hospital AKI occurrence, regardless of 7-day the time frame. Secondary outcomes were in-hospital mortality, 90-day mortality after hospital admission, hospital length of stay (LOS) and discharge to a care facility. In patients whose vital status at 90 days after hospital admission based on the institutional electronic medical record was unknown, the Social Security Death Index was used [18].
Statistical analysis
Continuous variables are reported as mean ± SD for normally distributed data and median (IQR) for non-normally distributed data. All categorical variables are reported as count with percentage. Baseline demographics and clinical characteristics were compared among the admission uric acid group, using analysis of variance (ANOVA) for continuous variables and the chi-square test for categorical variables. We categorized admission serum uric levels, based on six-quantile percentiles (10% | 25% | 50% | 75% | 90%): <3.4, 3.4–4.5, 4.5–5.8, 5.8–7.6, 7.6–9.4 and >9.4 mg/dL. The most common SUA level range (5.8–7.6 mg/dL) was selected as the reference group for outcome comparison (Table 1). We performed univariate analysis and then multivariate logistic regression analysis to evaluate the independent association between admission uric acid levels and AKI. Odds ratios (ORs) with 95% confidence intervals (CIs) are reported. The OR was adjusted for variables with statistically significant (P-value <0.05) differences between groups in univariate analysis. The adjusting variables were age, sex, body mass index (BMI), baseline SCr, principal diagnosis comorbidities and medications. Comorbidities were coronary artery disease (CAD), hypertension (HTN), diabetes mellitus (DM) and congestive heart failure (CHF). Medications were angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs) and diuretics. A two-tailed P-value <0.05 was considered statistically significant. All analyses were performed using JMP statistical software (version 10, SAS Institute, Cary, NC, USA).
Table 1.
Baseline clinical characteristics
| Variables | All | SUA level at hospital admission (mg/dL) |
||||||
|---|---|---|---|---|---|---|---|---|
| <3.4 | 3.4–4.5 | 4.5–5.8 | 5.8–7.6 | 7.6–9.4 | >9.4 | P-value | ||
| Patients (n) | 1435 | 135 | 223 | 337 | 374 | 216 | 150 | |
| Age (years) | 62.0 ± 16.0 | 58.3 ± 17.4 | 59.7 ± 17.3 | 60.9 ± 15.9 | 63.0 ± 15.8 | 64.8 ± 14.1 | 64.4 ± 15.3 | <0.001 |
| Male | 865 (60.3%) | 64 (47.4%) | 99 (44.4%) | 197 (58.5%) | 253 (67.6%) | 149 (70.0%) | 103 (68.7%) | <0.001 |
| Caucasian | 1311 (91.4%) | 126 (93.3%) | 207 (92.8%) | 303 (89.9%) | 347 (92.8%) | 195 (90.3%) | 133 (88.7%) | 0.44 |
| BMI (kg/m2) | 29.1 ± 7.3 | 26.6 ± 6.0 | 26.3 ± 6.8 | 28.2 ± 7.0 | 30.3 ± 6.8 | 30.5 ± 7.1 | 32.5 ± 8.4 | <0.001 |
