Abstract
Purpose
The clinical effects of sunitinib on human myeloid-derived suppressor cell (MDSC) subsets and correlation of the T-cell–mediated immune responses and clinical outcomes in patients with oligometastases treated by stereotactic body radiotherapy (SBRT) have been evaluated.
Experimental Design
The numbers of granulocytic and monocytic MDSC subsets, effector T cells, and regulatory T cells in the peripheral blood were evaluated pre- and post-sunitinib treatment and concurrent with SBRT. Correlations between MDSC, Treg, and T-cell responses and clinical outcomes were analyzed.
Results
Patients with oligometastases of various cancer types had elevated granulocytic MDSC and certain subsets of monocytic MDSC population. Sunitinib treatment resulted in a significant reduction in monocytic MDSC, phosphorylated STAT3, and arginase levels in monocytic MDSC (CD33+CD14+CD16+), and an increase in T-cell proliferative activity in cancer patients. Interestingly, the effects of sunitinib on reducing the accumulation and immune-suppressive function of MDSC were significantly correlated with Treg reduction, in responders but not in nonresponding patients. SBRT synergized the therapeutic effects of sunitinib, especially as related to decreased numbers of monocytic MDSC, Treg, and B cells, and augmented Tbet expression in primary CD4 and CD8 T cells. These effects were not observed in patients receiving radiation therapy alone. Most interestingly, the responders, defined by sunitinib-mediated reduction in CD33+CD11b+ myeloid cell populations, tend to exhibit improved progression-free survival and cause-specific survival.
Conclusions
Sunitinib treatment increased the efficacy of SBRT in patients with oligometastases by reversing MDSC and Treg-mediated immune suppression and may enhance cancer immune therapy to prevent tumor recurrence post-SBRT.
Introduction
Stereotactic body radiotherapy (SBRT) utilizes high doses of focused radiation which selectively spares adjacent healthy organs to safely ablate various primary and metastatic tumors (1). Patients with limited distant metastases or oligometastases, which were historically considered incurable, present a particularly attractive patient population for applying SBRT (2). Although SBRT for oligometastases can successfully control the majority of targeted tumors, the majority of patients eventually develop additional distant metastases.
Adding systemic therapy to radiation therapy has improved overall survival (OS) in various solid tumor types by enhancing locoregional control of the targeted tumors and by preventing distant metastases (3). Agents that enhance the response to radiation include cytotoxic chemotherapeutic agents and biologically targeted agents, such as EGFR inhibitors, immunotherapies, and angiogenesis inhibitors (4). Identification of an optimal reagent for enhancing systemic antitumor responses will significantly benefit SBRT therapy.
Sunitinib (Sutent), a multitargeted tyrosine kinase inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, c-kit, FLT3, and RET, is a well-studied angiogenesis inhibitor with an acceptable single-agent toxicity profile (5). Preclinical studies suggest that sunitinib and other angiogenesis inhibitors may enhance the antitumor responses of radiotherapy (6). Therefore, we initiated a phase I/II clinical trial to evaluate the safety and efficacy of concurrent sunitinib and SBRT for patients with oligometastases (7). Recently, we reported that the 4-year progression-free survival (PFS) and OS rates of patients with historically incurable oligometastases in this phase I/II clinical trial were 34% and 29%, respectively (8). Our data suggest that sunitinib treatment during radiotherapy may have a significant effect on micrometastases, thus preventing distant progression in a subset of patients with oligometastases (9). Several research groups, including ours, have demonstrated the robust effects of sunitinib on reducing myeloid-derived suppressor cells (MDSC; refs. 10, 11). However, correlation of this effect on MDSC with T-cell responses and clinical outcomes in SBRT patients has not been previously explored.
In humans, myeloid cell markers CD33, CD11b, and HLA-DR are used to characterize human MDSC and CD15 is specific for granulocytic MDSC. However, the absence of a universal marker makes proper identification of human monocytic MDSC more difficult and complicated. There are two monocytic populations in human blood that can be distinguished by the lipopolysaccharide coreceptor, CD14, and Fcγ-receptor, CD16. In healthy individuals, CD14++CD16− classical monocytes are the major population (~90%), whereas CD14+CD16+ nonclassical monocytes (proinflammatory monocytes) account for only 5% to 10% of circulating monocytes. Nevertheless, these proinflammatory monocytes (CD14+CD16+) are significantly increased in patients with systemic infections (12), and are associated with cardiovascular disease and atherosclerosis (13). In addition, it has been reported that CD14+CD16+ proinflammatory monocytes selectively upregulate Tie2 expression and may be involved in tumor infiltration and angiogenesis (14), and have biologic activities similar to M2-like macrophages. Recently, our group demonstrated that monocytic MDSC could further differentiate into an immunosuppressive M2 phenotype or a proinflammatory M1 phenotype dependent on the internal signaling of mouse PIRB or its human counterpart, LILRBs (leukocyte immunoglobulin-like receptors B; refs. 15, 16). In the tumor microenvironment, MDSC with the M2-like phenotype are dominant and produce large amounts of IL-10 and arginase, induce anergy of antitumor immune cells, and expand immunosuppressive regulatory T cells (Treg). Furthermore, promoting MDSC differentiation into the M1 phenotype has been hypothesized as an attractive strategy for preventing tumor growth and angiogenesis (15, 16). However, the specific immunologic effects of sunitinib treatment, in combination with SBRT, on CD14+CD16+ and CD14+CD16− monocytic subsets have not been elucidated in human cancer patients. We hypothesize that concurrent sunitinib and SBRT may modulate these patients' immune responses through reversion of an immune tolerance state to an immune activation state leading to an improvement in cause-specific survival (CSS) in patients. The present study evaluates immune endpoints following combination treatment with sunitinib and SBRT and correlates them with clinical outcomes.
Materials and Methods
Patient eligibility for phase I/II clinical trial
Between February 2007 and September 2010, 46 patients were enrolled in a phase I or II single institution clinical trial using sunitinib and SBRT to treat limited oligometastatic diseases. Details about clinical trial eligibility were described previously (7). Briefly, eligible patients had 1 to 5 sites of active metastatic disease measuring ≤ 6 cm based on whole body imaging (PET or CT scans of chest, abdomen and pelvis). Patients were treated with sunitinib or SBRT following established guidelines (see Drug administration and radiation guidelines). Among the patients enrolled in this clinical trial, the peripheral blood of 19 patients was collected and banked for immune studies at the indicated time points. Not all time points were collected for certain patients due to disease progression.
Patient clinical outcomes were defined as follows: PFS is the length of time after treatment that a patient lives with no evidence of disease progression; CSS is the length of time from the start of treatment to the date of death from disease; OS is the length of time from the start of treatment to the date of death from any cause.
