Significance
The cell wall is an essential protective structure for bacteria, and the enzymes required for its biogenesis are the targets for many potent antibiotics. However, there is little knowledge of how bacteria (particularly Gram-negative organisms) respond to damage to the cell wall caused by antibiotics or other agents. Here, we describe a signaling system (the histidine kinase/response regulator pair WigKR) in Vibrio cholerae, the cholera pathogen that is triggered by antibiotics that disrupt various steps in cell wall synthesis. Activation of WigR leads to increased expression of genes required for cell wall synthesis and enables the pathogen to recover from the spherical nondividing state induced by antibiotics. Collectively, our findings reveal a new signal transduction pathway controlling cell wall biosynthesis and antibiotic tolerance.
Keywords: peptidoglycan, stress response, antibiotic tolerance, two component system, cell envelope
Abstract
The bacterial cell wall is critical for maintenance of cell shape and survival. Following exposure to antibiotics that target enzymes required for cell wall synthesis, bacteria typically lyse. Although several cell envelope stress response systems have been well described, there is little knowledge of systems that modulate cell wall synthesis in response to cell wall damage, particularly in Gram-negative bacteria. Here we describe WigK/WigR, a histidine kinase/response regulator pair that enables Vibrio cholerae, the cholera pathogen, to survive exposure to antibiotics targeting cell wall synthesis in vitro and during infection. Unlike wild-type V. cholerae, mutants lacking wigR fail to recover following exposure to cell-wall–acting antibiotics, and they exhibit a drastically increased cell diameter in the absence of such antibiotics. Conversely, overexpression of wigR leads to cell slimming. Overexpression of activated WigR also results in increased expression of the full set of cell wall synthesis genes and to elevated cell wall content. WigKR-dependent expression of cell wall synthesis genes is induced by various cell-wall–acting antibiotics as well as by overexpression of an endogenous cell wall hydrolase. Thus, WigKR appears to monitor cell wall integrity and to enhance the capacity for increased cell wall production in response to damage. Taken together, these findings implicate WigKR as a regulator of cell wall synthesis that controls cell wall homeostasis in response to antibiotics and likely during normal growth as well.
Nearly all bacteria produce a sturdy cell wall located outside of the cytoplasmic membrane to maintain their shape and structural integrity (1). The cell wall is composed of peptidoglycan (PG), a remarkable macromolecule composed of strands of polymerized disaccharides cross-linked via short peptide sidechains to form a mesh. This complex polymer’s crucial role in bacterial cell integrity and consequent survival becomes dramatically apparent when PG synthesis is inhibited by antibiotics, which typically causes rapid cell lysis and death. For this reason, cell wall synthesis inhibitors (such as the penicillins) are among the most potent and widely used antibiotics (2, 3).
PG synthesis proceeds in three principal steps (4), each of which can be inhibited by antibiotics. Starting with the generation of disaccharide–oligopeptide precursors in the cytoplasm [the target of fosfomycin and d-cycloserine (5, 6)] and followed by precursor translocation across the cytoplasmic membrane (CM) [the target of several experimental drugs (7)], the cell wall is assembled outside the CM by membrane-bound enzymes called Penicillin Binding Proteins (PBPs), the targets of beta-lactams such as penicillins. High-molecular-weight PBPs, such as PBP1A and PBP1B, have both polymerizing (transglycosylation) and cross-linking (transpeptidation) activities and are found in large complexes that constitute cell wall assembly machines (8, 9). Cell wall biosynthesis and assembly are required both for bacterial elongation and for division. In addition, bacterial growth requires the activity of PG hydrolases (“autolysins”), enzymes that locally dissolve the meshwork to allow for the insertion of new cell wall material (10, 11). It is thought that an imbalance between synthetic and hydrolytic enzyme activities is ultimately responsible for the lysis phenotype that typically results from inhibition of cell wall synthesis.
Bacteria often use alternative sigma factors or two-component systems (TCS) to adapt gene expression in response to stressors (12). Two-component phosphorelay systems are composed of Histidine Kinase/Response Regulator (HK/RR) pairs. In HK/RR systems, which are ubiquitous in bacteria, the kinase typically relays extracellular information to the response regulator via autophosphorylation and subsequent phosphotransfer. This signal transmission results in changes in expression of stress-responsive transcripts.
Although several stress response systems are known to sense and respond to damaged/misfolded membrane proteins (13–15), there is a paucity of knowledge regarding systems that sense and ameliorate damage to the cell wall. Exceptions are TCS [e.g., WalRK and VraSR (16, 17)] found in several Gram-positive genera that respond to exposure to various cell-wall–acting antibiotics by activating regulons that mediate specialized cell wall synthesis/turnover functions. In addition, some Gram-negative genera encode an AmpR repressor that detects cell wall fragments generated by beta-lactam antibiotics and induces production of the AmpC beta-lactamase that degrades these compounds (18). Notably, TCS or other sensor/response systems that enable Gram-negative bacteria to induce PG synthesis after detection of PG damage (e.g., as a consequence of exposure to cell-wall–acting antibiotics) have not been described. In general, there is little knowledge of pathways that regulate cell wall biogenesis.
We have previously shown that the causative agent of cholera, Vibrio cholerae, is intrinsically tolerant to antibiotics targeting cell wall synthesis. Upon exposure to cell wall synthesis inhibitors, V. cholerae loses its cell wall and forms viable, nondividing spheres, which readily revert to the normal rod shape upon removal of the antibiotic (19). Here, we describe a two-component system, dubbed WigK and WigR (for wall integrity gauge kinase and regulator), which is critical for V. cholerae tolerance to cell-wall– acting antibiotics. Remarkably, the wigKR regulon, which includes the entire cell wall synthesis pathway, is induced by exposure to antibiotics that inhibit cell wall synthesis. Moreover, the deletion or overexpression of WigR caused changes in cell width, suggesting that the WigKR two-component system modulates cell wall homeostasis under normal growth conditions in the absence of antibiotics. Collectively, our findings reveal a new signal transduction pathway controlling PG biosynthesis and antibiotic tolerance.
Results
wigKR Locus Is Required for V. cholerae’s Tolerance to Inhibitors of Cell Wall Synthesis.
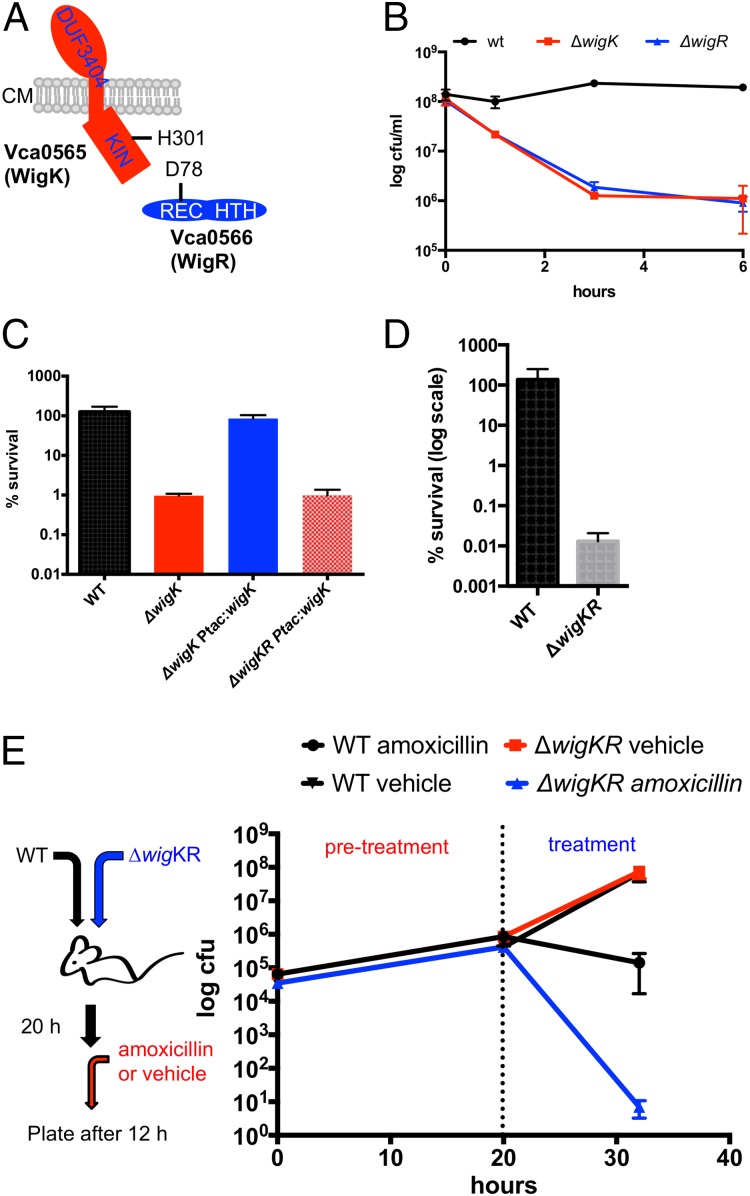
A TnSeq-based screen for V. cholerae factors required for recovery from penicillin exposure revealed that insertions in vca0565 and vca0566 were underrepresented in a transposon insertion library following treatment with penicillin. As noted above we renamed these genes WigKR (wall integrity gauge kinase and regulator) for reasons elaborated below. The two genes are arranged in an operon, in which the wigR reading frame overlaps with the 3′ end of the wigK-coding sequence. WigK codes for a histidine kinase (HK) of the BaeS family, whereas WigR encodes a response regulator (RR) in the OmpR family (Fig. 1A). WigK possesses a well-conserved kinase domain; however, the periplasmic portion of the protein lacks a recognizable, canonical HK signal-sensing domain and instead contains a domain of unknown function. The wigKR locus was recently reported to be a regulator of type VI secretion (20), but is otherwise uncharacterized.
