Abstract
Background
Chronic kidney disease (CKD) knowledge among patients newly referred to a nephrology clinic is limited. This study aimed to determine if CKD knowledge 1 year after initial consultation in a nephrology clinic improves with standard care.
Methods
Patients newly referred to a nephrology outpatient clinic received standard care from nephrologists, and had access to educational pamphlets, relevant internet sites and patient support groups. Those with estimated glomerular filtration rate <20 mL/min/1.73 m2 received individual education from a multi-disciplinary team. Knowledge was assessed by questionnaire at first visit and after 12 months.
Results
Of 210 patients at baseline, follow-up data were available at 12.7 (±1.7) months for 95. Median age was 70 [interquartile range (IQR) 60–76] years and 54% were male. Baseline median creatinine of the follow-up cohort was 137 (IQR 99–179) µmol/L. Eighty per cent had seen a nephrologist at least three times, 8% saw a CKD nurse, 50% reported collecting pamphlets and 16% reported searching the internet. At 12 months, fewer patients reported being uncertain why they had been referred (5 versus 20%, P = 0.002) and fewer reported being unsure of the meaning of CKD (37 versus 57%, P = 0.005). Unknown (44%) and alcohol (23%) remained the most common causes of CKD identified. Fewer patients responded ‘unsure’ regarding the treatment of CKD (38 versus 57%, P = 0.004).
Conclusions
After a year of standard care at nephrology outpatient clinics there were some minor improvements in patient knowledge; however, patient understanding of CKD remained poor.
Keywords: chronic kidney disease, education, kidney, knowledge, survey
Introduction
Health literacy describes an individual's ability to understand health information and engage in the healthcare process, and allows removal of barriers that otherwise prevent patient involvement [1]. Low health literacy among the general population is associated with poorer health outcomes and poorer use of health care services [2].
An estimated 23% of patients with chronic kidney disease (CKD) have limited health literacy [3]. In the Australian general population, awareness of risk factors of CKD among those with CKD was no greater than those without [4]. Our recent study has found poor knowledge and understanding of kidney disease in newly referred patients to a renal outpatient department [5]. Limited health literacy among people with kidney disease has been associated with lower socio-economic status, worse health outcomes [3], increased risk of missed dialysis sessions, hospitalization [6] and mortality among haemodialysis patients [7], and reduced likelihood of referral for transplantation [7].
Educational interventions are integral to chronic disease management. Most interventions in kidney disease have targeted diet and/or fluid management in dialysis patients using a multi-component education programme [8]. When compared with usual care, a 20-year follow-up study of a predialysis psychoeducational intervention showed increased survival of 2.25 years and an additional 8 months before commencement of dialysis [9]. Furthermore, formal patient education through participation in the National Kidney Foundation Kidney Early Evaluation Program (KEEP) was associated with higher pre-end stage kidney disease nephrologist care, peritoneal dialysis, pre-emptive transplant wait listing and transplantation [10].
CKD is very common and there is little published data examining the impact of educational interventions aimed at the early-stage CKD population [8]. Therefore, we assessed knowledge and understanding of kidney disease among patients 1 year after their initial consultation in a nephrology outpatient clinic to assess changes with standard care.
Materials and methods
This study is a longitudinal survey of kidney disease knowledge in adults (age ≥18 years) who were referred to a single nephrology outpatient clinic between 16 August 2010 and 31 October 2011. Selection methods, including inclusion and exclusion criteria, and baseline data have been published elsewhere [5]. In brief, baseline demographic data included age, gender, level of education, occupation and marital status. Other baseline data included referral source, body mass index, comorbidities, serum creatinine, estimated glomerular filtration rate (eGFR) and CKD stage. Prior to the first nephrology outpatient attendance, patients were asked open-ended questions to determine their perceived reason for referral; and understanding, causes, symptoms and treatment of CKD. Twelve months after the initial survey, subjects who were still actively attending the same nephrology clinic were approached to repeat the survey with the same open-ended questions. Written informed consent was obtained from all participants and the study was approved by the Prince Charles Hospital Human Research and Ethics Committee.
