Abstract
Stroke is the second most common cause of death and the leading cause of neurological disability worldwide, with huge economic costs and tragic human consequences. Both chronic kidney disease (CKD) and end-stage kidney disease are associated with a significantly increased risk of stroke. However, to date this has generated far less interest compared with the better-recognized links between cardiac and renal disease. Common risk factors for stroke, such as hypertension, hypercholesterolaemia, smoking and atrial fibrillation, are shared with the general population but are more prevalent in renal patients. In addition, factors unique to these patients, such as disorders of mineral and bone metabolism, anaemia and its treatments as well as the process of dialysis itself, are all also postulated to further increase the risk of stroke. In the general population, advances in medical therapies mean that effective primary and secondary prevention therapies are available for many patients. The development of specialist stroke clinics and acute stroke units has also improved outcomes after a stroke. Emerging therapies such as thrombolysis and thrombectomy are showing increasingly beneficial results. However, patients with CKD and on dialysis have different risk profiles that must be taken into account when considering the potential benefits and risks of these treatments. Unfortunately, these patients are either not recruited or formally excluded from major clinical trials. There is still much work to be done to harness effective stroke treatments with an acceptable safety profile for patients with CKD and those on dialysis.
Keywords: dialysis, end-stage kidney disease, prevention, stroke, treatment
Introduction
The term ‘stroke’ is not consistently defined in clinical practice or research. The World Health Organization, introduced in 1970, defines a stroke as ‘rapidly developing clinical signs of focal (or global) disturbance of cerebral function, lasting >24 h or leading to death, with no apparent cause other than that of vascular origin’ [1]. Transient ischaemic attacks were defined in 1975 as ‘an episode of temporary and focal dysfunction of vascular origin, which are variable in duration, commonly lasting 2–15 min, but occasionally lasting as long as a day (24 h). They leave no neurological ‘dysfunction’’ [2]. However, these definitions are now decades old and based solely on clinical criteria, and do not take into account developments in basic science, neuropathology and neuroimaging.
Taking these advances into account, the American Heart Association and American Stroke Association have redefined a stroke as ‘brain, spinal cord or retinal cell death attributable to ischaemia, based on pathological, imaging, other objective evidence, and/or clinical evidence’ [3]. The three main stroke subtypes, cerebral infarction or ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage have also been redefined based on these principles. These subtypes account for ∼87, 10 and 3% of all strokes in the general population, respectively [4]. Furthermore, the new definitions include the concept of ‘silent infarction/haemorrhage’ to take into account lesions discovered on neuroimaging or neuropathological examination without a history of acute neurological dysfunction attributed to the lesion [3]. Most subarachnoid haemorrhages occur as a consequence of aneurysmal rupture and will not be considered further in this article.
In 2010, stroke was globally the second commonest cause of death and the third commonest cause of life years lost due to premature mortality [5]. In addition, stroke is the leading cause of neurological disability worldwide [6]. In the USA alone, 700 000 new strokes happen every year costing an estimated $70 billion [7]. Furthermore, the suffering and negative consequences to patients, carers and society as a whole are incalculable. It is no wonder that most elderly patients fear a disabling stroke more than they fear death [8].
Chronic kidney disease (CKD) and end-stage kidney disease (ESKD) are associated with an increased risk of stroke [9, 10]. Both CKD and ESKD, and stroke, are associated with premature death, falls, cognitive impairment, dementia and decreased quality of life [5, 11–13]. However, this cerebro-renal link has attracted considerably less interest and research than the well-recognized cardio-renal connection. In this article, we will further examine the nature of the relationship between kidney disease and stroke, emphasizing areas in need of further research. We also highlight the rapidly evolving field of acute stroke treatment, especially regarding the recent trials on acute reperfusion treatments and the potential implications for nephrologists.
Renal impairment and the risk of stroke
A recent meta-analysis of over 2 million patients has confirmed the strong inverse relationship between renal function and stroke risk. For every 10 mL/min/1.73 m2 decline in renal function, stroke risk increases by 7% [9]. This meta-analysis also demonstrated a strong direct relationship between albuminuria and stroke risk. Any degree of albuminuria increases stroke risk by 68%, and for every 25 mg/mmol increase in albuminuria, stroke risk increases by 10% [9]. Interestingly, the effect of glomerular filtration rate (GFR) and albuminuria appear to be additive with no evidence of interaction [9]. These findings are consistent through categories of CKD and albuminuria, gender and patient risk groups (diabetes, hypertension and smoking) and types of stroke. Patients on dialysis have the highest stroke risk at 2–7 times higher than non-CKD patients, with a 3–5 times higher mortality [14–16]. Indeed, about two-thirds of patients on dialysis aged over 75 years will be dead within a year of a stroke [14].
Other risk factors for stroke are very similar to those for cardiac and peripheral vascular diseases. Increasing age, diabetes mellitus, male gender, positive family history and non-white ethnicity are all well-established non-modifiable risk factors for stroke [17]. In the general population, the major modifiable risk factors for ischaemic stroke are hypertension, smoking, hypercholesterolaemia and atrial fibrillation (AF) [17]. The risk for haemorrhagic stroke increases with higher blood pressure, high alcohol intake, bleeding diatheses and blood vessel wall fragility, as often seen in the elderly. All these risk factors are overrepresented in patients with CKD/ESKD. As in the cardio-renal syndrome, it is often very difficult to disentangle cause and effect [18]. In addition, there are a number of risk factors that are fairly unique to advanced CKD and ESKD patients including the mineral bone disorder associated with CKD (CKD-MBD), the dialysis process itself as well as anaemia and its treatment with erythropoietin stimulating agents (ESAs) [17, 19].
