Abstract
Background
Recent reports suggest that thymosin beta-4 (Tβ4) is a key regulator for wound healing and anti-inflammation. However, the role of Tβ4 in osteoclast differentiation remains unclear.
Purpose
The purpose of this study was to evaluate Tβ4 expression in H2O2-stimulated human periodontal ligament cells (PDLCs), the effects of Tβ4 activation on inflammatory response in PDLCs and osteoclastic differentiation in mouse bone marrow-derived macrophages (BMMs), and identify the underlying mechanism.
Methods
Reverse transcription-polymerase chain reactions and Western blot analyses were used to measure mRNA and protein levels, respectively. Osteoclastic differentiation was assessed in mouse bone marrow-derived macrophages (BMMs) using conditioned medium (CM) from H2O2-treated PDLCs.
Results
Tβ4 was down-regulated in H2O2-exposed PDLCs in dose- and time-dependent manners. Tβ4 activation with a Tβ4 peptide attenuated the H2O2-induced production of NO and PGE2 and up-regulated iNOS, COX-2, and osteoclastogenic cytokines (TNF-α, IL-1β, IL-6, IL-8, and IL-17) as well as reversed the effect on RANKL and OPG in PDLCs. Tβ4 peptide inhibited the effects of H2O2 on the activation of ERK and JNK MAPK, and NF-κB in PDLCs. Furthermore, Tβ4 peptide inhibited osteoclast differentiation, osteoclast-specific gene expression, and p38, ERK, and JNK phosphorylation and NF-κB activation in RANKL-stimulated BMMs. In addition, H2O2 up-regulated Wnt5a and its cell surface receptors, Frizzled and Ror2 in PDLCs. Wnt5a inhibition by Wnt5a siRNA enhanced the effects of Tβ4 on H2O2-mediated induction of pro-inflammatory cytokines and osteoclastogenic cytokines as well as helping osteoclastic differentiation whereas Wnt5a activation by Wnt5a peptide reversed it.
Conclusion
In conclusion, this study demonstrated, for the first time, that Tβ4 was down-regulated in ROS-stimulated PDLCs as well as Tβ4 activation exhibited anti-inflammatory effects and anti-osteoclastogenesis in vitro. Thus, Tβ4 activation might be a therapeutic target for inflammatory osteolytic disease, such as periodontitis.
Introduction
Bone loss associated with inflammatory diseases, such as rheumatoid arthritis, periodontal disease, and osteoporosis, and elevated osteoclast activity leads to bone destruction [1]. The most common osteolytic disease, periodontitis, is a multi-factorial irreversible and cumulative condition, initiated and propagated by bacteria and host factors [2]. Destruction of peridontal tissue is mediated via the expression of various tissue-destructive enzymes or inflammatory mediators such as interleukins-1 (IL-1), IL-6 and IL-8, tumor necrosis factor- α (TNF- α), nitric oxide (NO), and prostaglandin E2 (PGE2) [2]. Receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL) and osteoprotegerin (OPG) are critical for homeostatic control of osteoclast activity, suggesting that they have vital roles in the progression of bone loss in periodontitis [3, 4]. Therefore, resolution of inflammation and blocking osteoclast differentiation might be a potential therapeutic approach for the prevention and treatment of osteolytic inflammatory disease, such as periodontitis [5].
Thymosin beta-4 (Tβ4) is a water-soluble, 43-amino acid, and 4.9 kDa protein that was originally isolated from bovine thymus [6]. Since Tβ4 is the major actin-sequestering molecule in eukaryotic cells and is found in all cells [7], Tβ4 has multiple diverse cellular functions, including tissue development, migration, angiogenesis, and wound healing [7]. We previously reported that Tβ4-overexpressing transgenic mice, using a construct on the skin-specific keratin-5 promoter, have abnormal tooth development and enhanced stimulation of hair growth [8]. Moreover, exogenous Tβ4 has anti-inflammatory effects in the bleomycin-induced mouse model of lung fibrosis [9], tooth extraction sockets in rats [10], rat model of myocardial ischemia [11], corneal wound healing [12], wound healing of rat palatal mucosa [13], in vitro model of cultured human gingival fibroblasts [14], and cardiac fibroblasts [15]. However, the effects of Tβ4 over expression or inhibition on differentiation are controversial. Exogenous β4 peptide inhibited osteogenic differentiation but facilitated adipogenic differentiation in human bone marrow-derived-mesenchymal stem cells (MSCs) [16]. In contrast, Tβ4 inhibition by Tβ4 siRNA attenuated odontoblastic differentiation in the odontoblast-like cells, MDPC-23 [17]. Moreover, we recently demonstrated that odontoblastic differentiation was enhanced by activation of Tβ4 by Tβ4 peptide but was decreased by Tβ4 siRNA in human dental pulp cells (HDPCs) [18]. However, the effects of Tβ4 on osteoclastic differentiation have not been reported.
Moreover, Tβ4 concentration revealed wide variability, and it decreased in the gingival crevicular fluid (GCF) as periodontal disease progressed [19]. In contrast, Tβ4 mRNA expression was 3.76 fold higher in periodontitis-affected gingival tissue, compared with healthy individuals’ tissue obtained from public microarray data (GEO assession: GSE 23586) [20]. However, the Tβ4 mRNA level did not change in the periodontal-diseased gingival tissue (arbitrary units; 6.249) when compared with healthy tissue (arbitrary units; 6.242) (GEO assession: GSE 10334) [21]. Although Tβ4 exerts anti-inflammatory effects in vivo and in vitro, the precise role of Tβ4 in the inflammatory response remains unclear.
Therefore, this study was designed to investigate whether Tβ4 was up-regulated in patients with periodontitis, and this study was also designed to investigate whether Tβ4 inhibition or activation suppressed the osteoclastic differentiation in mouse bone marrow-derived macrophages (BMMs) and inflammatory response in periodontal ligament cells (PDLCs) as well as on their signaling pathways.
Materials and Methods
Cell culture
Established immortalized human PDLCs [22] that maintain the characteristics of primary PDLCs by transfecting human telomerase reverse transcriptase (hTERT) were used. These cell line were kindly provided by Professor Takashi Takata (Hiroshima University, Japan). Cells were cultured in α-MEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified atmosphere of 5% CO2 at 37°C. For the experiments, the cells were seeded into culture dishes and then cultured in α-MEM containing 10% FBS for 2 days until 70% confluent, and, then, the media was replaced by serum-free medium in order to minimize any serum-induced effects on PDLCs. Subsequently, the cells were exposed to H2O2 and human Tβ4 peptide (RegeneRx Biopharmaceuticals Inc., Rockville, MD). All treatments were performed in triplicate and approved by the local ethics committee.
Quantification of nitric oxide (NO) and prostaglandin E2 levels
Total NO production was determined as the nitrite concentration in the culture medium using a spectrophotometric assay based on the Griess reaction. The concentrations of PGE2 in culture supernatants were determined using an ELISA kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s recommendations.
Cell viability assay
The cytotoxicity was determined by the 3-(4,5-dimethylthiazolyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells seeded on 96-well microplates at 1×104 cells/well were incubated with H2O2 and Tβ4, for 48 hr. Medium was removed and then incubated with 100 μl MTT assay solution for 4 h. Absorbance was measured in an ELISA reader at 595 nm.
Osteoclast differentiation
Mouse bone marrow macrophage (BMMs) of 5-week-old female ICR mice (Charles River Laboratories, Seoul, South Korea) were used as previously described [23]. Animals were maintained in accordance with the National Institute of Toxicological Research of the Korea Food and Drug Administration guideline for the humane care and use of laboratory animals Institutional Animal Care and Use Committee (IACUC) approval was obtained from Kyung Hee University (Seoul, Korea). Briefly, bone marrow of tibiae and femurs of mice were flushed with α-MEM. After removing erythrocytes with hypotonic buffer, cells were cultured in α-MEM containing 10% FBS for 24 h and adherent cells were discarded. Non-adherent bone marrow cells were transferred onto 100-mm non-coated petri dishes at 5×106 cells per dish and cultured in the presence of M-CSF (30 ng/ml) for 3 days. Condition medium (CM) was obtained from HPDLCs treated with 200 μM H2O2 or Tβ4 (0.5, 1 and 5 μg/mL) for 2 days. To evaluate the osteoclastogenic activity of CM from HPDLCs, we added the CM mixture (60% CM plus 40% fresh α-MEM without M-CSF or RANKL) or rh-Tβ4 to pre-osteoclast-stage cells and further cultured the cells for up to 5 days to achieve mature osteoclast differentiation BMMs (1.5 × 105 cells/well) and PDLCs (1.5 × 104 cells/well) were co-cultured for 7 days in the presence of M-CSF (30 ng/ml), RANKL (100 ng/mL), H2O2 (200 μM) or Tβ4 (0.5, 1 and 5 μg/mL) in α-MEM, supplemented with10% in 48-well plates under 5% CO2 atmosphere.