Fig. 1.
V. cholerae requires the wigKR locus to survive inhibition of cell wall synthesis. (A) Predicted domain organization of WigK and WigR showing their predicted sites of phosphorylation. Kin: kinase domain; REC: receiver domain; HTH: helix-turn-helix motif; CM: cytoplasmic membrane. (B) Survival of WT V. cholerae N16961 and its ∆wigK and ∆wigR derivatives after exposure to penicillin G [100 µg/mL, 20x minimum inhibitory concentration (MIC)] for the indicated amount of time. Data are averages of three biological replicates; error bars represent SE. (C) Complementation of wigKR-dependent penicillin sensitivity. Strains carrying complementing genes inserted in a single copy in a neutral chromosomal locus under an isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible promoter were grown to late exponential phase in LB + inducer and then exposed to 100 µg/mL penicillin G for 3 h; values represent mean of three biological replicates, and error bars represent SDs. (D) Survival of a ∆wigKR mutant in the presence of fosfomycin (200 µg/mL, ∼4× MIC). (E) WigKR are required for V. cholerae survival of beta-lactam treatment in vivo. N16961 (lacZ−) parental and ∆wigKR (lacZ+) were coinoculated into infant mice. Twenty hours after inoculation, mice were treated orally with either 50 mg/kg amoxicillin or vehicle control (“treatment”), and 12 h later cfu of the parental and ∆wigKR strains were determined. Data points are averages of 9 mice (amoxicillin) or 10 mice (vehicle); error bars represent SE.
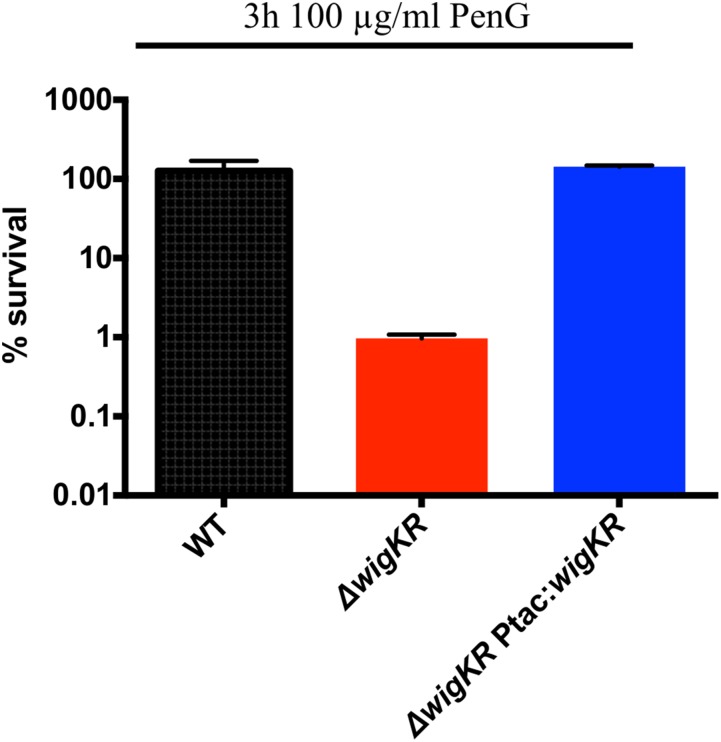
To explore the involvement of the wigKR locus in V. cholerae’s response to cell wall acting antibiotics, we measured the ability of defined mutants lacking wigK, wigR, or both to survive exposure to the beta-lactam antibiotic penicillin G. As previously observed (19), wild-type V. cholerae (El Tor isolate N16961) exhibited no reduction in colony-forming units in response to treatment. In contrast, the colony-forming capacity of the ∆wigK, ∆wigR, and ∆wigKR strains declined by ∼2–3 orders of magnitude in the presence of penicillin G (Fig. 1B and Fig. S1). Expression of wigK in the ∆wigK mutant was sufficient to restore wild-type levels of survival in the presence of penicillin G in vitro, excluding the possibility of polar effects and secondary site mutations (Fig. 1C). Likewise, expression of wigKR was able to complement the beta-lactam sensitivity of a ∆wigKR double mutant (Fig. S1). However, expression of wigK alone was insufficient to complement ∆wigKR (Fig. 1C), suggesting that WigK’s effect on survival requires the presence of its putative response regulator WigR.
Fig. S1.
Complementation of ∆wigKR survival in penicillin. The indicated strains were grown in penicillin G (100 μg/mL) for 3 h and then plated. Values represent averages of three biological replicates; error bars represent SE.
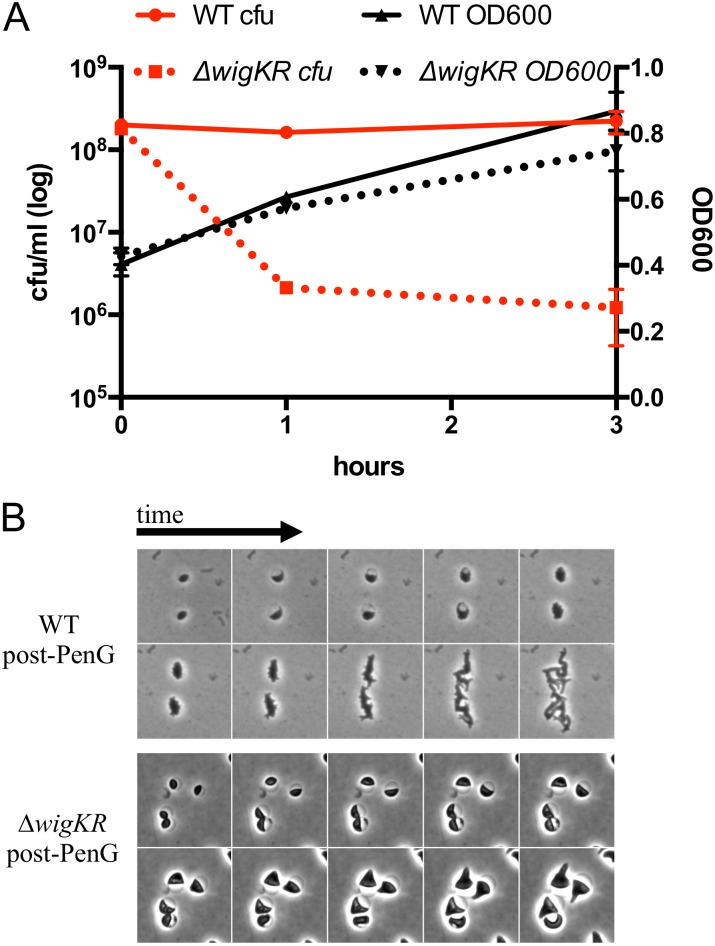
Exposure of the ∆wigKR mutant to penicillin G was not accompanied by a decrease in culture turbidity (Fig. S2A), suggesting that the absence of WigKR does not lead to cell lysis. Instead, we hypothesize that WigKR is required for recovery from damage induced by antibiotics that target cell wall synthesis. Consistent with this idea, we found that spherical cells formed by a ∆wigKR mutant after penicillin exposure failed to revert to rod shape after the antibiotic was removed (Fig. S2B). Additionally, we observed that the wigKR locus was critical for V. cholerae colony formation after exposure to fosfomycin (Fig. 1D), an antibiotic that inhibits MurA, which catalyzes the first step of synthesis of cell wall precursors (6). Thus, WigKR is required for V. cholerae to recover from the damage elicited by agents that inhibit distinct steps within PG biosynthesis, including cytoplasmic formation of cell wall precursors as well as cross-linking of PG precursors in the periplasm.
Fig. S2.
Absence of wigKR does not lead to enhanced lysis. (A) Survival (cfu) and cell culture density of WT and ∆wigKR V. cholerae after exposure to penicillin G (100 µg/mL) for the indicated amount of time. Data are averages of three biological replicates; error bars represent SE. (B) Dynamics of rod-shape recovery after exposure to penicillin G. Cells were exposed to the antibiotic for 3 h and then beta-lactamase (0.2 U) was added, followed by time-lapse imaging on an agarose pad/LB.