Stroke had been for many centuries considered a natural and inevitable consequence of aging [20]. However, with increasing understanding of the natural history and pathophysiology of the condition, the past few years have seen very rapid advances, not only in preventative strategies, but also in acute treatments aimed at improving outcomes and reducing disability [20]. These are discussed in the next two sections of this review with an emphasis on CKD and ESKD patients.
Primary and secondary prevention of stroke
Hypertension
In the general population, there is a well-established relationship between blood pressure and stroke with stroke mortality doubling for every 20 mmHg rise in systolic blood pressure [21]. The relationship between blood pressure and stroke in CKD/ESKD patients is less clear, with some studies reporting no association [17]. However, these observational studies are prone to significant biases [17]. Meta-analyses of randomized controlled trials in CKD/ESKD patients confirm that blood pressure reduction is associated with a significant fall in cardiovascular events [22, 23]. However, the optimum blood pressure range, or indeed even which blood pressure measurement (pre-, post-, intra- or inter-dialysis) to use is still far from clear and needs to be determined [24].
Hypercholesterolaemia
Lowering low-density lipoprotein cholesterol with statin-based treatment has repeatedly been shown to lower stroke risk in many populations [25]. In patients with CKD/ESKD, lipid lowering has been shown to reduce cardiovascular events and mortality [26]. However, the same meta-analysis found that stroke was not prevented by lipid-lowering treatment. There was marked heterogeneity between the studies reporting stroke as an outcome, and the negative results were mostly influenced by two studies of haemodialysis patients [27, 28] leading the authors to advise caution in the interpretation of their results in relation to stroke [26]. Indeed, one of these two studies [27] reported an increased risk of stroke with statin treatment, which instilled significant doubt over statin-based lipid-lowering therapy in haemodialysis patients. However, by far the largest trial examining the effect of lipid-lowering treatment in 9270 patients with CKD and ESKD found a significant reduction in ischaemic stroke [29]. There were comparable proportional benefits in patients already on dialysis at baseline and those that were not, with a predicted greater absolute benefit in dialysis patients due to their higher baseline risk [17, 29]. However, analysing both patients with CKD and ESKD together has drawn criticism from some commentators who do not accept that the case for the benefits of lipid lowering in patients with ESKD and feel it has yet been proved [30]. Thus, although there remains significant scepticism as to the role of lipid lowering in preventing stroke [31], and indeed cardiovascular events, in patients with advanced CKD and ESKD, it is clear that statin-based therapies are safe and should probably not be denied without good reason.
Atrial fibrillation
AF is the most common sustained cardiac arrhythmia with an estimated prevalence of >1.5–2% worldwide [32]. Patients with AF have a 5-fold increased risk of stroke, although this increased risk is not homogeneous and is dependent on the concomitant presence or absence of other risk factors [32]. The stroke risk associated with AF is significantly reduced with anticoagulation, although this is at the expense of an increased risk of bleeding [32]. Antiplatelet agents, even when used in combination, are not as effective as oral anticoagulation [33]. Several risk stratification scores, such as the CHADS2 and CHA2DS2-VASc scores, have been developed to identify patients at high risk of stroke who would derive the most net benefit from oral anticoagulation with vitamin K antagonists [32, 34]. The potential benefits of anticoagulation therapy need to be weighed against the increased bleeding risk associated with such therapy using scores such as HAS-BLED. This score adds a point for being a dialysis patient or transplant recipient or having a creatinine level of >200 μmol/L [34].
Having CKD/ESKD is associated with an increased prevalence of AF and vice versa [35]. The prevalence of AF in patients with advanced CKD has been reported at 4–21% and in dialysis patients at 7–27% [35]. The presence of AF confers a high risk of stroke in both patients with CKD and ESKD [35]. However, the inclusion of CKD as a risk factor as in the R2CHADS2 score or by adding 1 or 2 points to other established scores such as the CHA2DS2-VASc for stroke risk does not increase the predictive value of these scores [36–39].
Patients with advanced CKD and ESKD with AF have generally been excluded from anticoagulation trials. However, subgroup analyses of patients with early CKD suggest that these patients should be treated as per the general population [34]. The decision to anticoagulate patients with AF and ESKD is much more controversial given the associated increased bleeding risk [34]. Patients on haemodialysis are at particularly high risk of suffering a serious bleed, which may outweigh any stroke risk reduction conferred by anticoagulation [40]. Furthermore, the risk for vascular calcification may be enhanced by warfarin use [34]. As such, opinion on the use of anticoagulation in AF patients on dialysis is currently divided with commentators arguing that it is both safe and clinically beneficial [41] and others that the risks are not justified given the poorly documented benefits [42]. Indeed, two recent studies from Europe [43] and the USA [44] reported both no benefit [43] and a reduction [44] in ischaemic stroke with warfarin treatment in haemodialysis patients with AF. Randomized controlled trials examining the value of anticoagulation in ESKD patients with AF are warranted [44].
All the currently available non-vitamin-K oral anticoagulants have a degree of renal excretion, and all trials have excluded patients with advanced CKD [34]. Therefore, these agents should probably only be used as they were studied in their respective trials and should not be used in patients with advanced CKD (estimated GFR <30 mL/min/1.73 m2) [32].