TRAP staining and activity assay
Osteoclast differentiation was assessed by tartrate-resistant acid phosphatase (TRAP) staining and activity. After 5 days of culture, cells were stained for TRAP kit using a leukocyte acid phosphatase kit (Sigma Aldrich, St Louis, MO, USA). Cells with three or more nuclei were counted as multinucleated mature osteoclasts. To measure TRAP activity, cells were fixed with 10% formalin for 10 min and 95% ethanol for 1 min, and then 100 μl of citrate buffer (50 mM, pH 4.6) containing 10 mM sodium tartrate and 5 mM p-nitrophenylphosphate (Sigma-Aldrich) was added to the wells containing fixed cells in the 48-well plates. After incubation for 1 h, enzyme reaction mixtures in the wells were transferred to new plates containing an equal volume of 0.1 N NaOH. Absorbance was measured at 410 nm using a microplate reader.
Tβ4 or Wnt5a siRNA transfection
Silencing of the Tβ4 or Wnt5a gene was achieved by transfecting cells with small interfering RNA (siRNA). Cells were transfected with Tβ4 or Wnt5a siRNAs (30 nM) for 24 hours using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Cells were transfected with Silencer negative control siRNA using the same protocol.
RNA extraction, reverse transcriptase PCR
Total RNA was extracted from cells using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Reverse-transcription (RT)-PCR was performed using oligo deoxythymidine primer (Roche Diagnostics, Mannheim, Germany) in 20 μl volumes at 42°C for 60 min. The RT-PCR reaction was done with 1 μg of total RNA, 1 μl of 20 μM oligo dT primer, and 18 μl of reaction mixture by AccuPower RT/PCR PreMix (Bioneer, Daejeon, Korea). Then, PCR was performed in a 20 μl total mixture volume for 25 cycles at 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Primer sequences are detailed in Table 1. PCR products were subjected to electrophoresis on 1.5% agarose gels and visualized with ethidium bromide.
Table 1. Reverse transcriptase-polymerase chain reaction (RT-PCR) primers and conditions.
| Genes | Forward primer (5'-3') | Reverse primer (5'-3') | Annealing temperature (°C) | Cycle number | Product size (bp) |
|---|---|---|---|---|---|
| Tβ4 | 5'-CGCAGACCAGACTTCGCTCGTAC-3' | 5'-TCCTTCCCTGCCAGCCAGATAGAT-3' | 58 | 30 | 389 |
| COX-2 | 5'-GGAGAGACTATCAAGATAGTGATC-3' | 5'-ATGGTCAGTAGACTTTTACAGCTC-3' | 60 | 38 | 860 |
| iNOS | 5'-CCCTTCCGAAGTTTCTGGCAGCAGC-3' | 5'-GGCTGTCAGAGAGCCTCGTGGCTTTGG-3' | 61 | 35 | 497 |
| TNF-α | 5'- GGAAGACCCCTCCCA GAT AG -3' | 5'- CCCCAGGGACCTCTCTCTAA -3' | 52 | 35 | 413 |
| IL-1β | 5'- GGATATGGAGCAACAAGTGG -3' | 5'- ATGTACCAGTTGGGGAACTG -3' | 60 | 35 | 288 |
| IL-6 | 5'-ATGAACTCCTTCTCCACAAGC-3' | 5'-CTACATTTGCCGAAGAGCCC-3' | 55 | 34 | 639 |
| IL-8 | 5'-ATGACTTCCAAGCTGGCCGTGGCT-3' | 5'-TCTCAGCCCTCTTCAAAAACTTCTC-3' | 62 | 25 | 289 |
| IL-17 | 5'-CGATGACTCCTGGGAAGACCTC-3' | 5'-GTGTGGGCTCCCCAGAGCTCTTA-3' | 62 | 30 | 524 |
| RANKL | 5'-CCAGCATCAAAATCCCAAGT-3' | 5'-CCCCTTCAGATGATCCTTC-3' | 56 | 35 | 605 |
| OPG | 5'-TCAAGCAGGAGTGCAATCG-3' | 5'-AGAATGCCTCCTCACACAGG-3' | 59 | 31 | 342 |
| Wnt5a | 5'-CCGCGAGCGGGAGCGCATCCA CGCC-3' | 5'-GCCACATCAGCCAGGTTGTACACC-3' | 54 | 32 | 114 |
| Ror2 | 5'-ATCCAAGACCTGGACACAACAGA-3' | 5'-GAACCCCAGTGGCAGTGATG-3' | 60 | 30 | 85 |
| Frizzled | 5'-GCGAAGCCCTCATGAACAA-3' | 5'-TCCGTCCTCGGAGTGGTTCT-3' | 60 | 30 | 116 |
| GAPDH | 5'-CGGAGTCAACGGATTTGGTCGTAT-3' | 5'-AGCCTTCTCCATGGTGGTGAAGAC-3' | 62 | 25 | 306 |
| Mouse Cathepsin K | 5'-TGGATCTTTTAGCGTCTTGTTCC-3' | 5'-CCACGCTTGAGACAGGCTTA-3' | 60 | 30 | 226 |
| Mouse Calcitonin receptor | 5'-TGGTTGAGGTTGTGCCCAATGGAGA-3' | 5'-CTCGTGGGTTTGCCTCATCTTGGTC-3' | 65 | 32 | 503 |
| Mouse NFATc1 | 5'-TCGAGTTCGATCAGAGCGG-3' | 5'-TGGCTGAAGGAACAGCTGAG-3' | 59 | 34 | 168 |
| Mouse RANK | 5'-TCCTACCTCCGACAGTGTGT-3' | 5'-CCGTATCCTTGTTGAGCTGC-3' | 58 | 31 | 266 |
| Mouse c-fms | 5'-GGAAGAGGAGCCAGTGCAAC-3' | 5'-AAGAGTGGGCCGGATCTTTG-3' | 60 | 30 | 450 |
| Mouse GAPDH | 5'-GAGAGTGTTTCCTCGTCCCG-3' | 5'-ACTGTGCCGTTGAATTTGCC-3' | 59 | 28 | 201 |
Western blotting
Treated cells were washed with PBS and cytosolic protein extracts were prepared using 1X cell lysis buffer (Santa Cruz Biotechnology, CA) supplemented with protease inhibitor cocktail. Protein concentrations were determined using the Bradford assay (Bio-Rad, CA, USA) as per the manufacturer's protocol. Aliquots of protein lysates were separated on sodium dodecyl sulfate–10% polyacrylamide gels and Western blotting was performed. The proteins were transferred onto a polyvinylidene difluoride membrane (Bio-Rad, CA, USA) in transfer buffer (20 mm Tris, 150 mm glycine, 20% methanol, pH 8.0; TBS-T) at 4°C and 100 V for 1 hour. The membrane was blocked with 5% dry milk in TBS-T for 1 hour at room temperature and incubated with primary antibodies (1:1000) and horseradish peroxidase (HRP)-conjugated secondary antibodies. Protein bands were detected using an enhanced chemiluminescence (ECL) system (Amersham Biosciences, Backinghamshire, UK).
Statistical analysis
Differences among groups were analyzed using a one-way analysis of variance combined with the Bonferroni test. The relative intensities of mRNA and protein bands were assayed using Quantity-One software (Bio-Rad Co., Hercules, CA, USA); results were normalized to the mRNA and protein levels of beta-actin. All values were expressed as mean ± standard deviation. Differences were considered significant at p < 0.05.
Results
Effects of ROS on Tβ4 mRNA and protein expression in human PDLCs
As reactive oxygen species (ROS) have been implicated in the pathogenesis of periodontitis [24], we examined whether H2O2 could down-regulate or up-regulate Tβ4 expression in PDLCs. As shown in Fig 1A and 1B, Tβ4 mRNA and protein expressions were down-regulated by H2O2 in a time- and concentration-dependent manner. Because maximal Tβ4 mRNA and protein expressions were achieved with 200 μM H2O2 within 48 hours in PDLCs, this concentration was used in subsequent experiments.
Fig 1. Effects of H2O2 on mRNA and protein expression of Tβ4 in PDLCs.
