Abstract
Loin pain hematuria syndrome (LPHS), first described in 1967, is a rare pain syndrome, which is not well understood. The syndrome is characterized by severe intermittent or persistent flank pain, either unilateral or bilateral, associated with gross or microscopic hematuria. LPHS is a diagnosis of exclusion as there still is not a consensus of validated diagnostic criteria, though several criteria have been proposed. The wide differential diagnosis would suggest a meticulous yet specific diagnostic work-up depending on the individual clinical features and natural history. Several mechanisms regarding the pathophysiology of LPHS have been proposed but without pinpointing the actual causative etiology, the treatment remains symptomatic. Treatment modalities for LPHS are diverse including simple analgesia, opioid analgesic and kidney autotransplantation. This review article summarizes the current understanding regarding the pathophysiology of LPHS along with the steps required for proper diagnosis and a discussion of the different therapeutic approaches for LPHS.
Keywords: autotransplant, flank pain, hematuria, loin pain hematuria syndrome
Introduction
Loin pain hematuria syndrome (LPHS) was first described in 1967 by Little et al. [1]; the disorder is not well understood and is one in which patients experience severe unexplained chronic unilateral or bilateral flank pain, which is associated with gross or microscopic hematuria [1]. The absence of a primary kidney pathology is an important feature in LPHS, and the absence of consensus on the underlying etiology is reflected by different therapeutic options available to date for this condition with variable success rates.
Clinical features and natural history
The majority of patients affected are young females, in some series up to 70% [2], often white, with the majority showing symptoms by the third decade of life (ranging from the first to sixth decades) [3]. The loin pain varies in duration and frequency; the duration can range from minutes to a constant ache and the frequency can vary from once or twice a year to incessant [4–6]. The pain has also been described to radiate, in some cases, to the iliac fossa, anterior thigh or groin [7–10].
The natural history of the syndrome is not well described, but spontaneous resolution has been suggested. LPHS is not known to cause secondary kidney injury or increase mortality. In a series of 51 patients, LPHS resolved spontaneously in half the patients after several years (mean 3–5); the rest of the patient continued to have pain with the longest suffering for 17 years [6].
Epidemiology
Few data exist regarding the epidemiology of LPHS, which is an extremely rare disease with a prevalence of about 0.012%. LPHS is more common in women with as many as 70% of patients with LPHS being females [11].
Pathophysiology
Since Little et al. first described the condition in 1967 [1], several hypotheses have been proposed regarding the pathophysiology of LPHS. These include vascular disease of the kidney, complement activation on arterioles [12, 13], coagulopathy [14], venocalyceal fistula [15], abnormal ureteral peristalsis [16], hypersensitivity [12], psychopathology [17, 18], intratubular deposition of calcium [2, 15, 19, 20] and nephritis [16, 17]. Of these, nephritis (usually IgA) is the only one documented [21, 22].
When no acquired underlying glomerular disease is responsible for the LPHS, it is designated primary LPHS, as opposed to secondary LPHS when due to an underlying glomerular process, usually IgA nephropathy. The distinction can only be made by kidney biopsy [2].
Some studies have suggested that thin glomerular basement membrane (GBM) disease could be associated with or contribute to LPHS. In a study conducted by Herbert et al. seven patients were described with thin GBM, which was linked with LPHS [2, 23]. Renal biopsies on those patients showed red cells in the renal tubules, which indicated glomerular hematuria, the only glomerular abnormality present. These red cells were occluding the tubules, leading Herbert et al. to suggest that occlusion of a fraction of renal tubules could be behind the cause of the loin pain if back-leak of glomerular filtrate occurred resulting in expansion of the renal parenchymal volume and stretching of the renal capsule [23]. Thus, they concluded that thin GBM could be a cause of the flank pain and gross hematuria in some patients with LPHS [2, 23].
Another possible mechanism for LPHS is microcrystal formation in the renal tubules. Praga et al. conducted a study that noted high prevalence of hyperuricosuria, hypercalciuria and nephrolithiasis in patients with thin GBM neuropathy [19]. They suggested that the concomitant occurrence of factors that promote urolithiasis and glomerular hematuria may result in the formation of intratubular microcrystals. Combined with intratubular red blood cells, microcrystal formation could lead to increased intratubular obstruction and amplified flank pain [19]. It has since been found that most non-calcium oxalate stone formers make stones by tubular crystallization and do not have LPHS; although most stone formers do not have LPHS, many patients with LPHS have a history of kidney stones (up to 50%) [2, 20].