| Weight change in hospital (kg) | −0.9 ± 6.0 | −0.5 ± 4.8 | −0.8 ± 4.7 | −0.5 ± 6.0 | −0.9 ± 6.6 | −1.3 ± 6.1 | −2.1 ± 7.2 | 0.14 |
| Charlson comorbidity score | 2.0 ± 2.4 | 1.7 ± 2.3 | 2.2 ± 2.6 | 1.9 ± 2.3 | 2.0 ± 2.3 | 2.2 ± 2.4 | 2.0 ± 2.2 | 0.33 |
| Baseline serum creatinine (mg/dL) | 1.1 ± 0.4 | 0.9 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.5 | 1.3 ± 0.5 | <0.001 |
| eGFR (mL/min/1.73 m2) | 73.1 ± 26.1 | 89.5 ± 20.6 | 88.1 ± 21.9 | 79.3 ± 24.5 | 71.7 ± 24.8 | 58.6 ± 22.3 | 53.2 ± 21.8 | <0.001 |
| Comorbidities | ||||||||
| CAD | 273 (19.0%) | 18 (13.3%) | 21 (9.4%) | 54 (16.0%) | 78 (20.9%) | 53 (24.5%) | 49 (32.7%) | <0.001 |
| HTN | 699 (48.7%) | 55 (40.7%) | 83 (37.2%) | 140 (41.5%) | 214 (57.2%) | 125 (57.9%) | 82 (54.7%) | <0.001 |
| DM | 317 (22.1%) | 15 (13.3%) | 49 (22.0%) | 63 (18.7%) | 83 (22.2%) | 63 (29.2%) | 41 (27.3%) | 0.005 |
| CHF | 184 (12.8%) | 8 (5.9%) | 13 (5.8%) | 28 (8.3%) | 45 (12.0%) | 40 (18.5%) | 50 (33.3%) | <0.001 |
| Cirrhosis | 60 (4.2%) | 5 (3.7%) | 12 (5.4%) | 12 (3.6%) | 14 (3.7%) | 11 (5.1%) | 6 (4.0%) | 0.87 |
| Principal diagnosis | <0.001 | |||||||
| Cardiovascular | 487 (33.9%) | 15 (11.1%) | 38 (17.0%) | 101 (30.0%) | 145 (38.8%) | 103 (47.7%) | 85 (56.7%) | |
| Hematology/oncology | 378 (26.3%) | 57 (42.2%) | 74 (33.2%) | 92 (27.3%) | 88 (23.5%) | 45 (20.8%) | 22 (14.7%) | |
| Infectious disease | 53 (3.7%) | 12 (8.9%) | 9 (4.0%) | 13 (3.9%) | 9 (2.4%) | 6 (2.8%) | 4 (2.7%) | |
| Endocrine/metabolic | 94 (6.6%) | 14 (10.4%) | 14 (6.3%) | 18 (5.3%) | 23 (6.2%) | 14 (6.5%) | 11 (7.3%) | |
| Respiratory | 59 (4.1%) | 8 (6.0%) | 13 (5.8%) | 19 (5.6%) | 12 (3.2%) | 3 (1.4%) | 4 (2.7%) | |
| Gastrointestinal | 74 (5.1%) | 10 (7.4%) | 14 (6.3%) | 19 (5.6%) | 20 (5.3%) | 11 (5.1%) | 4 (2.7%) | |
| Other | 290 (20.2%) | 19 (14.1%) | 61 (27.4%) | 79 (23.4%) | 77 (20.6%) | 34 (15.7%) | 20 (13.3%) | |
| Medication | ||||||||
| ACEI/ARB | 535 (37.3%) | 32 (23.7%) | 49 (22.0%) | 109 (32.3%) | 163 (43.6%) | 95 (44.0%) | 87 (58%) | <0.001 |
| NSAID | 235 (16.4%) | 23 (17.0%) | 46 (20.6%) | 65 (19.3%) | 58 (15.5%) | 26 (12.0%) | 17 (11.3%) | 0.05 |
| Diuretic | 703 (49.0%) | 37 (27.4%) | 60 (26.9%) | 134 (39.8%) | 206 (55.1%) | 139 (64.4%) | 127 (84.7%) | <0.001 |
| Allopurinol | 219 (15.3%) | 21 (15.6%) | 26 (11.7%) | 49 (14.5%) | 58 (15.5%) | 35 (16.2%) | 30 (20%) | 0.40 |
| Shock | 153 (10.7%) | 8 (5.9%) | 19 (8.5%) | 40 (11.9%) | 40 (10.7%) | 25 (11.6%) | 21 (14.0%) | 0.24 |
Continuous data are presented as mean ± SD; categorical data are presented as n (%).
Results
A total of 76 719 adult patients were identified. After excluding 73 295 patients who lacked admission uric acid measurement, 390 patients with ESRD, 762 patients with AKI at presentation and 837 trauma patients, 1435 unique patients were enrolled (Supplementary data, Figure S1).