Antiplatelet agents
In general, guidelines recommend the use of antiplatelet agents (clopidogrel, aspirin or aspirin plus extended release dipyridamole) for secondary prevention after an ischaemic stroke based on robust evidence [45]. There have been no appropriately sized trials of aspirin, or indeed other antiplatelet agents, on stroke, or other cardiovascular end points, in patients with CKD/ESKD. A recent meta-analysis found that the evidence for antiplatelet agents in patients with CKD was of low quality, being frequently derived from post hoc analyses of trials of broader populations [46]. The review concluded that antiplatelet agents have uncertain effects on stroke and mortality and may increase bleeding risk. There have also been reports of worsening renal function in patients with CKD treated with aspirin [47]. In a meta-analysis by the Antithombotic Trialists Collaboration, aspirin was found to reduce serious vascular events, including stroke, in haemodialysis patients by 41% [48]. However, the number of subjects was low with only 1000 patients in each group and <40 events per group.
The potential bleeding hazards and lack of clear efficacy of antiplatelet agents for the prevention of ischaemic stroke need to be acknowledged in patients with CKD/ESKD and discussed in that context. As for anticoagulation in AF, the decision to treat with antiplatelet agents to prevent further ischaemic strokes is probably best individualized.
CKD-MBD
Arterial medial calcification of the aorta [49, 50] and intracranial arteries [51] increases in prevalence with age and worsening renal function, leading to increased arterial stiffness and systolic blood pressure, both of which are associated with an increased risk of stroke and subsequent mortality [49, 50]. Biochemical markers of CKD-MBD, especially raised serum phosphate, parathyroid hormone and fibroblast growth factor 23 [52, 53], have all been associated with increased mortality, although a causal role has still to be proved.
Dialysis
Although having ESKD in itself remains the largest risk factor associated with stroke incidence, several lines of evidence have suggested that the process of haemodialysis itself further increases the risk of stroke. Firstly, the risk of stroke is much higher in the first few weeks after starting dialysis [54, 55]. Secondly, strokes appear to be more common during a dialysis session [56, 57]. Thirdly, strokes also appear to be more common after the long (3-day) break from dialysis, when fluid and electrolyte abnormalities are at their peak [58]. These findings could potentially be explained by episodes of cardiovascular instability and cerebral hypoperfusion during fluid removal, and in the case of haemorrhagic stroke, as a result of the temporary anticoagulation required during dialysis [17]. These concepts have led to the suggestion that changes designed to increase haemodynamic stability and reduce circulatory stress could be beneficial to stroke risk and cerebral function.
The addition of convection therapy to dialysis (haemodiafiltration) reduces cardiovascular instability, and a trial has shown that it significantly reduces all-cause and cardiovascular mortality as well as stroke deaths [59]. Trials of daily dialysis and long-nocturnal dialysis have been small but have consistently been associated with improved surrogate markers of cardiovascular and cerebrovascular risks, including improved blood pressure control, less left ventricular hypertrophy and improved blood results, especially those related to CKD-MBD [60, 61]. Interestingly, a recently published small trial comparing cooled dialysate (5°C below core body temperature) with standard 37°C dialysate demonstrated a considerable reduction in radiological features of brain injury [62]. Whether these findings are reproducible in larger studies or translate into improved patient outcomes remains to be proved.
Anaemia and ESAs
Observational studies of anaemia and stroke risk provide conflicting evidence: in the general population [63] and in CKD patients, anaemia is associated with an increased risk of stroke [64], whereas in ESKD patients, it appears to be protective [54, 65]. As with all observational studies, caution in interpretation is required given the high likelihood of confounding by ill health and inflammation.
Interventional randomized controlled trials of ESAs in CKD patients have consistently demonstrated an increased risk of worse outcomes in the groups where a higher haemoglobin result had been targeted. In the TREAT study (4048 patients) using Aranesp (darbapoetin alpha), a doubling of stroke (both ischaemic and haemorrhagic) risk was observed in the higher (130 g/L) compared with the lower (90 g/L) targeted group [66]. In the CHOIR study (1432 patients) using epoietin alpha, the group randomized to achieve a higher haemoglobin (135 g/L) had a higher risk of the composite end point of death, myocardial infarction, heart failure and stroke, compared with the lower (113 g/L) group [67]. There was no difference in the rate of strokes when analysed separately. In the CREATE study (603 patients) using epoietin beta, there were no differences in outcomes between the higher-target (130–150 g/L) and lower-target (105–115 g/L) groups [68]. However, there was an increased incidence of hypertension and headaches in the higher-target group.
In dialysis patients, a randomized controlled trial using epoietin alpha had to be stopped early because targeting a normal haematocrit of 0.42 was associated with an increased risk of all-cause mortality and non-fatal myocardial infarction compared with targeting a lower haematocrit of 0.30 [69]. There was also a non-statistically significant increase in stroke deaths.
These studies reinforce the dangers of relying on observational studies to dictate clinical practice and the potential dangers of introducing new treatments proved in the general population to patients with CKD/ESKD or on the basis of observational studies, without robust evidence from large randomized controlled trials. This is particularly poignant in the context of the recent developments in the acute treatment of stroke discussed below.
Acute treatment of stroke
Stroke clinics
Early assessment in a dedicated stroke clinic and rapid initiation of treatments, including antiplatelet agents, antihypertensive agents, statins, oral anticoagulants for AF and endarterectomy for symptomatic stenosis after a minor stroke has been shown to reduce the 90-day stroke recurrence rate by 80% [70]. Thus, the success and efficacy of a rapid access stroke clinic depend heavily on the prompt identification of symptoms suggestive of an early stroke. Patients with advanced CKD or ESKD, especially those on haemodialysis, already have a very high symptom burden as a consequence of their dialysis treatment, including paraesthesiae, generalized weakness and headaches, making early identification of a stroke potentially problematic [71]. Ways of ensuring patients with advanced CKD/ESKD get rapid and equitable access to these clinics in the event of a stroke remains a challenge. However, before widespread changes in clinical practice are introduced and further burdens placed on these patients, evidence needs to be generated to prove that they would indeed benefit from this intervention. At present, no such evidence exists.