No causal relationship has been established between complement activation on arterioles and LPHS but several case reports have described an association. Miller et al. [12] described a case where the kidney biopsy showed microaneurysmal and glomeruloid angiomatous changes. The depositions of properdin, C5b-9 complex and C3 in arterioles would suggest complement activation. Both Naish et al. [24] and Miller et al. [12] described an association between C3 deposition and LPHS.
With regard to coagulopathy, even though platelet counts, prothrombin times, thrombin times and fibrinogen levels are usually all normal, several studies have shown more occult abnormalities. Abnormalities included below-normal heparin-thrombin clotting times [25], low factor XII levels [26], elevated plasma b-thromboglobulin levels with increased platelet aggregation by serotonin and adenosine diphosphate and higher than normal plasma C-reactive protein and D-dimer levels [27]. In another study, Siegler et al. hypothesized that abnormal platelet activation and fibrin deposition played a role in the pathogenesis of LPHS [14].
Although renal vascular disease was thought to be one of the underlying pathophysiological processes in LPHS early on [28–30], more recent renal arteriography studies of patients with LPHS disagree with this hypothesis as the renal vasculature more commonly appears normal [4, 27, 31, 32].
It is important to note that LPHS still remains a diagnosis of exclusion and can be the common presentation of a variety of pathologic processes as opposed to a single etiology.
Diagnosis
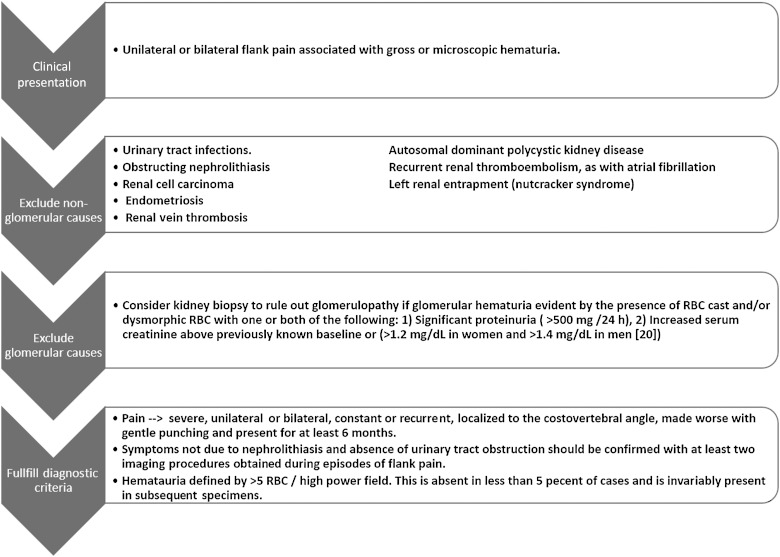
The diagnosis of LPHS remains one of exclusions [33]. Many patients with LPHS remain unrecognized for years and often shuttle from physician to physician in an effort to determine the cause of the pain and reach a diagnosis. A step-wise approach was adopted to reach the diagnosis of LPHS. After exclusion of non-glomerular causes for the hematuria, the minimal criteria to reach a diagnosis of LPHS include the documentation of hematuria and presence of pain for at least 6 months, which is proved not attributable to nephrolithiasis. Kidney biopsy is warranted to exclude secondary LPHS if there were any indications of glomerular disease as outlined in Figure 1 [2].
Fig. 1.
Clinical evaluation and diagnostic criteria. RBC, red blood cell.
A variety of conditions are often on the differential for patients with LPHS. These include ureteral obstruction [34], malignancy [35, 36], pyelonephritis, benign masses, recurrent renal thromboembolism, IgA nephropathy [37], arteriovenous fistula [15] or nutcracker syndrome.
Nutcracker phenomenon refers to compression of the left renal vein, commonly between the aorta and the superior mesenteric artery, leading to impaired blood flow and distention of the distal portion of the vein. Nutcracker syndrome is a clinical diagnosis of a complex of symptoms resembling those seen with nutcracker phenomenon. The symptoms of the syndrome include hematuria, which is due to rupture of thin-walled varices into the collecting system, left flank pain and other fatigue symptoms. It is important to rule out nutcracker syndrome when working up patients for hematuria and left flank pain [38].
