Abstract
The redox equilibrium is crucial for the maintenance of immune homeostasis. Here, we summarize recent data showing that oxidation regulates T-cell functions and that alterations of the redox equilibrium may play an important role in the pathogenesis of inflammatory conditions affecting the kidneys. We further discuss potential links between oxidation, T cells and renal diseases such as systemic lupus erythematosus, renal ischaemia/reperfusion injury, end-stage renal disease and hypertension. The basic understanding of oxidation as a means by which diseases are directly affected results in unexpected pathophysiological similarities. Finally, we describe potential therapeutic options targeting redox systems for the treatment of nephropathies affecting humans.
Keywords: acute kidney injury, autoimmunity, end-stage kidney disease, systemic lupus erythematosus (SLE), T-cell activation
Introduction
Recently, redox homeostasis has been the focus of intense investigations, especially in inflammatory conditions [1], and most recently in research of regulated necrosis [2–4]. An altered inflammatory response is the basis of many diseases, including allergy and systemic autoimmunity, which often affect the kidneys. Under particular circumstances, which are not yet completely understood, inflammation is not properly terminated, resulting in continuous activation of the immune cells and prolonged inflammation and tissue damage mainly mediated by T cells and macrophages. In this review, we summarize recent advances on how the redox balance regulates T-cell functions and we discuss the possible interplay between oxidation and T cells in inflammatory diseases affecting kidneys.
T-cell activation and differentiation
T cells are key orchestrators of the response against pathogens and are also fundamental in maintaining self-tolerance. A number of clinically important conditions have been described in which T-cell functions are altered, as in AIDS or upon immunosuppression for solid organ transplantation. T-cell progenitors differentiate in the thymus into immature T cells that acquire the expression of the T-cell receptor (TCR), which recognizes antigen peptides from pathogens presented along with major histocompatibility complex (MHC). In addition to the TCR, T cells are characterized by expression of the co-receptor molecules CD4 and CD8 on their cell surface. CD4+ T cells, also called T helper (Th) cells, recognize antigen/MHC-II complexes on antigen presenting cells (APCs) and coordinate the activation of other immune cells including B cells, macrophages, etc. Therefore, CD4+ T cells are crucial for coordination of the immune response and for the elimination of invading pathogens. On the other hand, CD8+ T cells, referred to as T cytotoxic cells, recognize antigen/MHC-I complexes and are responsible for the killing of pathogen-infected cells.
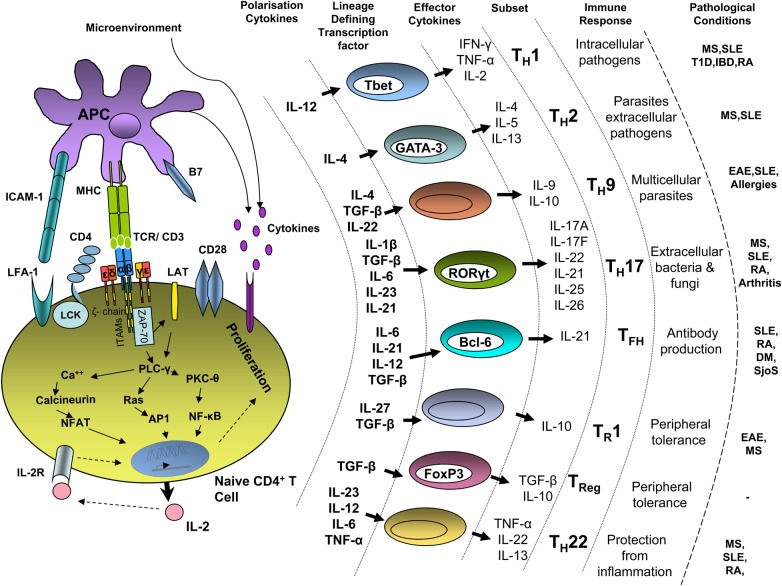
Recognition of MHC/peptide complexes by the TCR and the co-receptors results in T-cell activation (for a review, see [5]). Signalling via the TCR is further supported by co-stimulatory (e.g. CD28) and accessory (e.g. integrins) molecules. Upon TCR ligation, members of the Src family kinases Lck and Fyn phosphorylate the immunoreceptor tyrosine-based signalling motifs (ITAMs) located within the TCR-associated CD3 and ζ chains. This event results in the recruitment of the tyrosine kinase ζ chain–associated protein kinase of 70 kDa (ZAP-70) to the receptor. ZAP-70 is in turn activated and further phosphorylates the linker for activation of T cells (LAT), a transmembrane adaptor molecule that further assembles a complex leading to Ca2+ flux, Ras and protein kinase C (PKC) activation (Figure 1). These events ultimately culminate in gene transcription, proliferation and differentiation of T cells.
Fig. 1.
T-cell activation and T-cell subsets differentiation. Upon TCR ligation by MHC/peptide complexes on APCs, an activating signal is triggered within T cells leading to transcriptional activation, IL-2 production and proliferation. Additional receptors (e.g. CD4, CD28, integrins and cytokine receptors) also participate in this process. The orchestration of these signals results in Th differentiation into various effector and regulatory subsets. Cytokines inducing Th differentiation, lineage-defining transcription factors, effector cytokines, physiological function and implication in pathological conditions are indicated. RA, rheumatoid arthritis; MS, multiple sclerosis; SLE, systemic lupus erythematosus; T1D, diabetes mellitus type 1; IBD, inflammatory bowel disease; DM, juvenile dermatomyositis; SjoS, Sjogren's syndrome; EAE, experimental autoimmune encephalomyelitis.
T-cell activation and differentiation depends on APCs such as dendritic cells (DCs), macrophages and B cells. Among them, DCs are highly specialized in antigen presentation and in T-cell priming [6]. DCs act as sentinels in the body where they capture antigens. Danger signals such as microbial products or cytokines from injured tissue activate DCs, which in turn migrate to secondary lymphoid organs, where they allow initiation of the immune response [7]. The nature of the stimulus dictates which kind of immune response will be set in motion [8]. Therefore, depending on the insult affecting a given tissue, different subsets of DCs can be generated that in turn are able to coordinate the differentiation of a particular Th subset.
To date, the following Th subsets have been described: Th1, Th2, Th9, Th17, Th22, Tfh (follicular helper T cells), Tr1 (type 1 regulatory T cells) and Treg (regulatory T cells), each possessing a specific function in the elimination of pathogens. The development, the function and the involvement of Th subsets in human diseases are summarized in Figure 1 (for a review, see [9, 10]).
Redox equilibrium: an emerging new player in the regulation of T-cell differentiation
Reactive oxygen species (ROS) include the superoxide anion radical (), hydroxyl radical (HO·), hydrogen peroxide (H2O2) and hypochlorous acid [11]. It has been known for many years that ROS may have, in high concentrations (oxidative stress), deleterious effects on living organisms, as they can damage all major cell constituents, including lipids, proteins and DNA. Conversely, at lower concentrations, ROS participate in the regulation of signalling processes and cellular responses such as proliferation and differentiation [11, 12]. Therefore, in order to maintain the appropriate redox state, cells require a regulation system for the precise generation and elimination of ROS (redox homeostasis).