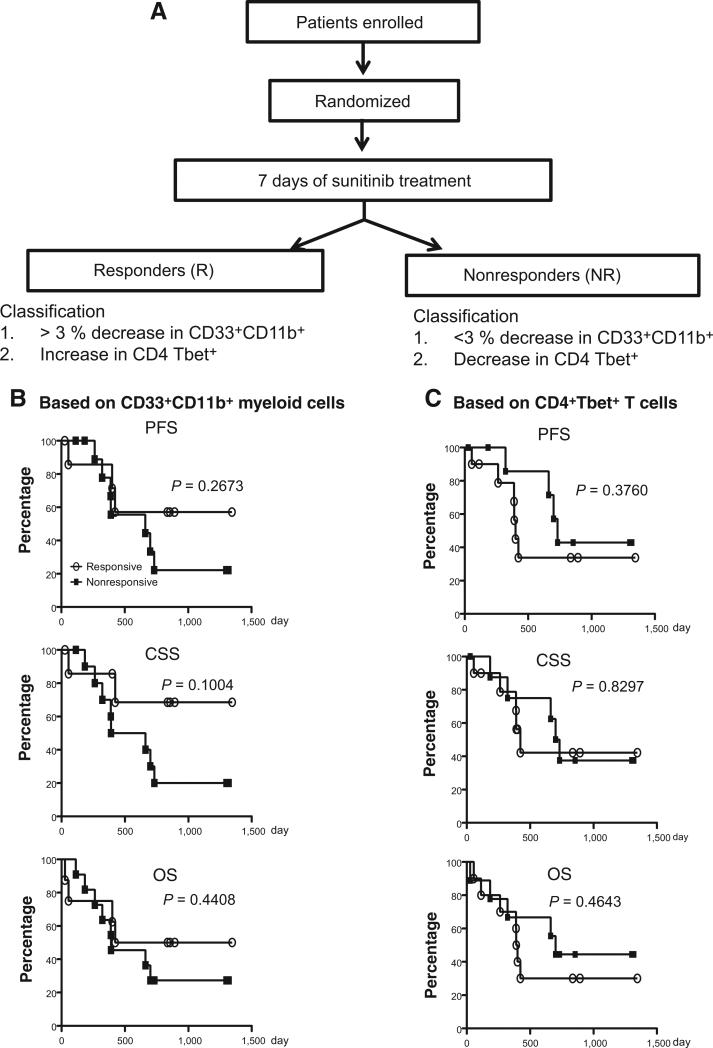
Nineteen pretreatment cancer patients with different types of cancers at the same tumor stage were recruited (Supplementary Table S1). All of the cancer patients had biopsy-confirmed diagnosis of a solid malignancy. On the basis of the extent of the decrease in the CD33+CD11b+ myeloid cell population in response to sunitinib, all clinical patients were classified as sunitinib-responders [R, if the decrease in percentage of CD33+CD11b+ myeloid cells was greater than the average of (time point A – time point B)] or sunitinib-nonresponders [NR, if the decrease in percentage of CD33+CD11b+ myeloid cells was less than the average of (time point A – time point B)]. Defining sunitinib-responders or sunitinib-nonresponders according to T-cell classification was based on increased or decreased Tbet+ expression in CD4 T cells after sunitinib treatment, respectively. Twenty-one healthy donor samples were obtained from various sources including blood bank samples and healthy volunteers without a known diagnosis of malignancy. A control cohort receiving radiation therapy alone was recruited from Mount Sinai Medical Center (New York, NY; n = 5) and the University of Pittsburgh Medical Center (Pittsburgh, PA; n = 4). This study was approved by both Institutional Review Boards and was conducted in accordance with federal and institutional guidelines.
Drug administration and radiation guidelines
The dose administration and radiation guidelines for the patients in this study were described previously (8). Sunitinib was administered orally once daily (37.5 mg) in 6-week cycles consisting of 4 weeks of treatment followed by 2 weeks without treatment. SBRT was administered concurrently with the first cycle of sunitinib on days 8 to 12 and 15 to 19. No treatment was administered on days 29 to 42. After completion of the first treatment cycle, the treating oncologists subsequently decided between further treatment with sunitinib or an alternative systemic therapy.
Antibodies, flow-cytometric analysis, and in vitro sunitinib effect
Antibodies to human CD4, CD8, CD5, CD19, CD33, CD206, CD14, CD15, CD16, HLA-DR, Foxp3, Tbet, and CD11b and Annexin V staining kit were purchased from eBioscience, anti-human phosphorylated-Stat3 and iNOS from BD Biosciences, and anti-human Arginase I antibodies from R&D Systems. For identification of human MDSC subsets, T cells, and B cells, we used several combination of staining sets, each set contains up to seven fluorochromes. Isotype-matched antibodies were used as controls. CD33+CD14+CD16− and CD33+CD14+CD16+ MDSC populations in fresh PBMC were sorted by BD FACSAriaII cell sorters. Sunitinib was obtained from Pfizer. Sorted cell populations or total PBMC were treated with sunitinib 250 nmol/L for 48 hours, followed by flow-cytometric analysis.
Peripheral blood mononuclear cell isolation
Peripheral blood (20 mL) was drawn from trial patients before sunitinib treatment (time point A), after 7 days of sunitinib treatment before SBRT (time point B), and after SBRT for 6 to 30 days (time point C). Peripheral blood was drawn into heparin-containing tubes. Peripheral blood mononuclear cells (PBMC) were purified and isolated within 2 hours of blood collection utilizing Ficoll–Paque PLUS (GE Healthcare), and stored in liquid nitrogen for subsequent analysis.
Determination of patient T-cell responses and MDSC suppression assay
Patient PBMC samples were stimulated with anti-CD3/CD28 (1 μg/mL) for 72 hours. To assay cellular proliferation, tritiated thymidine was added during the last 8 hours, and proliferative activity levels were determined using a beta counter, and presented as counts per minute (CPM). For the MDSC suppression assay, sorted cell populations were cocultured with T cells at 1:1, 0.5:1, 0.25:1, 0.125:1 ratios in the presence of anti-CD3/anti-CD28 (1 μg/mL) for 72 hours. Suppression index was calculated as (1 − (CPM of T cells cocultured with MDSCs/CPM of T cells alone)) × 100%.
Statistical analysis
Student t test was used to compare MDSC subsets from cancer patients and healthy donors. Paired t test was used to compare results from the same patient at the different time points (A, B, and C). The Log-rank test was used to compare the survival benefit between sunitinib responders and nonresponders. All statistical tests were one-tailed and all analyses were conducted using Prism and SPSS.
Results
Significantly more granulocytic MDSC and CD33+CD14+CD16+ nonclassical monocytes present in cancer patients
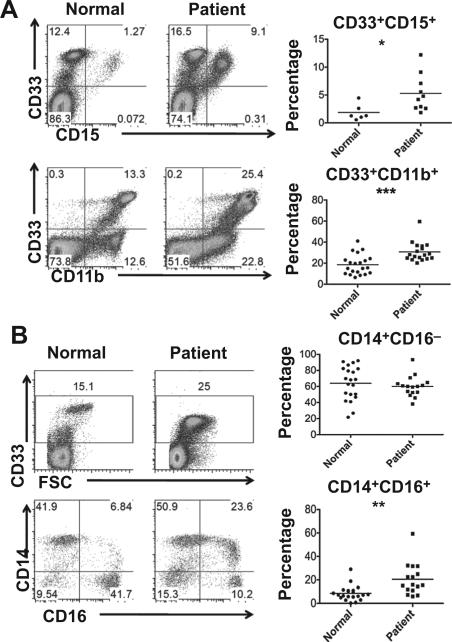
We compared PBMC from cancer patients and healthy donors using flow cytometry. On the basis of CD33+CD11b+ gating (Fig. 1A), the percentage of peripheral blood myeloid cells was significantly increased in cancer patients compared to healthy individuals (P < 0.001). There was also a significantly higher frequency of CD33+CD15+ granulocytic MDSC (P < 0.05) observed in cancer patients. When gating on CD33+ myeloid cells, a significant increase was observed in the percentage of nonclassical monocytic cells (CD14+CD16+; P < 0.01, Fig. 1B). On the other hand, the frequency of classical monocytic cells (CD14+CD16− ) varied and showed no significant differences between cancer patients and healthy donors.
Figure 1.
