Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) is increasingly recognized as a distinct entity with unique pathophysiology. In the Dietary Approaches to Stop Hypertension in Diastolic Heart Failure (DASH-DHF) study, the sodium-restricted Dietary Approaches to Stop Hypertension (DASH/SRD) diet was associated with improved blood pressure and cardiovascular function in 13 hypertensive patients with HFpEF. Using targeted metabolomics, we explored metabolite changes and their relationship with energy-dependent measures of cardiac function in DASH-DHF.
Methods and Results
Using chromatography and mass spectrometry, 152 metabolites including amino acids, free fatty acids, phospholipids, diglycerides, triglycerides, cholesterol esters and acyl carnitines were measured. Comparison of baseline and post-DASH/SRD samples revealed increases in short-chain acetyl, butryl and propionyl carnitines (p=0.02, 0.03, 0.03 respectively). Increases in propionyl carnitine correlated with ventricular–arterial coupling ratio (Ees:Ea; r=0.78; p =0.005) and ventricular contractility (maximum rate of change of pressure-normalized stress dσ*/dtmax; r=0.66; p=0.03). Changes in L-carnitine also correlated with Ees:Ea (r=0.62; p=0.04), dσ*/dtmax (r=0.60; p=0.05) and inversely with ventricular stiffness (k; r=−0.63, p=0.03).
Conclusions
Metabolite profile changes of patients with HFpEF during dietary modification with DASH/SRD suggest improved energy substrate utilization. Additional studies are needed to clarify connections between diet, metabolic changes, and myocardial function in HFpEF.
Keywords: heart failure with preserved ejection fraction, DASH diet, low sodium diet, metabolomics
Introduction
Heart failure with preserved ejection fraction (HFpEF) is rising in incidence as the population ages and as associated comorbidities become more common.1 As recent clinical trials have not identified evidence-based drug therapy specific for HFpEF, there is renewed interest in non-pharmacological management strategies.2 “Salt-sensitive” animal models suggest that dietary modification could improve cardiovascular function in HFpEF and impact pathophysiological mechanisms.3 In the Dietary Approaches to Stop Hypertension in Diastolic Heart Failure (DASH-DHF) pilot study, the sodium-restricted Dietary Approaches to Stop Hypertension (DASH/SRD) diet was associated with improved blood pressure and cardiovascular function in a small group of hypertensive patients with HFpEF.4, 5
Translational studies imply that HFpEF pathophysiology stems from systemic metabolic derangement rather than a primary cardiac insult.6 One recent publication outlined a metabolomic ‘fingerprint’ for HFpEF, noting substantial differences in specific carnitines, amino acids, and lipids when compared to age-matched controls and heart failure with reduced ejection fraction.7 We hypothesized that metabolic changes during DASH/SRD would be related to energy-dependent changes in cardiac function in patients with HFpEF, and explored this concept using targeted metabolomics in serum samples from the DASH-DHF study.
Methods
DASH-DHF pilot study design
The design and conduct of the DASH-DHF study have previously been published.4, 5 In brief, 13 patients with treated hypertension and stable HFpEF consumed the sodium-restricted (50 mmol/day) Dietary Approaches to Stop Hypertension (DASH/SRD) diet for 21 days (Supplementary Figure 1). Detailed medication histories, ambulatory blood pressure monitoring, transthoracic echocardiography, non-invasive vascular testing, blood samples, and 24-hour urinary collections were performed pre- and post-intervention. Baseline nutrient intake was estimated by Block Food Frequency Questionnaire and scored by NutritionQuest (Berkley, CA). Food diaries analyzed by Nutrition Data System for Research software estimated nutrient intake during the intervention (see Supplementary Tables 1 and 2).
Metabolite measurements: (please see Supplemental Methods for detailed description)
Short chain fatty acids and amino acids were measured using gas chromatography/mass spectrometry (GC/MS) in a targeted manner. Long chain fatty acid estimation and fatty acid species quantification of lipid fractions was achieved after separation using Thin Layer Chromatography. Lipids were then methylated using BF3-methanol (14% solution from Sigma) and analyzed by GC. Acyl carnitine levels were measured using targeted liquid chromatography/MS.
