Significance
The present work establishes a novel approach to the study of visual representations. This approach allows us to estimate the structure of human face space as encoded by high-level visual cortex, to extract image-based facial features from this structure, and to use such features for the purpose of facial image reconstruction. The derivation of visual features from empirical data provides an important step in elucidating the nature and the specific content of face representations. Further, the integrative character of this work sheds new light on the existing concept of face space by rendering it instrumental in image reconstruction. Last, the robustness and generality of our reconstruction approach is established by its ability to handle both neuroimaging and psychophysical data.
Keywords: image reconstruction, face space, reverse correlation
Abstract
The reconstruction of images from neural data can provide a unique window into the content of human perceptual representations. Although recent efforts have established the viability of this enterprise using functional magnetic resonance imaging (MRI) patterns, these efforts have relied on a variety of prespecified image features. Here, we take on the twofold task of deriving features directly from empirical data and of using these features for facial image reconstruction. First, we use a method akin to reverse correlation to derive visual features from functional MRI patterns elicited by a large set of homogeneous face exemplars. Then, we combine these features to reconstruct novel face images from the corresponding neural patterns. This approach allows us to estimate collections of features associated with different cortical areas as well as to successfully match image reconstructions to corresponding face exemplars. Furthermore, we establish the robustness and the utility of this approach by reconstructing images from patterns of behavioral data. From a theoretical perspective, the current results provide key insights into the nature of high-level visual representations, and from a practical perspective, these findings make possible a broad range of image-reconstruction applications via a straightforward methodological approach.
Face recognition relies on visual representations sufficiently complex to distinguish even among highly similar individuals despite considerable variation due to expression, lighting, viewpoint, and so forth. A longstanding conceptual framework, termed “face space” (1–6), suggests that individual faces are represented in terms of their multidimensional deviation from an “average” face, but the precise nature of the dimensions or features that capture these deviations, and the degree to which they preserve visual detail, remain unclear. Thus, the featural basis of face space along with the neural system that instantiate it remain to be fully elucidated. The present investigation aims not only to uncover fundamental aspects of neural representations but also to establish their plausibility and utility through image reconstruction. Concretely, the current study addresses the issues above in the context of two distinct challenges, first, by determining the visual features used in face identification and, second, by validating these features through their use in facial image reconstruction.
With respect to the first challenge, recent studies have demonstrated distinct sensitivity to local features (e.g., the size of the mouth) compared with configural features (e.g., the distance between the eyes and the mouth) in human face-selective cortex (7–10). Also, neurophysiological investigations (1, 11) of monkey cortex have found sensitivity to several facial features, particularly in the eye region of the face. However, most investigations consider only a few handpicked features. Thus, a comprehensive, unbiased assessment of face space still remains to be conducted. Furthermore, most studies target shape at the expense of surface features (e.g., skin tone) despite the relevance of the latter for recognition (12, 13).
With respect to the second challenge, a number of studies have taken steps toward image reconstruction from functional magnetic resonance imaging (fMRI) signals in visual cortex, primarily exploiting low-level visual features (14–16; but see ref. 17). The recent extension of this work to the reconstruction of face images (18) has demonstrated the promise of exploiting category-specific features (e.g., facial features) associated with activation in higher visual cortex. However, the substantial variability across individual faces in this latter study (due to race, age, image background, etc.) limits its conclusions with regard to facial identification and the representations underlying it. Moreover, this attempt deployed prespecified image features due to their reconstruction potential rather than as an argument for their biological plausibility.
The current work addresses the challenges above by adopting a broad, unbiased methodological approach. First, we map cortical areas that exhibit separable patterns of activation to different facial identities. We then construct confusability matrices from behavioral and neural data in these areas to determine the general organization of face space. Next, we extract the visual features accounting for this structure by means of a procedure akin to reverse correlation. And last, we deploy the very same features for the purpose of face reconstruction. Importantly, our approach relies on an extensive but carefully controlled stimulus set ensuring our focus on fine-grained face identification.
The results of our investigation show that (i) a range of facial properties such as eyebrow salience and skin tone govern face encoding, (ii) the broad organization of behavioral face space reflects that of its neural homolog, and (iii) high-level face representations retain sufficient detail to support reconstructing the visual appearance of different facial identities from either neural or behavioral data.
Pattern-Based Mapping of Facial Identity
Participants viewed a set of 120 face images (60 identities × 2 expressions), carefully controlled with respect to both high-level and low-level image properties (SI Text). Each image was presented at least 10 times per participant across five fMRI sessions using a slow event-related design (100-ms stimulus cue, followed by 900-ms stimulus presentation and 7-s fixation). Participants performed a one-back identity task across variation in expression (accuracy was high for each participant scoring above 92%).
Multivoxel pattern-based mapping (19) was carried out to localize cortical regions responding with linearly discriminable patterns to different facial identities. To this end, we separately computed at each location within a cortical mask the discriminability of every pair of identities using linear support vector machine (SVM) classification and leave-one-run-out cross-validation (Methods and SI Text). The resulting information-based map of each participant was normalized to a common space and analyzed at the group level to assess the presence of identity-related information and its approximate spatial location.
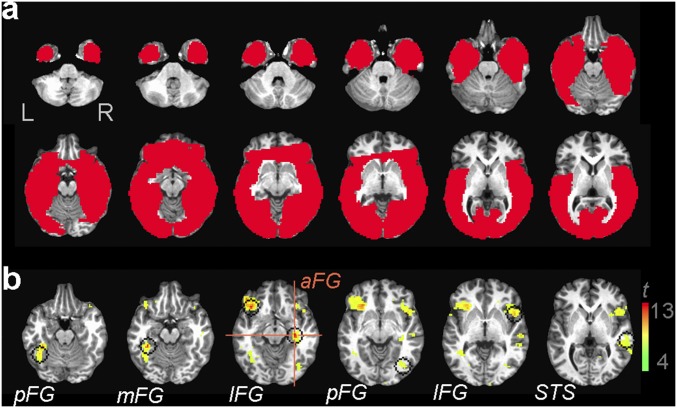
This analysis revealed multiple regions (Fig. S1) in the bilateral fusiform gyrus (FG), the inferior frontal gyrus (IFG), and the right posterior superior temporal sulcus (pSTS). Discrimination levels were compared against chance via one-sample two-tailed t tests across participants [false discovery rate (FDR)-corrected; q < 0.05]. Overall discriminability peaked in a region of interest (ROI) covering parts of the right anterior FG and the parahippocampal gyrus (t7 = 12.07, P < 10−5).
Fig. S1.
(A) Group-level cortical mask. (B) Group-level information-based map of face individuation (P < 10−3; q < 0.05). Crosshairs show the overall discrimination peak of the map in the raFG/PHG (Talairach coordinates, 31, –19, –9). Searchlight masks are centered on the peaks of additional regions (left posterior FG, –39, –49, –16; left middle FG, –34, –36, –14; left IFG, –36, 24, –9; right posterior FG, 39, –69, –4; right IFG, 46, 11, –1; and the right STS, 56, –21, –1). FG, fusiform gyrus; IFG, inferior frontal gyrus; PHG, parahippocampal gyrus; STS, superior temporal sulcus.
To ensure that other key regions were not missed, we included another region of potential interest for face processing localized in the anterior medial temporal gyrus (aMTG) (20) at a less conservative threshold (q < 0.10). Further, above-chance discrimination accuracy was confirmed in the bilateral fusiform face area (FFA) (21) in agreement with previous work (7, 22, 23) but not in the early visual cortex (EVC) (SI Text).
In summary, a total of eight ROIs localized through pattern-based mapping along with the bilateral FFA were found to be likely candidates for hosting representations of facial identity. Accordingly, these regions formed the basis for the investigation of neural representations reported below.
The Similarity Structure of Human Face Space
To evaluate the structure of the neural data relevant for identity representation, we extracted the discriminability values of all pairs of facial identities for each of 10 ROIs. Specifically, after mapping these ROIs in each participant, we separately stored, for each participant and ROI, all pairwise discrimination values corresponding to 1,770 identity pairs (i.e., all possible pairs derived from 60 identities).