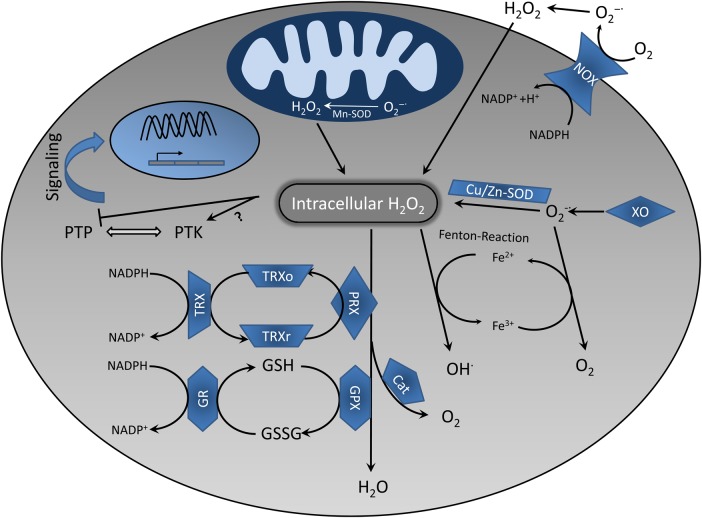
Superoxide is generated in the mitochondria when electrons ‘accidentally’ leak from the transport chain and reduce molecular oxygen (Figure 2) [12]. Additionally, cells also possess enzymatic sources of superoxide such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) and xanthine oxidase (XO) [11]. Superoxide is highly unstable and rapidly dismutates to H2O2, a process mediated by the superoxide dismutase (SOD) (Figure 2). H2O2 can further participate in the generation of HO· during Fenton reactions [12].
Fig. 2.
Redox homeostasis in T cells. The main sources of ROS are the mitochondrial electron transport chain, NOX and XO, which produce . either spontaneously or with the help of specific enzymes (i.e. SOD) is catalysed to H2O2. The latter can be further reduced to water by Cat or converted to OH· in the presence of transition metals (Fenton reaction). Different antioxidant systems have also been depicted. GSH is the most important cellular antioxidant. GPX mediates GSH oxidation by H2O2, which is converted to GSSG. GSSG is reconverted to GSH by GR via oxidation of NADPH. The TRX system is involved in cellular redox homeostasis as well. In this case, H2O2 oxidizes TRXr to TRXo, which is subsequently re-reduced by NADPH. H2O2 functions as a regulator of T-cell signalling via inhibition of PTPs or modulating the activity of PTKs. NOX, NADPH oxidase; XO, xanthine oxidase; , superoxide anion; SOD, superoxide dismutase; Cat, catalase; GSH, glutathione; GSSG, glutathione disulfide; TRX, thioredoxin; PTP, protein tyrosine phosphatase; PTK, protein tyrosine kinase; TRXr, reduced thioredoxin; TRXo, oxidized thioredoxin; PRX, peroxiredoxin, GR, glutathione reductase; GPX, glutathione peroxidase.
In order to protect cell constituents from oxidative damage and/or to ensure the appropriate intracellular concentration of ROS required for cell functions, cells have developed a complex defence system comprising a variety of substances (antioxidants), which neutralize the excess ROS (Figure 2). Antioxidants include enzymes such as SOD, glutathione peroxidase, catalase and non-enzymatic agents such as vitamin C, vitamin E, the tripeptide glutathione (γ-l-glutamyl-l-cysteinyl-glycine, GSH), carotenoids, flavonoids and also free amino acids, which can easily react with ROS (e.g. cysteine) [11, 12]. Whereas reduced GSH is one of the major intracellular redox buffers, the cysteine/cystine couple plays an important role in the regulation of extracellular redox homeostasis [13].
It is well established that TCR triggering results in the generation of ROS (for a review, see [14]). Different sources of ROS in T cells have been described, including NOX enzymes and mitochondria. Despite the fact that the mechanisms by which ROS regulate T-cell activation are not yet completely clear, an appropriate redox state is absolutely required for T-cell activation. Alterations in the redox equilibrium induced by TCR-generated ROS may influence the activation of several molecules and support signalling [14]. However, high levels of ROS (oxidative stress) are detrimental for T cells, as they may inhibit signalling or induce apoptosis. In fact, a decrease in intracellular GSH blocks TCR-mediated calcium flux and proliferation in human peripheral T cells [15, 16].
The same regulatory mechanism applies to a variety of cells in which ROS function as signalling molecules by inhibiting phosphatases, modulating the activity of kinases and regulating the activation of transcription factors [1, 11]. Nevertheless, the exact molecular mechanisms of how ROS regulate signalling are only partially understood, and we are far from understanding these mechanisms in disease settings of stress, sepsis, autoimmunity and acute kidney injury.
Accumulating evidence now suggests that T-cell differentiation is strongly redox dependent. Several in vitro experiments performed on isolated T cells have shown that the redox state regulates interleukin 2 (IL-2) and IL-4 production. Antioxidants inhibit IL-2 and IL-4 expression [17–19]. In agreement with these findings, a pro-oxidant situation induces the expression of IL-2 and IL-2R and also enhances proliferation [20–22]. Interestingly, inhibition of mitochondria-generated ROS in T cells isolated from patients suffering from atopic dermatitis also blocked TCR-mediated IL-4 production [17]. Therefore, in recent years, treatment with antioxidant has become a therapeutic option to cure inflammatory diseases (discussed below).
The data reported above suggest that IL-2 and IL-4 production in T cells requires ROS. The molecular mechanisms of ROS-mediated regulation of cytokine production are not yet clear. Although ROS have been directly implicated in the regulation of the activation of transcription factors, it appears that, in T cells, oxidative processes likely regulate proximal TCR signalling, which in turn affects the signal strength and the execution of the transcriptional programme [14].
In addition to the effects on cytokine production, different studies have assessed the role of the redox balance on Th differentiation in vitro. It appears that a pro-oxidation state induces T-cell polarization into the Th2 lineage. In fact, an increase in superoxide generation correlates with augmented IL-4, IL-5 and IL-13 production [23]. Importantly, antioxidants inhibit this effect. Similarly, treatment with N-acetyl cysteine (NAC), a GSH precursor, or GSH suppresses Th2 differentiation [24, 25]. In agreement with the latter studies, higher free thiol levels correlate with a skewed Th1/Th2 balance towards the Th1 lineage [26]. Also, data from NOX2-deficient mice suggest that naive CD4+ T cells with defective generation of ROS are biased towards the Th1 lineage [27, 28]. In the absence of NOX2, T cells display enhanced T-bet and reduced STAT5 and GATA3 activation and, accordingly, secreted more interferon γ (IFN-γ) but less IL-4 [29].