Different MDSC subpopulations increased in normal and cancer patients. Peripheral blood mononuclear cells were purified from normal healthy donors and cancer patients. A, cytometric (left) and statistical (right) analysis of CD33+CD11b+ myeloid cell and CD33+CD15+ granulocytic MDSC percentages. B, cytometric (left) and statistical (right) analysis of CD33+ myeloid cells and CD14+CD16− and CD14+CD16+ subpopulation (in CD33+ myeloid cells) percentages. (*, P < 0.05; **, P < 0.01; ***, P < 0.001, when compared with normal healthy donors).
Sunitinib induces apoptosis of mouse M2-like monocytic MDSC and decreases the suppressive activity of human CD33+CD14+CD16+ nonclassical monocytes
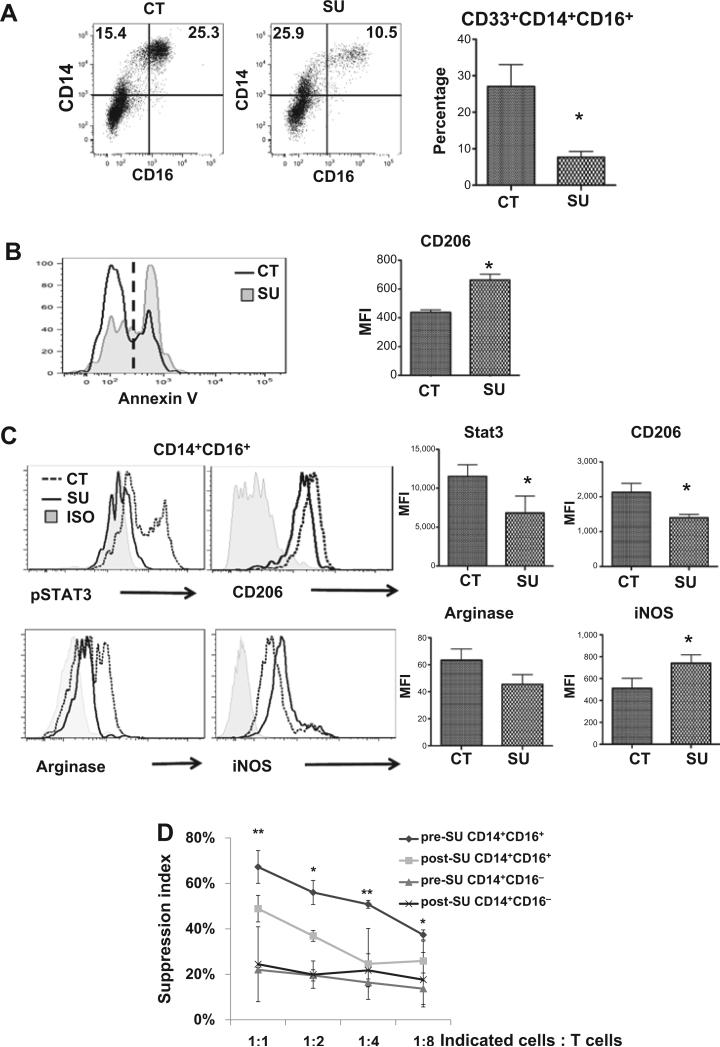
To characterize the immunomodulatory effect of sunitinib on MDSC,we treated human PBMC with sunitinib for 48 hours in vitro and found that sunitinib treatment sharply decreased the CD33+CD14+CD16+ population and increased apoptotic cells (Annexin V+) in PBMC (Fig. 2A and B). Interestingly, these apoptotic cells had higher CD206 expression, which is consistent with our observations in mice (Fig. 2B and Supplementary Fig. S5A). When we gated on viable cells, the percentage and mean fluorescent intensity of CD206 expression in CD33+CD14+CD16+ population were significantly decreased after sunitinib treatment (Supplementary Fig. S5B). The findings support our hypothesis that sunitinib treatment led to preferential apoptosis of the M2 but not M1 subset. Moreover, sunitinib treatment of CD33+CD14+CD16+ nonclassical monocytes from cancer patients resulted in significantly decreased levels of phosphorylated-STAT3, CD206, and arginase I, but significantly higher levels of iNOS, similar to the immunophenotype of M1-like cells. This population did not appear to arise from differentiation into classical macrophages, as the frequency of CD169+ cells was not significantly different between these two populations (data not shown). We also found that sorted CD33+CD14+CD16+ cells exhibited significant higher M2-associated factors (CD206, MRC1, and ARG2) and lower M1-assiciated factor (iNOS) than CD33+CD14+CD16− cells (Supplementary Fig. S5B and S5C). We further compared the immune-suppressive activities of sorted CD33+CD14+CD16+ and CD33+CD14+CD16− monocytes from cancer patients before and after sunitinib treatment. CD33+CD14+CD16+ cells exhibited stronger suppressive activity than CD33+CD14+CD16− cells and the suppressive activity was significantly decreased by sunitinib treatment (Fig. 2D) and inhibited by arginase inhibitor (Supplementary Fig. S5D). Similar to our findings, several studies reported that arginase inhibitor could significantly decrease the immunosuppressive activity of human myeloid suppressor cells against T-cell proliferation (17, 18). However, we cannot rule out the possibility that other factors, including ROS, PD-L1, TGFβ etc., are also involved. The results indicate that CD33+CD14+CD16+ nonclassical monocytes exhibited significantly greater immune-suppressive activity than CD33+CD14+CD16− classical monocytes on T-cell proliferation. Taken together, we found that the CD33+CD14+CD16+ population exhibited the characteristics of an M2-like phenotype and sunitinib treatment may promote differentiation into M1-like functional phenotype. Therefore, along with pathophysiological characteristics, we redefined CD33+CD14+CD16+ nonclassical monocytes (or so-called proinflammatory monocytes) based on the biologic functions of human monocytic MDSC in this study. Furthermore, following sunitinib treatment, human CD33+CD14+CD16+ monocytic MDSC lost a significant degree of their suppressive activity on T-cell proliferation, whereas sunitinib treatment had a less profound effect on CD33+CD14+CD16− cells.
Figure 2.
Sunitinib attenuates the M2-like phenotype in mouse MDSC and suppressive function in CD33+CD14+CD16+ nonclassical monocytes. Human PBMC or sorted human CD33+CD14+CD16+ nonclassical monocytes were left untreated (CT) or treated with sunitinib (SU, 250 nmol/L) for 48 hours followed by staining with indicated antibodies and isotype controls. A, total PBMCs were gated on CD33+ cells, and the dot plots of cell populations (left) and the statistical analyses of the percentages of CD33+CD14+CD16+ population (right) were presented. B, flow-cytometric histogram of Annexin V in total PBMC untreated (CT, black) or treated with sunitinib (SU, gray; left). Mean fluorescent intensity of CD206+ in Annexin V+CD33+CD14+CD16+ cells was shown in the right panel. C, flow-cytometric histograms (left) and mean fluorescent intensity (right) of pSTAT3+, CD206+, arginase+ (M2-like), and iNOS+ (M1-like) in sorted human CD33+CD14+CD16+ nonclassical monocytes. (*, P < 0.05, when compared with control group). D, human CD33+CD14+CD16− classical and CD33+CD14+CD16+ nonclassical monocytic populations were sorted from PBMC of patients before and after sunitinib treatment. Suppressive activity of CD33+CD14+CD16− and CD33+CD14+CD16+ populations in PBMC was assessed as described in Materials and Methods. (*, P < 0.05; **, P < 0.01, when compared with CD33+CD14+CD16− group before sunitinib treatment at indicated ratio.)
