Abstract
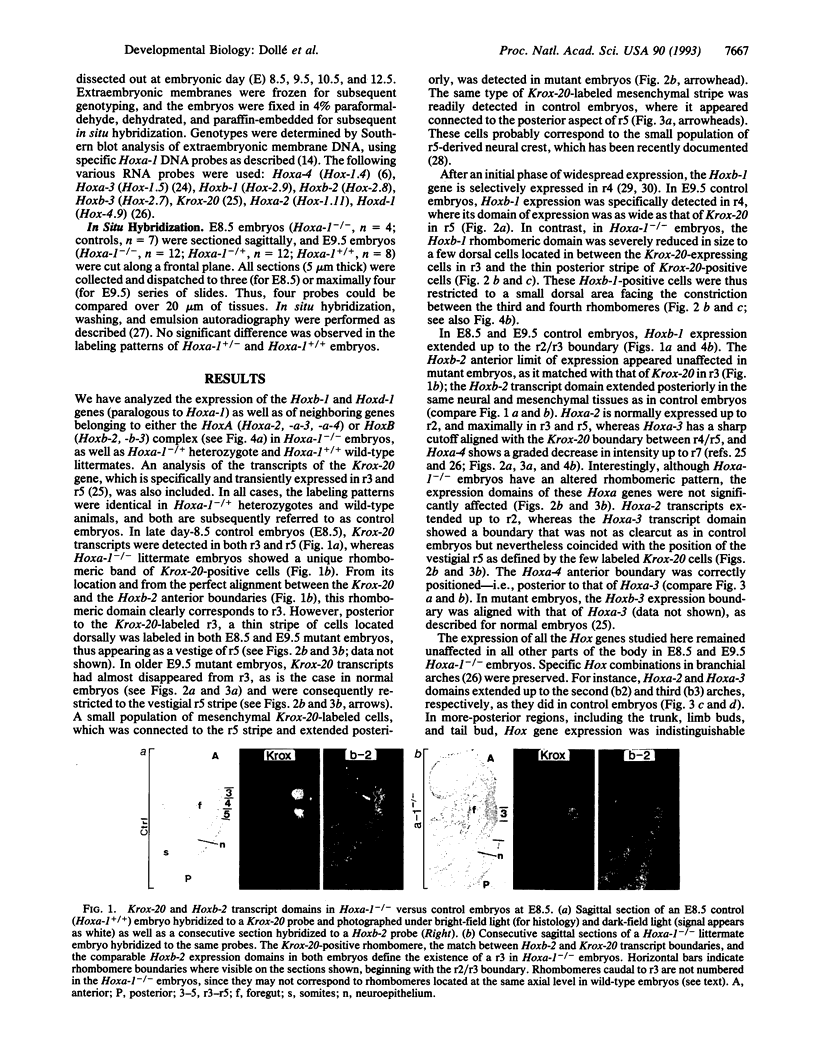
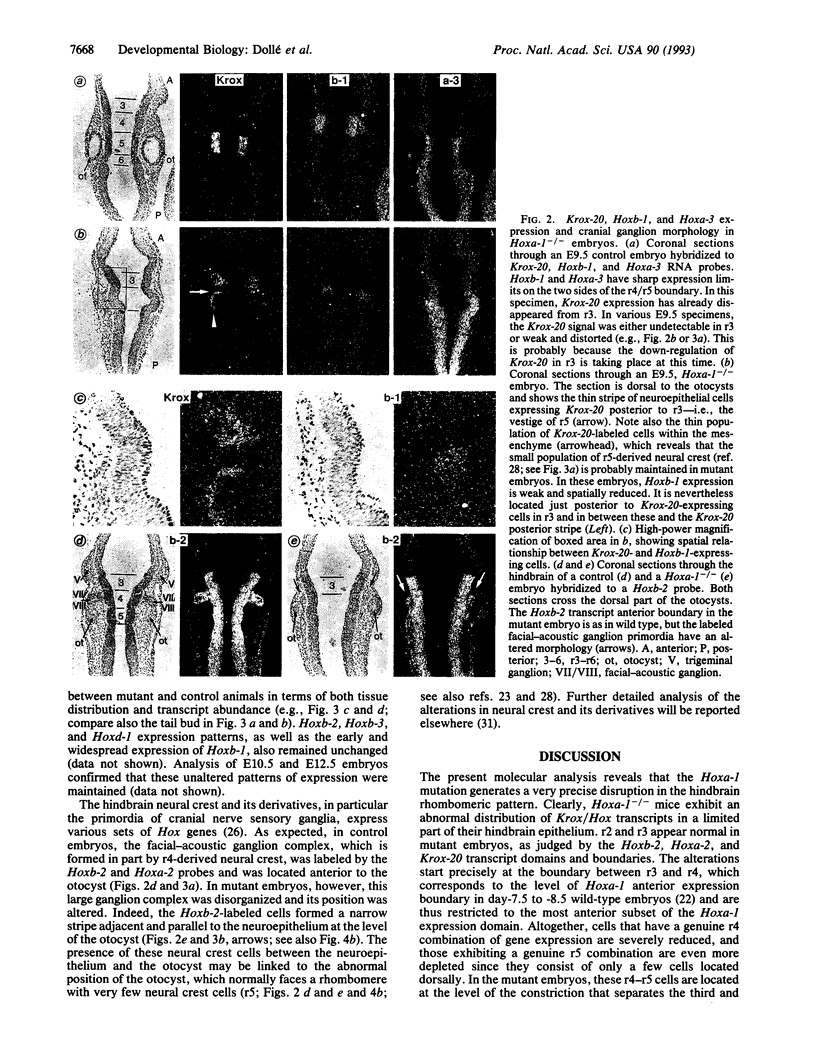
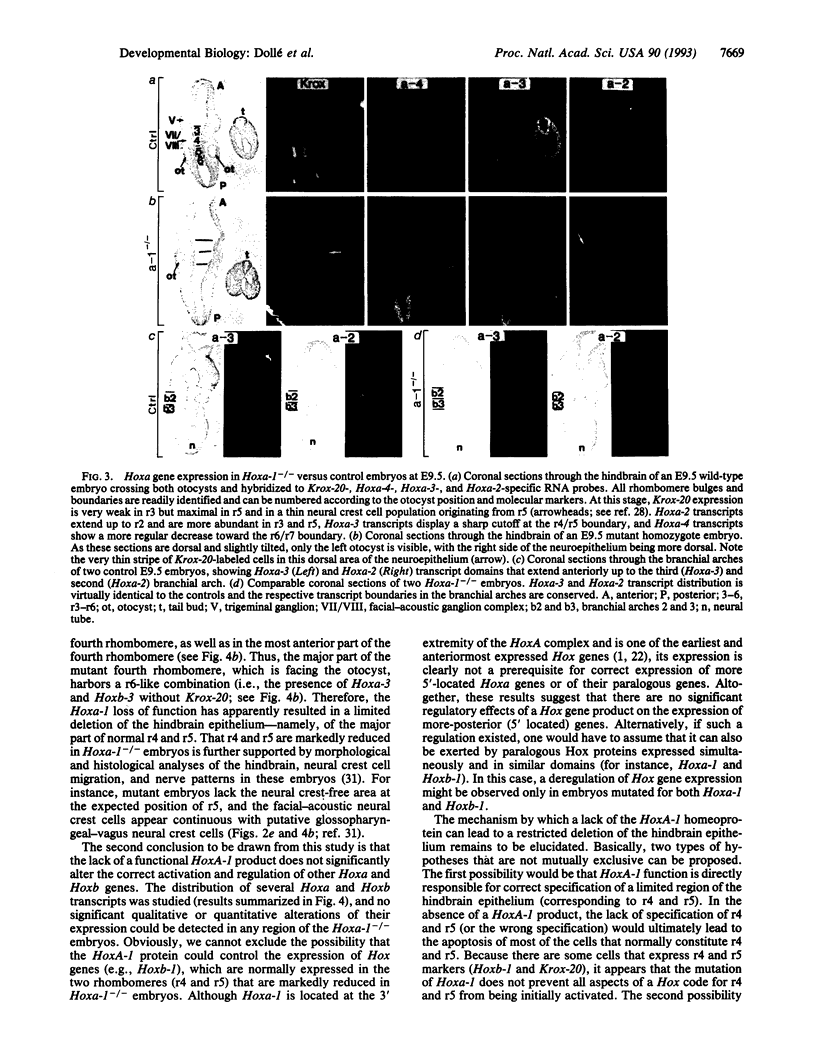
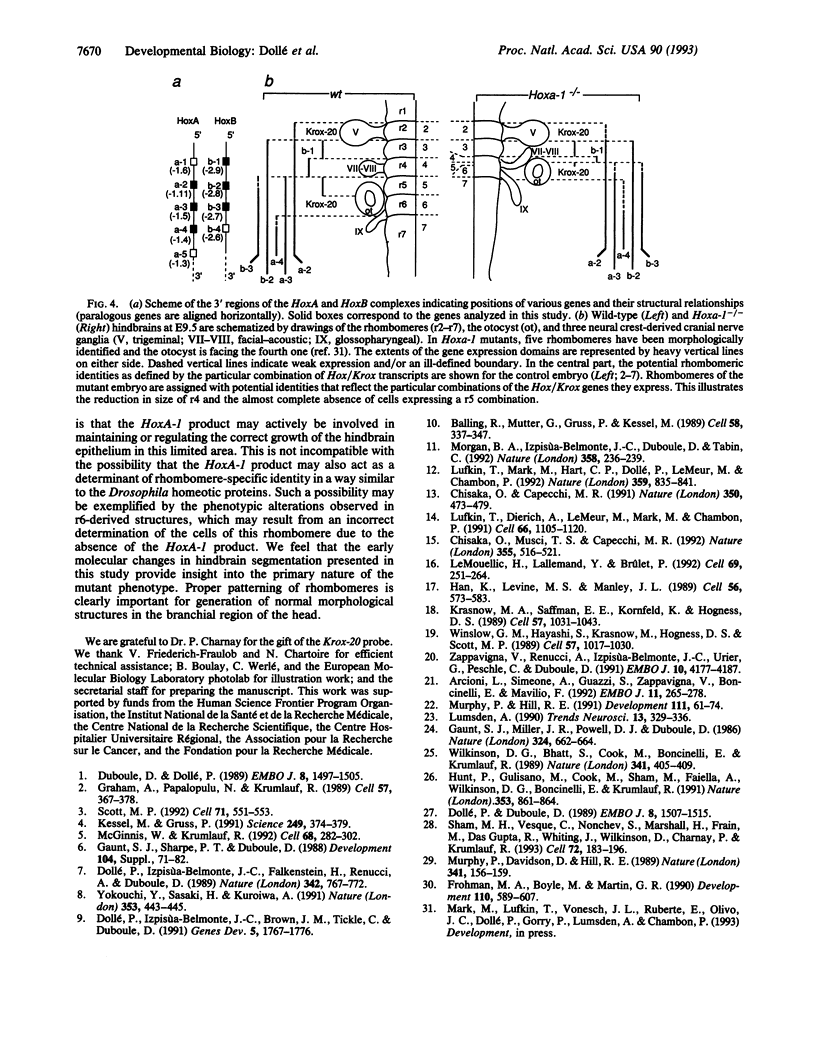
It is unknown whether cross-regulatory interactions between homeotic genes, which have been shown to play an important role in the maintenance of their expression domains during Drosophila development, are also important during mammalian development. We have analyzed here the expression of Hox genes in Hoxa-1 (Hox-1.6) null mutant embryos to investigate the possible existence of regulatory interactions between Hoxa-1 and other Hox genes. We show that the absence of a functional Hoxa-1 gene product does not globally interfere with the expression of other Hox genes in terms of both spatial boundaries and transcript abundance. However, a limited area of the hindbrain shows a strong reduction in Hoxb-1 (Hox-2.9) and Krox-20 transcripts, which most likely reflects a marked reduction in size of the former fourth and fifth rhombomeres. These alterations coincide with the region that is subsequently affected in Hoxa-1 null mutant mice and suggest that the primary defects in this mutation are spatially restricted deletions of some rhombomeric structures.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcioni L., Simeone A., Guazzi S., Zappavigna V., Boncinelli E., Mavilio F. The upstream region of the human homeobox gene HOX3D is a target for regulation by retinoic acid and HOX homeoproteins. EMBO J. 1992 Jan;11(1):265–277. doi: 10.1002/j.1460-2075.1992.tb05049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balling R., Mutter G., Gruss P., Kessel M. Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox-1.1 in transgenic mice. Cell. 1989 Jul 28;58(2):337–347. doi: 10.1016/0092-8674(89)90848-9. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Musci T. S., Capecchi M. R. Developmental defects of the ear, cranial nerves and hindbrain resulting from targeted disruption of the mouse homeobox gene Hox-1.6. Nature. 1992 Feb 6;355(6360):516–520. doi: 10.1038/355516a0. [DOI] [PubMed] [Google Scholar]
- Dollé P., Duboule D. Two gene members of the murine HOX-5 complex show regional and cell-type specific expression in developing limbs and gonads. EMBO J. 1989 May;8(5):1507–1515. doi: 10.1002/j.1460-2075.1989.tb03535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollé P., Izpisúa-Belmonte J. C., Brown J. M., Tickle C., Duboule D. HOX-4 genes and the morphogenesis of mammalian genitalia. Genes Dev. 1991 Oct;5(10):1767–1767. doi: 10.1101/gad.5.10.1767. [DOI] [PubMed] [Google Scholar]
- Dollé P., Izpisúa-Belmonte J. C., Falkenstein H., Renucci A., Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989 Dec 14;342(6251):767–772. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Boyle M., Martin G. R. Isolation of the mouse Hox-2.9 gene; analysis of embryonic expression suggests that positional information along the anterior-posterior axis is specified by mesoderm. Development. 1990 Oct;110(2):589–607. doi: 10.1242/dev.110.2.589. [DOI] [PubMed] [Google Scholar]