In addition to Th1/Th2 differentiation, ROS have also been implicated in the generation of Th17 cells. A recent study has shown that T cells from immediate early response gene X-1 (IEX-1)-deficient mice have increased mitochondrial ROS production upon CD3/CD28 stimulation and enhanced generation of Th17 cells [30, 31]. The specific involvement of ROS in the generation of Th17 cells was shown by the treatment with antioxidants that suppress Th17 differentiation in IEX-1-deficient mice. In addition, these mice are highly susceptible to develop collagen-induced arthritis.
Understanding how oxidation regulates Th polarization is an understudied area that needs further investigation. Indeed, recent studies emphasize that reprogramming of Th differentiation may have important therapeutic implications for the treatment of inflammation [32, 33].
Redox-dependent regulation of APC functions: the effects on Th differentiation
T cells are primed in lymphoid tissues and their differentiation depends on cytokines and signals provided by neighbouring cells, in particular APCs. Also APC functions can be regulated in a redox-dependent manner. Therefore, alterations of the redox state of APCs or of the microenvironment may affect the ability of APCs to support T-cell differentiation. In this section, we summarize recent data highlighting the importance of the redox state of APCs for T-cell differentiation and its potential implication in the development of human autoimmune diseases.
The role of GSH
It has been observed that an increase in intracellular GSH levels in LPS-stimulated human monocyte-derived DCs and thymic stromal lymphopoietin-activated myeloid DCs promoted Th1 (IFN-γ) but inhibited Th2 (IL-4, IL-13) responses [34]. When GSH levels are elevated, DCs release more IL-27 and IL-12, which in turn support Th1 differentiation. Indeed, blocking IL-12 by the addition of neutralizing anti-IL-12 monoclonal antibody or suppressing IL-27 by siRNA results in suppressed production of the Th1 cytokine IFN-γ. In addition, GSH levels are critical for the regulation of IL-12 production by APCs such as DCs and macrophages [35–38]. Collectively, these studies suggest that an oxidized intracellular milieu in APCs decreases the secretion of IL-12, thus skewing Th polarization to the Th2 lineage, whereas a reduced intracellular state in APCs favours Th1 differentiation.
Analysis of the molecular mechanisms involved in the expression of IL-12 revealed that GSH regulates IL-12 expression by inducing p38-MAPK but suppressing JNK-MAPK activation [39, 40]. GSH is not only involved in the regulation of redox homeostasis, but it also appears to control other aspects of cellular functions, including gene transcription and proliferation [40]. Therefore, it is not yet clear whether GSH regulates cytokine release in a redox-dependent manner. However, a study suggests that ROS produced upon LPS stimulation of human monocyte-derived DCs is required for cytokine production by DCs [41].
Altered GSH levels have been found in a variety of autoimmune diseases [42]. A correlation between decreased GSH levels and anti-thyroperoxidase antibodies has been found in Hashimoto's thyroiditis [43]. Moreover, a mutation in glutathione S-transferase, an enzyme facilitating the elimination of ROS by catalysing their conjugation to GSH, has been found to correlate with an elevated risk of developing anti-citrullinated protein antibody and rheumatoid arthritis (RA) [44]. Therefore, reduced GSH levels in autoimmunity may skew Th differentiation to the Th2 subset or even to the Th17 lineage [45], thus favouring autoantibody production.
The role of NOX2
ROS have also been directly implicated in antigen processing and in the generation of the MHC-II-restricted peptide repertoire in APCs. NOX2 appears to be involved in this process [46–48]. NOX2-mediated ROS inactivate cysteine cathepsines in the phagosome, thus modulating their activity. In the absence of ROS, cysteine cathepsines have altered substrate specificity that affects the processing of proteins. It has been shown that in myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE), NOX2-deficient APCs are unable to prime Th cells because of inefficient presentation of the MOG immunodominant epitope [46]. Consequently, NOX2-deficient mice are protected from EAE. However, these effects appear to be specific for certain proteins and for EAE. In fact, NOX2 deficiency results in the development of inflammatory diseases in both humans and mice (for a review, see [49–51]). As ROS production is strongly decreased in cells lacking a functional NOX2, the above findings contradicted the general idea that ROS promote inflammation. It is now clear that ROS have different effects depending on their levels, their source and when they are produced and, hence, under particular conditions, may promote hyperinflammatory responses, whereas in other cases they suppress autoimmunity. The molecular mechanisms regulating these two different outcomes remain unclear, but a necro-inflammatory auto-amplification loop may contribute to the pathogenesis [3]. One study has shown that NOX2-generated ROS are required to activate p38-MAPK signalling in IFN-γ/LPS-stimulated murine DCs [52]. This pathway in turn suppresses IL-12 expression. NOX2-deficient DCs secrete more IL-12 and skew CD4+ T-cell differentiation to the Th1 subset. It also appears that NOX2-generated ROS in Tregs is required for their suppression function [53]. In the absence of a functional NOX2 or upon antioxidant treatment, Tregs are less efficient in the suppression of effector cells. These data demonstrate that NOX2-derived ROS may regulate tolerance at different levels.
The role of oxidized lipids
Recent data have clearly highlighted that oxidation of low-density lipoproteins (LDLs) influences DC functions and plays a crucial role in atherosclerosis and autoimmunity. Atherosclerosis is a chronic inflammatory disease affecting the functionality of blood vessels [54]. It is also established that a correlation exists between autoimmunity [e.g. RA and systemic lupus erythematosus (SLE)] and the risk of developing cardiovascular disease [55]. Now possible links between atherosclerosis and autoimmunity have been proposed [56, 57]. LDLs function as lipid carriers in the blood [58]. When LDLs diffuse into the subendothelial area of the artery, they can undergo ROS-mediated oxidation. Oxidized LDLs (oxLDLs) function as inflammatory mediators via different pathways [59]. It is now evident that oxLDLs regulate DC function and Th polarization. In fact, oxLDLs stimulate DCs to polarize T cells into the Th1 lineage in both humans and mice [56]. Th1 cells in turn support and promote the disease, likely via IFN-γ [60]. In addition to promoting Th1 differentiation, new findings suggest that oxLDLs also support Th17 polarization both in vitro and in vivo [61]. oxLDLs induce IL-6 secretion by DCs upon binding to CD36 and TLR4 via MyD88, thus in turn favouring Th17-cell differentiation. Furthermore, this study demonstrates that oxLDLs enhance the pathogenicity of MOG-specific T cells and the severity of EAE. What is the exact temporal relation between atherosclerosis and autoimmunity is still unclear. It has been proposed that elevated levels of inflammatory cytokines in autoimmunity promote atherosclerosis [55]. However, on the basis of the above data, it is possible to speculate that increased levels of oxLDLs under atherosclerosis conditions may favour Th1 or Th17 differentiation, thus driving the progression of autoimmunity. How oxLDLs promote both Th1 and Th17 cells is not yet clear. It is possible that the discrimination between Th1 versus Th17 may depend on the chemical composition of the oxLDLs. DCs are able to recognize different species of modified lipids via different receptors (e.g. CD36, LOX-1) [56, 58]. Therefore, the integration of different signals downstream of these receptors will likely dictate the outcome of the DC-mediated Th developmental programme.