Education intervention
Patients received non-standardized explanation of their kidney disease from nephrologists during their outpatient visits as part of routine care. This was provided at the initial visit (typically a 45 min consultation) and then at subsequent visits (15–20 min consultations) as deemed necessary by the nephrologist. Education included some or all of the following: verbal explanation of causes, symptoms and treatment of CKD; verbal explanation of the individual patient's cause for CKD or reason for referral to clinic; provision of written material; direction to internet sites; direction to pamphlets; and referral to allied health staff or a CKD nurse educator as deemed necessary. The information provided by nephrologists was directed at the individual needs of each patient. Non-medical staff were unaware of what information was provided to each patient.
At each visit, patients had access to additional education material in the waiting room including posters and take-home pamphlets. Included within the pamphlets were the contact details of consumer support groups, Kidney Health Australia as well as the Kidney Support Network. Collection of pamphlets was at the discretion of each patient.
Patients with an eGFR <20 mL/min/1.73 m2 were all reviewed by a CKD nurse educator for one-on-one education during a single session followed by further education sessions with social work, dietetics and pharmacy as needed. Follow-up contact with the CKD nurse was arranged after the initial education sessions to clarify any questions.
Data collection at follow-up
Data were only collected for patients who continued to attend the nephrology clinic 12 months after their initial consultation. The survey was conducted prior to a clinic appointment, or if no clinic appointment was scheduled at the 12 month time-point, patients were contacted by telephone. If a patient was unable to be contacted after three telephone calls, they were recorded as un-contactable.
Patients self-reported details of collection of educational pamphlets, searching relevant internet sites and awareness of patient support groups. The numbers of visits to a nephrologist and/or CKD nurse educator during the study period were recorded from hospital databases.
Patients were re-surveyed with the same open-ended questions (Table 1) used in the initial questionnaire with no prompting for each question. The survey was delivered by renal nursing staff that had been trained for this project and had administered the baseline questionnaire. The nursing staff were blinded to the patients' diagnosis, any information provided by each nephrologist during consultations, as well as details of any pamphlets patients had collected or CKD nurse education sessions attended.
Table 1.
Open-ended questions asked at baseline and 12-month follow-up
|
Patients could provide multiple responses to each question, all of which were recorded. Data were summarized and tabulated as recurring themes. For example, responses of ‘not drinking enough water’ and ‘not drinking enough’ to the question ‘What sort of things do you think may lead to a person developing kidney disease?’ were grouped as ‘inadequate fluid intake’. The coding was performed by the investigators. The responses provided by each individual patient were not compared with that person's reason for referral, underlying cause of kidney disease or treatment plans.
Statistical analysis
For the demographic data, descriptive statistics were calculated as median and interquartile range (IQR) for continuous variables, or frequency (%) for categorical variables and analysed with chi squared tests for categorical values and unpaired t-tests for continuous variables.
Answers to open-ended questions could include multiple responses from each participant. Graphs of the most frequent responses are presented, with the percentage of patients using those responses compared between baseline and 12-month data and analysed using a paired t-test. Because alcohol was incorrectly but frequently identified in our baseline results as a cause for CKD, pre-specified analyses about perception of alcohol or diabetes as a cause for CKD were undertaken for those who self-reported collecting pamphlets and those who saw a nephrologist ≥5 times versus <5 times. Furthermore, a pre-specified analysis of those with diabetes as a comorbidity who nominated diabetes as a cause for CKD was undertaken and analysed by paired t-test. All analyses were conducted with Graph Pad Prism version 6 software.
Results
Of 210 patients surveyed at baseline, 125 (59.5%) were still actively under the care of the clinic after 12 months, 77 (36.7%) had been discharged and 8 (3.8%) had died. Of the 125 patients who met the inclusion criteria, 95 were included in the current analysis (representing 45.2% of baseline numbers and 76% of those still under care of the clinic); 19 (15.2%) were unable to be contacted to complete the 12-month survey, 7 (5.6%) declined to participate and 4 (3.2%) completed surveys which were misplaced. Mean (standard deviation) time to follow-up was 12.7 (1.7) months.