Analogous behavioral measurements were collected in a separate experiment in which the confusability of the stimuli was assessed. Briefly, pairs of faces with different expressions were presented sequentially for 400 ms, and participants were asked to perform a same/different identity task. Participants were tested with all facial identity pairs across four behavioral sessions before fMRI scanning. The average accuracy in discriminating each identity pair provided the behavioral counterpart of our neural pattern-discrimination data.
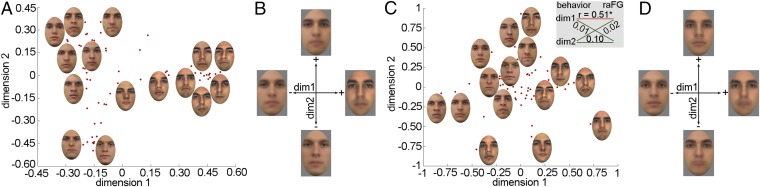
Next, metric multidimensional scaling (MDS) was applied to behavioral and neural discriminability vectors averaged across participants. This analysis forms a natural bridge between recent examinations of neural-based similarity matrices in visual perception (24) and traditional investigations of behavioral-based similarity in the study of face space (2, 25). The outcome of this analysis provides us with the locations of each facial identity in a multidimensional face space. Fig. 1 A and C illustrates the distribution of facial identities across the first two dimensions for behavioral data and for right anterior FG data; we focus on this ROI both because of the robustness of its mapping and because of its central role in the processing of facial identity (23). The first two MDS dimensions are particularly relevant, because, as detailed below, they contribute important information for reconstruction purposes.
Fig. 1.
Behavioral and neural face space topography estimated through MDS. Plots show the distribution of facial identities across the first two dimensions for (A) behavioral and (C) right anterior fusiform gyrus (raFG) data. Each dot represents a single identity (for simplicity only a subset of neutral images is shown in each plot). First-dimension coefficients corresponding to different facial identities correlate significantly across data types (C Inset, Pearson correlation, *P < 0.05). Pairs of opposing face templates are constructed for each dimension and data type (B, behavioral templates; D, raFG templates) for visualization and interpretation purposes. Images reproduced from refs. 46–50.
An examination of the results suggests an intuitive clustering of faces based on notable traits such as eyebrow salience. To facilitate the interpretation of these dimensions, faces were separately averaged on each side of the origin proportionally to their coefficients on each axis. This procedure yielded two opposing templates per dimension whose direct comparison informs the perceptual properties encoded by that particular dimension (Fig. 1 B and D). The comparison of these templates reveals a host of differences, such as eyebrow thickness, facial hair (i.e., stubble), skin tone, nose shape, and mouth height.
Further, the analysis of the behavioral data produced results similar to that of the fMRI data. To evaluate this correspondence, we correlated the coefficients of each facial identity across dimensions extracted for the two data types. This analysis confirmed the similarity between the organization of the first dimensions across behavioral and right aFG data (Fig. 1C, Inset); a broader evaluation of this correspondence is targeted by the assessment of image reconstructions below.
In sum, the present findings verify the presence of consistent structure in our data, assess its impact on the correspondence between behavior and neural processing, and account for this structure in terms of major visual characteristics spanning a range of shape and surface properties.
Derivation of Facial Features Underlying Face Space
The organization of face space is arguably determined by visual features relevant for identity encoding and recognition. An inspection of the results above (Fig. 1) suggests that a simple linear method can capture at least some of these features.
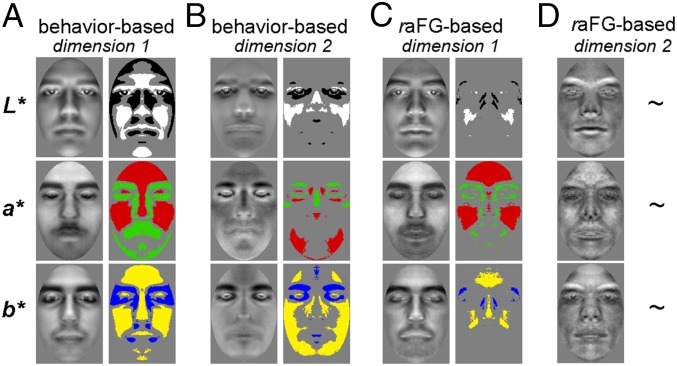
For this purpose, we deployed the following procedure. First, for each dimension, we subtracted each corresponding template from its counterpart, thereby obtaining another template akin to a classification image (CI) (26–28)—that is, a linear estimate of the image-based template that best accounts for identity-related scores along a given dimension (Methods). Then, this template was assessed pixel-by-pixel with respect to a randomly generated distribution of templates (i.e., by permuting the scores associated with facial identities) to reveal pixel values lower or higher than chance (two-tailed permutation test; FDR correction across pixels, q < 0.05). These analyses were performed separately for each color channel after converting images to CIEL*a*b* (where L*, a*, and b* approximate the lightness, red:green, and yellow:blue color-opponent channels of human vision). Examples of raw CIs and their analyses are shown in Fig. 2.
Fig. 2.
CIs derived from MDS analyses of behavioral (A and B) and raFG (C and D) data. Each pair shows a raw CI (Left) and its analysis (Right) with a permutation test (q < 0.05, FDR correction across pixels; ∼, not significant). Bright/dark, red/green, and yellow/blue regions in analyzed CIs mark areas of the face brighter (L*), redder (a*), or more yellow (b*) than chance for positive versus negative templates in Fig. 1. Results are shown separately for the first (A and C) and second (B and D) dimensions of the data.
Consistent with our observations regarding face clustering in face space, the CIs revealed extensive differences in surface properties such as eyebrow thickness and skin tone as well as in shape properties such as nose shape and relative mouth height. For instance, wide bright patches over the forehead and cheeks reflect sensitivity to color differences, whereas thinner patches along the length of the nose and the mouth provide information on shape differences. Also, these differences extend beyond lightness to chromatic channels, whether accompanied or not by similar L* differences. Further, behavioral CIs exhibited larger differences than their neural counterparts. However, most of the ROIs appeared to exhibit some sensitivity to image-based properties.
Thus, our methods were successful in using the similarity structure of neural and behavioral data to derive visual features that capture the topography of human face space.
Facial Image Reconstruction
An especially compelling way to establish the degree of visual detail captured by a putative set of face space features is to determine the extent to which they support identifiable reconstructions of face images. Accordingly, we carried out the following procedure (Fig. 3).
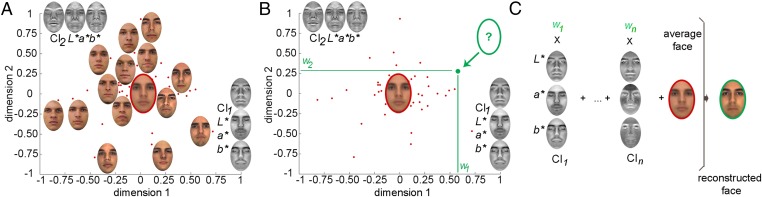
Fig. 3.
Steps involved in the reconstruction procedure: (A) We estimate a multidimensional face space associated with a cortical region, and we derive CI features for each dimension in CIEL*a*b* color space along with an average face (for simplicity, only two dimensions are displayed above); (B) we project a new face in this space based on its neural similarity with other faces, and we recover its coordinates; and (C) we combine CI features proportionately with the coordinates of the new face to achieve reconstruction. Thus, as long as we can estimate the position of a stimulus in face space we are able to produce an approximation of its visual appearance. Images reproduced from refs. 46–50.
First, we systematically left out each facial identity and estimated the similarity space for the remaining 59 identities. This space is characterized by a set of visual features corresponding to each dimension as well as an average face located at the origin of the space. Second, the left-out identity was projected into this space based on its neural or behavioral similarity with the other faces, and its coordinates were retrieved for each dimension. Third, significant features were weighted by the corresponding coordinates and linearly combined along with the average face to generate an image reconstruction. This procedure was carried out separately for behavioral data and for each ROI to generate exemplars with both emotional expressions. Last, reconstructions were combined across all ROIs to generate a single set of neural-based reconstructions (SI Text).