Baseline characteristics
Of 1435 patients, 865 (91.4%) patients were Caucasian and 865 (60.3%) were male (Table 1). The mean age was 62 ± 16 years. Patient age was positively correlated with SUA, whereas eGFR was inversely correlated with SUA. Patient comorbidities included HTN (48.7%), DM (22.1%), CAD (19.0%) and CHF (12.8%). Forty-nine per cent of the patients were taking diuretics, 37.3% were taking ACEIs or ARBs and 15.3% were taking allopurinol before admission. The distribution of admission serum uric levels was normally distributed (Supplementary data, Figure S2).
The principle admission diagnosis showed that patients with a diagnosis of cardiovascular diseases presented with high admission SUA, whereas patients with diagnoses of hematology/oncology, infectious, endocrine/metabolic and respiratory diseases presented with low admission SUA (Supplementary data, Figure S3).
Admission SUA and the incidence of AKI
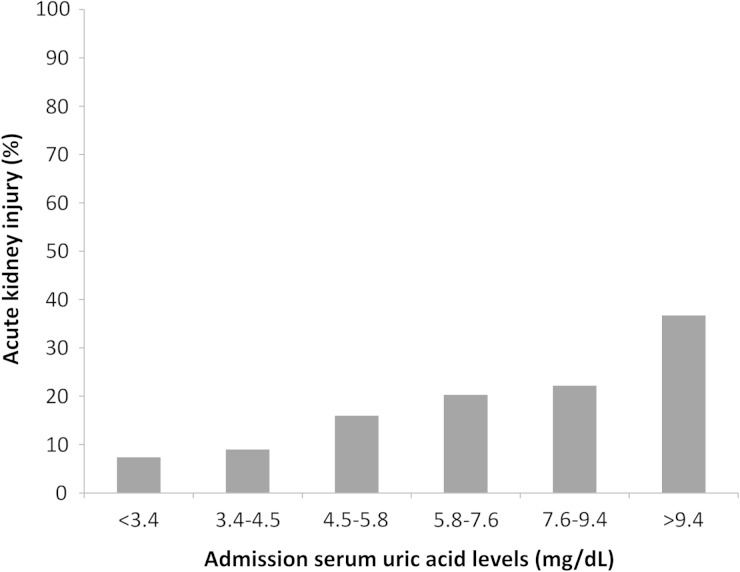
The incidence of AKI associated with admission SUA was linear (Figure 1). The lowest AKI incidence occurred when SUA levels were <3.4 mg/dL. Increasing SUA levels were correlated with higher incidences of all stages of AKI. Increasing SUA levels were also positively correlated with the need for dialysis, with the highest incidence in patients with SUA >9.4 mg/dL (Table 2). Despite minimal mortality, there was a trend of higher mortality with increasing admission SUA, with the highest mortality when SUA was >9.4 mg/dL. Both low admission SUA (<3.4 mg/dL) and high SUA (>9.4 mg/dL) were correlated with longer hospital LOS, whereas patients with admission SUA >9.4 mg/dL had the highest rate of discharge to a care facility.
Fig. 1.
In-hospital AKI within 7 days for various admission SUA levels.
Table 2.
Outcomes
| Outcome | SUA level at hospital admission (mg/dL) |
||||||
|---|---|---|---|---|---|---|---|
| <3.4 | 3.4–4.5 | 4.5–5.8 | 5.8–7.6 | 7.6–9.4 | >9.4 | P-value | |
| AKI | 10 (7.4%) | 20 (9.0%) | 54 (16.0%) | 76 (20.3%) | 48 (22.2%) | 55 (36.7%) | <0.001 |
| AKI stage | <0.001 | ||||||
| Stage 1 | 7 (5.2%) | 18 (8.1%) | 44 (13.1%) | 57 (15.2%) | 41 (19.0%) | 43 (28.7%) | |
| Stage 2 | 3 (2.2%) | 2 (0.9%) | 7 (2.1%) | 9 (2.4%) | 3 (1.4%) | 5 (3.3%) | |
| Stage 3 | 0 (0%) | 0 (0) | 3 (0.9%) | 10 (2.7%) | 4 (1.9%) | 7 (4.7%) | |
| Dialysis | 0 (0%) | 0 (0%) | 7 (2.1%) | 7 (1.9%) | 3 (1.4%) | 6 (4.0%) | 0.03 |
| In-hospital mortality | 3 (2.2%) | 4 (1.8%) | 8 (2.4%) | 8 (2.1%) | 4 (1.9%) | 4 (2.7%) | 0.99 |
| 90-day mortality | 25 (18.5%) | 29 (13.0%) | 42 (12.5%) | 38 (10.2%) | 21 (9.7%) | 16 (10.7%) | 0.14 |
| Hospital LOS (days), mean (range) | 7 (4–14) | 5 (3–10) | 5 (4–10) | 5 (3–8) | 6 (3–10) | 6.5 (4–11) | 0.03 |
| Discharge to care facilitya | 7 (7.9%) | 14 (8.3%) | 14 (5.5%) | 13 (4.6%) | 13 (7.9%) | 9 (9.3%) | 0.42 |
Values are given as n (%).