Baseline characteristics (month 0) of the original group at first visit to the nephrology clinic (n = 210) and the follow-up cohort (n = 95) are shown in Table 2. The follow-up group was generally similar to the baseline group with a median age of 70 years (IQR 60–76) and 54% age pensioners. The only significant difference between the baseline and follow-up groups was CKD stage, although there was no difference in serum creatinine [115 µmol/L (81–155) versus 137 µmol/L (99–179), P = 0.06]. Only 6% of the follow-up group had an eGFR <20 mL/min/1.73 m2. The median number of attendances with a nephrologist over the 12 months was 4 (IQR 3–6). Fifty-one per cent reported collecting pamphlets, 15.8% searched the internet for information on kidney disease and 8.4% saw a CKD nurse educator. Only 3.2% reported knowledge of Kidney Health Australia and 1.1% of the Kidney Support Network.
Table 2.
Baseline characteristics of initial and 12 month follow-up groups
| Characteristic | Initial survey (n = 210) | 12-month follow-up survey (n = 95) | P-value |
|---|---|---|---|
| Age in years (median, IQR) | 65.5 (52–77) | 70 (60–76) | 0.16 |
| Male gender | 104 (49.5%) | 51 (53.7%) | 0.76 |
| Level of education | 0.82 | ||
| Primary school | 37 (17.6%) | 20 (21.1%) | |
| Secondary school | 111 (52.9%) | 48 (50.5%) | |
| Tertiary education | 50 (23.8%) | 21 (22.1%) | |
| Other | 12 (5.7%) | 6 (6.3%) | |
| Occupation | 0.55 | ||
| Age pension | 94 (44.8%) | 51 (53.7%) | |
| Self-funded retiree | 15 (7.1%) | 8 (8.4%) | |
| Tradesperson | 7 (3.3%) | 2 (2.1%) | |
| Professional | 12 (5.7%) | 3 (3.2%) | |
| Student | 1 (0.5%) | 0 (0%) | |
| Unemployed | 11 (5.2%) | 2 (2.1%) | |
| Invalid/carer pensioner | 20 (9.5%) | 9 (9.5%) | |
| Other | 50 (23.9%) | 20 (21%) | |
| Aboriginal or Torres Strait Islander | 4 (1.9%) | 2 (2.1%) | 0.89 |
| Healthcare worker | 9 (4.3%) | 3 (3.2%) | 0.58 |
| Married/partner | 127 (60%) | 51 (53.7%) | 0.21 |
| Comorbidities | |||
| Diabetes | 66 (31.4%) | 36 (37.9%) | 0.17 |
| Hypertension | 131 (62.4%) | 63 (66.3%) | 0.43 |
| Ischaemic heart disease | 41 (19.5%) | 25 (26.3%) | 0.09 |
| Peripheral vascular disease | 18 (8.6%) | 7 (7.4%) | 0.67 |
| Chronic lung disease | 28 (13.3%) | 17 (17.9%) | 0.20 |
| Cerebrovascular disease | 12 (5.7%) | 5 (5.3%) | 0.85 |
| Smoker (current) | 34 (16.2%) | 17 (17.9%) | 0.65 |
| Smoker (former) | 64 (30.5%) | 29 (30.5%) | 1.0 |
| Body mass index (kg/m2) | 0.87 | ||
| Underweight (<18.5) | 5 (2.5%) | 3 (3.2%) | |
| Normal (18.5–24.9) | 45 (22.5%) | 22 (23.2%) | |
| Overweight (25.0–29.9) | 68 (34%) | 29 (30.5%) | |
| Obese (>30) | 82 (41%) | 38 (41%) | |