Reconstruction accuracy was quantified both objectively, with the use of a low-level L2 similarity metric, and behaviorally, by asking naïve participants to identify the correct identity of a stimulus from two different reconstructions using a two-alternative forced choice task.
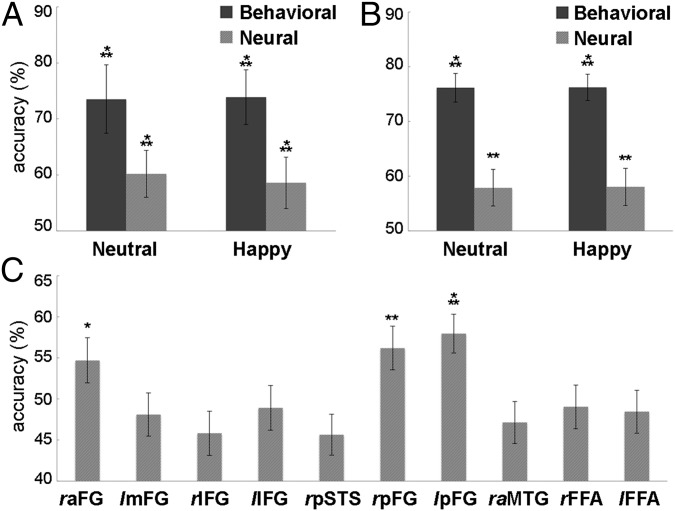
Overall, we found that reconstructions for each emotional expression were accurate above chance by either type of evaluation and emotional expression (one-sample t tests against chance) (see Fig. 4 for reconstruction exemplars and Fig. 5 A and B for accuracy estimates). Behavioral estimates surpassed their neural counterparts in accuracy for both evaluation metrics (P < 0.01, two-way analysis of variance across data types and emotional expression); no difference across expression and no interaction with data type were found. Additional analyses found significant variation in objective accuracy across the 10 ROIs (P < 0.01, one-way analysis of variance). Interestingly, further tests against chance-level performance showed that only three ROIs in the bilateral FG provided significant accuracy estimates (Fig. 5C).
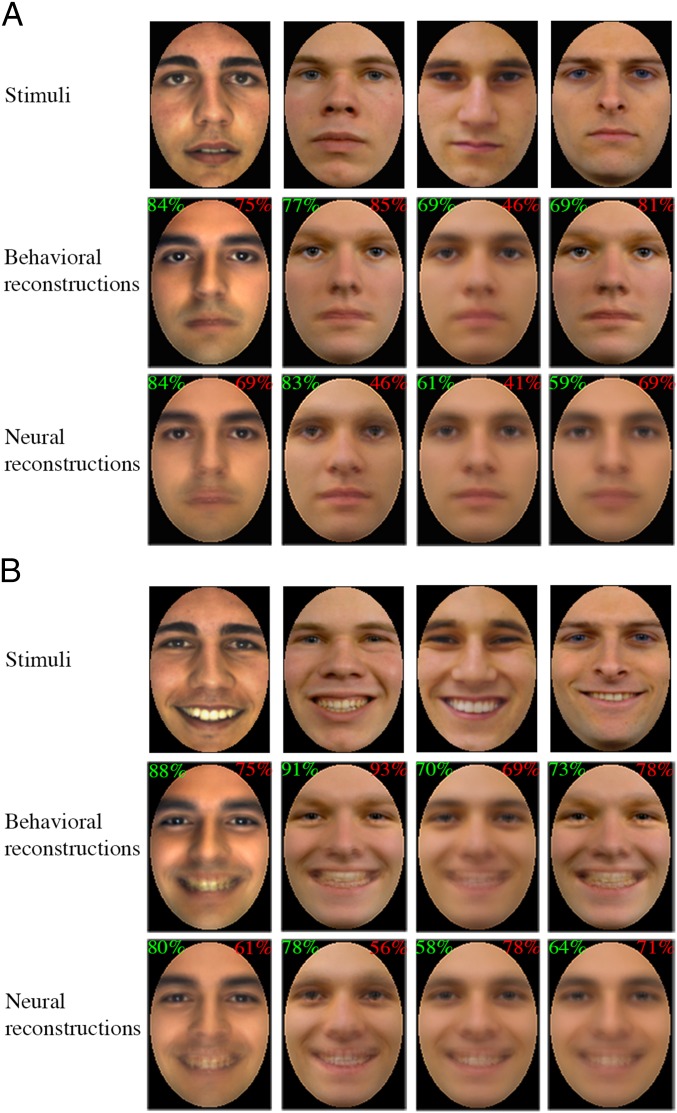
Fig. 4.
Examples of face stimuli and their reconstructions from behavioral and fMRI data across (A) neutral and (B) happy expressions. Numbers in the top corners of each reconstruction show its average experimentally based accuracy (green) along with its image-based accuracy (red). Images reproduced from refs. 48–50.
Fig. 5.
Reconstruction accuracy using (A) experimentally based and (B) image-based estimates of behavioral and neurally derived reconstructions. Image-based estimates are also separately shown for each ROI collapsed across expressions (C). Error bars show ±1 SE across (A) participants and (B and C) reconstructions (*P < 0.05; **P < 0.01; ***P < 0.001). FG, fusiform gyrus; IFG, inferior frontal gyrus; MTG, middle temporal gyrus; STS, superior temporal sulcus.
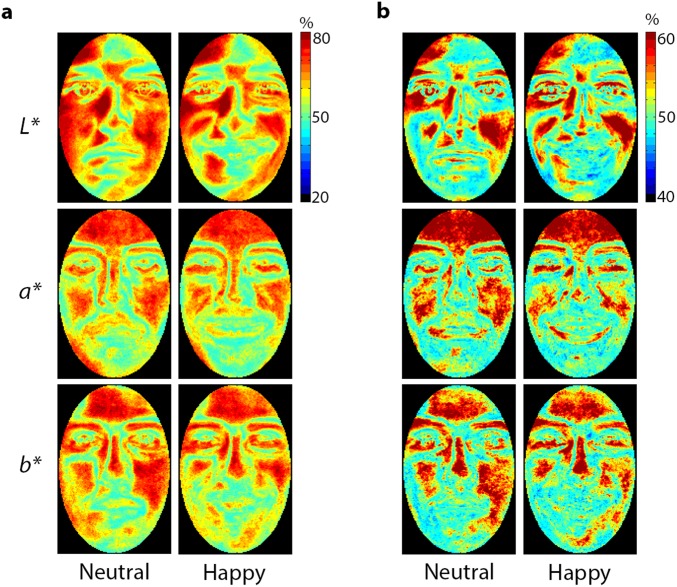
Next, we constructed pixelwise accuracy maps separately for each color channel and data type to quantify reconstruction quality across the structure of a face (Fig. S2). In agreement with our evaluation of visual features, the results suggest that a variety of shape and surface properties across all color channels contribute to reconstruction success.
Fig. S2.
Heat maps of pixelwise accuracy for behavioral (A) and neurally derived (B) reconstructions separately for neutral and happy faces. The color of each pixel indicates the proportion of times a pixel at that location accurately matched a stimulus with its reconstruction.
To compare behavioral and neural reconstructions, we related accuracy estimates for the two types of data across face exemplars. Overall, we found significant correlations for both experimentally derived estimates of accuracy (Pearson correlation; r = 0.39, P < 0.001) and for image-based estimates (r = 0.45, P < 0.001), confirming a general correspondence between the two types of data. Similar results were also confirmed by comparing image-based estimates of accuracy for the ROIs capable of supporting reconstruction and their behavioral counterpart (right aFG, r = 0.45; right pFG, r = 0.56; left pFG, r = 0.44; P < 0.001).