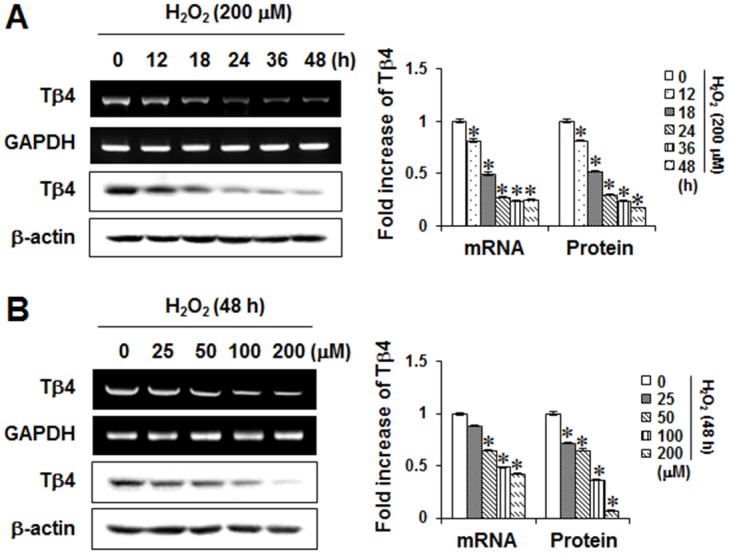
Cells were incubated for 48 hours with the indicated times with 200 μM H2O2 (A) and the indicated concentrations of H2O2 (B) for 48 hours. The mRNA and protein expressions were examined by RT-PCR and Western blotting, respectively. Data were representative of three independent experiments. The bar graph shows the fold increase in protein or mRNA expression compared with control cells. * Statistically significant differences compared with the control, p<0.05.
Effects of Tβ4 peptide on H2O2-induced inflammatory response in PDLCs
To determine the effects of Tβ4 peptide and H2O2 on cytotoxicity, its cell viability was evaluated. A 48-h exposure to 0.1–5 μg/mL Tβ4 peptide did not affect H2O2-mediated cell viabilities (Fig 2A). In order to examine whether Tβ4 peptide suppressed ROS-induced inflammatory mediators, the ability of Tβ4 peptide on production of NO and PGE2, and expressions of COX-2 and iNOS were measured by RT-PCR, Western blot, and ELISA. Pretreatment with Tβ4 peptide dose-dependently inhibited H2O2-induced mRNA and protein expressions of COX-2 and iNOS, and NO and PGE2 production (Fig 2B–2E).
Fig 2. Effect of Tβ4 peptide on H2O2-induced cytotoxicity (A), Tβ4, inducible nitric oxide (NO) synthase (iNOS) and cyclooxygenase-2 (COX-2) mRNA and protein expressions (B, C), NO and prostaglandin E2 (PGE2) secretion (D, E) in PDLCs.
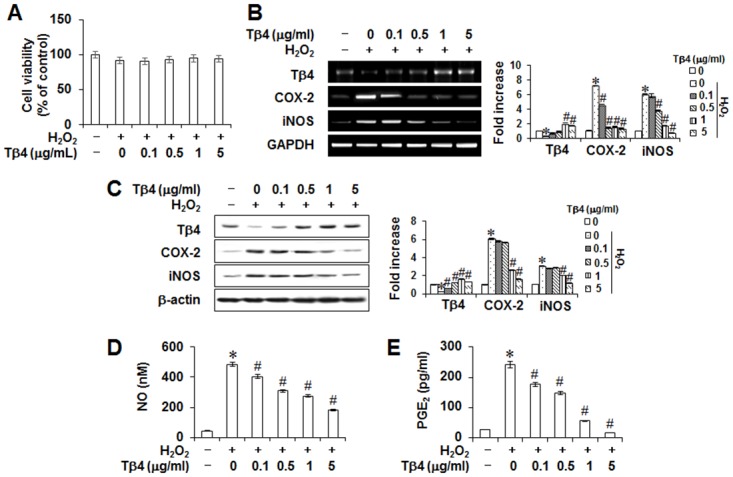
Cells were pretreated with indicated concentrations of Tβ4 peptide for 2 hours and then incubated with 200 μM H2O2 for 48 hours (A-E). Cell viability was measured by MTT assay (A). Protein and mRNA expressions were assessed by RT-PCR (B) and Western blot analysis (C), respectively. The production of NO (D) and PGE2 (E) were measured by Griess reaction and ELISA, respectively. Data replicated the quantifications of cytotoxicity, NO, and PGE2 with the standard deviation of at least three experiments (n = 4). The bar graph shows the fold increase in protein or mRNA expression compared with control cells. * Statistically significant differences compared with the control, p<0.05. # Statistically significant difference compared with the H2O2—treated group.
To examine whether suppression of inflammatory events by Tβ4 is specific to PDLCs, the anti-inflamamtory effects of Tβ4 peptide in gingival fibroblasts were verified. Tβ4 peptide also inhibited H2O2-induced mRNA and protein expressions of COX-2 and iNOS, and NO and PGE2 production in gingival fibroblasts (Fig 3A–3C).
Fig 3. Anti-inflammatory effect of Tβ4 peptide in gingival fibroblasts.
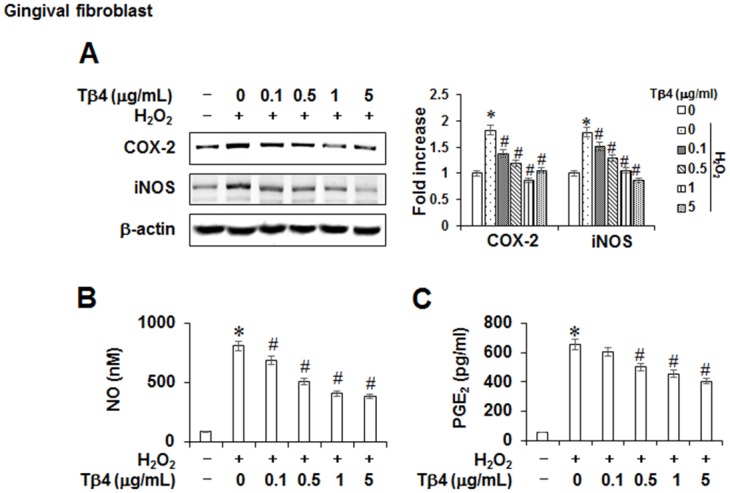
Cells were pretreated with indicated concentrations of Tβ4 peptide for 2 hours and then incubated with 200 μM H2O2 for 48 hours (A-C). Protein expressions were assessed by Western blot analysis (A). The production of NO (B) and PGE2 (C) were measured by Griess reaction and ELISA, respectively. Data replicated the quantifications of NO and PGE2 with the standard deviation of at least three experiments (n = 4). The bar graph shows the fold increase in protein expression compared with control cells. * Statistically significant differences compared with the control, p<0.05. # Statistically significant difference compared with the H2O2—treated group.
To further determine the potential anti-inflammatory effects of Tβ4 activation, expressions of proinflammatory or osteoclastogenic cytokines were measured by RT-PCR (Fig 4A). The TNF-α, IL-1β, IL-6, IL-8, and IL-17 mRNA levels increased in the H2O2- stimulated PDLCs, and these increases were significantly decreased in a concentration-dependent manner by treatment with the Tβ4 peptide. Since receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) are two important osteoclastogenic factors, we next explored the effects of Tβ4 peptide on RANKL and OPG expressions in PDLCs. Tβ4 peptide reduced H2O2-stimulated up-regulation of RANKL, with a reciprocal increase in OPG mRNA in a dose-dependent manner (Fig 4B).
Fig 4. Effect of Tβ4 peptide on H2O2-induced osteoclastogenic cytokines (A) and osteoclastogenic factors (B) in PDLCs.
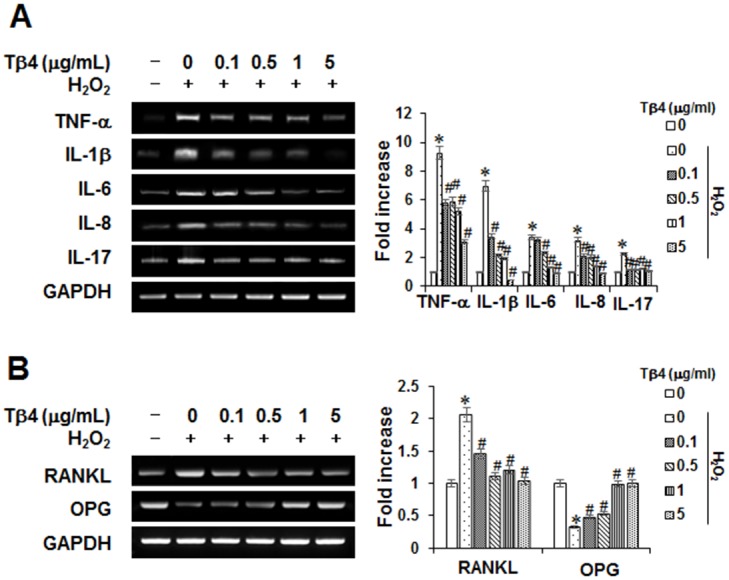
Cells were pretreated with indicated concentrations of Tβ4 peptide for 2 hours and then incubated with 200 μM H2O2 for 48 hours (A, B). The mRNAs expression was examined by RT-PCR analysis. This data were representative of three independent experiments. The bar graph shows the fold increase in mRNA expression compared with control cells. * Statistically significant differences compared with the control, p<0.05.
