Abstract
BACKGROUND
Arterial ischemic stroke occurs most frequently in term newborns than in the elderly, and brain immaturity affects mechanisms of ischemic injury and recovery. The susceptibility to injury of the brain was assumed to be lower in the perinatal period as compared to childhood. This concept was recently challenged by clinical studies showing marked motor disabilities after stroke in neonates, with the severity of motor and cortical sensory deficits similar in both perinatal and childhood ischemic stroke. The understanding of the triggers and the pathophysiological mechanisms of perinatal stroke has greatly improved in recent years, but many aspects remain still unclear.
METHODS
In this review, we will focus on the pathophysiology of perinatal stroke and on therapeutic strategies that can protect the immature brain from the consequences of stroke by targeting inflammation and brain microenvironment.
RESULTS
Studies in neonatal rodent models of cerebral ischemia have shown a potential role for soluble inflammatory molecules as important modulators of injury and recovery. A great effort has been made and is still in act to try neuroprotective molecules based on the new physiopatological acquisition.
CONCLUSION
In this review we aim to give a comprehensive view of new insights concerning pathophysiological mechanism of focal and global perinatal brain injury and its new therapeutic approaches.
Keywords: stroke, newborn, neuroprotection, brain repair, inflammation
INTRODUCTION
Perinatal stroke is defined as “a group of heterogeneous conditions in which there is focal disruption of cerebral blood flow secondary to arterial or cerebral venous thrombosis or embolization, between 20 weeks of fetal life through the 28th postnatal day, confirmed by neuroimaging or neuropathologic studies” 1. The term “focal” underlines the difference between this pathology and the more common neonatal hypoxic-ischemic encephalopathy (HIE), where injury is more often bilateral and may preferentially affect white or grey matter structures depending on regional and cell-type specific vulnerability at the time of the insult 2.
Perinatal arterial ischemic stroke (PAIS, Table 1) has an incidence of 1 in 2,300 to 5,000 births 3-7. It is a subset of perinatal ischemic stroke and it is associated with mortality and significant long-term neurologic morbidity. As opposed to white matter injuries, that affect typically preterm infants, PAIS occurs more frequently in term neonates. The clinical presentation of PAIS depends on the age at diagnosis: in newborns the main symptom is seizures, but also lethargy, hypotonia, poor feeding, irritability or apnea 8. Children who suffer PAIS typically develop long-term disabilities including motor deficits, epilepsy, cognitive and behavior disorders, deficits in language and vision. PAIS and HIE may share common risk factors and mechanisms, and can furthermore coexist in the same baby9. Most reported risk factors were derived from descriptive epidemiologic studies; thus, their causal relationship to perinatal stroke can only be assumed10.
Table 1.
Risk factors for PAIS.
| Maternal Factors | Fetal/neonatal factors | Others |
|---|---|---|
| Blood, homocysteine and lipid disorders | Inherited thrombophilias | Catheter-related complications |
| Thrombotic state during pregnancy | Twin tot twin transfusion | Male gender |
| Previous pregnancy related disorders | Systemic infections | Race and ethnicity |
| Primiparity, twin-twin pregnancy | Meningitis | Dehydration |
| Pre-eclampsia | Perinatal asphyxia | Trauma |
| Gestational diabetes | Congenital heart diseases | ECMO |
| Chorioamnionitis | Hypoglycemia (in preterm) | Emergency cesarean section |
| Labor and delivery complications | ELBW | |
| Autoimmune disorders | Polycythemia | |
| Drug abuse (cocaine) | ||
| Infertility and its treatment |
In this review, we will discuss data from rodent models of PAIS and hypoxic-ischemic (HI) injury to emphasize some of the common mechanisms of ischemic brain injury in the neonatal brain. We will describe potential therapeutic strategies considering that the sequence of key events in brain maturation is largely consistent between humans and rodents 11.
THE INTEREST OF RODENT MODELS
An elegant work 11 carefully marked the differences between rodents and humans in particular when comparing the maturational age of the CNS during normal and disrupted development. There is considerable cross-species alignment in terms of key developmental milestones, behavioural phenotypes and regional vulnerability to brain injury. It is now accepted that the maturation state of the brain, and in particular, specific processes of synaptogenesis and myelinisation, rather than chronological age, is the critical determinant of outcome after brain injury. Thus, comparisons can be made taking into consideration the timing of indices of neurobiological development, to gauge the impact of specific insults at different developmental stages, and best model the process of interest to the investigator. For a more detailed explanation of how the postnatal days in the animal model correlate with humans please refer to Table 2. Developmentally related differences are of importance not only to our understanding of the healthy brain during maturation, but also to predict differential responses to injury and potential therapeutics.
Table 2.
Summary of key developmental processes across comparable ages in humans and rodents.
| Human | Rodent | Developmental milestones | Reference (s) |
|---|---|---|---|
| 23–32 wk gestation (pre-term infant) |
pnd 1–3 | Oligodendrocyte maturation state changes—pre-dominance of mitotically active pre-OLsa. |
Craig et al. (2003), Lodygensky et al. (2010), Dean et al., 2011a and Dean et al., 2011b |
| Immune system development. | Holsapple et al. (2003) | ||
| Establishment of the blood-brain barrier. | Engelhardt (2003), Daneman et al. (2010) |
||
|
| |||
| 36–40 wk gestation (term infant) |
pnd 7– 10 |
Peak brain growth spurt. | Dobbing and Sands (1979), Bockhorst et al. (2008) |
| Peak in gliogenesis. | Catalani et al. (2002), Kriegstein and Alvarez-Buylla (2009) |
||
| Increasing axonal and dendritic density. | Cowan (1979), Bockhorst et al. (2008), Baloch et al. (2009) |
||
| Oligodendrocyte maturation state changes–switch to a pre-dominance of immature OLs. |
Craig et al. (2003), Dean et al., 2011a and Dean et al., 2011b |
||
| Consolidation of the immune system. | Holsapple et al. (2003) | ||
|
| |||
| 2–3 year old |
pnd 20– 21 |
Brain reaches 90–95% of adult weight. | Dobbing and Sands, 1973 and Dobbing and Sands, 1979, Dekaban et al. (1987), Giedd et al. (1999) |
| Peak in synaptic density at 50% > adult levels. |
Huttenlocher (1979), Micheva and Beaulieu (1996) |
||
| Peak in myelination rate. | Keshavan et al. (2002) | ||
| Neurotransmitter and receptor changes. | Hedner et al. (1986), Romijn et al. (1991) |
||
|
| |||
| 4–11 year old |
pnd 25– 35 |
Fractionation/specialization of prefrontal cortex neural networks (structural maturation). |
Tsujimoto (2008) |
| Maximum volume of grey matter and cortical thickness. |
Sowell et al. (1999), Bansal et al. (2008) |
||
In the literature there are sufficient evidences for selection of an age-appropriate rodent model that is predicated on biochemical and neuroanatomical changes during early postnatal development, as well as the emergence of age-specific behaviors. Ongoing in parallel research on cerebral development in both humans and rodents will provide a greater understanding of how all these factors interact, and how is the appropriate therapy for different injury and ages.
Another important aspect to be mentioned is the choice of the best model of hypoxia-ischemia. An ideal animal model, in addition to mimic the developmental stage of a human being, should also be reproducible and give the smallest variability. An important factor that influences variability in lesions obtained, and in related mortality and histopathological changes, is the cerebral blood flow supply. Across a wide range of species, two carotids and two vertebral arteries supply blood flow to the brain. However, the relative contribution of these large conductance vessels is highly variable. The cerebral circulation attempts to maintain constant cerebral perfusion despite changes in systemic conditions, due to its ability to autoregulate the blood flow. Occlusion of one carotid artery in rodents inconsistently results in brain ischemia unless combined with systemic hypotension. The combination of these insults in the P7 rat are needed to create a lesion. This is the principle used by the most important rodent models of systemic HI injury, which started with the Vannucci model in 1981 and that was further modified with the use of different ages and animal strands (Table 3). In contrast, a single permanent Middle Cerebral Artery (MCA) occlusion in the mouse appears sufficient to create an ischemic lesion. Heterogeneity within species observed in cerebral lesion size could be partly explained by collateral recruitment through the circle of Willis and/or through the cortical anastomoses between the vascular beds of the three terminal cerebral arteries. The lesion created by a single permanent artery occlusion is more similar to the lesion that we can have in PAIS, and for this reason is more and more used by scientists to investigate the effects of pure ischemia without hypoxia and the process of reperfusion injury 12.
Table 3.
Systemic hypoxia-ischemia (H-I) and ischemia-reperfusion rodent models
| HUMAN PATHOLOGIC CONDITION |
ANIMAL USED AND AGE OF THE ANIMAL |
TYPE OF DOMMAGE |
REFERENCE |
|---|---|---|---|
|
| |||
| H-I models | P7 Wistar rats | Permanent occlusion of the Middle Cerebral Artery (MCA), pMCAo + O2 8% |
Rice et al. 1981 |
| Rice’s model adapted to other rat’s ages |
“ | Derugin et al 1998, Oshima 2012 |
|
| Derugin 2005 | |||
| Mouse P7 | Blockade of the past External Carotid Artery- Internal Carotid Artery (ECA-ICA) bifurcation, external-internal carotid artery |
Oshima 2012 | |
| Mouse P8 | Permanent occlusion of the Middle Cerebral Artery (MCA), pMCAo + O2 8% |
Hagberg 2004 | |
| Mouse P10 | “ | Tsuji 2013 | |
| Mouse P12 | “ | ||
|
| |||
| Ischemia- Reperfusion Models (=Stroke-Like lesions) |
Wistar Rat P7 | pMCAo +bilateral transient Common Carotid Artery (tCCAo) occlusion |
Renolleau 1998 |
| Wistar Rat P10 | “ | Mitsufujii 1996 | |
| CB17 Mouse P12 | pMCAO | Tsuji 2013 | |
TIMING OF ONSET OF INJURY
Patterns of brain injury depend on the gestational age at which they occur 13: injury to the periventricular white matter in the preterm infant leads to permanent alterations in cerebral myelination, suggesting that oligodendrocytes are a target of injury. Oligodendrocytes progress through a phenotypic lineage comprised of successive stages of replication-capable progenitors that culminates with the postmitotic mature myelinating oligodendrocyte. Mature oligodendrocytes are relatively resistant to injury. During the window of vulnerability for periventricular white matter injury (gestational week, GW 23-32), before the onset of myelination, the subcortical white matter is populated predominantly by oligodendrocyte progenitors. Specifically, late oligodendrocyte progenitors (preoligodendrocytes) represent 90% of the total cells of the oligodendrocyte lineage present. Using a rodent model, it has been demonstrated that preoligodendrocytes manifest stage-specific vulnerability to one of the common insults occurring in early development, hypoxia-ischemia 14. Taken together this data suggests that injury specifically to preoligodendrocytes accounts for subsequent myelination defects after periventricular white matter injury in the preterm infant. Two mechanisms have been proposed for the selective vulnerability of oligodendrocyte progenitors: oxidative stress and excitotoxicity. Preoligodendrocyte sensitivity to oxidative stress has been demonstrated in vitro by glutathione depletion and exposure to exogenous free radicals, and exogenous antioxidants protect preoligodendrocytes from glutathione depletion 15,16. Mature oligodendrocytes, in comparison, are highly resistant to oxidative stress, in part because of differences in expression levels of antioxidant enzymes and proteins involved in programmed cell death 17. Cognitive and sensory impairments are associated with periventricular white matter injury and are observed with increased frequency with decreasing gestation 18. Cortical visual impairment is particularly common in infants with severe injury 19. These observations suggest widespread abnormalities of cortical development after periventricular white matter injury, especially of the posterior visual pathways. It was determined that subplate neurons, cells that play a critical role in normal visual thalamocortical development 20, are selectively vulnerable to neonatal hypoxia-ischemia in the postnatal day 2 (P2) rodent model 21. This rodent model is particularly relevant to the human preterm infant given the similarities in brain development 22, oligodendrocyte lineage progression 23, and the propensity to subcortical injury with HI insult 21 (Table 2). Subplate neurons are located beneath the developing neocortex 24, near areas of white matter signal abnormality observed on magnetic resonance imaging in preterm human infants 25 at risk for the diffuse type of periventricular white matter injury. Subplate neurons are a transient cell population that undergoes programmed cell death in the first postnatal week in mice 26. In humans, the subplate zone peaks at the onset of the developmental window of vulnerability to periventricular white matter injury (GW 24), undergoes dissolution during the third trimester, and is largely absent after 6 months of postnatal age 27. At its peak of development in human, the subplate zone is four times the width of the cortical plate 27. Subplate neurons are involved in the formation of area-specific thalamocortical connections 26, and early subplate neuron ablation in cat prevents visual thalamo- cortical innervation 28. However, periventricular white matter injury in human occurs after geniculocortical innervations 29. Subplate neurons become incorporated into mature synaptic networks in the developing neocortex 30, and later subplate neuron ablation is linked to a disruption in the functional maturation of visual cortical columns, including ocular dominance 31 and orientation selectivity 32. Late subplate neuron ablation leads to impaired synaptic transmission of visually driven activity from the lateral geniculate nucleus into cortical circuits 32, findings consistent with the cortical visual impairment observed in preterm humans with periventricular white matter injury 19. In the rodent model, as in human, selective subplate neuron cell death after early hypoxia- ischemia occurs after the development of the geniculocortical projection, so that visual thalamocortical innervations is not disrupted. Rodents do not have anatomically segregated eye-specific thalamic input to visual cortex, thus the effects of subplate neuron cell death on patterned visual cortical innervation cannot be assessed in this model. However, the development of the patterned somatosensory whisker representation is not affected 21. The mechanism of selective vulnerability of subplate neurons to early hypoxia-ischemia is not known. Because neurons undergo programmed cell death during normal development to a much greater extent than other cortical neurons 33, there may be an enhanced susceptibility to programmed cell death after appropriate triggers,a finding observed in certain immature cortical neurons 34. In addition, subplate neurons are known to mature earlier than other cortical neurons as assessed by the expression of a variety of markers 35, including a developmentally related increase in the NMDA-R1 glutamate receptor expression 36 and AMPA and kainite receptor 37.