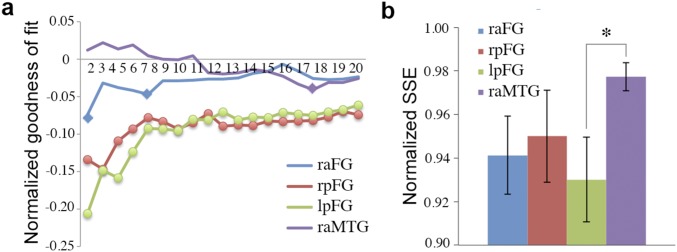
Further, a more thorough examination of the ROIs involved in reconstruction considered the similarity of their MDS spaces to their behavioral counterpart. Specifically, we computed the goodness of fit for Procrustes alignments between neural and behavioral spaces. This analysis found a systematic correspondence between the two types of space (Fig. S3A), especially for the bilateral posterior FG (P < 0.001, two-tailed permutation test). Also, fit estimates for FG regions, but not for a control ROI in the right aMTG, were adversely impacted by increases in space dimensionality as revealed by positive correlations between alignment error and the number of dimensions (right aFG, r = 0.67; right pFG, r = 0.71; left pFG, r = 0.81; P < 0.01). Last, a closer examination of 2D space alignments within each participant (Fig. S3B) showed significant variation in fit estimates across ROIs (P < 0.05, one-way analysis of variance); pairwise comparisons also found that the left pFG provided a better fit with the behavioral data than the right aMTG (t7 = 3.09, P < 0.05).
Fig. S3.
Goodness of fit for behavioral–neural face space alignment. (A) Normalized SSE as a function of space dimensionality. A permutation baseline was computed in each case and subtracted from the data so that the horizontal axis can indicate chance performance uniformly relative to each data point. Error is minimized for low-dimensional solutions in FG ROIs. Markers indicate lower-than-chance error (diamonds, P < 0.01; circles, P < 0.001; permutation test). (B) Normalized SSE for 2D space alignment across individual participants (no permutation-derived baseline was subtracted from the data in this case). Error bars show ±1 SE across participants (*P < 0.05; paired t test). FG, fusiform gyrus; MTG, middle temporal gyrus.
To conclude, visual features derived from neural or behavioral data were capable of supporting facial image reconstruction, with a good degree of agreement between the two, although neural reconstructions were driven primarily by bilateral FG activation.
Discussion
How is facial identity represented by the human visual system? To address this question, we undertook a comprehensive investigation that combines multiple, converging methods in the study of visual recognition, as detailed below.
Cortical Mapping of Facial Identity.
Growing sophistication in the analysis of neuroimaging data has facilitated the mapping of the neural correlates of face identification. The examination of face-selective cortex has implicated areas of the FG, STS, and the anterior temporal lobe (ATL) in identification (29–31). Recent investigations relying on multivoxel pattern analysis have extended this work by identifying regions responding with separable patterns of activation to different facial identities, regardless of whether they are accompanied by face selectivity (7, 19, 22, 23, 32).
In contrast to previous studies, which have explored the neural code associated with a relatively small number of facial identities, the present study examines the neural and psychological representations underlying an extensive, homogeneous set of unfamiliar faces. This constitutes an exacting test of identity mapping based on fine-grained sensitivity to perceptual differences.
Consistent with previous studies, our investigation found above-chance discrimination in multiple FG, IFG, and STS regions as well as in the FFA. However, the ability of a region to support identity discrimination does not necessarily imply that it encodes visual face representations. Higher level semantic information (33) or even a variety of unrelated task/stimulus properties may account for pattern discrimination (34). The latter possibility is a source of concern, especially given certain limitations of the fMRI signal in decoding facial identity (35).
The present findings address this concern by demonstrating that at least certain regions localized via pattern-based mapping contain visual information critical for facial identification. Specifically, three regions of the posterior and anterior FG were able to support identifiable reconstructions of face images. Interestingly, the FFA did not support similar results. However, recent work has shown that the FFA is particularly sensitive to templates driving face detection (36) and can even support the visual reconstruction of such templates (27). Thus, the current results agree with the involvement of the FFA primarily in face detection and, only to a lesser extent, in identification (37, 38). Also, the inability of more anterior regions of the IFG and aMTG to support image reconstruction is broadly consistent with their involvement in processing higher level semantic information (39).
Thus, our results confirm that facial identification relies on an entire network of cortical regions, and importantly, they point to multiple FG regions as responsible for encoding image-based visual information critical for the representation of facial identity.
Human Face Space and Its Visual Features.
What properties dominate the organization of human face space? To be clear, our investigation does not target the entirety of face space but rather a specific subdomain: young adult Caucasian males. Furthermore, we avoid large differences in appearance due to hair, outer contour, or aspect ratio, which are obvious, well-known cues to recognition (2, 40). Instead, we reason that understanding the structure of face representation in a carefully restricted domain challenges the system maximally and is instrumental in understanding recognition at its best. Our expectation, though, is that the principles revealed here generalize to face recognition as a whole.
A combination of MDS and CI analyses allowed us to assess and visualize the basic organization of face space in terms of both shape and surface properties. Our results reveal a host of visual properties across multiple areas of the face and across different color channels. Notably, we find evidence for the role of eyebrow salience, nose shape, mouth size, and positioning, as well as for the role of skin tone. Interestingly, these results agree with previous behavioral work; for instance, the critical role of the eyebrows in individuation has been specifically tested and confirmed (41). Also, several of the properties above appear to be reflected in the structure of both behavioral and neural data.
At the same time, we note that our analyses reveal only a handful of significant features relying on low-dimensional spaces, whereas human face space is believed to be high-dimensional (42). Critically, the number of significant dimensions recovered from the data depends on the signal-to-noise ratio (SNR) of the data as well as on the size of the stimulus set. Here, the restricted number of trials (e.g., 10 presentations per stimulus during scanning) imposes direct limitations on the SNR and allows only the estimation of the most robust features and of the dimensions associated with them. Hence, the current results do not speak directly to the dimensionality of face space, but they do open up the possibility of its future investigation with the aid of more advanced imaging techniques and designs.
Last, regarding the presence of visual information across multiple color channels, we note that, traditionally, the role of color in face identification has been downplayed (43, 44). However, recent work has pointed to the value of color in face detection (12) and even in identification (13). As previously suggested (45), color may aid identification when the availability of other cues is diminished. More generally, the difficulty of the task, as induced here by the homogeneity of the stimulus set, could lead to the recruitment of relevant color cues. From a representational standpoint, the current findings suggest that color information is included in the structure of face space and, thus, available when needed. However, additional investigations targeting this hypothesis are needed to ascertain its precise scope and its validity.
In sum, the present results establish the relevance of specific properties, their relative contribution to molding the organization of face space, and conversely, our ability to derive them from neural and behavioral data. More generally, we conclude that specific perceptual representations are encoded in high-level visual cortex and that these representations are fundamentally structured by the visual properties described here.
Facial Identity and Image Reconstruction.
The fundamental idea grounding our reconstruction method is that, as long as the relative position of an identity in face space is known, its visual appearance can be reconstructed from that of other faces in that space. In a way, our method validates the classic concept of face space (6) by making it instrumental in the concrete enterprise of image reconstruction. Also, from a practical perspective, the presumed benefit of this approach is a more efficient reconstruction method relying on empirically derived representations rather than on hypothetical, prespecified features. For instance, our reconstruction procedure involves only a handful of features (SI Text) whose relevance for representing facial identity is ensured by the process of their derivation and selection. The successful reconstruction of face images drawn from a homogeneous stimulus set provides strong support for this method.
Overall, the application of reconstruction to both behavioral and fMRI data has extensive theoretical and methodological implications. Theoretically, it points to the general correspondence of face space structure across behavioral and neural data; at the same time, it highlights the variation of face space across different cortical regions, only some of which contain relevant visual information. Methodologically, the generality and the robustness of the current approach allow its extension to other neuroimaging modalities as well as to data gleaned from patient populations (e.g., to examine distortions of visual representations in prosopagnosia or autism). Thus, our reconstruction results not only provide specific information about the nature of face space but also allow a wide range of future investigations into visual representations and their application to image reconstruction.