Specialized stroke units
The efficacy of specialized stroke units in decreasing the morbidity and mortality after a stroke has been demonstrated in multiple studies, with the positive effects being observable for several years after the event [72–74]. Regular communication, coordinated care as well as strict adherence to protocols and the best practice are all key features of stroke units [75]. Patients with advanced CKD or ESKD may preferentially be admitted to renal units for ‘specialist’ renal care, including dialysis, and, therefore, inadvertently be denied the best care for an acute stroke. Measures should be in place to ensure that patients who might potentially benefit have access to stroke units and that regular dialysis sessions have minimal impact on treatment and rehabilitation. Nevertheless, the demands placed on haemodialysis patients, both in terms of treatment time and the time taken to recover from a haemodialysis session, may make some interventions, for example, daily physiotherapy, not possible and thus negate some of the potential benefits stroke units offer.
Carotid endarterectomy/stenting
Carotid endarterectomy (CEA) is currently recommended as secondary prevention in patients with a symptomatic high-grade carotid artery stenosis of >70% and potentially in moderate stenosis (50–69%) depending on the severity of symptoms and individual patient characteristics [76]. Carotid artery stenting (CAS) has more recently been introduced as an alternative therapeutic intervention for patients with high risk of surgical complications [77]. Both CAS and CEA were deemed to be equally effective in the CREST study with a 4-year stroke or a death rate of 8% in the CAS group and 6.4% in the CEA group [78]. Stenting was shown to cause more non-disabling stroke peri-procedurally in patients at the age of >70 years, whereas endarterectomy carried a greater peri-procedural risk of acute myocardial infarction, cranial nerve palsies and access site haematoma [78, 79]. Long-term follow-up in the International Carotid Stenting Study revealed a significantly higher 5-year incidence of non-disabling stroke in patients who underwent CAS versus CEA (15.2 versus 9.4%; cumulative risk 8.9 versus 5.8%), although the incidence of disabling or fatal stroke did not differ significantly (6.4 versus 6.5%). Some studies also reported higher rates of severe restenosis or occlusion after treatment in the CAS arm [80].
The current body of evidence suggests that in over 90% of patients with asymptomatic carotid stenosis, intensive medical therapy in the form of antihypertensive treatment, lipid-lowering agents and lifestyle measures are preferable to and confer lower risk than vascular intervention in the form of CEA or stenting. This was confirmed in the SMART trial, which showed an annual risk of stroke of <1% with modern medical therapy alone [81]. Trials to determine any potential benefit of endarterectomy or stenting are still ongoing in asymptomatic patients [82].
To date, there has been no randomized trial focusing on the benefit of carotid revascularization in patients with CKD or ESKD. In a re-analysis of the North American Symptomatic Carotid Endarterectomy Trial (NASCET), which collected data on baseline creatinine, patients with high-grade stenosis and CKD were found to have a much higher 2-year risk of stroke compared with non-CKD patients (31.6 versus 19.3%, respectively) [83]. Treatment with CEA reduced this risk by 82% in CKD patients versus 51% in non-CKD patients. There was a higher risk of cardiac events in the peri-procedural period in the CKD group but similar rates of stroke and death.
Acute reperfusion therapy
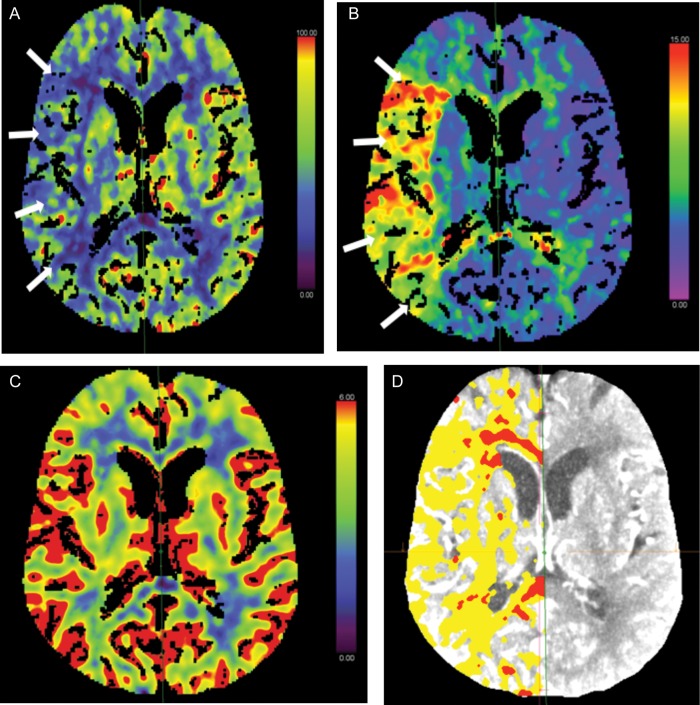
The aim of reperfusion therapy is to relieve the arterial occlusion (recanalization) and restore blood flow as soon as possible, to reduce tissue injury and death, and hence improve outcomes [6]. After an arterial occlusion, there is an area of hypo-perfused brain tissue, the ischaemic penumbra (Figure 1), which could potentially be prevented from progressing to irreversible infarction. With the passage of time, more ischaemic penumbra tissue infarcts, increasing the size of the unsalvageable ischaemic core (Figure 1) and diminishing the potential benefit of restoring perfusion and salvaging brain tissue [6].
Fig. 1.