aDenominator was hospital survivors.
Admission SUA and risk of AKI
To assess whether admission SUA levels contributed to AKI development, logistic regression models were built, using 5.8–7.6 mg/dL as a reference range. An unadjusted admission SUA >9.4 mg/dL was associated with an increased risk of AKI [OR 2.27 (95% CI 1.50–3.44)] (Table 3). Conversely, SUA <3.4 mg/dL was associated with a reduced risk of AKI [OR 0.31 (95% CI 0.15–0.60)]. An admission SUA of 3.4–4.5 mg/dL was associated with a reduced risk of AKI [OR 0.39 (95% CI 0.22–0.64)]. When adjusted for all variables including age, sex, BMI, baseline SCr, comorbidities and medications, these associations remained statistically significant. High admission SUA (>9.4 mg/dL) was associated with an increased risk of developing AKI [OR 1.79 (95% CI 1.79–2.82)] (Table 3). An SUA <3.4 mg/dL was associated with a decreased AKI [OR 0.38 (95% CI 0.17–0.75)] and an admission SUA of 3.4–4.5 mg/dL was associated with decreased AKI [OR 0.50 (95% CI 0.28–0.87)].
Table 3.
Odds ratios for association between admission SUA levels and in-hospital AKI occurrence within 7 days
| SUA level at hospital admission (mg/dL) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | Adjusteda OR (95% CI) | P-value | |
| <3.4 | 0.31 (0.15–0.60) | 0.001 | 0.38 (0.17–0.75) | 0.005 |
| 3.4–4.5 | 0.39 (0.22–0.64) | <0.001 | 0.50 (0.28–0.87) | 0.01 |
| 4.5–5.8 | 0.75 (0.51–1.10) | 0.14 | 0.86 (0.57–1.28) | 0.45 |
| 5.8–7.6 | 1 (ref) | 1 (ref) | ||
| 7.6−9.4 | 1.12 (0.74–1.68) | 0.59 | 1.03 (0.67–1.58) | 0.89 |
| >9.4 | 2.27 (1.50–3.44) | <0.001 | 1.79 (1.13–2.82) | 0.01 |
aAdjusted for age, gender, BMI, baseline SCr, comorbidities (including CAD, hypertension, diabetes, CHF), principal diagnosis and medication use (including angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers and diuretics).
Sensitivity analysis
These findings remained when AKI outcome was reanalyzed without the limitation of 7 days after admission (Supplementary data, Tables S4 and S5). The linear trend of higher in-hospital AKI with increasing SUA still existed, with the highest incidence in patients with admission SUA >9.4 mg/dL (Supplementary data, Table S4) with increased in-hospital AKI [OR 1.89 (95% CI 1.21–2.95)] (Supplementary data, Table S5).
Discussion
This study demonstrates that the admission SUA level is correlated with the incidence of AKI and the need for dialysis during hospitalization. This correlation occurs at all stages of AKI. There is also a positive correlation between admission SUA level and the risk of developing AKI, with the highest risk in the admission SUA >9.4 mg/dL patient group. The lowest risk group was admission SUA <3.4 mg/dL.