| Family history of kidney disease | 37 (17.6%) | 14 (14.7%) | 0.46 |
| CKD stage | 0.04 | ||
| CKD stage 5 (eGFR <15) | 1 (0.5%) | 0 (0%) | |
| CKD stage 4 (eGFR 15–30) | 44 (21%) | 29 (30.5%) | |
| CKD stage 3 (eGFR 30.1–60) | 89 (42.4%) | 44 (46.3%) | |
| CKD stage 1 & 2 (eGFR >60) | 74 (35.2%) | 22 (23.2%) | |
| Creatinine, µmol/L (median, IQR) | 115 (81–155) | 137 (99–179) | 0.06 |
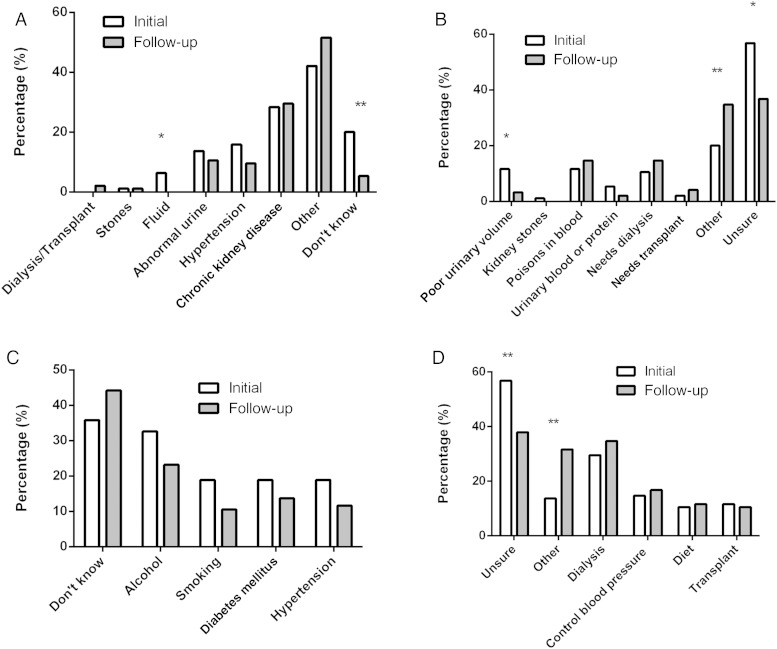
Figure 1 shows responses to the open-ended questions about kidney disease for the cohort of 95 that had both initial and 12-month responses available for analysis. Although there were improvements noted in some areas of knowledge, the results of follow-up data remained disappointing. Figure 1A shows a reduction in people responding ‘unsure’ as to the reason for referral (20% at baseline compared with 5% at follow-up, P = 0.002). The most common response among those who responded ‘other’ reason for referral was because of a recommendation by their general practitioner. Figure 1B shows responses to the question regarding patients' understanding of CKD. Although the percentage responding ‘unsure’ had reduced from 57 to 37% (P = 0.005), it remained the most common response. The next most common response was ‘other’, and among this group the most frequent answers were ‘(slow) death’ or ‘bad news’. The most common perceived causes of CKD listed in the follow-up data were similar to the initial data (Figure 1C). Disappointingly, the most common causes identified remained ‘unknown’ and ‘alcohol’. The follow-up of participants' understanding of CKD management (Figure 1D) showed fewer patients' responding ‘unsure’ at follow-up (57% at baseline, 38% at follow-up, P = 0.004), although it remained the most common response. In general, the most frequent responses for the management of CKD in follow-up data were comparable to initial data.
Fig. 1.
Patient responses to open-ended questions about kidney disease after attending routine clinic care for 12 months. Data presented as initial (n = 95) and follow-up (n = 95) response rates as a percentage. *P < 0.05, **P < 0.01. (A) Patient self-reported explanation of reason for initial referral to the nephrology clinic. (B) Patient self-reported explanation of their understanding of chronic kidney disease. (C) Patient self-reported explanation of causes of chronic kidney disease. (D) Patient self-reported explanation of treatment for chronic kidney disease.
Participants were asked what symptoms they associate with CKD. The vast majority of responses were categorized as ‘other’ which included ‘do not know’. The frequency of symptoms mentioned such as lethargy, reduced urine output or kidney pain was <10%.