Effects of Tβ4 peptide on H2O2-induced signal transduction pathway in PDLCs
To investigate the effect of Tβ4 peptide on H2O2-induced signaling cascades, the activation states of three mitogen-activated protein kinases (MAPKs; p38, c-Jun N-terminal kinase [JNK] and extracellular signal-related kinase [ERK]) as well as NF-κB p65 were examined in PDLCs. H2O2 treatment induced the phosphorylation of p38, ERK, and JNK MAPK(s) and the nuclear translocation of NF-κB p65 (Fig 5A). Treatment of cells with Tβ4 peptide blocked H2O2-induced nuclear translocation of NF-κB p65 and phosphorylation of ERK and JNK (Fig 5B).
Fig 5. Effect of H2O2-induced MAPK and NF-κB signaling pathways (A) and effect of Tβ4 peptide on H2O2-induced MAPK and NF-κB activation (B) in PDLCs.
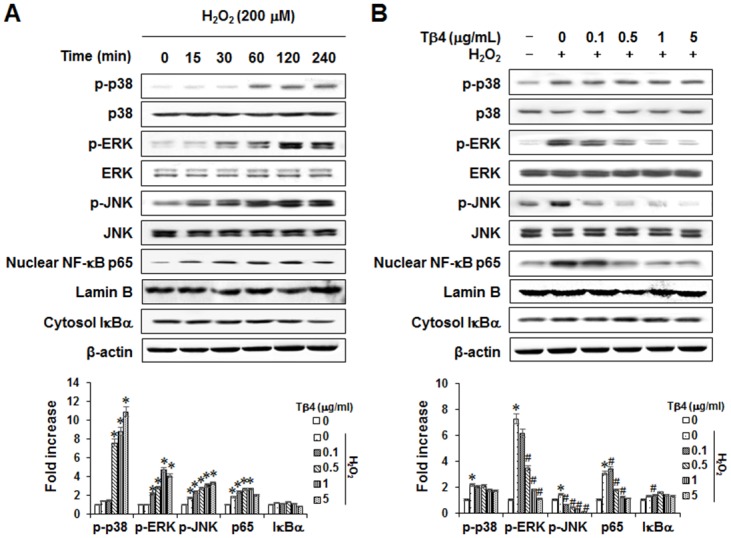
Cells were incubated with 200 μM H2O2 for indicated times (A). Cells were pretreated with indicated concentrations of Tβ4 peptide (0.1–5 μg/mL) for 2 hours and then incubated with 200 μM H2O2 for 60 minutes (B). Data were representative of three independent experiments. The bar graph shows the fold increase in protein expression compared with control cells * Statistically significant differences compared with the control, p<0.05. # Statistically significant difference compared with the H2O2—treated group.
Effects of Tβ4 peptide on osteoclastogenesis
To evaluate the indirect effect of Tβ4 peptide on RANKL-induced osteoclastogenesis, mouse BMMs were incubated with RANKL and CM, prepared from HPDLCs treated with H2O2 and different concentrations of Tβ4, and allowed to differentiate into osteoclasts. As shown in Fig 6, Tβ4 peptide dose-dependently decreased the number of osteoclasts and TRAP activity. To determine whether the reduction in osteoclast generation by Tβ4 could be due to effects of Tβ4 peptide on viability of the BMMs, a cytotoxicity assay was performed. The viability of BMMs was not significantly affected by Tβ4 peptide (data not shown).
Fig 6. Indirect effects of Tβ4 peptide on RANKL-induced osteoclastogenesis in mouse BMMs (A-C).
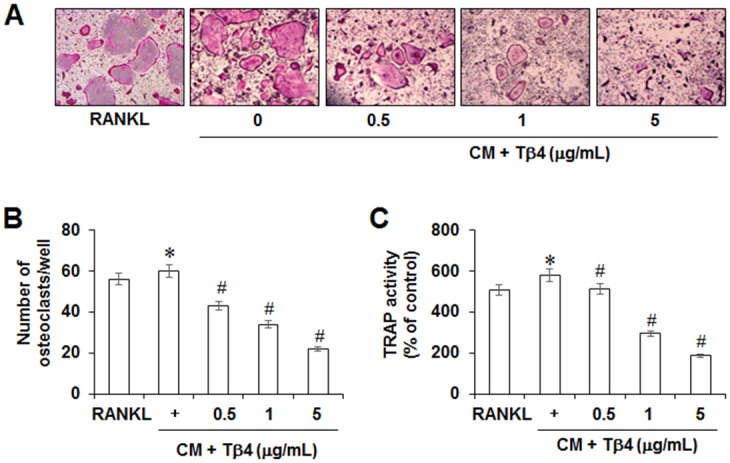
Cells were pretreated with indicated concentrations of Tβ4 peptide for 2 hours, post-incubated with 200 μM H2O2 for 48 hours, and then conditioned medium (CM) was collected. Mouse BMMs were cultured with CM in the presence of M-CSF (30 ng/mL) and RANKL (100 ng/mL), as described in Materials and methods. After 5 days, cells were fixed and stained for TRAP as a marker of osteoclasts (A), and the number of TRAP-positive multinucleated cells (MNCs) was scored (B). TRAP osteoclast activity was assayed using the TRAP cytochemical stain technique (C). * Statistically significant differences compared with the control, p<0.05. The data presented were representative of three independent experiments.
To determine the direct effect of Tβ4 peptide on osteoclastogenesis, mouse BMMs were directly exposed to Tβ4 peptide. Direct treatment with Tβ4 peptide also reduced the number of multinucleated TRAP-positive cells and TRAP activity in a dose-dependent manner (Fig 7A and 7B). Since Tβ4 downregulated H2O2-induced various cytokines expression, the indirect effect of Tβ4 on osteoclast formation through PDLC cells using co-culture system were investigated. After addition of Tβ4 peptide to the BMMs-PDLCs co-culture, the number of osteoclast and TRAP activity were also significantly decreased (Fig 7C and 7D).
Fig 7. Direct (A, B) or co-cultured (C, D) effects of Tβ4 peptide on RANKL-induced osteoclastogenesis in mouse BMMs (A-C).
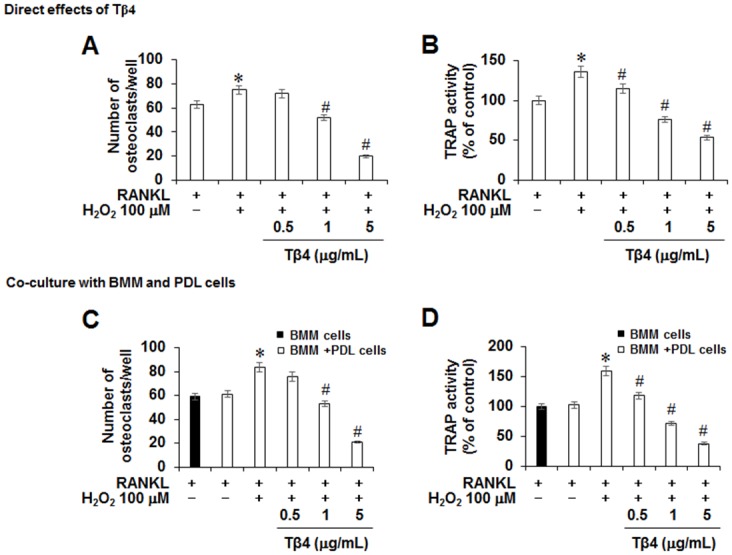
A and B; Mouse BMMs were cultured with 200 μM H2O2 and indicated concentrations of Tβ4 peptide in the presence of M-CSF (30 ng/mL) and RANKL (100 ng/mL). C and D; PDLCs were co-cultured with mouse BMMs in the presence of M-CSF, RANKL, 200 μM H2O2, and indicated concentrations of Tβ4 peptide. To monitor osteoclast differentiation, both TRAP activity and the number of TRAP multinucleated cells were examined. * Statistically significant difference compared with control, p<0.05. The data presented were representative of three independent experiments.
Effects of Tβ4 peptide on signaling pathways of osteoclastogenesis
To analyze the functional changes of osteoclastogenesis induced by Tβ4 peptide treatment, total RNA from BMMs was collected and RT-PCR for osteoclast-specific gene expression was performed. Tβ4 peptide reduced RANKL-induced cathepsin K, calcitonin receptor (CR), NFATc1, RANK, and c-fms up-regulations in a dose-dependent manner (Fig 8A).
Fig 8. Effects of Tβ4 peptide on the functional changes of osteoclastogenesis (A) and MAPK and NF-κB signaling pathways in mouse BMMs.
