Significance
Among the five basic tastes, sour is one of the least understood. Notably, the sour receptor remains to be identified, and molecular mechanisms by which sour stimuli are detected are largely not known. Previous work has shown that H+ ions can directly enter sour taste cells, eliciting a change in membrane potential and acidification of the cell cytosol. In the present work, we identify a second element of sensory transduction, a K+ channel, KIR2.1, which is inhibited by intracellular acidification. The presence of an acid-sensitive K+ channel in sour taste cells allows for amplification of the sensory response and may explain why weak acids that produce intracellular acidification, such as acetic acid, taste more sour than strong acids.
Keywords: proton channel, taste cell, gustatory, inward rectifier, potassium channel
Abstract
Sour taste is detected by a subset of taste cells on the tongue and palate epithelium that respond to acids with trains of action potentials. Entry of protons through a Zn2+-sensitive proton conductance that is specific to sour taste cells has been shown to be the initial event in sour taste transduction. Whether this conductance acts in concert with other channels sensitive to changes in intracellular pH, however, is not known. Here, we show that intracellular acidification generates excitatory responses in sour taste cells, which can be attributed to block of a resting K+ current. We identify KIR2.1 as the acid-sensitive K+ channel in sour taste cells using pharmacological and RNA expression profiling and confirm its contribution to sour taste with tissue-specific knockout of the Kcnj2 gene. Surprisingly, acid sensitivity is not conferred on sour taste cells by the specific expression of Kir2.1, but by the relatively small magnitude of the current, which makes the cells exquisitely sensitive to changes in intracellular pH. Consistent with a role of the K+ current in amplifying the sensory response, entry of protons through the Zn2+-sensitive conductance produces a transient block of the KIR2.1 current. The identification in sour taste cells of an acid-sensitive K+ channel suggests a mechanism for amplification of sour taste and may explain why weak acids that produce intracellular acidification, such as acetic acid, taste more sour than strong acids.
Sour taste is mediated by a subset of taste cells on the tongue and palate epithelium that respond to acids with trains of action potentials and transmitter release (1–3). Both strong acids, such as hydrochloric acid, and weak acids, such as acetic or citric acid, produce a sour sensation in humans and evoke sensory responses in nerve recordings in a variety of model organisms, including rat, mouse, and hamster (4–7). A number of molecules have been proposed to transduce sour taste, most recently the ion channel PKD2L1/PKD1L3 (8–12), but their role in taste transduction remains unclear as subsequent studies using knockout mouse strains have failed to identify significant effects on sour taste (13–15). Nonetheless, the Pkd2l1 gene serves as a useful marker for sour taste cells (also designated type III cells), which account for ∼10% of the ∼50–100 taste cells found in each taste bud (1, 9, 11, 16, 17). Previously, using a Pkd2l1-YFP mouse, we showed that sour cells express a unique Zn2+-sensitive proton conductance, of unknown identity, that is likely to mediate the initial event in taste transduction (16). Whether this conductance acts “alone” or in concert with other channels sensitive to changes in intracellular pH is not known. In this report, we provide evidence for a second component of the transduction cascade: a resting K+ current, mediated by KIR2.1 channels, which have an unexpected sensitivity to intracellular pH.
Several pieces of evidence argue for a second component of taste transduction, sensitive to intracellular acidification. First, it was demonstrated nearly a century ago (18) that weak acids, which can penetrate the cell membrane and acidify the cell cytosol, taste more sour than strong acids, at the same pH. Mirroring this effect, it is well established that the gustatory nerve response is greater when the tongue is stimulated with weak acids than with strong acids at the same pH, and varies both as a factor of pH and of the concentration of the undissociated acid (5, 19). Similarly, calcium responses from sour-sensitive cells in slice recording can be evoked with weak acids at a higher pH compared with strong acids (20). Moreover, we previously reported that action potentials can be elicited in sour taste cells in response to extracellular pH of 6.5–6.7, where the current carried by protons (2–3 pA) is unlikely to be sufficient to depolarize the cell (21). All of these phenomena can be explained if intracellular acidification increases membrane excitability of sour taste cells. Indeed, it has been proposed that two-pore domain K+ channels, several of which are expressed at high levels in sour taste cells, could serve as sensors of intracellular pH (22, 23). However, to date, there is no direct evidence showing that sour taste cells are activated by intracellular acidification, and the molecular mechanisms by which intracellular acidification could excite sour taste cells remain largely unexplored.
Here, using genetically identified sour taste cells, we show that intracellular acidification, in the absence of extracellular acidification, is sufficient to produce robust trains of action potentials in sour taste cells but not in taste cells that detect bitter, sweet, and umami. Intracellular acidification blocks an inwardly rectifying K+ current in sour taste cells, which we identify as KIR2.1 based on expression profiling and pharmacological analysis, and confirm using a newly developed, cell-type–specific KIR2.1-knockout model. We propose that block of the resting K+ current in sour taste cells contributes to the taste of weak acids and provides a mechanism for amplification of the sensory response.
Results
Sour Taste Cells Respond to Intracellular Acidification with Action Potentials.
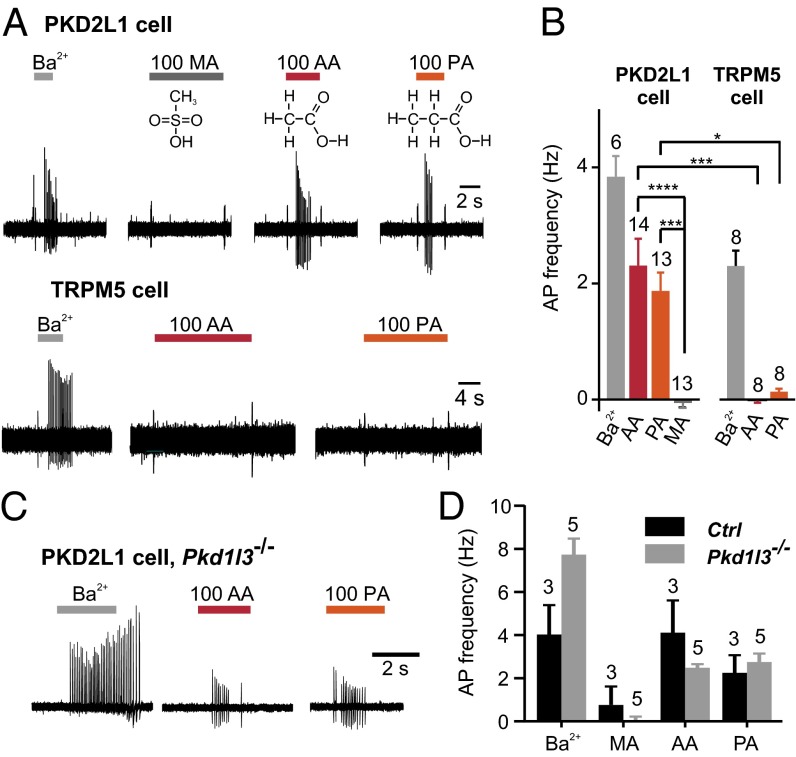
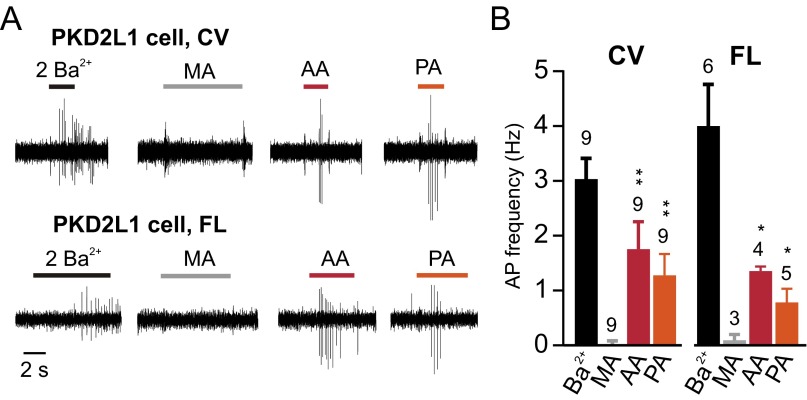
To determine whether sour taste cells respond electrically to intracellular acidification, we measured action potentials in cell-attached recording from freshly isolated taste receptor cells from mouse circumvallate papillae (CV). Sour taste cells were identified by expression of YFP from the Pkd2l1 promoter (PKD2L1 cells), and responses were compared with those obtained from nonsour taste cells, identified by GFP expression from the Trpm5 (transient receptor potential M5) promoter in a double-transgenic mouse (24, 25). Healthy, electrically excitable cells were identified using 2 mM Ba2+, which blocks resting K+ channels and elicits action potentials in both cell types (Fig. 1 A and B). To acidify the cell cytosol, we used acetic acid (AA) and propionic acid (PA), weak acids that partition in the lipid bilayer in their protonated form and are able to shuttle protons across the cell membrane (26–28). Each acid was used at a concentration of 100 mM and pH of 7.4, which generates ∼0.3 mM of the membrane permeable form. As can be seen in Fig. 1 A and B, the two weak acids evoked robust action potential firing in PKD2L1 cells, whereas no action potentials were elicited in response to the two weak acids in TRPM5 cells. As a control for removal of Cl−, we used methane sulfonic acid (MA), which is a strong acid and cannot shuttle protons across the cell membrane. MA was without effect on PKD2L1 cells (Fig. 1 A and B). We also measured responses from PKD2L1 cells isolated from foliate (FL) papillae on the sides of the tongue, and compared the results with those obtained using cells isolated from the CV (Fig. S1); no difference in responses were detected; thus, all additional experiments were done using cells from the CV. We conclude that intracellular acidification is sufficient to activate taste cells that express PKD2L1 and mediate sour taste, but not taste cells that express TRPM5 and mediate bitter, sweet, and umami taste.
Fig. 1.
Intracellular acidification evokes action potentials in dissociated PKD2L1 cells but not TRPM5 cells. (A) Action potentials were evoked in a PKD2L1 cell (Upper) but not in a TRPM5 cell (Lower) in response to 100 mM acetic acid (AA) and propionic acid (PA), adjusted to pH 7.4. Control stimuli were 100 mM methanesulfonic acid (MA), or 2 mM Ba2+. Structural formulae of acids are shown. (B) Average data from experiments as in A. The numbers of cells tested are indicated above the bars. By one-way ANOVA, there was a significant difference in the response of PKD2L1 cells to AA and PA compared with MA. Asterisks indicate P value from Tukey’s post hoc test. ***P < 0.001, ****P < 0.0001. By two-way ANOVA, there was a significant difference in the response to weak acids between cell types (P < 0.0001), but no difference between the response to the two weak acids (P = 0.70). Asterisks indicate P value from Tukey’s post hoc test. *P < 0.05, ***P < 0.001. (C) Action potentials evoked in a PKD2L1 cell from CV of a Pkd1l3−/− animal in response to 100 mM AA, 100 mM PA, or 2 mM Ba2+. (D) Average data from experiments as in C. By two-way ANOVA, there was no significant difference in response to acidic stimuli (MA, PA, and AA) across genotypes (P = 0.37).
Fig. S1.
Intracellular acidification evokes action potentials in dissociated PKD2L1 cells from both CV and foliate. (A) Action potentials were evoked in a PKD2L1 cells isolated from circumvallate and foliate papillae in response to 100 mM acetic acid (AA) and propionic acid (PA), adjusted to pH 7.4. Control stimuli were 100 mM methanesulfonic acid (MA), or 2 mM Ba2+. (B) Average data from experiments as in A. By two-way ANOVA, there was a significant difference in the response to different acids (MA, AA, and PA) (P < 0.01) but no difference between the two taste fields (P = 0.41). Asterisks indicate P value from Student’s test against MA in each cell type. *P < 0.05, **P < 0.01.
Intracellular Acidification Blocks Resting K+ Currents in PKD2L1 Cells.
Intracellular acidification could generate membrane depolarization either by activating excitatory, Na+- or Ca2+-permeable, channels, or by inhibiting K+ channels (3, 28). We previously tested whether weak acids could activate an inward Na+- or Ca2+-permeable current in PKD2L1 cells and failed to find any difference in the magnitude or reversal potential of the inward current evoked in response to pH 5 with or without acetic acid (16). We also tested whether the channel complex formed from PKD2L1/PKD1L3 contributes to the response to weak acids (29). Cells isolated from Pkd1l3−/− animals responded vigorously to 100 mM AA and 100 mM PA (pH 7.4), and no difference in response intensity could be detected between knockout animals and wild-type (WT) littermates (two-way ANOVA, P = 0.37; Fig. 1 C and D). Because PKD1L3 is required for membrane trafficking of the PKD2L1 (9), we conclude that PKD2L1/PKD1L3 does not contribute to the depolarization in response to intracellular acidification.