Sunitinib treatment significantly decreases different MDSC subpopulations and downregulates STAT3 phosphorylation and arginase expression in CD33+CD14+CD16+ monocytic MDSCs
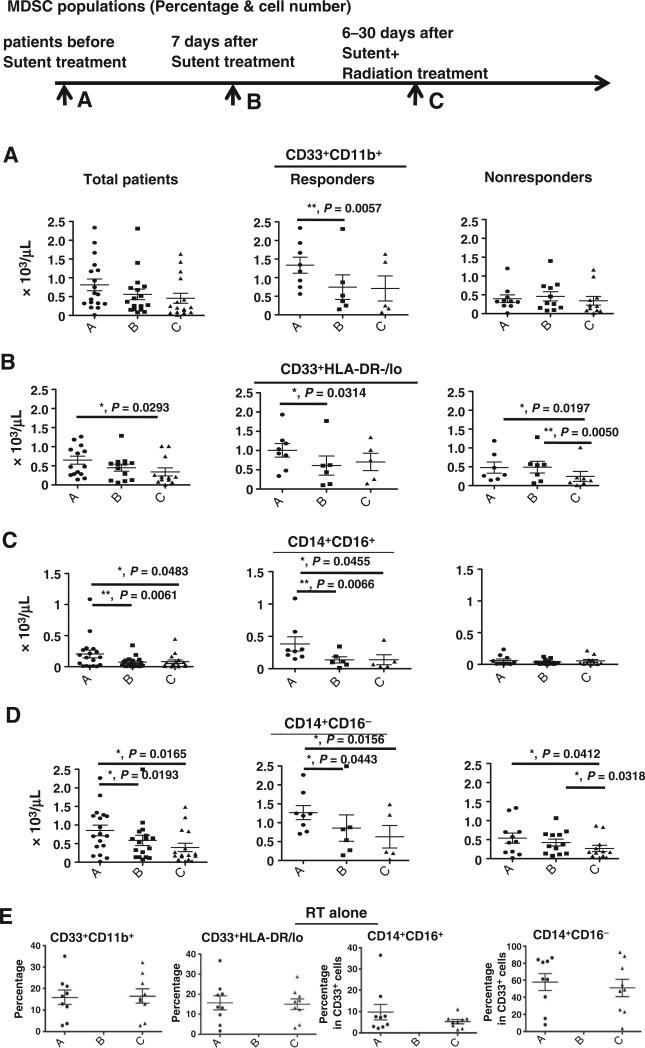
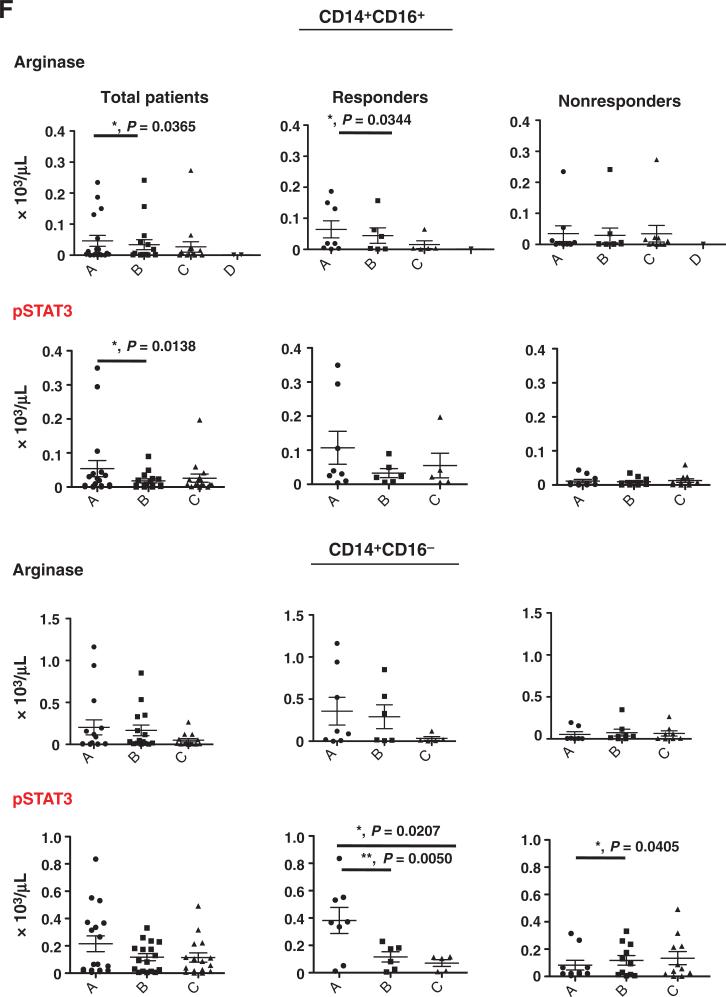
Given the effects observed with in vitro sunitinib treatment, we explored the in vivo effect by comparing PBMC from patients receiving sunitinib and SBRT with those from control patients receiving radiation therapy alone. In patients treated with sunitinib, when time point A (before taking sunitinib) was compared with time point B (7 days post-sunitinib treatment, but before SBRT), we found that sunitinib treatment significantly decreased the CD33+CD11b+ myeloid cells and general CD33+HLA-DR−/low MDSC (Fig. 3A and B and Supplementary Fig. S1A and S1B). When gating on the CD33+ population, human monocytic CD33+CD14+CD16+ MDSC were the major subpopulation significantly affected by sunitinib treatment, while only numbers of classical CD33+CD14+CD16− monocytes were decreased (Fig. 3C and D and Supplementary Fig. S1C and S1D). Comparing time point A and time point C (sunitinib + SBRT), CD33+HLA-DR−/low, CD33+CD14+CD16+ MDSC, and CD33+CD14+CD16− MDSC were significantly decreased (Fig. 3B–D). There were no significant differences in the CD33+CD11b+, CD33+HLA-DR−/Low, CD33+CD14+CD16+ MDSC, and CD33+CD14+CD16− classical monocytes in patients who received radiation alone (Fig. 3E). Interestingly, sunitinib treatment substantially decreased arginase+ and phosphorylated-STAT3+ cells in CD33+CD14+CD16+ MDSCs (Fig. 3F). The percentage changes in specific cell populations are presented in Supplementary Fig. S1. These results indicate that sunitinib treatment significantly decreased various MDSC populations while radiotherapy alone did not appear to significantly affect MDSC populations.
Figure 3.
Concurrent sunitinib treatment and SBRT leads to decreases in MDSC population frequencies. PBMCs were obtained from patients enrolled in the clinical trials at time point A (pre-sunitinib treatment), time point B (7 days post-sunitinib treatment), and time point C (6–30 days post-SBRT). The cell numbers of specific cell populations in total cancer patients (left) and responders (middle) or nonresponders (right) based on sunitinib responsiveness; CD33+CD11b+ (A), CD33+HLA-DR−/low (B), CD33+CD14+CD16+ (C), and CD33+CD14+CD16− (D). The radiotherapy alone control group (E) was also analyzed pretreatment (time point A) and after radiation therapy (time point C) (F) The numbers of arginase expressing (top) and pSTAT3+ (bottom) cells in the CD33+CD14+CD16+ and CD33+CD14+CD16− populations are shown. (*, P < 0.05; **, P < 0.01).