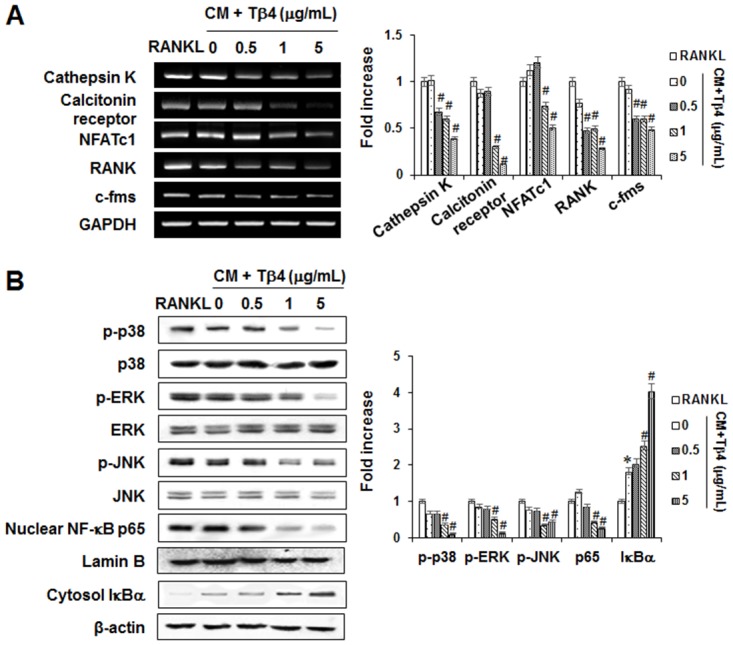
Mouse BMMs were cultured with M-CSF (30 ng/mL) and RANKL (100 ng/mL) or CM collected from PDLCs for 5 days (A) and 60 minutes (B). The mRNAs expression was determined by PCR analysis (A). The phosphorylation of MAPKs (p38, JNK, and ERK), and activation of NF-κB were determined by Western blot analysis (B). Data were representative of three independent experiments. The bar graph shows the fold increase in protein or mRNA expression compared with control cells * Statistically significant differences compared with the control, p<0.05.
To determine whether MAPK and NF-κB signaling pathways were involved in the anti-osteoclastogenic function of Tβ4, the effect of Tβ4 peptide on the phosphorylation levels of ERK, JNK, and p38 MAPK(s) as well as the nuclear translocation of NF-κB p65 in RANKL-stimulated BMMs were examined. As shown in Fig 8B, Tβ4 peptide inhibited the RANKL-induced phosphorylation of p38, ERK, and JNK and nuclear translocation of NF-κB p65.
Role of the Wnt-5a pathway in anti-inflammatory response and anti-osteoclastogenesis by Tβ4
Since Wnt5a expression is associated with rheumatoid arthritis and periodontitis [25, 26], expression of Wnt5a and its cell surface receptors, Frizzled and receptor tyrosine kinase-like orphan receptor 2 (Ror2), were examined. As shown in Fig 9A–9D, mRNA and protein expressions of Wnt5a and its receptors were increased by H2O2 in a time- and dose-dependent manner.
Fig 9. Effects of H2O2 on the expression of Wnt5a and its cell surface receptors in PDLCs.
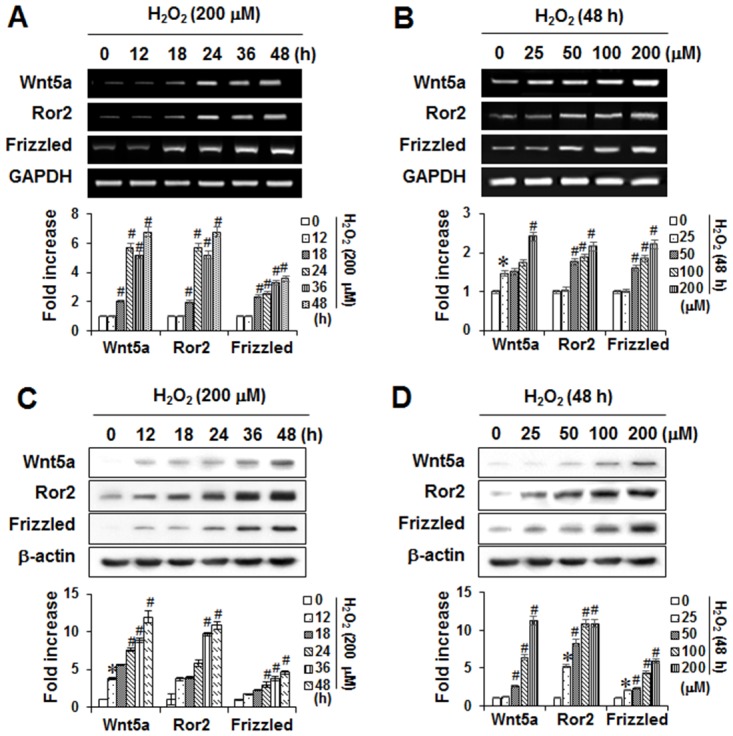
The mRNA and protein expressions were determined by PCR analysis (A) and Western blot analysis (B), respectively. The bar graph shows the fold increase in protein or mRNA expression compared with control cells * Statistically significant differences compared with the control, p<0.05. The data presented were representative of three independent experiments.
To explore whether Tβ4 peptide-induced anti-inflammatory and anti-osteoclastogenesis were dependent on the up-regulation of Wnt5a, the effects of recombinant human (rh) Wnt5a (500 ng/mL) and Wnt5a-specific siRNA were assessed. Pretreatment of Wnt5a siRNA reversed the inhibitory effects of Tβ4 peptide on H2O2-induced iNOS and COX-2 expressions, NO and PGE2 productions, osteoclastogenic cytokines, and RANKL expression (Fig 10A–10E). In contrast, pretreatment with rhWnt5a enhanced the anti-inflammatory effects of Tβ4 peptide whereas control siRNA showed no effect on PDLCs. In accordance with anti-inflammatory results, Tβ4 peptide-suppressed osteoclast number and TRAP activity in BMM cells were reversed by exogenous treatment with Wnt5a siRNA but enhanced by rh-Wnt5a (Fig 11A–11C).
Fig 10. Effects Wnt5a siRNA and Wnt5a peptide on the Tβ4 peptide-mediated inhibition of iNOS and COX-2 expressions (A), NO and PGE2 secretions (B, C), pro-inflammatory cytokines production (D) and osteoclastogenic factors (E) in PDLCs.
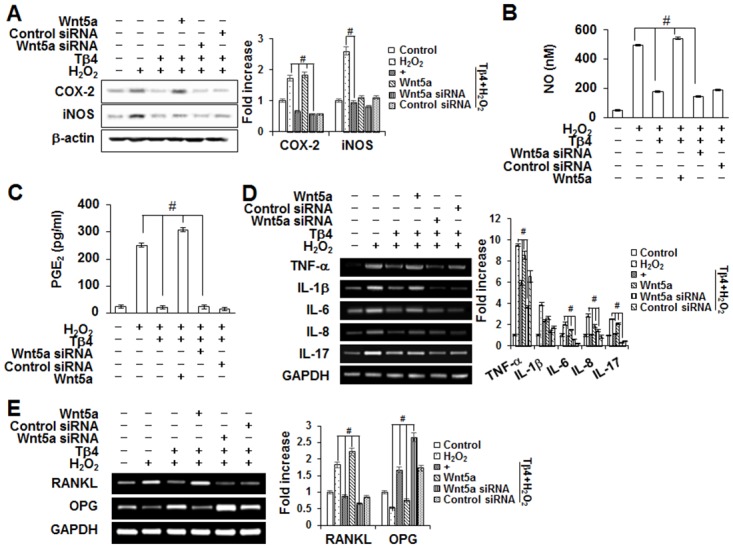
The PDLCs were pre-treated with Wnt5a siRNA (30 nM) or Wnt5 peptide (500 ng/mL) for 2 hours, post-incubated with Tβ4 peptide (1 μg/mL) and 200 μM H2O2 for 48 hours (A-E), and then conditioned medium (CM) was collected. The bar graph shows the fold increase in protein or mRNA expression compared with control. * Statistically significant differences compared with the control, p<0.05. # Statistically significant difference compared with the H2O2-treated group. The data presented were representative of three independent experiments.
Fig 11. Effects Wnt5a siRNA and Wnt5a peptide on the Tβ4 peptide-mediated RANKL-induced osteoclastogenesis in mouse BMMs (A-C).