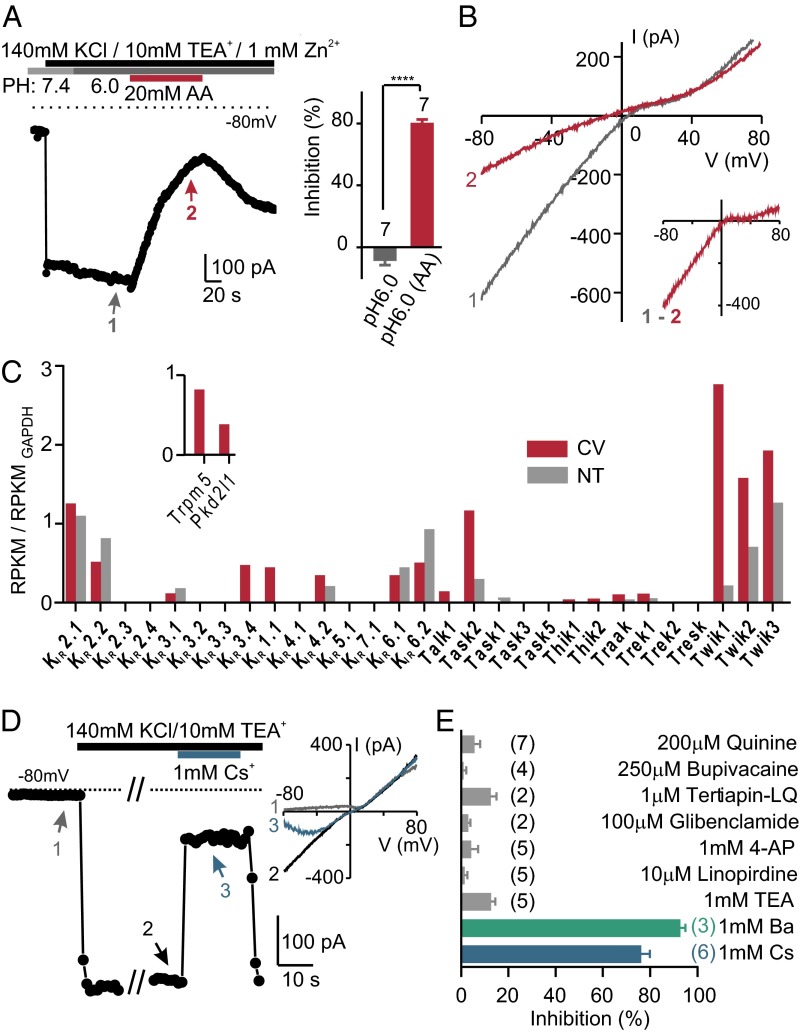
We next tested whether, instead, cytosolic acidification had an effect on the resting K+ currents in PKD2L1 cells, measured by elevating extracellular K+ to 50–140 mM. The inward K+ current (at −80 mV) was unaffected by lowering the pH of the extracellular solution (to pH 6.0), but was strongly inhibited by 20 mM acetic acid, pH 6.0, a condition that acidifies the cell cytosol (27) (Fig. 2 A and B). The block by acetic acid developed over tens of seconds and recovered over a similar time course (Fig. 2A), as expected if the protonated form of the acid must enter the cell to block the channel.
Fig. 2.
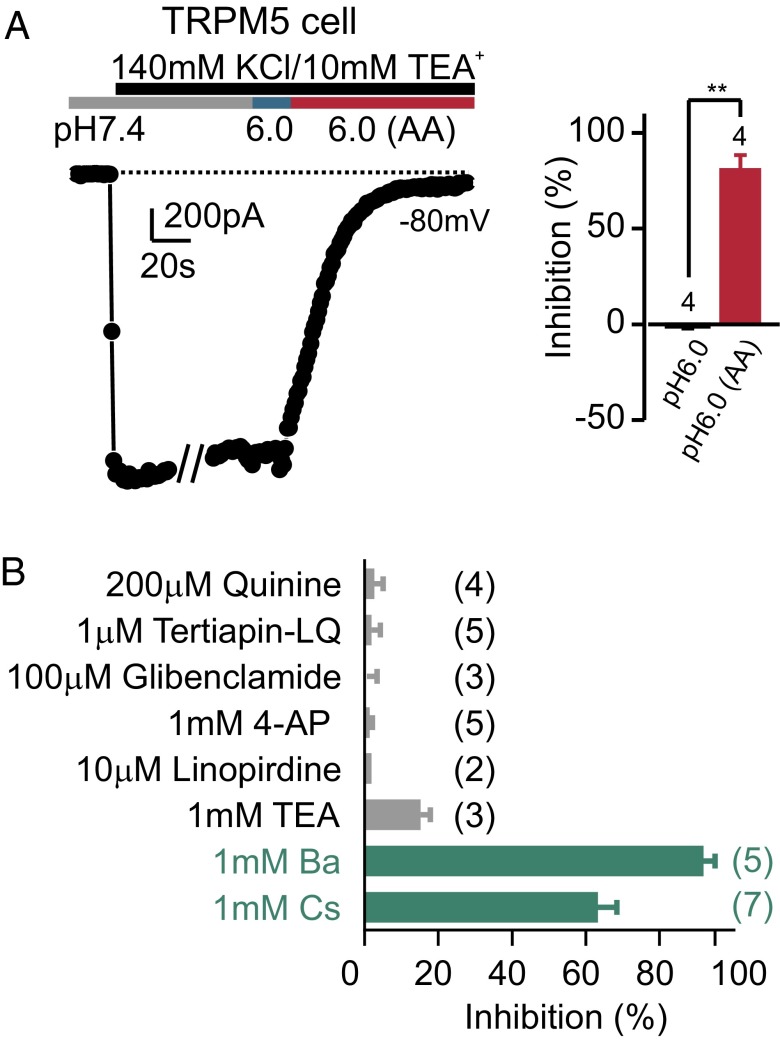
An inward rectifier K+ current in PKD2L1 cells is inhibited by intracellular acidification. (A) The inward K+ current measured in a PKD2L1 cell was reversibly inhibited by 20 mM acetic acid, pH 6.0, but not by pH 6.0 alone. Average data are shown in the Inset. ****P < 0.0001 using paired two-tailed Student’s t test. (B) I–V relation measure at the times indicated in A. Note that the difference current shows inward rectification. (C) The normalized expression levels of Kcnj (KIR) and Kcnk (K2P) transcripts from deep sequencing of taste and nontaste tissues (average of two samples each). The mapped reads were used to compute reads per kilobase per million reads (RPKM) for each gene, which was further normalized to the RPKM of GAPDH in each sample. Inset shows expression level of two taste cell markers, Trpm5 and Pkd2l1, which were detected only in taste tissue. (D) The inward K+ current from a PKD2L1 cell was reversibly blocked by 1 mM Cs+. Inset shows I–V relation measured at the time points indicated. (E) K+ channel blockers were tested against resting K+ currents in PKD2L1 cells in 100 mM NaCl, 50 mM KCl. The number of cells tested is indicated next to the bars. Of the compounds tested, only 1 mM Ba2+ and 1 mM Cs+ produced a significant block of the inward K+ current (P < 0.0001 by one-way ANOVA followed by Tukey’s post hoc analysis).
Identity of the Acid-Sensitive Resting K+ Current.
To identify candidates to mediate the resting K+ current in PKD2L1 cells, we analyzed the transcriptome of lingual epithelium containing circumvallate papillae and compared it with the transcriptome of nontaste epithelium (NT) (Fig. 2C). We focused on inward rectifier channels (KIR family) and two-pore domain K+ channels (K2P), based on our preliminary results, and known functions of these channels in mediating the resting potential in other cell types. Altogether, we detected moderate-to-high expression of eight KIR channels and five K2P channels in taste tissue (Fig. 2C); four additional K2P channels were detected at low levels and were not pursued further. The eight KIR channels (KIR2.1, 2.2, 3.1, 3.4, 1.1, 4.2, 6.1, and 6.2) included two that were previously identified in taste cells: KIR1.1 (ROMK1), which is expressed by type I taste cells (30), and KIR6.1, a component of a KATP channel, expressed by type II taste cells (31). Among the five K2P channels (Task2, Trek1, Twik1, Twik2, and Twik3), we observed particularly high expression of Twik1 (KCNK1) and lower expression of Task-2, which were previously reported to be expressed in taste tissue (22, 23). As a control, we examined expression of Pkd2l1 and Trpm5; both were highly and specifically expressed in taste tissue, as expected (Fig. 2C).
We next used pharmacological profiling to determine which among the 13 KIR and K2P channels mediates the resting K+ current in PKD2L1 cells. Of the K+ channel blockers tested (32, 33), the resting K+ current in PKD2L1 cells was sensitive only to 1 mM Ba2+ and 1 mM Cs+ (Fig. 2 D and E; one-way ANOVA, P < 0.0001). Notably, the current was insensitive to quinine (Fig. 2E), excluding contributions from Twik1-3 (sensitivity to Cs+ excludes the remaining K2P channels). The current was also insensitive to tertiapin-LQ (targets KIR1.1 and KIR3.1/3.4), glibenclamide (targets KIR6.1 and 6.2), tetraethylammonium (TEA), 4-aminopyridine (4-AP), and linopiridine (target Kv and KCNQ channels) (Fig. 2E). Of the channels expressed in taste tissue, only the inward rectifiers KIR2.1, KIR2.2, and KIR4.2 have this pharmacological profile.