We also tested whether V. cholerae requires wigKR to survive exposure to a beta-lactam antibiotic during infection in vivo. Following coinoculation of infant mice with wild-type (lacZ−) and ∆wigKR (lacZ+) strains, mice were treated at 20 h post infection with amoxicillin (a beta-lactam antibiotic suitable for oral administration) or a vehicle control. Subsequent enumeration of WT and ∆wigKR cells revealed that both strains were recovered at equal levels when the antibiotic was not present and thus showed that the ∆wigKR strain does not have an intrinsic colonization deficiency. However, there was a dramatic reduction (>10,000-fold) in the recovery of ∆wigKR vs. WT colonies after treatment with amoxicillin (Fig. 1E). Thus, WigKR contributes to V. cholerae’s beta-lactam tolerance in vivo.
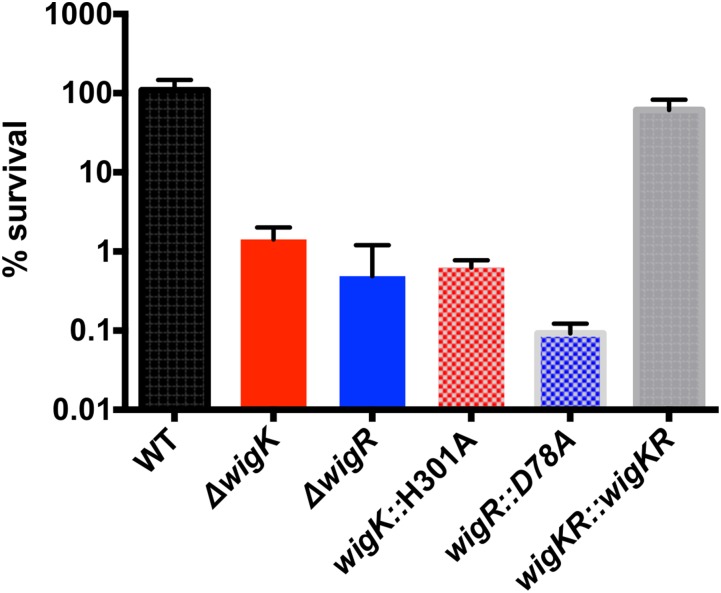
To begin to dissect the molecular requirements for WigKR function, we reconstituted a ∆wigKR strain with either wild-type wigKR or sequences producing point mutations in WigK’s and WigR’s predicted phosphorylation sites (H301A and D78A, respectively), which are expected to be inactivating. The strains with the H301A and D78A phospho-ablative mutations phenocopied the penicillin sensitivity of the ∆wigK and ∆wigR mutants (Fig. S3). These results suggest that WigKR’s contribution to survival of penicillin exposure relies on its ability to be phosphorylated, strongly suggesting that WigKR are part of a signaling cascade, the output of which ensures cell survival after cell wall damage.
Fig. S3.
Survival following penicillin G treatment depends on WigKR’s phosphorylation ability. Strains carrying chromosomal replacements of WigK and WigR with their putative phosphoablative mutants were grown to late exponential phase in LB and then exposed to 100 µg/mL penicillin G for 3 h; values represent mean of three biological replicates; error bars represent SDs.
WigR Regulon Includes the Full Set of Genes for Cell Wall Biosynthesis.
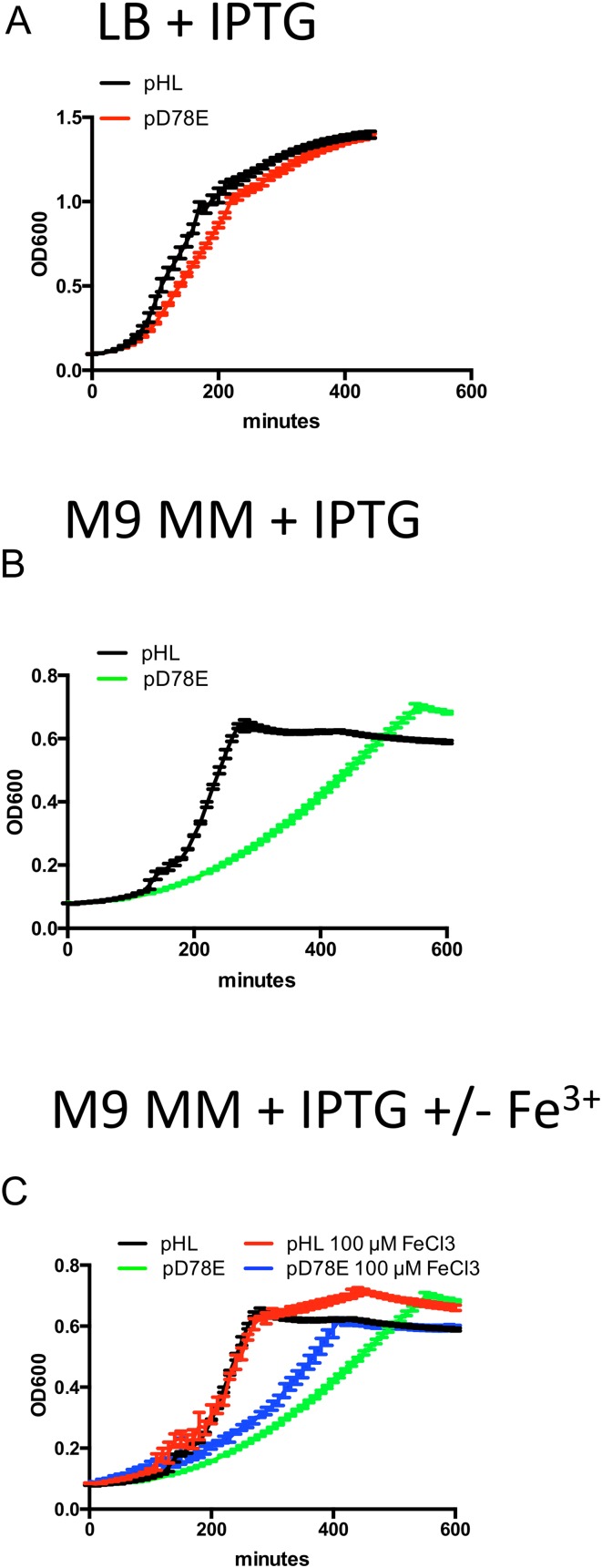
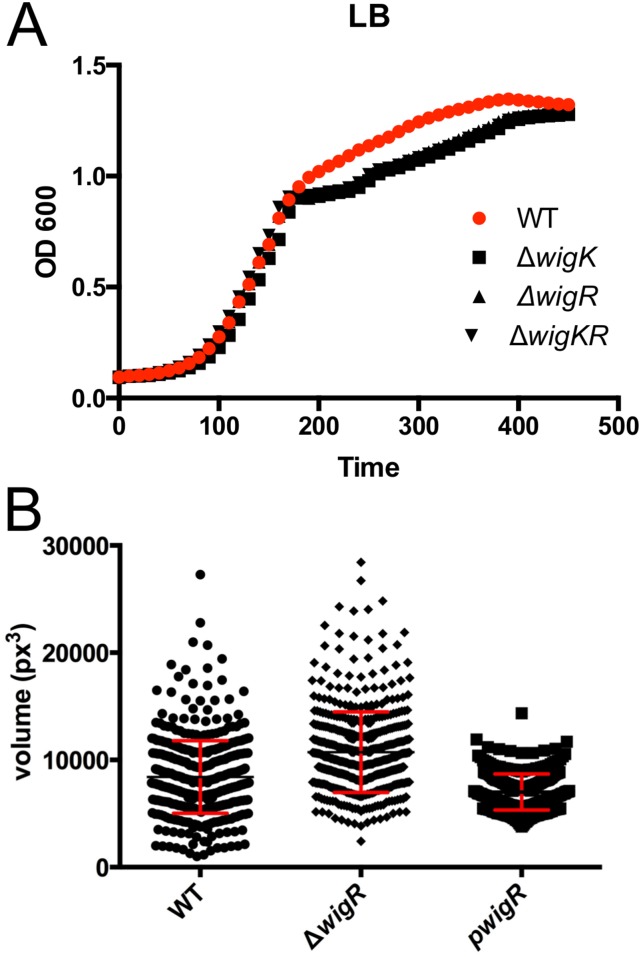
To begin to identify the genetic circuitry underlying WigKR-mediated beta-lactam tolerance, we used global RNA sequencing analysis to define the output of the WigKR regulon. For these analyses, we overexpressed a putative phosphomimetic version of WigR (D78E) to avoid heterogeneous phosphorylation states that might accompany overexpression of wild-type WigR. Overexpression of D78E caused a slight decrease in growth rate for cells grown in rich medium (LB) (Fig. S4A), but a much more pronounced decrease in M9 minimal medium (Fig. S4B). Consequently, RNASeq analyses were performed using V. cholerae cultured in LB.
Fig. S4.