Term human infants demonstrate a predilection for injury to thalamus and basal ganglia after hypoxia-ischemia in the neonatal rodent (P7), there are at least biphasic stages of neurodegeneration: early (1.5 to 3 hours) in forebrain and late (6 days) in striatum and thalamus 38. Recent anatomic studies demonstrate injury evolution and ongoing cell death through 168 hours 39 in rat cortex and striatum, as well as in mouse hippocampus 40. The nature of this cell death can be complex, and has recently been termed the “apoptotic-necrotic continuum “ 41. However, late cell death in the thalamus is programmed cell death and is selective for sensory thalamic nuclei (lateral geniculate and ventral basal) 42 well established that programmed cell death plays a prominent role in normal development 43, and this may account for an enhanced susceptibility to programmed cell death after injury in different regions of the developing brain. A role for programmed thalamic cell death in neonatal hypoxia-ischemia is supported by the observation of Fas death receptor expression, cytochrome c release, and cleavage of procaspase 8 44. Fas, a member of the tumor necrosis family of receptors, plays a central role in the programmed elimination of lymphocytes in the immune system, and upregulation of Fas ligand (Fas-L) has increasingly been recognized in neuronal cell death resulting from trophic factor deprivation, injury, or stress 45. Similar delayed programmed cell death in the thalamus is observed after mechanical injury to the developing visual cortex 46, suggesting that the cell death results from target deprivation. HIE in the term human infant is associated with selective injury to the deep gray nuclei, especially the basal ganglia. Within the basal ganglia, neuronal nitric oxide synthase (nNOS) expressing striatal neurons represent an example of selectively targeted cells, and these neurons are mechanistically involved in the selective vulnerability of nearby striatal projection neurons that do not express nNOS. nNOS-containing interneurons throughout the central nervous system produce nitric oxide (NO) dependent on the coupling and activation of the NMDA receptor and calcium entry 47. The enzyme is maximally expressed in regions where the immature NMDA-R is expressed, especially the basal ganglia 48. When NO is produced in excessive amounts during periods of oxidative stress in these regions of abundance, nitric oxide is converted to peroxynitrite, a potent mediator of free radical injury 49. nNOS-expressing neurons are resistant to both hypoxia-ischemia an NMDA-mediated excitotoxicity 50,51, but this selective sparing of nNOS-expressing striatal neurons to NMDA agonists is limited to young ages (P7 in rodent) and as the brain matures this resistance is lost 51. On the other hand, nNOS-expressing neurons are vulnerable to AMPA agonists 52. Thus the selective vulnerability of striatal projection neurons to neonatal hypoxia-ischemia may result from a bystander effect attributable to their proximity to this enriched population of nNOS-expressing neurons. This hypothesis is supported by the selective ablation of nNOS-expressing neurons with AMPA agonists before hypoxia-ischemia; a manipulation resulting in reduced injury from HI insult53. The susceptibility to injury of the immature brain was assumed to be lower in the perinatal period as compared to childhood 54,55. This concept was recently challenged by clinical studies showing marked motor disabilities after stroke in neonates 54,56, with the severity of motor and sensory deficits similar in both perinatal and childhood ischemic stroke. Since PAIS results mostly from occlusion of the middle cerebral artery (MCA), the functions dependent on the brain regions supplied by this vessel (e.g. motor cortex) are more frequently affected 57-59. Furthermore, in preterm infants, the structural immaturity of blood vessels, which appear thin walled, may predispose to bleeding 60. Previously some studies have shown that the immature brain has a large potential to compensate for perinatal injury to the motor system 61, 62, but recently this concept has questioned by several authors63,64
PATHOPHYSIOLOGICAL MECHANISMS OF PERINATAL ISCHEMIC BRAIN INJURY
Cellular injury and neuronal cell death
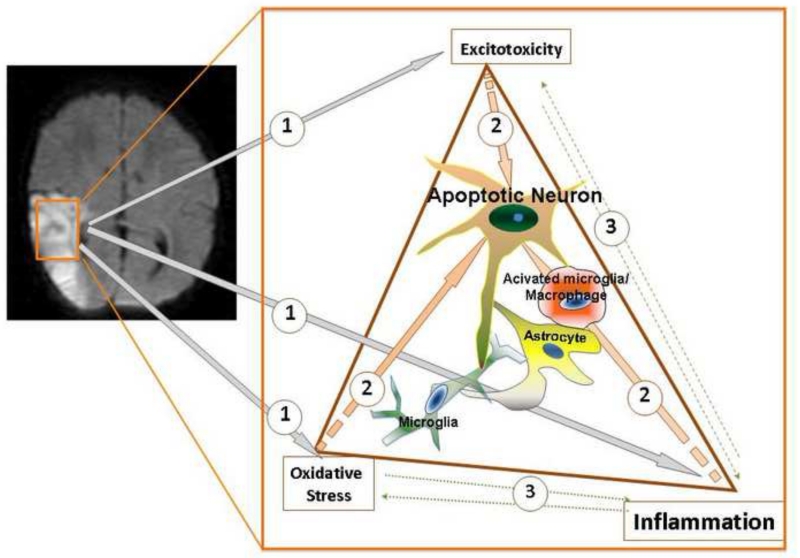
Excitotoxicity, free radical formation and activation of the inflammatory cascades are the main mechanisms of ischemic brain injury at term (Figure 1). Each of these injury components elicits injury independently and they act in concert to aggravate injury.
Figure 1. The mechanisms of injury in perinatal stroke and HIE.
A. A representative MRI demonstrates a focal ischemic lesion in perinatal stroke. B. Schematic representation of the major mechanisms contributing to neuronal death in post-ischemic neonatal brain. Excitotoxicity, oxidative damage and neuroinflammatory processes are the main mechanisms of injury (shown as (1)). These mechanisms of injury can independently lead to spectra of neuronal death mechanisms (2), but also feed and potentiate each others effects (3), further exacerbating brain injury.
Failure of ATP dependent calcium pumps results in increased intracellular calcium concentration, which is directly toxic to the mitochondria and activates several DNAses, proteases and lipases 65. Failure of intracellular metabolism, particularly ATP depletion, results in neuronal depolarization and release of glutamate, which activates post-synaptic NMDA receptors, and other glutamate receptors, allowing for a greater influx of calcium and further cellular injury 65-68. Higher levels of glutamate receptor expression 69, a different composition of individual NMDA receptor subunits 70,71, and intrinsic differences in the GABAergic system, which is immature and excitatory during early postnatal brain development, also contribute to excitotoxic injury 69,72. Nitric oxide synthase (NOS), superoxide dismutase (SOD) and NADPH oxidase activated by ischemia form potent reactive oxygen species (ROS) 65,67,68.
In rat, ionotropic glutamate receptors undergo rapid maturational changes: NMDAR density peaks late in the first postnatal week in many forebrain structures, including hippocampus and the neocortex 73, whereas AMPAR density peaks in the second postnatal week at around P10 73. Both NMDA receptors and AMPA receptors overshoot adult expression levels, resulting in heightened glutamate-mediated plasticity 74,75. Maturational regulation of glutamate receptor subunit composition also enhances their ability to mediate activity-dependent synaptic plasticity in early postnatal life. The maturational regulation of AMPA receptor composition and function also enhances glutamate-mediated plasticity in early postnatal life. The ratio of GluR2 expression to that of other AMPA receptor subunits is significantly lower in immature neocortex and hippocampus compared to the adult 76,77, and AMPA receptors that lack a GluR2 subunit exhibit higher Ca2+ permeability than those that contain GluR2 78-81. The increased Ca2+ influx through AMPA receptors in immature neurons may trigger mechanisms of plasticity that would not be triggered in the mature brain. In addition, the presence of such receptors may provide potential mechanisms for excitotoxicity. Glutamate receptors are also developmentally regulated on non-neuronal cells. In particular, olygodendrocytes express functional glutamate receptors in vitro, and these are exclusively of the non-NMDA subtype 82-84. Glutamate has been shown to be toxic to oligodendroglia in vitro by receptor-independent 16,85-87, and receptor-mediated mechanisms 88-91.
Reperfusion and associated reoxygenation of the ischemic brain tissue following focal ischemic stroke causes a second wave of ROS formation that occurs in activated peripheral leukocytes and in multiple cell types in injured brain regions 65,67,68. Inflammation associated with ROS triggers production and release of various toxic mediators, including cytokines, amplifying injury. As a result, necrotic neuronal death begins occurring almost immediately in the “core” of the ischemic region whereas neuronal apoptosis and several intermediate cell death states that exhibit features of both necrosis and apoptosis, occur within hours to days following ischemia, mostly affecting cells in the penumbral regions 92,93.
Zhou et al 94 showed that pyramidal neurons engaged in cortico-cortical connectivity in limbic cortex are vulnerable to denervation lesions. At least one trigger of this transsynaptic degenerative phenomenon is the activation of inhibitory interneurons in layer I, which are induced to upregulate neuronal nitric oxide synthase (nNOS) and release NO.
The improvement of transsynaptic degeneration with AMPA antagonist confirm that transsynaptic apoptosis and anoxic/ischemic neuronal necrosis are both glutamatergic events that involve the synthesis and release of NO 95. Although the apoptotic or necrotic outcome is related to levels of superoxide anion generated with NO release (i.e., apoptosis involves a lesser degree of free radical burden 96), the two mechanism have distinct neuropathology. In its classical formulation, anoxic-ischemic injury is a necrotic phenomenon that involves an early activation of NMDA receptors and the downstream induction of nNOS and intracellular toxic release of NO. In contrast, transsynaptic cell death is an apoptotic phenomenon that appears to involve the interaction of two neurons, one of which upregulates nNOS in response to AMPA signaling and releases NO to the toxic effects of which it is resistant because of a concomitant induction of the manganese isoform of SOD, a known superoxide anion scavenger in vivo 95,97,98.
On the other hand, the success of therapy with AMPA receptor antagonist in HIE white matter injury in P7 rat does not exclude that the transsynaptic mechanism may be involved in HI injury, too.
Both caspase -dependent 99,100 and –independent 100,101 pathways contribute to neuronal death after brain injury. The abundance of caspases in the neonatal brain, in particular caspase-3, may in part account for a more marked caspase-3-dependent cell death observed after neonatal brain injury than after similar injury in the adult. Neuronal death and injury are preferentially reduced following pharmacological inhibition or genetic deletion of PARP-1 in male pups 102, but following caspase-3 inhibition in female pups 100. Hagberg’s work suggests that the degree of PAR accumulation during early (1–4 h) post-HI reperfusion was similar in females and males, whereas the drop in NAD+ was only found in males and in consequence the degree of mitochondrial impairment may depend on gender. PARP-1-dependent neuronal injury in vitro has also been shown to rely on translocation of apoptosis inducing factor (AIF) from mitochondria to the nucleus103; furthermore, the genes for AIF, as well as for several other proteins involved in perinatal hypoxia- ischemia that may be related to PARP-1 (e.g.: X-linked inhibitor of apoptosis), are localized on the X-chromosome and may, in addition to NAD+, be differentially expressed in males and females. On the other hand, Renolleau et al. showed that concentration of cytochrome c release did not differ between males and females but it appeared as a sharp peak at 12 h post-ischaemia in males and then rapidly decreased at 24 h, whereas a regular increase from 0 h up to 16 h followed by a slight decrease at 24 h was observed in females. This might represent a mitochondrial dysfunction in males, but not in females. Recent data demonstrated that male neurons displayed a more pronounced translocation of apoptosis-inducing factor (AIF) and female neurons a stronger activation and cleavage of caspase 3 104,105. Accordingly, it is also interesting to note a gender-specific neuroprotection by iminobiotin, an inhibitor of nitric oxide synthases, via the cytochrome c and caspase 3 after HI insult in female P7 rats104, suggesting that the intrinsic apoptotic pathway might predominate in females. Sex differences have also been reported for hypothermia, which provides more effective long-term neuroprotection in female than in male 7-day-old rats 106.
It also to be considered the importance of calcium in ischemic cell death, calcium antagonists came into the focus of ischemic neuroprotection 107. Magnesium prevents cellular calcium influx and excitatory aminoacid release in neurons 108 by blockade of N-type and L-type calcium channels 109, prevents cellular calcium entry through NMDA-receptor channels 110, reduces calcium-induced mitochondrial dysfunction 111 and preserves cellular energy metabolism 112. By these mechanisms, magnesium may inhibit or delay ischemic cell death during and after cerebral ischemic events 113. However, despite promising results in animal models, magnesium was not found to reduce excitotoxicity following adult stroke. A multicenter phase III trial was conducted to determine the effect of magnesium therapy on outcome in aneurysmal subarachnoid hemorrhage. A total of 1,204 patients were administered intravenous magnesium sulfate (64 mmol/day) or placebo and evaluated for outcome on the modified Rankin Scale for up to 90 days. No improvement in outcome was seen with magnesium treatment versus controls. In addition, a retrospective analysis of 2,047 patients from previous trials was also performed and similarly concluded that magnesium has no benefit in the treatment of aneurysmal subarachnoid hemorrhage114,115.
Blood-brain barrier (BBB) disruption and contribution of peripheral immune cells
BBB breakdown is a key injury factor in stroke. Both the structure and function of the BBB are influenced by a number of relatively independent pathophysiological processes, including oxidative/nitrosative endothelial cell damage, cytokine-dependent activation of the endothelium, altered phosphorylation and cellular localization of endothelial tight junction proteins, and degradation of individual BBB components by activated proteases 116-120. Transmigration of peripheral immune cells and retraction of astrocyte endfeet further affect functional integrity of the BBB 121. Paradoxically, functional integrity of the BBB is better preserved after acute neonatal stroke than after adult stroke in the rat122. Compared to acute adult stroke, the expression of several tight junction proteins (occludin, ZO-1 and claudin-5) is better preserved after acute neonatal stroke, which would support the integrity of tight junctions in injured neonates 122. However, it is unknown if phosphorylation or subcellular distribution of the tight junction proteins following stroke differs between the two ages. The higher resistance of the BBB to stroke in neonates is likely related to the maturation-dependent leukocyte-vascular interaction after injury. Compared to acute adult stroke, infiltration of neutrophils and monocytes is low after neonatal HI injury and focal stroke despite markedly increased leukocyte chemoattractant levels in injured neonatal brain 122,123.