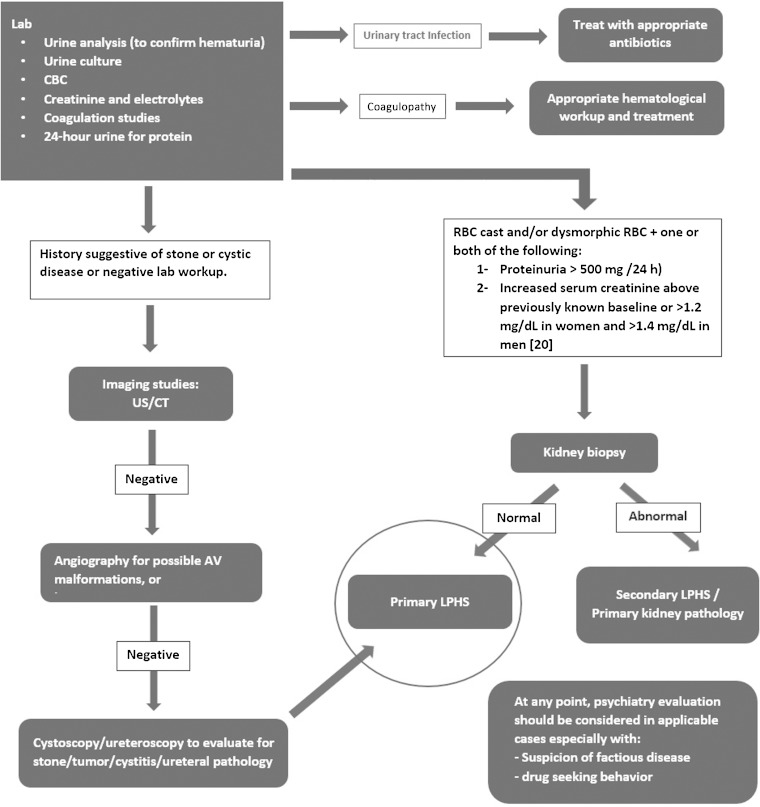
In order to reach a diagnosis of LPHS and exclude other options on the differential diagnosis, a variety of tests should be conducted [39–43]. Urine culture should be done to rule out infection whereas a urinalysis should be done to check for glomerular disease. A cystoscopy and/or computed tomography (CT) can be done to check for kidney stones or other tumors and cysts. Angiography or CT angiography can be done to rule out A-V malformation and hemangioma whereas flexible ureteroscopy can be done to check for ureteral pathology [40–43]. Coagulation studies should be ordered to check for problems with coagulopathy. Additionally, a psychiatric evaluation should be undertaken to rule out any factious disease (Figure 2) [17, 18, 44, 45].
Fig. 2.
Diagnostic work-up for LPHS. AV, arteriovenous; CBC, complete blood count; CT, computed tomography; LPHS, loin pain hematuria syndrome; RBS, red blood cell; US, ultrasound.
Exam findings
Physical exam and laboratory findings from LPHS vary. Many patients have unremarkable physical exam findings. Numerous patients report low-grade fevers and the majority have normal renal function, judged by creatinine clearance and serum creatinine. Studies have shown that there are no abnormal 24-h urine concentrations of calcium, phosphorus, uric acid, oxalate and cystine. Coagulation studies in these patients are frequently normal, including partial thromboplastin time, prothrombin time, bleeding time, hematocrit and platelet count [2, 3, 21].
Hematuria in LPHS is typically glomerular in origin characterized by dysmorphic red cells with or without red blood cell casts. However, as described earlier, intratubular crystal depositions could play a role in the pathogenesis of LPHS and the absence of typical findings to suggest glomerular origin of hematuria does not exclude LPHS.
Hematuria can be either microscopic or macroscopic. Episodes of macroscopic hematuria are usually associated with worsening pain but in between episodes urine analysis may show microscopic hematuria or in some patients, the hematuria clears completely even though the pain persists [2].
Proteinuria is not a feature of LPHS. One series of 34 patients showed the protein excretion to be above the upper limit of the normal of 150 mg/day in 11 patients and above 500 mg/day in only two patients.
Treatment and prognosis
Medical treatments for LPHS are varied and have been derived from the various theories of the pathophysiology of LPHS. Treatments targeting the coagulopathy etiology hypothesis are largely outdated and no longer indicated. These approaches included low-dose aspirin (ineffective [23]), warfarin (temporary partial relief), dipyridamole (discontinued due to epistaxis), antiplatelet therapy (ineffective) and discontinuation of birth control (with some positive results) [46]. Additional therapies, such as aminocaproic acid to treat hematuria and beta-blockers to treat the vaso-constrictive etiology were also ineffective [3, 33].