Growth phenotypes of strains overexpressing phosphomimetic WigR (D78E). (A) Overexpression in LB broth. (B) Overexpression in M9 minimal medium. (C) Toxicity of WigR(D78E) overexpression is partially alleviated by the addition of iron. Cells were grown in M9 minimal medium + glucose and inducer either with or without the addition of 100 µM FeCl3. Values are averages of four technical replicates; error bars represent SD.
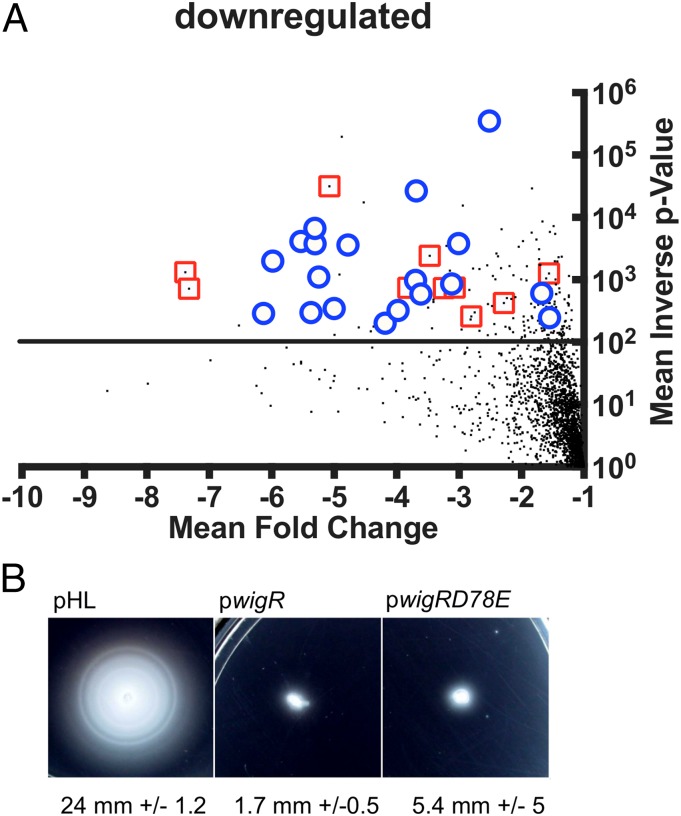
These transcriptomic analyses of the consequences of D78E expression vs. empty control plasmid revealed differential expression (defined as greater than twofold differences with a P value of < 0.01) of 320 genes (Dataset S1). Of these, 100 genes were down-regulated (Fig. 2A), many of which (28%) are associated with either motility (e.g., flagella biosynthesis) or iron acquisition (heme import and siderophore production). Consistent with these data, overexpression of WT WigR or WigR(D78E) resulted in a complete loss of motility in a soft agar swarming assay (Fig. 2B). In addition, we explored whether the poor growth of the WigR(D78E) overexpressing strain in M9 medium might reflect the absence of added iron in this media coupled with the down-regulation of iron acquisition genes. We found that addition of Iron(III) partially alleviated the growth defect caused by D78E overexpression (Fig. S4C). This result suggests that iron availability can limit growth when the WigR regulon is induced, likely due to down-regulation of siderophore biosynthesis genes.
Fig. 2.
Overexpression of phosphomimetic WigR leads to the down-regulation of motility and iron acquisition genes. (A) Volcano plot representation of down-regulated genes from comparison of transcript abundance in cells overexpressing phosphomimetic WigR(D78E) with empty vector control. Horizontal line indicates P = 0.01 (t test), the cutoff for significance. Red squares are genes involved in iron acquisition; blue circles indicate genes involved in motility. The full dataset is given in Dataset S1. (B) Overexpression of WigR or WigR(D78E) represses motility. Growing cultures were stabbed into soft agar plates and incubated overnight. Average radii of swarming zones were measured from five independent colonies for each strain.
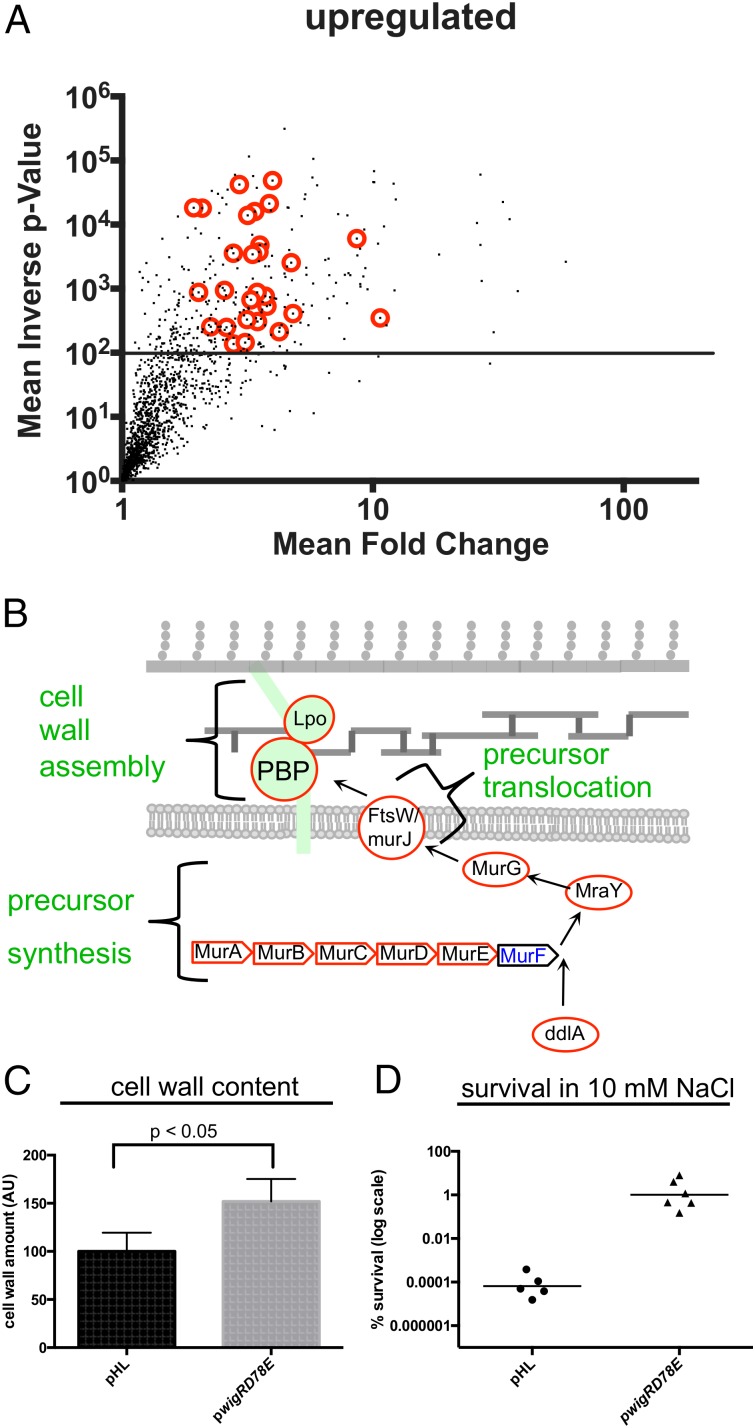
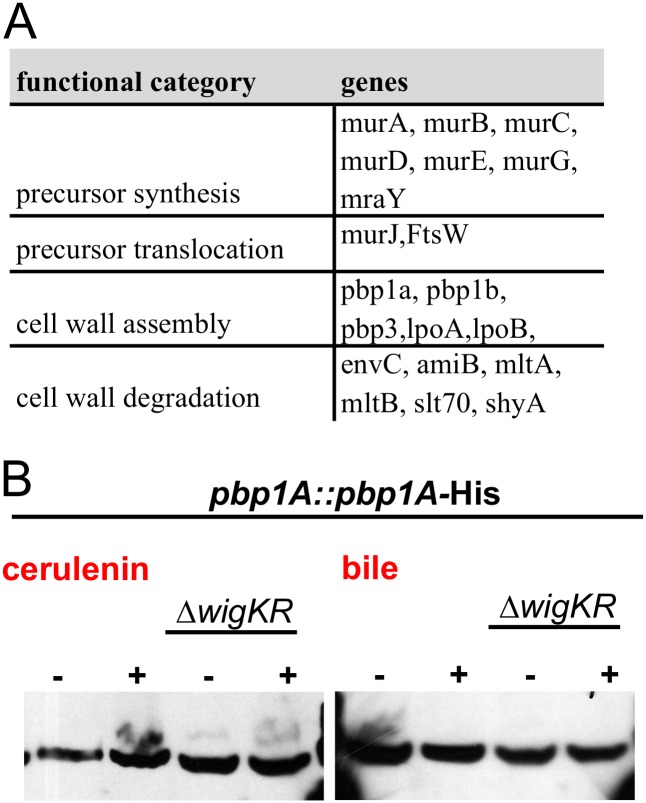
In addition to the genes negatively regulated by WigR, we identified 220 genes that were up-regulated as a consequence of D78E overexpression (Fig. 3A). Strikingly, 66 (30%) of the up-regulated genes coded for proteins related to the cell envelope, such as outer membrane proteins, envelope stress responses, and phospholipid biosynthesis. Remarkably, this list also encompassed the entire peptidoglycan biogenesis pathway, including precursor synthesis, precursor translocation, and cell wall assembly, as well as several factors associated with cell wall modification and degradation (Fig. 3B and Fig. S5A). The increase in the expression of cell wall synthesis genes was accompanied by a significant 1.5-fold increase in cell wall content (Fig. 3C) and by a marked (10,000-fold) increase in survival following hypo-osmotic shock (Fig. 3D). Thus, induction of the WigR regulon appears to enhance cell envelope strength, probably due to alterations in PG synthesis and/or structure. We did not observe increased expression of most of the type VI secretion genes previously found to be regulated by WigR (20); however, these secretion systems have been found to be expressed by only a small subset of V. cholerae strains (21).
