Abstract
In many developing countries in the South Asian region, screening for chronic diseases in the community has shown a widely varying prevalence. However, certain geographical regions have shown a high prevalence of chronic kidney disease (CKD) of unknown etiology. This predominantly affects the young and middle-aged population with a lower socioeconomic status. Here, we describe the hotspots of CKD of undiagnosed etiology in South Asian countries including the North, Central and Eastern provinces of Sri Lanka and the coastal region of the state of Andhra Pradesh in India. Screening of these populations has revealed cases of CKD in various stages. Race has also been shown to be a factor, with a much lower prevalence of CKD in whites compared to Asians, which could be related to the known influence of ethnicity on CKD development as well as environmental factors. The difference between developed and developing nations is most stark in the realm of healthcare, which translates into CKD hotspots in many regions of South Asian countries. Additionally, the burden of CKD stage G5 remains unknown due to the lack of registry reports, poor access to healthcare and lack of an organized chronic disease management program. The population receiving various forms of renal replacement therapy has dramatically increased in the last decade due to better access to point of care, despite the disproportionate increase in nephrology manpower. In this article we will discuss the nephrology care provided in various countries in South Asia, including India, Bangladesh, Pakistan, Nepal, Bhutan, Sri Lanka and Afghanistan.
Keywords: CKD, diabetes mellitus, glomerulonephritis, glomerulosclerosis, pediatrics
Introduction
Chronic kidney disease (CKD) is an important cause of increasing global morbidity and mortality that constitutes a major public health priority worldwide. Globally, the number of CKD stage G5 patients receiving renal replacement therapy (RRT) is estimated to be >1.4 million, with an annual growth rate of 8% [1]. The burden is very high in developing countries of South Asia, Eastern Europe and Latin America. Diabetes mellitus, hypertension, lower socioeconomic status, environmental factors and intrauterine growth retardation are among the predisposing factors for CKD in developing countries in South Asia. In many low and middle income countries (LMICs) there is a scarcity of infrastructure and personnel, which limits high-quality screening for early detection and prevention of CKD.
Pediatric CKD: India
In the context of pediatric CKD, India has emerged as a ‘hotspot’ that is probably representative of the burden of CKD that exists in South Asia. The pediatric CKD registry of India [2] (n = 1287) reveals chronic glomerulonephritis (32.6%) and undetermined etiology (10.6%) as major contributors, with nearly 45% of the children presenting with CKD stage G4 or G5. However, according to our observations, fewer than half of the children presenting with end-stage renal disease (ESRD) undertake chronic dialysis. Why Indian children are more susceptible to CKD and how this susceptibility and progression differ from the developed world are critical issues that require a special focus.
Low nephron mass
When exploring the concept of adult diseases that have their roots in childhood, we discovered that South Asian adults are at high risk for premature severe CKD [3, 4] and harbor a greater propensity to third trimester growth restriction, which has an impact on the developing kidney leading to reduced kidney volume [1]. Maternal nutrition, including vitamin A status, could influence nephrogenesis and renal volume in the newborn [5]. With an incidence of 40%, India has the highest rate of low birthweight (LBW) babies in the developing world [6]. LBW is a well-known risk factor for hypertension and CKD in early adulthood [7]. We conducted a prospective longitudinal cohort study (manuscript in progress) on the assessment of renal growth and function during the dynamic phase of body growth and glomerular functional maturation in infancy based on neonatal birthweight and gestational age. We observed that renal growth, which is in accordance with body growth, is suboptimal in LBW and small for gestational age neonates compared with normal and appropriate for gestational age neonates from birth to late infancy (18–24 months). However, we discovered that renal function is comparable between LBW and normal birthweight neonates, and similarly between small and appropriate for gestational age neonates in late infancy. Nevertheless, this significant catch-up in glomerular filtration rate despite lower kidney volumes in infants who are born small does not appear to translate into glomerular hyperfiltration in the form of microalbuminuria during infancy. Therefore, it appears that children from India with LBW or small for gestational age are extremely vulnerable and at risk of developing renal injury with subtle and trivial insults.