The active pathophysiological role of the systemic inflammation was demonstrated in experimental stroke in the adult 124. In newborns with ischemic brain injury, several clinical studies showed an increase in cytokine and chemokine serum levels 125. Hu et al 126 showed a hypoxia/ischemia-induced alteration of cortical development by proteome analysis of the cortex 2h after HI. Of the altered proteins, 14-3-3ɛ and TUC-2, both playing an important role in the development of the CNS, decrease after HI events, consistent with an early disturbance of cortical development. These observations suggested that changes in peripheral levels of individual inflammatory molecules may potentially serve as indicators of both cerebral damage and prognosis.
Glial cells and neuroinflammation
Endogenous brain macrophages—microglial cells—are the main cell type that provides immuno-surveillance in the brain, but these cells have been traditionally considered deleterious after stroke due to production of cytokines, chemokines, ROS, induction of proteases in response to injury and ultimate activation of cell death mechanisms and degradation of BBB components. However, microglial cells are the source of growth factors, and several known mediators produced in microglia/macrophages, such as MMPs, can exert dual effects: they can harm during the initial injury stages but enhance neural repair through remodeling of the extracellular matrix and the assembly of a neurogenic niche during the recovery phase 127.
The microglia/macrophage population is heterogeneous. Microglia is capable of acquiring diverse phenotype in response to injury: M1 (classically activated microglia with cytotoxic properties in response to infections), M2a (alternate activation and involvement in repair and regeneration), M2b (immunoregolatory phenotype), M2c (acquired-deactivating phenotype). Functions are known to overlap and be context dependent128,129. It was considered that cytotoxic phenotype develops due to insult/injury and the same cell shift to an M2-repair/regenerative phenotype over time130 and depends on the combination of specific signals received from the local microenvironment 131. However the precence of an early M2 phenotype (preceding M1) has recently been reported in a after hypoxic/ischemic insult in vivo132. Furthermore, brain macrophages that originate from invading monocytes may affect injury progression differently than activated microglia. It remains still unknown if the phenotypical composition of the microglia/macrophage population is the same during early postnatal development and adulthood.
Interestingly, selective depletion of microglia (by intracranial administration of liposomes containing clodronate) in neonatal rats does not improve, but worsens brain injury during the sub-acute phase after MCAO 133. A larger infarct size in these animals is associated with additional accumulation of several pro-inflammatory cytokines and chemokines, including TNFα, MCP-1 and MIP-1a 133 and expression of these mediators, which are typically produced by microglia, is overcompensated by production in other cells (astrocytes, neurons and endothelial cells) when microglia are absent 133. These data indicate that at least a subpopulation of microglial cells can exert beneficial effects in injured neonatal brain, likely by acting as a “buffering” component in the brain inflammatory response.
Astrocytes actively participate in several pathophysiological stroke mechanisms, including excitotoxicity through regulation of the extracellular glutamate accumulation, oedema formation through increased aquaporin-4 expression, inflammation through cytokine production, and changes in BBB stability due to retraction of astrocyte endfeet from the vascular surface 134,135. Compared to astrocyte functions in adult stroke, relatively little is known about the role of these cells in injury in the neonate. The finding that expression of several cytokines and chemokines in astrocytes is increased after focal ischemia in neonatal rats with pre-depleted microglia 133 suggests that astrocytes modulate the inflammatory response early after injury. However, genetic deletion of GAFP and attenuated reactive gliosis in the developing brain does not affect infarct volume after sub-chronic HI insult 136.
Receptor-mediated and intracellular signalling pathways
Death receptors
Death receptors, such as TNF-α receptor 1 (TNFR1), Fas, and TNF-related apoptosis inducing ligand (TRAIL) receptors, respond to inflammatory cytokines and initiate the extrinsic apoptotic pathway through the oligomerization of the adaptor molecule Fas-associated protein with death domain (FADD) with the initiator caspase, caspase-8 137. Activated caspase-8 then cleaves and activates effector caspases. Upregulation of Fas receptor after neonatal HI injury is concomitant with increased cleavage of Casp-8, -9 and -3 and neuronal apoptosis 42,138. Genetic deletion of Fas protects 138, in part via increased expression of its counteracting protein c-FLIP 139.
Toll-like receptors (TLRs) and scavenger receptor CD36
TLRs are a receptor superfamiliy that mediate innate immune responses and activation of microglia in response to changed local microenvironment140. TLR intracellular signalling is complex and involves the activation of NF-kB and the subsequent expression of different gene sets 140. TLR2 and TLR4 are the two most studied members of the TLR superfamily in the context of brain ischemia. While TLR4 seems to be purely injurious, TLR2 may elicit context- and tissue-dependent effects—injurious 141,142 or beneficial 143—based on the types of heterodimers that it forms with other TLRs. Administration of selective TLR2 and TLR4 agonists to neonatal rats impairs normal postnatal myelination and induces hippocampal neuronal death even without stroke 144, indicating that activation of innate immunity is sufficient to interfere with normal postnatal development of the white matter. However, the role of TLR4 on progression of neonatal HI injury is unclear, since deletion of its intracellular effector protein, Myd88, does not result in improve neuroprotection 145. At the same time, TLR2 deletion does reduce injury 146, suggesting that the particular contribution of each TLR subtype to injury differs between adults and neonates.
The scavenger class B receptor CD36 can signal independently as well as in cooperation with TLRs. Genetic deletion of CD36 protects against acute injury after MCAO in the adult 147,148 but worsens injury in the neonate 149. While the exact mechanisms of such striking age-dependant differences are largely unknown, the pattern of changes for CD36 and its downstream effectors may be important injury modifiers. Genetic deletion of CD36 is associated with diminished engulfment and phagocytosis of apoptotic neurons that are abundant in injured neonatal brain and results in additional accumulation of cleaved caspase-3 149. CD36 toxicity depends on superoxide produced through NADPH oxidase (Nox) activation, but the relative contribution of individual Nox isoforms 150 and superoxide utilization 151 in injured neonates differs from that after focal stroke in adults. A markedly increased superoxide accumulation in microglia/macrophages in acutely injured adult brain 148, but not in neonatal brain 149, may activate distinct CD36-dependent intracellular signalling pathways.
Nuclear factor kappa B (NF-kB)
NF-kB is a ubiquitously expressed transcription factor that regulates expression of genes involved in inflammation, cell survival and apoptosis and plays a major role in regulation of neuronal death and injury in the ischemic brain 152. Activation of several signalling pathways after neonatal brain injury converges in the activation of NF-kB. Upon stimulation, dissociation of NF-kB from its endogenous inhibitor protein IkB allows NF-kB to translocate from the cytoplasm to the nucleus and induce an array of genes in a cell-type and stimulus-specific way. NF-kB activation leads to the production of inflammatory mediators, but also induces the expression of pro-survival factors including antioxidants, growth factors and antiapoptotic molecules 153,154. This dual role of NF-kB may underlie the time-dependent and sometimes opposite effects of NF-kB inhibition observed after neonatal HI insult. NF-kB inhibition during the early phase of activation (0-3 h) after HI injury limits neuroinflammation, prevents up-regulation and accumulation of NF-kB in the nucleus, reduces caspase-3 activation, and leads to marked long-term functional neuroprotection 155 and improved cognitive outcome 156. In contrast, inhibition over a more extended time period after HI events abolishes the protective effect on neuronal apoptosis and exacerbates injury 155. These opposing results suggest that the time window of NF-kB inhibition needs to be considered carefully in order to translate these results to the clinic.
Individual mediators of injury
Reactive oxygen species (ROS)
Production of superoxide, hydrogen peroxide and other ROS is a part of normal brain function 157,158 but excessive accumulation of these species and the subsequent formation of hydroxyl radicals, lipid peroxides and peroxynitrate contribute to ischemic injury by altering cellular components and intracellular signaling, and by disrupting BBB integrity 159,160. The neonatal brain is particularly susceptible to ROS accumulation due to a limited activity of endogenous antioxidants and anti-oxidative enzymes 161,162. Consistently, HI injury is diminished in transgenic mice over-expressing glutathione peroxidase-1 (GPx) 163,164, or by antioxidants 165,166. However, in contrast to a functional neuroprotection in adult stroke, overexpression of SOD-1 exacerbates injury following neonatal HI insult 151. These opposite effects are due to a further increase in H2O2 in injured neonatal brain because of insufficient catalase and GPx activity and the consequent metabolism of accumulated H2O2 produced via SOD-1 activation.
NO has dual effects on ischemic injury: it mediates vasodilation and neuroprotection when produced by endothelial NOS (eNOS), but is a major mediator of oxidative/nitrosative damage when produced by neuronal (nNOS) and inducible NOS (iNOS) 159,167. Genetic deletion and pharmacological inhibition of iNOS and nNOS reduce injury associated with HI insult 159,168-172. NO produced by iNOS also forms toxic peroxynitrate and increases activity of inducible cyclooxygenase (COX-2) 173,174, further propagating neonatal brain injury.
Cytokines
Increased cytokine production has been traditionally related to inflammatory states and injury progression following brain ischemia in adults. Several cytokines have been shown to modulate injury after neonatal HI events and focal stroke, as shown in Table 4. Expression of IL-1β is rapidly and locally upregulated after HI injury and transient MCAO in neonatal rodents 175,176. Increased CSF levels of IL-1β have been identified as a marker of severe injury during the first 24 hours after asphyxia in babies 177,178, but the effects of this cytokine in injured neonatal brain are complex. While IL-1β contributes to injury after neonatal HI insult 179, genetic deletion of IL-1α or IL-1β, alone or in combination (IL-1αβ knockout), does not protect one week after HI events 180, whereas administration of IL-1 receptor antagonist (IL-1Ra) protects 175,181. Furthermore, the data that brain levels of IL-1β remain elevated when pharmacological neuroprotective effects are achieved 182,183, suggests that a decrease in IL-1β levels, induced by injury, is not necessarily required for protecting brain from focal/global injury. TNF-α and IL-6 mRNA and protein expression are also up-regulated after neonatal HI injury 175,184. TNF-α induces biological activity via stimulation of the tumor necrosis factor receptor (TNFR) 185,186.
Table 4.
Pro- and anti-inflammatory mediators involved in the ischemic cascades.
| Mediators | Post ischemia | Neuroprotection | Treatment | CK target | Effect |
|---|---|---|---|---|---|
|
| |||||
| IL-1 | upregulated in H-I in mice |
IL1Ra ↓ brain injury | |||
|
| |||||
| IL-18 | upregulated in H-I in mice |
IL18 KO mice ↓ brain injury in neonate |
|||
|
| |||||
| TNFα |
|
? | R-7050 | TNFα | Attenuate neurovascolar injury |
|
| |||||
| IL-2 | ? | ? | |||
|
| |||||
| IL-6 | upregulated in H-I in mice |
? | |||
|
| |||||
| IL-8 | ? | ? | |||
|
| |||||
| IL-9 | induce histamine release→cell injury |
inhibit post-mitotic neuronal apoptosis in the newborn mouse cortex |
|||
|
| |||||
| IL-10 | ? | exogenous IL10 therapy |
|||
|
| |||||
| IL-4 | ? | in adult stroke | |||
|
| |||||
| INFγ | ? | IL4/ INFγ ↓ apoptosis in oligodendrocyte progenitors and astrocytes |
|||
|
| |||||
| NFkB | dual role pro-anti apoptotic |
Precoce (0-3 h) inibhition ↓ neuroinflammation and Casp 3 activation |
Minocycline | NF-kB | Attenuation of the postischaemic inflammatory response and ↓ in white matter damage in the immature rodent brain (HI model) |
|
| |||||
| MIP | upregulated in H-I in mice |
Estradiol | MIP-2; CCR7 | MIP-2; CCR7 | |
|
| |||||
| MCP/CCL2 | upregulated in H-I in mice |
Genetic deletion/ pharmacological inhibition |
vMIP-II chemokine analogue peptide acting as antagonist against several chemokines and chemokine receptors. |
Various chemokines (CCL2, CCL3) and chemokine receptors (CCR1,2,3,4,5,8; CXCR3,4). |
Attenuation of brain infarction in mice |
|
Dimemorfan sigma-1 receptor agonist. |
CCL2 | ↓ of infarct size and glutamate- mediated excitotoxicity in rats |
|||
|
Rosiglitazone agonists of ligand- activated transcription factors (PPAR)-γ. |
CCL2; CXCL8 | ↓ of infarct size and improved functional outcomes in mice |
|||
|
| |||||
| RANTES | upregulated in H-I in mice |
? | |||
|
| |||||
| H2O2 | injure neonate mice/rats after H-I |
? | N-acetylcysteine | Oxygen radical scavenger |
↓ in cerebral oxidative stress and cerebral injury; improvement in myelin expression and neurological outcome in neonatal rats (combined with hypothermia) (HI model) |
| Melatonin |
Oxygen radical scavenger |
↓ in white matter damage and promotion of repair by induction of axonal regrowth or sprouting in newborn mice (ibotenate- mediated excitotoxic brain injury). ↓ in oxidative damage mediators in 7- day-old rats (HI model) |
|||
|
| |||||
| NO | nNOS, iNOS | eNOS inhibition of nNOS, i- NOS |
2-iminobiotin | Selective nNOS and iNOS inhibitor |
Improvement in cerebral energy state, ↓ in vasogenic oedema and neuronal death (HI model) |
| 7-nitroindazole | Selective nNOS inhibitor |
Suppression of both two peaks of NO metabolites (in hypoxia and re-oxygenation period) (HI model) |
|||
| Aminoguanidine | Selective iNOS inhibitor |
Suppression only theNOmetabolites peak in the re- oxygenation period. ↓ in cerebral injury in a neonatal rats (HI model) |
|||
|
| |||||
| COX-2 | upregulated in H-I | COX2 inhibition ? | |||
|
| |||||
| CXC | increased in periphery and in the brain in neonatal MCAO; protect BBB early after injury |
CXC12L→improvement of neurogenesis, neuroblast migration, vasculogenesis |
JWH-133 Synthetic cannabinoid 2 receptor agonist |
CXCL2 | Inhibition neutrophil migration CXCL2 - mediated in ischaemic brain in mice |
|
Candesartan angiotensin AT1 receptor blockers. |
CXCL1 | Down regulation of CXCL1 and TNF- α expression and reduction of cerebral infarct size in rats |
|||
|
Pioglitazone thiazolidinediones |
|||||
|
| |||||
| Galectin-3 | upregulated in H-I in mice |
Gal3 KO mice ↓ injury in neonate |
|||
|
| |||||
| Caspases | markedly upregulated; cross- talk between inflammatory mediators and apoptosis pathways |
|
|||
Beneficial effects of therapeutic agents against HI damge are often associated with reduced brain levels of TNF-α, but the outcomes of inhibition of this mediator and the relative contribution of signaling via TNFR1 and TNFR2 remain largely undefined. TNF-α damages oligodendrocyte progenitors in vitro 187,188, but genetic deletion of TNF-α leads to impaired differentiation of myelinating oligodendrocytes during the postnatal period 189. Recently R-7050, a novel cell-permeable triazoloquinoxaline, has been shown to attenuate neurovascular injury and improve neurobehavior sequels,by selectively inhibiting TNF-α induced cellular signaling 190.