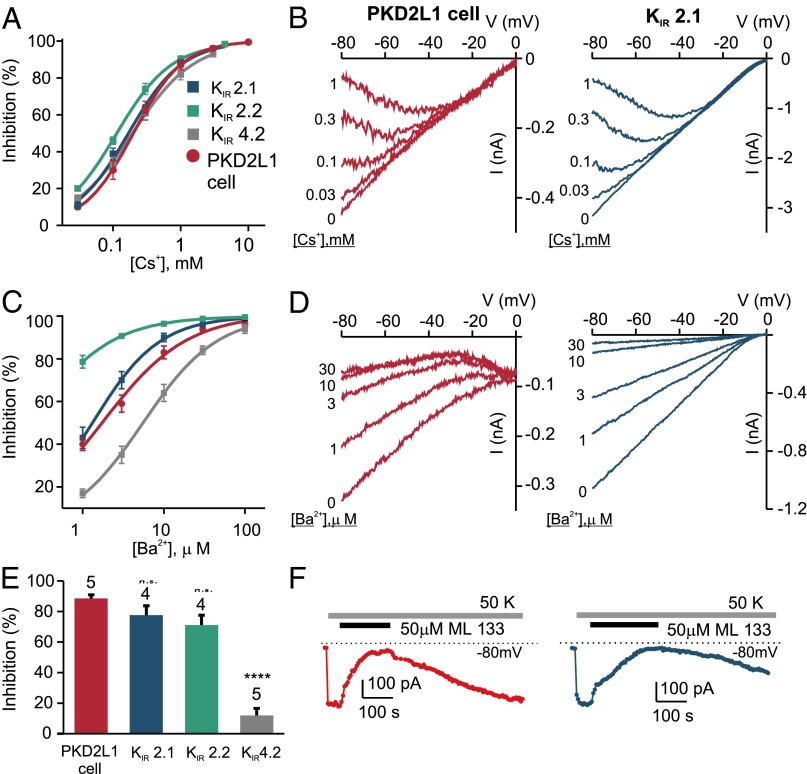
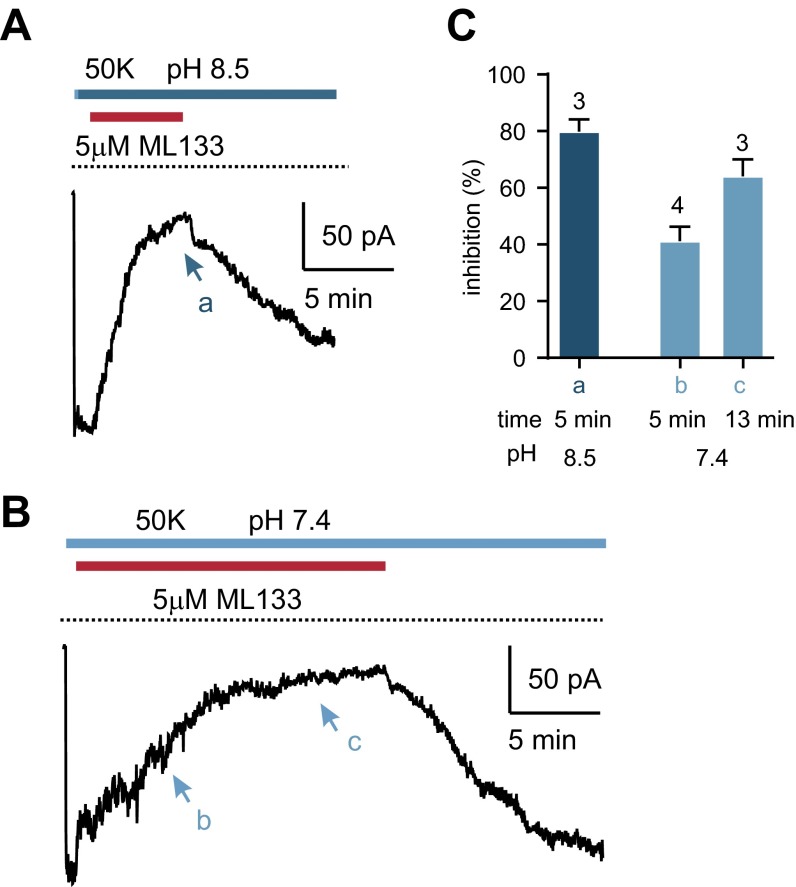
To narrow down the candidates further, we assessed the sensitivity of the resting K+ current in PKD2L1 cells to block by Ba2+ and Cs+ (Fig. 3 A–D). Block of the current by Cs+ was strongly voltage dependent (Figs. 2D and 3 A and B) with an IC50 of 211 ± 35 μM measured at −80 mV. A similar sensitivity to Cs+ was measured for heterologously expressed KIR2.1 and 4.2 (IC50 values of 169 ± 8 and 194 ± 19 μM, respectively), whereas heterologously expressed KIR2.2 showed a significantly higher sensitivity to Cs+ (118 ± 9; P < 0.05 by one-way ANOVA followed by Tukey’s multiple-comparison test). Sensitivity to Ba2+ was even more informative. Ba2+ blocked the K+ current in PKD2L1 cells with an IC50 of 2.1 ± 0.4 μM (measured at −80 mV), which was not significantly different from the IC50 for inhibition of KIR2.1 (1.4 ± 0.2 μM; Fig. 3 C and D), but considerably different from the IC50 of either KIR2.2 or KIR4.2 (0.23 ± 0.06 and 5.8 ± 0.8 μM, respectively; P < 0.0001 and P < 0.01 by one-way ANOVA followed by Tukey’s multiple-comparison test). Finally, we tested the KIR2-specific blocker ML133, which has a reported IC50 of 1.9 μM for KIR2.1 (34). ML133 (50 μM) blocked the resting K+ current in PKD2L1 cells by ∼90%, similar to its effect on KIR2.1 and KIR2.2, whereas KIR4.2 was virtually insensitive to ML133 (Fig. 3 E and F). Moreover, we observed an increase in the rate of inhibition by ML133 when the pH of the extracellular solution was raised to 8.5, as previously described for KIR2.1 (Fig. S2) (34). Thus, based on sensitivity to Ba2+, Cs+, and ML133, we conclude that the resting K+ current in PKD2L1 cells is mediated by KIR2.1.
Fig. 3.
Pharmacological profile of the resting K+ current in PKD2L1 cells implicates KIR2.1. (A–D) The dose dependence of Cs+ and Ba2+ block of inward K+ currents at −80 mV in PKD2L1 cells compared with that of KIR2.1, KIR2.2, and KIR4.2 expressed in HEK 293 cells (mean ± SEM of four to seven cells). (A) By two-way ANOVA, there was a significant difference in sensitivity to Cs+ block between currents in PKD2L1 cells and HEK cells transfected with the KIR channels (P < 0.05). Tukey’s post hoc analysis showed significant difference in Cs+ block between PKD2L1 cells and KIR2.2 at a concentration of 30 μM (P < 0.05). (B) The I–V relationships of the inward K+ current in PKD2L1 cells or HEK cells transfected with KIR2.1 measured in the presence of the indicated concentration of Cs+. Vm was −80 mV, and the voltage was ramped to +80 mV (1 V/s). Note that block is strongly voltage dependent. (C) Two-way ANOVA showed a significant difference (P < 0.0001) in sensitivity to Ba2+ block between currents recorded from PKD2L1 cells and HEK cells transfected with the KIR channels. Tukey’s post hoc analysis showed that KIR2.1 and PKD2L1 currents differed only in response to 3 μM (P < 0.01); at all other concentrations, there was no significant difference. (D) I–V relationships from experiments as in C. (E) Percentage block by ML133 (50 μM) of the inward K+ current in PKD2L1 cells and HEK cells heterologously expressing and KIR channels. By one-way ANOVA, there was a significant difference in ML133 block efficiencies across K+ channel types (P < 0.0001). Asterisks indicate P value compared with PKD2L1 cell using Tukey’s multiple-comparisons test. ****P < 0.0001. (F) Representative data showing the time course of the block by ML133 of the inward K+ current in a PKD2L1 cell and a cell heterologously expressing KIR2.1.
Fig. S2.
Block by ML133 of the resting K+ current in PKD2L1 taste cells is pH dependent. (A and B) The block of resting K+ current in PKD2L1 cells by ML133 was extracellular pH dependent. At pH 8.5 (A), the block by 5 μM ML133 was faster than that at pH 7.4 (B). (C) Average data of cells shown in A and B. The time points the block efficiency was measured are arrowed in the trace in A and B.
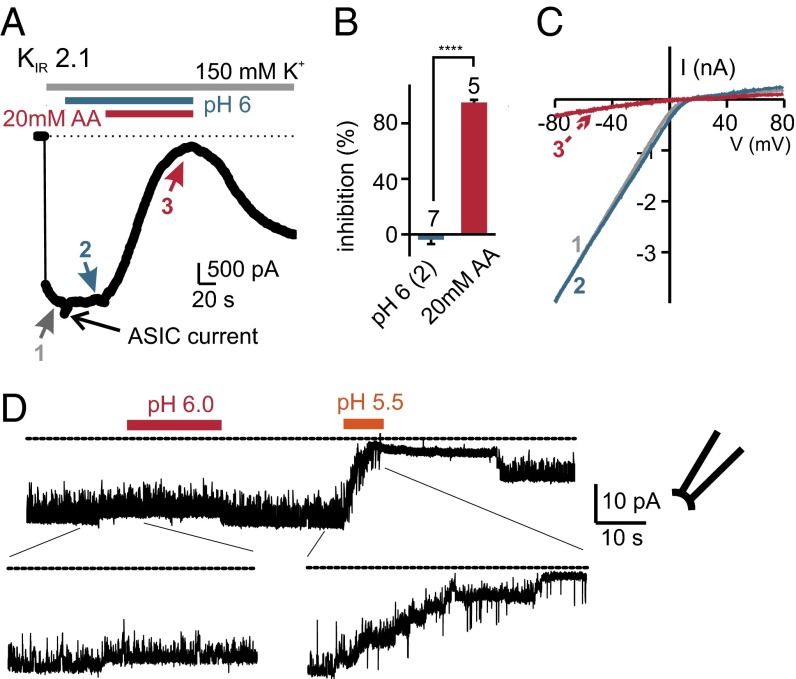
Sensitivity to intracellular acidification is not a well-documented feature of KIR2.1 as it is for other KIR and K2P channels (35, 36). We therefore tested whether heterologously expressed KIR2.1 channels could be blocked by intracellular acidification. Indeed, KIR2.1 currents were potently inhibited by 20 mM acetic acid, pH 6, and to a similar degree as currents in PKD2L1 cells (Fig. 4A; compare with Fig. 2A), but not by extracellular acid alone (Fig. 4 A–C). Moreover, in excised patches of cells heterologously expressing KIR2.1 channels, we found that exposure of the cytoplasmic surface to acidic pH (pH <6) produced a rapid and partially reversible block of channel activity (Fig. 4D). Thus, among K+ channels expressed in taste epithelium, KIR2.1, with a pharmacological profile nearly identical to that of the resting K+ current in PKD2L1 cells, including a similar sensitivity to intracellular acidification, is a strong candidate for mediating the resting K+ current of PKD2L1 cells.
Fig. 4.
KIR2.1 is inhibited by intracellular acidification. (A) The current in a HEK cell heterologously expressing KIR2.1 is inhibited by 20 mM acetic acid at pH 6, but not by pH 6 alone. Note that the pH 6 solution evokes an inward current carried by acid-sensing ion channels (ASIC). (B) Average data from seven cells as in A normalized against the current magnitude before the application of acid. ****P < 0.0001 using paired two-tailed Student’s t test. (C) The I–V relationship of the KIR2.1 current at times indicated in A. (D) Excised patch recording from a KIR2.1 transfected HEK cell in response to acids. The bath solution contained 150 mM KCl, 1 mM EGTA, and 1 mM EDTA. The membrane was held at −80 mV. The application of pH 5.5 completely inhibited channel activity, and channel activity was partially restored by return to neutral pH. Similar results were observed in five cells.
Tissue-Specific Knockout Confirms the Contribution of KIR2.1 to the Resting K+ Current.
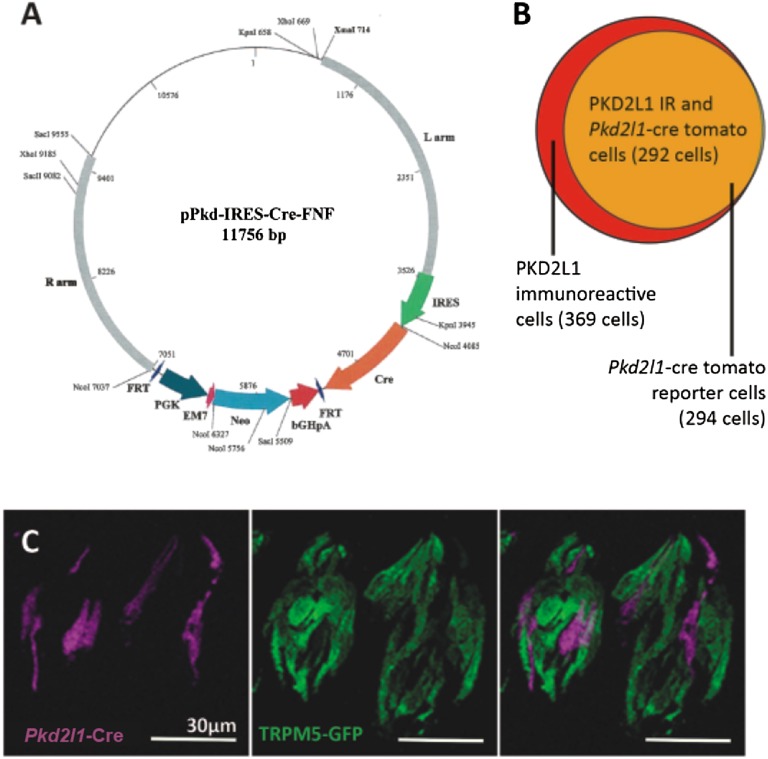
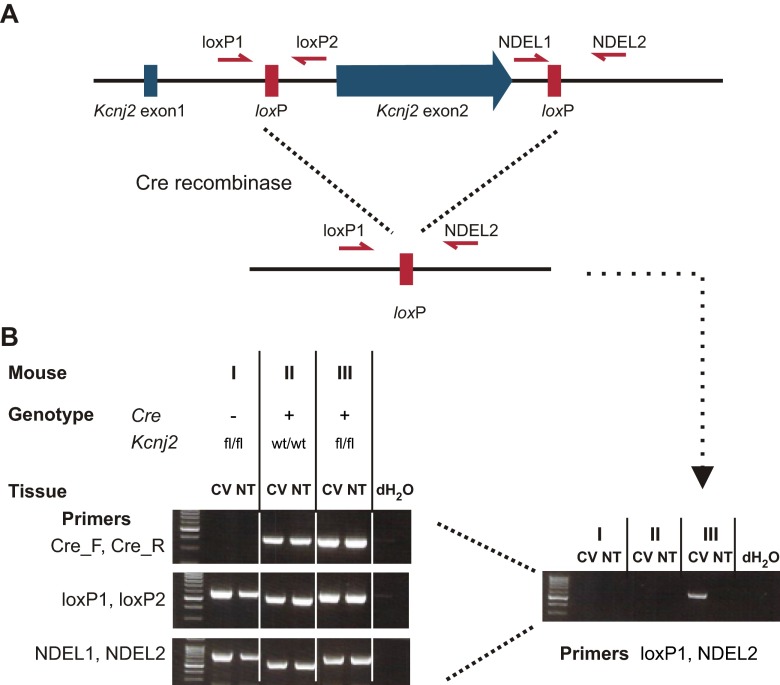
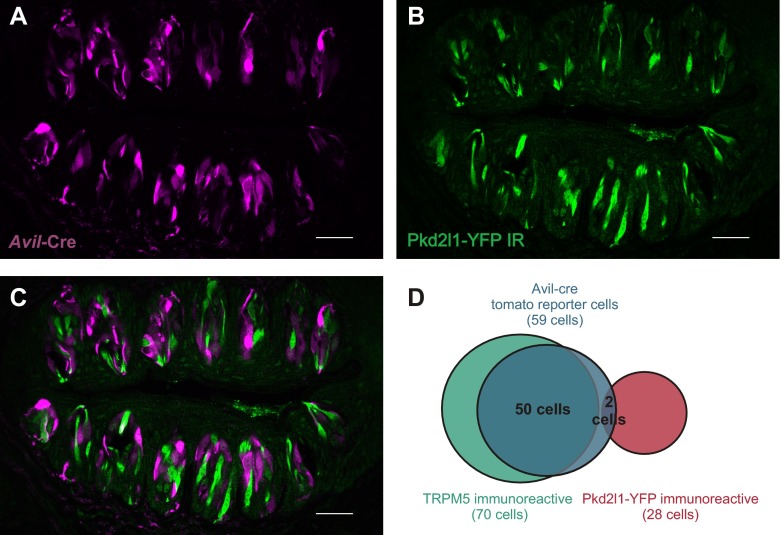
To further determine whether KIR2.1 accounts for the resting K+ current in PKD2L1 cells, we measured currents in cells isolated from mice carrying a targeted deletion of the gene. Because knockout of KIR2.1 is neonatal lethal (37) and it is difficult to identify sour taste cells by expression of YFP at birth, we chose to use a tissue-specific gene deletion strategy. To this end, we used a mouse strain carrying a floxed allele of Kcnj2 (Methods) and crossed it to a mouse strain in which the Pkd2l1 promoter drives expression of Cre recombinase. Based on use of a floxed Tdt reporter, Cre is expected to be active in ∼79% of the Pkd2l1-expressing cells, and only in Pkd2l1-expressing cells in the circumvallate papillae (Fig. 5A and Fig. S3). We confirmed that Kcnj2 was inactivated in taste tissue using a PCR strategy designed to detect the deletion event (Fig. S4).
