Summary
How did humans sleep before the modern era? Because the tools to measure sleep under natural conditions were developed long after the invention of the electric devices suspected of delaying and reducing sleep, we investigated sleep in three preindustrial societies[1–3]. We find that all three show similar sleep organization, suggesting that they express core human sleep patterns, likely characteristic of pre-modern era Homo sapiens. Sleep periods, the times from onset to offset, averaged 6.9–8.5-h, with sleep durations of 5.7–7.1-h, amounts near the low end of those industrial societies[4–7]. There was a difference of nearly 1-h between summer and winter sleep. Daily variation in sleep duration was strongly linked to time of onset, rather than offset. None of these groups began sleep near sunset, onset occurring, on average, 3.3-h after sunset. Awakening was usually before sunrise. The sleep period consistently occurred during the nighttime period of falling environmental temperature, was not interrupted by extended periods of waking and terminated, with vasoconstriction, near the nadir of daily ambient temperature. The daily cycle of temperature change, largely eliminated from modern sleep environments, may be a potent natural regulator of sleep. Light exposure, was maximal in the morning greatly decreasing at noon, indicating that all three groups seek shade at midday and that light activation of the suprachiasmatic nucleus is maximal in the morning. Napping occurred on <7% of days in winter and <22% of days in summer. Mimicking aspects of the natural environment might be effective in treating certain modern sleep disorders.
Results
It has been argued that the invention of the electric light, followed by the development of television, the Internet and related technologies, along with increased caffeine usage, has greatly shortened sleep duration from “natural” levels, and disrupted its evolved timing. The purported reduction in sleep duration has been linked to obesity, mood disorders and a host of other physical and mental illnesses thought to have increased recently (http://www.healthypeople.gov/2020/topics-objectives/topic/sleep-health#eight), although complaints about reduced sleep time in the “modern world” were made at least as far back as the 1880s[8, 9].
In the current paper we examine sleep duration, timing, relation to natural light, ambient temperature and seasons, in three preindustrial human societies (Fig. 1a.). The Hadza live in northern Tanzania, 2 degrees south of the equator, in woodland-savannah habitats around Lake Eyasi. The Hadza in this study were wholly dependent on hunting and gathering each day for wild foods. Until the recent past, the Kalahari San were also nomadic hunter-gatherers. The Ju/'hoansi (Ju/’hoan language group) San that we studied live in the Den/ui village, 20 degrees south of the equator, are currently not migratory, but are isolated from surrounding villages, and continue to live as hunter-gatherers. Genetic studies indicate that the Kalahari San genome is the most variable of those yet sequenced, much more variable within this group than in the descendants of the small groups that migrated out of Africa to populate Europe, Asia and the Americas[2]. The Tsimané, living near the furthest reaches of the human migration out of Africa, close to the Maniqui River in Bolivia, 15 degrees south of the equator are hunter-horticulturalists. Extensive health studies of the Tsimané have found that although child mortality is higher than in “modern” societies, largely due to infectious diseases, adults have lower levels of blood pressure and atherosclerosis and higher levels of physical fitness than industrial populations[10]. Many live into their 60s, 70s, 80s and beyond. Similar health findings have been reported in the Hadza[1, 11] and San[12, 13].
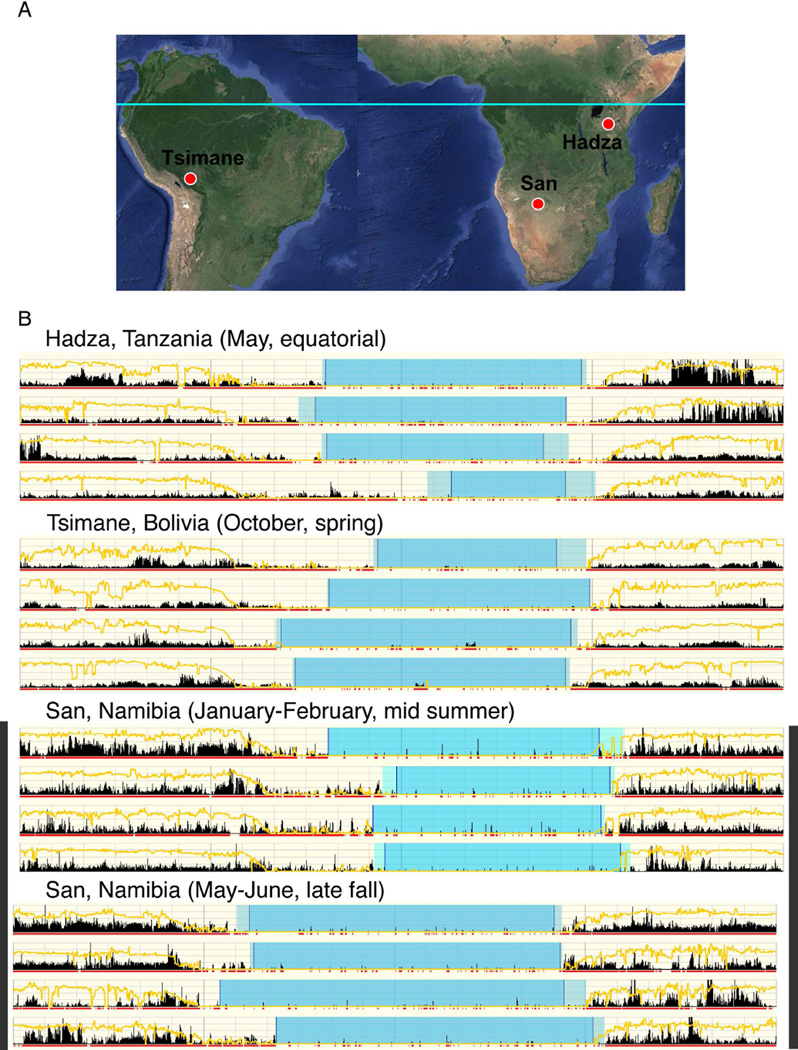
Fig 1. Location of recording sites.