In conclusion, our work sheds light on the representational basis of face recognition regarding its cortical locus, its underlying features, and their visual content. Our findings reveal a range of shape and surface properties dominating the organization of face space, they show how to synthesize these properties into image-based features of facial identity, they establish a general method for using these features in image reconstruction, and last, they validate their behavioral and neural plausibility. More generally, this work demonstrates the strengths of a multipronged multivariate paradigm that brings together functional mapping, investigations of behavioral and neural similarity space, as well as feature derivation and image reconstruction.
Methods
Stimuli and Design.
A total of 120 images of adult Caucasian males (60 identities × 2 expressions) were selected from multiple face databases and further processed to ensure their homogeneity.
Eight right-handed Caucasian adults participated in nine 1-h experimental sessions (four behavioral and five fMRI). During behavioral sessions, participants viewed pairs of faces, presented in succession, and judged whether they represented the same/different individuals. During fMRI scans, participants performed a continuous one-back version of the same task using a slow event-related design (8-s trials). We imaged 27 oblique slices covering the ventral cortex at 3T [2.53 mm voxels, 2 s time-to-repeat (TR)]. Informed consent was obtained from all participants, and all procedures were approved by the Institutional Review Board of Carnegie Mellon University.
Pattern-Based Brain Mapping.
Multivoxel pattern-based mapping was performed by walking a spherical searchlight voxel-by-voxel across a cortical mask (see Fig. S1A for a group mask). At each location, a single average voxel pattern was extracted per run for every facial identity. To estimate neural discriminability, linear SVM classification was applied across these patterns for each identity pair using leave-one-run-out cross-validation. Then, participant-specific maps were constructed by voxel-wise averaging of discrimination estimates across identity pairs. For the purpose of group analysis, all maps were brought into Talairach space, and statistical effects were computed across participants (two-tailed t test against chance).
Similarity Structure Analyses.
Behavioral estimates of pairwise face (dis)similarity were computed based on the average discrimination accuracy of each participant during behavioral sessions. Homologous neural-based estimates were computed with the aid of pattern classification in different ROIs based on average discrimination sensitivity. For both data types, this procedure yielded a vector of 1,770 pairwise discrimination values.
Further, discrimination vectors were encoded as facial dissimilarity matrices. These matrices were then averaged across participants and analyzed by metric MDS. To interpret the perceptual variation encoded by MDS dimensions (Fig. 1 A and C), individual faces were averaged on each side of the origin proportionally to their dimension-specific coefficients. The resulting templates were assessed using a reverse correlation approach (26). Concretely, each pair of templates thus obtained were subtracted from each other to derive a CI summarizing the perceptual differences specific to that dimension. Each CI was next analyzed, pixel-by-pixel, by comparison with a group of randomly generated CIs (t test; q < 0.05 correction across pixels). This analysis was separately conducted for the L*, a*, and b* components of face images.
Image Reconstruction Method.
For every facial identity, an independent estimate of face space was constructed through MDS using all other identities. Then, the left-out identity was projected in this space via Procrustes alignment. Concretely, the MDS solution derived for all 60 identities was mapped onto the first solution, providing us with the coordinates of the target face in the original face space. The resulting coordinates are next used to weight the contribution of significant CIs in the reconstruction process; relevant CIs are selected based on the presence of significant pixels in any of three color channels via a permutation test (FDR correction across pixels; q < 0.10). The linear combination of significant CIs along with that of an average face is used to approximate the visual appearance of the target face. This method was conducted separately for 10 ROIs and for behavioral data. Last, a single set of neural reconstructions was derived through the linear combination of ROI-specific reconstructions via an L2 regularized regression model and a leave-one-identity-out procedure.
Neural and Behavioral Face Space Correspondence.
The global correspondence between the two types of face space was assessed by bringing ROI-specific spaces into alignment with behavioral space. Goodness of fit was then estimated via sum of squared errors (SSE) between Procrustes-aligned versions of neural space and behavioral space. Fit estimates were compared with chance across systematic differences in MDS-derived space dimensionality (from 2 to 20 dimensions) via permutation tests. Last, fit estimates across ROIs were compared with each other through parametric tests across participants.
Participants
Ten right-handed Caucasian adults (five female; age, 20–34 y), with normal or corrected-to-normal vision and no history of neurological disorder, volunteered for the study. However, one participant exhibited excessive head movements, and one was unable to complete all nine experimental sessions; hence, analyses were carried out on data from the remaining eight participants.
Stimuli
Color face images were extracted from four different databases: FERET (46, 47), FEI (48), AR (49), and Radboud (50). (Portions of the research in this paper use the FERET database of facial images collected under the FERET program, sponsored by the Department of Defense Counterdrug Technology Development Program Office.) These images displayed front views of Caucasian young adult males with frontal gaze, frontal lighting, and no facial hair, salient features, or other accessories. All individuals had short hair that did not obscure the forehead and they displayed two different expressions: neutral and happy (open-mouth smile). These images were spatially normalized with the position of the eyes, were cropped to eliminate background/hair, and were normalized with the same mean and root-mean-square contrast separately for each color channel in CIEL*a*b* color space. In all, 120 images were thus obtained.
Behavioral and fMRI Procedures
Before scanning, participants completed four sessions of behavioral testing. On each trial, participants viewed pairs of faces and judged whether they displayed the same or different individuals. Each participant was presented with all possible pairs of facial identities across the course of the experiment. Of note, each pair of faces consisted of a neutral version of one individual and the happy expression of the same or another individual; the use of different expressions aimed to preclude a single-image matching strategy and, thus, to minimize reliance on simple low-level cues.
Concretely, on any given trial, a fixation cross was displayed first for 100 ms, and then the two face images in a stimulus pair were presented sequentially, each for 400 ms, with no interstimulus lag. The two stimuli appeared on different sides of fixation and subtended a visual angle of 3.1° × 4.6°. Participants were asked to make a response as soon as the second stimulus in a pair appeared by pressing one of two designated keys. In all, 2,360 trials (including 480 “same” trials) were collected for each participant across 20 experimental blocks (five blocks per behavioral session). Although the homogeneity of the dataset contributed to task difficulty, the performance of the subjects was still relatively high (d’ ± 1 SE, 2.82 ± 0.20).
During fMRI scanning, participants performed a one-back identity task compatible with a slow event-related design. Each event started with a bright fixation cross, displayed for 100 ms, followed by a single image presented centrally for 900 ms, followed by a dimmer fixation cross for another 7 s. Any given run contained a sequence of 60 such events, preceded by 8 s of fixation (for a total of 488 s). On 10% of all trials, the previous image of an individual was followed by the other image of the same individual; facial identities used on the same events were counterbalanced across scans. In all, 20 (or 22) such runs were conducted across five 1-h imaging sessions for a total of 1,200 (or 1,320) trials.
In addition, one standard localizer run was included in each session. Localizer scans contained blocks of images grouped by category: faces, common objects, houses, and scrambled images. Each block consisted of back-to-back presentations of 16 stimuli for a total of 16 s. Stimulus blocks were separated by 8 s of fixation and were preceded by an 8-s fixation interval at the beginning of each run. Any given localizer run contained 12 stimulus blocks, three per stimulus category, and had a total duration of 296 s. Last, one anatomical scan was collected in each session for the purpose of coregistration and cross-session alignment.