An additional study further emphasizes the importance of lipid oxidation in DC maturation and in turn on Th differentiation [62]. Mice lacking lipoxygenase (LO), an enzyme oxygenating free and esterified polyunsaturated fatty acids, show enhanced DC maturation and increased Th17 differentiation. Moreover, these mice also display a more severe EAE, thus indicating the importance of LO and LO-derived oxidized lipids in autoimmunity. Mechanistically, LO participates in regulation of the activation of the transcription factor NRF2, which in turn inhibits DC maturation. LO also seems to inhibit IL-23 transcription, which is required for Th17 polarization.
On top of these molecularly defined pathways, cell death in a form of regulated necrosis, referred to as ferroptosis, has recently been associated with a defined lipid peroxidation signature [63] that depends on glutathione peroxidase 4 activity and GSH levels [64]. Since regulated necrosis triggers necroinflammation [65], it is conceivable that also these processes of ROS-driven parenchymal damage may contribute to overall organ damage, obviously with a predominantly important function in the kidney [3, 63]. Very recently, ferroptosis has also been described in T cells in immunity to infection [66]. Therefore, pharmacological targeting of ROS differentiation and ferroptosis by means of the same compounds, e.g. ferrostatins, may provide a promising therapeutic option. Today, however, broad clinical application of such inhibitors is precluded by the lack of mechanistic insights.
The interplay between oxidation, Th cells and kidney diseases
The aetiology of many kidney diseases is still largely not well understood. In particular, little is known about the interplay between oxidation, Th cells and tissue damage. As mentioned in the section “T-cell activation and differentiation”, under some inflammatory conditions, alteration of Th differentiation is one of the factors contributing to disease development or progression. In this paragraph, we summarize recent data in which a link between oxidation, alterations of T-cell function and renal disease has been proposed and we also discuss potential therapeutic implications (see Table 1).
Table 1.
Potential therapeutic approaches targeting redox homeostasis in kidney diseases
| Disease | Treatment | Effects | Species | Ref. |
|---|---|---|---|---|
| SLE lupus nephritis | Rapamycin | Restores T-cell activation, ameliorates disease | Human | [67] |
| NAC | Suppresses anti-DNA antibody, modestly improves survival | Mouse | [68] | |
| Cysteamine | Inhibits renal insufficiency, markedly improves survival | Mouse | [68] | |
| NAC | Blocks mTOR, restores T-cell functions, ameliorates disease | Human | [69] | |
| Transmethylation micronutrients | Reduce CD40L expression on T cells, ameliorate disease | Mouse | [70] | |
| Antroquinonol | Inhibits conventional T-cell activation, enhances Treg suppression, reduces inflammation | Mouse | [71] | |
| Epigallocatechin-3-gallate | Reduces oxidative stress, enhances Treg suppression activity, prevents renal disease | Mouse | [72] | |
| Curcumin | Reduces immune complex deposition, decreases inflammation | Mouse | [73] | |
| IRI | Ligustrazine | Reduces oxidative stress, reduces immune cell infiltration, protects from kidney injury | Mouse | [74] |
| β-Carotene | Protects from oxidative stress | Rat | [75] | |
| NAC/ebselen | Reduce oxidation, prevent kidney damage | Rat | [76] | |
| EPC-K1 | Reduces oxidative stress, attenuates disease | Rat | [77] | |
| Green tea polyphenols | Reduce oxidative stress, reduce infiltration of CD8+ T cells, reduce apoptosis, reduce renal injury | Rabbit | [78, 79] | |
| Ferrostatins | Reduce lipid peroxidation in IRI in epithelial and immune cells | Mouse | [3, 63] | |
| ESRD | Zinc | Reduces oxidative stress, decreases inflammation, improve immune status | Human | [80, 81] |
| Ginkgo biloba extract | Partially reverse thrombogenic coagulation | Human | [82] | |
| Renal diseases mediated by oxLDLs | Quercetin | Reduces NOX2 and NOX4 activation and oxidative stress | In vitro | [83] |
| Coenzyme Q10 | Reduces NOX2 activation and ROS generation | In vitro | [84] | |
| Ubiquinol | Reduces ROS production, ameliorates renal function | Rat | [85] | |
| Ellagic acid | Inhibits NOX2-mediated superoxide production, enhances antioxidant defences | In vitro | [86] | |
| Ginkgo biloba extract | Inhibits NOX2 activation, the expression of inflammatory genes and protein nitrosylation | In vitro | [87] | |
| Epigallocatechin-3-gallate | Inhibits NOX2 activation, ROS generation and the expression of inflammatory genes | In vitro | [88] | |
| Resveratrol | Protects from oxidative damage | In vitro | [89] | |
| Lithiasis | Coenzyme Q10 | Improves renal function | Human | [90] |
| Hypertension | NAC | Enhances reduced GSH level, improves renal functions | Rat | [91] |
| Epoxyeicosatrienoic acid analogue | Reduces oxidative stress and inflammation, protects kidneys | Rat | [92] |
SLE is one of the most well-known kidney diseases in which oxidative stress is increased [93, 94]. Recent data point out that oxidation inhibits T-cell signalling, leading to Erk activation and DNA methyltransferase expression, thus in turn resulting in DNA demethylation, overexpression of immune genes and autoimmunity [95]. Additional studies have shown that oxidation of PKCδ, which results in its inactivation, is responsible for the defective Erk activation and lupus development in mice [96, 97]. Therefore, antioxidants may represent beneficial co-adjuvants for the treatment of SLE. Indeed, a study has shown that non-enzymatic antioxidants (e.g. NAC and cysteamine) improve survival in a mouse model of SLE [68]. An important advance in the therapy of SLE using dietary supplements has been recently provided. It has been shown that a diet rich in transmethylation micronutrients can ameliorate SLE in a lupus mouse model [70]. This study has further shown that the dietary methyl donor content directly correlates with increased methylation and decreased expression of the CD40lg gene. CD40L is expressed on T cells and contributes to disease pathogenesis by stimulating antibody production upon engagement with CD40 expressed on B cells [98].