(A) (Left to right, Tsimané, San, Hadza). (B) Representative Actograms from Hadza, Tsimané and San subjects (the bottom 2 sets show the same San participant in summer (upper set) and winter (lower set)). Sleep onset time is highly variable and occurred several hours after sunset in all groups. Awakening time was relatively regular and occurred before sunrise, except in the San in summer. Naps may have occurred on up to 7% of days in winter and up to 22% of days in summer. Extended periods of nocturnal waking were rare. Yellow line is a log plot of light level; red, 1-min intervals with movement; black number of movements in each 1-min interval; light blue=Actogram scored rest; dark blue=Actogram scored sleep period. Sleep period, defined as interval between sleep onset and offset is greater than sleep time, defined as sleep period minus waking after sleep onset (WASO). Sleep efficiency (sleep time divided by “bed” time) was between 81 and 86%, similar to that in industrial populations. See Table S1).
In these societies, electricity and its associated lighting and entertainment distractions are absent, as are cooling and heating systems. Individuals are exposed, from birth, to sunlight and a continuous seasonal and daily variation in temperature within the thermoneutral range for much of the daylight period, but above thermoneutral temperatures in the afternoon, and below thermoneutrality at night. By examining 3 such groups in two continents over long periods of time, we were able to evaluate common elements and differences that provide insights into the nature of human sleep under natural conditions.
Sleep Duration
Sleep time in the Hadza, San and Tsimané groups (Fig. 1b) was similar, between 5.7 and 7.1-h, with the sleep period duration (time between sleep onset and offset) of from 6.9 to 8.5-h (Table S1). Sleep parameters were determined with Actiwatch-2 devices, which have been extensively validated with polysomnography (Fig. 1b.; see Supplementary Materials). The standard deviation of sleep onset times exceeded the standard deviation of sleep offset times in all San individuals [N=27, p=7.4E-5, Binomial test) and in all Tsimané individuals (N=45, p=2.0E-08], with a similar trend in the more limited data set from the Hadza. Therefore, sleep duration was much more strongly correlated with sleep onset time than with sleep offset time in both summer and winter. Sleep onset and offset times were very weakly correlated with each other (Table S1).
BMI
Mean BMIs of the three groups were between 18.3 and 26.2 (Table S2), with none of the participants having BMIs >30, in keeping with prior anthropological observations of a lack of obesity in these populations[11].
Summer vs. winter durations
The Tsimané and San live far enough south of the equator to have substantial seasonal changes in day length and temperature. Tsimané participants recorded in the winter slept 56 min longer than those in summer (Fig. 2a) [t=2.1, df=19, p=0.05]. In the San, we recorded the same group of 13 participants in winter and summer. Sleep times in the winter were longer than in the summer by an average of 53 min (Fig. 2b, Table S2) [t=3.7, df=20, p=0.001].
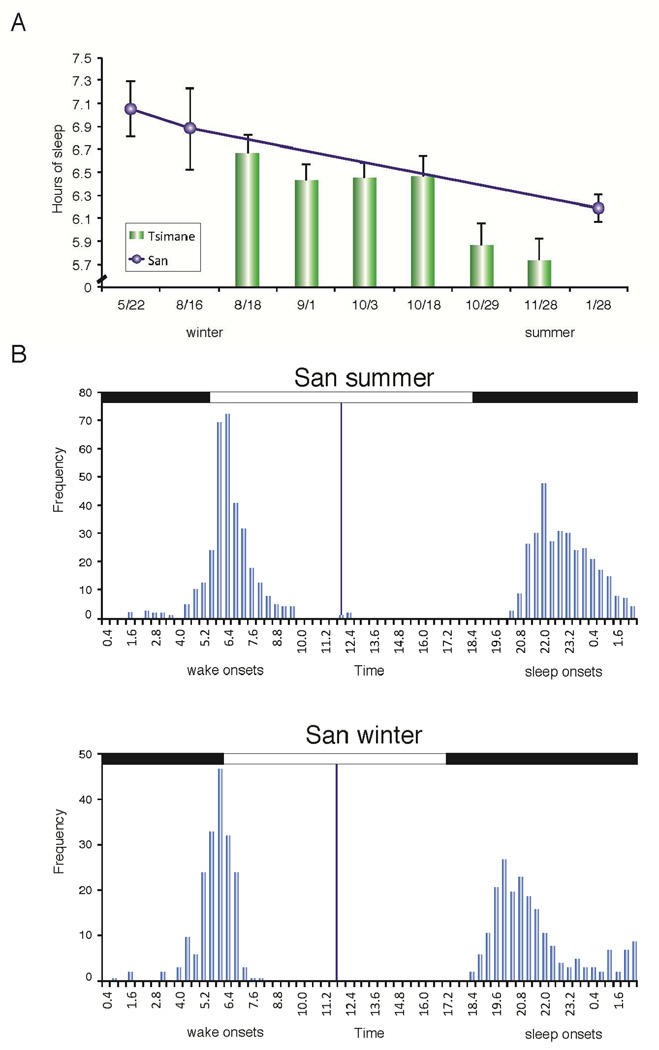
Fig. 2. Seasonal effects on sleep.