Consanguinity and genetics
Consanguineous marriage is a cultural phenomenon that is associated with kidney disease and is prevalent in many Asian countries [8], including rural India. Consanguinity and genetic predisposition add to the risk of congenital anomalies of the kidney and urinary tract (CAKUT), reflux nephropathy and urinary tract obstruction, which are major contributors to CKD. Specific renal diseases known to be prevalent in India, such as polycystic kidney disease and primary hyperoxaluria, which can progress to CKD, share an autosomal recessive inheritance. Focal segmental glomerulosclerosis is a major cause of CKD, and we have shown the variation in the genetic polymorphisms among Indian children [9] with recurrence, post-renal transplant [10] being an additional economic burden.
The AKI-CKD continuum
The incidence of pediatric acute kidney injury (AKI) in hospitalized Indian children [11] is as high as 36%, and the etiological spectrum ranges from tropical systemic infections, sepsis with multiorgan dysfunction and snake bite envenomation to primary renal diseases. The use of alternative forms of medicine poses an additional challenge. Neonates who survive sepsis or asphyxia with neonatal AKI [12], including urinary tract obstruction, are at high risk of developing CKD. Systematic monitoring of the clinical syndrome of AKI-CKD is the most urgent need of the hour. Sensitization and active assessment of possible sequelae of AKI, as has been proposed by the ASSESS-AKI study [13], must be implemented in neonates and children in our country.
Besides the three major facets discussed above, many other challenges contribute to the limitations in CKD care: late detection of renal disease, late referral to tertiary centers, limited access to healthcare facilities, suboptimal validation of laboratory performance across the country, delayed interventions, limited opportunities for focused training, lack of active national registries and governmental or insurance support. To conclude, as long as we fail to protect our children by detecting the roots of CKD in childhood, we in India, as well those in many other South Asian regions, will remain hotspots of the dreadful health burden of CKD.
CKD in two coastal districts of Andhra Pradesh, India
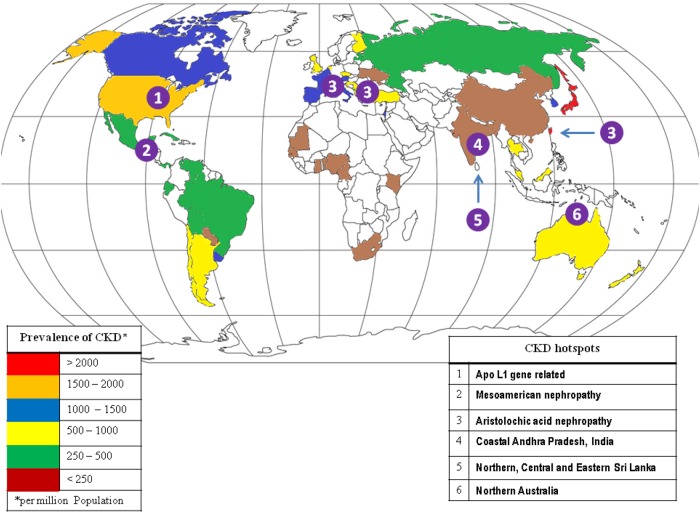
In the coastal regions of the Srikakulam district and Chimakurthy mandal (30–40 km from the coast) in the Prakasham district of Andhra Pradesh, India (known as the Uddanam area), 60% of the local population has been found to have CKD. Nearly 4000 villagers have died of CKD in the last decade, and almost a third of the population in Uddanam suffers from CKD. Of the 1500 people from 13 villages in the Prakasham district evaluated, 27% had serum creatinine levels >1.5 mg/dL. Medical experts and the local population have speculated that contaminated drinking water may hold the clue to the etiology. As groundwater is the only source of drinking water for these two regions, major ions and trace elements were measured in the water from different sources to identify the causative element(s), if any. Comparison of the hydrochemical data indicated that the groundwater in the Srikakulam coastal region is less mineralized than in the Prakasham region, which may be due to geological, hydrological and climatic factors. However, the concentrations of various inorganic chemicals are within permissible limits, and these inorganic chemicals are unlikely to be the cause of high the incidence of CKD in this region. With continuing suspicion that kidney damage could be due to contaminants in the drinking water, it becomes evident that further investigation of other organic and inorganic chemicals associated with kidney damage is needed (Figure 1) [13].