Computed Tomography perfusion brain parametric maps. Clockwise from left: (A) CBF map demonstrating a large area of reduced perfusion in the right MCA territory (arrows). (B) MTT map shows prolongation within the right MCA territory (arrows), corresponding with reduced CBF in the same region. (C) CBV map demonstrating no abnormality. This represents a CBV/MTT mismatch or ‘ischaemic penumbra’ (salvageable brain tissue). (D) Map demonstrating region of ‘ischaemic penumbra’ (seen in yellow). This patient would be amenable to acute reperfusion therapy in the form of thrombolysis or thrombectomy. CBF, cerebral blood flow; MCA, middle cerebral artery; MTT, mean transit time; CBV, cerebral blood volume.
Reperfusion treatments broadly fall into two categories: intravenous thrombolysis with recombinant tissue plasminogen activator (IV rtPA) and intra-arterial approaches to directly remove the clot.
Intravenous thrombolysis
A systematic review and meta-analysis of 12 randomized controlled trials of IV rtPA given within 6 h of an ischaemic stroke in over 7000 patients confirmed that, in the first 7 days, IV rtPA significantly increased symptomatic intracranial haemorrhage (5.8%), fatal intracranial haemorrhage (2.9%) and death (2.5%) [84]. However, by the end of the studies (1–6 months), there was a significantly increased survival free of dependency (4.2%), improved outcome on the modified Rankin Scale (5.5%) and no significant difference in mortality (19.1% IV rtPA versus 18.5% control) [84]. These findings have been replicated in large observational studies [85, 86], confirming the trial findings and cost effectiveness [87] of IV rtPA in clinical practice. As such, thrombolysis with IV rtPA has become the standard of care in many countries for patients with an acute ischaemic stroke.
However, studies have reported an increased bleeding risk in stroke patients with advanced CKD compared with patients with normal renal function after thrombolysis [88–91]. These studies were all observational and included only small numbers of patients with CKD. There is no good-quality data on the use of IV rtPA in dialysis patients.
Despite the proven benefits of IV rtPA in the general population, there is still significant room for improving outcomes. This is especially true when proximal arterial occlusions are considered. These account for about one-third of all ischaemic strokes and typically result in more severe neurological disability [6]. After IV rtPA alone only 10–15% of internal carotid artery occlusions and 25–50% of proximal middle cerebral artery occlusions recanalize; only 35–40% of patients achieve functional independence [92, 93]. This has led to the development and study of techniques to improve recanalization rates with more direct intra-arterial therapies.
Intra-arterial treatments
Early studies quickly established that endovascular treatments were effective in rapidly re-establishing blood flow after an ischaemic stroke. However, the three first randomized controlled trials of endovascular recanalization published in 2013 failed to demonstrate any improvement in patient outcomes [93–95]. Several factors have been postulated to be responsible for these failures [96]. Firstly, these trials were performed using intra-arterial thrombolysis or first-generation clot retrieval devices. Secondly, patients with mild to moderate stroke severity were included and may have been less likely to benefit from endovascular reperfusion. Thirdly, patients with occlusion of smaller intracranial vessels were included, whereas larger vessel occlusions have been demonstrated to be more resistant to recanalization by IV rtPA and more likely to benefit from endovascular treatment.
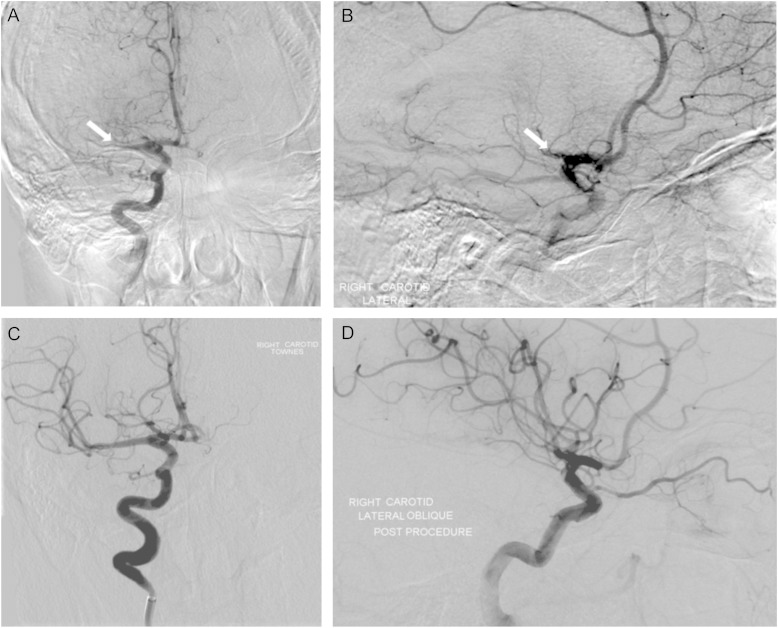
Five studies with stricter inclusion criteria and using third-generation stent-retriever devices published in 2015 [97–101] reported significant clinical benefits of endovascular mechanical therapy (Table 1), with more studies presenting similar results in subsequent international stroke meetings [96]. Figure 2 demonstrates the successful recanalization of the right middle cerebral artery using a third-generation stent-retriever device. It is interesting that although only two of these studies [100, 101] specifically excluded patients with significant renal impairment, only one published baseline renal function and even then only in the supplementary material accompanying the main article (Table 1) [99]. Furthermore, the average age of patients included in four of the five studies was <70 years. Whether patients with advanced age and CKD/ESKD were not recruited into these studies by the investigators because of concerns regarding general frailty or the potential renal toxicity from contrast agents is unclear. Thus, whether these interventions will have the same benefit to harm ratio in patients with CKD/ESKD remains to be determined in a randomized controlled trial. This not only relates to the potential differences in haemorrhagic risk but also to the risk of acute kidney injury associated with the use of contrast media.
Table 1.