(A) Sleep duration decreased from winter to summer. (Note that the Hadza, San and Tsimane live in the southern hemisphere.) The Tsimané data were from 6 separate groups recorded over the 4 month period. Each group consisted of 7–12 individuals recorded for 7 days. A parallel study in the San recorded from 10 individuals, each for 21 days in May-June. An additional 5 San individuals were recorded for 11 days in August and 13 of the initial 15 were recorded for 28 days in Jan-February of the next year (two of the original 10 participants had migrated out of Den/ui). (B) Change in sleep onset and offset times across the seasons. The same San individuals were sampled for a 28 day period in summer and a 21 day period in winter (a total of 1260 sleep onsets and offsets). Note the much later sleep onset in the summer and the later wake onset in the summer relative to winter, despite shorter sleep times. Bin size is 0.4-h (24 min). Blue vertical line marks solar noon.
Napping
It has long been known that “modern” humans experience an extended dip in midafternoon alertness, which is not due to food intake[14, 15]. It has been speculated that under natural conditions, a nap would occur during this period, and that this nap has been suppressed by industrial lifestyles. An automated Actogram analysis using the Actogram program (see “Actiwatch-2 devices” in Supplementary Materials) on the data from the San scored no afternoon naps in 210 days of recording in the winter. It scored 10 naps on 364 days in the summer (3% of days) (see Table S3). Nocturnal awakenings were also infrequent (see Table S3). The Actograms of the Tsimané and Hadza showed a similar dearth of potential napping and nocturnal waking intervals (Fig. 1b). Because Actiwatches have not been as thoroughly validated against polygraphic recording for naps as they have for nighttime sleep [4, 16–18] we conducted a second quantification of naps using visual scoring of the Actiwatch records to identify periods with motor activity reduced to levels seen within the nighttime sleep for periods of 15 min or longer. We saw such episodes in only 7% of the recording afternoons in the San winter data. This should be considered the maximum incidence of napping, since we cannot exclude the possibility that some or all of these were waking rest periods. In the summer, 22% of days had potential naps [comparing summer and winter frequencies, t=3.5, df=25, p=0.0007] (Fig. 1b). Nap duration using the longer summer visually scored putative nap periods averaged 32 min. Thus if all potential napping time was considered sleep time, it would raise the average daily sleep duration in summer by 7 min. It remains possible that naps shorter than 15 min occur, but EEG recording would be necessary to identify them.
Insomnia
Since insomnia is a complaint and does not closely correspond to sleep time[19, 20], we investigated the prevalence of this complaint in the Tsimane and San groups. At the time of application of Actiwatches on the Tsimane, GY and a Tsimané translator visited the participants in their homes early in the morning to conduct an interview on fatigue and sleep quality. A similar interview was done by JS on the San group. Neither group has a word for insomnia in their language, so we explained the concept in terms of sleep onset insomnia and sleep maintenance insomnia not due to illness. Five percent of the participants said they sometimes had sleep onset problems and 9% sometimes had sleep maintenance problems. Less than one third of these participants said that they had these problems regularly, i.e. more than once a year (1.5% and 2.5% of the total number of participants). These numbers are far lower than the 10–30% chronic insomnia rate reported in industrial societies[19, 20].
Light
Average sleep onset across groups occurred between 2.5 and 4.4-h after sunset (mean=3.3 h) (Table S2, Fig. 3). At the latitudes of the participant populations, the duration of evening and morning civil twilight ranges from 24–28 min. Therefore the participants remained awake long after darkness had fallen. The 3 groups often had small fires, but the Actiwatch-measured light levels remained below 5.0 lux (the lower limit of the actiwatch 2 sensor) throughout the night (Fig. 3). Awakening occurred on average an hour before sunrise in the Tsimane and Hadza, well before civil twilight (Table S2), but awakening was much closer to sunrise than sleep onset was to sunset. Awakening was also well before civil twilight in the winter in the San (Table S2). But in the summer awakenings in the San occurred 1-h after sunrise, on average [t=2.4, df=20, p=0.02] (Figs. 2–4). The shorter sleep duration in the summer was completely a result of later sleep onset not of earlier awakening (mean sleep onset time=22:39 summer vs. 21:16 winter) [t=5.0, df=20, p=6.8E-5]. So, neither sleep onset nor offset were tightly linked to solar light level. A striking feature of the light exposure in all three groups was that it decreased from a maximum level at approximately 9 AM, to a lower level at noon, despite the doubling of ambient light levels over this period. This occurred in winter as well as in summer, indicating that all three groups sought shade from the midday sun (Fig. 3).