Significant reductions in CD33+CD14+CD16+ monocytic MDSC and CD4+CD25+CD127− Treg correlated with sunitinib responsiveness
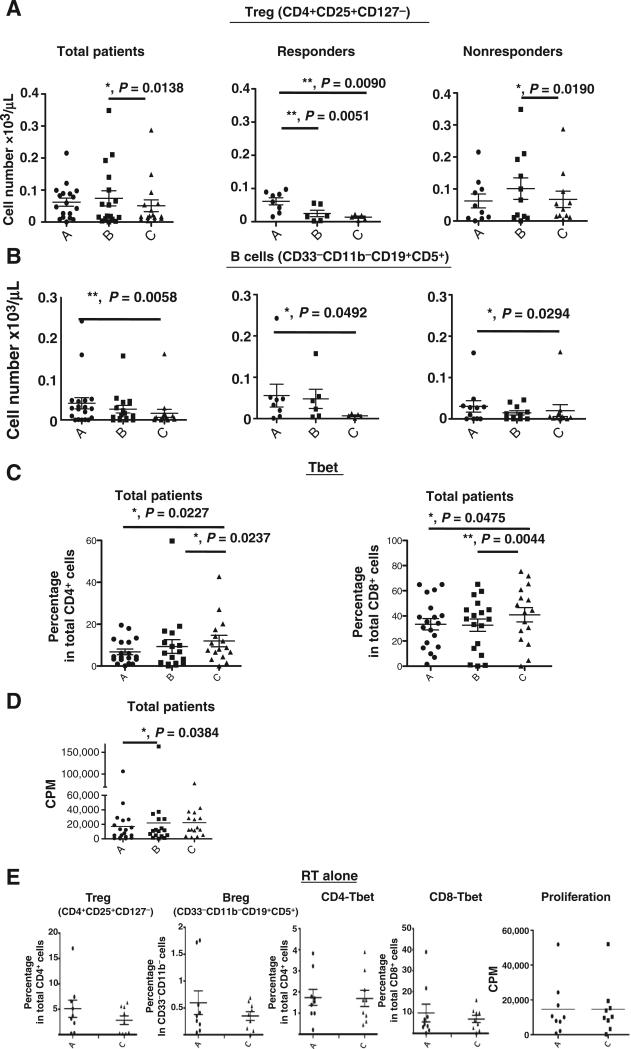
We further divided sunitinib trial patients into responders (R, n = 8) and nonresponders (NR, n = 11), based on whether a decrease in CD33+CD11b+ myeloid cells was observed from time point A (prior sunitinib) to time point B (one week after sunitinib) as described in Materials and Methods. Sunitinib treatment resulted in the following changes: a significant reduction in CD33+CD11b+ myeloid cells and CD33+HLA-DR−/low MDSC in the R group, but not the NR group (Fig. 3A and B), and marked decreases in CD33+CD14+CD16+ monocytic MDSC and arginase+ and phosphorylated STAT3+ cells in this population in R group (Fig. 3C and F and Supplementary Fig. S1C). On the other hand, CD33+CD14+CD16− classical monocytes (Fig. 3D) and phosphorylated STAT3+ cells (Fig. 3F) in this population were significantly decreased in the R group (P = 0.0443 and 0.0050, respectively). There is a significant reduction in CD33+HLA-DR−/low and CD33+CD14+CD16− MDSC (before sunitinib treatment vs. sunitinib + SBRT, and sunitinib treatment alone vs. sunitinib + SBRT) in total and NR group (Fig. 3B and D). In addition to STAT3 phosphorylation, we also observed that phosphorylated-STAT1+ cells between CD14+CD16+ and CD14+CD16− monocytes were noticeably downregulated in the R group, but not the NR group (Supplementary Fig. S2). In our previous study, we found that sunitinib skewed a tolerogenic tumor microenvironment by reducing the Treg and MDSC populations in tumor-bearing mice (10). In this study, we also observed that sunitinib induced a favorable phenotypic switch of human monocytic MDSC to M1-like macrophages, which may also contribute to the observed loss of Treg activation (10). It has been suggested that Treg (regulatory T cells) and Breg (regulatory B cells) are important immunosuppressive cell types in cancer patients. Therefore, we also determined the profiles of CD4+CD25+CD127− Treg and CD33−CD11b−CD19+CD5+ B cells in this study. The results indicate that there are no substantial changes in these parameters pre- and post-sunitinib treatment, but there is a marked decrease in Treg (P = 0.0138) in all cancer patients treated with sunitinib before and after SBRT (Fig. 4A). However, interestingly, after we stratified the patients into sunitinib-responsive and nonresponsive groups by the extent of the reduction in CD33+CD11b+ MDSC, we found that sunitinib-responders showed a significant decrease in Treg cell population post-sunitinib treatment and further reduction in post sunitinib + SBRT treatment (Fig. 4A). In contrast with the above findings, sunitinib-nonresponders exhibited less effects on Treg post-sunitinib treatment (Fig. 4A) and sunitinib + SBRT treatment (Supplementary Fig. S1E). This suggests that SBRT may potentially synergize the immune-modulatory effect of sunitinib on Treg suppression in sunitinib-responders, but not in sunitinib-nonresponders. Moreover, these results may suggest that there is a high correlation between reductions in CD33+CD14+CD16+ MDSC and Treg populations, which were found to be beneficial responses from sunitinib treatment. The combination of sunitinib and SBRT also considerably decreased the CD19/CD5 double positive B cells in all patients (both sunitinib-responders and sunitinib-nonresponders; Fig. 4B).
Figure 4.
Sunitinib responders had a markedly reduced Treg population. The total cell number of CD4+CD25+CD127low Treg (A) and CD33−CD11b−CD19+CD5+ B cells (B) in PBMC from all patients, sunitinib-responders, and sunitinib-nonresponders based on the myeloid cell-based classification were presented. C, the percentages of Tbet-expressing CD4 (left) or CD8 (right) T cells were analyzed by flow cytometry. D, proliferative responses of PBMC obtained from patients at different time points. E, the radiotherapy alone control group was also analyzed before treatment (time point A) and after radiotherapy (time point C). (*, P < 0.05; **, P < 0.01).
Sunitinib treatment promotes the development of IFNγ-producing effector T cells in clinical responders
We hypothesized that patients who undergo treatment with sunitinib and SBRT would likely also have changes in effector T-cell function. To investigate this possibility, we analyzed the expression of Tbet (T-box expressed in T cells), a key transcriptional factor that initiates the transcription of IFNγ in both CD4 and CD8 effector T cells. We found a significant increase in the expression of Tbet, but not RORγt, a key factor driving development of Th17 (data not shown), in both CD4+ and CD8+ T cells present in patient PBMC following concurrent sunitinib and radiotherapy (Fig. 4C). We analyzed the proliferative activity of T cells isolated from trial patients resulting from in vitro CD3/CD28 stimulation for 72 hours. The results showed that sunitinib significantly increased the proliferative activity of T cells in all patients (Fig. 4D, A to B, P = 0.0384). Furthermore, we did not observe significant changes in Treg, B cells, CD4, or CD8 Tbet+ cells or any increases in T-cell proliferation in PBMC from patients treated with radiation therapy alone (Fig. 4E). The results suggest that the increases in Tbet expression and proliferative activity of T cells can be attributed to sunitinib-mediated immune-modulatory activity.