There are several plausible explanations for the increased AKI risk in patients with elevated SUA values. Uric acid has been proposed to play a role in AKI via crystal-independent mechanisms, as well as crystal-dependent pathways [5]. Elevated SUA can induce renal vasoconstriction and impair autoregulation, which results in reduced renal blood flow and GFR [5, 8, 19]. Sanchez-Lozada et al. [20] showed that even a mild elevation of SUA can cause renal vasoconstriction in rats without evidence of intratubular crystal precipitation. Furthermore, hyperuricemia has been shown to worsen renal injury via pro-inflammatory pathways involving chemokine expression with leukocyte infiltration, as well as proliferation of vascular smooth muscle cells and inhibition of endothelial function [8, 21–23]. AKI-related crystal-dependent pathways can also occur in renal stones and acute urate nephropathy associated with tumor lysis syndrome [19, 24–27].
Previous reports have demonstrated that elevated SUA is a risk factor for developing AKI in specific circumstances. Preoperative and postoperative hyperuricemia has been linked to a higher incidence of postoperative AKI, especially in cardiovascular surgery settings [8–12]. Moreover, hyperuricemia has also been shown to increase the risk of contrast-induced AKI after percutaneous coronary interventions [28, 29]. Hyperuricemia at baseline has also been shown to be an important long-term predictor of AKI and mortality [30], especially in the elderly with CKD [31]. In cases of acute paraquat intoxication, baseline SUA has been proposed as a clinical marker of AKI and mortality [32]. Ongoing research to identify novel biomarkers for AKI diagnosis and risk stratification has the potential to reduce delays in the diagnosis of AKI in the hospital setting [33]. SUA measurement has been proposed as a novel marker for early detection of AKI [6, 7]. The results presented in our study are the first to demonstrate that SUA at the time of admission is an important predictor of developing in-hospital AKI in the general hospitalized patient.
Recently Lapsia et al. [8] demonstrated a J-shaped relationship between preoperative SUA and postoperative AKI. The proposed explanation was that AKI-associated hypouricemia is due to oxidative stress, as uric acid can act as both an antioxidant and pro-oxidant agent, depending on the SUA level [21, 34, 35]. However, the results of our study of hospitalized patients reveal a positive linear correlation between admission SUA level and the risk of developing AKI. This difference may be due to different study populations and settings.
The results of our study demonstrate a prognostic effect of admission SUA level on AKI development. Previous attempts to identify effective interventions to prevent AKI have been largely unsuccessful [2, 3]. Using the admission SUA level in clinical practice may help identify patients with a high risk of AKI during hospitalization in order to promptly prevent AKI events. For example, hyperuricemia-related AKI has been reported in patients with non-steroidal anti-inflammatory drug use. Thus discontinuation or avoidance of such nephrotoxic agents in patients with elevated admission SUA should be considered. Several clinical trials have examined the efficacy of uric acid–lowering agents, such as allopurinol, in cardiovascular surgery and found a reduction in the production of reactive oxygen species [36, 37]. Recently, long-term follow-up of a randomized clinical trial showed that long-term treatment with allopurinol may slow the rate of progression of kidney disease and decrease cardiovascular risks [38]. Rasburicase has also been studied in a prospective, double-blind, placebo-controlled, randomized trial of 26 hyperuricemic patients undergoing cardiac surgery [39]. Despite no observed benefit on postoperative SCr, markers of structural renal injury such as urine neutrophil-associated lipocalin (uNGAL) tended to be lower in rasburicase-treated patients.
This study has several limitations. First, this is a single-center, retrospective study. Second, the patient population in this study is relatively homogeneous (predominantly Caucasian). Further studies with a more heterogeneous population are desirable to ascertain the clinical effects of admission SUA on AKI in a broad patient population. A multicenter, prospective study is ultimately required to address these limitations. (3) Our study demonstrated that the lowest SUA level group (<3.4 mg/dL) was correlated with the longest hospital LOS. Principal diagnoses likely played important roles for this association since those patients had more diagnoses of hematology/oncology or infectious diseases, as indicated in Table 1. Malnutrition and inflammation were suggested to be important factors for lower SUA levels and worse outcome. However, the data regarding C-reactive protein and albumin were limited since they were not commonly measured in hospitalized patients. Finally, there is potential selection bias, as those patients who had admission SUA measurements may have had different clinical characteristics from others who did not have admission SUA measurements. A multicenter, prospective study is ultimately required to address these limitations.