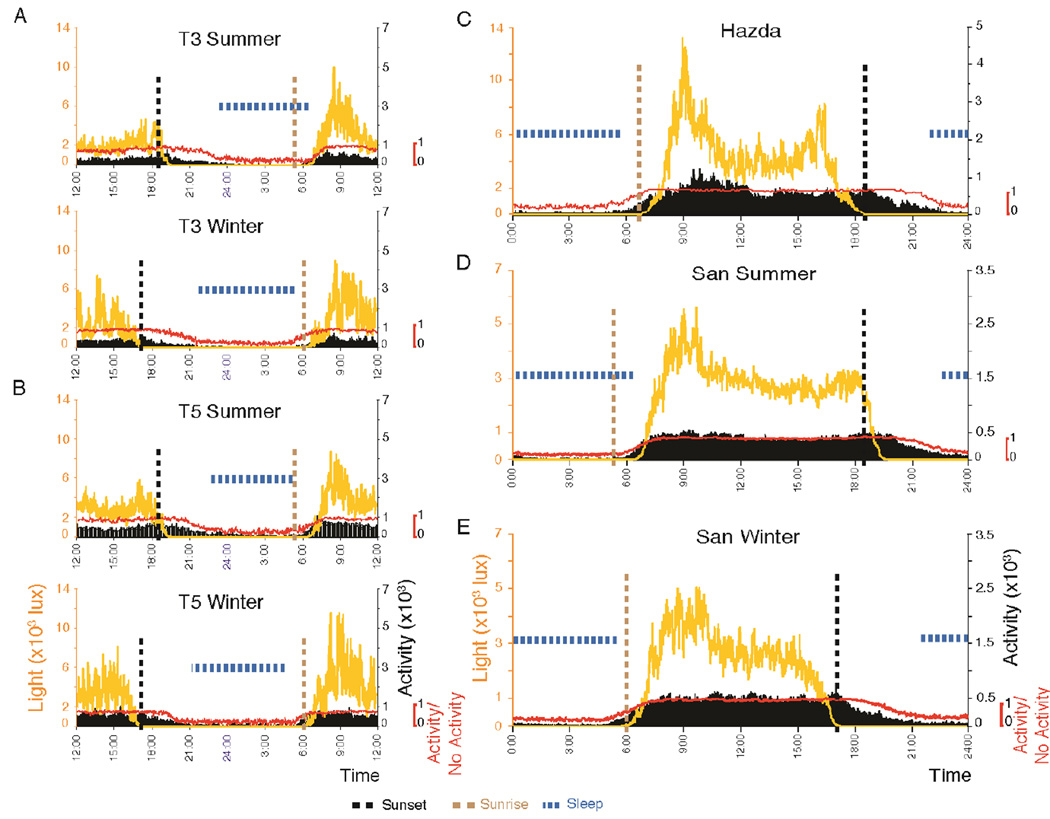
Fig. 3. Light and activity plots.
(A, B) Average light and activity level in plots centered at midnight: Two participants (T3 and T5) are shown. Both have shorter sleep time in the summer despite later awakening. Participant data is their average sleep parameters over the summer or winter recording periods. Yellow line indicates subject light exposure as measured by the Actiwatch. Sunset, identifiable by the vertical interrupted black line, is not tightly linked to sleep onset. Interrupted blue bars indicates sleep periods. Red line at the bottom of each graph plots average of 1 min epochs with (+1) and without (0) activity. Note maintained and even increased activity (Black) with sunset, location of inactivity linked to sleep at the end of the dark period, awakening before dawn in winter, lack of period of activity within sleep and differences between duration of summer and winter nighttime inactivity period. The duration of these inactivity epochs are used in the algorithm that identifies sleep (Fig. 1). Sleep onset occurs from 2.5 to 4.4-h after sunset in all of the groups examined (mean = 3.3h). (C) Staying out of the midday sun. Plots centered at noon. Light levels recorded by Actiwatch drop steeply and consistently at midday, despite the increase of the ambient light level from morning (9 AM) to noon levels. Figure shows average of 60 days of data from the 10 Hadza recorded in Tanzania. It shows the reduction in light exposure during the afternoon, a lack of reduction in afternoon activity to sleep levels consistent with the lack of regular napping and the reduction in activity throughout the sleep period. No regular period of activity was seen in the night, consistent with the lack of a “second sleep” scored by the algorithm (also see fig S1). (D, E) Averaged data across all San recorded in summer and winter. Note the consistent pattern across groups and seasons. Time is local clock time.