The sources of ROS in SLE are not known. Nevertheless, a possible involvement of mitochondria in the generation of ROS in T cells from SLE patients has been previously proposed [99]. T cells from SLE patients exhibit mitochondrial dysfunctions such as elevated mitochondrial transmembrane potential, reduced ATP production and enhanced generation of ROS. It is believed that these alterations diminish activation-induced apoptosis and sensitize T cells for necrosis, a process that may contribute to the establishment of the inflammatory milieu in SLE. Recently, it has been shown that these alterations in the mitochondrial functions activate mechanistic target of rapamycin (mTOR), which in turn drives IL-4 production and necrotic T-cell death in SLE [100]. According to the data presented above, inhibition of mTOR using rapamycin reduces disease activity and restores T-cell activation in SLE patients [67]. Interestingly, a more recent work has demonstrated that the antioxidant NAC blocks mTOR activation in lupus patients, restores T-cell functions and ameliorates disease [69]. How NAC affects mTOR activation is not yet clear. It is possible that NAC neutralizes excessive ROS or may influence mitochondrial functions.
The effects of other antioxidants, such as vitamins A, C and E, were also tested in SLE patients [101]. In these studies, it appears that the nutrient intake of regular antioxidants is not associated with a decreased risk of developing SLE [101]. Thus, NAC and rapamycin, perhaps also used in combination, are promising therapeutic interventions to reduce oxidative stress and restore T-cell functions in SLE patients. Other compounds displaying antioxidant and anti-inflammatory activities showed efficacy in the treatment of glomerulonephritis in mouse models. For example, antroquinonol inhibited the activation of conventional T cells but enhanced the suppressive capability of Tregs and also reduced renal inflammation [71]. Similarly, epigallocatechin-3-gallate treatment reduced oxidative stress in the kidney and enhanced Treg function, thus preventing lupus nephritis [72]. Finally, treatment with the well-known plant antioxidant compound curcumin decreased renal inflammation and immune complex deposition in the glomeruli via a mechanism likely involving Tregs [73].
Ischaemia/reperfusion kidney injury (IRI) is another well-known disease leading to kidney failure. ROS also play an important role in the pathogenesis of this disease. Recent data suggest that stanniocalcin-1 (STC1), an intracrine protein crucial for tubular epithelial survival [102], inhibits IRI by regulating the expression of mitochondrial uncoupling protein 2 by negatively regulating superoxide generation and by reducing the infiltration of macrophages and T cells in the kidney [103]. Thus, in addition to superoxide scavengers such as SOD, which protects from IRI [104], targeting STC1 may represent a therapeutic option for this disease. Also, several antioxidants such as ligustrazine, β-carotene, NAC/ebselen, EPC-K1 and green tea polyphenols have been shown to be effective in attenuating IRI in different animal models [74–79]. Long-term dialysis is a procedure required for the treatment of patients suffering from end-stage renal disease (ESRD). It is known that ESRD patients undergoing long-term dialysis display increased oxidative stress [105]. Recent studies have found an association between oxidative stress and altered levels of essential trace elements in long-term dialysis patients [80, 106]. In particular, an elevated Cu/Zn ratio was observed in these patients. Zn has antioxidants and anti-inflammatory properties and thus zinc supplementation may represent a therapeutic tool for the treatment of dialysis patients. Indeed, clinical studies have shown that zinc supplementation intake ameliorates oxidative stress, inflammation and immune status in dialysis patients [80, 81]. Among immune cells, Tregs isolated from long-term dialysis display reduced suppressive capacity, cell-cycle arrest and undergo apoptosis [107]. These alterations appear to be mediated by increased levels of oxLDL in ESRD patients. oxLDLs strongly contribute to endothelial dysfunctions and are responsible for secondary cardiovascular defects associated with a variety of diseases characterized by oxidative stress, including renal diseases [108]. As mentioned in the paragraph above, oxLDLs induce alterations in Th differentiation and thus oxLDLs may further contribute to autoimmunity and tissue damage. A number of in vitro studies have shown that different compounds, including quercetin, coenzyme Q10, ellagic acid, gingko biloba extract, epigallocatechin-3-gallate and resveratrol, are capable of counteracting or attenuating oxLDL-mediated dysfunctions in vitro [83, 84, 86–89]. Some data regarding the in vivo efficacy of these compounds are also available. For example, it has been demonstrated that coenzyme Q10 administration improves renal function in patients with lithiasis undergoing extracorporeal shockwave lithotripsy [90]. Similarly, reduced coenzyme Q10 ameliorates renal function in animal models [85]. Moreover, gingko biloba extract also exerted beneficial effects on the thrombogenic coagulation profile in ERSD patients [82].
It has been shown that in both animal models and humans, T cells also participate in the pathogenesis of kidney disease induced during hypertension (reviewed in [109]). In an animal model of hypertension, T cells infiltrate the kidney where they contribute to tissue damage, likely by releasing ROS (via NOX2) and other inflammatory mediators [110]. Compounds with antioxidant and anti-inflammatory properties such as NAC and an epoxyeicosatrienoic acid analogue attenuate kidney damage and hypertension in animal models [91, 92].
Future perspective
Changing the redox homeostasis of the microenvironment or modulating more selectively the T cell and APC redox state may represent a therapeutic approach for the treatment of inflammatory diseases. During recent years, studies have been undertaken in an attempt to modulate DC functions or to reprogramme Th differentiation [111–113]. It will be important for the future to assess whether reprogramming of DCs or Th cells upon modulation of the redox equilibrium in vitro will be helpful in the treatment of inflammatory kidney diseases. As reported above, alterations of redox homeostasis affect DC functions, thus in turn altering the adaptive immune response. Surprisingly, little is known about redox-mediated alterations affecting DCs in kidney diseases. Therefore, further steps aimed at dissecting the role of DCs in renal pathologies are required for the development of new pharmacological strategies.
A number of studies, summarized in Table 1, have also assessed the efficacy of different antioxidants and anti-inflammatory compounds as potential therapeutic agents in the prevention and cure of inflammatory kidney diseases. To date, the question remains open as to whether available compounds, especially those having antioxidant activity, are beneficial or not for patients suffering from nephropathies. We hope that by improving the limited bioavailability of these compounds, it will be possible to enhance their efficacy and their therapeutic effects.
Of crucial importance will be identification of the molecular targets of ROS in T cells and DCs. This will reveal important new insights into ROS-regulated pathways and will lead to the identification of oxidation targets. The aim of this avenue of research is the development of immunomodulatory compounds for the treatment of inflammation. During the last decade, advances in the field of proteomics have led to the development of new tools to analyse protein thiol oxidation [114–116]. These new methods have been shown to be useful for the identification of oxidation targets in cell lines [117–119].
Funding
This work was supported by grants from the German Research Foundation (DFG) to L.S. (SFB854, project B19; SPP1710, project SI861-3/1), Else Kröner-Fresenius-StiftungElse Kröner-Forschungskolleg Magdeburg, TP6, and Cluster of Excellence ‘Inflammation at Interfaces’ (EXC306) to A.L.
Conflict of interest statement
None declared.