Fig. 1.
Global CKD hotspots and prevalence.
Nepal
Nepal is a country with a population of 28 million and currently 30 nephrologists. Until 1980, there were no facilities for renal replacement therapy in Nepal. In the early 1980s, intermittent peritoneal dialysis and renal biopsies were initiated by the late Dr. Puskar Raj Satyal at Bir Hospital, Kathmandu. In 1986, with the help of the government of India, the first hemodialysis (HD) unit was established in the same hospital with two functioning HD machines. In 1996, a second dialysis unit was established in Tribhuvan University Teaching Hospital, subsequently followed by a few other private hospitals and dialysis centers within Kathmandu Valley and outside the capital.
Dr. Rishi Kafle has built up a huge dialysis unit, which started on a small scale in 1997, that provides free or subsidized dialysis to a large section of the Nepali CKD population with the support of the Nepal Kidney Foundation. Dr. Sanjib Sarma's outstanding work on early detection and prevention of kidney disease in Dharan in eastern Nepal was recognized by the International Society of Nephrology.
The first successful renal transplantation was performed at Tribhuvan University Teaching Hospital in August 2008 by the transplant surgeon Dr. David Francis, supported by the Nepalese surgeon and nephrologist Dr. Dibya Singh Shah. To date, >270 renal transplantations, have been performed. Two more centers also perform renal transplantation, with a total of ∼ 400 transplantations having been performed in the country to date.
Continuous ambulatory peritoneal dialysis (CAPD) service has now also been initiated, and ∼100 patients are on CAPD. Approximately 1500 patients are on HD [14], in 41 HD centers with 252 HD machines. The government of Nepal now provides up to $1890 remuneration to renal transplant patients towards transplant surgery and immunosuppressive drugs and up to $2460 for HD and CAPD patients [15].
Still, the current nephrology services are far from sufficient and must be expanded throughout the country. So far, only the largest cities have the facilities for optimum nephrology care. Nephrology service needs to be made much more affordable and accessible to all. As our program offers only living related donor renal transplantation, it is now the right time for the introduction of a deceased donor transplantation program in Nepal. Finally, awareness, early detection and timely management of kidney disease would help to decrease the burden of this disease in Nepal.
On 25 April 2015, a catastrophic earthquake of 7.8 magnitude rocked Nepal, killing 8800 and injuring >22 000 people. The epicenter was ∼80 km northwest of the capital, Kathmandu. The quake occurred on a Saturday when most of the dialysis centers were closed, and only a few of the 16 service providers in Kathmandu were able to open the next day. It took ∼40 h to resume operations at the National Kidney Center (NKC) due to damage to the water treatment plant. By then, there was a significant backlog of patients requiring dialysis, and partial sessions were provided in an attempt to accommodate as many patients as possible. The service gradually increased, and normal operations resumed ∼2 weeks later. The powerful aftershock measuring 7.3 in magnitude, which occurred on 12 May 2015, was a major setback, invoking safety and security concerns and also forcing one service provider to close its doors due to extensive structural damage. Surprisingly, in spite of the magnitude of the catastrophe, there were only a few cases of acute kidney injury from trauma and crush injuries. It is estimated that ∼100 new patients were treated, with 20 succumbing to their injuries and the rest recovering after receiving dialysis and supportive care.