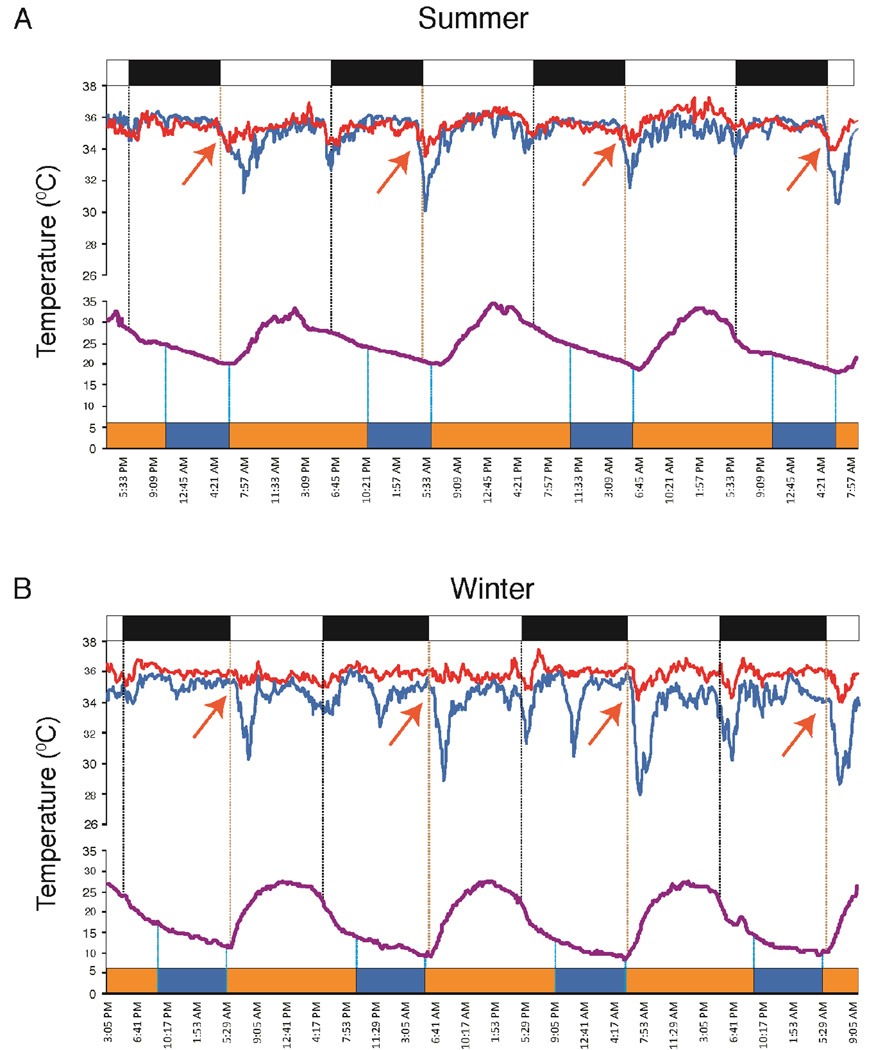
Fig. 4. Relation of sleep to ambient temperature and skin temperature.
Sleep offset, averaged across all subjects and all days, consistently occurs near the nadir of daily environmental temperature, in both summer and winter. In the San recorded in the summer, the temperature nadir occurred after sunrise, as did awakening. In the winter the nadir occurred near sunrise with awakening preceding sunrise. Note that the ambient temperature has a gradual fall at night and a rapid rise starting at sunrise, with sleep occurring during the period of slowly falling temperature. Vasoconstriction is seen upon awakening in both summer and winter. In the winter there are additional vasoconstrictions occurring during the day. These are likely related to food preparation or other similar activities exposing the hands to cold. Violet lines are environmental temperature, red line, abdominal temperature, blue line, finger temperature. Also see Fig. S2 for an example of individual subject data. All temperatures recorded by iButtons are synchronized to the actiwatch time ±2 min. Black bar=night, orange=waking, blue=sleep. Vertical lines at top of figures indicate light-dark transitions; those at bottom indicate sleep-wake transitions. Sleep measures are averages of 15 participants recorded in the summer and 13 of these participants recorded in winter (See Fig. 1, Table S1). Red arrows indicate onset of drop in finger temperature starting near the temperature nadir, indicative of peripheral vasoconstriction, serving to warm proximal regions with awakening.
Temperature
Because we noticed that the Hadza Tsimane and San did not initiate sleep at sunset and that their sleep was confined to the latter portion of the dark period, we investigated the role of temperature. We found that the nocturnal sleep period in the Hadza was always initiated during a period of falling ambient temperature (Fig. S1) and we saw a similar pattern in the Tsimané. Therefore, we precisely measured ambient temperature at the sleeping sites along with finger temperature and abdominal temperature in our studies of the San[21]. Figs. 3, 4 and S2 show that sleep in both the winter and summer occurred during the period of decreasing ambient temperature and that wake onset occurred near the nadir of the daily temperature rhythm. A strong vasoconstriction occurred at wake onset in both summer and winter (Fig. 4, S2), presumably functioning to aid thermogenesis in raising the brain and core temperature for waking activity. See Supplementary Material for a discussion of the use of iButtons to measure vasoconstriction and vasodilation. The presence of vasoconstriction at awakening, indicates that the subjects were not vasoconstricted prior to awakening.
In the Tsimané, summer wake times were earlier and sleep onset times were later than in the winter, accounting for their reduced sleep duration; however, the San, despite their shorter sleep duration in the summer, as in the Tsimane, the time of awakening was significantly later, with the decreased sleep times being entirely the result of later sleep onset. The San the participants awakened, on average, 1.0 h after sunrise in the summer. The Tsimane awakened 1.4 h before sunrise in the summer (Table S2). The 2.4 h difference in awakening times, with respect to sunrise, was significant (t=8.4, df=22, p=1.2E-08). This was not due to differences in day/night length at the two recording locations. The summer observation period in the San had 11 h nights and 13 h days. The summer observation period in the Tsimane had 11.1 h nights and 12.9 h days. The difference between the sleep offset times in these two populations, despite the similar light conditions, may be due to the much cooler morning temperatures (by 6° C on average), and the shifting of the temperature nadir into the light period (Fig. 4) in the San’s environment, paralleling the effect of winter on changes in sleep duration.
