Abstract
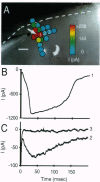
An approach for high-spatial-resolution mapping of functional circuitry in living mammalian brain slices has been developed. The locations of neurons making functional synaptic connections to a single neuron are revealed by photostimulation of highly restricted areas of the slice (50-100 microns in diameter) while maintaining a whole-cell recording of the neuron of interest. Photostimulation is achieved by bathing brain slices in a molecularly caged form of the neurotransmitter glutamate [L-glutamic acid alpha-(4,5-dimethoxy-2-nitrobenzyl) ester], which is then converted to the active form by brief pulses (< 1 ms in duration) of ultraviolet irradiation. Direct activation of receptors on recorded neurons in rat hippocampus and ferret visual cortex demonstrates that photostimulation is reliable and reproducible and can be repeated at the same site at least 30 times without obvious decrement in neuronal responsiveness. Photostimulation of presynaptic neurons at sites distant to the recorded neuron evoked synaptic responses in hippocampal and cortical cells at distances of up to several millimeters from the recorded neuron. Stimulation of 25-100 distinct presynaptic sites while recording from a single postsynaptic neuron was easily achieved. Caged glutamate-based photostimulation eliminates artifacts and limitations inherent in conventional stimulation methods, including stimulation of axons of passage, desensitization, and poor temporal resolution of "puffer" pipettes, and current artifacts of iontophoretic application. This approach allows detailed physiological investigation and manipulation of the complex intrinsic circuitry of the mammalian brain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Farber I. C., Grinvald A. Identification of presynaptic neurons by laser photostimulation. Science. 1983 Dec 2;222(4627):1025–1027. doi: 10.1126/science.6648515. [DOI] [PubMed] [Google Scholar]
- Friauf E., Shatz C. J. Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. J Neurophysiol. 1991 Dec;66(6):2059–2071. doi: 10.1152/jn.1991.66.6.2059. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Clustered intrinsic connections in cat visual cortex. J Neurosci. 1983 May;3(5):1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Gurney A. M., Lester H. A. Light-flash physiology with synthetic photosensitive compounds. Physiol Rev. 1987 Apr;67(2):583–617. doi: 10.1152/physrev.1987.67.2.583. [DOI] [PubMed] [Google Scholar]
- Görne-Tschelnokow U., Hucho F., Naumann D., Barth A., Mäntele W. Fourier transform infrared (FTIR) spectroscopic investigation of the nicotinic acetylcholine receptor (nAChR). Investigation of agonist binding and receptor conformational changes by flash-induced release of 'caged' carbamoylcholine. FEBS Lett. 1992 Sep 7;309(2):213–217. doi: 10.1016/0014-5793(92)81097-6. [DOI] [PubMed] [Google Scholar]
- Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol. 1979;13(3):277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Somlyo A. P. Flash photolysis of caged compounds: new tools for cellular physiology. Trends Neurosci. 1989 Feb;12(2):54–59. doi: 10.1016/0166-2236(89)90136-7. [DOI] [PubMed] [Google Scholar]
- Katz L. C. Local circuitry of identified projection neurons in cat visual cortex brain slices. J Neurosci. 1987 Apr;7(4):1223–1249. doi: 10.1523/JNEUROSCI.07-04-01223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Douglas R., Wehmeier U. Visibility of synaptically induced conductance changes: theory and simulations of anatomically characterized cortical pyramidal cells. J Neurosci. 1990 Jun;10(6):1728–1744. doi: 10.1523/JNEUROSCI.10-06-01728.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Tov A., Miller J. P., Burke R. E., Rall W. Factors that control amplitude of EPSPs in dendritic neurons. J Neurophysiol. 1983 Aug;50(2):399–412. doi: 10.1152/jn.1983.50.2.399. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Whitteridge D. Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol. 1984 Aug;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray J. A., Trentham D. R. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- Milburn T., Matsubara N., Billington A. P., Udgaonkar J. B., Walker J. W., Carpenter B. K., Webb W. W., Marque J., Denk W., McCray J. A. Synthesis, photochemistry, and biological activity of a caged photolabile acetylcholine receptor ligand. Biochemistry. 1989 Jan 10;28(1):49–55. doi: 10.1021/bi00427a008. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U., Singer W. Prominent excitatory pathways in the cat visual cortex (A 17 and A 18): a current source density analysis of electrically evoked potentials. Exp Brain Res. 1978 Nov 15;33(3-4):371–394. doi: 10.1007/BF00235560. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M., Brayton R. K., Miller J. P., Segev I., Rinzel J., Rall W. Signal enhancement in distal cortical dendrites by means of interactions between active dendritic spines. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2192–2195. doi: 10.1073/pnas.82.7.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Thio L. L., Zorumski C. F., Fischbach G. D. Rapid desensitization of glutamate receptors in vertebrate central neurons. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4562–4566. doi: 10.1073/pnas.85.12.4562-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C. F., Thio L. L. Properties of vertebrate glutamate receptors: calcium mobilization and desensitization. Prog Neurobiol. 1992 Sep;39(3):295–336. doi: 10.1016/0301-0082(92)90020-f. [DOI] [PubMed] [Google Scholar]