All nine experimental sessions, behavioral and fMRI, were conducted on separate days for each participant.
fMRI Acquisition
MRI data were acquired using a Siemens Verio 3T scanner with a 32-channel coil at Carnegie Mellon University. An echoplanar imaging sequence was used to acquire 27 oblique slices covering the ventral cortex with 2.5-mm isotropic voxels; TR, 2 s; time-to-echo, 30 ms; GRAPPA (generalized autocalibrating partial parallel acquisition) acceleration factor, 2 (with 30% phase oversampling); flip angle, 79°; and field of view, 240 × 240 mm2. An MPRAGE (magnetization prepared rapid acquisition gradient echo) anatomical scan was acquired at the end of each session, consisting of 176 axial slices covering the entire brain with 1-mm isotropic voxels; TR, 2.3 s; time-to-echo, 1.97 ms; flip angle, 9°; and field of view, 256 × 256 mm2.
fMRI Data Preprocessing and Standard Univariate Analysis
Functional scans were slice scan time-corrected, motion-corrected, coregistered to the same anatomical image, and normalized to percentage of signal change using AFNI (51). Functional localizer data were smoothed with a Gaussian kernel of 7.5 mm FWHM (full width at half maximum). No spatial smoothing was performed on the rest of the data to allow multivariate analysis to exploit high-frequency information. Slow event-related data were linearly detrended before pattern analyses. Last, the results of subject-specific analyses were brought into Talairach space for the purpose of further group analyses.
After completing all preprocessing steps, we discarded the first three volumes of each run to allow hemodynamics to achieve a steady state. Next, we fitted each type of block, by stimulus category, with a boxcar predictor and convolved it with a gamma hemodynamic response function. A general linear model was used to estimate the coefficient of each predictor separately for each voxel. Statistical maps were computed by pairwise contrasts between different block types. Correction for multiple comparisons was implemented using the FDR.
Pattern-Based Brain Mapping
Multivoxel pattern-based mapping was carried out across a ventral cortical mask constructed separately for each participant. The mask was derived by intersecting a coarse cortical mask with an SNR mask including only voxels that deviated from baseline on at least half of the trials (see Fig. S1A for the corresponding conjunction-based group-level mask). Concretely, a spherical searchlight with a 5-voxel radius was centered on each voxel within the mask and intersected with the mask to restrict the analysis to cortical/informative voxels. As suggested by previous research (23), the present size of the searchlight (e.g., 12.5 mm) represents a compromise between maximizing its informational content and maintaining adequate regional specificity (e.g., posterior, middle, and anterior parts of the FG can be captured by different, nonoverlapping searchlights).
To construct identity-specific patterns, activation values corresponding to each stimulus presentation were extracted at three different time points (4, 6, and 8 s after stimulus onset) for each voxel within a searchlight. Further, to boost the SNR of the resulting patterns, we computed their average both in time (i.e., across the three time points mentioned above) and across expressions separately for each facial identity; that is, the two patterns elicited during the same functional run by different images of an individual were combined into a single pattern. This procedure produced 10 or 11 different observations per facial identity for each participant. To estimate identity discriminability, we applied multiclass linear SVM classification across these patterns using a one-against-one approach; that is, each facial identity was compared with every other identity separately, one at a time. Leave-one-run-out cross-validation was carried out for each pair of facial identities, and identity discriminability was estimated for each pair. Specifically, each of 10 (or 11) presentations of an identity pair was left out one at a time for cross-validation purposes, and classification results were encoded as sensitivity estimates (d’).
Next, information maps were constructed for each participant by voxelwise averaging of sensitivity estimates across all identity pairs. For the purpose of group analysis, all such maps were brought into Talairach space, and a single group map was obtained by averaging participant-specific maps. Statistical effects were computed by means of a two-tailed one-sample t test against chance (50% accuracy), and results were assessed after thresholding the map following multiple comparisons correction (FDR correction; q < 0.05).
The mapping procedure above found seven main regions exhibiting above-chance levels of discriminability in the bilateral FG and IFG and the right STS (Fig. S1B). An additional ROI was found in the aMTG (Talairach coordinates, 28, 1, –36) after replacing the original threshold with a more liberal one (q < 0.10). Next, the resulting eight ROIs in the group map were identified again in each participant (q < 0.05) for the purpose of further analyses.
The procedure above was conducted for three additional ROIs: the bilateral FFA and the EVC. To render classification methods comparable, a spherical mask with a 5-voxel radius was placed at the selectivity peak of the FFA (as defined by a face–house contrast from our univariate localizer). A similar mask was centered in the calcarine sulcus of each participant (at the peak of a scrambled objects–objects contrast), and the resulting ROI was used as a rough approximation of EVC. Classification analyses confirmed above-chance discrimination estimates in the bilateral FFA (one-sample t test against chance; P < 0.05) but not in the EVC (P > 0.05). Thus, all subsequent analyses considered the bilateral FFA in addition to areas localized through multivariate mapping for a total of 10 distinct ROIs.
Face Space–Similarity Structure Analyses and Feature Derivation
Briefly, the face space framework proposes that distinct faces can be conceptualized as points in a multidimensional metric space organized around an average face lying at the origin of this space. The distance of an individual face from the origin immediately accounts for its distinctiveness, whereas its angle within a given coordinate system is representative of a particular combination of facial properties. Thus, faces lying on the same line crossing the origin would only vary across a set combination of properties proportionally with their respective distance from the origin. Although the general characterization of face space is largely accepted, the precise topography of the space, its dimensionality, and the nature of the features defining its dimensions remain topics of active research.
The present work focuses on a specific set of features underlying an image-based visual subspace. More clearly, we investigate visual features of face space sufficiently robust to be captured via a linear image-based approach. For this purpose, first, we derive face space estimates from behavioral and neural data, and second, we assess the possibility of extracting relevant features from such estimates as detailed below.
Behavioral estimates of pairwise face (dis)similarity were computed based on the average accuracy of each participant at discriminating facial identities across variation in expression during behavioral sessions. In turn, neural estimates of face dissimilarity, based on fMRI data, were computed with the aid of pattern discrimination in different ROIs (see Pattern-Based Brain Mapping in the Supporting Information above). For both behavioral and fMRI data, this procedure yields a vector of 1,770 values corresponding to all possible identity pairs. The resulting vectors were encoded then as identity dissimilarity matrices, averaged across participants, and analyzed using metric MDS (assuming pairwise distances could be reasonably approximated with a Euclidean metric).
The 2D MDS solutions were plotted to provide a coarse visualization of the organization of face space (Fig. 1 A and C). To interpret the perceptual variation encoded by MDS dimensions, dimension-specific coefficients were, first, normalized to z scores; second, separated into negative and positive values; and, third, used to construct average face templates by combining individual face stimuli proportionally to their corresponding coefficients. Then, the difference of each pair of templates was treated as a CI. To be clear, “classification” here bears no conceptual relationship with pattern classification as used in our multivoxel localization procedure. Instead, it refers to the reverse correlation approach, as applied to the study of vision, of classifying and combining images based on the empirical responses that they elicit. In the present case, CIs serve to account for the organization of behavioral and neural face space as revealed by MDS analyses.
Each CI was next analyzed, pixel-by-pixel, by comparison with a group of randomly generated CIs. Specifically, all 60 identities are permuted with respect to their coefficients on each dimension, and a CI is computed for each of 103 such permutations. Then, the value of every pixel in the original CI is compared with the distribution of values corresponding to that pixel location in random CIs (two-tailed t test; FDR correction across pixels, q < 0.05). This analysis was separately conducted for the L*, a*, and b* components of face images. Examples of raw CIs and their analysis are shown in Fig. 2 for behavioral and right aFG data.
Image Reconstruction—Procedure
The reconstruction approach proposed here takes advantage of the conceptual framework of face space and rests on the idea that the appearance of a face can be approximated from that of other faces within a relevant visual face subspace. For this purpose, three types of information are needed: (i) a robust set of visual features corresponding to one or more dimensions of face space, (ii) an approximation of an average face at the origin of the space, and (iii) the coordinates of the target face within the relevant subspace. This information was acquired as follows.