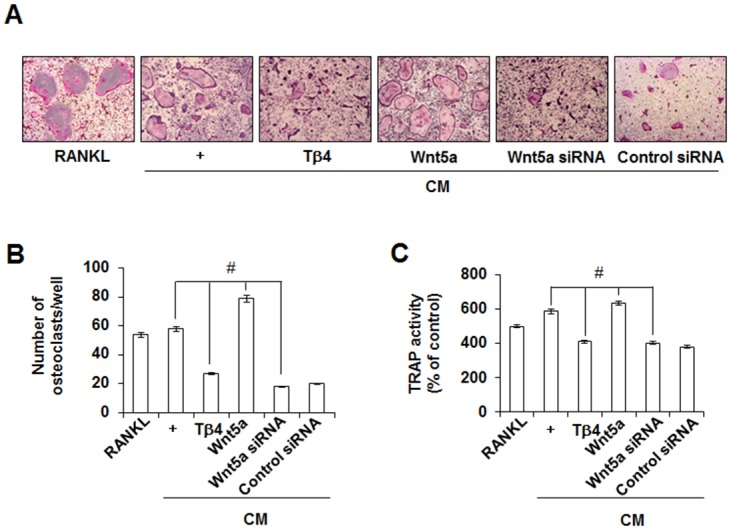
The BMM cells were incubated with M-CSF (30 ng/mL) and RANKL (100 ng/mL) or CM collected from PDLCs. * Statistically significant differences compared with the control, p<0.05. # Statistically significant difference compared with the each group. The data presented were representative of three independent experiments.
Discussion
Oxidative stress is characterized by an accumulation of ROS and plays a key role in the progression of periodontal diseases [24]. Damage of tissues in inflammatory periodontal disease can be mediated by ROS resulting from the physiological activity of PMN during the phagocytosis of periodontopathic bacteria [27]. In addition, LPS from Porphyromonas gingivalis as well as hypoxia induces a NOX4-dependent increase in H2O2 release in PDLCs [28]. Furthermore, ROS such as H2O2 are small, diffusible, and ubiquitous molecules, can affect human PDLCs and gingival fibroblasts cell injury indirectly by enhancing pro-inflammatory factors such as cytokines, NO, PGE2, and ROS [29–31]. This ROS is known to stimulate osteoclast differentiation and participate in early signaling events associated with osteoclast activation for bone resorption [32]. Since LPS from P. gingivalis increases oxidative stress in PDLCs and contributes to periodontitis [28], human PDLCs treated with H2O2 may serve as an in vitro model relevant to periodontitis.
Tβ4 is the major monomeric actin-sequestering peptide in human tissues, and can bind globular actin (G-actin) in a 1:1 ratio and consequently involved in cytoskeletal regulation by inhibiting the polymerization of G-actin into fibrous actin (F-actin) [7]. In addition, Tβ4 is an ubiquitous naturally occurring molecule and is found at concentrations of 1 × 10−5 to 5.6 × 10−1 M in a variety of tissues and cell types, yet, no receptors for the protein have been identified [33]. A recent study suggests that internalization of exogenous Tβ4 is essential for its subsequent cellular functions [34]. Moreover, Tβ4 has been shown to be associated with, wound healing, hair growth, immunomodulation, and angiogenesis [7–9].
Tβ4 is not a thymus-specific peptide but also present in most tissue and all cells except red blood cells [35]. High amounts of Tβ4 were detected in human white blood cells, especially in neutrophils and in macrophages [34], expressed in developing mandible (embryonic day 12) [36] and hair follicles (HF) of mice [37]. In addition, the peptide is also detected outside cells, in blood plasma and in wound and blister fluids [34]. Although the mechanism(s) of action of exogenous Tβ4 on anti-inflammatory effects remains unclear, the high levels of Tβ4 present in human wound fluid (13 μg/mL) suggest its importance in wound healing or anti-inflammation [38]. However, the level of Tβ4 is variable (unchanged, decreased, and increased) in GCF or biopsied gingival tissue of periodontal patients [20, 21]. Based on the observations that Tβ4 has anti-inflammatory effects [11–14], the hypothesis is that Tβ4 regulates inflammatory mediators and osteoclastogenesis in osteolytic bone disease, such as periodontitis.
In this study, Tβ4 mRNA down-regulation was detected in in vitro in PDLCs stimulated with the ROS. This down-regulation of Tβ4 was also observed in GCF of periodontitis patient [19] and endotoxin-induced septic shock of rats [39]. ROS were generated predominantly by polymorphonuclear leukocytes (PMN) during an inflammatory response and involved in tissue destruction associated with periodontal diseases [40]. Thus, we chose to use ROS-stimulated PDLCs in this study since ROS, such as superoxide and H2O2, have been proposed as key players in bone resorption [41] and implicated in the pathogenesis of rheumatoid arthritis and periodontitis [29].
Exogenous Tβ4 can function like a hormone on cells in terms of its ability to modulate their biological behavior. Since one of the primary roles of Tβ4 in cells is the sequestration of actin monomers, and the protein is not secreted, previously indicated that it was unlikely that Tβ4 could have a hormonal function [42]. However, other studies have shown that the intracellular level of Tβ4 or its mRNA can be significantly and rapidly altered by external stimuli and that change in the level of Tβ4 often are correlated with cell differentiation [18, 43]. In the present study, exogenous Tβ4 peptide activate intracellular Tβ4, which results suggested that exogenous Tβ4 spontaneously enter the cytoplasm through rapid internalization, and acts their functions same as endogenous one [8, 18].
The full-length Tβ4 polypeptide has been shown to be effective in reducing inflammation [44]. It is also reported that only the 4-AA, amino-terminal peptide of Tβ4, known as Ac-SDKP, can block inflammation [45]. In this study, we used a synthetically human peptide produced copy of a naturally occurring, highly conserved 43-amino acid (MW = 4964 Da) water soluble acidic peptide, originally isolated from bovine thymus tissue [46]. This peptide is produced by Fmoc solid-phase peptide synthesis in accordance with the current Good Manufacturing Practice (cGMP) regulations (21 CFR 210 and 211) of the FDA [47]. An effective healer, Tβ4 can be administered topically on the surface of cells and systemically, through injection [9–11]. In this study, Tβ4 activation by Tβ4 peptide inhibited H2O2-induced production of NO and PGE2, expression of COX-2 and iNOS, and mRNA expression of TNF- α, IL-1β, -6, -8, and -17 in cultured PDLCs. These findings suggested that Tβ4 activation possessed anti-inflammatory activity in PDLCs. These results were consistent with previous in vivo and in vitro studies [9–15]. MAPK is a proline-directed serine/threonine kinase consisting of three-enzyme modules; its targets, inducing ERK, JNK and p38 kinases, are important in cellular signal transduction pathways and exert an anti-inflammatory response [48, 49]. NF-κB is a major transcription factor involved in the release of proteins that mediate the inflammatory response, and the degradation and phosphorylation of Iκ-Bα are necessary to release NF-κB from the cytoplasmic NF-κB/Iκ-Bα complex and allow its subsequent translocation to the nucleus of the cell [50]. In this study, Tβ4 peptide down-regulated the H2O2-triggered activation of the ERK and JNK MAPKs and the NF-κB in PDLCs. These results suggested that the ERK and JNK MAPKs and the NF-κB pathway may be involved in the anti-inflammatory effects of Tβ4 activation in PDLCs. Consistent with our findings, Tβ4 treatment decreased TNF-α-induced NF-κB activation in human corneal epithelial cells [51].
The RANKL and OPG have been identified as a key regulatory component of alveolar bone loss associated with inflammatory periodontal disease [52]. Moreover, PDLCs were shown to express several osteoclastogenic cytokines, including both OPG and RANKL [30, 31]. Our data demonstrated that Tβ4 peptide abolished H2O2-induced RANKL expression and restored OPG expression. Osteoclasts, bone-resorptive multinucleated cells derived from hematopoietic stem cells, are associated with osteolytic diseases. Furthermore, NFATc1, a master modulator of osteoclastogenesis, regulates target genes, such as cathepsin K and calcitonin receptor or Calcr [53]. In our in vitro study using BMMs, Tβ4 peptide directly and indirectly inhibited RANKL-induced osteoclast differentiation and expression of osteoclast markers, such as cathepsin-K, calcitonin receptor or Calcr, NFATc1, and RANK in BMM cells. These results indicated that Tβ4 was a key therapeutic target in controlling inflammation-induced bone loss.
MAPKs and NF-κB played pivotal roles in the development of osteoclasts downstream of RANK signaling [54]. In this study, we demonstrated that Tβ4 activation by Tβ4 peptide inhibited RANKL-induced p38, ERK, JNK MAPK, and NF-κB signaling pathways. These results suggested that Tβ4 activation might inhibit osteoclast differentiation via inhibition of the signaling cascades MAPK/NF-κB/NFATc1.