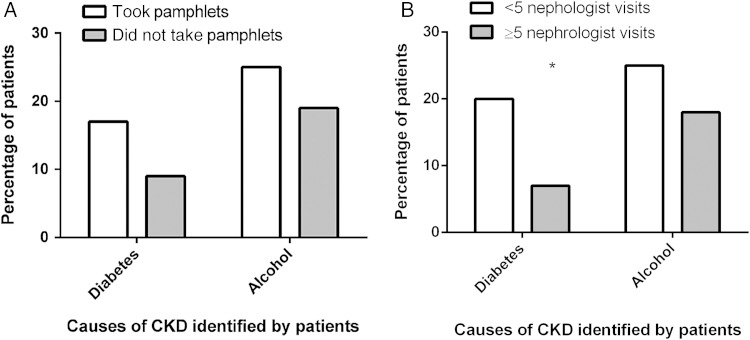
A subgroup analysis showed no difference in the numbers who identified ‘alcohol’ or ‘diabetes’ as causes for CKD among participants who reported collecting education pamphlets (n = 48) compared with those who did not (n = 47) (Figure 2). There was also no difference among those who saw a nephrologist ≥5 times (n = 44) compared with those with <5 visits (n = 51) who identified ‘alcohol’ as a cause, and a worse outcome at follow-up for those identifying diabetes (P = 0.01) (Figure 2). There was no difference in the number of patients with diabetes (n = 36) who nominated diabetes as a cause for CKD at baseline (n = 12) and follow-up (n = 10).
Fig. 2.
Frequency of diabetes and alcohol being identified as causes for CKD at follow-up (n = 95) and association with uptake of pamphlet education or more frequent nephrologist visits. *P = 0.01
Discussion
Despite attending a nephrology outpatient clinic for 12 months with several nephrologist visits and easy access to education materials at the clinic, patient knowledge about kidney disease remained limited with only small changes compared with baseline results.
Although 80% of our respondents visited nephrologists at least three times, their kidney disease knowledge and understanding remained inadequate. These results are supported by a study of patients with CKD stage 3–5, where although perceived knowledge improved with the frequency of nephrology visits, only half of patients who had seen a nephrologist at least four times reported knowledge of haemodialysis, peritoneal dialysis or transplantation [11]. The reasons for inadequate education by nephrologists are uncertain but may include time constraints. Recognizing the inadequate education delivered by nephrologists has led to the development of a physician-delivered education intervention whereby a one-page educational worksheet reviewed with the patient was associated with higher patient kidney disease knowledge [12].
The clinic area had a large display of kidney disease pamphlets with information on diet, medications, disease management and support groups. Although this information was readily available, only 50.5% collected pamphlets to read. Given that the information was free we thought the uptake of this information may have been higher. It is possible that participants under-reported the collection of pamphlets when surveyed at 12 months. However, it seems likely that many did not collect pamphlets at all. In an Australian study on CKD mineral and bone disorder, only 18% of patients received information about phosphate from written material [13].
The information gained by those who did collect pamphlets seems inadequate. We were unable to show a difference in knowledge when comparing participants who had and had not collected information material. Furthermore, participants' awareness of community support groups was poor, suggesting that although pamphlets were available detailing these groups, participants did not read or comprehend the information. The Department of Education has reported that half of the adult population of the USA has difficulty using commonly available print materials to accomplish everyday tasks. More than 1000 studies conducted since the 1960s indicate that health materials for the public and patients are generally written at levels of complexity beyond the reading skills of high-school graduates [14].
People with CKD often suffer multiple medical problems, many of which cause greater morbidity than CKD. It is possible that people prioritize their medical conditions. Diabetic patients with multiple comorbidities ranked diabetes and hypertension among their top three important concerns, but none of the patients reported renal disease when asked of ‘other health concern(s)’. Patients were likely to focus on symptomatic conditions such as pain, depression and breathing problems [15]. Our population, in general, had mild CKD and hence they may not see kidney disease as a major issue.