Fig. 3.
Overexpression of phosphomimetic WigR leads to the up-regulation of cell wall synthesis genes. (A) Up-regulated genes from transcriptomic analysis described in Fig. 2A; note that, unlike Fig. 2A, the x axis is plotted on a logarithmic scale. Red circles indicate cell wall synthesis genes. (B) Schematic showing WigR-up-regulated cell wall synthesis genes. Black font indicates genes at least twofold up-regulated after WigR(D78E) overexpression. (C) Comparison of cell wall content in a strain overexpressing WigR(D78E) or empty vector control. Cell wall content was measured by ultra performance liquid chromatography (UPLC)-mediated quantification of digested cell wall material derived from cultures normalized to the same cfu/mL. (D) Osmoresistance caused by overexpression of WigR(D78E). Cells were grown to exponential phase in LB (200 mM NaCl), pelleted, and resuspended in 10 mM NaCl for 5 min, followed by adjustment to 200 mM NaCl. Individual data points of six biological replicates are shown.
Fig. S5.
(A) Cell wall synthesis/remodeling genes up-regulated at least twofold after WigR(D78E) overexpression. (B) Western blot of PBP1A-His levels in response to envelope stress factors. Cells were grown to late exponential phase and then exposed to cerulenin (∼10× MIC) for 3 h or to 2% bile for 30 min before SDS-PAGE followed by Western blotting.
WigKR Regulon Is Activated by Antibiotic-Induced Cell Wall Damage.
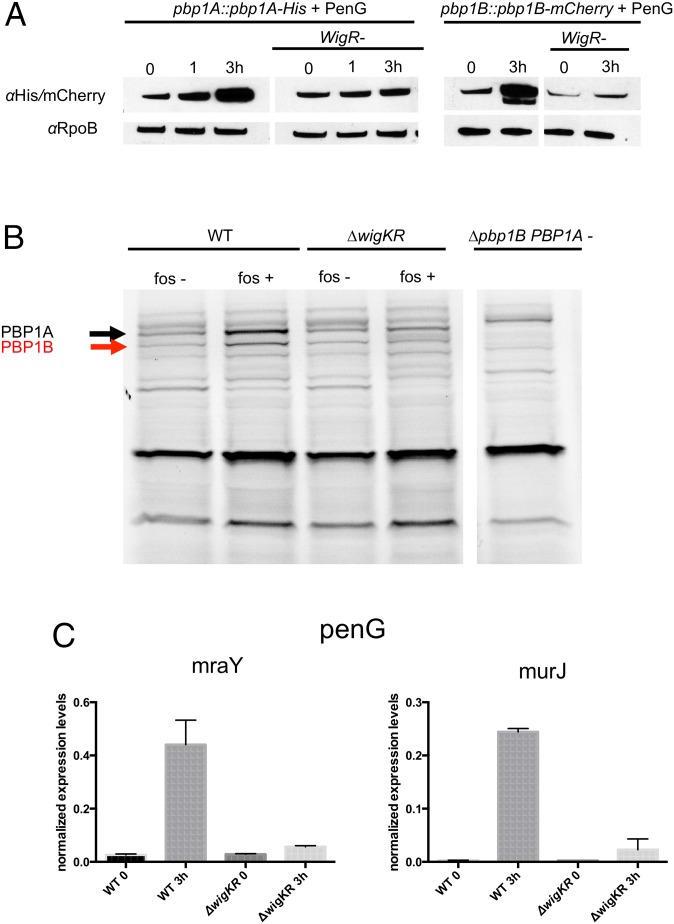
Given WigR’s regulation of cell wall synthesis, coupled with V. cholerae’s reguirement for wigKR to survive beta-lactam exposure, we hypothesized that beta-lactams activate the WigKR-signaling pathway and thereby lead to WigKR-dependent induction of cell wall synthesis genes. To test this possibility, we compared accumulation of epitope-tagged forms of V. cholerae’s two major cell wall synthases, PBP1A and PBP1B, following penicillin exposure of cells containing or lacking wigR. Strikingly, penicillin induced the expression of both PBPs approximately four- to fivefold (Fig. 4A) in a largely wigR-dependent fashion.
Fig. 4.
Cell-wall–acting antibiotics induce expression of cell wall synthesis genes in a WigKR-dependent fashion. (A) Western blot analysis of His- or mCherry-tagged PBP1A or PBP1B, respectively, expressed from their native chromosomal loci. Cells were grown to late exponential phase and exposed to penicillin G (100 µg/mL). (B) Bocillin-stained membrane proteins from the indicated strains, grown in the absence or presence of fosfomycin. (C) qRT-PCR analysis of cell wall precursor synthesis/translocation genes. Values are means of biological triplicates; error bars represent SD. Expression levels were normalized to an internal control transcript, rpoC.
WigKR-dependent accumulation of PBP1A and PBP1B following inhibition of cell wall synthesis was also observed with untagged membrane proteins that were visualized using the fluorescent penicillin derivative Bocillin-FL (22). Bocillin, like penicillin, covalently binds to the active site of PBPs, and thus one precludes binding of the other; consequently, for this assay we inhibited cell wall synthesis with fosfomycin, which does not directly interfere with bocillin binding. As with penicillin, we found that the abundance of PBP1A and PBP1B increased in a largely wigKR-dependent manner when cells were treated with fosfomycin (Fig. 4B). Thus, inhibition of distinct steps in cell wall synthesis can induce WigR-dependent production of PBPs. The minor increase in PBP1A levels following antibiotic exposure in the absence of WigKR suggests that PBPs can also be up-regulated by another, unidentified factor.
To gather additional evidence for penicillin induction of the WigR regulon, we measured transcripts from two other cell wall synthesis genes, mraY (lipid2 biosynthesis) and murJ (lipid2 flippase), using qRT-PCR. Expression of both genes was strongly (17- and 88-fold) induced by penicillin in a wigKR-dependent fashion (Fig. 4C). Thus, taken together, our findings reveal that wigKR activates multiple steps of the cell wall biogenesis process in response to cell wall synthesis inhibitors.
We then asked whether WigKR-dependent induction of cell wall synthesis was associated only with cell wall damage or with assaults on the cell envelope in general. To test this, we exposed our PBP1A-His reporter strain to two unrelated cell envelope stressors, cerulenin (an inhibitor of fatty acid biosynthesis) and crude bile, which acts as a general membrane-damaging agent. Neither agent induced a significant up-regulation of PBP1A expression (Fig. S5B), suggesting that the WigKR regulon is not induced by cell envelope damage in general.
wigKR Controls Its Own Expression in Response to beta-Lactams.
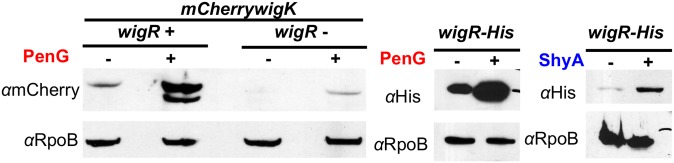
Many HK/RR systems are known to autoregulate their expression; i.e., these systems are expressed constitutively at low levels but increase their own expression upon signal recognition (23). Indeed, wigK was up-regulated in response to expression of WigR(D78E) in our RNASeq dataset (Dataset S1). We tested whether beta-lactams induce WigKR in conjunction with its regulon by measuring protein levels of an mCherry-WigK translational fusion and of C-terminally His-tagged WigR-His (both encoded at their native chromosomal loci) after exposure to antibiotics. Baseline WigK and WigR levels were very low, but increased dramatically upon addition of penicillin (Fig. 5). The baseline level and penicillin-induced increase in WigK abundance were markedly reduced in a wigR mutant, confirming that the wigKR operon autoregulates. Increased WigR levels were likewise observed after exposure to fosfomycin and to d-cycloserine (Fig. S6), an inhibitor of d-Ala-d-Ala ligase (5), which catalyzes a later cytoplasmic step in cell wall synthesis. Notably, WigR levels were also increased upon overexpression of the PG endopeptidase ShyA (Fig. 5), a housekeeping enzyme required for PG degradation in the context of cell elongation (24). Thus, therapeutics that target various steps in cell wall synthesis, as well as endogenous agents of cell wall damage, can all induce WigR.