Without definite understanding of the underlying pathophysiological process in LPHS, the goal of management has been limited to symptomatic relief and pain management. As some studies have shown that 25–50% of LPHS patients enter a period of spontaneous remission within 3–5 years [2, 6], a gradual approach in treatment progressing from conservative management to more invasive measures would be reasonable.
There is limited evidence that angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker may reduce both the frequency and severity of both gross hematuria and loin pain. The presumed mechanism is through reduced intraglomerular pressure secondary to efferent arteriolar dilation. The decreased pressure in turn decreases the chance of glomerular rupture and hematuria especially when accompanied by underlying thin GBM pathology [23]. One report of seven patients receiving enalapril for 7–48 months reported fewer severe episodes of both hematuria and pain [23].
Patients are normally started with non-opioid analgesics and progress to parenteral opioid analgesics, which require hospitalization. Furthermore, many of these patients end up becoming addicted to the high-dose narcotics [46].
Other treatments including transcutaneous electrical nerve stimulation and regional nerve blocks have shown success in some cases of LPHS but none was effective in long-term pain relief [11].
Local capsaicin infusion has not been proved to be clinically justifiable [47–51]. Retrograde infusion of capsaicin into the ureter and renal pelvis has been performed in an attempt to block the sympathetic C fibers responsible for pain signal transmission [47–52]. Capsaicin provides short-term pain relief to patients with LPHS, which can last from 2–17 weeks. One series reported complications including severe bladder pain, urinary tract infection, worsening renal pain and, most importantly, deterioration of renal function. The pathophysiology of nephrotoxicity is secondary to inflammation of the collecting system after intrarenal reflux, pelvi-ureteric inflammation, edema and subsequent stricture and scar tissue formation [48]. The series reviewed reports from 52 cases of LPHS of which long-term data on renal function were available for 17 patients. Five of these patients underwent significant loss of kidney function and eventually nephrectomies. The high complication and nephrectomy rate of 20–67% should be weighed against the benefits [48]. Most authors have abandoned the use of capsaicin in treating LPHS, and recommend that patients should be adequately counseled on its potential side-effects, including nephrotoxicity and high rate of nephrectomy [47–52].
A different approach to treating LPHS is surgical renal denervation. This can be achieved through several modalities including stripping the renal capsule from the kidney (capsulotomy), stripping the renal nerves from the renal artery (neurectomy), percutaneous catheter-based radiofrequency ablation of the renal sympathetic nerves or by renal autotransplantation [29, 32, 53–56].
Surgical renal denervation has been found to be helpful with a temporary complete relief from pain in some patients with LPHS, but this was sustained for a maximum of 2 years [53]. Three large series have reported results on renal denervation in LPHS. Reports for a total of 84 procedures performed in 65 patients with LPHS showed success rates ranging from 25 to 33% depending on the follow-up period, which varied from 8 to 54 months. The recurrence rate of ipsilateral pain was reported in 67–75% of the patients within a median of 6–11 months of the procedure. Sheil et al. reported mild wound infection in 8% of the procedures; otherwise, no postoperative complications were reported [29, 53, 57]. The recurrence of pain is assumed to be secondary neuronal regeneration. Surgical renal denervation should not be attempted in patients for whom renal autotransplantation is being considered as dense scarring related to the procedure might affect the quality and quantity of vessels harvested for the autotransplantation [53].
Gambaro et al. reported a patient with LPHS and hypertension successfully treated with catheter-based radiofrequency ablation of the renal sympathetic nerve. The patient remained pain-free and normotensive at 6 months post follow-up [54]. The limited evidence regarding this form of renal denervation along with the danger of publication bias (with only positive results being reported) and in addition to the large placebo effect in this poorly understood syndrome, all warrant that a clinical trial should be performed producing solid evidence before catheter-based renal denervation can be recommended for the treatment of LPHS [58].