Fresenius Medical Care responded to the NKC's request for help to cope with damage inflicted by the earthquake, along with support from the international nephrology community, providing donated dialysis machines along with portable reverse osmosis systems and consumables, which were distributed to the various centers providing care after the earthquake (https://commons.wikimedia.org/wiki/File%3ANepal_relief_location_map.jpg).
Sri Lanka
Diabetes and hypertension is increasing in Sri Lanka, as predicted by the World Health Organization (WHO) as a part of the South Asian epidemic. These patients are finally being treated in dialysis and transplantation centers in the government and private sector (see Table 1). The bigger issue is that of chronic kidney disease of unknown origin (CKDu), as is detailed below. The exact prevalence of CKDu remains unknown.
Table 1.
Transplantation centers in the government and private sector, 2015
| Government sector | Private sector | |
|---|---|---|
| Hemodialysis | ||
| Centers | 25 | 11 |
| HD machines | 186 | 65 |
| Transplantation | ||
| Centers | 8 | 5 |
| Kidney transplant teams | 12 | 15 |
Sri Lanka, an island nation with a population of 21 million inhabitants, has seen a decade-long epidemic of CKDu in two of its nine provinces (North Central Province and North West Province), with some spillover into two other provinces (Uva and Eastern Province).
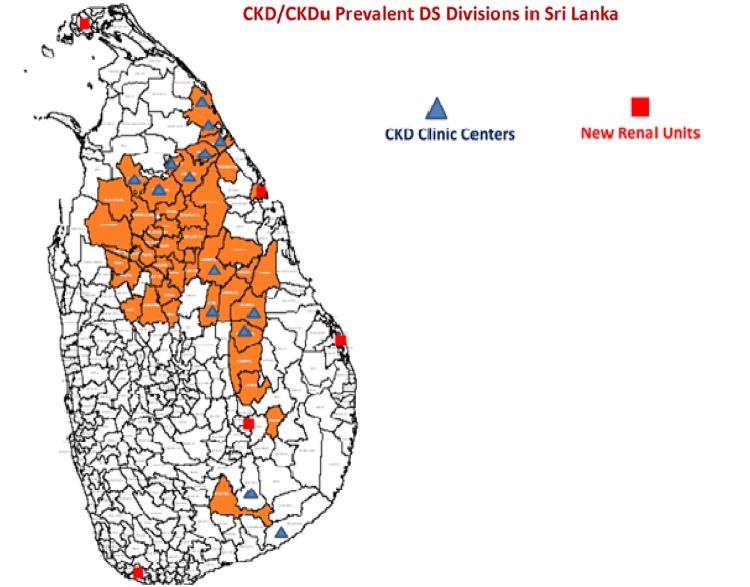
In the nonaffected areas, CKD due to diabetes, hypertension, stone disease, etc. shows a prevalence of 0.4/1000 population, while the average in the affected provinces is 1.8/1000 population. CKD is uniformly distributed in all provinces while CKDu is restricted to a few provinces as illustrated in Figure 2.
Fig. 2.
CKD/CKDu prevalence in Sri Lanka.
Of the 21 million population, nearly 1.7 million people live in the affected areas and 69 258 patients are currently attending clinics (CKD G1–G5). Studies show the following distribution: CKD G3 (31.8%), CKD G4 (40.0%) and CKD G5 (24.5%).
Contaminated surface water seems to be an important factor, as prevalence of CKDu is low (1.5%) where water is obtained from deep wells, natural springs and pipe-borne town water and the prevalence is high (7.7%) in those areas using shallow dug wells, stream water and water tanks.
Research has identified heavy metals (Cd, As, Pb), pesticide residues, glyphosate from herbicides, hardness of water, fluoride in the water, superphosphates, arsenic-contaminated fertilizers and cyanogens from algae as possible etiological factors. There is much controversy in the scientific forum as to the importance of each of the above contaminants. Organic farming with lower yields has been encouraged, for which the government offers a higher guaranteed price per kilogram. Further research is needed to elucidate the exact etiology (Table 2).