Chemokines
Several studies have suggested an important role for chemokines in cerebral damage in models of ischemic stroke, HI and excitotoxic injury 191. In P7 rats, secretion of a macrophage inflammatory protein-1α and MIP-1β mediates inflammatory cell recruitment and activation after HI insult 192. Another chemokine, the macrophage chemo-attractant protein (MCP)-1/CCL2, is a potent mediator of ischemic cerebral injury in adults 193,194 and neonates 195, likely by attracting circulating monocytes. MCP-1 also mediates excitotoxic injury in the neonatal rat brain 196, and the genetic 197 or pharmacological 198 inhibition of MCP-1-dependent pathways reduces cerebral damage, supporting a role for this chemokine in the early phases of the ischemic insult.
The CXC chemokine CINC-1/KC has been recently shown to paradoxically contribute to both BBB integrity and reduction of brain injury after MCAO in neonatal rats 122. Changing the balance between the CINC-1 levels in plasma and in the brain increases transmigration of neutrophils, disrupts vascular function and extends injury 122.
CXCL12 (SDF-1), a chemokine that is upregulated in astrocytes and brain vessels in the peri-infarct area following adult stroke, has been shown to mediate the recruitment of circulating leukocytes into the brain 199,200. In the neonatal brain, reduced activation of the CXCL12 receptor, CXCR4, by dexamethasone, leads to decreased lesion size following HI injury 201. Importantly, the sustained induction of SDF-1 after stroke in adult mice but not in neonatal mice 202 may indicate a shorter temporal window for SDF-1 -mediated repair in the neonatal brain.
MEDIATORS OF CELL SURVIVAL AND RECOVERY
Growth factors and neurotrophins
The pharmacological and histologial neuroprotective effects of Brain Derived Neurtrophic Factor have been firmly established in adult experimental stroke 203-205 and at least several underlying mechanisms have been identified, including activation of pro-survival mechanisms 206, modulation of local inflammation 207, and reduction of excitotoxic injury 204,208. In neonates, it protects against HI injury via ERK activation and blockade of caspase-3 activation 209,210. BDNF is known to promote endothelial cell survival and mediate neurogenesis in ischemic tissue in the adult 211 and is likely to contribute angiogenesis in injured neonates.
Angiogenesis is essential for long-term repair, but recent findings show that in contrast to a relatively rapid induction of angiogenesis in the adult brain after MCAO, within days, in the neonate, increase in angiogenesis does not seem to occur for at least 10-14 days after MCAO 212. Experimental studies from the adult brain suggest that the “early” up-regulation (between 1 h and 3 h post-MCAO) of VEGF could be associated with alterations in BBB permeability and contribute to exacerbating injury 213. In both neonatal and adult models following hypoxia, intracerebroventricularly injection of VEGF 48h after Ischemia resulted in reduced brain injury, decreased infarct volume and decreased apoptotic cells without increasing BBB permeability. Both studies suggest this is related to the activation of the Akt/ERK pathway 214, 215. Inhibition of VEGFR-2 has also been shown to decrease endothelial cell proliferation, increase cell death and worsen injury following neonatal stroke in rodents 216. Taken together, these results indicate that VEGF-dependent angiogenesis is important for recovery of a neonatal stroke.
Another key hypoxia inducible gene is erythropoietin (EPO) that increases the capacity of red blood cells to supply oxygen following hypoxia 217,218. Hypoxic up-regulation of EPO is regulated by HIF-1α and HIF-2α in vivo and in vitro 217,219,. EPO is widely expressed in the brain by astrocytes, neurons, microglia, and endothelial cells 218,220. Following cerebral ischemia, endothelial cells are the first to increase EPO expression, which could implicate that EPO mediates angiogenesis, probably by stimulating the expression of VEGF and its receptors on endothelial cells 221,222. In vitro, EPO can also modulate angiogenesis by stimulating endothelial cell migration and proliferation 223. Suggested to be neuroprotective following hypoxia, increased EPO expression has also been shown to increase anti-apoptotic gene expression and promote survival in oligodendrocytes, neurons, astrocytes, and microglia 217,218. Treatment with recombinant human EPO following focal hypoxia-ischemia in neonatal rats results in enhanced revascularization, neurogenesis, endothelial cell and neuronal survival and increased Glut-1, Tie-1, and angpt-2 expression which resulted in enhanced neurovascular unit repair 221,224. EPO treatment following neonatal stroke in rats has also shown significant neuroprotection ,225-227, after neonatal HI injury 228 and MCAO 229,230.
Mediators with dual effects
Some of the mediators that are upregulated and are injurious during the acute injury phase, such as NO, MMPs, MIP-1a, MCP-1, and complement, may be beneficial and mediate the repair. Activation of MMPs, MMP-9 in particular, after stroke is thought to contribute to acute brain injury 231, but MMPs are critically involved in the remodelling of extracellular matrix and migration of immature neurons from the SVZ into the striatum 232. Galectin-3 (Gal-3), a multifunctional carbohydrate-binding protein that controls numerous cell functions, has been identified as an angiogenic factor in adult stroke 233. Like MMP-9, Gal-3 may have dual roles after stroke—harm initially but promote long-term repair. Anti-inflammatory agents may protect in part by reducing a subpopulation of microglia/macrophages with upregulated Gal-3. Genetic deletion of Gal-3 exacerbates injury and increases apoptosis after MCAO in the adult 234 but reduces hippocampal injury after HI insult 235. Microglia-mediated IGF-1 production 234 and increased endothelial proliferation 233 were suggested as mechanisms of Gal-3-mediated remodeling after adult stroke. Gal-3 expression is rapidly increased after HI injury in the neonate 235, but its role in brain repair has not yet been explored in neonates.
TRANSLATIONAL ASPECTS AND TREATMENTS
In the past two decades, a broad range of therapeutic agents were used in neonatal ischemic brain injury models to target the excitotoxic, oxidative and inflammatory injury components, but, like in adult stroke, the results of the protective efforts have been mixed. Both broad-spectra and relatively selective anti-inflammatory treatments, including allopurinol 166, deferoxamine 236, N-acetylcysteine 237, melatonin 238-241, and minocycline 242, demonstrated beneficial effects but many studies revealed several limits in neuroprotection. As an example, the neuroprotective effect by minocycline is either short-lived or contingent on genetic background 183,243. Targeting NF-kB signalling with peptide-based inhibitors showed that while sustainable neuroprotection can be achieved, the timing of administration and the length of inhibition are crucial and that prolonged treatment can exacerbate injury 155,244. Non-psychotropic cannabinoids have been considered as therapeutics based on numerous reports that demonstrated neuroprotection against neurodegenerative conditions 245,246, including not only neonatal rodent models 182 but HIE models in larger animals, newborn piglets and fetal lambs 247-250.
The HI white matter injury at P7 rats could be significantly attenuated by post-insult treatment with the AMPA receptor antagonist 6-nitro-7-sulfamoylbenzo(f)quinoxaline-2,3-dione (NBQX)251. Likewise, intracerebral injections of AMPA (in combination with the N-methyl-d-aspartate receptor antagonist MK-801) demonstrated greater susceptibility of olygodendrocytes to injury at P7 than in younger or older pups and this injury was attenuated by systemic pretreatment with the AMPA antagonist NBQX. The AMPA receptor antagonist NBQX was effective at attenuating immature white matter injury in vivo, due either to direct receptor activation or hypoxia/ischemia. NBQX blocked the injury at P7 due to AMPA injections, consistent with the results of others in adult brain 88,91,252, and suggesting a receptor-mediated cause of injury. Notably, the acute seizures and the long-term enhanced seizure susceptibility are blocked by systemic administration of the AMPA receptor antagonist NBQX, whereas there is no effect of NMDA receptor antagonists (MK-801), GABA agonists (lorazepam and phenobarbital), or the conventional AED phenytoin 253.
The overactivation of NMDA receptor in immature rats are used as target of therapy of white matter injury 254: the uncompetitive NMDA antagonist memantine attenuates in vivo acute loss of the developing oligodendrocytes cell surface marker O1 and the mature oligodendrocyte marker MBP(myelin basic protein), and also prevents the long-term reduction in cerebral mantle thickness seen at postnatal day 21. These protective doses of memantine do not affect normal myelination or cortical growth as previously observed for uncompetitive MK-801.
Attempts to protect the neonatal brain by inhibiting caspase-3 dependent apoptosis also showed mixed results. Pancaspase inhibitors and casp-3-selective inhibitors showed pharmacological neuroprotective effects in several 255-257 but not all 258,259 rodent studies of neonatal brain injury. While the age at the time of insult and the limited ability of individual inhibitors to distribute within the brain might affect injury outcome 259,260, caspase inhibition may activate caspase-independent cell death pathways, undermining neuroprotective efforts. Yet, targeting individual caspases upstream of Casp-3 may be promising 261, as was demonstrated by the potent neuroprotective role of a Casp2 inhibitor against perinatal ischemic brain damage in rodents 262.
Recently, the pluripotent capacity of stem cells from the human umbilical cord blood provides simultaneous targeting of multiple neuropathologic events initiated by a HI insult. Umbilical cord blood contains a mixture of mononuclear cells and other blood components, including red blood cells and platelets. The mononuclear cell fraction contains white blood cells, as well as progenitor and stem cells at an amount comparable to or exceeding that in bone marrow. Progenitor cells are defined as cells that can divide, producing more than one type of cell. Stem cells are defined as dividing cells that can differentiate into more mature cell types. Studies on human blood samples showed that, compared with bone marrow-derived stem cells, umbilical cord blood cells are reported to display lower immunogenicity and risk of rejection 263 and an eightfold greater proliferative potential 264,265. These features are advantageous for transplantation, and furthermore their acquisition does not require painful donor extraction procedures.
HIE neonatal models that have received systemic injections of umbilical cord blood cells commonly display human cells in the lesioned side of the brain and variable improvement in morphologic or functional outcome 266. Pimentel-Coelho et al. 267 described a region-specific effect of mononuclear umbilical cord blood cells in a neonatal rat model of HIE. Is not clear if neurofunctional improvements are related to an anti-inflammatory effect of cord blood cell transplantation, as suggested by decreased microglial activation in the ischemic cortex at 7 days after injury, or they are due to cytokine or growth factor release from human cord blood cells. Further improvement in functional outcomes after brain injury has been achieved via adjunctive therapies268-272. EPO administration enhances neurogenesis and promotes functional recovery after neonatal HI injury 228 and MCAO 229,230 in rodents. While a single EPO dose has been found to be beneficial over a short time period, multi-dose EPO treatment markedly improves structural and functional outcomes over several months 230. Its safety profile and beneficial effects via activation of multiple pathways makes EPO a good candidate as a treatment of newborns with brain injury. However, the combination of EPO with hypothermia showed no benefit over EPO alone after HI insult in neonatal rats 270, reinforcing the importance of proper timing for adjunctive therapies. BDNF and IGF-1 seem to be promising therapeutic approaches, but few data are available to date.
More recently, cell based therapies, including mesenchymal stem/progenitor cells (MSCs), have been shown to improve, in rats, functional outcomes after stroke 273-278 and traumatic brain injury 279 in the adult and after HI event in the neonate 280-284. Intravenously administered MSC reduce apoptosis, promote endogenous cell proliferation 273, and reduce the expression of inhibitory factors in astrocytes, including a broad array of glycoproteins 285, but they increase production of VEGF and BDNF 274. MSC may stimulate angiopoietin1 and VEGF signaling and amplify angiogenesis, a process necessary for vessel remodeling and neuroblast migration 274. Intranasal MSC administration in mice was shown to markedly reduce infarct size, facilitate formation of new neurons and oligodendrocytes, and improve sensorimotor outcome following HI injury 281,282. Although initially long-lasting neuroprotection by MSC against HI damage was attributed to replacement of dead neurons, the survival of MSC appeared to sharply decline to only 1% by 18 days after delivery whereas the beneficial effects continued 283. This data, along with marked effects of MSC on gene expression of growth factors and inflammatory molecules 283, has suggested that engrafted cells stimulate the microenvironment, which, in turn, permits remodelling and improvement of neurological function.
While further proof of the latter mechanism is needed, data that MSC that overexpress BDNF are even more potent in repair than MSC themselves supports the notion of a critical role of the changing microenvironment by MSC. One recent work 286 showed that Intranasal delivery of MSC- and MSC-BDNF significantly reduces infarct size and gray matter loss in comparison with vehicle-treated rats without any significant difference between MSC- and MSC-BDNF– treatments. Treatment with MSC-BDNF significantly reduced white matter loss with no significant difference between MSC- and MSC-BDNF–treatment. Motor deficits were also improved by MSC treatment when compared with vehicle-treated rats. MSCBDNF–treatment resulted in an additional significant improvement of motor deficits 14 days after MCA occlusion, but there was no significant difference between MSC or MSC-BDNF 28 days after occlusion.