Summary of recently published intra-arterial thrombectomy trials
| Trial acronym, n | Baseline NIHSS (range) |
mRS 0–2 at 90 days |
Mortality |
Mean age (years) | Upper age limit (years) | Baseline renal function | Renal exclusion criteria | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Treated | Control (%) | Treated (%) | Control (%) | Treated (%) | |||||
| MR CLEAN 500 [97] | 18 (14–21) | 17 (14–22) | 19 | 33 | 19 | 18 | 65 | None | Not reported | None specified |
| EXTEND IA 70 [98] (stopped early) | 13 (9–19) | 17 (13–20) | 40 | 71 | 20 | 9 | 69 | None | Not reported | None specified |
| ESCAPE 315 [99] (stopped early) | 17 (12–20) | 16 (13–20) | 29 | 53 | 19 | 10 | 71 | None | Control: 84 (SD 27) Treated: 84 (SD 28) | None specified |
| SWIFT PRIME 196 [100] (stopped early) | 17 (13–19) | 17 (13–20) | 36 | 60 | 12 | 9 | 66 | 85 | Not reported | Creatinine >176.8 μmol/L or GFR <30 mL/min/1.73 m2 or on dialysis |
| REVASCAT 206 [101] | 17 (12–19) | 17 (14–20) | 28 | 44 | 16 | 18 | 66 | 85 | Not reported | Creatinine >265.2 μmol/L |
NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin scale; SD, standard deviation; GFR, glomerular filtration rate.
Fig. 2.
Successful intra-arterial treatment for an acute thromboembolic stroke involving the right middle cerebral artery (MCA). Pre-treatment digital subtraction angiogram—anterior (A) and lateral (B) views demonstrate proximal M1 occlusion of the right MCA. Defects are shown by arrows. Post-treatment—anterior (C) and lateral (D) views demonstrate recanalization of the proximal MCA and restoration of flow in its distal branches.
Conclusions
Stroke remains a major cause of disability and mortality worldwide. The increased risk found in CKD/ESKD patients is being increasingly recognized, as is the fact that the current thought processes about risk are insufficient to explain the pathophysiology, which remains poorly understood. In keeping with most conditions associated with CKD/ESKD, there is a distinct lack of good-quality large randomized controlled trials with which to guide treatment. Proven effective therapies for reversing such a sizeable stroke risk in patients with CKD, and especially ESKD, do not exist. This situation needs to be addressed urgently, especially in light of the rapid development of interventions to both reduce the stroke risk and the functional impact following stroke in the general population. History has taught us that blindly extrapolating the results of trials in the general population to patients with CKD/ESKD is fraught with danger [102]. Let us not repeat these mistakes again.
Conflict of interest statement
None declared.
Acknowledgments
We are grateful to the patients who granted us permission to use their anonymized images in this review article. We would also like to acknowledge and thank Dr Satheesh Ramalingam and Dr Kurdow Nader of the Queen Elizabeth Hospital, Birmingham Neuroradiology Department for their input in selecting and interpreting images for use in this article.
References
- 1.Aho K, Harmsen P, Hatano S, et al. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ 1980; 58: 113–130 [PMC free article] [PubMed] [Google Scholar]
- 2.A classification and outline of cerebrovascular diseases. II. Stroke 1975; 6: 564–616 [DOI] [PubMed] [Google Scholar]
- 3.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 2064–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010; 121: 948–954 [DOI] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA 2015; 313: 1451–1462 [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–e322 [DOI] [PubMed] [Google Scholar]
- 8.Solomon NA, Glick HA, Russo CJ, et al. Patient preferences for stroke outcomes. Stroke 1994; 25: 1721–1725 [DOI] [PubMed] [Google Scholar]
- 9.Masson P, Webster AC, Hong M, et al. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 2015; 30: 1162–1169 [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Perales C, Vazquez E, Garcia-Cortes MJ, et al. Ischaemic stroke in incident dialysis patients. Nephrol Dial Transplant 2010; 25: 3343–3348 [DOI] [PubMed] [Google Scholar]
- 11.Wolfe CD. The impact of stroke. Br Med Bull 2000; 56: 275–286 [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 13.Silverwood RJ, Richards M, Pierce M, et al. Cognitive and kidney function: results from a British birth cohort reaching retirement age. PLoS One 2014; 9: e86743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Renal Data System. USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethseda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 15.Wang HH, Hung SY, Sung JM, et al. Risk of stroke in long-term dialysis patients compared with the general population. Am J Kidney Dis 2014; 63: 604–611 [DOI] [PubMed] [Google Scholar]
- 16.Ovbiagele B. Chronic kidney disease and risk of death during hospitalization for stroke. J Neurol Sci 2011; 301: 46–50 [DOI] [PubMed] [Google Scholar]
- 17.Herrington W, Haynes R, Staplin N, et al. Evidence for the prevention and treatment of stroke in dialysis patients. Semin Dial 2015; 28: 35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moody WE, Chue CD, Inston NG, et al. Understanding the effects of chronic kidney disease on cardiovascular risk: are there lessons to be learnt from healthy kidney donors? J Hum Hypertens 2012; 26: 141–148 [DOI] [PubMed] [Google Scholar]
- 19.Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol 2014; 13: 823–833 [DOI] [PubMed] [Google Scholar]
- 20.Demarin V, Zikic M, Zikic TR. Stroke: a historical overview and contemporary management. Curr Top Neurol Pyschiatr Relat Discip 2011; 19: 15–23 [Google Scholar]
- 21.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913 [DOI] [PubMed] [Google Scholar]