Along the lines of renal denervation is renal autotransplantation. Renal autotransplantation was first described in 1982 and involves removing the kidney and inserting it to the ipsilateral iliac fossa [6]. It has been shown that the loin pain due to LPHS terminates immediately after the autotransplantation. It is postulated this happens due to the fact that all the nerves around the kidney are severed completely [32, 55, 56]. After autotransplantation, most patients are weaned off of narcotics and can go back to their daily routines within a few weeks. Although this approach may lead to decreased morbidity and more rabid improvement, autotransplantation should not be first-line therapy and should not be offered to all patients with LPHS. Selection criteria for suitable candidates for surgery should include patients with severe pain requiring high doses of analgesics to control pain for whom extensive nonsurgical therapies have been found unsuitable [55]. Also, patients should not be counseled about surgery early on in the course of the disease as the knowledge of the availability of a surgical option may discourage them from proper use of pain clinic management [17].
Several series have reported encouraging results for kidney autotransplantaion in LPHS. Chin et al. reported a series of 26 procedures in 22 LPHS patients over a 12-year period with a median follow-up of 78.5 months in which 12 of the 26 autotransplanted kidneys in the 22 patients remained pain-free for a minimum follow-up of 5 years. Overall, they reported that 69% of the kidney autotransplant procedures resulted in significant pain relief for a follow-up period of 10 years, where most patients could be weaned off analgesics and back to normal daily activities [43]. The longest reported period of pain relief following autotransplant was reported to be 21 years [59]. One study reported the incidence of postoperative complications associated with autotransplantaion as high as 30% in 46 procedures in patients with LPHS. Complications included nephrectomy in four patients, infarction of the autotransplanted kidney, wound infection and nerve entrapment [29]. Another study reported nephrectomy of the autotransplanted kidney in 3 of 26 kidneys due to arterial or venous thrombosis [60].
While there are many benefits to renal autotransplantation, some studies have shown there to be some recurrence of pain (between 4 and 10 months) [31, 61, 62]. While the reason for the recurrence of pain is unknown, some postulate it is due to the regeneration of autonomic nerves. Canine studies have shown that autonomic nerves do regenerate after renal autotransplantation. A minority of patients may require graft nephrectomy to combat the pain [40, 63, 64]. A last resort for pain relief comes via nephrectomy, but the risks must be balanced with the benefits; nephron-sparing therapy is desired [65, 66].
One study has shown promising results for neuromodulation of the lumbar sympathetic plexus in four patients with LPHS. Goroszeniuk et al. showed that patients had significant reduction in pain allowing them to return to activities of daily living with less analgesic dependency [67]. They used a low-frequency stimulation on the autonomic system to control the pain. While the mechanism of this approach is not well defined, beneficial effects of low-frequency stimulation have been shown in clinical applications and basic science studies. Still, more experience is needed with this technique before it can be recommended for the management of LPHS outside of the experimental setting [67].
Conclusion
LPHS is a complex condition that is not well understood; diagnosis continues to be one of exclusion. It is a condition that causes severe discomfort for patients and can lead to many limitations on the activities of daily living. No consensus exists for the appropriate sequential approach in treating LPHS. While autotransplantation appears to provide promising results, we still strongly find that conservative measures for the treatment of LPHS should be used first. The risk and benefits of surgical procedures must be carefully weighed and compared with more conservative alternatives and each patient should be evaluated individually. Going forward, it would be very beneficial to have more basic science and controlled clinical studies conducted on LPHS to help determine the definitive pathophysiology of the syndrome and how to diagnose and treat the condition.
Conflict of interest statement
None declared.