To date, all these translational treatments have been tested only in animal models.
As of today, hypothermia is the only neuroprotective treatments of proven efficacy in humans for injury resulting from perinatal HIE. Recent multicenter clinical trials demonstrated the effectiveness of hypothermia, when initiated within the first 6 hours in neonates with moderate HIE, eventually reducing the risk of major neurological disabilities 287,288. However, approximately 40% of cooled infants died or survived with significant impairments. Selective brain cooling was shown to potentially induce anti-inflammatory effects 289. The beneficial effects of hypothermia seen in experimental models of ischemia are the result of a wide range of biological effects, as outlined in Table 5. Hypothermia can protect by preserving energy metabolism 290, reducing proteolysis 291,292 and ROS production 292,293, as well as by affecting vascular integrity and neuroinflammation 294,295. It has the potential to minimize secondary injury to vulnerable areas 296,297. Although clinical and experimental studies show functional improvement after hypothermia in injured neonates, there is still the need to better understand the optimal depth, timing and duration of hypothermia in order to maximize beneficial effects and reduce long-term neurologic morbidity.
Table 5.
Mechanisms of action by which hypothermia can limit ischemic damage.
| Reduced metabolic demand |
| Reduced proteolysis |
| Cell membrane stabilization |
| Inhibited spreading depolarization |
| Decreased excitotoxic damage |
| Lower lactate and tissue acidosis |
| Reduced free radical and reactive oxygen species formation |
| Altered proapoptotic signals |
| Reduced neuronal calcium influx and toxicity |
| Reduced ischemia-associated gene expression |
| Inhibited inflammation and cytokine production |
| Reduced disruption of the blood brain barrier |
| Preserved cerebral autoregulation |
CONCLUSIONS
Global/Focal perinatal brain injury represents a complex disease, frequently occurring in the perinatal period and resulting in neurological sequelae. The various clinical and pathological outcomes in neonates could be associated with either post-ischemic processes or other insults occurring during neural development. The data is emerging that neuroinflammation plays a role not only in short-term injury outcomes, but in modulating the long-term repair after perinatal stroke and enhancing the effects of adjunctive therapies. Pharmacological inhibitors, in particular glutamate-receptor antagonists and caspase inhibitors that target neuro-inflammatory mediators, and cell based therapies, in association with hypothermia represent the most promising therapeutic options for trials in humans in the near future.
Footnotes
All authors disclose any potential conflict of interest
REFERENCES
- 1.Raju TN, Nelson KB, Ferriero D, Lynch JK. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics. 2007;120:609–16. doi: 10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- 2.McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatric neurology. 2004;30:227–35. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Perlman JM, Rollins NK, Evans D. Neonatal stroke: clinical characteristics and cerebral blood flow velocity measurements. Pediatric neurology. 1994;11:281–4. doi: 10.1016/0887-8994(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 4.Schulzke S, Weber P, Luetschg J, Fahnenstich H. Incidence and diagnosis of unilateral arterial cerebral infarction in newborn infants. J Perinat Med. 2005;33:170–5. doi: 10.1515/JPM.2005.032. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. Jama. 2005;293:723–9. doi: 10.1001/jama.293.6.723. [DOI] [PubMed] [Google Scholar]
- 6.Estan J, Hope P. Unilateral neonatal cerebral infarction in full term infants. Arch Dis Child Fetal Neonatal Ed. 1997;76:F88–93. doi: 10.1136/fn.76.2.f88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laugesaar R, Kolk A, Tomberg T, et al. Acutely and retrospectively diagnosed perinatal stroke: a population-based study. Stroke; a journal of cerebral circulation. 2007;38:2234–40. doi: 10.1161/STROKEAHA.107.483743. [DOI] [PubMed] [Google Scholar]
- 8.Kirton A, Armstrong-Wells J, Chang T, et al. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics. 2011;128:e1402–10. doi: 10.1542/peds.2011-1148. [DOI] [PubMed] [Google Scholar]
- 9.Ramaswamy V, Miller SP, Barkovich AJ, Partridge JC, Ferriero DM. Perinatal stroke in term infants with neonatal encephalopathy. Neurology. 2004;62:2088–91. doi: 10.1212/01.wnl.0000129909.77753.c4. [DOI] [PubMed] [Google Scholar]
- 10.Harteman JC, Groenendaal F, Kwee A, Welsing PM, Benders MJ, de Vries LS. Risk factors for perinatal arterial ischaemic stroke in full-term infants: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F411–6. doi: 10.1136/archdischild-2011-300973. [DOI] [PubMed] [Google Scholar]
- 11.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Progress in neurobiology. 2013 doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charriaut-Marlangue C, Bonnin P, Leger PL, Renolleau S. Brief update on hemodynamic responses in animal models of neonatal stroke and hypoxia-ischemia. Experimental neurology. 2013;248:316–20. doi: 10.1016/j.expneurol.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Krageloh-Mann I. Imaging of early brain injury and cortical plasticity. Experimental neurology. 2004;190(Suppl 1):S84–90. doi: 10.1016/j.expneurol.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:455–63. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:6241–53. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1993;13:1441–53. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernardo A, Greco A, Levi G, Minghetti L. Differential lipid peroxidation, Mn superoxide, and bcl-2 expression contribute to the maturation-dependent vulnerability of oligodendrocytes to oxidative stress. Journal of neuropathology and experimental neurology. 2003;62:509–19. doi: 10.1093/jnen/62.5.509. [DOI] [PubMed] [Google Scholar]
- 18.Piecuch RE, Leonard CH, Cooper BA, Kilpatrick SJ, Schlueter MA, Sola A. Outcome of infants born at 24-26 weeks’ gestation: II. Neurodevelopmental outcome. Obstetrics and gynecology. 1997;90:809–14. doi: 10.1016/S0029-7844(97)00429-8. [DOI] [PubMed] [Google Scholar]
- 19.Cioni G, Fazzi B, Coluccini M, Bartalena L, Boldrini A, van Hof-van Duin J. Cerebral visual impairment in preterm infants with periventricular leukomalacia. Pediatric neurology. 1997;17:331–8. doi: 10.1016/s0887-8994(97)00152-5. [DOI] [PubMed] [Google Scholar]
- 20.Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annual review of neuroscience. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- 21.McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:3308–15. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheldon RA, Chuai J, Ferriero DM. A rat model for hypoxic-ischemic brain damage in very premature infants. Biology of the neonate. 1996;69:327–41. doi: 10.1159/000244327. [DOI] [PubMed] [Google Scholar]
- 23.Craig A, Ling Luo N, Beardsley DJ, et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Experimental neurology. 2003;181:231–40. doi: 10.1016/s0014-4886(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 24.Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–44. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- 25.Maalouf EF, Duggan PJ, Counsell SJ, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107:719–27. doi: 10.1542/peds.107.4.719. [DOI] [PubMed] [Google Scholar]
- 26.McQuillen PS, DeFreitas MF, Zada G, Shatz CJ. A novel role for p75NTR in subplate growth cone complexity and visual thalamocortical innervation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:3580–93. doi: 10.1523/JNEUROSCI.22-09-03580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. The Journal of comparative neurology. 1990;297:441–70. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–81. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- 29.Hevner RF. Development of connections in the human visual system during fetal mid-gestation: a DiI-tracing study. Journal of neuropathology and experimental neurology. 2000;59:385–92. doi: 10.1093/jnen/59.5.385. [DOI] [PubMed] [Google Scholar]
- 30.Friauf E, Shatz CJ. Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. Journal of neurophysiology. 1991;66:2059–71. doi: 10.1152/jn.1991.66.6.2059. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh A, Shatz CJ. Involvement of subplate neurons in the formation of ocular dominance columns. Science. 1992;255:1441–3. doi: 10.1126/science.1542795. [DOI] [PubMed] [Google Scholar]
- 32.Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–5. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- 33.Price DJ, Aslam S, Tasker L, Gillies K. Fates of the earliest generated cells in the developing murine neocortex. The Journal of comparative neurology. 1997;377:414–22. [PubMed] [Google Scholar]
- 34.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annual review of neuroscience. 1990;13:171–82. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 35.Chun JJ, Shatz CJ. The earliest-generated neurons of the cat cerebral cortex: characterization by MAP2 and neurotransmitter immunohistochemistry during fetal life. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1989;9:1648–67. doi: 10.1523/JNEUROSCI.09-05-01648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catalano SM, Chang CK, Shatz CJ. Activity-dependent regulation of NMDAR1 immunoreactivity in the developing visual cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:8376–90. doi: 10.1523/JNEUROSCI.17-21-08376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furuta A, Martin LJ. Laminar segregation of the cortical plate during corticogenesis is accompanied by changes in glutamate receptor expression. J Neurobiol. 1999;39:67–80. [PubMed] [Google Scholar]
- 38.Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early Neurodegeneration after Hypoxia-Ischemia in Neonatal Rat Is Necrosis while Delayed Neuronal Death Is Apoptosis. Neurobiology of disease. 2001;8:207–19. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 39.Nakajima W, Ishida A, Lange MS, et al. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:7994–8004. doi: 10.1523/JNEUROSCI.20-21-07994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheldon RA, Hall JJ, Noble LJ, Ferriero DM. Delayed cell death in neonatal mouse hippocampus from hypoxia-ischemia is neither apoptotic nor necrotic. Neuroscience letters. 2001;304:165–8. doi: 10.1016/s0304-3940(01)01788-8. [DOI] [PubMed] [Google Scholar]
- 41.Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 42.Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:1931–8. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–74. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- 44.Northington FJ, Ferriero DM, Martin LJ. Neurodegeneration in the thalamus following neonatal hypoxia-ischemia is programmed cell death. Developmental neuroscience. 2001;23:186–91. doi: 10.1159/000046141. [DOI] [PubMed] [Google Scholar]
- 45.Raoul C, Pettmann B, Henderson CE. Active killing of neurons during development and following stress: a role for p75(NTR) and Fas? Current opinion in neurobiology. 2000;10:111–7. doi: 10.1016/s0959-4388(99)00055-0. [DOI] [PubMed] [Google Scholar]
- 46.Repici M, Atzori C, Migheli A, Vercelli A. Molecular mechanisms of neuronal death in the dorsal lateral geniculate nucleus following visual cortical lesions. Neuroscience. 2003;117:859–67. doi: 10.1016/s0306-4522(02)00968-5. [DOI] [PubMed] [Google Scholar]
- 47.Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9030–3. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Black SM, Bedolli MA, Martinez S, Bristow JD, Ferriero DM, Soifer SJ. Expression of neuronal nitric oxide synthase corresponds to regions of selective vulnerability to hypoxia-ischaemia in the developing rat brain. Neurobiology of disease. 1995;2:145–55. doi: 10.1006/nbdi.1995.0016. [DOI] [PubMed] [Google Scholar]
- 49.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:6368–71. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferriero DM, Arcavi LJ, Sagar SM, McIntosh TK, Simon RP. Selective sparing of NADPH-diaphorase neurons in neonatal hypoxia-ischemia. Annals of neurology. 1988;24:670–6. doi: 10.1002/ana.410240512. [DOI] [PubMed] [Google Scholar]
- 51.Ferriero DM, Arcavi LJ, Simon RP. Ontogeny of excitotoxic injury to nicotinamide adenine dinucleotide phosphate diaphorase reactive neurons in the neonatal rat striatum. Neuroscience. 1990;36:417–24. doi: 10.1016/0306-4522(90)90437-9. [DOI] [PubMed] [Google Scholar]
- 52.Ferriero DM, Simon RP. Neonatal striatal NADPH-diaphorase neurons are vulnerable to quisqualate and its analogue alpha-amino-3-hydroxy-5-methyl-4-isoxazole proprionate (AMPA) Neuroscience letters. 1991;126:52–6. doi: 10.1016/0304-3940(91)90369-5. [DOI] [PubMed] [Google Scholar]
- 53.Ferriero DM, Sheldon RA, Black SM, Chuai J. Selective destruction of nitric oxide synthase neurons with quisqualate reduces damage after hypoxia-ischemia in the neonatal rat. Pediatric research. 1995;38:912–8. doi: 10.1203/00006450-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 54.Hartel C, Schilling S, Sperner J, Thyen U. The clinical outcomes of neonatal and childhood stroke: review of the literature and implications for future research. Eur J Neurol. 2004;11:431–8. doi: 10.1111/j.1468-1331.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 55.Titomanlio L, Zanin A, Sachs P, et al. Pediatric ischemic stroke: acute management and areas of research. J Pediatr. 2013;162:227–35. e1. doi: 10.1016/j.jpeds.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 56.Boardman JP, Ganesan V, Rutherford MA, Saunders DE, Mercuri E, Cowan F. Magnetic resonance image correlates of hemiparesis after neonatal and childhood middle cerebral artery stroke. Pediatrics. 2005;115:321–6. doi: 10.1542/peds.2004-0427. [DOI] [PubMed] [Google Scholar]
- 57.Benders MJ, Groenendaal F, De Vries LS. Preterm arterial ischemic stroke. Semin Fetal Neonatal Med. 2009;14:272–7. doi: 10.1016/j.siny.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Lynch JK. Epidemiology and classification of perinatal stroke. Semin Fetal Neonatal Med. 2009;14:245–9. doi: 10.1016/j.siny.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 59.de Vries LS, Groenendaal F, Eken P, van Haastert IC, Rademaker KJ, Meiners LC. Infarcts in the vascular distribution of the middle cerebral artery in preterm and fullterm infants. Neuropediatrics. 1997;28:88–96. doi: 10.1055/s-2007-973679. [DOI] [PubMed] [Google Scholar]
- 60.Volpe JJ. Intraventricular hemorrhage and brain injury in the premature infant. Diagnosis, prognosis, and prevention. Clinics in perinatology. 1989;16:387–411. [PubMed] [Google Scholar]
- 61.Holmstrom L, Vollmer B, Tedroff K, et al. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Developmental medicine and child neurology. 2010;52:145–52. doi: 10.1111/j.1469-8749.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- 62.Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain: a journal of neurology. 2002;125:2222–37. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- 63.Huang Z, Song L, Wang C, Liu JQ, Chen C. Hypoxia-ischemia upregulates TRAIL and TRAIL receptors in the immature rat brain. Developmental neuroscience. 2011;33:519–30. doi: 10.1159/000334475. [DOI] [PubMed] [Google Scholar]