Echocardiographic analysis
Standard measures of ventricular structure and function were obtained as per American Society of Echocardiography recommendations for clinical studies. In addition, as described in our previous publication,5 we measured in blinded fashion: 1) ventricular diastolic function with the Parametrized Diastolic Formalism method, which has been invasively validated in patients with diastolic dysfunction8 and yields stiffness (k, spring constant), and relaxation/viscoelastic (c, damping constant) diastolic function components; 2) ventricular contractility as the maximum rate of change of pressure-normalized stress (dσ*/dtmax), previously invasively validated and shown to predict prognosis in HFpEF;9 and 3) ventricular–arterial (V–A) coupling ratio (Ees:Ea) using previously published single-beat methods.10
Statistical analysis
All pre and post metabolite concentrations were log transformed before performing paired 2 sample t tests. Correlation between changes in metabolite levels and changes in echocardiographic parameters were determined using Pearson’s correlation. Nominal p values <0.15 were considered to indicate trends and <0.05 statistical significance. Heat Maps were generated using the MetaboAnalyst platform. All other statistical analysis used SAS software.
Results
The primary results of the DASH-DHF study have been previously published. As in other HFpEF cohorts, participants were primarily obese post-menopausal women (age 72±10 years, 12/13 female, BMI 36±8 kg/m2) with multiple medical comorbidities (100% hypertension, 43% diabetes mellitus, 100% with stage II/III chronic kidney disease). Estimated total fat, cholesterol, and sodium intake decreased while fiber and potassium intake increased on the study diet (Supplementary Table 2). The DASH/SRD was associated with reduced 24-hour ambulatory systolic blood pressure (130±4 to 123±4 mmHg, p=0.02), improved ventricular diastolic stiffness and relaxation (k: 252±115 to 170±37 g/s2; p=0.03 and c: 24.3±5.3 to 22.7±8.1 g/s; p=0.03), and increased ventricular contractility and V-A coupling (dσ*/dtmax: 1.6±0.5 to 1.8±0.5 s−1; p=0.01, and Ees:Ea: 1.5±0.3 to 1.7±0.4; p=0.04).4, 5
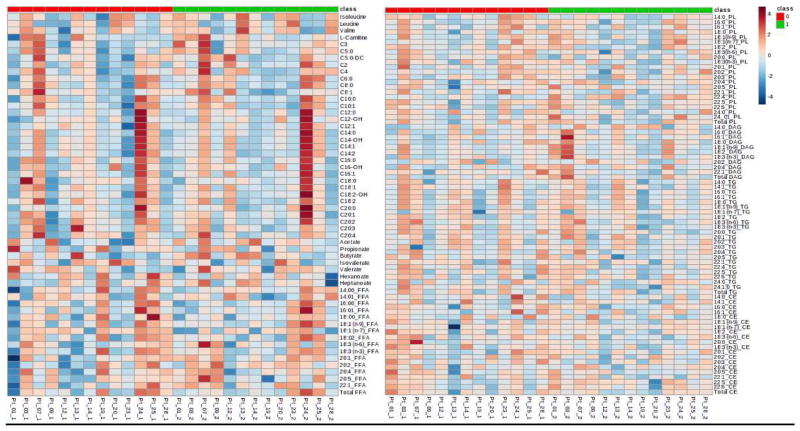
Pre-post changes in metabolites are shown in Figure 1 and Supplementary Table 3. The DASH/SRD was associated with trend towards decreased triglycerides and cholesterol esters and increased diglycerides. The total free fatty acid levels also trended lower, particularly saturated long-chain fatty acids. Short-chain fatty acids like acetate (C2), butyrate (C4), valerate (C5) and Heptanoate (C7) tended to increase, while propionate (C3) and isovalerate, a branched 5-carbon fatty acid, significantly decreased.
Figure 1. Metabolites pre- and post-DASH/SRD intervention.