References
- 1.Little PJ, Sloper JS, de Wardener HE. A syndrome of loin pain and haematuria associated with disease of peripheral renal arteries. Q J Med 1967; 36: 253–259 [PubMed] [Google Scholar]
- 2.Spetie DN, Nadasdy T, Nadasdy G, et al. Proposed pathogenesis of idiopathic loin pain-hematuria syndrome. Am J Kidney Dis 2006; 47: 419–427 [DOI] [PubMed] [Google Scholar]
- 3.Dube GK, Hamilton SE, Ratner LE, et al. Loin pain hematuria syndrome. Kidney Int 2006; 70: 2152–2155 [DOI] [PubMed] [Google Scholar]
- 4.Reifsteck JE, Holder JC, Liu GC, et al. Loin pain hematuria syndrome. Urol Radiol 1987; 9: 155–157 [DOI] [PubMed] [Google Scholar]
- 5.Hutchison SM. Loin pain and haematuria syndrome. Br Med J 1987; 295: 391–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheil AG, Ibels LS, Thomas MA, et al. Renal autotransplantation for severe loin-pain/haematuria syndrome. Lancet 1985; 2: 1216–1217 [DOI] [PubMed] [Google Scholar]
- 7.Taguchi Y. The loin pain-hematuria syndrome. Can J Surg 1996; 39: 93. [PMC free article] [PubMed] [Google Scholar]
- 8.Winearls CG, Bass C. The loin pain haematuria syndrome. Nephrol Dial Transplant 1994; 9: 1537–1539 [PubMed] [Google Scholar]
- 9.Habte W, Dobbie JW, Boulton-Jones M. The loin pain-haematuria syndrome in males. Scott Med J 1981; 26: 118–120 [DOI] [PubMed] [Google Scholar]
- 10.Sherwood T. Loin pain/haematuria syndrome. Lancet 1979; 1: 1033–1034 [DOI] [PubMed] [Google Scholar]
- 11.Taba Taba Vakili S, Alam T, Sollinger H. Loin pain hematuria syndrome. Am J Kidney Dis 2014; 64: 460. [DOI] [PubMed] [Google Scholar]
- 12.Miller F, Lane BP, Kirsch M, et al. Loin pain-hematuria syndrome with a distinctive vascular lesion and alternative pathway complement activation. Arch Pathol Lab Med 1994; 118: 1016–1019 [PubMed] [Google Scholar]
- 13.Pollock CA, Ibels LS, Eckstein RP, et al. Afferent arteriolar C3 disease—a distinct pathological entity. Am J Kidney Dis 1989; 14: 31–38 [DOI] [PubMed] [Google Scholar]
- 14.Siegler RL, Brewer ED, Hammond E. Platelet activation and prostacyclin supporting capacity in the loin pain hematuria syndrome. Am J Kidney Dis 1988; 12: 156–160 [DOI] [PubMed] [Google Scholar]
- 15.Low AI, Matz LR. Haematuria and renal fornical lesions. Br J Urol 1972; 44: 681–691 [DOI] [PubMed] [Google Scholar]
- 16.Woolfson RG, Lewis CA, Hill PD, et al. Ureteric peristalsis studies in loin pain and haematuria syndrome: another diagnostic disappointment. Br J Urol 1993; 72: 291–292 [DOI] [PubMed] [Google Scholar]
- 17.Kelly B. Psychiatric issues in the “loin pain and haematuria syndrome”. Aust N Z J Psychiatry 1994; 28: 302–306 [DOI] [PubMed] [Google Scholar]
- 18.Lucas PA, Leaker BR, Neild GH. Psychiatric aspects of loin pain/haematuria syndrome. Lancet 1992; 340: 1038. [DOI] [PubMed] [Google Scholar]
- 19.Praga M, Martinez MA, Andres A, et al. Association of thin basement membrane nephropathy with hypercalciuria, hyperuricosuria and nephrolithiasis. Kidney Int 1998; 54: 915–920 [DOI] [PubMed] [Google Scholar]
- 20.Coe FL, Evan AP, Worcester EM, et al. Three pathways for human kidney stone formation. Urol Res 2010; 38: 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith HS, Bajwa ZH. Loin pain hematuria syndrome-visceral or neuropathic pain syndrome? Clin J Pain 2012; 28: 646–651 [DOI] [PubMed] [Google Scholar]
- 22.Jefferies ER, Phull JS, Gallegos CR. Comment on: Loin pain haematuria syndrome. Ann R Coll Surg Engl 2010; 92: 360; author reply 360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hebert LA, Betts JA, Sedmak DD, et al. Loin pain-hematuria syndrome associated with thin glomerular basement membrane disease and hemorrhage into renal tubules. Kidney Int 1996; 49: 168–173 [DOI] [PubMed] [Google Scholar]