- 64.Kichev A, Rousset CI, Baburamani AA, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling and cell death in the immature central nervous system after hypoxia-ischemia and inflammation. The Journal of biological chemistry. 2014;289:9430–9. doi: 10.1074/jbc.M113.512350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–8. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 67.Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol. 2006;26:1057–83. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mergenthaler P, Dirnagl U, Meisel A. Pathophysiology of stroke: lessons from animal models. Metab Brain Dis. 2004;19:151–67. doi: 10.1023/b:mebr.0000043966.46964.e6. [DOI] [PubMed] [Google Scholar]
- 69.Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol. 2005;15:234–40. doi: 10.1111/j.1750-3639.2005.tb00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang X, Zhu D, Okagaki P, et al. N-methyl-D-aspartate and TrkB receptor activation in cerebellar granule cells: an in vitro model of preconditioning to stimulate intrinsic survival pathways in neurons. Ann N Y Acad Sci. 2003;993:134–45. doi: 10.1111/j.1749-6632.2003.tb07522.x. discussion 59-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knox R, Zhao C, Miguel-Perez D, et al. Enhanced NMDA receptor tyrosine phosphorylation and increased brain injury following neonatal hypoxia-ischemia in mice with neuronal Fyn overexpression. Neurobiology of disease. 2012 doi: 10.1016/j.nbd.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jensen FE. The role of glutamate receptor maturation in perinatal seizures and brain injury. Int J Dev Neurosci. 2002;20:339–47. doi: 10.1016/s0736-5748(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 73.Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- 74.Swann JW, Pierson MG, Smith KL, Lee CL. Developmental neuroplasticity: roles in early life seizures and chronic epilepsy. Advances in neurology. 1999;79:203–16. [PubMed] [Google Scholar]
- 75.Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–6. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- 76.Pellegrini-Giampietro DE, Bennett MV, Zukin RS. Differential expression of three glutamate receptor genes in developing rat brain: an in situ hybridization study. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:4157–61. doi: 10.1073/pnas.88.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez RM, Jensen FE. Maturational aspects of epilepsy mechanisms and consequences for the immature brain. Epilepsia. 2001;42:577–85. doi: 10.1046/j.1528-1157.2001.12000.x. [DOI] [PubMed] [Google Scholar]
- 78.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–98. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 79.Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994;12:1281–9. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 80.McBain CJ, Dingledine R. Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. The Journal of physiology. 1993;462:373–92. doi: 10.1113/jphysiol.1993.sp019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Washburn MS, Numberger M, Zhang S, Dingledine R. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:9393–406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallo V, Patneau DK, Mayer ML, Vaccarino FM. Excitatory amino acid receptors in glial progenitor cells: molecular and functional properties. Glia. 1994;11:94–101. doi: 10.1002/glia.440110204. [DOI] [PubMed] [Google Scholar]
- 83.Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–71. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 84.Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends in neurosciences. 2001;24:224–30. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- 85.Yoshioka A, Bacskai B, Pleasure D. Pathophysiology of oligodendroglial excitotoxicity. Journal of neuroscience research. 1996;46:427–37. doi: 10.1002/(SICI)1097-4547(19961115)46:4<427::AID-JNR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 86.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:1302–12. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yonezawa M, Back SA, Gan X, Rosenberg PA, Volpe JJ. Cystine deprivation induces oligodendroglial death: rescue by free radical scavengers and by a diffusible glial factor. Journal of neurochemistry. 1996;67:566–73. doi: 10.1046/j.1471-4159.1996.67020566.x. [DOI] [PubMed] [Google Scholar]
- 88.Yoshioka A, Hardy M, Younkin DP, Grinspan JB, Stern JL, Pleasure D. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors mediate excitotoxicity in the oligodendroglial lineage. Journal of neurochemistry. 1995;64:2442–8. doi: 10.1046/j.1471-4159.1995.64062442.x. [DOI] [PubMed] [Google Scholar]
- 89.Matute C, Sanchez-Gomez MV, Martinez-Millan L, Miledi R. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8830–5. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McDonald JW, Levine JM, Qu Y. Multiple classes of the oligodendrocyte lineage are highly vulnerable to excitotoxicity. Neuroreport. 1998;9:2757–62. doi: 10.1097/00001756-199808240-00014. [DOI] [PubMed] [Google Scholar]
- 91.Tekkok SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:4237–48. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blomgren K, Zhu C, Hallin U, Hagberg H. Mitochondria and ischemic reperfusion damage in the adult and in the developing brain. Biochem Biophys Res Commun. 2003;304:551–9. doi: 10.1016/s0006-291x(03)00628-4. [DOI] [PubMed] [Google Scholar]
- 93.Northington FJ, Zelaya ME, O’Riordan DP, et al. Failure to complete apoptosis following neonatal hypoxia-ischemia manifests as “continuum” phenotype of cell death and occurs with multiple manifestations of mitochondrial dysfunction in rodent forebrain. Neuroscience. 2007;149:822–33. doi: 10.1016/j.neuroscience.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou Y, Zhou L, Chen H, Koliatsos VE. An AMPA glutamatergic receptor activation-nitric oxide synthesis step signals transsynaptic apoptosis in limbic cortex. Neuropharmacology. 2006;51:67–76. doi: 10.1016/j.neuropharm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 95.Koliatsos VE, Dawson TM, Kecojevic A, Zhou Y, Wang YF, Huang KX. Cortical interneurons become activated by deafferentation and instruct the apoptosis of pyramidal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14264–9. doi: 10.1073/pnas.0404364101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7162–6. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keller JN, Kindy MS, Holtsberg FW, et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:687–97. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim GW, Kondo T, Noshita N, Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: implications for the production and role of superoxide radicals. Stroke; a journal of cerebral circulation. 2002;33:809–15. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- 99.Zhu C, Wang X, Huang Z, et al. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ. 2007;14:775–84. doi: 10.1038/sj.cdd.4402053. [DOI] [PubMed] [Google Scholar]
- 100.Renolleau S, Fau S, Goyenvalle C, et al. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. Journal of neurochemistry. 2007;100:1062–71. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 101.Wang X, Carlsson Y, Basso E, et al. Developmental shift of cyclophilin D contribution to hypoxic-ischemic brain injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:2588–96. doi: 10.1523/JNEUROSCI.5832-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hagberg H, Wilson MA, Matsushita H, et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. Journal of neurochemistry. 2004;90:1068–75. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 103.Yu SW, Wang H, Poitras MF, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–63. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 104.Nijboer CH, Groenendaal F, Kavelaars A, Hagberg HH, van Bel F, Heijnen CJ. Gender-specific neuroprotection by 2-iminobiotin after hypoxia-ischemia in the neonatal rat via a nitric oxide independent pathway. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:282–92. doi: 10.1038/sj.jcbfm.9600342. [DOI] [PubMed] [Google Scholar]
- 105.Zhu C, Xu F, Wang X, et al. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. Journal of neurochemistry. 2006;96:1016–27. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- 106.Bona E, Hagberg H, Loberg EM, Bagenholm R, Thoresen M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatric research. 1998;43:738–45. doi: 10.1203/00006450-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 107.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–89. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iseri LT, French JH. Magnesium: nature’s physiologic calcium blocker. American heart journal. 1984;108:188–93. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 109.Peruche B, Krieglstein J. Mechanisms of drug actions against neuronal damage caused by ischemia--an overview. Progress in neuro-psychopharmacology & biological psychiatry. 1993;17:21–70. doi: 10.1016/0278-5846(93)90032-n. [DOI] [PubMed] [Google Scholar]
- 110.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–5. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 111.Kristal BS, Dubinsky JM. Mitochondrial permeability transition in the central nervous system: induction by calcium cycling-dependent and -independent pathways. Journal of neurochemistry. 1997;69:524–38. doi: 10.1046/j.1471-4159.1997.69020524.x. [DOI] [PubMed] [Google Scholar]
- 112.Schanne FA, Gupta RK, Stanton PK. 31P-NMR study of transient ischemia in rat hippocampal slices in vitro. Biochimica et biophysica acta. 1993;1158:257–63. doi: 10.1016/0304-4165(93)90023-2. [DOI] [PubMed] [Google Scholar]
- 113.Westermaier T, Stetter C, Kunze E, et al. Magnesium treatment for neuroprotection in ischemic diseases of the brain. Experimental & translational stroke medicine. 2013;5:6. doi: 10.1186/2040-7378-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dorhout Mees SM, Algra A, Vandertop WP, et al. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomised placebo-controlled trial. Lancet. 2012;380:44–9. doi: 10.1016/S0140-6736(12)60724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Majid A. Neuroprotection in stroke: past, present, and future. ISRN neurology. 2014;2014:515716. doi: 10.1155/2014/515716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. The Journal of biological chemistry. 2000;275:20520–6. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- 117.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–57. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 118.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–40. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke; a journal of cerebral circulation. 1998;29:1020–30. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 120.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 121.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke; a journal of cerebral circulation. 2011;42:3323–8. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fernandez-Lopez D, Faustino J, Daneman R, et al. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:9588–600. doi: 10.1523/JNEUROSCI.5977-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Denker S, Ji S, Lee SY, et al. Macrophages are Comprised of Resident Brain Microglia not Infiltrating Peripheral Monocytes Acutely after Neonatal Stroke. Journal of neurochemistry. 2007;100:893–904. doi: 10.1111/j.1471-4159.2006.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2010;24:708–23. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 125.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Annals of neurology. 1998;44:665–75. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 126.Hu X, Rea HC, Wiktorowicz JE, Perez-Polo JR. Proteomic analysis of hypoxia/ischemia-induced alteration of cortical development and dopamine neurotransmission in neonatal rat. Journal of proteome research. 2006;5:2396–404. doi: 10.1021/pr060209x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao BQ, Wang S, Kim HY, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nature medicine. 2006;12:441–5. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 128.Chhor V, Le Charpentier T, Lebon S, et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain, behavior, and immunity. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 130.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 131.Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends in neurosciences. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 132.Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke; a journal of cerebral circulation. 2012;43:3063–70. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 133.Faustino JV, Wang X, Johnson CE, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:12992–3001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mae M, Armulik A, Betsholtz C. Getting to know the cast - cellular interactions and signaling at the neurovascular unit. Curr Pharm Des. 2011;17:2750–4. doi: 10.2174/138161211797440113. [DOI] [PubMed] [Google Scholar]
- 135.Turner DA, Adamson DC. Neuronal-astrocyte metabolic interactions: understanding the transition into abnormal astrocytoma metabolism. Journal of neuropathology and experimental neurology. 2011;70:167–76. doi: 10.1097/NEN.0b013e31820e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jarlestedt K, Rousset CI, Faiz M, et al. Attenuation of reactive gliosis does not affect infarct volume in neonatal hypoxic-ischemic brain injury in mice. PLoS One. 2010;5:e10397. doi: 10.1371/journal.pone.0010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Andera L. Signaling activated by the death receptors of the TNFR family. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153:173–80. doi: 10.5507/bp.2009.029. [DOI] [PubMed] [Google Scholar]
- 138.Graham EM, Sheldon RA, Flock DL, et al. Neonatal mice lacking functional Fas death receptors are resistant to hypoxic-ischemic brain injury. Neurobiology of disease. 2004;17:89–98. doi: 10.1016/j.nbd.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 139.Matsumori Y, Northington FJ, Hong SM, et al. Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70. Stroke; a journal of cerebral circulation. 2006;37:507–12. doi: 10.1161/01.STR.0000199057.00365.20. [DOI] [PubMed] [Google Scholar]
- 140.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–71. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abe T, Shimamura M, Jackman K, et al. Key role of CD36 in Toll-like receptor 2 signaling in cerebral ischemia. Stroke; a journal of cerebral circulation. 2010;41:898–904. doi: 10.1161/STROKEAHA.109.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ziegler G, Harhausen D, Schepers C, et al. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun. 2007;359:574–9. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]
- 143.Hua F, Ma J, Ha T, et al. Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res. 2009;1262:100–8. doi: 10.1016/j.brainres.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Du X, Fleiss B, Li H, et al. Systemic Stimulation of TLR2 Impairs Neonatal Mouse Brain Development. PLoS One. 2011;6:e19583. doi: 10.1371/journal.pone.0019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang X, Stridh L, Li W, et al. Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a MyD88-dependent manner. J Immunol. 2009;183:7471–7. doi: 10.4049/jimmunol.0900762. [DOI] [PubMed] [Google Scholar]