In conclusion, this study demonstrates that elevated admission SUA is associated with an increased risk for in-hospital AKI.
Authors’ contributions
All the authors had access to the data and a role in writing the manuscript.
Supplementary data
Supplementary data are available online at http://ndt.oxfordjournals.org.
Conflict of interest statement
None declared.
Supplementary Material
Acknowledgements
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
References
- 1.Thongprayoon C, Cheungpasitporn W, Akhoundi A, et al. Actual versus ideal body weight for acute kidney injury diagnosis and classification in critically Ill patients. BMC Nephrol 2014; 15: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng X, Tong J, Hu Q, et al. Meta-analysis of the effects of preoperative renin-angiotensin system inhibitor therapy on major adverse cardiac events in patients undergoing cardiac surgery. Eur J Cardiothorac Surg 2015; 47: 958–966 [DOI] [PubMed] [Google Scholar]
- 3.Yacoub R, Patel N, Lohr JW, et al. Acute kidney injury and death associated with renin angiotensin system blockade in cardiothoracic surgery: a meta-analysis of observational studies. Am J Kidney Dis 2013; 62: 1077–1086 [DOI] [PubMed] [Google Scholar]
- 4.Garg AX, Kurz A, Sessler DI, et al. Perioperative aspirin and clonidine and risk of acute kidney injury: a randomized clinical trial. JAMA 2014; 312: 2254–2264 [DOI] [PubMed] [Google Scholar]
- 5.Ejaz AA, Dass B, Kambhampati G, et al. Lowering serum uric acid to prevent acute kidney injury. Med Hypotheses 2012; 78: 796–799 [DOI] [PubMed] [Google Scholar]
- 6.Lisowska-Myjak B. Serum and urinary biomarkers of acute kidney injury. Blood Purif 2010; 29: 357–365 [DOI] [PubMed] [Google Scholar]
- 7.Kosmadakis G, Viskaduraki M, Michail S. The validity of fractional excretion of uric acid in the diagnosis of acute kidney injury due to decreased kidney perfusion. Am J Kidney Dis 2009; 54: 1186–1187 [DOI] [PubMed] [Google Scholar]
- 8.Lapsia V, Johnson RJ, Dass B, et al. Elevated uric acid increases the risk for acute kidney injury. Am J Med 2012; 125: 302 e9–17 [DOI] [PubMed] [Google Scholar]
- 9.Ejaz AA, Beaver TM, Shimada M, et al. Uric acid: a novel risk factor for acute kidney injury in high-risk cardiac surgery patients? Am J Nephrol 2009; 30: 425–429 [DOI] [PubMed] [Google Scholar]
- 10.Moguel-Gonzalez B, Wasung-de-Lay M, Tella-Vega P, et al. Acute kidney injury in cardiac surgery. Rev Invest Clin 2013; 65: 467–475 [PubMed] [Google Scholar]
- 11.Ejaz AA, Kambhampati G, Ejaz NI, et al. Post-operative serum uric acid and acute kidney injury. J Nephrol 2012; 25: 497–505 [DOI] [PubMed] [Google Scholar]
- 12.Hillis GS, Cuthbertson BH, Gibson PH, et al. Uric acid levels and outcome from coronary artery bypass grafting. J Thorac Cardiovasc Surg 2009; 138: 200–205 [DOI] [PubMed] [Google Scholar]
- 13.Bihorac A, Baslanti TO, Cuenca AG, et al. Acute kidney injury is associated with early cytokine changes after trauma. J Trauma Acute Care Surg 2013; 74: 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251 [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO). Acute Kidney Injury Work Group: KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Kidney Int Suppl 2012; 2: 1–138 [Google Scholar]
- 17.Zavada J, Hoste E, Cartin-Ceba R, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant 2010; 25: 3911–3918 [DOI] [PubMed] [Google Scholar]
- 18.Wentworth DN, Neaton JD, Rasmussen WL. An evaluation of the Social Security Administration master beneficiary record file and the National Death Index in the ascertainment of vital status. Am J Public Health 1983; 73: 1270–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conger JD, Falk SA. Intrarenal dynamics in the pathogenesis and prevention of acute urate nephropathy. J Clin Invest 1977; 59: 786–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Lozada LG, Tapia E, Santamaria J, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int 2005; 67: 237–247 [DOI] [PubMed] [Google Scholar]