Discussion
A striking finding is the uniformity of sleep patterns across groups despite their ancient geographic isolation from each other. This suggests that the observed patterns are not unique to their particular environmental or cultural conditions but rather are central to the physiology of humans living in the tropical latitudes near the locations of the San and Hadza groups, where our species evolved.
In some ways the sleep in these traditional human groups is more similar to sleep in industrial societies than has been assumed. They do not sleep more than most individuals in industrial [1, 4–7, 22]. The traditional groups do not regularly awaken for extended periods in the middle of the night (see supplementary data) despite anecdotal reports[23]. Sleep is strongly modulated by the seasons, averaging 53–56 min longer in the winter, coincident with a 1.2 or 2-h increase in the night duration in the San and Tsimane respectively [Table S2, Fig. 2]. In contrast, no seasonal effect on sleep durations has been reported in most studies in industrial societies. Kleitman [24] summarizes some of the early, conflicting data on seasonal changes in sleep (p192). A recent large scale study of seasonal sleep changes reported an 18-min difference between summer and winter. This study investigated participants in Berlin, (latitude +53°) where night duration changes f rom 7-h 39-min in summer to 16-h 21-min in winter)[25]. Of course, the Berlin participants were not as directly exposed to changes in light and temperature as were our participants.
Light has been shown to be a major factor in human sleep and circadian rhythm control, partially mediated by light’s effects on the melanopsin system[19, 26–28]. Consistent with this, we show here that sleep occurs almost entirely during the dark period in these traditional societies. In contrast, sleep typically continues well after sunrise in industrial populations[27]. A recent study has shown a striking difference in the sleep onset and offset times as a function of light exposure in a comparison of two closely related traditional Argentinian hunter-gatherer populations[26]. Three other studies showed the rapid regularization of human sleep patterns created by moving “modern” subjects into more natural lighting situations [28–30].
Our finding that hunter-gatherers get maximal light exposure in the morning, rather than at noon is consistent with behavioral thermoregulation to avoid afternoon heat. It also may explain the greater effectiveness of morning light [31, 32] in the reversal of depression, since such treatments tend to restore the evolved pattern of human exposure to light.
Of the 10 groups we studied, the only group in our study that awakened after sunrise was the San in the summer. The Tsimane always arouse before dawn. At the end of November - beginning of December (two weeks from the summer solstice), they awakened more than 1 h 20 mi before sunrise, whereas the San at approximately the same season, with nearly indistinguishable seasonal light levels (13 vs. 12.9 h of light) awakened nearly an hour after sunrise. Our data suggest that ambient temperature may be responsible for the difference between these groups and may be a major determinant of sleep timing and duration, independent of light level.
Historical evidence suggests that “until the close of the early modern era, Western Europeans experienced two major intervals of sleep bridged by up to an hour or more of quiet wakefulness[30, 33].” Our results suggest that the bimodal sleep pattern that may have existed in Western Europe is not today present in traditional equatorial groups, and by extension, was probably not present before humans migrated into such areas. Rather, this pattern may have been a consequence of longer winter nights in higher latitudes. In this view, the “recent” disappearance of bimodal sleep was not a pathological development caused by restricted sleep duration, but rather a return to a pattern still seen today in the groups we studied, enabled by the electric lights and temperature control that restored aspects of natural conditions in the tropical latitudes.
We found that nocturnal sleep in all groups occurred towards the end of the night, during the period of lowest ambient temperatures. In nature, the daily rhythm of environmental temperature is tightly locked to the rhythm of sunset and sunrise. However, in most industrial societies the seasonal and circadian temperature rhythms are greatly attenuated by insulated buildings and artificial heating and cooling. The synchronization that we observed between the reduction in ambient temperature at night and sleep under traditional conditions, with its associated decline in core temperature[34], may have evolved to save energy by reducing the temperature differential between body and environment and consequent heat loss. Being active during the late night period of lowest temperatures would be metabolically costly. Individuals in groups like those we observed may be less vulnerable to insomnia because they are exposed to a falling ambient temperature at the time of sleep onset and do not have to actively shed heat to achieve the body temperature reduction that accompanies sleep onset[35–37]. The daily reduction in light is followed by the daily reduction in temperature. The delayed melatonin response to darkness is adaptive in facilitating sleep after darkness[27] bringing the entire sleep period in synchrony with the lowest nighttime temperatures.
Our findings indicate that sleep in industrial societies has not been reduced below a level that is normal for most of our species' evolutionary history. Recreating aspects of the environments we observed in preindustrial societies might have beneficial effects on sleep and insomnia in industrial populations.
Methods
Methods summary
Sleep was quantified with Actiwatch-2 devices worn for 6–28 days. Sleep states were scored by the Actogram program. We extracted the light and acceleration data and statistically compared these data with temperature, solar and seasonal variables. The San participants wore iButton temperature recorders on the middle fingers of both hands and on the abdomen for 4 days at the start of recording periods in the summer and winter periods. iButton devices were also placed near the participants’ sleeping sites to accurately measure environmental temperature and humidity at 4 min intervals. (See Supplementary Materials for details.)
Supplementary Material
Highlights.
Hunter-gatherers in Africa and Bolivia show similar sleep amounts and timing.