Table 2.
CKDu etiology
| Province | District | DS division | Population | Estimated CKDu patients |
|---|---|---|---|---|
| NCP | Anuradhapura | All divisions | 856 232 | 34 249 |
| Polonnaruwa | All divisions | 403 335 | 16 133 | |
| Uva | Badulla | Giranduru Kotte | 41 811 | 1672 |
| Moneragala | Wellawaya, Ridimaliyadda | Under investigation | ||
| EP | Ampara | Dehiatta Kandiya | 59 628 | 2385 |
| Trincomalee | Padavi Sripura, Gomarankadawala | 58 499 | 2339 | |
| NWP | Kurunegala | Polpithigama, Galgamuwa, Mahawa, Giribawa, Nikawaratiya | 258 537 | 10 341 |
| Central | Matale | Wilgamuwa | 29 550 | 1182 |
| NP | Mulathiv | Weli oya | 6949 | 277 |
| Vaunya | Vaunya South | 17 000 | 680 | |
| SP | Hambathota | Thissamaharamaya Angunakola Palassa | Under investigation | |
| Total | 1 731 451 | 69 258 | ||
Presently, much attention is being paid to the supply of clean water for drinking and cooking. Farmers reluctantly take a few gallons of water to work, but as they work under the hot sun, excessive perspiration leads to dehydration. Fortunately, attention is now being focused on these factors. A kidney made vulnerable due to chronic toxic exposure can be adversely affected by recurrent dehydration. To add insult to injury, there is the intermittent consumption of plain tea with large amounts of sugar added for energy, which may also contribute to the loss of renal function. Dehydration in combination with sucrose as causative factors of AKI on top of prior CKD due to contaminated water has been extensively studied, and currently this theory seems to be favored in South America.
Geographic information systems mapping by the WHO has provided much information on the source of water-related CKDu.
There are several different relief plans to aid in increasing the clean water supply:
Town water supplies and bowser supplies
Reverse osmosis water in schools and temples
Rainwater harvesting in homes
CKD clinics and dialysis centers have been set up in high prevalence areas, and there is now training and human resources for dialysis and transplantation.
A doctorate study from Rajarata University in the North West Province by Channa Jayasumana has presented a well-researched theory that a complex lattice of heavy metals such as arsenic in amounts greater than cadmium, hard water, fluoride, glyphosate and pesticide residues is absorbed and arsenic is released into the acid-producing tubular segments of the nephron, leading to chronic interstitial nephritis. This cycle of repeated AKI due to contaminants, dehydration and sucrose (or fructose) increases the damage. This theory, although attractive, has yet to be proven by bench research, and many academic chemists do not endorse this view. However, this research has led to a glyphosate ban in the area of rice production in particular.
CKDu is a major economic issue for the government, as it affects, for the most part, those who are poor. Clinics and dialysis and transplantation centers are being set up that the country can ill afford. Providing pure water supplies to a large population is a costly exercise. The present president has secured foreign aid from China to set up a 500-bed renal hospital to aid those affected in the North Central Province of the country. Prevention is better than the cure, but more research into the exact etiology will help lead us forward down the correct path.
Bangladesh
In Bangladesh, the causes of CKD G5 among 954 patients who were on HD in 2012–13 were chronic glomerulonephritis [251 (25.5%)], diabetic nephropathy [403 (41%)] and hypertensive renal disease, [324 (33%)]. In 1998, glomerulonephritis (40%), diabetic nephropathy (31%), hypertension (15%), obstructive uropathy (8%) and undetermined (10%) were among the causes of CKD G5. In 1994, 24% of the patients presented with diabetic nephropathy, in 1998, 31% (an increase of 7%) and in 2013, 41% (a further increase of 10% compared with 1998). The causes of primary glomerulonephritis could not be determined because the patients presented with CKD G5 with proteinuria and bilateral contracted kidneys. However, the causes of glomerulonephritis studied from 1990 to 2004 were (n = 1238) minimal change disease (10%), mesangial proliferative glomerulonephritis (32%), membranous nephropathy (21%), membranoproliferative glomerulonephritis (17%), IgA nephropathy (10%), focal segmental glomerulosclerosis (11%), lupus nephritis (12%) and postinfectious glomerulonephritis (3.4%) [16].