Fig. 5.
Tissue-specific knockout of Kcnj2 in PKD2L1 taste cells confirms that KIR2.1 contributes to the inward K+ current. (A) Confocal image of circumvallate taste buds from a Pkd2l1-Cre::Rosa26tdT mouse. Tomato reporter expression is displayed in magenta. PKD2L1 immunoreactivity is displayed in green. Cre expression efficiency, as determined by the tomato reporter fluorescence, is ∼79% in circumvallate taste tissue. (All scale bars: 30 μm.) (B) A representative recording from a PKD2L1 cell from a Pkd2l1-Cre::Pkd2l1-YFP::Kcnj2fl/fl mouse (cKO; Upper) and a recording from a control cell, from a mouse where Kcnj2 is not floxed (Lower). In the cKO, the K+ current induced by 50 mM K+ is small, and not sensitive to 1 or 10 μM Ba2+. (C) The I–V curves at indicated time points in B. (D) K+ current magnitudes in PKD2L1 taste cells from cKO mice and controls. Black symbols indicate the current was sensitive to 1 μM Ba2+ (>15% block); red indicates not sensitive (<5% block); gray are cells not tested with Ba2+. One-way ANOVA followed by Tukey’s post hoc test, **P < 0.01, ***P < 0.001. (E) Same data as in D showing the distribution of Ba2+-sensitive currents across genotypes. The difference is significant by one-tailed χ2 test (P < 0.05).
Fig. S3.
Pkd2l1-Cre mouse strain. (A) Map of the Pkd2l1-cre plasmid used to generate the transgenic mouse strain. (B) A Venn diagram of count data comparing PKD2L1 immunoreactivity (red circle) to Pkd2l1-Cre tomato reporter positive cells (yellow circle). Overlap shown in orange. Cre reporter efficiency, as determined by the tomato reporter fluorescence, is ∼79% in the circumvallate taste tissue. (C) Confocal image z stacks of circumvallate taste buds from a triple-transgenic Pkd2l1-Cre, RosaTdt, Trpm5-GFP mouse. Tomato reporter expression is displayed in magenta, and Trpm5-driven GFP fluorescence is displayed in green. Tomato reporter fluorescence and Trpm5-driven GFP fluorescence do not coincide. (All scale bars: 30 µm.)
Fig. S4.
Floxed Kcnj2 is specifically excised in Cre-expressing tissue. (A) The design for Cre-loxP dependent tissue-specific knockout of Kcnj2, the gene that encodes KIR2.1. Exon2 of Kcnj2, which covers the entire coding sequence, was floxed. Primers that were used to genotype animals are indicated. (B) PCR of genomic DNA from circumvallate (CV) and nontaste (NT) tissues of mice representing the three genotypes indicated. The left panel shows the results of genotyping the mice using the indicated primer pairs. Note that, for this analysis, the source of the genomic DNA is irrelevant. The right panel shows the results of a PCR designed to test for the deletion of exon 2, which is only expected to occur in taste tissue from Pkd2l1-Cre::Kcnj2fl/fl mice. The same template DNA was used as in the right panel, which therefore serves as a control for this experiment.
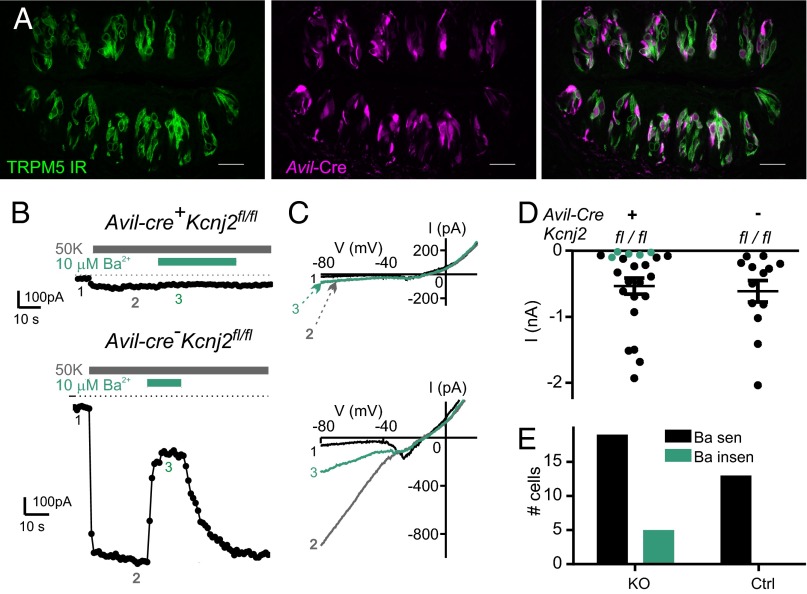
The resting K+ current in animals carrying two floxed alleles of Kcnj2 and one allele of Pkd2l1-Cre (cKO) was compared with that in littermates that carried either the floxed allele or the Pkd2l1-Cre driver alone. As shown in Fig. 5 B–D, the average magnitude of the resting K+ current in the cKO animals was significantly reduced compared with either control group (one-way ANOVA followed by Tukey’s post hoc test, P < 0.001 compared with Cre+ and P < 0.01 compared with Kcnj2fl/fl). We also measured sensitivity of the current to 1 μM Ba2+, a concentration that is expected to produce ∼50% block of KIR2.1. Ba2+ produced a substantial (>15%) block of the inward K+ current in all cells isolated from WT mice (18 of 18), whereas in a significant proportion cells from the cKO, the inward current was Ba2+-insensitive (4 of 18; P < 0.05 by one-tailed χ2 test; Fig. 5E). These cells tended to be the ones with the smallest inward current, and thus likely represent cases where the Kcnj2 gene was completely excised and the KIR2.1 protein eliminated. In the remaining cells, the observation that the residual current retained sensitivity to Ba2+ indicates that it is not a product of a compensatory increase in the expression of a different subtype of ion channel, but instead likely represents incomplete elimination of the Kcnj2 gene product. Thus, tissue-specific knockout of Kcnj2 significantly reduces the magnitude of the resting K+ current in PKD2L1 cells and eliminates the current in a subset of cells, lending support to the conclusion that KIR2.1 mediates this current.
The Magnitude of the K+ Current Determines Sensitivity to Weak Acids.
These data argue that, in response to weak acids, inhibition of KIR2.1 by intracellular acidification produces membrane depolarization that drives action potentials in PKD2L1 cells. To directly test this hypothesis would require replacing KIR2.1 with an acid-insensitive variant, which is not currently possible. Instead, we reasoned that there should be a correlation between sensitivity to weak acids and expression of KIR2.1, such that a cell that is insensitive to weak acids should not express KIR2.1 or a similar acid-sensitive K+ channel. TRPM5 taste cells are insensitive to weak acids (Fig. 1), and we therefore measured the resting K+ current in these cells. To our surprise, we found that the resting K+ current in TRPM5 cells was inhibited by exposure to weak acids (Fig. 6A), to a similar extent to that of the current in PKD2L1 cells. Moreover, among known blockers of K+ channels tested, the resting K+ current in TRPM5 cells was sensitive to only Cs+ and Ba2+, like the resting K+ current PKD2L1 cells (Fig. 6B; compare with Fig. 2A), indicating that it is likely mediated by KIR2.1. To directly test this hypothesis, we generated a cell type-specific knockout of Kcnj2, this time using the Advillin promoter (38), which drove expression of Cre in ∼71% of TRPM5 cells (Fig. 7A and Fig. S5). We then measured the resting K+ current in cKO and control cells (Fig. 7 B and C). Although there was no change in the average magnitude of the current (Fig. 7D), the Ba2+-sensitive resting K+ current was eliminated in a subpopulation of cKO cells (5 of 24) but not in any control cells (0 of 13; P < 0.05; one-tailed χ2 test) (Fig. 7E). This supports the interpretation that KIR2.1 contributes to the resting K+ current in TRPM5 cells.
Fig. 6.
The resting K+ current in TRPM5 cells is sensitive to intracellular acidification. (A) Inward K+ currents measured at −80 mV from a TRPM5 cell are sensitive to intracellular acidification (pH 6.0 solution containing 20 mM acetic acid) but not extracellular acidification (6.0 solution buffered with 10 mM Mes). Average data are shown in the right panel. **P < 0.01 using paired two-tailed Student’s t test. (B) The pharmacological profiling of the resting K+ current in TRPM5 taste cells. The inhibition efficiency was tested in 50 mM K+ solutions at −80 mV as for the PKD2L1 cells. Of the blockers tested, only 1 mM Ba2+ and 1 mM Cs+ produced a significant block of the inward K+ current (P < 0.0001 by one-way ANOVA followed by Tukey’s post hoc analysis).
Fig. 7.
Tissue-specific knockout of Kcnj2 in TRPM5 taste cells. (A) Confocal image of circumvallate taste buds from an Avil-Cre::Rosa26tdT::Pkd2l1-YFP mouse. Tomato reporter expression is displayed in magenta. TRPM5 immunoreactivity is displayed in green. Cre is expressed in ∼71% of TRPM5 cells as determined by expression of the tomato reporter fluorescence in TRPM5-immunoreactive cells. (Scale bar: 30 μm.) (B) A representative PKD2L1 cell from a Avil-Cre::Trpm5-GFP::Kcnj2fl/fl mouse (cKO; Upper) and a Cre− control cell. In the taste cell from the cKO mouse, the K+ current is small, and insensitive to 10 μM Ba2+. (C) I–V curves at time points indicated in B. (D) Magnitude of the K+ current in TRPM5 taste cells from cKO mice and WT controls. Black symbols indicate the current was sensitive to 10 μM Ba2+ (>40% block); green indicates that the current was not sensitive (<25% block). (E) Same data as in D showing the distribution of Ba2+-sensitive currents across genotypes (P < 0.05; one-tailed χ2 test).