References
- 1.Lei Y, Wang K, Deng L, et al. Redox regulation of inflammation: old elements, a new story. Med Res Rev 2015; 35: 306–340 [DOI] [PubMed] [Google Scholar]
- 2.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012; 149: 1060–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linkermann A, Skouta R, Himmerkus N, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA 2014; 111: 16836–16841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 2014; 15: 135–147 [DOI] [PubMed] [Google Scholar]
- 5.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol 2009; 27: 591–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell 2001; 106: 263–266 [DOI] [PubMed] [Google Scholar]
- 7.Vega-Ramos J, Roquilly A, Asehnoune K, et al. Modulation of dendritic cell antigen presentation by pathogens, tissue damage and secondary inflammatory signals. Curr Opin Pharmacol 2014; 17: 64–70 [DOI] [PubMed] [Google Scholar]
- 8.Macagno A, Napolitani G, Lanzavecchia A, et al. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol 2007; 28: 227–233 [DOI] [PubMed] [Google Scholar]
- 9.Raphael I, Nalawade S, Eagar TN, et al. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015; 74: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt N, Ueno H. Regulation of human helper T cell subset differentiation by cytokines. Curr Opin Immunol 2015; 34: 130–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Droge W. Free radicals in the physiological control of cell function. Physiol Rev 2002; 82: 47–95 [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84 [DOI] [PubMed] [Google Scholar]
- 13.Yan Z, Banerjee R. Redox remodeling as an immunoregulatory strategy. Biochemistry 2010; 49: 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simeoni L, Bogeski I. Redox regulation of T-cell receptor signaling. Biol Chem 2015; 396: 555–568 [DOI] [PubMed] [Google Scholar]
- 15.Staal FJ, Anderson MT, Staal GE, et al. Redox regulation of signal transduction: tyrosine phosphorylation and calcium influx. Proc Natl Acad Sci USA 1994; 91: 3619–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suthanthiran M, Anderson ME, Sharma VK, et al. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci USA 1990; 87: 3343–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaminski MM, Sauer SW, Klemke CD, et al. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J Immunol 2010; 184: 4827–4841 [DOI] [PubMed] [Google Scholar]
- 18.Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013; 38: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li-Weber M, Giaisi M, Treiber MK, et al. Vitamin E inhibits IL-4 gene expression in peripheral blood T cells. Eur J Immunol 2002; 32: 2401–2408 [DOI] [PubMed] [Google Scholar]
- 20.Roth S, Droge W. Regulation of T-cell activation and T-cell growth factor (TCGF) production by hydrogen peroxide. Cell Immunol 1987; 108: 417–424 [DOI] [PubMed] [Google Scholar]
- 21.Los M, Schenk H, Hexel K, et al. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J 1995; 14: 3731–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hehner SP, Breitkreutz R, Shubinsky G, et al. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J Immunol 2000; 165: 4319–4328 [DOI] [PubMed] [Google Scholar]
- 23.King MR, Ismail AS, Davis LS, et al. Oxidative stress promotes polarization of human T cell differentiation toward a T helper 2 phenotype. J Immunol 2006; 176: 2765–2772 [DOI] [PubMed] [Google Scholar]
- 24.Jeannin P, Delneste Y, Lecoanet-Henchoz S, et al. Thiols decrease human interleukin (IL) 4 production and IL-4-induced immunoglobulin synthesis. J Exp Med 1995; 182: 1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bengtsson A, Lundberg M, Avila-Carino J, et al. Thiols decrease cytokine levels and down-regulate the expression of CD30 on human allergen-specific T helper (Th) 0 and Th2 cells. Clin Exp Immunol 2001; 123: 350–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann FW, Hashimoto AC, Shafer LA, et al. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr 2010; 140: 1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snelgrove RJ, Edwards L, Rae AJ, et al. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur J Immunol 2006; 36: 1364–1373 [DOI] [PubMed] [Google Scholar]
- 28.Jackson SH, Devadas S, Kwon J, et al. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol 2004; 5: 818–827 [DOI] [PubMed] [Google Scholar]
- 29.Shatynski KE, Chen H, Kwon J, et al. Decreased STAT5 phosphorylation and GATA-3 expression in NOX2-deficient T cells: role in T helper development. Eur J Immunol 2012; 42: 3202–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akilov OE, Ustyugova IV, Zhi L, et al. Enhanced susceptibility to Leishmania infection in resistant mice in the absence of immediate early response gene X-1. J Immunol 2009; 183: 7994–8003 [DOI] [PubMed] [Google Scholar]
- 31.Zhi L, Ustyugova IV, Chen X, et al. Enhanced Th17 differentiation and aggravated arthritis in IEX-1-deficient mice by mitochondrial reactive oxygen species-mediated signaling. J Immunol 2012; 189: 1639–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noval Rivas M, Burton OT, Wise P, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 2015; 42: 512–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagliani N, Vesely MC, Iseppon A, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015; 523: 221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamide Y, Utsugi M, Dobashi K, et al. Intracellular glutathione redox status in human dendritic cells regulates IL-27 production and T-cell polarization. Allergy 2011; 66: 1183–1192 [DOI] [PubMed] [Google Scholar]
- 35.Murata Y, Amao M, Yoneda J, et al. Intracellular thiol redox status of macrophages directs the Th1 skewing in thioredoxin transgenic mice during aging. Mol Immunol 2002; 38: 747–757 [DOI] [PubMed] [Google Scholar]
- 36.Dobashi K, Aihara M, Araki T, et al. Regulation of LPS induced IL-12 production by IFN-gamma and IL-4 through intracellular glutathione status in human alveolar macrophages. Clin Exp Immunol 2001; 124: 290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuppner MC, Scharner A, Milani V, et al. Ifosfamide impairs the allostimulatory capacity of human dendritic cells by intracellular glutathione depletion. Blood 2003; 102: 3668–3674 [DOI] [PubMed] [Google Scholar]
- 38.Kim HJ, Barajas B, Chan RC, et al. Glutathione depletion inhibits dendritic cell maturation and delayed-type hypersensitivity: implications for systemic disease and immunosenescence. J Allergy Clin Immunol 2007; 119: 1225–1233 [DOI] [PubMed] [Google Scholar]
- 39.Utsugi M, Dobashi K, Ishizuka T, et al. c-Jun N-terminal kinase negatively regulates lipopolysaccharide-induced IL-12 production in human macrophages: role of mitogen-activated protein kinase in glutathione redox regulation of IL-12 production. J Immunol 2003; 171: 628–635 [DOI] [PubMed] [Google Scholar]