Fig. 5.
wigKR expression is autoregulated and increased in response to antibiotics targeting cell wall synthesis. Cells harboring either mCherry-WigK or a WigR-His fusion were grown to late exponential phase and exposed to penicillin G for 3 h or overexpression of ShyA for 1 h, followed by Western blotting using antibodies specific to mCherry (for WigK detection) or His (for WigR detection).
Fig. S6.
Cells harboring a chromosomal WigR-His fusion were grown to late exponential phase and exposed to fosfomycin or d-cycloserine (both 200 µg/mL, ∼4× MIC), followed by Western blotting using antibodies specific to His6.
wigKR Locus Plays a Role in Cell Wall Homeostasis During Normal Growth.
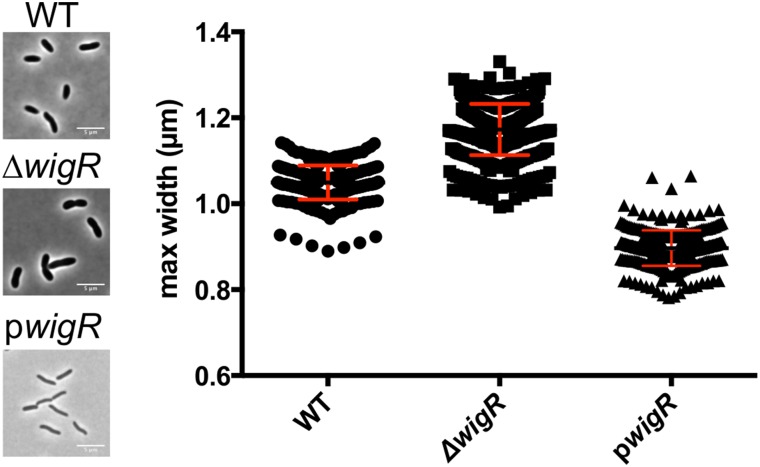
Given the large set of genes in the WigR regulon that mediate cell wall biogenesis, we speculated that wigKR might modulate cell wall synthesis/degradation during normal growth, as well as in response to antibiotics that target cell wall synthesis. Although mutants lacking wigK and/or wigR exhibited only minor growth defects, which interestingly were restricted to the late exponential phase (Fig. S7A), we observed a marked increase in cell diameter (Fig. 6) and cell volume (Fig. S7B) in mutants lacking wigR. Conversely, overexpression of WigR caused a reduction in cell width from 1 µm on average to ∼0.8 µm (Fig. 6). Because cell width is determined by the cell wall and is typically tightly controlled (25, 26), these striking aberrations suggest a fundamental role for wigR in cell wall homeostasis, the molecular details of which warrant further investigation.
Fig. S7.
(A) Growth of ∆wig mutants in LB medium. (B) Cell volume of strains lacking or overexpressing wigR.
Fig. 6.
WigKR regulates V. cholerae cell size during exponential phase growth. Phase contrast images and comparison of cell widths in WT and ∆wigR V. cholerae and in V. cholerae overproducing WigR during exponential phase growth in LB.
Discussion
WigKR is an unusual two-component system that responds to damage caused by antibiotics that target cell wall biosynthesis and to endogenously generated stimuli, such as those elicited by overexpression of the ShyA endopeptidase. Similar cell wall damage response systems have not been described in Gram-negative bacteria. The VraSR and WalRK two-component systems in Staphylococcus aureus and Bacillus subtilis are thought to respond to cell wall stress induced by antibiotics, but, in marked contrast to the WigKR system, the regulons controlled by these Gram-positive two-component systems primarily include specialized, single PBPs and modulators of autolysin activity, and not the entire pathway for PG biogenesis. WigKR’s control of the complete PG synthesis pathway explains why this system is critical for V. cholerae tolerance to antibiotics that target the cell wall. Treatment of V. cholerae with cell-wall–acting antibiotics results in formation of PG-deficient spheres; WigR and its regulon, which are induced by these agents, are required for V. cholerae recovery presumably because they enable V. cholerae to regenerate its cell wall and restore its rod shape.
Although WigK is conserved only in the Vibrionaceae, it is possible that other bacteria have evolved analogous stress response systems to fulfill similar functions. For example, the Escherichia coli Rcs phosphorelay system (homologs of which are absent from V. cholerae) has been shown to be required for recovery from a spherical state induced by lysozyme treatment in an osmotically stabilized medium (27) (a state that resembles V. cholerae after treatment with beta-lactam antibiotics). Moreover, Pseudomonas aeruginosa and Acinetobacter baumannii, like V. cholerae, fail to lyse in the presence of cell wall synthesis inhibitors under some conditions (19, 28). These pathogens lack Rcs systems as well, but may harbor specialized stress response systems functionally equivalent to WigKR that aide in recovery from cell wall damage. Inhibition of such cell wall stress response systems may be a promising avenue for discovery of adjuvants that enhance the efficacy of antibiotics that target cell wall synthesis.
In addition to mediating antibiotic tolerance, wigKR likely contributes to cell wall homeostasis in the absence of antibiotics. The ∆wigR strain exhibited increased cell width, whereas wigR overexpression led to cell slimming. WigR(D78E)-induced up-regulation of the major PBPs and their activators (PBP1A/B and LpoA/B, respectively) is of particular interest. Cell wall assembly in the periplasm was thought to be a constitutive housekeeping process until the discovery of outer membrane-localized activators of PBP1A and PBP1B activity (8, 9). Our data suggest that, in addition to posttranslational regulation, at least some bacteria are also capable of modulating transcription of cell wall synthesis pathways. Very few transcriptional regulators of PBPs have been described to date, especially in Gram-negative bacteria. E. coli regulates the expression of low-molecular-weight PBPs (which are thought to play roles in PG modification rather than synthesis) via the transcriptional regulator BolA in response to an unknown signal upon entry into stationary phase (29). Neisseria gonorrheae’s principal PBP, encoded by the ponA gene, is under control of a repressor also controlling expression of type IV pili and a multidrug efflux pump (30). The WigKR system is unusual as it regulates the entire cell wall synthesis pathway, including precursor synthesis, precursor translocation, and cell wall assembly (PBPs and PBP activators) as well as PG hydrolases, which are also required for growth. By governing the complete pathway for PG synthesis, WigKR presumably allows for control of total cell wall content. Consistent with this idea, we found that cells overexpressing phosphomimetic wigR had increased PG content, which likely accounts for their markedly increased resistance to osmotic stress. Thus, the WigKR system is a bona fide regulator of total cell wall content.
A key challenge for future investigation is identification of the signal that activates the WigKR regulon. It is formally possible that WigK directly senses antibiotics or depletion of PG synthesis precursors, which has recently been described as a lethal consequence of exposure to beta-lactam antibiotics (31). However, direct sensing of antibiotics is highly unlikely because structurally diverse agents (beta-lactams, d-cycloserine, fosfomycin) induce WigR and WigR governs cell width even in the absence of antibiotics. Sensing of precursor depletion is unlikely because WigR is also induced by overexpression of ShyA, an endopeptidase that cuts cross-links in the PG meshwork, which in theory should not affect precursor availability. ShyA preferentially releases short PG chains from the PG network and is primarily responsible for cell wall degradation after inhibition of cell wall synthesis (19, 24); consequently, WigK may be activated by endogenous cell wall fragments. Such fragments could be the product of normal growth processes; result from the action of antibiotics; or potentially be induced by phage or type VI effector attack, both of which can damage the cell wall.
Materials and Methods
Strains, Plasmids, and Oligos.
Strains, plasmids, and oligos are summarized in Dataset S2. All V. cholerae strains are derivatives of N16961, an El Tor clinical isolate (32). For details on strain construction, media, and growth conditions, see SI Materials and Methods.
Killing and Osmoresistance Assays.
For time-dependent antibiotic survival experiments, cells were grown with shaking at 37 °C to midexponential phase in 3 mL LB broth in 10-mL glass tubes, followed by addition of antibiotic. At designated time points, cfu/mL were enumerated by serial dilution and spot-plating.
For the osmoresistance assay, strains were grown to midexponential phase (OD600 ∼0.3) in LB (∼200 mM NaCl), pelleted, and resuspended in 10 mM NaCl for 5 min, followed by addition of 1/10 volume of 10× PBS (1.37 M NaCl, 27 mM KCl, 100 mM Na2HPO4, 18 mM KH2PO4) and then serial-diluted and spot-plated onto LB agar plates.
RNAseq Analysis.
RNA was extracted using the TRIzol method (33) (for details see SI Materials and Methods). RNAseq and differential gene expression analysis was conducted by Genewiz.
Bocillin-FL Staining.