First, any given facial identity was left out and an MDS solution was estimated based on the similarity matrix corresponding to the remaining 59 identities (across both expressions). Second, CI-based features for each expression were computed for the first 20 dimensions (typically accounting for more than 95% of the variance in the data). The resulting features were analyzed for the presence of significant pixels in any of the three color channels via the permutation test described above. Significant features could thus be selected to define a restricted visual subspace relevant for reconstruction purposes. Third, an average face was computed in this subspace from a weighted average of all 59 faces, where the weights were inversely proportional to the distance of each face from the origin. Fourth, a separate MDS solution was constructed for all 60 faces and aligned with the original one via Procrustes analysis. The alignment provides us with a mapping between the two spaces that allows us to retrieve the coordinates of the target face in the original space. Last, we computed a weighted sum of features, proportionally to the coordinates above, and added the result to the average face characterizing the origin of the space. The outcome of this procedure is used to approximate the visual appearance of the target face (converted to standard RGB color space for visualization purposes).
The method above was applied, in turn, to all ROIs and to behavioral data to reconstruct the visual appearance of both neutral and happy face exemplars. Also, for the purpose of combining ROI-specific reconstructions into a unique set of neural reconstructions, we deployed an L2 linear regularized regression model using a leave-one-identity-out schema (i.e., each model was fit using 59 identities × 2 expressions and was used to predict the reconstructions of the remaining two images of the same individual).
Importantly, we note here the significance of selecting relevant features along with the subspaces that they define. Overall, we found that behavioral reconstructions relied on a relatively small number of features (mean ± 1 SD, 3.26 ± 1.01) and so did neural reconstructions from ROIs able to support this task (right aFG, 3.31 ± 0. 90; right pFG, 2.03 ± 0.73; left pFG, 1.7 ± 0.67). As an alternative, one could consider a simplified version of the method. Specifically, target face images can simply be estimated as a weighted average of other faces within the entire face space, thus bypassing completely the process of feature computation and selection. However, not only is such a procedure uninformative about the features that enter the reconstruction process but also, by virtue of including additional, irrelevant information across all MDS dimensions, it leads to noisier, poorer estimates.
Last, we note that the visual features as well as the average faces used for the purpose of reconstructing any target face are derived from similarity data that exclude the target face. Thus, we ensure that dependency does not affect our reconstruction approach.
Image Reconstruction—Evaluation
Reconstruction results were assessed both objectively, with the use of a low-level image similarity metric, and behaviorally, with the use of additional psychophysical testing.
Objectively, we assessed image similarity between reconstructed images and the original stimuli with a pixel-wise L2 image metric. Specifically, any given stimulus was compared with its corresponding reconstruction and all other reconstruction of the same expression, one at a time. The results of each such comparison were considered correct when the similarity between the stimulus and its corresponding reconstruction was higher than the similarity between the stimulus and the alternative reconstruction. Next, for each set of reconstructions, the results of these comparisons, averaged across identities, were compared against chance (one-sample t tests). Last, we note that although other image metrics are common for measuring low-level image similarity, such as the L1 distance (52), their application did not significantly change the pattern of results reported here (Fig. 5).
Behaviorally, an analog of the estimation process above was conducted in a separate psychophysical experiment. For this purpose, a new group of 16 participants (7 female; age range, 18–23 y) was asked to identify the correct identity of a stimulus from two different reconstructions using a two-alternative forced choice task. On each trial, participants viewed a display containing the target stimulus (at the top) and two reconstructions (at the bottom), one of the same stimulus and one of a different stimulus. Both reconstructions were of the same type (either behavioral or fMRI-based) and showed the same expression. Each display was visible for 4 s or until the subject made a response. Participants were tested in separate blocks of trials with four complete sets of reconstructed stimuli corresponding to the type of underlying data (behavioral/fMRI) and the type of facial expression (neutral/happy) for a total of eight experimental blocks. Each experimental session was completed over the course of 1 h.
The Relationship Between Neural and Behavioral Face Representations
The correspondence between neural and behavioral data was assessed by comparing directly reconstruction accuracy across face exemplars for the two types of data via Pearson correlation (see Facial Image Reconstruction). However, this analysis only considers visual information that our methods can capture from face space structures via reverse correlation.
A different approach rests on comparing MDS dimensions one by one across the two data types (e.g., per Fig. 1C, Inset). However, the information contained by a single dimension in behavioral face space can be distributed across multiple dimensions in neural face space and vice versa. Further, an exhaustive comparison of all dimensions for all ROI data versus behavioral data may obscure the precise extent of the correspondence.
To address the limitations of the approaches above, another analysis was conducted aiming to match entire spaces to each other via Procrustes alignment rather than singled-out dimensions. Specifically, neural spaces were brought into alignment with behavioral space for a variable number of dimensions, from 2 to 20, and the goodness of fit was assessed by SSE. To allow the comparison of the fit across dimensions, SSE-based fit was further normalized by the sum of squared elements of a centered version of the target space—that is, behavioral space in the present case. Then, statistical significance of normalized alignment was assessed via a permutation test; the labels of each point in neural face space were randomly permuted before alignment. This analysis targeted the three FG ROIs involved in reconstruction and included, as a control, the right aMTG, an ROI that did not support reconstruction. Statistical significance was assessed for each ROI and for each number of dimensions (two-tailed permutation test, 103 iterations). Fig. S3A displays the results of this analysis. For convenience, permutation-based fit was subtracted from actual fit at each point so that the horizontal axis (i.e., a value of 0) corresponds to chance in every case.
Further, to compare the goodness of fit for different ROIs, Procrustes alignment was conducted separately for each participant by matching neural spaces onto their corresponding behavioral space. Because fit estimates were, overall, better for low-dimensional spaces (Fig. S3A) and, also, because reconstruction primarily relied on such spaces (Image Reconstruction—Procedure), this analysis focused on 2D MDS solutions. Normalized SSE-based fits were then compared across ROIs via paired comparisons (Fig. S3B).
All analyses reported above were carried out in Matlab with the aid of the Parallel Processing Toolbox running on a ROCKS+ multiserver environment.
Acknowledgments
This research was supported by the Natural Sciences and Engineering Research Council of Canada (A.N.), by a Connaught New Researcher Award (to A.N.), by National Science Foundation Grant BCS0923763 (to M.B. and D.C.P.), and by Temporal Dynamics of Learning Center Grant SMA-1041755 (to M.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514551112/-/DCSupplemental.