Heat map of log transformed and auto scaled to standard deviation of each metabolite concentration demonstrating individual subject levels before DASH/SRD diet in the red section and after the diet in the green section. Panel A: Branched chain amino acids, acyl carnitines, short chain fatty acids and long chain fatty acids. Panel B: Phospholipid, diglycerides, triglycerides and cholesterol esters
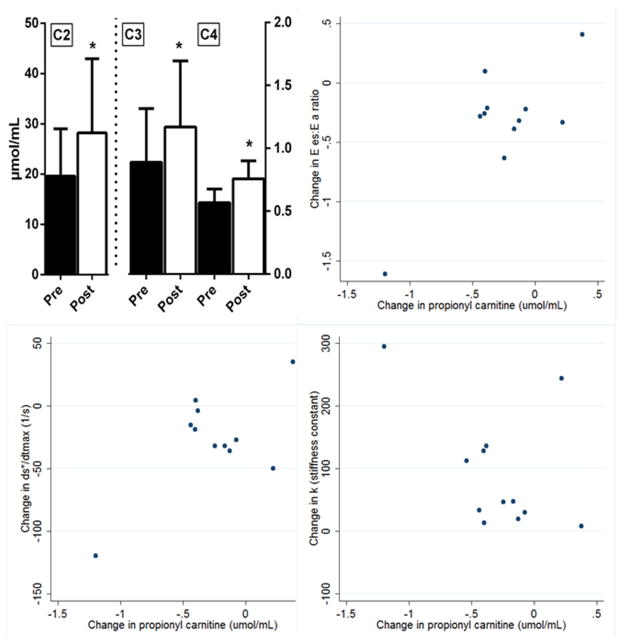
The total carnitine and the individual acyl carnitines increased following DASH/SRD, in particular short-chain carnitines (C2/acetyl, C3/propionyl, and C4/butryl; Figure 2, top left). The branched-chain amino acids leucine, isoleucine, and valine, substrates for formation of odd-carbon short-chain acyl carnitines like propionyl carnitine and C5 acyl carnitine trended higher.
Figure 2. Short-chain carnitines and relationship of C3 carnitine with cardiac function.
Abbreviations: C2, acetyl carnitine; C3, propionyl carnitine; C4, butryl carnitine; Ees:Ea ratio, ventricular-arterial coupling ratio; ds*/dtmax, maximum rate of change of pressure-normalized stress; k, ventricular stiffness constant
* denotes p<0.05
The change in propionyl carnitine (C3) was significantly related (Figure 2) to changes in Ees:Ea (r=0.78; p =0.005) and dσ*/dtmax (r=0.66; p=0.03); there was a trend toward inverse relationship with changes in ventricular stiffness k (r=−0.47, p=0.13) but no relationship with the relaxation constant c (r=−0.18, p=0.57). Overall, L-carnitine levels did not significantly change following DASH/SRD. However, on an individual basis changes in propionyl carnitine were closely related to changes in L-carnitine (r=0.83, p<0.001), which also correlated with changes in Ees:Ea (r=0.62; p =0.04), dσ*/dtmax (r=0.60; p=0.05), and k (r=−0.63, p=0.03). Changes in these echocardiographic parameters were unrelated to changes in body weight, 24-hour urinary sodium or potassium excretion (all p>0.6). Blood pressure changes correlated with changes in c (r=0.68, p=0.01), but not other echocardiographic parameters.
Discussion
In 13 hypertensive patients with HFpEF, 3 weeks of the DASH/SRD increased short-chain acyl carnitines. In turn, diet-induced changes in L-carnitine and propionyl carnitine correlated with improved left ventricular function, suggesting improved entry of these acyl residues into the mitochondria for myocardial energy utilization.
Results are broadly consistent with previous studies demonstrating increased acyl carnitine metabolites in HFpEF,7 and suggest improved short-chain acyl residue availability after the DASH/SRD diet. Short acyl residues could be derived from short-chain fatty acid production in the intestines as a result of increased fiber intake or as a result of branched chain amino acid oxidation. Branched chain amino acids isoleucine, leucine and valine generally increased following DASH/SRD, but some of the short-chain fatty acids decreased; the exact source of the short acyl residues remains unclear.