Fig. S5.
Distribution of Avil-Cre in taste cells. (A–C) Confocal images of the same field of circumvallate taste buds from an Avil-Cre::Rosa26tdT::Pkd2l1-YFP mouse as in Fig. 7. The reporter expression is displayed in magenta, and YFP immunoreactivity, which reflects the expression pattern of Pkd2l1, is displayed in green. Note that there is very little overlap between Cre reporter expression and and YFP immunoreactivity (C). (Scale bar: 30 μm.) (D) A Venn diagram of count data showing TRPM5-immunoreactive cells (green circle, as in Fig. 7A), pkd2l1-YFP–immunoreactive cells (blue circle), and Avil-Cre tomato reporter-positive cells (red circle). The Cre reporter was detected in ∼71% of TRPM5 cells and 8% of PKD2L1 cells.
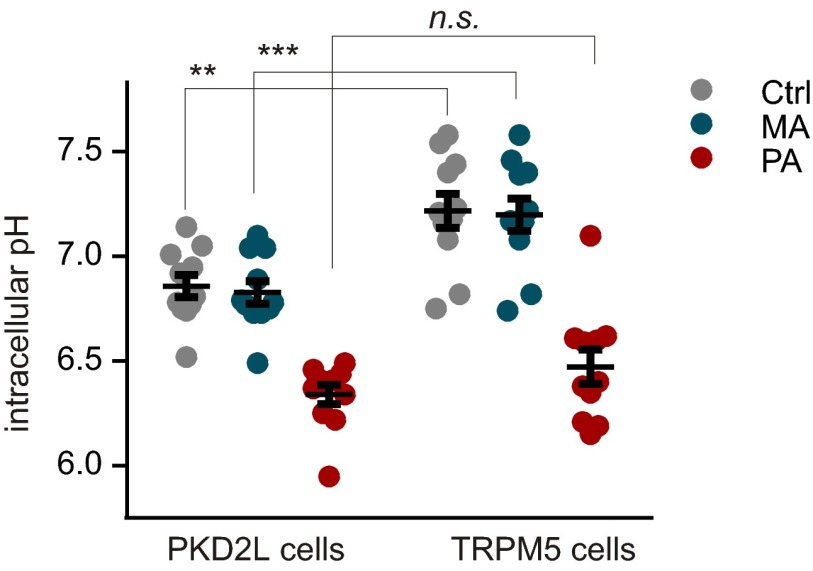
We next determined whether TRPM5 cells and PKD2L1 cells acidified to the same extent in response to the weak acid stimuli. Freshly isolated cells were loaded with the pH-sensitive dye phrodo red, and fluorescent intensity was monitored under the same conditions used to measure action potentials in cell-attached recording. As we previously reported, PKD2L1 cells had an initial starting pH that was acidified compared with that of TRPM5, a phenomenon that can be attributed to proton leak through ungated proton channels in these cells (21). There was, however, no difference in the final pH reached in response to the weak acid solutions between cell types (pH 6.47 ± 0.08 in TRPM5 cells versus 6.34 ± 0.05 in PKD2L1 cells, P = 0.43 by two-way ANOVA followed by Sidak’s multiple-comparison test; Fig. S6).
Fig. S6.
The intracellular pH to which TRPM5 cells are acidified by weak acids is similar to that of PKD2L1 cells. Scatter plot of intracellular pH from imaging of cells loaded with pHrodo Red. Cells were sequentially exposed to bath solution (Tyrode’s), 100 mM methanesulfonic acid (MA), and 100 mM propionic acid (PA), all pH 7.4. Intracellular pH was calibrated as described in Methods. By two-way ANOVA, there was a significant difference across the conditions (P < 0.0001) and across cell types (P < 0.01). Post hoc analysis with Sidak’s test corrected for multiple comparisons showed no significant difference between cell types in response to the weak acid, PA (P = 0.43). As previously reported (21), the resting pH of the two cell types was significantly different as was the pH in the presence of MA. Note that MA was used as a control and was not expected to cause a change in pH from resting conditions. **P < 0.01, ***P < 0.001.
Thus, TRPM5 cells express a resting K+ channel sensitive to intracellular pH and show a drop in intracellular pH in response to weak acids, similar to that in PKD2L1 cells. Why then do they not fire action potentials to weak acids? In contemplating this question, we noticed that there was one important difference between the resting K+ currents in PKD2L1 and TRPM5 cells—the K+ currents were severalfold larger in TRPM5 cells compared with PKD2L1 cells (Fig. 8 A and B). The larger K+ current is expected to more effectively clamp the resting potential of TRPM5 cells, reducing sensitivity to K+ channel blockers such as intracellular acidification. To test this hypothesis, we used a low concentration of Ba2+ (10 μM) to reduce the magnitude of the resting K+ current in the TRPM5 cells to the level observed in PKD2L1 cells (Fig. 8 A and B). We then asked whether under these conditions TRPM5 cells would fire action potentials to weak acids. Indeed, when primed with 10 μM Ba2+, which alone did not stimulate action potentials, the TRPM5 cells fired action potentials to weak acid stimulation (Fig. 8 C and D). These data are consistent with the hypothesis that the acid-sensitive KIR2.1 channels mediate responses to weak acids in PKD2L1 cells; the small magnitude of this current, relative to other conductances, and not its identity, restricts acid sensitivity to these cells.
Fig. 8.
Reduction in the magnitude of the resting K+ currents makes TRPM5 cells sensitive to intracellular acidification. (A) K+ (100 mM) elicits significantly larger currents in TRPM5 cells than in PKD2L1 cells and that 10 μM Ba2+ reduces the current to a similar magnitude as in PKD2L1 cells. (B) Scatter plot of current magnitudes from cells as shown in A. Tests for significant difference of TRM5 cells with or without Ba2+ were against PKD2L1 cells. **P < 0.01 using two-tailed Student’s t test. (C) With the preapplication of 10 μM Ba2+, robust action potentials were elicited by 100 mM propionic acid at pH 7.4. (D) The average data of cells as in C. **P < 0.01 using paired one-tailed Student’s t test.
The Resting K+ Current Is Blocked Downstream of H+ Entry Through a Zn2+-Sensitive Conductance.
The pH sensitivity of KIR2.1 makes it well suited to be part of the sour transduction machinery, acting to transduce cellular acidification into a change in membrane potential. Intracellular acidification can be elicited in PKD2L1 cells when protons enter through an apical conductance. In the previous experiments, we took safeguards to prevent proton entry through the proton conductance, which would have generated an increase in the inward current and confounded interpretation of the results. Thus, whenever the extracellular solution was acidified, we added Zn2+ (2 mM) to block the proton current (16, 21). To determine whether entry of protons through the proton conductance could block the resting K+ current in PKD2L1 cells, we simply measured the response to extracellular acidification in the absence of Zn2+. As seen in Fig. 9 A and B, the resting K+ current was inhibited when the pH of the extracellular solution was lowered to 6.0 in the absence of Zn2+ (48 ± 7% at 120 s), but not in its presence (13 ± 5%). A similar block of the K+ current in TRPM5 cells was not observed (Fig. 9A), consistent with the observation that these cells do not have a proton current (16, 21).
Fig. 9.
Block of the resting K+ current by proton entry through a proton conductance provides a mechanism for amplification of the sour sensory response. (A) The inward K+ current in a PKD2L1 cell, but not a TRPM5 cell, was inhibited by extracellular solution adjusted to pH 6 when 2 mM Zn2+, a blocker of the taste cell proton conductance, was omitted from the solution, but not when it was included. (B) Average data from experiments as in A at time points indicated by the arrows, normalized against the current magnitude before the application of the pH 6 solution. Asterisks indicate the significant difference compared with pH 6 plus 2 mM Zn2+ in PKD2L1 cells, or pH 6 alone in TRPM5 cells. (C) Action potential recorded in cell-attached mode from a PKD2L1 taste cell in response to the indicated stimuli. Neither the pH 7.0 stimulus nor a low concentration of AA (0.1 mM of protonated acid) could effectively evoke action potentials, but together they elicited robust firing. (D) Average data from experiments as in C. By two-way ANOVA, there was a significant difference between Ctrl and AA groups (P < 0.01). Asterisks indicate results of Sidak’s multiple-comparison test. *P < 0.05, ***P < 0.001.
These data suggest that there are two components to the sensory response to sour stimuli: H+ entry through a proton channel and intracellular acidification, which blocks resting K+ channels. To determine whether the two components act synergistically, we tested the response to two subthreshold stimuli: mild extracellular acidification (pH 7.0) and a low concentration of acetic acid (0.1 mM of protonated acid; Methods) either alone or in combination. Neither alone was sufficient to stimulate action potentials, but together they strongly activated PKD2L1 cells (Fig. 9 C and D). At more acidic pH (6.6), action potentials were evoked even in the absence of the weak acid as we previously reported (21), and addition of the weak acid did not produce a significant change in firing rate.
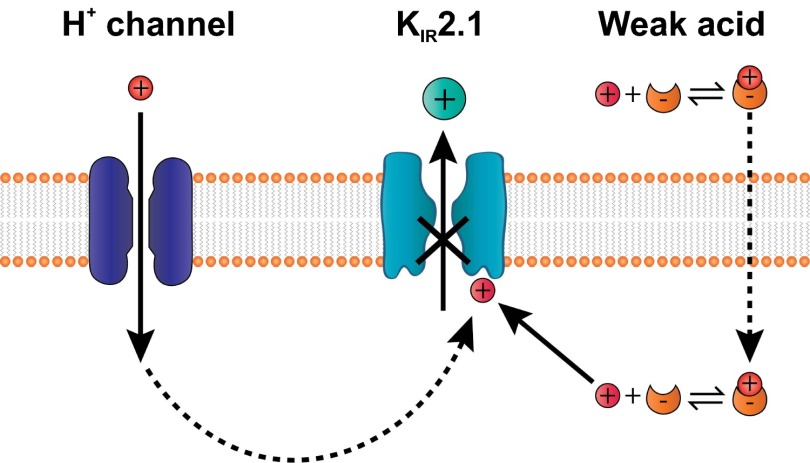
Together, these results suggest that protons serve not only as charge carriers to generate membrane depolarization but also as second messengers that amplify the primary sensory response (Fig. 10).
Fig. 10.
A two-component mechanism for sour taste transduction. In the proposed model, H+ entering through an H+ channel inhibits the K+ channel. The K+ channel can also be inhibited by H+ that is shuttled by weak acids. Both the proton entry and the block of the K+ channel contribute to membrane depolarization, which drives action potentials and release of neurotransmitter.
Discussion
Based on the observation that many of the most potent sour stimuli are weak acids, it has long been speculated that intracellular acidification plays an important role in the transduction of sour taste (5, 19, 39). Consistent with this possibility, here we show for the first time (to our knowledge) that intracellular acidification alone, in the absence of extracellular acidification, can excite sour taste cells. We identify the mechanism by which intracellular acidification excites sour taste cells, as block of a resting K+ current, whose molecular identity we reveal to be KIR2.1. Surprisingly, KIR2.1 is not specifically expressed by sour taste cells, and thus sensitivity to acids is not conferred by the expression of the channel alone. Instead, we find that sensitivity to weak acids can be attributed to the small magnitude of the current in the PKD2L1 cells compared with cells that are acid insensitive, such as taste cells responsive to bitter/sweet and umami (TRPM5 cells). Finally, we show that block of the resting K+ current can also be elicited by elevation of protons following entry through a Zn2+-sensitive conductance, which is the first step in the transduction of strong acids (16). This provides a mechanism for amplification of the sensory response. This mechanism is very different from a recently proposed mechanism for detection of acids by chemosensory neurons in the retrotrapezoid nucleus, which use a proton-activated receptor, GPR4, in combination with a K2P channel sensitive to extracellular pH (TASK-2) (40).
Acid-Sensitive Resting K Current Is Mediated by KIR2.1, Not KCNK1.
Block of K+ channels that are open at the resting membrane potential (resting K+ channels) has previously been suggested as a potential mechanism for sour transduction, although the specific elements of such a pathway have been uncertain. Using the mudpuppy, it was found that extracellular protons block apically located K+ channels (41, 42), but this mechanism does not appear to apply to other more conventional model systems. More recently, K+ (K2P) channels, which are sensitive to either intracellular or extracellular protons and thus are well suited to play a role in sour transduction were identified in rodent taste cells (22, 23). However, although electrophysiological properties of each type of taste cell have been described (43, 44), before our study, very little was known about the molecular nature of the K+ channels that set the resting potential in taste cells and their sensitivity to intracellular or extracellular acidification.