Membrane fractions were obtained by differential centrifugation (see SI Materials and Methods for details). PBPs in the membrane fractions were stained using 50 µM Bocillin-FL (Invitrogen) for 20 min at 37 °C.
PG Quantification.
For PG quantification and analysis, cultures were normalized to the same cfu/mL, and muropeptides were isolated following previously described methods (34, 35).
Microscopy and Image Analysis.
Cells were imaged on 0.8% agarose pads using a Zeiss Axiovision light microscope and 100× objective. Cell width/volume in cells grown in LB to OD600 = 0.5 was determined using MicrobeTracker software (36).
For additional methods, see SI Materials and Methods.
SI Materials and Methods
Strains, Plasmids, and Oligos.
Strains, plasmids, and oligos are summarized in Dataset S2. Unmarked deletions were generated by allele replacement via homologous recombination using the suicide plasmid pCVD442 (37). For the experiments using the PBP1B::PBP1BmCherry and the WigK::mCherryWigK strains, WigR− is an insertional disruption of WigR using a kanamycin-resistant derivative of the suicide plasmid pGP704 carrying a 300-bp internal fragment of WigR. Insertional disruptions generated with this plasmid exhibited the same phenotype as the deletion in all assays. To generate mutations in the predicted phosphorylation sites of WigK and WigR, the whole wigKR region was amplified and inserted into pCVD442 using isothermal assembly (38). The product was used as a template for site-directed mutagenesis (using the Agilent QuikChange kit according to the manufacturer’s protocol). The resulting plasmids were used for the introduction of the respective alleles (or an unmutagenized plasmid for reconstitution of wild type) into a ∆wigKR mutant.
For complementation assays, wigK, wigR, or the entire wigKR operon were cloned into pHL100 (kanR, laciQ-Ptac:MCS) using isothermal assembly. To generate strains expressing complementing genes from a neutral chromosomal locus, the respective ORFs (including lacIq and the Ptac promoter) were amplified from pHL100 and cloned into pJL1, a suicide plasmid permitting allele insertion into V. cholerae lacZ.
pHL100-expressing phosphomimetic WigR (D78E) was generated using site-directed mutagenesis as described above. All constructs were verified by sequencing.
Media and Growth Conditions.
Experiments were conducted in LB broth (Fisher) unless noted otherwise. Antibiotics (Sigma) were used at the following concentrations: streptomycin (200 µg/mL), kanamycin (50 µg/mL), carbenicillin (50 µg/mL), penicillin G (100 µg/mL), fosfomycin (200 µg/mL), and d-cycloserine (200 µg/mL). Growth curves were conducted on a Biotek Growth Curve machine in 100-well honeycomb plates.
For in vivo survival experiments, infant mice were inoculated with ∼105 cfu each of a mixture of WT (lacZ−) and ∆wigKR (lacZ+) strains (n = 20). After 20 h, mice were orally inoculated either with 50 mg/kg amoxicillin in LB (Sigma) (n = 9) or with LB only (n = 10). After an additional 12 h, mice were killed and their small intestines homogenized, serially diluted, and plated onto LB agar containing streptomycin and X-gal (60 µg/mL) for enumeration of lacZ+ vs. lacZ− cells.
RNAseq Analysis.
RNA was prepared from cells growing in LB medium (biological triplicates) using the TRIzol method. Five milliliters of exponential phase cultures expressing (shaking at 37 °C for 2 h, 250 µM IPTG added at OD600 ∼0.05) either empty plasmid or pHLwigRD78E were pelleted, resuspended in TRIzol reagent (Ambion), and left at ambient temperature for 5 min. Next, 260 µL of chloroform was added, followed by shaking and centrifugation (11,500 × g, 5 min). The upper (aqueous) phase was transferred to a fresh tube, mixed with an equal volume of chloroform, and centrifuged. The upper phase was then transferred to 600 µL of isopropanol, vortexed, and incubated at room temperature for 10 min, followed by centrifugation (12,000 × g, 15 min, 4 °C). The pellet (total RNA) was washed twice with 75% (vol/vol) ethanol and then resuspended in 100 µL of nuclease-free water (Ambion). DNA was removed using the Turbo DNase kit (Ambion). RNAseq and differential gene expression analysis was done by Genewiz, as described below.
NGS sequencing.
RNA samples were quantified using Qubit 2.0 Fluorometer (Life Technologies), and the RNA integrity was checked with RNA6000 Nano Assay using the Agilent 2100 Bioanalyzer (Agilent Technologies). cDNA library preparation and sequencing reactions were conducted by Genewiz. Illumina TruSeq Stranded Total RNA Library Prep Kit, clustering, and sequencing reagents were used throughout the process following the manufacturer’s recommendations. The cDNA library was quantified using Qubit 2.0 Fluorometer (Life Technologies) and by qPCR. The samples were clustered on a flow cell using the cBOT. After clustering, the samples were loaded for sequencing of 1 × 50 single read on the Illumina HiSEq. 2500 instrument.
Data analysis.
Raw sequence data generated from Illumina HiSEq. 2500 was converted into fastq files and de-multiplexed using Illumina CASSAVA 1.8.4 program. Fastq files from each sample were imported into CLC Genomics Workbench 7.5. Sequence reads were trimmed to remove bases with low quality at ends. Then sequence reads were aligned to the reference genome for Vibrio cholerae N16961 using a strand-specific method. Sequence read account and reads per kilobase of transcript per million mapped reads (RPKM) values were calculated for genes. Quantile normalization and log2 transformation were performed for the RPKM values. Principal component analysis and hierarchical clustering analysis were performed to examine sample separation between the two groups. Then Student’s t-test was conducted to compare gene expression between the control samples and experimental samples, and a list of 366 genes with an false discovery rate of <0.05 were selected as differentially expressed genes. These genes were further prioritized by excluding those that were less than twofold differentially regulated.
Bocillin-FL Staining.
Membrane fractions were isolated from cells grown in LB at OD600 = 0.5 to obtain membrane fractions and 400 mL of culture were pelleted, washed 1× in TBS, and resuspended in 5 mL cold TBS [+ HALT protease inhibitor/EDTA (Fisher) and 5 mg/mL lysozyme]. Cells were disrupted by sonication (Sonic Dismembrator, Fisher, 3- × 10-s pulses at power level 16), and then cell debris were pelleted by centrifugation (35 min 13,000 × g, 4 °C). The supernatant fraction (i.e., membranes) were subjected to ultracentrifuation (110,000 × g, 3 min, 4 °C), and the membrane pellet washed 1× in TBS, then resuspended in TBS/10% (vol/vol) glycerol, and stored at −80 °C. Membrane fractions were adjusted to the same total protein content (quantified using Qubit) by addition of TBS.
For PBP staining, Bocillin-FL was added to 75 µL of membrane suspension to a 50-µM final concentration and incubated at 37 °C for 20 min. The reaction was stopped by the addition of 1% SDS and boiling. Bocillin-stained fractions were subjected to SDS-PAGE (14% Tris–glycine gel), followed by visualization of bands using a Fuji fluorescent gel scanner (437 nm exc, FITC filter, 1,000 V). As a control, we subjected a strain deleted in PBP1A and PBP1B (with PBP1A expressed from an IPTG-inducible promoter integrated in single copy into the chromosome) grown in the absence of inducer. Depletion of PBP1A in this background leads to sphere formation (35), which was verified by light microscopy.
Western Blotting.
Cells were grown to OD600 ∼0.3, pelleted, washed 1× with Tris-buffered saline (TBS, 50 mM Tris⋅HCl, pH 7.5, 150 mM NaCl), and then resuspended in 100 µL TBS. Following addition of 2× lysis buffer [50 mM Tris, 2% (wt/vol) SDS, 0.8% (vol/vol) beta-mercaptoethanol], suspensions were boiled for 2 min at 95 °C and then sonicated 2× (Sonic Dismembrator) at medium power for 5 s each. Lysates were normalized to total protein content (measured by Qubit) and then loaded onto a 4–12% Bis–Tris gel (Novagen) and subjected to SDS-PAGE. Gels were blotted onto nitrocellulose using an iBlot system (Fisher). Membranes were blocked for 1 h in fat-free milk (0.5%) and then blotted using anti-mCherry (Pierce) or anti-His6 (Pierce) antibodies. RpoB antibody served as loading control.
RT-PCR.
RNA was prepared as described above for RNAseq analyses. RT-PCR was conducted with RNA extracted from biological triplicates. cDNA was synthesized using the SuperScript III reverse transcriptase system (Fisher) according to the manufacturer’s protocol. RT-PCR was conducted using FAST SYBR Green mastermix (ThermoFisher) in technical triplicate for each biological replicate using rpoC as internal control.
PG Quantification.