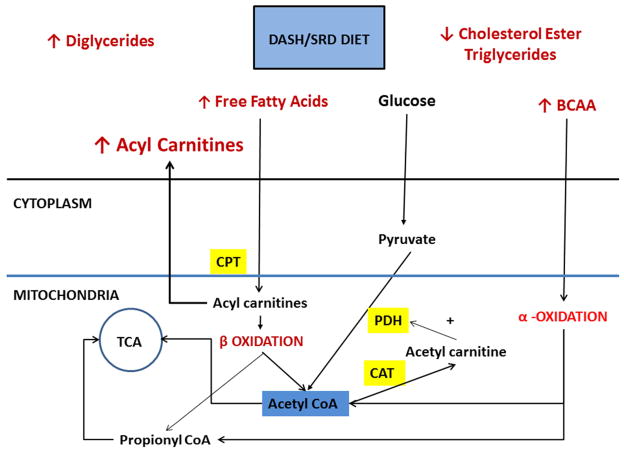
Of the short acyl carnitines, propionyl carnitine showed the highest correlation to altered echo parameters. Cardiomyocytes derive energy primarily through fatty acid beta-oxidation, but progressing heart failure promotes a shift to other energy substrates like glucose, amino acids and ketone bodies11. Propionyl carnitine has a high affinity for carnitine transferase; it increases cellular carnitine content, thereby allowing free fatty acid transport into the mitochondria. Carnitines decrease the acetyl CoA-to-CoA ratio, resulting in decreased inhibition of pyruvate dehydrogenase complex, the rate limiting step to glucose oxidation by conversion of acetyl CoA to acetyl carnitines. Moreover, propionyl-L-carnitine improves efficiency of the Krebs cycle during hypoxia by providing it with an easily usable substrate, propionate, which is rapidly transformed into succinate without energy consumption 12 (Figure 3).
Figure 3. Metabolic changes during DASH/SRD and potential relation to cardiac energetics.
Abbreviations: BCAA: branched-chain amino acids; CAT: carnitine acyl transferase; CPT: Carnitine palmitoyltransferase; PDH: Pyruvate dehydrogenase; TCA: tricarboxylic acid cycle
Schematic diagram of effects of DASH/SRD diet on metabolic profile including decreased triglycerides and cholesterol esters and increased diglycerides is shown in Figure 3. Increased circulating acyl carnitines, free fatty acids and branched chain amino acids (BCAA) enter the cell for further oxidation. The fatty acids enter the mitochondria after conversion in to acyl carnitines, excess acyl carnitines escape in to the circulation. Beta oxidation produces acetyl Co A from even chain acyl residues and propionyl CoA from odd chain residues which then enter the Tricarboxylic cycle (TCA). As more carnitine is made available, acetyl CoA to acetyl carnitine conversion by carnitine acetyl transferase (CAT) favors stimulation of pyruvate dehydrogenase (PDH) and entry in to the TCA cycle. BCAA also is oxidized to acetyl and propionyl residues that enter the TCA cycle as well. Thus DASH/SRD diet appears to promote energy utilization through entry of acyl carnitines.
Propionyl carnitine has been explored as supplement to improve exercise capacity in heart failure. However, a previous randomized trial of propionyl carnitine in 537 heart failure patients with reduced ejection fraction did not demonstrate increased exercise capacity. In an exploratory post-hoc analysis of this trial, exercise capacity improved in patients with left ventricular ejection fraction 30–40%, suggesting that carnitine supplementation might benefit patients with more preserved ventricular systolic function.13 In a recent pilot study, L-carnitine supplementation improved diastolic function and reduced dyspnea in patients with mild HFpEF.14 In the Dahl salt-sensitive rat, an animal model of HFpEF, L-carnitine dietary supplementation reduces ventricular fibrosis, improves cardiac function, and increases survival despite continued high-sodium intake.15
There are several limitations to our study including the small size, predominantly female study subjects, single-arm design, and the lack of power to demonstrate strong trends. Our study used a targeted approach of multiple metabolomic assays (10–30 metabolites in each) and is primarily hypothesis generating. We did not adjust for false discovery rate, as the main aim was to detect trends with dietary modification that would be worthy of further study in larger cohorts. Similar strategy was applied in the only other previously published metabolomic study in patients with HFpEF.7 Interestingly, short-chain acyl-carnitines were key differentiators between HFpEF and controls in this prior study. Our exploratory findings need to be confirmed after accounting for the false discovery rate in future studies.
To our knowledge, this is the first exploration of diet-related metabolite changes and their relation to cardiac functional parameters in HFpEF. Our results suggest connections between dietary changes, energy utilization, and myocardial function that should be further explored in larger studies.
Conclusions
In hypertensive patients with HFpEF, short-term dietary modification with DASH/SRD produced potentially beneficial metabolic changes, including increases in short-chain acyl carnitines that correlated with improvements in cardiac function. Additional studies are needed to clarify connections between diet, metabolic changes, and myocardial function in HFpEF.
Supplementary Material
Article highlights.