A multi-disciplinary team (MDT) may be a better model to improve patient knowledge. A randomized controlled trial in patients with progressive CKD has shown that additional educational and social worker interventions improved discussion and active pursuit of living donor kidney transplantation compared with usual care [16]. A systematic review of 22 randomized trials involving multi-component structured educational and psychological care with usual care revealed significant improvement of at least one of the outcomes (diet and/or fluid) in a majority of pre-dialysis and dialysis studies [8]. MDT care has been associated with a lower mean annual decline in eGFR compared with usual nephrology care in CKD stage 3 [17]. In the adult population, MDT care has been shown to be cost effective for patients with CKD stage 3 and 4 mainly due to reduced hospitalizations [18]. Furthermore, MDT care has been shown to reduce costs in the first 6 months after commencement of haemodialysis [19].
Repetitive education may be effective in maintaining knowledge. In early-stage CKD, an educational intervention covering management of CKD increased knowledge at 6 months but it had fallen again by 12 months [20]. Pre-dialysis education increased time to dialysis in Canada, but required a one-on-one interactive educational session, booklet and importantly a phone call every 3 weeks [21]. This was far more intense than provided by standard care in our model. In transplant patients, an intervention consisting of five one-to-one sessions had both short- and long-term (6 months post-transplant) benefits [22].
Education programmes and participation in a voluntary community kidney disease programme were associated with improved outcomes in end-stage kidney disease adults who participated in the National Kidney Foundation Kidney Early Evaluation Program [10]. In the USA, this finding is particularly important for those with CKD stage 4 who are eligible for the Medicare education intervention [23] consisting of up to six education sessions. In Australia, there is no funding for CKD education and for this reason an eGFR cut-off for nurse-provided education in our unit is <20 mL/min/1.73 m2 due to financial constraints.
There are a number of limitations to our study. The survey questions were not validated prior to the study. Following commencement of our study, a kidney-specific knowledge questionnaire has been validated, the Kidney Knowledge Survey [24]. However, this survey has some limitations including only being validated in a predominantly white and educated population, and there is a need to develop CKD stage-specific knowledge surveys [25]. Secondly, due to our limited resources, only those with advanced CKD (8.4%) received more individualized education by a CKD nurse and MDT. Thirdly, our study population does not reflect the multicultural population in other areas in Australia by excluding non-English speakers, and only 2% were indigenous. Lastly, we relied on patient recall for determining collection of pamphlets and searching the internet.
In summary, our study has shown that after a year of attending a single nephrology outpatient clinic, standard care and access to pamphlets are insufficient for improving kidney disease knowledge. A more structured, individualized and repetitive education programme delivered by a multi-disciplinary team may be more effective and hopefully lead to better health outcomes. The cost-effectiveness of this educational intervention remains to be proven.
Conflict of interest statement
The authors have no competing interests. These results have not been published previously in whole or in part, except in abstract form.
Acknowledgements
The authors thank Wishlist, Sunshine Coast Health Foundation for financial support.
References
- 1.Raynor DK. Health literacy. BMJ 2012; 344: e2188. [DOI] [PubMed] [Google Scholar]
- 2.Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an update systematic review. Ann Intern Med 2011; 155: 97–107 [DOI] [PubMed] [Google Scholar]
- 3.Fraser SD, Roderick PJ, Casey M, et al. Prevalence and associations of limited health literacy in chronic kidney disease: a systematic review. Nephrol Dial Transplant 2013; 28: 129–137 [DOI] [PubMed] [Google Scholar]
- 4.White SL, Polkinghorne KR, Cass A, et al. Limited knowledge of kidney disease in a survey of AusDiab study participants. Med J Aust 2008; 188: 204–208 [DOI] [PubMed] [Google Scholar]