They do not sleep more than modern humans, with average durations of 5.7–7.1-h
They go to sleep several hours after sunset and typically awaken before sunrise.
Temperature appears to be a major regulator of human sleep duration and timing.
Blurb.
Yetish et al. find that hunter-gatherers sleep 6.4-h/day on average, 1-h more in winter than in summer. Sleep onset is about 3.3-h after sunset and sleep occurs during the nightly period of falling temperature. Onset times are irregular, but offset time is very regular. Little napping is seen. Light exposure is maximal in the morning, not at noon.
Acknowledgments
We thank Oma Tsamkgao for translation, assistance with the Actiwatch and iButton application and various associated tasks, Adhil Bhagwandin, Leigh-Anne Dell and Josh Davimes for assistance with set-up of studies in Namibia, Alfredo Maito Nosa and Basilio Vie Tayo for translation, long hours of hard work, and comprehensive field guidance in Bolivia, Keng-Tee Chew and M.F. Wu for help with data analysis, Eus Van Someren for advice on the use of iButtons and Craig Heller for advice on ambient temperature recording. JMS was supported by MH064109 and DA034748. MG, HK and GY were supported by NIH/NIA #R01AG024119. PRM was supported by funding from the National Research Foundation of South Africa. BW received support from the National Science Foundation #1062879.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts to disclose.
An abstract of this work was submitted to the World Federation of Sleep Research Societies meeting on April 6, 2015.
Author Contributions: GY, JMS, PM and HK conceived the study, GY collected the data in Bolivia, BW and HP collected the data in Tanzania, JMS and PM collected the data in Namibia, GY, CW, RM and JMS analyzed the data, all authors assisted in writing the manuscript.
Reference List
- 1.Marlowe F. The Hadza Hunter-Gatherers of Tanzania. Berkley and Los Angeles: University of California Press; 2010. [Google Scholar]
- 2.Schuster SC, Miller W, Ratan A, Tomsho LP, Giardine B, Kasson LR, Harris RS, Petersen DC, Zhao F, Qi J, et al. Complete Khoisan and Bantu genomes from southern Africa. Nature. 2010;463:943–947. doi: 10.1038/nature08795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasunilashorn S, Crimmins EM, Kim JK, Winking J, Gurven M, Kaplan H, Finch CE. Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. Am J Hum. Biol. 2010;22:731–740. doi: 10.1002/ajhb.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwell T, Ancoli-Israel S, Redline S, Stone KL. Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. J Clin Sleep Med. 2011;7:357–367. doi: 10.5664/JCSM.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heeren M, Sojref F, Schuppner R, Worthmann H, Pflugrad H, Tryc AB, Pasedag T, Weissenborn K. Active at night, sleepy all day: Sleep disturbances in patients with hepatitis C virus infection. Journal of Hepatology. 2014;60:732–740. doi: 10.1016/j.jhep.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Natale V, Leger D, Martoni M, Bayon V, Erbacci A. The role of actigraphy in the assessment of primary insomnia: a retrospective study. Sleep Medicine. 2014;15:111–115. doi: 10.1016/j.sleep.2013.08.792. [DOI] [PubMed] [Google Scholar]
- 7.Robillard R, Naismith SL, Smith KL, Rogers NL, White D, Terpening Z, Ip TK, Hermens DF, Whitwell B, Scott EM, et al. Sleep-wake cycle in young and older persons with a lifetime history of mood disorders. PLoS. ONE. 2014;9:e87763. doi: 10.1371/journal.pone.0087763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horne JA. Sleepfaring: The Secrets and Science of a Good Night's Sleep. Oxford University Press; 2007. [Google Scholar]
- 9.Horne J. The end of sleep: Sleep debt versus biological adaptation of human sleep to waking needs. Biological Psychology. 2011;87:1–14. doi: 10.1016/j.biopsycho.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Gurven M, Kaplan H, Winking J, Eid RD, Vasunilashorn S, Kim JK, Finch C, Crimmins E. Inflammation and infection do not promote arterial aging and cardiovascular disease risk factors among lean horticulturalists. PLoS. ONE. 2009;4:e6590. doi: 10.1371/journal.pone.0006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pontzer H, Raichlen DA, Wood BM, Mabulla AZ, Racette SB, Marlowe FW. Hunter-gatherer energetics and human obesity. PLoS. ONE. 2012;7:e40503. doi: 10.1371/journal.pone.0040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchengast S. Differential reproductive success and body size in !Kung San people from northern Namibia. Coll. Antropol. 2000;24:121–132. [PubMed] [Google Scholar]
- 13.Fernandes-Costa FJ, Marshall J, Ritchie C, van Tonder SV, Dunn DS, Jenkins T, Metz J. Transition from a hunter-gatherer to a settled lifestyle in the !Kung San: effect on iron, folate, and vitamin B12 nutrition. The American Journal of Clinical Nutrition. 1984;40:1295–1303. doi: 10.1093/ajcn/40.6.1295. [DOI] [PubMed] [Google Scholar]
- 14.Carskadon MA, Dement WC. Normal human sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: W.B. Saunders; 2005. pp. 13–23. [Google Scholar]