- 24.Naish PF, Aber GM, Boyd WN. C3 deposition in renal arterioles in the Loin pain and haematuria syndrome. Br Med J 1975; 3: 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burden RP, Dathan JR, Etherington MD, et al. The loin-pain/haematuria syndrome. Lancet 1979; 1: 897. [DOI] [PubMed] [Google Scholar]
- 26.Higgins PM, Aber GM. Renal pain and haematuria. Br J Urol 1974; 46: 601–608 [DOI] [PubMed] [Google Scholar]
- 27.Leaker BR, Gordge MP, Patel A, et al. Haemostatic changes in the loin pain and haematuria syndrome: secondary to renal vasospasm? Q J Med 1990; 76: 969–979 [PubMed] [Google Scholar]
- 28.Fletcher P, Al-Khader AA, Parsons V, et al. The pathology of intrarenal vascular lesions associated with the loin-pain haematuria syndrome. Nephron 1979; 24: 150–154 [DOI] [PubMed] [Google Scholar]
- 29.Sheil A, Chui A, Verran D, et al. Evaluation of the loin pain/hematuria syndrome treated by renal autotransplantation or radical renal neurectomy. Am J Kidney Dis 1998; 32: 215–220 [DOI] [PubMed] [Google Scholar]
- 30.Burden RP, Booth LJ, Ockenden BG, et al. Intrarenal vascular changes in adult patients with recurrent haematuria and loin pain—a clinical, histological and angiographic study. Q J Med 1975; 44: 433. [PubMed] [Google Scholar]
- 31.Dimski DS, Hebert LA, Sedmak D, et al. Renal autotransplantation in the loin pain-hematuria syndrome: a cautionary note. Am J Kidney Dis 1992; 20: 180–184 [DOI] [PubMed] [Google Scholar]
- 32.Hutchison SM, Doig A, Jenkins AM. Recurrence of loin pain/haematuria syndrome after renal autotransplantation. Lancet 1987; 1: 1501–1502 [DOI] [PubMed] [Google Scholar]
- 33.Vince HB, Tomson CR, Loveday EJ, et al. Nutcracker phenomenon presenting as loin pain haematuria syndrome. NDT Plus 2011; 4: 418–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sayeed R, Nyamekye I, Kinder R. Unsuspected rectal adenocarcinoma causing a urinoma. Int J Urol 1997; 4: 99–100 [DOI] [PubMed] [Google Scholar]
- 35.Janane A, Hachi H, Tijami F, et al. Kidney cancer: report of 47 cases. Ann Urol (Paris) 2003; 37: 57–60 [DOI] [PubMed] [Google Scholar]
- 36.Maughan EO, Wilson J, Wilde JT. Spontaneous resolution of acquired factor V inhibitor associated with ovarian carcinoma. Int J Lab Hematol 2007; 29: 316–319 [DOI] [PubMed] [Google Scholar]
- 37.Ducloux D, Queffeulou G, Faucher C, et al. IgA nephropathy and loin pain haematuria syndrome associated with acute renal failure. Nephrol Dial Transplant 1994; 9: 584. [DOI] [PubMed] [Google Scholar]
- 38.Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc 2010; 85: 552–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bass CM, Parrott H, Jack T, et al. Severe unexplained loin pain[loin pain haematuria syndrome]: management and long-term outcome. QJM 2007; 100: 369–381 [DOI] [PubMed] [Google Scholar]
- 40.Pukenas BA, Zaslau S. Loin pain hematuria syndrome: case series. W V Med J 2003; 99: 192–193 [PubMed] [Google Scholar]
- 41.Ghanem AN. Features and complications of nephroptosis causing the loin pain and hematuria syndrome. A preliminary report. Saudi Med J 2002; 23: 197–205 [PubMed] [Google Scholar]
- 42.Bhandari A, Ellias M. Loin pain hematuria syndrome: pain control with RFA to the splanchanic plexus. Pain Clinic 2000; 12: 323–327 [Google Scholar]
- 43.Chin JL, Kloth D, Pautler SE, et al. Renal autotransplantation for the loin pain-hematuria syndrome: long-term followup of 26 cases. J Urol 1998; 160: 1232–1235; discussion 1235–1236 [PubMed] [Google Scholar]
- 44.Lucas PA, Leaker BR, Murphy M, et al. Loin pain and haematuria syndrome: a somatoform disorder. QJM 1995; 88: 703–709 [PubMed] [Google Scholar]
- 45.Kelly B. Psychological aspects of loin-pain/haematuria syndrome. Lancet 1992; 340: 1294. [DOI] [PubMed] [Google Scholar]
- 46.Coffman KL. Loin pain hematuria syndrome: a psychiatric and surgical conundrum. Curr Opin Organ Transplant 2009; 14: 186–190 [DOI] [PubMed] [Google Scholar]