- Gaunt S. J., Miller J. R., Powell D. J., Duboule D. Homoeobox gene expression in mouse embryos varies with position by the primitive streak stage. Nature. 1986 Dec 18;324(6098):662–664. doi: 10.1038/324662a0. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Han K., Levine M. S., Manley J. L. Synergistic activation and repression of transcription by Drosophila homeobox proteins. Cell. 1989 Feb 24;56(4):573–583. doi: 10.1016/0092-8674(89)90580-1. [DOI] [PubMed] [Google Scholar]
- Hunt P., Gulisano M., Cook M., Sham M. H., Faiella A., Wilkinson D., Boncinelli E., Krumlauf R. A distinct Hox code for the branchial region of the vertebrate head. Nature. 1991 Oct 31;353(6347):861–864. doi: 10.1038/353861a0. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Krasnow M. A., Saffman E. E., Kornfeld K., Hogness D. S. Transcriptional activation and repression by Ultrabithorax proteins in cultured Drosophila cells. Cell. 1989 Jun 16;57(6):1031–1043. doi: 10.1016/0092-8674(89)90341-3. [DOI] [PubMed] [Google Scholar]
- Le Mouellic H., Lallemand Y., Brûlet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992 Apr 17;69(2):251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Mark M., Hart C. P., Dollé P., LeMeur M., Chambon P. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature. 1992 Oct 29;359(6398):835–841. doi: 10.1038/359835a0. [DOI] [PubMed] [Google Scholar]
- Lumsden A. The cellular basis of segmentation in the developing hindbrain. Trends Neurosci. 1990 Aug;13(8):329–335. doi: 10.1016/0166-2236(90)90144-y. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Morgan B. A., Izpisúa-Belmonte J. C., Duboule D., Tabin C. J. Targeted misexpression of Hox-4.6 in the avian limb bud causes apparent homeotic transformations. Nature. 1992 Jul 16;358(6383):236–239. doi: 10.1038/358236a0. [DOI] [PubMed] [Google Scholar]