Our data show that there is an acid-sensitive resting K+ current in sour cells, but that this current is not mediated by the K2P channel Twik1/KCNK1 (22, 23). Although we found that KCNK1 was expressed at significantly higher levels than other K2P or KIR channels, we were unable to detect a current with the pharmacological profile of KCNK1. We calculate that KCNK1 contributes no more than 1% to the resting K+ current (Fig. S7).
Fig. S7.
KCNK1 does not contribute to the resting K+ current in PKD2L1 cells. (A) The current recorded from a KCNK1-AA-GFP–transfected HEK cell under conditions designed to unmask the current. Note that in 130 mM Rb+ (with 20 mM TEA), the current magnitude was much larger than that in 130 mM K+ extracellular solution (also with 20 mM TEA) as previously reported (57). Cs+ was not an effective blocker of KCNK1 current (58). (B) Same experiments as in A performed with a KIR2.1-transfected cell. Note that the current in Rb+ is much smaller than that in K+ solutions, and that Cs+ blocks the current effectively. (C) The current recorded in a PKD2L1 taste cell. Note that the current magnitude in Rb+ is smaller than that in K+, similar to the KIR2.1-mediated current. (D) The ratios of current in 130 mM Rb+ (IRb+) to that in 130 mM K+ (IK+) for KCNK1-AA, KIR2.1, and PKD2L1 taste cells. Using these data, we estimated the contribution of KCNK1 to the taste cell K+ current by rearranging a set of linear functions assuming that the inward currents in high Rb+ and K+ are generated solely from the contributions of KCNK1 and KIR2.1 (SI Methods). This calculation showed that KCNK1 accounts for no more than 1% of the resting K+ current.
Instead, pharmacological and molecular profiling indicate that KIR2.1 channels mediate the resting, acid-sensitive K+ current in sour taste cells. This is supported by the observation that the resting K+ currents in PKD2L1 cells from a tissue-specific KIR 2.1 KO mouse are significantly reduced in magnitude, and in a subset of PKD2L1 cells, the K+ current is completely eliminated. We did not expect to observe a complete elimination of the current in all cells because a reporter for the driver showed activity in only 70% of PKD2L1 cells. The same argument applies to TRPM5 cells, where the promoter that we used to drive expression of Cre, is effective at most in ∼70% of cells and fewer if one considers only cells that show strong expression of the reporter (∼50%). Moreover because the Pkd2l1 Cre driver is expressed by mature neurons, it is only expected to excise the floxed allele at a time when there may already be significant expression of ion channels; under these circumstances, elimination of the current would only be observed if the existing protein has turned over and both alleles of Kcnj2 were inactivated. Given the short lifetime of taste cells (∼22 d) (45), the likelihood that all these events occur before a cell dies is low. Nonetheless, the significant reduction in the resting K+ current in the tissue-specific KO mice, together with our pharmacological and expression profiling data, provide strong evidence that KIR2.1 is required for the resting K+ current in sour taste cells.
KIR2.1 is an unexpected player in sour taste transduction, as the channel was not previously known to be expressed in taste cells or to be associated with sensitivity to intracellular acidification. Notably, KIR2.1 is much less sensitive to intracellular acidification than other inward rectifiers, such as KIR2.3 and KIR1.1 (35). Nonetheless, KIR2.1 can be inhibited at a sufficiently low intracellular pH (≤5.5; our data and ref. 35). Moreover, we find that KIR2.1 can be blocked in whole-cell recording by weak acids that activate sour taste cells. Under these conditions, the block is slow because the concentration of the protonated form of the weak acid is low, and protons can diffuse out of the cell and into the pipette. We speculate that the reduced sensitivity of KIR2.1 to intracellular pH may be advantageous in the context of the sour taste cell, which is acidified at rest (21). Indeed, we do not know the extent of intracellular acidification that occurs in intact taste cells during sensory stimulation, but assuming that proton entry occurs through channels located on sensory microvilli, it is likely that local concentrations of protons could be very high (46). Under these conditions, a less sensitive channel, like KIR2.1, would be better able to sense dynamic changes in intracellular pH.
The Magnitude of the Current, and Not the Identity, Determines Sensitivity to Acids.
Our experiments show that, among taste cells, only those that mediate sour taste respond to intracellular acidification with action potentials. However, quite unexpectedly this response cannot be attributed to the expression of a highly specialized ion channel whose distribution is restricted to sour taste cells. Instead, sensitivity to intracellular acidification is conferred by a relatively ubiquitous ion channel, KIR2.1. KIR2.1 (IRK1/KCNJ2) was the first member of the family of inward rectifier ion channels to be identified (47), and it is expressed broadly in the brain, heart, vascular smooth muscle cells, skeletal muscle, and throughout the body (47). Our data also indicate that KIR2.1 mediates the resting K+ current in TRPM5 cells, which are unresponsive to weak acids that stimulate action potentials in PKD2L1 cells. In an attempt to reconcile these findings, we found one way in which the resting K+ currents in TRPM5 and PKD2L1 cells differed substantially—in their magnitude. As previously reported (44), the resting K+ currents in TRPM5 cells are severalfold larger than those of PKD2L1 cells. This difference in the magnitude of the K+ current can completely account for the difference in sensitivity to intracellular acidification between the two cells types as inhibition of the K+ currents in TRPM5 cells to the level observed in PKD2L1 cells rendered the TRPM5 cells responsive to intracellular acidification. Thus, a smaller K+ current, as found in PKD2L1 cells, makes the cells more responsive to intracellular acidification.
To gain a quantitative appreciation of this phenomenon, we consider two hypothetical cells that vary only in the magnitude of their resting K+ current. The first cell (representing the PKD2L1 cell) has a mixed cation conductance of 0.25 nS and a resting K+ conductance of 1 nS. A resting potential of −67 mV is calculated from the equation: Vm = [(GK*EK) + (GC*EC)]/(GK + GC), where GK and EK are the K+ conductance and equilibrium potential, GC and EC are the cation conductance and cation equilibrium potential, and the K+ concentration is assumed to be 5 mM outside the cell and 140 mM inside it (48). The same equation can be used to show that it would be necessary to block 60% of the K+ current to reach threshold for firing action potentials (assumed to be −50 mV). Now consider another cell (a TRPM5 cell), with a similar resting cation conductance, but a threefold larger K+ conductance (3 nS). The resting potential of this cell is calculated to be −77 mV. Block of 50% of the current will depolarize the cell to −72 mV. In this cell, it is necessary to block 87% of the current to depolarize the cell to a threshold of −50 mV. Thus, one can see that, in moving from a cell with a small resting K+ current to one with a larger current, the fractional block of the current required to reach threshold has increased substantially, from 60% to 87%. If one further assumes a single-site binding site model for block of the channel, this translates into a need for a ∼4.5-fold higher concentration of intracellular protons to bring the TRPM5 cell to threshold compared with the PKD2L1 cell (Methods). Thus, without considering other conductances, the smaller K+ current in PKD2L1 cells is expected to make the cells much more sensitive to intracellular acidification compared with TRPM5 cells.
It is also worth noting that, among a variety of cells tested, only PKD2L1 cells have an inward proton conductance, which can act upstream of the acid-sensitive K+ channel (21). Thus, in other cells in the taste bud, the only way that the cytosol can be acidified is by penetration of weak acids. Although these factors make it unlikely that in vivo TRPM5 taste cells will fire action potentials to acids, we cannot rule out the possibility that intracellular acidification might modulate the response of TRPM5 cells to bitter, sweet, and umami stimuli. Moreover, because we looked only at the aggregate behavior of TRPM5 cells, it is possible that a small subset of TRPM5 cells responsive to one taste quality could show heightened sensitivity to intracellular acidification, and possibly even fire action potentials under some conditions.
Does This Explain the Sour Taste of Weak Acids?
It was noted nearly a century ago that at the same pH, organic weak acids evoke a more intense sour sensation than strong mineral acids (18). This has been attributed to the ability of weak acids, in the protonated form, to penetrate the cell membrane, and upon entering the cell, release a proton (28). Cytosolic acidification could then affect membrane excitability and/or transmitter release. Consistent with this hypothesis, a previous study found that the gustatory nerve response was better correlated with the ability of sour stimuli to acidify the cytosol, rather than the absolute concentration of free protons they contained (5). Similar results were found in recordings from a slice preparation of lingual epithelium, where focal application of sour stimuli elicited calcium responses in taste cells that correlated with the degree of intracellular acidification (20) To our knowledge, our experiments are the first to directly show that intracellular acidification alone, in the absence of extracellular acidification, increases cellular excitability in identified sour taste cells. For these experiments, we used two organic weak acids, acetic acid and propionic acid, which due to their small size and relatively high pKa values (4.76 and 4.88, respectively) can acidify the cell cytosol when used at neutral pH (27). Application of these acids at neutral pH caused a similar degree of intracellular acidification in both TRPM5 and PKD2L1 cells, but only PKD2L1 cells fired action potentials. However, it is worth noting that, in the intact epithelium where taste buds are surrounded by a relatively impenetrable barrier, weak acids may not enter taste cells unimpeded (49). Alternatively, weak acids may be effective sour stimuli because they buffer pH, protecting protons from absorption by salivary proteins. The extent to which penetration of weak acids into taste cells and the buffering capacity of weak acids contributes to the intensity of sour taste perception remains to be determined.
Role of KIR2.1 in Amplification of the Sensory Response.
The ability of KIR2.1 to respond to an elevation of protons makes it well suited to participate in sour transduction, downstream of the previously characterized Zn2+-sensitive proton conductance (Fig. 10). Indeed, we find that the KIR2.1 current in sour taste cells can be blocked by extracellular acidification, when protons are allowed to enter the cell through the Zn2+- sensitive conductance. Block of resting K+ channels would reduce the extent of proton entry needed to depolarize the sour taste cell to threshold, and thereby enhance the detection of small signals. This may explain how taste cells can respond to bath acidification as mild as pH 6.7, which produces a proton current of only a few picoamperes (21). Moreover, it is possible that KIR2.1 channels are localized in close proximity to proton channels, facilitating interaction between the two molecules.
Amplification is a common theme in sensory biology and is usually associated with signaling by G-protein–coupled receptors that activate a cascade of intracellular events (50, 51). Our proposed mechanism, on the other hand, is most similar to the mechanism of amplification observed in the olfactory system where calcium entering through cyclic-nucleotide gated ion channels acts on chloride channels, which further depolarize the cell membrane (52, 53). This mechanism allows the cell to respond to odorants under circumstances where extracellular ions are limited. Similarly, the use of an intracellular amplification step for sour taste transduction is expected to enhance signaling while limiting the need for excessive proton entry that might be cytotoxic.
Taken together, our experiments provide evidence for the participation of the inward rectifier K+ channel, KIR2.1, as a key element in sour taste transduction. Notably, we find that KIR2.1 is blocked by intracellular acidification and acts downstream of proton channels to enhance electrical excitability. Working in tandem, the proton conductance and KIR2.1 channel constitute an elegant mechanism for amplification of the sensory response to sour stimuli.
Methods
Animals.
All methods of mouse handling were approved by the University of Southern California Institutional Animal Care and Use Committee. Mouse strains Pkd2l1-YFP, Pkd1l3−/− were previously described (14, 16). Mouse strains Kcnj2fl/fl, Pkd2l1-Cre are described in SI Methods. Avil-Cre mice were a kind gift from the Peter Heppenstall Laboratory, European Molecular Biology Laboratory, Monterotondo, Rome (38).
Taste Cell Isolation.
Taste cells were isolated from adult mice (6–10 wk old) as previously described (16, 21). In brief, Tyrode’s solution containing 1 mg/mL elastase (Worthington), 2.5 mg/mL dispase II (Roche), and 1 mg/mL trypsin inhibitor (Sigma-Aldrich) was injected between the epithelium and the muscle of the isolated tongue, which was incubated for 20 min in Tyrode's solution bubbled with 95% O2/5% CO2. The epithelium was then peeled off from the tongue. The small piece of epithelium containing the papillae was further trimmed and incubated in the enzyme mixture for 20 min at room temperature. The reaction was stopped by washing the tissue in Ca2+-free saline. Single cells were isolated by trituration in Tyrode’s solution with a fire-polished Pasteur pipette and were used within 6 h.