Correlation between immune responses and clinical outcomes based on changes in CD33+CD11b+ MDSC or Tbet+ T cells
In our previous study, we found that sunitinib treatment increased the production of the Th1 cytokine IFNγ (10), which is driven by the key transcriptional factor Tbet. Therefore, we further stratified the 19 trial patients into responders (n = 10) and nonresponders (n = 9) based on increases or decreases in the percentage of Tbet+ cells in CD4+ T cells following sunitinib treatment (see Materials and Methods). Similar to the CD33+CD11b+ myeloid cell-based classification, we found significant reductions in different monocytic MDSC subsets CD33+CD11b+, CD33+HLA-DR−/Low (Supplementary Fig. S3A), CD33+CD14+CD16+, and CD33+CD14+CD16− cells (Supplementary Fig. S3B). Sunitinib treatment markedly increased the Tbet+ population among both CD4 and CD8 T cells after SBRT, regardless of the Tbet+ T-cell– or CD33+CD11b+ myeloid cell–based classification (Supplementary Fig. S4A and S4B). Furthermore, the Tbet+ population in CD4 T cells was augmented from time points A to C in responders, but abated from time points A to B in nonresponders (Supplementary Fig. S4B). The proliferative activity of T cells was also substantially increased after sunitinib treatment in responders (Supplementary Fig. S4C). On the basis of the Tbet+ T-cell–based classification, there was a moderate reduction in Treg numbers after SBRT in responders (P = 0.0281) and in nonresponders (P = 0.0417), but no significant changes were detected when comparing the before and after sunitinib treatment time points (Supplementary Fig. S4D).
We further evaluated whether the effects of 7-day sunitinib treatment on MDSC or T cells could be used as an immune endpoint that would predict clinical outcomes. We correlated the myeloid cell- or T-cell–based classifications of responses to sunitinib treatment with clinical outcomes. When we analyzed the survival across all patients, there were no significant differences in PFS, CSS, or OS between sunitinib-responsive and nonresponsive groups based on the two different classifications (Fig. 5). This could be due to the limited sample sizes. Of note, somewhat surprisingly, patients who were considered responders based on T-cell criteria might exhibit worse PFS, CSS, and OS at an early phase in the trial (less than 500 days). In contrast, responders based on myeloid cell criteria tended to show improved PFS and CSS outcomes compared with nonresponders. The results from these analyses suggest that the myeloid cell-based classification may be more useful for prognosis, as it more effectively predicts those who are likely to benefit from sunitinib treatment clinically. However, further investigation incorporating longer follow-up periods and greater patient numbers may be required to draw significant conclusions.
Figure 5.
Sunitinib-responsive patients based on CD33+CD11b+ myeloid cells exhibited prolonged clinical survival. Patients were divided into sunitinib-responders and nonresponders based on changes in their CD33+CD11b+ myeloid cell population, (Responders, n = 8; Non-responders, n = 11; A) or Tbet+ T-cell population (Responders, n = 10; Nonresponders, n = 9; B), as described in the Materials and Methods. Kaplan–Meier analysis on PFS (top), CSS (middle), and OS (bottom) stratified by sunitinib responsiveness were presented.
Discussion
Previous studies using an animal model (19) and human tumor cell lines (20) have demonstrated the synergistic antitumor effects of sunitinib and SBRT (21), but the underlying mechanisms remain unclear. In a preclinical evaluation of sunitinib as a radiosensitizer for human prostate cancer, Brooks and colleagues concluded that sunitinib and radiation therapy do not interact directly to radiosensitize PC3 tumor cells in vivo (22). However, the fact that there was additional delay in tumor growth suggested that sunitinib may be acting on the tumor stroma by suppressing its ability to sustain regrowth from the irradiated tumor. In our previous study, we demonstrated that sunitinib modulated the function and numbers of MDSC and Treg in the tumor micro-environment and promoted antitumor immune responses (10). In our clinical trial, we established that sunitinib could be an immune modulator in patients receiving the combination of sunitinib treatment and SBRT. The mechanism we propose can also explain the results from previous studies where sunitinib was combined with SBRT.
The presence and frequency of circulating and infiltrating MDSC have been correlated with the promotion of angiogenesis, clinical cancer staging, and metastatic status (23, 24), thus providing a potential new target for treating cancer. However, a universal marker for human MDSC, particularly monocytic MDSC, has not been identified. Human monocytes can be classified into two subsets based on CD14 and CD16 expression levels: intermediate CD14++CD16+ and nonclassical CD14+CD16++ (25, 26). CD14+CD16+ human monocytes have been reported to be proinflammatory monocytes (27) and are highly associated with various human diseases, including acute liver failure (28), generalized pustular psoriasis (29), chronic hepatitis B (30), inflammation and microbial infections (31). In this study, we found the CD33+CD14+CD16+ population to be significantly augmented in cancer patients with high phosphorylated-STAT3 and arginase expression levels and elevated immune-suppressive activity, which lead us to identify them as human monocytic MDSC. Most importantly, sunitinib treatment effectively decreased the frequency and suppressive activity of CD33+CD14+CD16+ cells.
The variability of clinical outcomes in response to treatment with tyrosine kinase inhibitors may be due to a variety of biologic parameters including FLT3-internal tandem duplication mutations (32), polymorphisms (33), and miRNA expression signature (34). It is known that some tumor-derived factors, including Bv8, SDF-1α, G-CSF, can stimulate myelopoiesis, initiate and expand myeloid cell mobilization to circulating blood (35), effects which may not be completely controlled by sunitinib. To investigate the immune-regulatory role of sunitinib, we divided all patients in the clinical trials for this study according to their relative reduction in CD11b+CD33+ myeloid cells in response to sunitinib treatment. Interestingly, we found that sunitinib-mediated reductions in CD33+HLA-DR−/low MDSC and CD33+CD14+CD16+ monocytic MDSC were observed in the sunitinib-responders, but not in the sunitinib-nonresponders. In addition, a reduction in the Treg population was coincident with the above observations, supporting our previous finding that MDSC can mediate Treg induction and activation (36). It has been reported that CD19+CD5+ B cells may have higher FoxP3 expression (37), and IL10 expression (38), which could possibly represent the Breg (B10) population. However, additional markers to characterize the human Treg/Breg should be further investigated. Most importantly, the overall results were highly correlated with longer PFS and CSS and suggest that a reduction in myeloid cell numbers may provide a reliable prognostic parameter for predicting clinical outcomes.
We further investigated the molecular mechanism by which sunitinib modulates MDSC accumulation and function. MDSCs represent an immature monocyte population that retains potential for further differentiation into the immune-suppressive M2 phenotype or the proinflammatory M1 phenotype. Many studies in animal models have shown that MDSC can acquire antitumor effects upon differentiation into the M1 phenotype (15, 16). Sunitinib, a multitargeted tyrosine kinase inhibitor, has the potential to drive MDSC toward the M1 phenotype via multiple mechanisms. Sunitinib-mediated inhibition of STAT3 phosphorylation could be the underlying mechanism on myeloid cell survival (39) and MDSC-suppressive function (40), as well as arginase expression (17). On the other hand, phosphorylation of STAT1 plays an important role in macrophage activation, monocyte differentiation, and proinflammatory cytokine/chemokine production via IFNγ (41), IL-27 (42), or IL-10 signaling cascades (43). In this study, we found that phosphorylation of STAT1 was downregulated in CD33+CD14+CD16+ and CD33+CD14+CD16− cells after sunitinib treatment in total patients and the Responder group (Supplementary Fig. S2). This suggests that sunitinib may hamper the inflammatory status of individuals by suppressing differentiation of inflammatory monocytes, characterized as the CD33+CD14+CD16+ population, or secretion of proinflammatory cytokines in human monocytes. Therefore, sunitinib may magnify the radiotherapy-induced cytotoxic effect on immunosuppressive cells, and modulate myeloid differentiation into M1 versus M2 functional phenotypes. In addition, it has been reported that the ionizing radiation, as an immunologic adjuvant, can increase damage-associated molecular patterns emitted from dying tumor cells with high immunogenicity, including calreticulin, high-mobility group box 1 protein, and ATP (44, 45). This radiotherapy-induced immunogenic tumor cell death can potentially synergize with the antitumor and M1-polarizing effects of sunitinib. This study was conducted through the use of a limited cohort of heterogenous patients with one to five radiographically apparent distant metastases. The cancer types included lung carcinoma, head and neck squamous cell carcinoma, prostate adenocarcinoma, hepatocellular carcinoma, colorectal adenocarcinoma, renal cell carcinoma, melanoma, thyroid carcinoma, and salivary gland carcinoma (Supplementary Table S1). The diverse carcinoma types of recruited patients enabled us to demonstrate the beneficial effects of sunitinib treatment in combination with SBRT for multiple cancer types. In contrast, most previous sunitinib clinical trials were focused on a single cancer type. In a previous study, Ko and colleagues (46) found that sunitinib-based therapy had the potential to modulate antitumor immunity by reversing MDSC-mediated immunosuppression in renal cell carcinoma patients.