- Murphy P., Davidson D. R., Hill R. E. Segment-specific expression of a homoeobox-containing gene in the mouse hindbrain. Nature. 1989 Sep 14;341(6238):156–159. doi: 10.1038/341156a0. [DOI] [PubMed] [Google Scholar]
- Murphy P., Hill R. E. Expression of the mouse labial-like homeobox-containing genes, Hox 2.9 and Hox 1.6, during segmentation of the hindbrain. Development. 1991 Jan;111(1):61–74. doi: 10.1242/dev.111.1.61. [DOI] [PubMed] [Google Scholar]
- Sham M. H., Vesque C., Nonchev S., Marshall H., Frain M., Gupta R. D., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993 Jan 29;72(2):183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Cook M., Boncinelli E., Krumlauf R. Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature. 1989 Oct 5;341(6241):405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]
- Winslow G. M., Hayashi S., Krasnow M., Hogness D. S., Scott M. P. Transcriptional activation by the Antennapedia and fushi tarazu proteins in cultured Drosophila cells. Cell. 1989 Jun 16;57(6):1017–1030. doi: 10.1016/0092-8674(89)90340-1. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y., Sasaki H., Kuroiwa A. Homeobox gene expression correlated with the bifurcation process of limb cartilage development. Nature. 1991 Oct 3;353(6343):443–445. doi: 10.1038/353443a0. [DOI] [PubMed] [Google Scholar]
- Zappavigna V., Renucci A., Izpisúa-Belmonte J. C., Urier G., Peschle C., Duboule D. HOX4 genes encode transcription factors with potential auto- and cross-regulatory capacities. EMBO J. 1991 Dec;10(13):4177–4187. doi: 10.1002/j.1460-2075.1991.tb04996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]