Recent reports have stated that inhibitors of Wnt signaling have emerged as promising strategies for bone disease and inflammatory diseases [26, 55]. Wnt5a, one of the most common Wnt molecules that activate the non-canoical pathway, binds to Fzd and its co-receptor, Ror2 [56]. In synoviocytes from rheumatoid arthritis patients, the expressions of Wnt5a and Frizzled5 (Fzd5) were significantly enhanced [25] and their blockades inhibited synoviocyte activation [55]. Recently, Wnt5a was highly expressed in synovial tissues in a mouse model of rheumatoid arthritis where inhibition of Wnt5a-Ror2 signaling suppressed bone loss [57]. Our data demonstrated that ROS up-regulated Wnt5a and its cell surface receptors, Frizzled and Ror2, as well as mRNA and protein expressions in time- and dose-dependent manners in PDLCs.
These results were in agreement with previous studies that showed Wnt5a expression can be induced in activated macrophages, endothelial cells, and bone marrow mesenchymal stem cells (BMSCs) after inflammatory stimulation [58, 59]. In addition, we found that the effects of Tβ4 peptide on H2O2-mediated induction of pro-inflammatory cytokines (NO, PGE2, TNF-α, IL-1β, IL-6, IL-8, and IL-17), the expression of inflammatory mediators (iNOS and COX-2), osteoclastogenic cytokines (cathepsin-K, calcitonin receptor or Calcr, NFATc1, and RANK), and osteoclastic differentiation, were reversed by exogenous treatment with Wnt5a siRNA but enhanced by rh-Wnt5a, suggesting that the anti-inflammatory and anti-osteoclastogenetic effects of Tβ4 activation were involved the Wnt5a-dependent signaling pathway. Similar to our results, Wnt5a knock-down markedly reduced cytokine/chemokine production induced by TNF in HDPCs [60].
Recently, therapeutic biomolecules such as growth factors provide great potential as an alternative therapeutic approach to traditional periodontal wound healing [61]. However, because of the short half-lives of growth factors and polynucleotides in the body and the necessity to deliver to specific target sites, those medicinal substances do not always exhibit the anticipated therapeutic potency and outcomes [62]. Thus, optimized delivery regimes and well-defined release kinetics appear to be logical prerequisites for safe and efficacious clinical application of biomolecules. For considering the application of Tβ4 in clinical trials, target cells of exogenous Tβ4 should be restricted to cells in the periodontal tissue.
In conclusion, this study is the first study to demonstrate that down-regulation of Tβ4 was observed in an in vitro model of H2O2-stimulated PDLCs. Tβ4 activation had anti-inflammatory effects via MAPK and NF-κB pathways in PDLCs and anti-osteoclastogenic effects via MAPK, NF-κB, and Wnt5a pathways in BMMs. These findings supported the fact that Tβ4 peptide could possibly be used in the development of a therapeutic drug for periodontitis and osteolytic disease.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No, 2012R1A5A2051384).
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2012R1A5A2051384).
References
- 1.Vattakuzhi Y, Abraham SM, Freidin A, Clark AR, Horwood NJ. Dual-specificity phosphatase 1–null mice exhibit spontaneous osteolytic disease and enhanced inflammatory osteolysis in experimental arthritis. Arthritis Rheum. 2012; 64: 2201–2210. 10.1002/art.34403 . [DOI] [PubMed] [Google Scholar]
- 2.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003; 74: 391–401. . [DOI] [PubMed] [Google Scholar]
- 3.Chen B, Wu W, Sun W, Zhang Q, Yan F, Xiao Y. RANKL expression in periodontal disease: where does RANKL come from? Biomed Res Int. 2014; 2014: 731039 10.1155/2014/731039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araújo AA, Souza TO, Moura LM, Brito GA, Aragão KS, Araújo LS, et al. Effect of telmisartan on levels of IL-1, TNF-α, down-regulated COX-2, MMP-2, MMP-9 and RANKL/RANK in an experimental periodontitis model. J Clin Periodontol. 2013; 40: 1104–1111. 10.1111/jcpe.12160 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jimi E, Aoki K, Saito H, D'Acquisto F, May MJ, Nakamura I, et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nature Medicine 2004; 10: 617–624. . [DOI] [PubMed] [Google Scholar]
- 6.Yu FX, Lin SC, Morrison-Bogorad M, Yin HL. Effects of thymosin beta 4 and thymosin beta 10 on actin structures in living cells. Cell Motil Cytoskeleton. 1994; 27: 13–25. . [DOI] [PubMed] [Google Scholar]
- 7.Philp D, Goldstein AL, Kleinman HK. Thymosin beta 4 promotes angiogenesis, wound healing, and hair follicle development. Mech Ageing Dev. 2004; 125: 113–115. . [DOI] [PubMed] [Google Scholar]
- 8.Cha HJ, Philp D, Lee SH, Moon HS, Kleinma HK, Nakamura T. Over-expression of thymosin beta 4 promotes abnormal tooth development and stimulation of hair growth. Int J Dev Biol. 2010; 54: 135–140. 10.1387/ijdb.082735hc . [DOI] [PubMed] [Google Scholar]
- 9.Conte E, Genovese T, Gili E, Esposito E, Iemmolo M, Fruciano M, et al. Protective effects of thymosin β4 in a mouse model of lung fibrosis. Ann N Y Acad Sci. 2012; 1269: 69–73. 10.1111/j.1749-6632.2012.06694.x . [DOI] [PubMed] [Google Scholar]
- 10.Matsuo K, Akasaki Y, Adachi K, Zhang M, Ishikawa A, Jimi E, et al. Promoting effects of thymosin β4 on granulation tissue and new bone formation after tooth extraction in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114: 17–26. 10.1016/j.tripleo.2011.05.025 . [DOI] [PubMed] [Google Scholar]
- 11.Bao W, Ballard VL, Needle S, Hoang B, Lenhard SC, Tunstead JR, et al. Cardioprotection by systemic dosing of thymosin beta four following ischemic myocardial injury. Front Pharmacol. 2013; 4: 149 10.3389/fphar.2013.00149 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan H, Ma C, Moinet L, Sato N, Martins-Green M. Reversal of second-hand cigarette smoke-induced impairment of corneal wound healing by thymosin beta 4 combined with anti-inflammatory agents. Invest Ophthalmol Vis Sci. 2010; 51: 2424–2435. 10.1167/iovs.09-3692 . [DOI] [PubMed] [Google Scholar]
- 13.Zhu T, Park HC, Son KM, Kwon JH, Park JC, Yang HC. Effects of thymosin β4 on wound healing of rat palatal mucosa. Int J Mol Med. 2014; 34: 816–821. 10.3892/ijmm.2014.1832 . [DOI] [PubMed] [Google Scholar]
- 14.Reti R, Kwon E, Qiu P, Wheater M, Sosne G. Thymosin beta 4 is cytoprotective in human gingival fibroblasts. Eur J Oral Sci. 2008; 116: 424–430. 10.1111/j.1600-0722.2008.00569.x . [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Gupta S. Thymosin beta 4 prevents oxidative stress by targeting antioxidant and anti-apoptotic genes in cardiac fibroblasts. PLoS One 2011; 6: e26912 10.1371/journal.pone.0026912 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho JH, Ma WH, Su Y, Tseng KC, Kuo TK, Lee OK. Thymosin beta-4 directs cell fate determination of human mesenchymal stem cells through biophysical effects. J Orthop Res. 2010; 28: 131–138. 10.1002/jor.20956 . [DOI] [PubMed] [Google Scholar]
- 17.Choi BD, Yun SH, Jeong SJ, Wang G, Kim HJ, Lim DS, et al. Expression of thymosin β4 in odontoblasts during mouse tooth development. Int J Mol Med. 2012; 29: 841–847. 10.3892/ijmm.2012.913 . [DOI] [PubMed] [Google Scholar]
- 18.Lee SI, Kim DS, Lee HJ, Cha HJ, Kim EC. The role of thymosin beta 4 on odontogenic differentiation in human dental pulp cells. PLoS One 2013; 8: e61960 10.1371/journal.pone.0061960 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon E, Jacobs LC, Wheater M. Gingival crevicular fluid levels of thymosin beta 4 in periodontal health and disease. J Adv Oral Research. 2013; 4: 1–6. [Google Scholar]
- 20.Abe D, Kubota T, Morozumi T, Shimizu T, Nakasone N, Itagaki M, et al. Altered gene expression in leukocyte transendothelial migration and cell communication pathways in periodontitis-affected gingival tissues. J Periodontal Res. 2011; 46: 345–353. 10.1111/j.1600-0765.2011.01349.x . [DOI] [PubMed] [Google Scholar]
- 21.Demmer RT, Behle JH, Wolf DL, Handfield M, Kebschull M, Celenti R, et al. Transcriptomes in healthy and diseased gingival tissues. J Periodontol. 2008; 79: 2112–2124. 10.1902/jop.2008.080139 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitagawa M, Kudo Y, Iizuka S, Ogawa I, Abiko Y, Miyauchi M, et al. Effect of F-spondin on cementoblastic differentiation of human periodontal ligament cells. Biochem Biophys Res Commun. 2006; 349: 1050–1056. . [DOI] [PubMed] [Google Scholar]