- 22.Blood Pressure Lowering Treatment Trialists Collaboration, Ninomiya T, Perkovic V, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. BMJ 2013; 347: f5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heerspink HJ, Ninomiya T, Zoungas S, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet 2009; 373: 1009–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntyre CW, Goldsmith DJ. Ischemic brain injury in hemodialysis patients: which is more dangerous, hypertension or intradialytic hypotension? Kidney Int 2015; 87: 1109–1115 [DOI] [PubMed] [Google Scholar]
- 25.Cholesterol Treatment Trialists Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376: 1670–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer SC, Craig JC, Navaneethan SD, et al. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157: 263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353: 238–248 [DOI] [PubMed] [Google Scholar]
- 28.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360: 1395–1407 [DOI] [PubMed] [Google Scholar]
- 29.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377: 2181–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berns JS. A sharp study, but with blunted conclusions. Semin Dial 2011; 24: 684–685 [DOI] [PubMed] [Google Scholar]
- 31.Massy ZA, de Zeeuw D. LDL cholesterol in CKD—to treat or not to treat? Kidney Int 2013; 84: 451–456 [DOI] [PubMed] [Google Scholar]
- 32.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33: 2719–2747 [DOI] [PubMed] [Google Scholar]
- 33.Sherzai AZ, Elkind MS. Advances in stroke prevention. Ann N Y Acad Sci 2015; 1338: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng KP, Edwards NC, Lip GY, et al. Atrial fibrillation in CKD: balancing the risks and benefits of anticoagulation. Am J Kidney Dis 2013; 62: 615–632 [DOI] [PubMed] [Google Scholar]
- 35.Boriani G, Savelieva I, Dan GA, et al. Chronic kidney disease in patients with cardiac rhythm disturbances or implantable electrical devices: clinical significance and implications for decision making-a position paper of the European Heart Rhythm Association endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace 2015; 17: 1169–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friberg L, Benson L, Lip GY. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J 2015; 36: 297–306 [DOI] [PubMed] [Google Scholar]
- 37.Banerjee A, Fauchier L, Vourc'h P, et al. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. J Am Coll Cardiol 2013; 61: 2079–2087 [DOI] [PubMed] [Google Scholar]
- 38.Roldan V, Marin F, Fernandez H, et al. Renal impairment in a “real-life” cohort of anticoagulated patients with atrial fibrillation (implications for thromboembolism and bleeding). Am J Cardiol 2013; 111: 1159–1164 [DOI] [PubMed] [Google Scholar]
- 39.Apostolakis S, Guo Y, Lane DA, et al. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur Heart J 2013; 34: 3572–3579 [DOI] [PubMed] [Google Scholar]
- 40.Shah M, Avgil Tsadok M, Jackevicius CA, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 2014; 129: 1196–1203 [DOI] [PubMed] [Google Scholar]
- 41.Qamar A, Bhatt DL. Anticoagulation therapy: balancing the risks of stroke and bleeding in CKD. Nat Rev Nephrol 2015; 11: 200–202 [DOI] [PubMed] [Google Scholar]
- 42.Schlieper G, Floege J. Challenging the use of warfarin in patients on dialysis with atrial fibrillation. Nat Rev Nephrol 2015; 11: 450. [DOI] [PubMed] [Google Scholar]
- 43.Findlay MD, Thomson PC, Fulton RL, et al. Risk factors of ischemic stroke and subsequent outcome in patients receiving hemodialysis. Stroke 2015; 46: 2477–2481 [DOI] [PubMed] [Google Scholar]
- 44.Shen JI, Montez-Rath ME, Lenihan CR, et al. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis 2015; 66: 677–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.JBS3 Board. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014; 100 (Suppl 2): ii1–ii67 [DOI] [PubMed] [Google Scholar]
- 46.Palmer SC, Di Micco L, Razavian M, et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev 2013; 2: CD008834. [DOI] [PubMed] [Google Scholar]
- 47.Kim AJ, Lim HJ, Ro H, et al. Low-dose aspirin for prevention of cardiovascular disease in patients with chronic kidney disease. PLoS One 2014; 9: e104179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324: 71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chue CD, Townend JN, Steeds RP, et al. Arterial stiffness in chronic kidney disease: causes and consequences. Heart 2010; 96: 817–823 [DOI] [PubMed] [Google Scholar]
- 50.Ferro CJ, Chue CD, Steeds RP, et al. Is lowering phosphate exposure the key to preventing arterial stiffening with age? Heart 2009; 95: 1770–1772 [DOI] [PubMed] [Google Scholar]
- 51.Bugnicourt JM, Chillon JM, Massy ZA, et al. High prevalence of intracranial artery calcification in stroke patients with CKD: a retrospective study. Clin J Am Soc Nephrol 2009; 4: 284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 2011; 305: 1119–1127 [DOI] [PubMed] [Google Scholar]
- 53.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray AM, Seliger S, Lakshminarayan K, et al. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol 2013; 24: 1166–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eckardt KU, Gillespie IA, Kronenberg F, et al. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int 2015; 88: 1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toyoda K, Fujii K, Fujimi S, et al. Stroke in patients on maintenance hemodialysis: a 22-year single-center study. Am J Kidney Dis 2005; 45: 1058–1066 [DOI] [PubMed] [Google Scholar]
- 57.Sozio SM, Armstrong PA, Coresh J, et al. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: the choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis 2009; 54: 468–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foley RN, Gilbertson DT, Murray T, et al. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011; 365: 1099–1107 [DOI] [PubMed] [Google Scholar]
- 59.Maduell F, Moreso F, Pons M, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013; 24: 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Group FHNT, Chertow GM, Levin NW, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010; 363: 2287–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 2007; 298: 1291–1299 [DOI] [PubMed] [Google Scholar]