- 40.Utsugi M, Dobashi K, Koga Y, et al. Glutathione redox regulates lipopolysaccharide-induced IL-12 production through p38 mitogen-activated protein kinase activation in human monocytes: role of glutathione redox in IFN-gamma priming of IL-12 production. J Leukoc Biol 2002; 71: 339–347 [PubMed] [Google Scholar]
- 41.Yamada H, Arai T, Endo N, et al. LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. Life Sci 2006; 78: 926–933 [DOI] [PubMed] [Google Scholar]
- 42.Perricone C, De Carolis C, Perricone R. Glutathione: a key player in autoimmunity. Autoimmun Rev 2009; 8: 697–701 [DOI] [PubMed] [Google Scholar]
- 43.Rostami R, Aghasi MR, Mohammadi A, et al. Enhanced oxidative stress in Hashimoto's thyroiditis: inter-relationships to biomarkers of thyroid function. Clin Biochem 2013; 46: 308–312 [DOI] [PubMed] [Google Scholar]
- 44.Mikuls TR, Gould KA, Bynote KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther 2010; 12: R213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang G, Wang J, Fan X, et al. Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: dose- and time-response studies in female MRL+/+ mice. Toxicology 2012; 292: 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allan ER, Tailor P, Balce DR, et al. NADPH oxidase modifies patterns of MHC class II-restricted epitopic repertoires through redox control of antigen processing. J Immunol 2014; 192: 4989–5001 [DOI] [PubMed] [Google Scholar]
- 47.Rybicka JM, Balce DR, Chaudhuri S, et al. Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner. EMBO J 2012; 31: 932–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi H, Bordy MD, Sharma V, et al. Hyperthyroidism in patients with Down's syndrome. Clin Pediatr (Phila) 1979; 18: 273–275 [DOI] [PubMed] [Google Scholar]
- 49.Sareila O, Kelkka T, Pizzolla A, et al. NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal 2011; 15: 2197–2208 [DOI] [PubMed] [Google Scholar]
- 50.Schappi MG, Jaquet V, Belli DC, et al. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Semin Immunopathol 2008; 30: 255–271 [DOI] [PubMed] [Google Scholar]
- 51.Hultqvist M, Olsson LM, Gelderman KA, et al. The protective role of ROS in autoimmune disease. Trends Immunol 2009; 30: 201–208 [DOI] [PubMed] [Google Scholar]
- 52.Jendrysik MA, Vasilevsky S, Yi L, et al. NADPH oxidase-2 derived ROS dictates murine DC cytokine-mediated cell fate decisions during CD4T helper-cell commitment. PLoS One 2011; 6: e28198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Efimova O, Szankasi P, Kelley TW. Ncf1 (p47phox) is essential for direct regulatory T cell mediated suppression of CD4+ effector T cells. PLoS One 2011; 6: e16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999; 340: 115–126 [DOI] [PubMed] [Google Scholar]
- 55.Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med 2013; 64: 249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perrin-Cocon L, Diaz O, Andre P, et al. Modified lipoproteins provide lipids that modulate dendritic cell immune function. Biochimie 2013; 95: 103–108 [DOI] [PubMed] [Google Scholar]
- 57.Ryu H, Chung Y. Regulation of IL-17 in atherosclerosis and related autoimmunity. Cytokine 2015; 74: 219–227 [DOI] [PubMed] [Google Scholar]
- 58.Dieckmann M, Dietrich MF, Herz J. Lipoprotein receptors—an evolutionarily ancient multifunctional receptor family. Biol Chem 2010; 391: 1341–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 2011; 12: 204–212 [DOI] [PubMed] [Google Scholar]
- 60.Mallat Z, Gojova A, Brun V, et al. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation 2003; 108: 1232–1237 [DOI] [PubMed] [Google Scholar]
- 61.Lim H, Kim YU, Sun H, et al. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity 2014; 40: 153–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothe T, Gruber F, Uderhardt S, et al. 12/15-Lipoxygenase-mediated enzymatic lipid oxidation regulates DC maturation and function. J Clin Invest 2015; 125: 1944–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 2014; 16: 1180–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014; 156: 317–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mulay SR, Linkermann A, Anders HJ. Necroinflammation in kidney disease. J Am Soc Nephrol 2015; doi:10.1681/ASN.2015040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsushita M, Freigang S, Schneider C, et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med 2015; 212: 555–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernandez D, Bonilla E, Mirza N, et al. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum 2006; 54: 2983–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suwannaroj S, Lagoo A, Keisler D, et al. Antioxidants suppress mortality in the female NZB × NZW F1 mouse model of systemic lupus erythematosus (SLE). Lupus 2001; 10: 258–265 [DOI] [PubMed] [Google Scholar]
- 69.Lai ZW, Hanczko R, Bonilla E, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2012; 64: 2937–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strickland FM, Hewagama A, Wu A, et al. Diet influences expression of autoimmune-associated genes and disease severity by epigenetic mechanisms in a transgenic mouse model of lupus. Arthritis Rheum 2013; 65: 1872–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jardin F, Delorme G, Hardy A, et al. Reevaluation of hemodynamic consequences of positive pressure ventilation: emphasis on cyclic right ventricular afterloading by mechanical lung inflation. Anesthesiology 1990; 72: 966–970 [DOI] [PubMed] [Google Scholar]
- 72.Tsai PY, Ka SM, Chang JM, et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med 2011; 51: 744–754 [DOI] [PubMed] [Google Scholar]
- 73.Lee H, Kim H, Lee G, et al. Curcumin attenuates lupus nephritis upon interaction with regulatory T cells in New Zealand Black/White mice. Br J Nutr 2013; 110: 69–76 [DOI] [PubMed] [Google Scholar]
- 74.Feng L, Ke N, Cheng F, et al. The protective mechanism of ligustrazine against renal ischemia/reperfusion injury. J Surg Res 2011; 166: 298–305 [DOI] [PubMed] [Google Scholar]
- 75.Hosseini F, Naseri MK, Badavi M, et al. Effect of beta carotene on lipid peroxidation and antioxidant status following renal ischemia/reperfusion injury in rat. Scand J Clin Lab Invest 2010; 70: 259–263 [DOI] [PubMed] [Google Scholar]
- 76.Kizilgun M, Poyrazoglu Y, Oztas Y, et al. Beneficial effects of N-acetylcysteine and ebselen on renal ischemia/reperfusion injury. Ren Fail 2011; 33: 512–517 [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto S, Hagiwara S, Hidaka S, et al. The antioxidant EPC-K1 attenuates renal ischemia-reperfusion injury in a rat model. Am J Nephrol 2011; 33: 485–490 [DOI] [PubMed] [Google Scholar]
- 78.Li YW, Zhang Y, Zhang L, et al. Protective effect of tea polyphenols on renal ischemia/reperfusion injury via suppressing the activation of TLR4/NF-kappaB p65 signal pathway. Gene 2014; 542: 46–51 [DOI] [PubMed] [Google Scholar]
- 79.Rah DK, Han DW, Baek HS, et al. Protection of rabbit kidney from ischemia/reperfusion injury by green tea polyphenol pretreatment. Arch Pharm Res 2007; 30: 1447–1454 [DOI] [PubMed] [Google Scholar]