In a single-arm study in 13 patients with hypertensive heart failure and preserved ejection fraction (HFpEF), 3 weeks of the sodium-restricted Dietary Approaches to Stop Hypertension diet (DASH/SRD) improved energy-dependent indices of cardiac function.
In addition to expected trends in lipid subfractions, consumption of the DASH/SRD increased short-chain acyl carnitine levels
Increases in propionyl carnitine and L-carnitine correlated with changes in ventricular contractility and ventricular-vascular coupling, suggesting that connections between metabolites and myocardial function in HFpEF should be further explored
Acknowledgments
Dr. Hummel is supported by NIH grant HL1091976; metabolomic analyses were performed by the University of Michigan Metabolomics Center, supported in part by NIH grants DK089503, DK097153 and DK081943. Dr. Mitchell Seymour is supported by NIH grant UL1TR000433.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction.[see comment] N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Senni M, Paulus WJ, Gavazzi A, Fraser AG, Díez J, Solomon SD, Smiseth OA, Guazzi M, Lam CSP, Maggioni AP, Tschöpe C, Metra M, Hummel SL, Edelmann F, Ambrosio G, Stewart Coats AJ, Filippatos GS, Gheorghiade M, Anker SD, Levy D, Pfeffer MA, Stough WG, Pieske BM. New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. 2014 doi: 10.1093/eurheartj/ehu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive dahl rats: Focus on heart failure with preserved ejection fraction. See comment. Hypertension. 2006;47:901–911. doi: 10.1161/01.HYP.0000215579.81408.8e. [DOI] [PubMed] [Google Scholar]
- 4.Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, Rosenblum HR, Wells JM, Weder AB. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction/novelty and significance. Hypertension. 2012;60:1200–1206. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovacs SJ, Kolias TJ. Low-sodium dash diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail. 2013;6:1165–1171. doi: 10.1161/CIRCHEARTFAILURE.113.000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 7.Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, Bouatra S, Anderson T, Oudit GY, Wishart DS, Dyck JR. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS One. 2015;10:e0124844. doi: 10.1371/journal.pone.0124844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisauskas JB, Singh J, Bowman AW, Kovács SJ. Chamber properties from transmitral flow: Prediction of average and passive left ventricular diastolic stiffness. J Appl Physiol. 2001;91:154–162. doi: 10.1152/jappl.2001.91.1.154. [DOI] [PubMed] [Google Scholar]
- 9.Zhong L, Ng KKC, Sim LL, Allen JC, Lau YH, Sim DKL, Lee RKK, Poh KK, Chua TSJ, Kassab GS, Kwok BWK, Tan RS. Myocardial contractile dysfunction associated with increased 3-month and 1-year mortality in hospitalized patients with heart failure and preserved ejection fraction. Int J Cardiol. 2013;168:1975–1983. doi: 10.1016/j.ijcard.2012.12.084. [DOI] [PubMed] [Google Scholar]
- 10.Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease: Insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolwicz SC, Jr, Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. 2013;113:603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari R, Merli E, Cicchitelli G, Mele D, Fucili A, Ceconi C. Therapeutic effects of l-carnitine and propionyl-l-carnitine on cardiovascular diseases: A review. Ann N Y Acad Sci. 2004;1033:79–91. doi: 10.1196/annals.1320.007. [DOI] [PubMed] [Google Scholar]
- 13.Study on propionyl-l-carnitine in chronic heart failure. Eur Heart J. 1999;20:70–76. doi: 10.1053/euhj.1998.1271. [DOI] [PubMed] [Google Scholar]
- 14.Serati AR, Motamedi MR, Emami S, Varedi P, Movahed MR. L-carnitine treatment in patients with mild diastolic heart failure is associated with improvement in diastolic function and symptoms. Cardiology. 2010;116:178–182. doi: 10.1159/000318810. [DOI] [PubMed] [Google Scholar]
- 15.Omori Y, Ohtani T, Sakata Y, Mano T, Takeda Y, Tamaki S, Tsukamoto Y, Kamimura D, Aizawa Y, Miwa T, Komuro I, Soga T, Yamamoto K. L-carnitine prevents the development of ventricular fibrosis and heart failure with preserved ejection fraction in hypertensive heart disease. J Hypertens. 2012;30:1834–1844. doi: 10.1097/HJH.0b013e3283569c5a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.