Taste Cell Recording.
Taste cells were prepared for patch-clamp recording as previously described (16, 21). Data were collected with an Axopatch 200B amplifier using pClamp software suite (Axon Instruments), and time course measurements and I–V curve were graphed with Origin 6 (OriginLab). For cell-attached recording, we selected for cells that were healthy and able to fire action potentials, using 2 mM Ba2+, which elicited action potentials more reliably in both PKD2L1 and TRPM5 cells compared with high K+ (16). In addition, we selected for cells with intact apical processes, based on the flask-like shape of the cell, and presence of apically located YFP. Firing rates were measured within the interval of 0.4 to 4 s following application of stimuli unless otherwise stated. The evoked frequency was calculated after subtraction of the background frequency, measured during the 10 s before application of the stimulus. Note that the amplitude of the spikes measured in a single cell varied during recordings; this can be attributed to changes in the availability of Na+ channels, which may not fully recover from inactivation when resting K+ channels are blocked to changes in the seal resistance, which can affect spike amplitude. For whole-cell recording, the membrane potential was held at −80 mV, the voltage was ramped from −80 to +80 mV (1 V/s), and the current was measured at −80 mV. For testing the dose dependence of inhibition by Ba2+ and Cs+ of the resting K+ current (Fig. 3 A–D), series resistance compensation was performed.
Solutions.
Solutions for electrophysiology were exchanged using a piezo-driven stepper motor system (SF-77B; Warner Instruments). The pipette solution in all experiments except those shown in Figs. 2A, 4A, 6A, and 9A contained the following (in mM): 120 KAsp, 7 KCl, 8 NaCl, 10 Hepes, 2 MgATP, 0.3 GTP-Tris, 5 EGTA, 2.4 CaCl2 (∼100 nM free Ca2+), pH 7.4 with KOH. For the experiments shown in Figs. 2A, 4A, and 6A, to minimize contributions due to changes in intracellular Ca2+ as a result of competition between H+ and Ca2+ for binding to EGTA, a Ca2+-free pipette solution was used containing the following (in mM): 120 KAsp, 20 KCl, 10 Hepes, 2 MgATP, 0.3 GTP-Tris, 0.5 EGTA, pH 7.3; in Fig. 9A, Hepes concentration was 10 μM. Tyrode’s solution contained the following (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, and 10 d-glucose, pH 7.4. Sodium solution was as follows (in mM): 150 NaCl, 10 Hepes, and 2 CaCl2, pH 7.4. For recording action potentials, the tested solutions contained the indicated concentration of acid (in mM), which replaced equimolar Cl−, and the pH was adjusted with NaOH. In Fig. 9 C and D, the pH 7.4, pH 7.0, and pH 6.6 solutions have 43.7, 17.5, and 7 mM overall acetic acid, respectively, to generate a final concentration of 0.1 mM protonated form. For whole-cell recordings, 50 mM KCl (Figs. 2E, 3 E and F, 5, 6B, 7, 9 A and B), 100 mM KCl (Fig. 3 A–D and 8 A and B), or 140 mM KCl (Figs. 2 A and D, and 6A) and labeled concentrations of TEA replaced equimolar NaCl in the extracellular solution. For solutions of pH ≤6.6, Mes replaced Hepes. In excised patch, the pipette solution was as follows (in mM): 150 KCl, 2 CaCl2, 10 Hepes, pH 7.4. The extracellular solution was 150 KCl, 10 Hepes or 10 Mes, 1 EDTA, 1 EGTA, pH 7.4 with KOH or HCl.
Chemicals.
Glibenclamide and Tertiapin-LQ, bupivacaine, and linopirdine were purchased from Tocris; ML133 was a gift of Craig Lindsley, Vanderbilt University, Nashville, TN. All other chemicals used for pharmacological profiling were from Sigma-Aldrich.
Statistics and Analysis.
Bar and summary scatter plots were prepared in Prism (GraphPad Software). Significance was determined with Student’s t test or ANOVA as indicated. In all cases, data represent the mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Heterologous expression, mouse genetics, immunocytochemistry, transcriptome analysis, intracellular pH imaging, and other experimental details are described in SI Methods.
SI Methods
Animals.
Mouse handling, genotyping, and methods of killing were approved by the University of Southern California Institutional Animal Care and Use Committee. Mouse strains Pkd2l1-YFP, Pkd1l3−/− and Trpm5-GFP were previously described (14, 16, 25). Mouse strains Kcnj2fl/fl, Pkd2l1-Cre are described below. Avil-Cre mice were a kind gift from the Peter Heppenstall Laboratory, European Molecular Biology Laboratory, Monterotondo, Rome (38). For the validation of the cell-specific expression of Cre recombinase, the two strains were each bred into a Rosa26Tdt strain (The Jackson Laboratory; stock 007908) (54), and immunocytochemistry (see below) was used to identify the Td-Tomato–expressing cell population. To monitor expression of Cre from the Pkd2l1 promoter in nonsour taste cells, the Pkd21l-Cre::Rosa26Tdt strain was bred into a Trpm5-GFP strain (25). To monitor expression of Cre from the Avil promoter in sour taste cells, the Avil-Cre::Rosa26Tdt was bred into a pkd211-YFP strain (16).
Pkd2l1-Cre Mouse Strain.
Gene targeting was used to insert an IRES-Cre transgene immediately after the stop codon of Pkd2l1 gene in the mouse genome. The targeting vector was constructed by cloning a 5.2-kb genomic fragment encompassing the last exon of Pkd2l1 gene into pBluescript and inserting IRES-Cre immediately after the stop codon. A floxed-neomycin resistance cassette was cloned at a site immediately downstream of the IRES-Cre. The targeting vector was linearized by XmaI digestion and electroporated into mouse ES cells (EC7.1 line). Recombinant ES cells were selected by G418 resistance in culture, and 282 clones were isolated for PCR screening to identify correct targeting events. Two correctly targeted clones were isolated and were used for microinjection to generate germ-line chimeras, and through standard breeding, the F1 generation of mice. To monitor expression in nonsour taste cells, the Pkd21l-Cre::Rosa26Tdt strain was bred into a Trpm5-GFP strain (25), and confocal images were taken, as shown in Fig. S3C.
Floxed Kcnj2 Mouse Strain.
Floxed Kcnj2 mice were generated by the M.T.N. Laboratory (University of Vermont, Burlington, VT) and will be described elsewhere in more detail. In brief, the second exon of Kcnj2 was flanked by LoxP sites and a downstream Neo cassette was flanked by FRT sites. The neo cassette was removed by breeding to a FLP-deleter mouse (The Jackson Laboratory). To generate a Kcnj2 sour taste cell-specific knockout in a background of Pkd2l1-YFP, the Pkd2l1-Cre and Pkd2l1-YFP strains were each bred with the Kcnj2fl strain and the offspring were further bred to generate Pkd2l1-YFP, Pkd2l1-Cre, Kcnj2fl/fl progeny. To generate a knockout of Kcnj2 in TRPM5 cells in a background of Trpm5-GFP, the Avil-Cre strain and Trpm5-GFP strain were each bred with the Kcnj2fl strain, and the offspring were further bred to generate Trpm5-GFP, Avil-Cre, Kcnj2fl/fl progeny. Genotyping methods for Pkd2l1-YFP strain were previously described (16). The primers for detecting Cre, Tdt, and Kcnj2fl are as follows: Cre (GCACTGATTTCGACCAGGTT, GAGTCATCCTTAGCGCCGTA; 420-bp product), Rosa26Tdt (CTGTTCCTGTACGGCATGG, GGCATTAAAGCAGCGTATCC; 196-bp product), Kcnj2 distal loxP (TCCGATGACACTGAGAACCTGGA, TGGATGCTTCCGAGAACCTTGG; WT allele: 520 bp; floxed allele: 600 bp), Kcnj2 neo deletion (CTGACTGAACACACAGGTCCAGGG, GGGACCATCAAGCCCTGGTAATGG; floxed allele: 800 bp; WT allele: 600 bp).
Subcloning and Heterologous Expression.
KIR2.1 was kindly provided by Zhe Lu, University of Pennsylvania, Philadelphia; KIR2.2, KIR4.2 were amplified from mouse skeleton muscle and kidney cDNA. The amplified fragments were inserted into pcDNA3 vector with the In-Fusion method (Clontech). KCNK1 was mutated to increase membrane trafficking (KCNK1-I293A,I294A; KCNK1-AA) (55) and was fused to GFP by removing the stop codon and inserting the gene upstream in frame with the GFP coding sequence. Clones used for recording were fully sequenced (Genewiz).
HEK 293 cells were cultured with DMEM containing 10% (vol/vol) FBS and 0.05% gentamicin. KIR2.1, KIR2.2, and KIR4.2 were cotransfected with GFP (ratio 20:1), whereas KCNK1-GFP and KCNK1-AA-GFP were transfected alone, using TransIT-293 Transfection Reagent (Mirus Bio). Cells were lifted with trypsin-EDTA 24 h after transfection and used for recording within 6 h. All cell culture reagents were from Life Technologies.
Calculation of the Blocker Concentration Needed to Produce an Equivalent Magnitude Current in TRPM5 Cells as in PKD2L1 Cells.
We assumed a single-site binding model for block of the K+ channel by intracellular protons:
Solving this equation for the case of the PKD2L1 cell where Iblocked/Iinitial = 0.60 yields a value of [H+]i = 1.5*IC50 and solving for the case of the TRPM5 cell where Iblocked/Iinitial = 0.87 yields a value of [H+]i = 6.7*IC50. The ratio of the two is 4.5.
Estimation of the Contribution of KCNK1 to the Taste Cell K+ Current.
We rearranged a set of linear functions assuming that the Rb+ and K+ inward currents are generated solely from the contributions of KCNK1 and KIR2.1, and that the ratio of Rb+ to K+ permeability was preserved. Rearranging to solve for IKKCNK1, the potassium current contributed by KCNK1, results in the following equation:
where IKtaste is the resting K+ current amplitude in PKD2L1 taste cells, and r is the ratio of Rb+ to K+ current for KCNK1, KIR2.1, or PKD2L1 taste cell as indicated in subscript.
RNAseq.
A small region of the tongue containing circumvallate papillae and control regions devoid of taste buds was microdissected, and RNA was extracted with TRIzol (Life Technologies). Library construction and sequencing were carried out at the Norris Cancer Center Next Gen sequencing core. RNA was visualized on a Bio-Rad Experion to ensure RNA Quality Index values were greater than 8. For library construction, the Illumina TruSeq, version 2, mRNA kit was used. The protocol was followed according to manufacturer’s instructions except the final number of PCR cycles was 12 and not 15. Following construction, libraries were visualized by Bioanalyzer (Agilent) using the High Sensitivity Chip and quantified for pooling and sequencing using Kapa Biosystems qPCR quantitation kit according to manufacturer’s instructions. For sequencing, libraries were diluted to 16 pM, and then applied to a V3 flowcell using the Illumina cBot according to manufacturer’s instructions. Sequencing was carried out on the HiSeq 2000 using HSCS, version 1.5.15.1. Image analysis and base calling were carried out using RTA 1.13.48.0, and deconvolution and fastq file production were performed with CASAVA 1.8.2. Reads were mapped to the mouse genome (mm9) summed and converted to reads per kilobase per million reads (RPKM) using the equation: RPKM = [# of mapped reads]/([length of transcript]/1,000)/([total reads]/10^6).
Immunohistochemistry and Cell Counting.
For measuring the expression of the PKD2L1-Cre reporter, circumvallate taste tissue was incubated in 4% paraformaldehyde for 1.5 h before being cryoprotected in 20% sucrose solution at 4 °C overnight. Tissue was embedded in OCT at −60 °C and sectioned at 12 µm. Slides were blocked with 2% normal donkey serum in buffer containing 0.3% Triton for 1 h before being incubated in primary antibody overnight at 4 °C and secondary antibody for 3 h at room temperature (RT). Slides were mounted with Fluoromount-G (SouthernBiotech) and imaged on a Leica confocal microscope. Hiroaki Matsunami, Duke University, Durham, NC generously provided the primary anti-PKD2L1 1:500, which was previously described (9). All z-stack images were acquired on a Leica confocal. To quantify immunohistochemical images, we defined a cell as a distinct shape featuring: (i) a clear nuclear region and (ii) an elongation along the pore-to-base axis of the taste bud. We thresholded images using a modified Otsu method (56) and counted positive cells by eye, tracing each through the image stack via optical dissection.