References
- 1.Freiwald WA, Tsao DY, Livingstone MS. A face feature space in the macaque temporal lobe. Nat Neurosci. 2009;12(9):1187–1196. doi: 10.1038/nn.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston RA, Milne AB, Williams C, Hosie J. Do distinctive faces come from outer space? An investigation of the status of a multidimensional face-space. Vis Cogn. 1997;4:59–67. [Google Scholar]
- 3.Leopold DA, O’Toole AJ, Vetter T, Blanz V. Prototype-referenced shape encoding revealed by high-level aftereffects. Nat Neurosci. 2001;4(1):89–94. doi: 10.1038/82947. [DOI] [PubMed] [Google Scholar]
- 4.Loffler G, Yourganov G, Wilkinson F, Wilson HR. fMRI evidence for the neural representation of faces. Nat Neurosci. 2005;8(10):1386–1390. doi: 10.1038/nn1538. [DOI] [PubMed] [Google Scholar]
- 5.O’Toole AJ. In: The Oxford Handbook of Face Perception. Calder AJ, Rhodes G, Johnson M, Haxby JV, editors. Oxford Univ Press; Oxford: 2011. pp. 15–30. [Google Scholar]
- 6.Valentine T. A unified account of the effects of distinctiveness, inversion, and race in face recognition. Q J Exp Psychol A. 1991;43(2):161–204. doi: 10.1080/14640749108400966. [DOI] [PubMed] [Google Scholar]
- 7.Goesaert E, Op de Beeck HP. Representations of facial identity information in the ventral visual stream investigated with multivoxel pattern analyses. J Neurosci. 2013;33(19):8549–8558. doi: 10.1523/JNEUROSCI.1829-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris A, Aguirre GK. Neural tuning for face wholes and parts in human fusiform gyrus revealed by FMRI adaptation. J Neurophysiol. 2010;104(1):336–345. doi: 10.1152/jn.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Harris A, Kanwisher N. Perception of face parts and face configurations: An FMRI study. J Cogn Neurosci. 2010;22(1):203–211. doi: 10.1162/jocn.2009.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer D, et al. Neural correlates of processing facial identity based on features versus their spacing. Neuropsychologia. 2007;45(7):1438–1451. doi: 10.1016/j.neuropsychologia.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Issa EB, DiCarlo JJ. Precedence of the eye region in neural processing of faces. J Neurosci. 2012;32(47):16666–16682. doi: 10.1523/JNEUROSCI.2391-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bindemann M, Burton AM. The role of color in human face detection. Cogn Sci. 2009;33(6):1144–1156. doi: 10.1111/j.1551-6709.2009.01035.x. [DOI] [PubMed] [Google Scholar]
- 13.Nestor A, Plaut DC, Behrmann M. Face-space architectures: Evidence for the use of independent color-based features. Psychol Sci. 2013;24(7):1294–1300. doi: 10.1177/0956797612464889. [DOI] [PubMed] [Google Scholar]
- 14.Miyawaki Y, et al. Visual image reconstruction from human brain activity using a combination of multiscale local image decoders. Neuron. 2008;60(5):915–929. doi: 10.1016/j.neuron.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Nishimoto S, et al. Reconstructing visual experiences from brain activity evoked by natural movies. Curr Biol. 2011;21(19):1641–1646. doi: 10.1016/j.cub.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thirion B, et al. Inverse retinotopy: Inferring the visual content of images from brain activation patterns. Neuroimage. 2006;33(4):1104–1116. doi: 10.1016/j.neuroimage.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 17.Naselaris T, Prenger RJ, Kay KN, Oliver M, Gallant JL. Bayesian reconstruction of natural images from human brain activity. Neuron. 2009;63(6):902–915. doi: 10.1016/j.neuron.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowen AS, Chun MM, Kuhl BA. Neural portraits of perception: Reconstructing face images from evoked brain activity. Neuroimage. 2014;94:12–22. doi: 10.1016/j.neuroimage.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104(51):20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Der Heide RJ, Skipper LM, Olson IR. Anterior temporal face patches: A meta-analysis and empirical study. Front Hum Neurosci. 2013;7:17. doi: 10.3389/fnhum.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anzellotti S, Fairhall SL, Caramazza A. Decoding representations of face identity that are tolerant to rotation. Cereb Cortex. 2014;24(8):1988–1995. doi: 10.1093/cercor/bht046. [DOI] [PubMed] [Google Scholar]
- 23.Nestor A, Plaut DC, Behrmann M. Unraveling the distributed neural code of facial identity through spatiotemporal pattern analysis. Proc Natl Acad Sci USA. 2011;108(24):9998–10003. doi: 10.1073/pnas.1102433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kriegeskorte N, et al. Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron. 2008;60(6):1126–1141. doi: 10.1016/j.neuron.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dailey MN, Cottrell GW, Padgett C, Adolphs R. EMPATH: A neural network that categorizes facial expressions. J Cogn Neurosci. 2002;14(8):1158–1173. doi: 10.1162/089892902760807177. [DOI] [PubMed] [Google Scholar]
- 26.Murray RF. Classification images: A review. J Vis. 2011;11(5):11. doi: 10.1167/11.5.2. [DOI] [PubMed] [Google Scholar]
- 27.Nestor A, Vettel JM, Tarr MJ. Internal representations for face detection: An application of noise-based image classification to BOLD responses. Hum Brain Mapp. 2013;34(11):3101–3115. doi: 10.1002/hbm.22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith ML, Gosselin F, Schyns PG. Measuring internal representations from behavioral and brain data. Curr Biol. 2012;22(3):191–196. doi: 10.1016/j.cub.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 29.Fox CJ, Moon SY, Iaria G, Barton JJ. The correlates of subjective perception of identity and expression in the face network: An fMRI adaptation study. Neuroimage. 2009;44(2):569–580. doi: 10.1016/j.neuroimage.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 31.Rotshtein P, Henson RN, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nat Neurosci. 2005;8(1):107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- 32.Natu VS, et al. Dissociable neural patterns of facial identity across changes in viewpoint. J Cogn Neurosci. 2010;22(7):1570–1582. doi: 10.1162/jocn.2009.21312. [DOI] [PubMed] [Google Scholar]
- 33.Çukur T, Huth AG, Nishimoto S, Gallant JL. Functional subdomains within human FFA. J Neurosci. 2013;33(42):16748–16766. doi: 10.1523/JNEUROSCI.1259-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todd MT, Nystrom LE, Cohen JD. Confounds in multivariate pattern analysis: Theory and rule representation case study. Neuroimage. 2013;77:157–165. doi: 10.1016/j.neuroimage.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Dubois J, de Berker AO, Tsao DY. Single-unit recordings in the macaque face patch system reveal limitations of fMRI MVPA. J Neurosci. 2015;35(6):2791–2802. doi: 10.1523/JNEUROSCI.4037-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilad S, Meng M, Sinha P. Role of ordinal contrast relationships in face encoding. Proc Natl Acad Sci USA. 2009;106(13):5353–5358. doi: 10.1073/pnas.0812396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nestor A, Vettel JM, Tarr MJ. Task-specific codes for face recognition: How they shape the neural representation of features for detection and individuation. PLoS One. 2008;3(12):e3978. doi: 10.1371/journal.pone.0003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong F, Nakayama K, Moscovitch M, Weinrib O, Kanwisher N. Response properties of the human fusiform face area. Cogn Neuropsychol. 2000;17(1):257–280. doi: 10.1080/026432900380607. [DOI] [PubMed] [Google Scholar]
- 39.Simmons WK, Reddish M, Bellgowan PS, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 2010;20(4):813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mondloch CJ, Le Grand R, Maurer D. Configural face processing develops more slowly than featural face processing. Perception. 2002;31(5):553–566. doi: 10.1068/p3339. [DOI] [PubMed] [Google Scholar]
- 41.Sadr J, Jarudi I, Sinha P. The role of eyebrows in face recognition. Perception. 2003;32(3):285–293. doi: 10.1068/p5027. [DOI] [PubMed] [Google Scholar]
- 42.Sirovich L, Meytlis M. Symmetry, probability, and recognition in face space. Proc Natl Acad Sci USA. 2009;106(17):6895–6899. doi: 10.1073/pnas.0812680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruce V, Young A. In the Eye of the Beholder: The Science of Face Perception. Oxford Univ Press; New York: 1998. [Google Scholar]
- 44.Kemp R, Pike G, White P, Musselman A. Perception and recognition of normal and negative faces: The role of shape from shading and pigmentation cues. Perception. 1996;25(1):37–52. doi: 10.1068/p250037. [DOI] [PubMed] [Google Scholar]
- 45.Yip AW, Sinha P. Contribution of color to face recognition. Perception. 2002;31(8):995–1003. doi: 10.1068/p3376. [DOI] [PubMed] [Google Scholar]
- 46.Phillips JP, Moon H, Rizvi SA, Rauss PJ. The FERET evaluation methodology for face-recognition algorithms. IEEE Trans Pattern Anal Mach Intell. 2000;22(10):1090–1104. [Google Scholar]
- 47.Phillips JP, Wechsler H, Huang J, Rauss PJ. The FERET database and evaluation procedure for face-recognition algorithms. Image Vis Comput. 1998;16(5):295–306. [Google Scholar]
- 48.Thomaz CE, Giraldi GA. A new ranking method for principal component analysis and its application to face image analysis. Image Vis Comput. 2010;28(6):902–913. [Google Scholar]
- 49.Martinez AR, Benavente R. 1998. The AR Face Database, CVC Technical Report #24. Available at www.cat.uab.cat/Public/Publications/1998/MaB1998/CVCReport24.pdf. Accessed December 18, 2015.
- 50.Langner O, et al. Presentation and validation of the Radboud Faces Database. Cogn Emotion. 2010;24(8):1377–1388. [Google Scholar]
- 51.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 52.Sinha P, Russell R. A perceptually based comparison of image similarity metrics. Perception. 2011;40(11):1269–1281. doi: 10.1068/p7063. [DOI] [PubMed] [Google Scholar]