Manpower in nephrology
There are 120 nephrologists working in various hospitals in Bangladesh. Twelve professors, 18 associate professors and 21 assistant professors are working in various public and private institutes across the country. A total of 28 doctors are undergoing resident training in four public institutes for postdoctorate degrees in nephrology as of 2013. The Bangladesh College of Physicians and Surgeons also awards postdoctorate degrees in nephrology, as do four of the institutes.
RRT
Approximately 150–200 patients per million population (PMP) reach ESRD each year in Bangladesh (hospital-based survey). The total population in 2000 was 132 million, 143 million in 2005 and 151 million in 2010 [17]. A number of surveys were carried out in urban (n = 1200), rural (n = 2000) and disadvantaged populations (n = 1000) in 2005–6. The data showed that 17–18 million of the adult population suffered from CKD G1–5; of these, 11–12% were CKD G3–G5 [18–20].
Renal disease care, including dialysis, is provided by 11 of 21 government-run medical college hospitals, one nephrology institute and one medical university. A transplant facility is available only in the postgraduate medical university. On the other hand, 86 private hospitals are providing care for renal patients with dialysis and 8 centers are performing kidney transplantation.
There are 95 HD centers in Bangladesh; of these, 52 centers are in the capital city Dhaka, population 15.391 million. In Chittagong, with a population of 5.2 million, there are 16 centers, in Sylhet there are 10 centers (population 0.98 million) Khulna 5 centers (population 1.78 million) and Rajshahi 11 centers (population 0.932 million). The age distribution is 0–18 years (32%), 15–54 years (56.8%) and >55 years (11%). The health expenditure is 3.7% of the gross domestic product (GDP) (2011 estimate): The physicians per population ratio is 0.36 physicians per 1000 population and the hospital bed ratio is 0.6 beds per 1000 population [21].
On 31 December 2013, the number of prevalent patients on RRT, including all modalities of RRT, was 18 900 (119 PMP). Of these, 17 458 (113 PMP) were on hospital HD, 43% on 8 h of dialysis per week and 55% on 12 h of dialysis per week. The mean age of the patients on RRT was 46 years (range 12–76 years). The mean age of patients on HD was 41 years, CAPD 52 years and transplant 34 years.
Bhutan
Bhutan currently has a population of 708 241, with a per capita GDP of $2068.37 in 2014. For a small country with a large population, Bhutan is witnessing a rampant rise in the number of patients with CKD. Today, there are 140 maintenance HD patients in Bhutan. Chronic kidney diseases have become the greatest health burden and challenge in Bhutan, accounting for 62% of the total death due to illness. This has led to increased costs in healthcare, as Bhutan must refer transplant treatment abroad.
Additionally, the prevalence of non-communicable diseases (NCDs) such as hypertension and diabetes (the top two leading factors causing CKD in Bhutan) poses an even greater threat. Records show that the number of patients with diabetes has increased from 2541 in 2008 to 4097 in 2012, while the incidence of hypertension has increased from 20 347 in 2008 to 27 023 in 2012.
This year, hospitals across the country have experienced a dramatic increase in CKD, with ∼12 patients diagnosed each month until June 2015. Today, there are 140 CKD patients undergoing dialysis in three different hospitals. Jigme Dorji Wangchuck National Referral Hospital, in the capital city of Thimphu, has eight HD machines, Mongar Regional Referral Hospital in Eastern Bhutan has three and Gelephu Regional Referral Hospital in Southern Bhutan has two.