- 23.Lee SI, Kim GT, Kim HJ, Park SH, Kim EC. NOD2 Mediates odontoblast differentiation and RANKL Expression. J Dent Res. 2014; 93: 678–684. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canakçi CF, Ciçek Y, Canakçi V. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry (Mosc). 2005; 70: 619–628. . [DOI] [PubMed] [Google Scholar]
- 25.Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci USA. 2000; 97: 2791–2796. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanbara H, Wara-aswapati N, Nagasawa T, Yoshida Y, Yashiro R, Bando Y, et al. Modulation of Wnt5a expression by periodontopathic bacteria. PLoS One. 2012; 7: e34434 10.1371/journal.pone.0034434 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000. 2007; 43: 160–232. . [DOI] [PubMed] [Google Scholar]
- 28.Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G, Götz W, Frede S. LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediators Inflamm. 2014; 2014: 986264 10.1155/2014/986264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000; 6: 138–151. . [DOI] [PubMed] [Google Scholar]
- 30.Lee HJ, Pi SH, Kim Y, Kim HS, Kim SJ, Kim YS, et al. Effects of nicotine on antioxidant defense enzymes and RANKL expression in human periodontal ligament cells. J Periodontol. 2009; 80: 1281–1288. 10.1902/jop.2009.090098 . [DOI] [PubMed] [Google Scholar]
- 31.Lee SK, Chung JH, Choi SC, Auh QS, Lee YM, Lee SI, et al. Sodium hydrogen sulfide inhibits nicotine and lipopolysaccharide-induced osteoclastic differentiation and reversed osteoblastic differentiation in human periodontal ligament cells. J Cell Biochem. 2013; 114: 1183–1193. 10.1002/jcb.24461 . [DOI] [PubMed] [Google Scholar]
- 32.Hall TJ, Schaeublin M, Jeker H, Fuller K, Chambers TJ. The role of reactive oxygen intermediates in osteoclastic bone resorption. Biochem Biophys Res Commun. 1995; 207: 280–287. . [DOI] [PubMed] [Google Scholar]
- 33.Hannappel E, van Kampen M. Determination of thymosin beta 4 in human blood cells and serum. J Chromatogr. 1987; 397: 279–285. . [DOI] [PubMed] [Google Scholar]
- 34.Ho JH, Chuang CH, Ho CY, Shih YR, Lee OK, Su Y. Internalization is essential for the antiapoptotic effects of exogenous thymosin beta-4 on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007; 48: 27–33. . [DOI] [PubMed] [Google Scholar]
- 35.Goldstein AL, Slater FD, White A, Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci USA. 1966; 56: pp. 1010–1017. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaza H, Matsuo K, Kiyoshima T, Shigemura N, Kobayashi I, Wada H, et al. Detection of differentially expressed genes in the early developmental stage of the mouse mandible. Int J Dev Biol. 2001; 45: 675–680. . [PubMed] [Google Scholar]
- 37.Philp D, St-Surin S, Cha HJ, Moon HS, Kleinman HK, Elkin M. Thymosin beta 4 induces hair growth via stem cell migration and differentiation. Ann N Y Acad Sci. 2007; 1112: 95–103. 10.1196/annals.1415.009 . [DOI] [PubMed] [Google Scholar]
- 38.Frohm M, Gunne H, Bergman AC, Agerberth B, Bergman T, Boman A, et al. Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem. 1996; 237: 86–92. . [DOI] [PubMed] [Google Scholar]
- 39.Badamchian M, Fagarasan MO, Danner RL, Suffredini AF, Damavandy H, Goldstein AL. Thymosin beta(4) reduces lethality and down-regulates inflammatory mediators in endotoxin-induced septic shock. Int Immunopharmacol. 2003; 3: 1225–1233. . [DOI] [PubMed] [Google Scholar]
- 40.Moseley R, Waddington RJ, Embery G, Rees SG. The modification of alveolar bone proteoglycans by reactive oxygen species in vitro. Connect Tissue Res. 1998; 37: 13–28. . [DOI] [PubMed] [Google Scholar]
- 41.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990; 85: 632–639. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nachmias VT. Small actin-binding proteins: The beta-thymosin family. Curr Opin Cell Biol. 1993; 5: 56–62. . [DOI] [PubMed] [Google Scholar]
- 43.Shimamura R, Kudo J, Kondo H, Dohmen K, Gondo H, Okamura S, et al. Expression of the thymosin beta 4 gene during differentiation of hematopoietic cells. Blood 1990; 76: 977–984. . [PubMed] [Google Scholar]
- 44.Sosne G, Szliter EA, Barrett R, Kernacki KA, Kleinman H, Hazlett LD. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002; 74: pp. 293–299. . [DOI] [PubMed] [Google Scholar]
- 45.Yang F, Yang XP, Liu YH, Xu J, Cingolani O, Rhaleb NE, et al. Ac-SDKP reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension. 2004; 43: pp. 229–236. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Low TL, Hu SK, Goldstein AL. Complete amino acid sequence of bovine thymosin β4: a thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc Natl Acad Sci USA. 1981; 78: 1162–1166. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crockford D. Development of thymosin beta4 for treatment of patients with ischemic heart disease. Ann N Y Acad Sci. 2007; 1112: 385–395. [DOI] [PubMed] [Google Scholar]
- 48.Park GJ, Kim YS, Kang KL, Bae SJ, Baek HS, Auh QS, et al. Effects of sirtuin 1 activation on nicotine and lipopolysaccharide-induced cytotoxicity and inflammatory cytokine production in human gingival fibroblasts. J Periodontal Res. 2013; 48: 483–492. 10.1111/jre.12030 . [DOI] [PubMed] [Google Scholar]
- 49.Lim HD, Kim YS, Ko SH, Yoon IJ, Cho SG, Chun YH, et al. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J Pineal Res. 2012; 53: 225–237. 10.1111/j.1600-079X.2012.00991.x . [DOI] [PubMed] [Google Scholar]
- 50.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000; 18: 621–663. . [DOI] [PubMed] [Google Scholar]
- 51.Sosne G, Qiu P, Christopherson PL, Wheater MK. Thymosin beta 4 suppression of corneal NF-κB: A potential anti-inflammatory pathway. Exp Eye Res 2007; 84: 663–669. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Töz H, et al. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol. 2007; 34: 370–376. . [DOI] [PubMed] [Google Scholar]
- 53.So H, Rho J, Jeong D, Park R, Fisher DE, Ostrowski MC, et al. Microphthalmia transcription factor and PU.1 synergistically induce the leukocyte receptor osteoclast-associated receptor gene expression. J Biol Chem. 2003; 278: 24209–24216. . [DOI] [PubMed] [Google Scholar]
- 54.Zhao Q, Wang X, Liu Y, He A, Jia R. NFATc1: functions in osteoclasts. Int J Biochem Cell Biol. 2010; 42: 576–579. 10.1016/j.biocel.2009.12.018 . [DOI] [PubMed] [Google Scholar]
- 55.Sen M, Chamorro M, Reifert J, Corr M, Carson DA. Blockade of Wnt-5A/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001; 44: 772–781. . [DOI] [PubMed] [Google Scholar]
- 56.Bolzoni M, Donofrio G, Storti P, Guasco D, Toscani D, Lazzaretti M, et al. Myeloma cells inhibit non-canonical wnt co-receptor ror2 expression in human bone marrow osteoprogenitor cells: effect of wnt5a/ror2 pathway activation on the osteogenic differentiation impairment induced by myeloma cells. Leukemia. 2013; 27: 451–463. 10.1038/leu.2012.190 . [DOI] [PubMed] [Google Scholar]
- 57.Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, et al. WNT5A-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012; 18: 405–412. 10.1038/nm.2653 . [DOI] [PubMed] [Google Scholar]
- 58.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008; 28: 504–510. 10.1161/ATVBAHA.107.157438 . [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y, Wang CL, Li RM, Hui TQ, Su YY, Yuan Q, et al. Wnt5a promotes inflammatory responses via nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells. J Biol Chem. 2014; 289: 21028–21039. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rauner M, Stein N, Winzer M, Goettsch C, Zwerina J, Schett G, et al. WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J Bone Miner Res. 2012; 27: 575–585. 10.1002/jbmr.1488 . [DOI] [PubMed] [Google Scholar]
- 61.Giannobile WV, Somerman MJ. Growth and amelogenin-like factors in periodontal wound healing. A systematic review. Ann Periodontol 2003; 8: 193–204. . [DOI] [PubMed] [Google Scholar]
- 62.Chen FM, Shelton RM, Jin Y, Chapple IL. Localized delivery of growth factors for periodontal tissue regeneration: role, strategies, and perspectives. Med Res Rev. 2009; 29: 472–513. 10.1002/med.20144 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