- 47.Ahmed M, Acher P, Deane AM. Ureteric bupivicaine infusion for loin pain haematuria syndrome. Ann R Coll Surg Engl 2010; 92: 139–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uzoh CC, Kumar V, Timoney AG. The use of capsaicin in loin pain-haematuria syndrome. BJU Int 2009; 103: 236–239 [DOI] [PubMed] [Google Scholar]
- 49.Ghanem AN. Re: Early experience of intraureteric capsaicin infusion in loin-pain haematuria syndrome. BJU Int 2000; 86: 911–914 [DOI] [PubMed] [Google Scholar]
- 50.Ghanem AN. Intra-ureteric capsaicin in loin pain haematuria syndrome: efficacy and complications. BJU Int 2003; 91: 429–430 [DOI] [PubMed] [Google Scholar]
- 51.Playford D, Kulkarni H, Thomas M, et al. Intra-ureteric capsaicin in loin pain haematuria syndrome: efficacy and complications. BJU Int 2002; 90: 518–521 [DOI] [PubMed] [Google Scholar]
- 52.Bultitude MI. Capsaicin in treatment of loin pain/haematuria syndrome. Lancet 1995; 345: 921–922 [PubMed] [Google Scholar]
- 53.Greenwell TJ, Peters JL, Neild GH, et al. The outcome of renal denervation for managing loin pain haematuria syndrome. BJU Int 2004; 93: 818–821 [DOI] [PubMed] [Google Scholar]
- 54.Gambaro G, Fulignati P, Spinelli A, et al. Percutaneous renal sympathetic nerve ablation for loin pain haematuria syndrome. Nephrol Dial Transplant 2013; 28: 2393. [DOI] [PubMed] [Google Scholar]
- 55.Chin JL. Loin pain-hematuria syndrome: role for renal autotransplantation. J Urol 1992; 147: 987–989 [DOI] [PubMed] [Google Scholar]
- 56.Sheil AG, Ibels LS, Pollock C, et al. Treatment of loin pain/haematuria syndrome by renal autotransplantation. Lancet 1987; 2: 907–908 [DOI] [PubMed] [Google Scholar]
- 57.Andrews BT, Jones NF, Browse NL. The use of surgical sympathectomy in the treatment of chronic renal pain. Br J Urol 1997; 80: 6–10 [DOI] [PubMed] [Google Scholar]
- 58.de Beus E, Blankestijn PJ, Fox JG, et al. Catheter-based renal denervation as a novel treatment for loin pain haematuria syndrome. Nephrol Dial Transplant 2013; 28: 2197. [DOI] [PubMed] [Google Scholar]
- 59.Turini D, Barbanti G, Beneforti P, et al. Autotransplantation for intractable loin pain: report of a case with longterm followup. J Urol 1995; 153: 389–391 [DOI] [PubMed] [Google Scholar]
- 60.Burke JR, Hardie IR. Loin pain haematuria syndrome. Pediatr Nephrol 1996; 10: 216. [DOI] [PubMed] [Google Scholar]
- 61.Karvelas JP, Ramsey EW. Renal autotransplantation in patients with loin pain-hematuria syndrome. Can J Surg 1996; 39: 121–125 [PMC free article] [PubMed] [Google Scholar]
- 62.Harney J, Rodgers E, Campbell E, et al. Loin pain-hematuria syndrome: how effective is renal autotransplantation in its treatment? Urology 1994; 44: 493–496 [DOI] [PubMed] [Google Scholar]
- 63.Casingal VP, Asolati M, Hunter D, et al. Emergent autotransplantation of a renal allograft. Clin Transplant 2005; 19: 563–565 [DOI] [PubMed] [Google Scholar]
- 64.Spitz A, Huffman JL, Mendez R. Autotransplantation as an effective therapy for the loin pain-hematuria syndrome: case reports and a review of the literature. J Urol 1997; 157: 1554–1559 [PubMed] [Google Scholar]
- 65.Talic RF, Parr N, Hargreave TB. Anephric state after graft nephrectomy in a patient treated with renal autotransplantation for bilateral metachronous loin pain/hematuria syndrome. J Urol 1994; 152: 1194–1195 [DOI] [PubMed] [Google Scholar]
- 66.Aber GM, Higgins PM. The natural history and management of the loin pain/haematuria syndrome. Br J Urol 1982; 54: 613–615 [DOI] [PubMed] [Google Scholar]
- 67.Goroszeniuk T, Khan R, Kothari S. Lumbar sympathetic chain neuromodulation with implanted electrodes for long-term pain relief in loin pain haematuria syndrome. Neuromodulation 2009; 12: 284–291 [DOI] [PubMed] [Google Scholar]