For PG quantification and analysis, cultures were harvested at the exact same cfu/mL, and muropeptides were isolated following previously described methods (34, 35). In brief, samples were boiled in SDS 10% (wt/vol) for 4 h, and sacculi were repeatedly washed with MilliQ water by ultracentrifugation (110,000 × g, 10 min, 20 °C). Samples were treated with Pronase E (100 µg/mL, 1 h, 60 °C) for Braun’s lipoprotein removal. The reaction was heat-inactivated, and sacculi were further washed by ultracentrifugation as described above. Samples were digested with muramidase (100 µg/mL) for 16 h at 37 °C and heat-inactivated. Coagulated proteins were removed by 10 min of centrifugation at 14,000 × g. For sample reduction, samples were first adjusted to pH 8.5–9.0 with borate buffer, and then a freshly prepared NaBH4 2M solution wash was added to a final concentration of 10 mg/mL. After 30 min at room temperature, samples were adjusted to pH 3.5 with phosphoric acid.
UPLC separation of muropeptides was performed on a Waters UPLC system equipped with an ACQUITY UPLC BEH C18 Column, 130 Å, 1.7 μm, 2.1 mm × 150 mm (Waters) and a dual wavelength absorbance detector. Elution of muropeptides was detected at 204 nm. Muropeptides were separated at 35 °C using a linear gradient from buffer A (phosphate buffer 50 mM, pH 4.35) to buffer B [phosphate buffer 50 mM, pH 4.95 methanol 15% (vol/vol)] in a 20-min run with a 0.25 mL/min flow.
Relative PG amounts were calculated by comparison of the total intensities of the chromatograms (total area) from three biological replicas normalized to the same cfu/mL and extracted with the same volumes because there is a direct correlation between muropeptide content and detected intensity. The injection volume was optimized to ensure that the signal was within the detection limits of the system.
Supplementary Material
Acknowledgments
We thank Stavroula Hatzios for assistance with illustrations. Research in the M.K.W. laboratory is supported by Howard Hughes Medical Institute and NIH Grant R37 AI–042347. Research in the F.C. laboratory is supported by the Molecular Infection Medicine Sweden (MIMS), the Knut and Alice Wallenberg Foundation, and the Swedish Research Council. L.A. is supported by Grant JCK-1326 from the Kempe Foundation (Kempestiftelserna).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520333113/-/DCSupplemental.
References
- 1.Höltje JV. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev. 1998;62(1):181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis K. Platforms for antibiotic discovery. Nat Rev Drug Discov. 2013;12(5):371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 3.Schneider T, Sahl HG. An oldie but a goodie: Cell wall biosynthesis as antibiotic target pathway. Int J Med Microbiol. 2010;300(2-3):161–169. doi: 10.1016/j.ijmm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Typas A, Banzhaf M, Gross CA, Vollmer W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol. 2012;10(2):123–136. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert MP, Neuhaus FC. Mechanism of D-cycloserine action: Alanine racemase from Escherichia coli W. J Bacteriol. 1972;110(3):978–987. doi: 10.1128/jb.110.3.978-987.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahan FM, Kahan JS, Cassidy PJ, Kropp H. The mechanism of action of fosfomycin (phosphonomycin) Ann N Y Acad Sci. 1974;235(0):364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- 7.Huber J, et al. Chemical genetic identification of peptidoglycan inhibitors potentiating carbapenem activity against methicillin-resistant Staphylococcus aureus. Chem Biol. 2009;16(8):837–848. doi: 10.1016/j.chembiol.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Paradis-Bleau C, et al. Lipoprotein cofactors located in the outer membrane activate bacterial cell wall polymerases. Cell. 2010;143(7):1110–1120. doi: 10.1016/j.cell.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Typas A, et al. Regulation of peptidoglycan synthesis by outer-membrane proteins. Cell. 2010;143(7):1097–1109. doi: 10.1016/j.cell.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vollmer W. Bacterial growth does require peptidoglycan hydrolases. Mol Microbiol. 2012;86(5):1031–1035. doi: 10.1111/mmi.12059. [DOI] [PubMed] [Google Scholar]
- 11.Uehara T, Bernhardt TG. More than just lysins: Peptidoglycan hydrolases tailor the cell wall. Curr Opin Microbiol. 2011;14(6):698–703. doi: 10.1016/j.mib.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capra EJ, Laub MT. Evolution of two-component signal transduction systems. Annu Rev Microbiol. 2012;66:325–347. doi: 10.1146/annurev-micro-092611-150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bury-Moné S, et al. Global analysis of extracytoplasmic stress signaling in Escherichia coli. PLoS Genet. 2009;5(9):e1000651. doi: 10.1371/journal.pgen.1000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raivio TL. Everything old is new again: An update on current research on the Cpx envelope stress response. Biochim Biophys Acta. 2014;1843(8):1529–1541. doi: 10.1016/j.bbamcr.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Majdalani N, Gottesman S. The Rcs phosphorelay: A complex signal transduction system. Annu Rev Microbiol. 2005;59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- 16.Dubrac S, Bisicchia P, Devine KM, Msadek T. A matter of life and death: Cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol Microbiol. 2008;70(6):1307–1322. doi: 10.1111/j.1365-2958.2008.06483.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuroda M, et al. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol Microbiol. 2003;49(3):807–821. doi: 10.1046/j.1365-2958.2003.03599.x. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs C, Frère JM, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible beta-lactam resistance in gram-negative bacteria. Cell. 1997;88(6):823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 19.Dörr T, Davis BM, Waldor MK. Endopeptidase-mediated beta lactam tolerance. PLoS Pathog. 2015;11(4):e1004850. doi: 10.1371/journal.ppat.1004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng AT, Ottemann KM, Yildiz FH. Vibrio cholerae response regulator VxrB controls colonization and regulates the type VI secretion system. PLoS Pathog. 2015;11(5):e1004933. doi: 10.1371/journal.ppat.1004933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho BT, Dong TG, Mekalanos JJ. A view to a kill: The bacterial type VI secretion system. Cell Host Microbe. 2014;15(1):9–21. doi: 10.1016/j.chom.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao G, Meier TI, Kahl SD, Gee KR, Blaszczak LC. BOCILLIN FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob Agents Chemother. 1999;43(5):1124–1128. doi: 10.1128/aac.43.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitrophanov AY, Groisman EA. Signal integration in bacterial two-component regulatory systems. Genes Dev. 2008;22(19):2601–2611. doi: 10.1101/gad.1700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dörr T, Cava F, Lam H, Davis BM, Waldor MK. Substrate specificity of an elongation-specific peptidoglycan endopeptidase and its implications for cell wall architecture and growth of Vibrio cholerae. Mol Microbiol. 2013;89(5):949–962. doi: 10.1111/mmi.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taheri-Araghi S, et al. Cell-size control and homeostasis in bacteria. Curr Biol. 2015;25(3):385–391. doi: 10.1016/j.cub.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang KC, Mukhopadhyay R, Wen B, Gitai Z, Wingreen NS. Cell shape and cell-wall organization in Gram-negative bacteria. Proc Natl Acad Sci USA. 2008;105(49):19282–19287. doi: 10.1073/pnas.0805309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranjit DK, Young KD. The Rcs stress response and accessory envelope proteins are required for de novo generation of cell shape in Escherichia coli. J Bacteriol. 2013;195(11):2452–2462. doi: 10.1128/JB.00160-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monahan LG, et al. Rapid conversion of Pseudomonas aeruginosa to a spherical cell morphotype facilitates tolerance to carbapenems and penicillins but increases susceptibility to antimicrobial peptides. Antimicrob Agents Chemother. 2014;58(4):1956–1962. doi: 10.1128/AAC.01901-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos JM, Lobo M, Matos AP, De Pedro MA, Arraiano CM. The gene bolA regulates dacA (PBP5), dacC (PBP6) and ampC (AmpC), promoting normal morphology in Escherichia coli. Mol Microbiol. 2002;45(6):1729–1740. doi: 10.1046/j.1365-2958.2002.03131.x. [DOI] [PubMed] [Google Scholar]
- 30.Folster JP, Dhulipala V, Nicholas RA, Shafer WM. Differential regulation of ponA and pilMNOPQ expression by the MtrR transcriptional regulatory protein in Neisseria gonorrhoeae. J Bacteriol. 2007;189(13):4569–4577. doi: 10.1128/JB.00286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho H, Uehara T, Bernhardt TG. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell. 2014;159(6):1300–1311. doi: 10.1016/j.cell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heidelberg JF, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406(6795):477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 34.Desmarais SM, De Pedro MA, Cava F, Huang KC. Peptidoglycan at its peaks: How chromatographic analyses can reveal bacterial cell wall structure and assembly. Mol Microbiol. 2013;89(1):1–13. doi: 10.1111/mmi.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dörr T, et al. A novel peptidoglycan binding protein crucial for PBP1A-mediated cell wall biogenesis in Vibrio cholerae. PLoS Genet. 2014;10(6):e1004433. doi: 10.1371/journal.pgen.1004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sliusarenko O, Heinritz J, Emonet T, Jacobs-Wagner C. High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Mol Microbiol. 2011;80(3):612–627. doi: 10.1111/j.1365-2958.2011.07579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donnenberg MS, Kaper JB. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59(12):4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson DG, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.