The novelty of this study is the correlation of the immune-modulation effects of sunitinib and radiation therapy and the frequencies of MDSC in responders versus nonresponders with survival prognosis of multiple cancer types. A number of studies have reported that elevated frequencies of MDSC defined by different markers, CD14+HLA-DR−/lo (47) or lineage-1 (lin-1)−HLA-DR−/lo CD33+CD11b+ (48) or CD14+CD11b+HLA-DR−/lo (49), were associated with poor survival rates or varying clinical tumor stages (23). In terms of the clinical impact of T cells in cancer patients, it has been suggested that Tbet+ and Th1 T cells in tumor tissues are correlated with better survival rates (50, 51). Ladoire and colleagues (52) reported that a trastuzumab-docetaxel-based treatment, but not an anthracyclines-based regimen, could induce a significant T-bet induction in peritumoral lymphoid tissues that predicts a better survival prognosis in breast cancer patients. These findings suggest that the induction of Tbet+ T cells varies, depending on the treatments. Importantly, a single factor, Tbet in T cells, may not be sufficient to predict cancer prognosis of patients. In this study, we observed that sunitinib treatment alone did not significantly induce Tbet in CD4 or CD8 T cells of the entire cohort of cancer patients in the trial (Fig. 4C) The presence of Tbet+ CD4 or CD8 T cells in peripheral blood did not by itself lead to a clinical benefit in cancer patients treated with sunitinib. These findings strongly suggest that the immunologic effect of sunitinib may be predominantly mediated through modulation of myeloid cells. However, more factors may need to be included to address the roles of various T-cell subsets, Th1 and Tc, in the prognosis of cancer patients treated with sunitinib only (51). Taken together, CD33+CD11b+ myeloid cells or CD33+CD14+CD16+ monocytic MDSC can potentially provide a better immunologic indicator than Tbet+ T cells for predicting the clinical outcome of sunitinib responsiveness as well as concurrent sunitinib treatment with SBRT.
Although a relatively small population of patients was investigated in this study, the sunitinib-responders, which had greater sunitinib-related reductions in the frequency of MDSC, tended to exhibit improvement in PFS and CSS. This reduction in MDSC frequency was not observed in patients treated with SBRT alone. This suggests that classifying immune responsiveness of patients based on reductions in MDSC frequency could potentially be used by physicians to predict the likelihood that an individual patient would benefit from sunitinib treatment in combination with radiation therapy. Similar to our findings, Meyer and colleagues suggested that the low frequency of Lin−CD14+HLA-DR− monocytic MDSC might be used as a predictive biomarker of patient response to ipilimumab treatment (53).
This is the first study to investigate the therapeutic and immunologic effects of concurrent sunitinib and SBRT on various immunologic parameters, including human granulocytic MDSC and different subsets of monocytic MDSC, T cells, and B cells in patients with oligometastases and to correlate the different measurements with clinical outcome. In conclusion, concurrent therapy using the multitargeted tyrosine kinase inhibitor sunitinib and SBRT in patients with oligometastases has the potential to modulate antitumor immunity by reversing MDSC-mediated tumor-induced immune suppression, which may be beneficial to cancer immunotherapies.
Supplementary Material
Translational Relevance.
In the present study, we have identified human CD33+CD14+CD16+ monocytes as a monocytic MDSC subset with potent immunosuppressive bioactivities in patients of multiple types of cancers. As a follow-up study for a previous clinical trial of concurrent sunitinib and stereotatic body radiotherapy (SBRT), we suggested that sunitinib might sensitize host antitumor immunities to SBRT in patients with oligometastases by reversing MDSC and Treg-mediated immune suppression. Surprisingly, peripheral MDSCs, other than T cells, can plausibly be a candidate of predictive biomarkers for early patient stratification, cancer progression, and survival prognosis of patients based on their immune responsiveness to sunitinib treatment. The results show that concurrent therapy, using the multitargeted tyrosine kinase inhibitor sunitinib and SBRT in patients with oligometastases, has the potential to modulate antitumor immunity by reversing MDSC-mediated tumor-induced immune suppression, which may be beneficial to cancer immunotherapies.
Acknowledgments
The authors thank Marcia Meseck for article editing and Drs. Andrew Sikora and Matthew Galsky for constructive discussions.
Grant Support
This work was supported in part by grants from the National Cancer Institute, and Pfizer research fund (to S.-H. Chen), grant support from NCI and Susan G. Komen Breast Cancer Foundation (to P.-Y. Pan).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Authors' Contributions
Conception and design: G. Ma, S. Eisenstein, B.A. Coakley, C. Divino, M. Schwartz, M. Sung, R. Ferris, J. Kao, P.-Y. Pan, E.C. Ko, S.-H. Chen
Development of methodology: S. Eisenstein, C. Divino, M. Sung, R. Ferris, E.C. Ko, S.-H. Chen, L.-H. Wang
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): H.-M. Chen, G. Ma, N. Gildener-Leapman, S. Eisenstein, B.A. Coakley, J. Ozao, C. Divino, M. Schwartz, M. Sung, R. Ferris, E.C. Ko, S.-H. Chen
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): H.-M. Chen, S. Eisenstein, J. Ozao, J. Mandeli, C. Divino, M. Schwartz, R. Ferris, J. Kao, E.C. Ko, S.-H. Chen
Writing, review, and/or revision of the manuscript: H.-M. Chen, S. Eisenstein, C. Divino, M. Schwartz, M. Sung, R. Ferris, P.-Y. Pan, E.C. Ko, S.-H. Chen, L.-H. Wang
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): C. Divino, M. Sung, R. Ferris, E.C. Ko, S.-H. Chen
Study supervision: C. Divino, M. Sung, S.-H. Chen
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
J. Kao reports receiving commercial research grants from Pfizer and OSI, speakers bureau honoraria from Sanofi Aventis, and is a consultant/advisory board member for Bayer. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Tree AC, Khoo VS, Eeles RA, Ahmed M, Dearnaley DP, Hawkins MA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28–37. doi: 10.1016/S1470-2045(12)70510-7. [DOI] [PubMed] [Google Scholar]
- 2.Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–86. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 3.Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm–general principles. Nat Clin Pract Oncol. 2007;4:86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- 4.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roskoski R., Jr Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun. 2007;356:323–8. doi: 10.1016/j.bbrc.2007.02.156. [DOI] [PubMed] [Google Scholar]