- 5.Burke MT, Kapojos J, Sammartino C, et al. Kidney disease health literacy among new patients referred to a nephrology outpatient clinic. Intern Med J 2014; 44: 1080–1086 [DOI] [PubMed] [Google Scholar]
- 6.Green JA, Mor MK, Shields AM, et al. Associations of health literacy with dialysis adherence and health resource utilization in patients receiving maintenance hemodialysis. Am J Kidney Dis 2013; 62: 73–80 [DOI] [PubMed] [Google Scholar]
- 7.Cavanaugh KL, Wingard RL, Hakim RM, et al. Low health literacy associates with increased mortality in ESRD. J Am Soc Nephrol 2010; 21: 1979–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason J, Khunti K, Stone M, et al. Educational interventions in kidney disease care: a systematic review of randomized trials. Am J Kidney Dis 2008; 51: 933–951 [DOI] [PubMed] [Google Scholar]
- 9.Devins GM, Mendelssohn DC, Barré PE, et al. Predialysis psychoeducational intervention extends survival in CKD: a 20-year follow-up. Am J Kidney Dis 2005; 46: 1088–1098 [DOI] [PubMed] [Google Scholar]
- 10.Kurella Tamura M, Li S, Chen SC, et al. Educational programs improve the preparation for dialysis and survival of patients with chronic kidney disease. Kidney Int 2014; 85: 686–692 [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein FO, Story K, Firanek C, et al. Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int 2008; 74: 1178–1184 [DOI] [PubMed] [Google Scholar]
- 12.Wright Nunes J, Greene JH, Wallston K, et al. Pilot study of a physician-delivered education tool to increase patient knowledge about CKD. Am J Kidney Dis 2013; 62: 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toussaint ND, Pedagogos E, Beavis J, et al. Improving CKD-MBD management in haemodialysis patients: barrier analysis for implementing better practice. Nephrol Dial Transplant 2011; 26: 1319–1326 [DOI] [PubMed] [Google Scholar]
- 14.Rudd RE. Improving Americans’ health literacy. N Engl J Med 2010; 363: 2283–2285 [DOI] [PubMed] [Google Scholar]
- 15.Zulman DM, Kerr EA, Hofer TP, et al. Patient-provider concordance in the prioritization of health conditions among hypertensive diabetes patients. J Gen Intern Med 2010; 25: 408–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulware LE, Hill-Briggs F, Kraus ES, et al. Effectiveness of educational and social worker interventions to activate patients’ discussion and pursuit of preemptive living donor kidney transplantation: a randomized controlled trial. Am J Kidney Dis 2013; 61: 476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayliss EA, Bhardwaja B, Ross C, et al. Multidisciplinary team care may slow the rate of decline of renal function. Clin J Am Soc Nephrol 2011; 6: 704–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins RB, Garg AX, Levin A, et al. Cost-effectiveness analysis of a randomized trial comparing care models for chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 1248–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu-Jen Y, I-Wen W, Chun-Yu H, et al. Multidisciplinary predialysis education reduced the inpatient and total medical costs of the first 6 months of dialysis in incident hemodialysis patients. PLoS One 2014; 9: e112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yen M, Huang JJ, Teng HL. Education for patients with chronic kidney disease in Taiwan: a prospective repeated measures study. J Clin Nurs 2008; 17: 2927–2934 [DOI] [PubMed] [Google Scholar]
- 21.Devins GM, Mendelssohn DC, Barré PE, et al. Predialysis psychoeducational intervention and coping styles influence time to dialysis in chronic kidney disease. Am J Kidney Dis 2003; 42: 693–703 [DOI] [PubMed] [Google Scholar]
- 22.Urstad KH, Øyen O, Andersen MH, et al. The effect of an educational intervention for renal recipients: a randomized controlled trial. Clin Transplant 2012; 26: 246–253 [DOI] [PubMed] [Google Scholar]
- 23.Young HN, Chan MR, Yevzlin AS, et al. The rationale, implementation, and effect of the Medicare CKD education benefit. Am J Kidney Dis 2011; 57: 381–386 [DOI] [PubMed] [Google Scholar]
- 24.Wright JA, Wallston KA, Elasy TA, et al. Development and results of a kidney disease knowledge survey given to patients with CKD. Am J Kidney Dis 2011; 57: 387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon EJ, Lash JP. A timely change in CKD delivery: promoting patient education. Am J Kidney Dis 2011; 57: 375–377 [DOI] [PMC free article] [PubMed] [Google Scholar]