Government intervention and support
The government has maintained a system of completely free healthcare, not only for Bhutanese citizens but also for all those who reside in the country. The Ministry of Health is the central government institution responsible for ensuring a healthy and happy nation through a dynamic professional healthcare system. For every transplant, the Health Ministry spends ∼$12 100–$13 600 per patient, including donor expenditures. The government also spends $380 per patient as an additional expenditure.
Afghanistan
Currently the population of the Islamic Republic of Afghanistan is estimated to be 33 million. Although Afghanistan is a democracy, it is constantly affected by strife and conflict that affects the provision of renal healthcare in different parts of the country. Afghanistan has 10 nephrologists and 200 HD machines, which are concentrated in Kabul, Jalalbad and Mazar-e Sharif.
CKD is widely prevalent in Afghanistan. Patients travel to Peshawar in Northwest Pakistan and some to New Delhi, India for advanced renal care such as CAPD and transplantation.
Pakistan
Pakistan is a country that does not spend more than 0.6% of its gross national product (GNP) on healthcare and only 1.9% on education. Education is essential for proper healthcare as well, to afford a better understanding of the appropriate measures necessary for disease prevention and treatment, especially in regard to the need for prolonged treatment, as in CKD. In the province of Sindh, with a population of 9800 inhabitants, 22 districts were screened, and the resulting etiology is as follows: diabetes 715, hypertension 1911, stone diseases 343, congenital diseases 337, unknown causes 305 (Table 3).
Table 3.
Causes of ESRD
| Diagnosis available | No. of patients | % |
|---|---|---|
| Diabetic nephropathy | 2719 | 37.45 |
| Hypertensive renal failure | 2876 | 39.61 |
| Chronic glomerulonephritis | 658 | 9.06 |
| Calculus disease | 287 | 3.95 |
| Autosomal dominant polycystic kidney disease | 373 | 5.14 |
| Other/unknown | 347 | 4.78 |
In Pakistan, disease awareness and education are communicated to the community through lectures to the general public. Preventive measures are emphasized, such as cessation of smoking, adherence to a low salt diet and weight reduction through dieting. According to the 2013 Renal Registry of Pakistan, there were 1159 HD machines and 7260 dialysis patients, of which 4841 had dialysis twice a week, 1537 three times a week and 882 at irregular intervals.
Transplantation can be divided into ethical and nonethical transplants. Nonethical transplants involve the transplantation of kidneys from unrelated ‘voluntary’ kidney donors, often for monetary compensation. This is unregulated and illegal. Nearly 4000 ethical transplantations have been performed from live related donors with full government and community support. These transplantations are performed free of cost. Dialysis does receive 20% government support.
In conclusion, in the South Asian developing countries of India, Bangladesh, Pakistan, Nepal, Bhutan, Sri Lanka and Afghanistan, CKD prevalence is increasing. There is also a strikingly high prevalence of CKD of unknown etiology in some geographical regions (CKD hotspots). This has an important socioeconomic impact. The difference in the availability of healthcare resources from the developed nations is patently obvious. The burden of CKD G5 remains unknown in this region. However, in recent times, improvement in access to point of care and the introduction of partial governmental support has led to a dramatic increase in the number of patients receiving RRT.
Conflict of interest statement
None declared.