- 6.Kleibeuker EA, Griffioen AW, Verheul HM, Slotman BJ, Thijssen VL. Combining angiogenesis inhibition and radiotherapy: a double-edged sword. Drug Resist Updat. 2012;15:173–82. doi: 10.1016/j.drup.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Kao J, Packer S, Vu HL, Schwartz ME, Sung MW, Stock RG, et al. Phase 1 study of concurrent sunitinib and image-guided radiotherapy followed by maintenance sunitinib for patients with oligometastases: acute toxicity and preliminary response. Cancer. 2009;115:3571–80. doi: 10.1002/cncr.24412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao J, Chen CT, Tong CC, Packer SH, Schwartz M, Chen SH, et al. Concurrent sunitinib and stereotactic body radiotherapy for patients with oligometastases: final report of a prospective clinical trial. Target Oncol. 2014;9:145–53. doi: 10.1007/s11523-013-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong CC, Ko EC, Sung MW, Cesaretti JA, Stock RG, Packer SH, et al. Phase II trial of concurrent sunitinib and image-guided radiotherapy for oligometastases. PLoS ONE. 2012;7:e36979. doi: 10.1371/journal.pone.0036979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 12.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–7. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, et al. CD14+CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60:1512–20. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, et al. SuperSAGE evidence for CD14+CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 15.Ma G, Pan PY, Eisenstein S, Divino CM, Lowell CA, Takai T, et al. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34:385–95. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenstein S, Coakley BA, Briley-Saebo K, Ma G, Chen HM, Meseck M, et al. Myeloid-derived suppressor cells as a vehicle for tumor-specific oncolytic viral therapy. Cancer Res. 2013;73:5003–15. doi: 10.1158/0008-5472.CAN-12-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–9. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–45. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 19.Zhang HP, Takayama K, Su B, Jiao XD, Li R, Wang JJ. Effect of sunitinib combined with ionizing radiation on endothelial cells. J Radiat Res. 2011;52:1–8. doi: 10.1269/jrr.10013. [DOI] [PubMed] [Google Scholar]
- 20.El Kaffas A, Al-Mahrouki A, Tran WT, Giles A, Czarnota GJ. Sunitinib effects on the radiation response of endothelial and breast tumor cells. Microvasc Res. 2014;92:1–9. doi: 10.1016/j.mvr.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Dalhaug A, Haukland E, Nieder C. Leptomeningeal carcinomatosis from renal cell cancer: treatment attempt with radiation and sunitinib (case report). World J Surg Oncol. 2010;8:36. doi: 10.1186/1477-7819-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks C, Sheu T, Bridges K, Mason K, Kuban D, Mathew P, et al. Preclinical evaluation of sunitinib, a multi-tyrosine kinase inhibitor, as a radiosensitizer for human prostate cancer. Radiat Oncol. 2012;7:154. doi: 10.1186/1748-717X-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicincyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS ONE. 2013;8:e57114. doi: 10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castagna A, Polati R, Bossi AM, Girelli D. Monocyte/macrophage proteomics: recent findings and biomedical applications. Expert Rev Proteomics. 2012;9:201–15. doi: 10.1586/epr.12.11. [DOI] [PubMed] [Google Scholar]
- 26.Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 27.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 28.Abeles RD, McPhail MJ, Sowter D, Antoniades CG, Vergis N, Vijay GK, et al. CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14(hi)/CD16(neg) monocytes: expansion of CD14(hi)/CD16(pos) and contraction of CD14(lo)/CD16(pos) monocytes in acute liver failure. Cytometry A. 2012;81:823–34. doi: 10.1002/cyto.a.22104. [DOI] [PubMed] [Google Scholar]
- 29.Fujisawa T, Murase K, Kanoh H, Takemura M, Ohnishi H, Seishima M. Adsorptive depletion of CD14(+)CD16(+) proinflammatory monocyte phenotype in patients with generalized pustular psoriasis: clinical efficacy and effects on cytokines. Ther Apher Dial. 2012;16:436–44. doi: 10.1111/j.1744-9987.2012.01108.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang JY, Zou ZS, Huang A, Zhang Z, Fu JL, Xu XS, et al. Hyper-activated pro-inflammatory CD16 monocytes correlate with the severity of liver injury and fibrosis in patients with chronic hepatitis B. PLoS ONE. 2011;6:e17484. doi: 10.1371/journal.pone.0017484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 32.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11:856–61. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. doi: 10.3389/fonc.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kancha RK, Grundler R, Peschel C, Duyster J. Sensitivity toward sorafenib and sunitinib varies between different activating and drug-resistant FLT3-ITD mutations. Exp Hematol. 2007;35:1522–6. doi: 10.1016/j.exphem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Erdem L, Giovannetti E, Leon LG, Honeywell R, Peters GJ. Polymorphisms to predict outcome to the tyrosine kinase inhibitors gefitinib, erlotinib, sorafenib and sunitinib. Curr Top Med Chem. 2012;12:1649–59. doi: 10.2174/156802612803531333. [DOI] [PubMed] [Google Scholar]
- 36.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–28. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noh J, Choi WS, Noh G, Lee JH. Presence of Foxp3-expressing CD19(+) CD5(+) B cells in human peripheral blood mononuclear cells: human CD19(+)CD5(+)Foxp3(+) regulatory B cell (Breg). Immune Netw. 2010;10:247–9. doi: 10.4110/in.2010.10.6.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hua F, Ji L, Zhan Y, Li F, Zou S, Wang X, et al. Pulsed high-dose dexamethasone improves interleukin 10 secretion by CD5+ B cells in patients with primary immune thrombocytopenia. J Clin Immunol. 2012;32:1233–42. doi: 10.1007/s10875-012-9714-z. [DOI] [PubMed] [Google Scholar]
- 39.de Koning JP, Soede-Bobok AA, Ward AC, Schelen AM, Antonissen C, van Leeuwen D, et al. STAT3-mediated differentiation and survival and of myeloid cells in response to granulocyte colony-stimulating factor: role for the cyclin-dependent kinase inhibitor p27(Kip1). Oncogene. 2000;19:3290–8. doi: 10.1038/sj.onc.1203627. [DOI] [PubMed] [Google Scholar]
- 40.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coccia EM, Del Russo N, Stellacci E, Testa U, Marziali G, Battistini A. STAT1 activation during monocyte to macrophage maturation: role of adhesion molecules. Int Immunol. 1999;11:1075–83. doi: 10.1093/intimm/11.7.1075. [DOI] [PubMed] [Google Scholar]
- 42.Kalliolias GD, Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J Immunol. 2008;180:6325–33. doi: 10.4049/jimmunol.180.9.6325. [DOI] [PubMed] [Google Scholar]
- 43.Rahimi AA, Gee K, Mishra S, Lim W, Kumar A. STAT-1 mediates the stimulatory effect of IL-10 on CD14 expression in human monocytic cells. J Immunol. 2005;174:7823–32. doi: 10.4049/jimmunol.174.12.7823. [DOI] [PubMed] [Google Scholar]
- 44.Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol. 2012;2:88. doi: 10.3389/fonc.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25:11–7. doi: 10.1016/j.semradonc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 47.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 48.Idorn M, Køllgaard T, Kongsted P, Sengeløv L, Thor Straten P. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother. 2014;63:1177–87. doi: 10.1007/s00262-014-1591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res. 2014;20:1601–9. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 50.Chen LJ, Zheng X, Shen YP, Zhu YB, Li Q, Chen J, et al. Higher numbers of T-bet(+) intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol Immunother. 2013;62:553–61. doi: 10.1007/s00262-012-1358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–71. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 52.Ladoire S, Arnould L, Mignot G, Apetoh L, Rebe C, Martin F, et al. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival. Br J Cancer. 2011;105:366–71. doi: 10.1038/bjc.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63:247–57. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.