- 62.Eldehni MT, Odudu A, McIntyre CW. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 2015; 26: 957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah RV, Rubenfire M, Brook RD, et al. Heterogeneity in statin indications within the 2013 American college of cardiology/American heart association guidelines. Am J Cardiol 2015; 115: 27–33 [DOI] [PubMed] [Google Scholar]
- 64.Abramson JL, Jurkovitz CT, Vaccarino V, et al. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: the ARIC Study. Kidney Int 2003; 64: 610–615 [DOI] [PubMed] [Google Scholar]
- 65.Tripepi G, Mattace-Raso F, Rapisarda F, et al. Traditional and nontraditional risk factors as predictors of cerebrovascular events in patients with end stage renal disease. J Hypertens 2010; 28: 2468–2474 [DOI] [PubMed] [Google Scholar]
- 66.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 67.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 68.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 69.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339: 584–590 [DOI] [PubMed] [Google Scholar]
- 70.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007; 370: 1432–1442 [DOI] [PubMed] [Google Scholar]
- 71.Power A, Edwards C, Sawyer J, et al. Screening for transient ischemic attacks in hemodialysis patients. J Nephrol 2013; 26: 919–924 [DOI] [PubMed] [Google Scholar]
- 72.Stroke Units Tralists Collaboration. How do stroke units improve patient outcomes? A collaborative systematic review of the randomized trials. Stroke Unit Trialists Collaboration. Stroke 1997; 28: 2139–2144 [DOI] [PubMed] [Google Scholar]
- 73.Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013; 9: CD000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKinney JS, Cheng JQ, Rybinnik I, et al. Comprehensive stroke centers may be associated with improved survival in hemorrhagic stroke. J Am Heart Assoc 2015; 4: e001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947 [DOI] [PubMed] [Google Scholar]
- 76.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37: 577–617 [DOI] [PubMed] [Google Scholar]
- 77.Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med 2008; 358: 1572–1579 [DOI] [PubMed] [Google Scholar]
- 78.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363: 11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bonati LH, Dobson J, Featherstone RL, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet 2015; 385: 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol 2008; 7: 893–902 [DOI] [PubMed] [Google Scholar]
- 81.den Hartog AG, Achterberg S, Moll FL, et al. Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease. Stroke 2013; 44: 1002–1007 [DOI] [PubMed] [Google Scholar]
- 82.Spence JD. Management of asymptomatic carotid stenosis. Neurol Clin 2015; 33: 443–457 [DOI] [PubMed] [Google Scholar]
- 83.Mathew A, Eliasziw M, Devereaux PJ, et al. Carotid endarterectomy benefits patients with CKD and symptomatic high-grade stenosis. J Am Soc Nephrol 2010; 21: 145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012; 379: 2364–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275–282 [DOI] [PubMed] [Google Scholar]
- 86.Shobha N, Buchan AM, Hill MD, et al. Thrombolysis at 3–4.5 hours after acute ischemic stroke onset—evidence from the Canadian Alteplase for Stroke Effectiveness Study (CASES) registry. Cerebrovasc Dis 2011; 31: 223–228 [DOI] [PubMed] [Google Scholar]
- 87.Tan Tanny SP, Busija L, Liew D, et al. Cost-effectiveness of thrombolysis within 4.5 hours of acute ischemic stroke: experience from Australian stroke center. Stroke 2013; 44: 2269–2274 [DOI] [PubMed] [Google Scholar]
- 88.Agrawal V, Rai B, Fellows J, et al. In-hospital outcomes with thrombolytic therapy in patients with renal dysfunction presenting with acute ischaemic stroke. Nephrol Dial Transplant 2010; 25: 1150–1157 [DOI] [PubMed] [Google Scholar]
- 89.Lyrer PA, Fluri F, Gisler D, et al. Renal function and outcome among stroke patients treated with IV thrombolysis. Neurology 2008; 71: 1548–1550 [DOI] [PubMed] [Google Scholar]
- 90.Naganuma M, Koga M, Shiokawa Y, et al. Reduced estimated glomerular filtration rate is associated with stroke outcome after intravenous rt-PA: the Stroke Acute Management with Urgent Risk-Factor Assessment and Improvement (SAMURAI) rt-PA registry. Cerebrovasc Dis 2011; 31: 123–129 [DOI] [PubMed] [Google Scholar]
- 91.Power A, Epstein D, Cohen D, et al. Renal impairment reduces the efficacy of thrombolytic therapy in acute ischemic stroke. Cerebrovasc Dis 2013; 35: 45–52 [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez RG, Furie KL, Goldmacher GV, et al. Good outcome rate of 35% in IV-tPA-treated patients with computed tomography angiography confirmed severe anterior circulation occlusive stroke. Stroke 2013; 44: 3109–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013; 368: 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013; 368: 904–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013; 368: 914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khatri P, Hacke W, Fiehler J, et al. State of acute endovascular therapy: report from the 12th thrombolysis, thrombectomy, and acute stroke therapy conference. Stroke 2015; 46: 1727–1734 [DOI] [PubMed] [Google Scholar]
- 97.Berkhemer OA, Majoie CB, Dippel DW, et al. Endovascular therapy for ischemic stroke. N Engl J Med 2015; 372: 2363. [DOI] [PubMed] [Google Scholar]
- 98.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018 [DOI] [PubMed] [Google Scholar]
- 99.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030 [DOI] [PubMed] [Google Scholar]
- 100.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295 [DOI] [PubMed] [Google Scholar]
- 101.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306 [DOI] [PubMed] [Google Scholar]
- 102.Ng KP, Townend JN, Ferro CJ. Randomised-controlled trials in chronic kidney disease—a call to arms! Int J Clin Pract 2012; 66: 913–915 [DOI] [PubMed] [Google Scholar]