- 80.Guo CH, Wang CL. Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int J Med Sci 2013; 10: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rashidi AA, Salehi M, Piroozmand A, et al. Effects of zinc supplementation on serum zinc and C-reactive protein concentrations in hemodialysis patients. J Ren Nutr 2009; 19: 475–478 [DOI] [PubMed] [Google Scholar]
- 82.Kim SH, Lee EK, Chang JW, et al. Effects of Ginkgo biloba on haemostatic factors and inflammation in chronic peritoneal dialysis patients. Phytother Res 2005; 19: 546–548 [DOI] [PubMed] [Google Scholar]
- 83.Hung CH, Chan SH, Chu PM, et al. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol Nutr Food Res 2015; 59: 1905–1917 [DOI] [PubMed] [Google Scholar]
- 84.Tsai KL, Chen LH, Chiou SH, et al. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol Nutr Food Res 2011; 55 (Suppl 2): S227–S240 [DOI] [PubMed] [Google Scholar]
- 85.Ishikawa A, Kawarazaki H, Ando K, et al. Renal preservation effect of ubiquinol, the reduced form of coenzyme Q10. Clin Exp Nephrol 2011; 15: 30–33 [DOI] [PubMed] [Google Scholar]
- 86.Lee WJ, Ou HC, Hsu WC, et al. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J Vasc Surg 2010; 52: 1290–1300 [DOI] [PubMed] [Google Scholar]
- 87.Ou HC, Hsieh YL, Yang NC, et al. Ginkgo biloba extract attenuates oxLDL-induced endothelial dysfunction via an AMPK-dependent mechanism. J Appl Physiol 2013; 114: 274–285 (1985) [DOI] [PubMed] [Google Scholar]
- 88.Ou HC, Song TY, Yeh YC, et al. EGCG protects against oxidized LDL-induced endothelial dysfunction by inhibiting LOX-1-mediated signaling. J Appl Physiol 2010; 108: 1745–1756 [DOI] [PubMed] [Google Scholar]
- 89.Guo H, Chen Y, Liao L, et al. Resveratrol protects HUVECs from oxidized-LDL induced oxidative damage by autophagy upregulation via the AMPK/SIRT1 pathway. Cardiovasc Drugs Ther 2013; 27: 189–198 [DOI] [PubMed] [Google Scholar]
- 90.Carrasco J, Anglada FJ, Campos JP, et al. The protective role of coenzyme Q10 in renal injury associated with extracorporeal shockwave lithotripsy: a randomised, placebo-controlled clinical trial. BJU Int 2014; 113: 942–950 [DOI] [PubMed] [Google Scholar]
- 91.Hye Khan MA, Neckar J, Manthati V, et al. Orally active epoxyeicosatrienoic acid analog attenuates kidney injury in hypertensive Dahl salt-sensitive rat. Hypertension 2013; 62: 905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tian N, Rose RA, Jordan S, et al. N-acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J Hypertens 2006; 24: 2263–2270 [DOI] [PubMed] [Google Scholar]
- 93.Perl A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat Rev Rheumatol 2013; 9: 674–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shah D, Mahajan N, Sah S, et al. Oxidative stress and its biomarkers in systemic lupus erythematosus. J Biomed Sci 2014; 21: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strickland FM, Li Y, Johnson K, et al. CD4(+) T cells epigenetically modified by oxidative stress cause lupus-like autoimmunity in mice. J Autoimmun 2015; 62: 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gorelik G, Sawalha AH, Patel D, et al. T cell PKCdelta kinase inactivation induces lupus-like autoimmunity in mice. Clin Immunol 2015; 158: 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gorelik GJ, Yarlagadda S, Patel DR, et al. Protein kinase Cdelta oxidation contributes to ERK inactivation in lupus T cells. Arthritis Rheum 2012; 64: 2964–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Desai-Mehta A, Lu L, Ramsey-Goldman R, et al. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest 1996; 97: 2063–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gergely P, Jr, Grossman C, Niland B, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum 2002; 46: 175–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai ZW, Borsuk R, Shadakshari A, et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol 2013; 191: 2236–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Costenbader KH, Kang JH, Karlson EW. Antioxidant intake and risks of rheumatoid arthritis and systemic lupus erythematosus in women. Am J Epidemiol 2010; 172: 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang L, Belousova T, Pan JS, et al. AKI after conditional and kidney-specific knockdown of stanniocalcin-1. J Am Soc Nephrol 2014; 25: 2303–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang L, Belousova T, Chen M, et al. Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int 2012; 82: 867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest 1984; 74: 1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karamouzis I, Sarafidis PA, Karamouzis M, et al. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am J Nephrol 2008; 28: 397–404 [DOI] [PubMed] [Google Scholar]
- 106.Guo CH, Chen PC, Yeh MS, et al. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin Biochem 2011; 44: 275–280 [DOI] [PubMed] [Google Scholar]
- 107.Meier P, Golshayan D, Blanc E, et al. Oxidized LDL modulates apoptosis of regulatory T cells in patients with ESRD. J Am Soc Nephrol 2009; 20: 1368–1384 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Gradinaru D, Borsa C, Ionescu C, et al. Oxidized LDL and NO synthesis—biomarkers of endothelial dysfunction and ageing. Mech Ageing Dev 2015; 151: 101–113 [DOI] [PubMed] [Google Scholar]
- 109.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 2014; 307: F499–F508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Miguel C, Guo C, Lund H, et al. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 2011; 300: F734–F742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Adema GJ. Dendritic cells from bench to bedside and back. Immunol Lett 2009; 122: 128–130 [DOI] [PubMed] [Google Scholar]
- 112.Delamarre L, Mellman I. Harnessing dendritic cells for immunotherapy. Semin Immunol 2011; 23: 2–11 [DOI] [PubMed] [Google Scholar]
- 113.Cosmi L, Maggi L, Santarlasci V, et al. T helper cells plasticity in inflammation. Cytometry A 2014; 85: 36–42 [DOI] [PubMed] [Google Scholar]
- 114.Poole LB. Measurement of protein sulfenic acid content. Curr Protoc Toxicol 2008; Chapter 17: Unit 17.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lindemann C, Leichert LI. Quantitative redox proteomics: the NOxICAT method. Methods Mol Biol 2012; 893: 387–403 [DOI] [PubMed] [Google Scholar]
- 116.Ckless K. Redox proteomics: from bench to bedside. Adv Exp Med Biol 2014; 806: 301–317 [DOI] [PubMed] [Google Scholar]
- 117.Seo YH, Carroll KS. Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies. Proc Natl Acad Sci USA 2009; 106: 16163–16168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kettenhofen NJ, Wood MJ. Formation, reactivity, and detection of protein sulfenic acids. Chem Res Toxicol 2010; 23: 1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leonard SE, Reddie KG, Carroll KS. Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem Biol 2009; 4: 783–799 [DOI] [PubMed] [Google Scholar]