For measuring expression of the Avil-Cre reporter, circumvallate papillae from triple transgenic Avil-Cre::Rosa26Tdt::Pkd2l1-YFP mice were dissected and incubated in 4% paraformaldehyde for 30 min at RT. The tissue was cryoprotected in 30% sucrose at 4 °C overnight, embedded in OCT at −80 °C and sectioned at 14 μm. Immunohistochemistry was performed by permeabilizing the tissue with 0.1% Triton X-100 for 15 min, blocking with BlockAid (Life Technologies) plus 0.1% Triton X-100 for 1 h and incubating in primary antibody overnight at 4 °C or RT for 1 h and secondary antibody at RT for 1 h. Slides were mounted with Fluoroshield (Sigma-Aldrich). The primary antibodies were chicken anti-GFP (1:1,000) (Aves Labs), guinea pig anti-TRPM5 (1:500) (24); the secondary antibodies were goat anti-chicken Alexa Fluor 488 and goat anti-guinea pig Alexa Fluor 546 (Life Technologies). All z-stack images were acquired on an Olympus confocal. To quantify immunohistochemical images, two observers independently scored cells as Tdt+, TRPM5+, or PKD2L1-GFP+.
Solutions for Recording KCNK1 Currents (Fig. S7).
The pipette solution contained the following (in mM): 120 KAsp, 7 KCl, 8 NaCl, 10 Hepes, 2 MgATP, 0.3 GTP-Tris, 5 EGTA, 2.4 CaCl2 (∼100 nM free Ca2+), pH 7.4 with KOH. The standard bath solution contained the following (in mM):130 NaCl, 20 TEA-Cl, 10 Hepes, 2 CaCl2, 1 MgCl2, pH 7.4. Rb solution contained the following (in mM): 130 RbCl, 20 TEA-Cl, 10 Hepes, 2 CaCl2, 1 MgCl2, pH 7.4. K solution contained the following (in mM): 130 KCl, 20 TEA-Cl, 10 Hepes, 2 CaCl2, 1 MgCl2, pH 7.4. CsCl was added at the concentrations indicated.
pH Imaging.
Dissociated taste cells were loaded with the intracellular pH indicator pHrodo Red AM using PowerLoad concentrate accordingly to the manufacturer’s instructions (Molecular Probes). TRPM5-GFP and PKD2L1-YFP cells were distinguished as previously described (21). During pH imaging, cells were excited every 5 s with light of 560 nm, and emission at 630 nm was detected by a Hamamatsu digital CCD camera attached to an Olympus IX71 microscope using a U-N31004 Texas Red/Cy3.5 filter cube (Chroma Technologies). After exposure to MA and PA (both pH balanced to 7.4 using NaOH), intracellular pH was calibrated with high K+ solution containing the following (in mM): 140 KCl, 4.5 NaCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 Mes or 10 Hepes, and 0.02 nigericin (Molecular Probes), pH adjusted with NaOH to pH 6.0, 6.7, or 7.4. PHrodo Red fluorescence intensity at baseline, when exposed to MA, and when exposed to PA was converted to pHi using linear calibration curves constructed for each cell using fluorescence intensity data in the presence of calibration solutions under the assumption that pHi = pHo.
Acknowledgments
This work was supported by National Institute of Deafness and Communications Disorders (NIDCD) Grants R21 DC012747 and R01 DC013741 (to E.R.L.); NIDCD Grant F32 DC013971-01 (to J.D.B.); NIDCD Grant R01 DC012555 (to S.C.K.); and NIDDKD Grant R37 DK-053832, NHLBI Grants R01 HL044455, HL121706, and P01 HL-095488, and grants from Fondation Leducq and the Totman Medical Research Trust (to M.T.N.). C.E.W. was supported by NIH Grant T32 HD041697, and W.S.C. was supported by NINDS Grant P30 NS048154. The generation of Pkd2l1-Cre mice was supported by Grants P30 NS048154 and P30 DC004657.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 246.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514282112/-/DCSupplemental.
References
- 1.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442(7105):934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190(3):285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liman ER, Zhang YV, Montell C. Peripheral coding of taste. Neuron. 2014;81(5):984–1000. doi: 10.1016/j.neuron.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas CJ, Lawless HT. Astringent subqualities in acids. Chem Senses. 1995;20(6):593–600. doi: 10.1093/chemse/20.6.593. [DOI] [PubMed] [Google Scholar]
- 5.Lyall V, et al. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol. 2001;281(3):C1005–C1013. doi: 10.1152/ajpcell.2001.281.3.C1005. [DOI] [PubMed] [Google Scholar]
- 6.Dickman JD, Smith DV. Response properties of fibers in the hamster superior laryngeal nerve. Brain Res. 1988;450(1-2):25–38. doi: 10.1016/0006-8993(88)91541-7. [DOI] [PubMed] [Google Scholar]
- 7.Barretto RP, et al. The neural representation of taste quality at the periphery. Nature. 2015;517(7534):373–376. doi: 10.1038/nature13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin W, Ogura T, Kinnamon SC. Acid-activated cation currents in rat vallate taste receptor cells. J Neurophysiol. 2002;88(1):133–141. doi: 10.1152/jn.2002.88.1.133. [DOI] [PubMed] [Google Scholar]
- 9.Ishimaru Y, et al. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci USA. 2006;103(33):12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens DR, et al. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature. 2001;413(6856):631–635. doi: 10.1038/35098087. [DOI] [PubMed] [Google Scholar]
- 11.LopezJimenez ND, et al. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J Neurochem. 2006;98(1):68–77. doi: 10.1111/j.1471-4159.2006.03842.x. [DOI] [PubMed] [Google Scholar]
- 12.Ugawa S, et al. Receptor that leaves a sour taste in the mouth. Nature. 1998;395(6702):555–556. doi: 10.1038/26882. [DOI] [PubMed] [Google Scholar]
- 13.Richter TA, Dvoryanchikov GA, Roper SD, Chaudhari N. Acid-sensing ion channel-2 is not necessary for sour taste in mice. J Neurosci. 2004;24(16):4088–4091. doi: 10.1523/JNEUROSCI.0653-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson TM, et al. Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem Senses. 2010;35(7):565–577. doi: 10.1093/chemse/bjq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horio N, et al. Sour taste responses in mice lacking PKD channels. PLoS One. 2011;6(5):e20007. doi: 10.1371/journal.pone.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang RB, Waters H, Liman ER. A proton current drives action potentials in genetically identified sour taste cells. Proc Natl Acad Sci USA. 2010;107(51):22320–22325. doi: 10.1073/pnas.1013664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kataoka S, et al. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008;33(3):243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey RB. The relation between the total acidity, the concentration of the hydrogen ion, and the taste of acid solutions. J Am Chem Soc. 1920;42(4):712–714. [Google Scholar]
- 19.Ogiso K, Shimizu Y, Watanabe K, Tonosaki K. Possible involvement of undissociated acid molecules in the acid response of the chorda tympani nerve of the rat. J Neurophysiol. 2000;83(5):2776–2779. doi: 10.1152/jn.2000.83.5.2776. [DOI] [PubMed] [Google Scholar]
- 20.Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol. 2003;547(Pt 2):475–483. doi: 10.1113/jphysiol.2002.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushman JD, Ye W, Liman ER. A proton current associated with sour taste: Distribution and functional properties. FASEB J. 2015;29(7):3014–3026. doi: 10.1096/fj.14-265694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter TA, Dvoryanchikov GA, Chaudhari N, Roper SD. Acid-sensitive two-pore domain potassium (K2P) channels in mouse taste buds. J Neurophysiol. 2004;92(3):1928–1936. doi: 10.1152/jn.00273.2004. [DOI] [PubMed] [Google Scholar]
- 23.Lin W, Burks CA, Hansen DR, Kinnamon SC, Gilbertson TA. Taste receptor cells express pH-sensitive leak K+ channels. J Neurophysiol. 2004;92(5):2909–2919. doi: 10.1152/jn.01198.2003. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27(21):5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008;586(Pt 12):2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER. A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol. 2011;137(6):493–505. doi: 10.1085/jgp.201110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roper SD. Signal transduction and information processing in mammalian taste buds. Pflugers Arch. 2007;454(5):759–776. doi: 10.1007/s00424-007-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii S, et al. Acetic acid activates PKD1L3-PKD2L1 channel—a candidate sour taste receptor. Biochem Biophys Res Commun. 2009;385(3):346–350. doi: 10.1016/j.bbrc.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 30.Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N. Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol. 2009;517(1):1–14. doi: 10.1002/cne.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA. 2011;108(13):5431–5436. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hibino H, et al. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol Rev. 2010;90(1):291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 33.Enyedi P, Czirják G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol Rev. 2010;90(2):559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 34.Wang HR, et al. Selective inhibition of the K(ir)2 family of inward rectifier potassium channels by a small molecule probe: The discovery, SAR, and pharmacological characterization of ML133. ACS Chem Biol. 2011;6(8):845–856. doi: 10.1021/cb200146a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu Z, et al. Identification of a critical motif responsible for gating of Kir2.3 channel by intracellular protons. J Biol Chem. 1999;274(20):13783–13789. doi: 10.1074/jbc.274.20.13783. [DOI] [PubMed] [Google Scholar]
- 36.Masia R, Krause DS, Yellen G. The inward rectifier potassium channel Kir2.1 is expressed in mouse neutrophils from bone marrow and liver. Am J Physiol Cell Physiol. 2015;308(3):C264–C276. doi: 10.1152/ajpcell.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ Res. 2000;87(2):160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- 38.Zurborg S, et al. Generation and characterization of an Advillin-Cre driver mouse line. Mol Pain. 2011;7:66. doi: 10.1186/1744-8069-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyall V, et al. Intracellular pH modulates taste receptor cell volume and the phasic part of the chorda tympani response to acids. J Gen Physiol. 2006;127(1):15–34. doi: 10.1085/jgp.200509384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar NN, et al. PHYSIOLOGY. Regulation of breathing by CO₂ requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science. 2015;348(6240):1255–1260. doi: 10.1126/science.aaa0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinnamon SC, Roper SD. Membrane properties of isolated mudpuppy taste cells. J Gen Physiol. 1988;91(3):351–371. doi: 10.1085/jgp.91.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinnamon SC, Dionne VE, Beam KG. Apical localization of K+ channels in taste cells provides the basis for sour taste transduction. Proc Natl Acad Sci USA. 1988;85(18):7023–7027. doi: 10.1073/pnas.85.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci. 2003;23(7):2608–2617. doi: 10.1523/JNEUROSCI.23-07-02608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romanov RA, Kolesnikov SS. Electrophysiologically identified subpopulations of taste bud cells. Neurosci Lett. 2006;395(3):249–254. doi: 10.1016/j.neulet.2005.10.085. [DOI] [PubMed] [Google Scholar]
- 45.Perea-Martinez I, Nagai T, Chaudhari N. Functional cell types in taste buds have distinct longevities. PLoS One. 2013;8(1):e53399. doi: 10.1371/journal.pone.0053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang J, et al. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol. 2010;20(3):189–197. doi: 10.1016/j.cub.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 47.Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- 48.Filosa JA, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 49.Dando R, et al. A permeability barrier surrounds taste buds in lingual epithelia. Am J Physiol Cell Physiol. 2015;308(1):C21–C32. doi: 10.1152/ajpcell.00157.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardie RC, et al. Molecular basis of amplification in Drosophila phototransduction: Roles for G protein, phospholipase C, and diacylglycerol kinase. Neuron. 2002;36(4):689–701. doi: 10.1016/s0896-6273(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 51.Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- 52.Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366(6452):283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- 53.Kurahashi T, Yau KW. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363(6424):71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- 54.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feliciangeli S, et al. Potassium channel silencing by constitutive endocytosis and intracellular sequestration. J Biol Chem. 2010;285(7):4798–4805. doi: 10.1074/jbc.M109.078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otsu N. Threshold selection method from gray-level histograms. IEEE T Syst Man Cyb. 1979;9(1):62–66. [Google Scholar]
- 57.Ma L, Xie YP, Zhou M, Chen H. Silent TWIK-1 potassium channels conduct monovalent cation currents. Biophys J. 2012;102(8):L34–L36. doi: 10.1016/j.bpj.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou M, et al. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci. 2009;29(26):8551–8564. doi: 10.1523/JNEUROSCI.5784-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]