- 15.Roehrs T, Carskadon M, Dement W, Roth T. Daytime sleepiness and alertness. In: Kryger M, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine. Missouri: Elsevier, Saunders; 2011. pp. 42–53. [Google Scholar]
- 16.Basner M, Dinges DF, Mollicone DJ, Savelev I, Ecker AJ, Di AA, Jones CW, Hyder EC, Kan K, Morukov BV, et al. Psychological and behavioral changes during confinement in a 520-day simulated interplanetary mission to mars. PLoS. ONE. 2014;9:e93298. doi: 10.1371/journal.pone.0093298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basner M, Dinges DF, Mollicone D, Ecker A, Jones CW, Hyder EC, Di Antonio A, Savelev I, Kan K, Goel N, et al. Mars 520-d mission simulation reveals protracted crew hypokinesis and alterations of sleep duration and timing. PNAS. 2013;110:2635–2640. doi: 10.1073/pnas.1212646110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kandy JC, Drummund SPA, Mednick S. Actigraphic assessment of a polysomnographic-recorded nap: a validation study. J Sleep Res. 2011;20:214–222. doi: 10.1111/j.1365-2869.2010.00858.x. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ. Insomnia. JAMA. 2013;309:706–716. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 21.Romeijn N, Raymann R, Most E, Te Lindert B, Van Der Meijden W, Fronczek R, Gomez-Herrero G, Van Someren E. Sleep, vigilance, and thermosensitivity. Pflugers Arch - Eur J Physiol. 2012;463:169–176. doi: 10.1007/s00424-011-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans DS, Snitker S, Wu SH, Mody A, Njajou OT, Perlis ML, Gehrman PR, Shuldiner AR, Hsueh WC. Habitual sleep/wake patterns in the Old Order Amish: heritability and association with non-genetic factors. Sleep. 2011;34:661–669. doi: 10.1093/sleep/34.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worthman CM. After dark: The evolutionary ecology of human sleep. In: Trevathan WR, Smith EOMJJ, editors. Evolutionary Medicine and Health. Oxford: Oxford University Press; 2008. pp. 291–313. [Google Scholar]
- 24.Kleitman N. Sleep and Wakefulness. Chicago: University of Chicago Press; 1963. [Google Scholar]
- 25.Lehnkering H, Siegmund R. Influence of chronotype, season, and sex of subject on sleep behavior of young adults. Chronobiol. Int. 2007;24:875–888. doi: 10.1080/07420520701648259. [DOI] [PubMed] [Google Scholar]
- 26.de-la-Iglesia HO, Fernandez-Duque E, Golombek DA, Lanza N, Duffy JF, Czeisler CA, Valeggia CR. Access to Electric light is associated with shorter sleep duration in a traditionally hunter-gatherer community. J. Biol. Rhythms. 2015 doi: 10.1177/0748730415590702. 0748730415590702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santhi N, Thorne HC, van der Veen DR, Johnsen S, Mills SL, Hommes V, Schlangen LJM, Archer SN, Dijk DJ. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. Journal of Pineal Research. 2012;53:47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 28.Wright K, McHill A, Birks B, Griffin B, Rusterholz T, Chinoy E. Entrainment of the Human Circadian Clock to the Natural Light-Dark Cycle. Current Biology. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piosczyk H, Landmann N, Holz J, Feige B, Riemann D, Nissen C, Voderholzer U. Prolonged sleep under Stone Age conditions. J Clin Sleep Med. 2014;10:719–722. doi: 10.5664/jcsm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehr TA, Aeschbach D, Duncan WC., Jr Evidence for a biological dawn and dusk in the human circadian timing system. J Physiol. 2001;535:937–951. doi: 10.1111/j.1469-7793.2001.t01-1-00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kripke DF, Elliott JA, Welsh DK, Youngstedt SD. Photoperiodic and circadian bifurcation theories of depression and mania. F1000Res. 2015;4:107. doi: 10.12688/f1000research.6444.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uzoma HN, Reeves GM, Langenberg P, Khabazghazvini B, Balis TG, Johnson MA, Sleemi A, Scrandis DA, Zimmerman SA, Vaswani D, et al. Light treatment for seasonal Winter depression in African-American vs Caucasian outpatients. World J Psychiatry. 2015;5:138–146. doi: 10.5498/wjp.v5.i1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekirch A. At Day's Close, Night in Times Past. New York: W.W. Norton and Company; 2005. [Google Scholar]
- 34.Krauchi K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med. Rev. 2007;11:439–451. doi: 10.1016/j.smrv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Fronczek R, Overeem S, Lammers GJ, van Dijk JG, van Someren EJ. Altered skin-temperature regulation in narcolepsy relates to sleep propensity. Sleep. 2002;29:1444–1449. doi: 10.1093/sleep/29.11.1444. [DOI] [PubMed] [Google Scholar]
- 36.Raymann RJEM, Swaab DF, Van Someren EJW. Skin deep: enhanced sleep depth by cutaneous temperature manipulation. Brain. 2008;131:500–513. doi: 10.1093/brain/awm315. [DOI] [PubMed] [Google Scholar]
- 37.Van DeWerken M, Gimenez MC, De VB, Beersma DG, van Someren EJ, Gordijn MC. Effects of artificial dawn on sleep inertia, skin temperature, and the awakening cortisol response. J Sleep Res. 2010;19:425–435. doi: 10.1111/j.1365-2869.2010.00828.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.