- 21.Roncal CA, Mu W, Croker B, et al. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 2007; 292: F116–F122 [DOI] [PubMed] [Google Scholar]
- 22.Shimada M, Dass B, Ejaz AA. Paradigm shift in the role of uric acid in acute kidney injury. Semin Nephrol 2011; 31: 453–458 [DOI] [PubMed] [Google Scholar]
- 23.Durante P, Romero F, Perez M, et al. Effect of uric acid on nephrotoxicity induced by mercuric chloride in rats. Toxicol Ind Health 2010; 26: 163–174 [DOI] [PubMed] [Google Scholar]
- 24.Cameron JS, Simmonds HA. Uric acid, gout and the kidney. J Clin Pathol 1981; 34: 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lameire NH, Flombaum CD, Moreau D, et al. Acute renal failure in cancer patients. Ann Med 2005; 37: 13–25 [DOI] [PubMed] [Google Scholar]
- 26.Hsu HH, Chen YC, Tian YC, et al. Role of serum sodium in assessing hospital mortality in cancer patients with spontaneous tumour lysis syndrome inducing acute uric acid nephropathy. Int J Clin Pract 2009; 63: 751–756 [PubMed] [Google Scholar]
- 27.Preitner F, Laverriere-Loss A, Metref S, et al. Urate-induced acute renal failure and chronic inflammation in liver-specific Glut9 knockout mice. Am J Physiol Renal Physiol 2013; 305: F786–F795 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Tan N, Chen J, et al. The relationship between hyperuricemia and the risk of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with relatively normal serum creatinine. Clinics 2013; 68: 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SH, Shin WY, Lee EY, et al. The impact of hyperuricemia on in-hospital mortality and incidence of acute kidney injury in patients undergoing percutaneous coronary intervention. Circ J 2011; 75: 692–697 [DOI] [PubMed] [Google Scholar]
- 30.Ben-Dov IZ, Kark JD. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: the Jerusalem Lipid Research Clinic cohort study. Nephrol Dial Transplant 2011; 26: 2558–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heras M, Fernandez-Reyes MJ, Guerrero MT, et al. Acute renal failure predictors in elderly patients with chronic kidney disease. Nefrologia 2012; 32: 819–823 [DOI] [PubMed] [Google Scholar]
- 32.Kim JH, Gil HW, Yang JO, et al. Serum uric acid level as a marker for mortality and acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant 2011; 26: 1846–1852 [DOI] [PubMed] [Google Scholar]
- 33.Lieske JC, Chawla L, Kashani K, et al. Biomarkers for acute kidney injury: where are we today? Where should we go? Clin Chem 2014; 60: 294–300 [DOI] [PubMed] [Google Scholar]
- 34.Kaneko K, Taniguchi N, Tanabe Y, et al. Oxidative imbalance in idiopathic renal hypouricemia. Pediatr Nephrol 2009; 24: 869–871 [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Lozada LG, Soto V, Tapia E, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol 2008; 295: F1134–F1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarkka MR, Vuolle M, Kaukinen S, et al. Effect of allopurinol on myocardial oxygen free radical production in coronary bypass surgery. Scand Cardiovasc J 2000; 34: 593–596 [DOI] [PubMed] [Google Scholar]
- 37.Castelli P, Condemi AM, Brambillasca C, et al. Improvement of cardiac function by allopurinol in patients undergoing cardiac surgery. J Cardiovasc Pharmacol 1995; 25: 119–125 [DOI] [PubMed] [Google Scholar]
- 38.Goicoechea M, Garcia de Vinuesa S, Verdalles U, et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis 2015; 65: 543–549 [DOI] [PubMed] [Google Scholar]
- 39.Ejaz AA, Dass B, Lingegowda V, et al. Effect of uric acid lowering therapy on the prevention of acute kidney injury in cardiovascular surgery. Int Urol Nephrol 2013; 45: 449–458 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.