References
- 1.Roderick PJ, Jeffrey RF, Yuen HM, et al. Smaller kidney size at birth in South Asians: findings from the Born in Bradford birth cohort study. Nephrol Dial Transplant 2015; doi:10.1093/ndt/gfv274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2nd Annual Report CKD Registry of India. Indian Society of Nephrology., 2007. www.ckdri.org (May 2014, date last accessed)
- 3.Chandie Shaw PK, Vandenbroucke JP, Tjandra YI, et al. Increased end-stage diabetic nephropathy in Indo-Asian immigrants living in the Netherlands. Diabetologia 2002; 45: 337–341 [DOI] [PubMed] [Google Scholar]
- 4.Ball S, Lloyd J, Cairns T, et al. Why is there so much end stage renal failure of undetermined cause in the Indo-Asian population? QJM 2001; 94: 187–193 [DOI] [PubMed] [Google Scholar]
- 5.Goodyer P, Kurpad A, Rekha S, et al. Effects of maternal vitamin A status on kidney development: a pilot study. Pediatr Nephrol 2007; 22: 209–214 [DOI] [PubMed] [Google Scholar]
- 6.UNICEF. Progress for children. A report card on nutrition New York, NY: UNICEF, 2006, pp. 10–11 [Google Scholar]
- 7.White SL, Perkovic V, Cass A, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 2009; 54: 248–261 [DOI] [PubMed] [Google Scholar]
- 8.Barbari A, Stephan A, Masri M, et al. Consanguinity-associated kidney diseases in Lebanon: an epidemiological study. Mol Immunol 2003; 39: 1109–1114 [DOI] [PubMed] [Google Scholar]
- 9.Vasudevan A, Siji A, Raghavendra A, et al. NPHS2 mutations in Indian children with sporadic early steroid resistant nephrotic syndrome. Indian Pediatr 2012; 49: 231–233 [DOI] [PubMed] [Google Scholar]
- 10.Vasudevan A, Iyengar A, Phadke K. Outcome of renal transplantation in Indian children with primary FSGS-a single centre experience. Nephrol Rev 2010; 2: e6 [Google Scholar]
- 11.Mehta P, Sinha A, Sami A, et al. Incidence of acute kidney injury in hospitalized children. Indian Pediatr 2012; 49: 537–542 [DOI] [PubMed] [Google Scholar]
- 12.Subramanian S, Agarwal R, Deorari AK, et al. Acute renal failure in neonates. Indian J Pediatr 2008; 75: 385–391. [DOI] [PubMed] [Google Scholar]
- 13.Reddy DV, Gunasekar A. Chronic kidney disease in two coastal districts of Andhra Pradesh, India: role of drinking water. Environ Geochem Health 2013; 35: 439–454 [DOI] [PubMed] [Google Scholar]
- 14.Abraham G. The challenges of renal replacement therapy in Asia. Nat Clin Pract Nephrol 2008; 4: 643. [DOI] [PubMed] [Google Scholar]
- 15.Reddy YNV, Abraham G, Reddy Y, et al. Evolution of deceased-donor transplantation in India with decline of commercial transplantation: a lesson for developing countries. Kidney Int Suppl 2013; 3: 190–194 [Google Scholar]
- 16.Khanam A, Alam MR, Islam S, et al. Histological pattern of glomerulonephritis in a teaching hospital. Bangladesh Renal J 2004; 23: 14–18 [Google Scholar]
- 17.National Institute of Population Research and Training (NIPORT), Mitra and Associates, and ICF International, 2015. Bangladesh Demographic and Health Survey 2014: Key Indicators. Dhaka, Bangladesh, and Rockville, Maryland, USA: NIPORT, Mitra and Associates, and ICF International
- 18.Alam KS, Huda MN, Rashid HU, et al. Prevalence of diabetes mellitus, hypertension, proteinuria and association of these risk factors with estimated glomerular filtration rate (eGFR) in adult disadvantaged population. Bangladesh Renal J 2010; 29: 1–6 [Google Scholar]
- 19.Faroque MO, Rashid HU, Rahman MH, et al. Prevalence of diabetes mellitus, hypertension and proteinuria in a rural area of Bangladesh. Bangladesh Renal J 2010; 29: 7–11 [Google Scholar]
- 20.Hadiuzzaman KBM, Rahman MH, Alam MR, et al. Prevalence of diabetes mellitus and hypertension in health service providers. Bangladesh Renal J 2010; 29: 12–15 [Google Scholar]
- 21.Roy S. Determinants of healthcare expenditure on human capital and economic growth in Bangladesh: a longitudinal data analysis from 1995–2010. Asian J Pharm Res Health Care 2014; 6: 6–10 [Google Scholar]