- 146.Stridh L, Smith PL, Naylor AS, Wang X, Mallard C. Regulation of Toll-like receptor 1 and -2 in neonatal mice brains after hypoxia-ischemia. J Neuroinflammation. 2011;8:45. doi: 10.1186/1742-2094-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kunz A, Abe T, Hochrainer K, et al. Nuclear factor-kappaB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:1649–58. doi: 10.1523/JNEUROSCI.5205-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cho S, Park EM, Febbraio M, et al. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:2504–12. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Woo MS, Wang X, Faustino JV, et al. Genetic deletion of CD36 enhances injury after acute neonatal stroke. Annals of neurology. 2012;72:961–70. doi: 10.1002/ana.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Doverhag C, Keller M, Karlsson A, et al. Pharmacological and genetic inhibition of NADPH oxidase does not reduce brain damage in different models of perinatal brain injury in newborn mice. Neurobiology of disease. 2008;31:133–44. doi: 10.1016/j.nbd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 151.Fullerton HJ, Ditelberg JS, Chen SF, et al. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Annals of neurology. 1998;44:357–64. doi: 10.1002/ana.410440311. [DOI] [PubMed] [Google Scholar]
- 152.Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience. 2009;158:995–1006. doi: 10.1016/j.neuroscience.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 153.Papa S, Bubici C, Zazzeroni F, et al. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–29. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- 154.Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006;13:730–7. doi: 10.1038/sj.cdd.4401830. [DOI] [PubMed] [Google Scholar]
- 155.Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. A dual role of the NF-kappaB pathway in neonatal hypoxic-ischemic brain damage. Stroke; a journal of cerebral circulation. 2008;39:2578–86. doi: 10.1161/STROKEAHA.108.516401. [DOI] [PubMed] [Google Scholar]
- 156.van der Kooij MA, Nijboer CH, Ohl F, et al. NF-kappaB inhibition after neonatal cerebral hypoxia-ischemia improves long-term motor and cognitive outcome in rats. Neurobiology of disease. 2010;38:266–72. doi: 10.1016/j.nbd.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 157.Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7:1140–9. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- 158.Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14:2013–54. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:9157–64. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain: a journal of neurology. 1997;120(Pt 3):435–44. doi: 10.1093/brain/120.3.435. [DOI] [PubMed] [Google Scholar]
- 161.Khan JY, Black SM. Developmental changes in murine brain antioxidant enzymes. Pediatric research. 2003;54:77–82. doi: 10.1203/01.PDR.0000065736.69214.20. [DOI] [PubMed] [Google Scholar]
- 162.Lafemina MJ, Sheldon RA, Ferriero DM. Acute hypoxia-ischemia results in hydrogen peroxide accumulation in neonatal but not adult mouse brain. Pediatric research. 2006;59:680–3. doi: 10.1203/01.pdr.0000214891.35363.6a. [DOI] [PubMed] [Google Scholar]
- 163.Sheldon RA, Jiang X, Francisco C, et al. Manipulation of antioxidant pathways in neonatal murine brain. Pediatric research. 2004;56:656–62. doi: 10.1203/01.PDR.0000139413.27864.50. [DOI] [PubMed] [Google Scholar]
- 164.Sheldon RA, Christen S, Ferriero DM. Genetic and pharmacologic manipulation of oxidative stress after neonatal hypoxia-ischemia. Int J Dev Neurosci. 2008;26:87–92. doi: 10.1016/j.ijdevneu.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Spanggord H, Sheldon RA, Ferriero DM. Cysteamine eliminates nitric oxide synthase activity but is not protective to the hypoxic-ischemic neonatal rat brain. Neuroscience letters. 1996;213:41–4. doi: 10.1016/0304-3940(96)12848-2. [DOI] [PubMed] [Google Scholar]
- 166.Peeters-Scholte C, Braun K, Koster J, et al. Effects of allopurinol and deferoxamine on reperfusion injury of the brain in newborn piglets after neonatal hypoxia-ischemia. Pediatric research. 2003;54:516–22. doi: 10.1203/01.PDR.0000081297.53793.C6. [DOI] [PubMed] [Google Scholar]
- 167.Tsuji M, Higuchi Y, Shiraishi K, Kume T, Akaike A, Hattori H. Protective effect of aminoguanidine on hypoxic-ischemic brain damage and temporal profile of brain nitric oxide in neonatal rat. Pediatric research. 2000;47:79–83. doi: 10.1203/00006450-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 168.Higuchi Y, Hattori H, Kume T, Tsuji M, Akaike A, Furusho K. Increase in nitric oxide in the hypoxic-ischemic neonatal rat brain and suppression by 7-nitroindazole and aminoguanidine. European journal of pharmacology. 1998;342:47–9. doi: 10.1016/s0014-2999(97)01524-0. [DOI] [PubMed] [Google Scholar]
- 169.Peeters-Scholte C, Koster J, Veldhuis W, et al. Neuroprotection by selective nitric oxide synthase inhibition at 24 hours after perinatal hypoxia-ischemia. Stroke; a journal of cerebral circulation. 2002;33:2304–10. doi: 10.1161/01.str.0000028343.25901.09. [DOI] [PubMed] [Google Scholar]
- 170.Rao S, Lin Z, Drobyshevsky A, et al. Involvement of neuronal nitric oxide synthase in ongoing fetal brain injury following near-term rabbit hypoxia-ischemia. Developmental neuroscience. 2011;33:288–98. doi: 10.1159/000327241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tsuji M, Higuchi Y, Shiraishi K, Kume T, Akaike A, Hattori H. Protective effect of aminoguanidine on hypoxic-ischemic brain damage and temporal profile of brain nitric oxide in neonatal rat [In Process Citation] Pediatric research. 2000;47:79–83. doi: 10.1203/00006450-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 172.Charriaut-Marlangue C, Nguyen T, Bonnin P, et al. Sildenafil mediates blood-flow redistribution and neuroprotection after neonatal hypoxia-ischemia. Stroke; a journal of cerebral circulation. 2014;45:850–6. doi: 10.1161/STROKEAHA.113.003606. [DOI] [PubMed] [Google Scholar]
- 173.Yang L, Sameshima H, Yamaguchi M, Ikenoue T. Expression of inducible nitric oxide synthase and cyclooxygenase-2 mRNA in brain damage induced by lipopolysaccharide and intermittent hypoxia-ischemia in neonatal rats. J Obstet Gynaecol Res. 2005;31:185–91. doi: 10.1111/j.1341-8076.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 174.Yin W, Signore AP, Iwai M, et al. Preconditioning suppresses inflammation in neonatal hypoxic ischemia via Akt activation. Stroke; a journal of cerebral circulation. 2007;38:1017–24. doi: 10.1161/01.STR.0000258102.18836.ca. [DOI] [PubMed] [Google Scholar]
- 175.Hagberg H, Gilland E, Bona E, et al. Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats. Pediatric research. 1996;40:603–9. doi: 10.1203/00006450-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 176.Denker SP, Ji S, Dingman A, et al. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. Journal of neurochemistry. 2007;100:893–904. doi: 10.1111/j.1471-4159.2006.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Aly H, Khashaba MT, El-Ayouty M, El-Sayed O, Hasanein BM. IL-1beta, IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev. 2006;28:178–82. doi: 10.1016/j.braindev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 178.Oygur N, Sonmez O, Saka O, Yegin O. Predictive value of plasma and cerebrospinal fluid tumour necrosis factor-alpha and interleukin-1 beta concentrations on outcome of full term infants with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1998;79:F190–3. doi: 10.1136/fn.79.3.f190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu XH, Kwon D, Schielke GP, Yang GY, Silverstein FS, Barks JD. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19:1099–108. doi: 10.1097/00004647-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 180.Hedtjarn M, Mallard C, Iwakura Y, Hagberg H. Combined deficiency of IL-1beta18, but not IL-1alphabeta, reduces susceptibility to hypoxia-ischemia in the immature brain. Developmental neuroscience. 2005;27:143–8. doi: 10.1159/000085986. [DOI] [PubMed] [Google Scholar]
- 181.Girard S, Kadhim H, Larouche A, Roy M, Gobeil F, Sebire G. Pro-inflammatory disequilibrium of the IL-1 beta/IL-1ra ratio in an experimental model of perinatal brain damages induced by lipopolysaccharide and hypoxia-ischemia. Cytokine. 2008;43:54–62. doi: 10.1016/j.cyto.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 182.Fernandez-Lopez D, Faustino J, Derugin N, et al. Reduced infarct size and accumulation of microglia in rats treated with WIN 55,212-2 after neonatal stroke. Neuroscience. 2012;207:307–15. doi: 10.1016/j.neuroscience.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fox C, Dingman A, Derugin N, et al. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:1138–49. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Szaflarski J, Burtrum D, Silverstein FS. Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke; a journal of cerebral circulation. 1995;26:1093–100. doi: 10.1161/01.str.26.6.1093. [DOI] [PubMed] [Google Scholar]
- 185.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 186.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 187.McLaurin J, D’Souza S, Stewart J, et al. Effect of tumor necrosis factor alpha and beta on human oligodendrocytes and neurons in culture. Int J Dev Neurosci. 1995;13:369–81. doi: 10.1016/0736-5748(95)00012-6. [DOI] [PubMed] [Google Scholar]
- 188.Ye P, D’Ercole AJ. Insulin-like growth factor I protects oligodendrocytes from tumor necrosis factor-alpha-induced injury. Endocrinology. 1999;140:3063–72. doi: 10.1210/endo.140.7.6754. [DOI] [PubMed] [Google Scholar]
- 189.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–22. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 190.King MD, Alleyne CH, Jr., Dhandapani KM. TNF-alpha receptor antagonist, R-7050, improves neurological outcomes following intracerebral hemorrhage in mice. Neuroscience letters. 2013;542:92–6. doi: 10.1016/j.neulet.2013.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mirabelli-Badenier M, Braunersreuther V, Viviani GL, et al. CC and CXC chemokines are pivotal mediators of cerebral injury in ischaemic stroke. Thromb Haemost. 2011;105:409–20. doi: 10.1160/TH10-10-0662. [DOI] [PubMed] [Google Scholar]
- 192.Bona E, Andersson AL, Blomgren K, et al. Chemokine and inflammatory cell response to hypoxia-ischemia in immature rats. Pediatric research. 1999;45:500–9. doi: 10.1203/00006450-199904010-00008. [DOI] [PubMed] [Google Scholar]
- 193.Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:308–17. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 194.Schilling M, Strecker JK, Schabitz WR, Ringelstein EB, Kiefer R. Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice. Neuroscience. 2009;161:806–12. doi: 10.1016/j.neuroscience.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 195.Xu H, Barks JD, Schielke GP, Silverstein FS. Attenuation of hypoxia-ischemia-induced monocyte chemoattractant protein-1 expression in brain of neonatal mice deficient in interleukin-1 converting enzyme. Brain Res Mol Brain Res. 2001;90:57–67. doi: 10.1016/s0169-328x(01)00087-0. [DOI] [PubMed] [Google Scholar]
- 196.Galasso JM, Miller MJ, Cowell RM, Harrison JK, Warren JS, Silverstein FS. Acute excitotoxic injury induces expression of monocyte chemoattractant protein-1 and its receptor, CCR2, in neonatal rat brain. Experimental neurology. 2000;165:295–305. doi: 10.1006/exnr.2000.7466. [DOI] [PubMed] [Google Scholar]
- 197.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke; a journal of cerebral circulation. 2007;38:1345–53. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 198.Minami M, Satoh M. Chemokines and their receptors in the brain: pathophysiological roles in ischemic brain injury. Life Sci. 2003;74:321–7. doi: 10.1016/j.lfs.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 199.Malik M, Chen YY, Kienzle MF, Tomkowicz BE, Collman RG, Ptasznik A. Monocyte migration and LFA-1-mediated attachment to brain microvascular endothelia is regulated by SDF-1 alpha through Lyn kinase. J Immunol. 2008;181:4632–7. doi: 10.4049/jimmunol.181.7.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kantele JM, Kurk S, Jutila MA. Effects of continuous exposure to stromal cell-derived factor-1 alpha on T cell rolling and tight adhesion to monolayers of activated endothelial cells. J Immunol. 2000;164:5035–40. doi: 10.4049/jimmunol.164.10.5035. [DOI] [PubMed] [Google Scholar]
- 201.Felszeghy K, Banisadr G, Rostene W, Nyakas C, Haour F. Dexamethasone downregulates chemokine receptor CXCR4 and exerts neuroprotection against hypoxia/ischemia-induced brain injury in neonatal rats. Neuroimmunomodulation. 2004;11:404–13. doi: 10.1159/000080151. [DOI] [PubMed] [Google Scholar]
- 202.Miller JT, Bartley JH, Wimborne HJ, et al. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Galvin KA, Oorschot DE. Continuous low-dose treatment with brain-derived neurotrophic factor or neurotrophin-3 protects striatal medium spiny neurons from mild neonatal hypoxia/ischemia: a stereological study. Neuroscience. 2003;118:1023–32. doi: 10.1016/s0306-4522(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 204.Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 2011;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 205.Yanamoto H, Nagata I, Sakata M, et al. Infarct tolerance induced by intra-cerebral infusion of recombinant brain-derived neurotrophic factor. Brain Res. 2000;859:240–8. doi: 10.1016/s0006-8993(00)01966-1. [DOI] [PubMed] [Google Scholar]
- 206.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual review of neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nature medicine. 2002;8:1363–8. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 209.Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:5775–81. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Han BH, D’Costa A, Back SA, et al. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiology of disease. 2000;7:38–53. doi: 10.1006/nbdi.1999.0275. [DOI] [PubMed] [Google Scholar]
- 211.Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med. 2007;17:140–3. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fernandez-Lopez D, Faustino J, Derugin N, Vexler ZS. Acute and chronic vascular responses to experimental focal arterial stroke in the neonate rat. Translational Stroke Research. 2012 doi: 10.1007/s12975-012-0214-5. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Baburamani AA, Ek CJ, Walker DW, Castillo-Melendez M. Vulnerability of the developing brain to hypoxic-ischemic damage: contribution of the cerebral vasculature to injury and repair? Frontiers in physiology. 2012;3:424. doi: 10.3389/fphys.2012.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kaya D, Gursoy-Ozdemir Y, Yemisci M, Tuncer N, Aktan S, Dalkara T. VEGF protects brain against focal ischemia without increasing blood--brain permeability when administered intracerebroventricularly. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:1111–8. doi: 10.1038/sj.jcbfm.9600109. [DOI] [PubMed] [Google Scholar]
- 215.Feng Y, Rhodes PG, Bhatt AJ. Neuroprotective effects of vascular endothelial growth factor following hypoxic ischemic brain injury in neonatal rats. Pediatric research. 2008;64:370–4. doi: 10.1203/PDR.0b013e318180ebe6. [DOI] [PubMed] [Google Scholar]
- 216.Shimotake J, Derugin N, Wendland M, Vexler ZS, Ferriero DM. Vascular endothelial growth factor receptor-2 inhibition promotes cell death and limits endothelial cell proliferation in a neonatal rodent model of stroke. Stroke; a journal of cerebral circulation. 2010;41:343–9. doi: 10.1161/STROKEAHA.109.564229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain research reviews. 2009;62:99–108. doi: 10.1016/j.brainresrev.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 218.Marti HH. Erythropoietin and the hypoxic brain. The Journal of experimental biology. 2004;207:3233–42. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- 219.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:9471–81. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Castillo-Melendez M, Yan E, Walker DW. Expression of erythropoietin and its receptor in the brain of late-gestation fetal sheep, and responses to asphyxia caused by umbilical cord occlusion. Developmental neuroscience. 2005;27:220–7. doi: 10.1159/000085995. [DOI] [PubMed] [Google Scholar]
- 221.Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke; a journal of cerebral circulation. 2007;38:2795–803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 222.Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:1620–43. doi: 10.1038/jcbfm.2009.100. [DOI] [PubMed] [Google Scholar]
- 223.Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5978–82. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Keogh CL, Yu SP, Wei L. The effect of recombinant human erythropoietin on neurovasculature repair after focal ischemic stroke in neonatal rats. The Journal of pharmacology and experimental therapeutics. 2007;322:521–8. doi: 10.1124/jpet.107.121392. [DOI] [PubMed] [Google Scholar]
- 225.Gonzalez FF, McQuillen P, Mu D, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Developmental neuroscience. 2007;29:321–30. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 226.Chang YS, Mu D, Wendland M, et al. Erythropoietin improves functional and histological outcome in neonatal stroke. Pediatric research. 2005;58:106–11. doi: 10.1203/01.PDR.0000163616.89767.69. [DOI] [PubMed] [Google Scholar]
- 227.van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, van Bel F. Combination of deferoxamine and erythropoietin: therapy for hypoxia-ischemia-induced brain injury in the neonatal rat? Neuroscience letters. 2009;451:109–13. doi: 10.1016/j.neulet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 228.Iwai M, Stetler RA, Xing J, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke; a journal of cerebral circulation. 2010;41:1032–7. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gonzalez F, McQuillen PS, Mu D, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Developmental neuroscience. 2007;29:321–30. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 230.Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Developmental neuroscience. 2009;31:403–11. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–39. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 232.Lee SR, Kim HY, Rogowska J, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:3491–5. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yan YP, Lang BT, Vemuganti R, Dempsey RJ. Galectin-3 mediates post-ischemic tissue remodeling. Brain Res. 2009;1288:116–24. doi: 10.1016/j.brainres.2009.06.073. [DOI] [PubMed] [Google Scholar]
- 234.Lalancette-Hebert M, Weng YC, Sato S, Kriz J. Role of galectin-3 in microglial activation after cerebral ischemia. Society for Neuroscience. 2009;2009 [Google Scholar]
- 235.Doverhag C, Hedtjarn M, Poirier F, et al. Galectin-3 contributes to neonatal hypoxic-ischemic brain injury. Neurobiology of disease. 2010;38:36–46. doi: 10.1016/j.nbd.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 236.Papazisis G, Pourzitaki C, Sardeli C, Lallas A, Amaniti E, Kouvelas D. Deferoxamine decreases the excitatory amino acid levels and improves the histological outcome in the hippocampus of neonatal rats after hypoxia-ischemia. Pharmacological research: the official journal of the Italian Pharmacological Society. 2008;57:73–8. doi: 10.1016/j.phrs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 237.Jatana M, Singh I, Singh AK, Jenkins D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatric research. 2006;59:684–9. doi: 10.1203/01.pdr.0000215045.91122.44. [DOI] [PubMed] [Google Scholar]
- 238.Husson I, Mesples B, Bac P, Vamecq J, Evrard P, Gressens P. Melatoninergic neuroprotection of the murine periventricular white matter against neonatal excitotoxic challenge. Annals of neurology. 2002;51:82–92. doi: 10.1002/ana.10072. [DOI] [PubMed] [Google Scholar]
- 239.Signorini C, Ciccoli L, Leoncini S, et al. Free iron, total F-isoprostanes and total F-neuroprostanes in a model of neonatal hypoxic-ischemic encephalopathy: neuroprotective effect of melatonin. J Pineal Res. 2009;46:148–54. doi: 10.1111/j.1600-079X.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 240.Van Bel F, Shadid M, Moison RM, et al. Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, and electrical brain activity. Pediatrics. 1998;101:185–93. doi: 10.1542/peds.101.2.185. [DOI] [PubMed] [Google Scholar]
- 241.Gunes T, Ozturk MA, Koklu E, Kose K, Gunes I. Effect of allopurinol supplementation on nitric oxide levels in asphyxiated newborns. Pediatric neurology. 2007;36:17–24. doi: 10.1016/j.pediatrneurol.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 242.Buller KM, Carty ML, Reinebrant HE, Wixey JA. Minocycline: a neuroprotective agent for hypoxic-ischemic brain injury in the neonate? Journal of neuroscience research. 2009;87:599–608. doi: 10.1002/jnr.21890. [DOI] [PubMed] [Google Scholar]
- 243.Tsuji M, Wilson MA, Lange MS, Johnston MV. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Experimental neurology. 2004;189:58–65. doi: 10.1016/j.expneurol.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 244.Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke; a journal of cerebral circulation. 2008;39:2129–37. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]
- 245.Martinez-Orgado J, Fernandez-Lopez D, Lizasoain I, Romero J. The seek of neuroprotection: introducing cannabinoids. Recent Pat CNS Drug Discov. 2007;2:131–9. doi: 10.2174/157488907780832724. [DOI] [PubMed] [Google Scholar]
- 246.van der Stelt M, Di Marzo V. Cannabinoid receptors and their role in neuroprotection. Neuromolecular Med. 2005;7:37–50. doi: 10.1385/NMM:7:1-2:037. [DOI] [PubMed] [Google Scholar]
- 247.Castillo A, Tolon MR, Fernandez-Ruiz J, Romero J, Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol Dis. 2010;37:434–40. doi: 10.1016/j.nbd.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 248.Fernandez-Lopez D, Martinez-Orgado J, Nunez E, et al. Characterization of the neuroprotective effect of the cannabinoid agonist WIN-55212 in an in vitro model of hypoxic-ischemic brain damage in newborn rats. Pediatric research. 2006;60:169–73. doi: 10.1203/01.pdr.0000228839.00122.6c. [DOI] [PubMed] [Google Scholar]
- 249.Fernandez-Lopez D, Pazos MR, Tolon RM, et al. The cannabinoid agonist WIN55212 reduces brain damage in an in vivo model of hypoxic-ischemic encephalopathy in newborn rats. Pediatric research. 2007;62:255–60. doi: 10.1203/PDR.0b013e318123fbb8. [DOI] [PubMed] [Google Scholar]
- 250.Pazos MR, Cinquina V, Gomez A, et al. Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology. 2012;63:776–83. doi: 10.1016/j.neuropharm.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 251.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:9235–41. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nature medicine. 1998;4:291–7. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- 253.Jensen FE, Blume H, Alvarado S, Firkusny I, Geary C. NBQX blocks acute and late epileptogenic effects of perinatal hypoxia. Epilepsia. 1995;36:966–72. doi: 10.1111/j.1528-1157.1995.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 254.Manning SM, Talos DM, Zhou C, et al. NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:6670–8. doi: 10.1523/JNEUROSCI.1702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hagberg H, Mallard C, Rousset CI, Xiaoyang W. Apoptotic mechanisms in the immature brain: involvement of mitochondria. J Child Neurol. 2009;24:1141–6. doi: 10.1177/0883073809338212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Cheng Y, Deshmukh M, D’Costa A, et al. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998;101:1992–9. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Han BH, Xu D, Choi J, et al. Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic-ischemic brain injury. The Journal of biological chemistry. 2002;277:30128–36. doi: 10.1074/jbc.M202931200. [DOI] [PubMed] [Google Scholar]
- 258.Joly LM, Mucignat V, Mariani J, Plotkine M, Charriaut-Marlangue C. Caspase inhibition after neonatal ischemia in the rat brain. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:124–31. doi: 10.1097/01.WCB.0000100061.36077.5F. [DOI] [PubMed] [Google Scholar]
- 259.Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Annals of neurology. 2009;66:378–89. doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- 260.Karatas H, Aktas Y, Gursoy-Ozdemir Y, et al. A nanomedicine transports a peptide caspase-3 inhibitor across the blood-brain barrier and provides neuroprotection. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:13761–9. doi: 10.1523/JNEUROSCI.4246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Carlsson Y, Schwendimann L, Vontell R, et al. Genetic inhibition of caspase-2 reduces hypoxic-ischemic and excitotoxic neonatal brain injury. Annals of neurology. 2011;70:781–9. doi: 10.1002/ana.22431. [DOI] [PubMed] [Google Scholar]
- 262.Chauvier D, Renolleau S, Holifanjaniaina S, et al. Targeting neonatal ischemic brain injury with a pentapeptide-based irreversible caspase inhibitor. Cell Death Dis. 2011;2:e203. doi: 10.1038/cddis.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sirchia G, Rebulla P. Placental/umbilical cord blood transplantation. Haematologica. 1999;84:738–47. [PubMed] [Google Scholar]
- 264.Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:3828–32. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Buzanska L, Jurga M, Domanska-Janik K. Neuronal differentiation of human umbilical cord blood neural stem-like cell line. Neuro-degenerative diseases. 2006;3:19–26. doi: 10.1159/000092088. [DOI] [PubMed] [Google Scholar]
- 266.Verina T, Fatemi A, Johnston MV, Comi AM. Pluripotent possibilities: human umbilical cord blood cell treatment after neonatal brain injury. Pediatric neurology. 2013;48:346–54. doi: 10.1016/j.pediatrneurol.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 267.Pimentel-Coelho PM, Magalhaes ES, Lopes LM, deAzevedo LC, Santiago MF, Mendez-Otero R. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: functional outcome related to neuroprotection in the striatum. Stem cells and development. 2010;19:351–8. doi: 10.1089/scd.2009.0049. [DOI] [PubMed] [Google Scholar]
- 268.Barks JD, Liu YQ, Shangguan Y, Silverstein FS. Phenobarbital augments hypothermic neuroprotection. Pediatric research. 2010;67:532–7. doi: 10.1203/PDR.0b013e3181d4ff4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Carlsson Y, Wang X, Schwendimann L, et al. Combined effect of hypothermia and caspase-2 gene deficiency on neonatal hypoxic-ischemic brain injury. Pediatric research. 2012;71:566–72. doi: 10.1038/pr.2012.15. [DOI] [PubMed] [Google Scholar]
- 270.Fang AY, Gonzalez FF, Sheldon RA, Ferriero DM. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia-ischemia. Pediatric research. 2013;73:12–7. doi: 10.1038/pr.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke; a journal of cerebral circulation. 2008;39:1307–13. doi: 10.1161/STROKEAHA.107.499822. [DOI] [PubMed] [Google Scholar]
- 272.Liu Y, Barks JD, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke; a journal of cerebral circulation. 2004;35:1460–5. doi: 10.1161/01.STR.0000128029.50221.fa. [DOI] [PubMed] [Google Scholar]
- 273.Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. Journal of neuroscience research. 2003;73:778–86. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 274.Zacharek A, Chen J, Cui X, et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1684–91. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhang J, Li Y, Chen J, et al. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004;1030:19–27. doi: 10.1016/j.brainres.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 276.Horie N, Pereira MP, Niizuma K, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–85. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Daadi MM, Davis AS, Arac A, et al. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke; a journal of cerebral circulation. 2010;41:516–23. doi: 10.1161/STROKEAHA.109.573691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiology of disease. 2010;37:275–83. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21:33–9. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- 280.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatric research. 2010;68:419–22. doi: 10.1203/PDR.0b013e3181f1c289. [DOI] [PubMed] [Google Scholar]
- 281.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:9603–11. doi: 10.1523/JNEUROSCI.1835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun. 2010;24:387–93. doi: 10.1016/j.bbi.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 283.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav Immun. 2011;25:1342–8. doi: 10.1016/j.bbi.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 284.van Velthoven CT, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatric research. 2012;71:474–81. doi: 10.1038/pr.2011.64. [DOI] [PubMed] [Google Scholar]
- 285.Shen LH, Li Y, Gao Q, Savant-Bhonsale S, Chopp M. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008;56:1747–54. doi: 10.1002/glia.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.van Velthoven CT, Sheldon RA, Kavelaars A, et al. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke; a journal of cerebral circulation. 2013;44:1426–32. doi: 10.1161/STROKEAHA.111.000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 289.Perrone S, Szabo M, Bellieni CV, et al. Whole body hypothermia and oxidative stress in babies with hypoxic-ischemic brain injury. Pediatric neurology. 2010;43:236–40. doi: 10.1016/j.pediatrneurol.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 290.Thoresen M, Penrice J, Lorek A, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatric research. 1995;37:667–70. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- 291.Feigin V, Anderson N, Gunn A, Rodgers A, Anderson C. The emerging role of therapeutic hypothermia in acute stroke. Lancet Neurol. 2003;2:529. doi: 10.1016/s1474-4422(03)00500-3. [DOI] [PubMed] [Google Scholar]
- 292.Ji X, Luo Y, Ling F, et al. Mild hypothermia diminishes oxidative DNA damage and pro-death signaling events after cerebral ischemia: a mechanism for neuroprotection. Front Biosci. 2007;12:1737–47. doi: 10.2741/2185. [DOI] [PubMed] [Google Scholar]
- 293.Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke; a journal of cerebral circulation. 1996;27:913–8. doi: 10.1161/01.str.27.5.913. [DOI] [PubMed] [Google Scholar]
- 294.Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:3921–8. doi: 10.1523/JNEUROSCI.22-10-03921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lyden PD, Krieger DW, Yenari MA. Therapeutic hypothermia for acute stroke. Int J Stroke. 2005 doi: 10.1111/j.1747-4949.2005.00011.x. in press. [DOI] [PubMed] [Google Scholar]
- 296.Corbett D, Thornhill J. Temperature modulation (hypothermic and hyperthermic conditions) and its influence on histological and behavioral outcomes following cerebral ischemia. Brain Pathol. 2000;10:145–52. doi: 10.1111/j.1750-3639.2000.tb00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg. 2001;94:90–6. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]