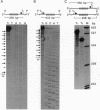
Abstract
Slipped mispairing between repeated sequences during DNA replication is an important mutagenic event. It is one of several suggested mechanisms thought to be responsible for generating polymorphic regions and large-scale deletions found in mammalian mitochondrial DNA. In the porcine mitochondrial genome, a domain carrying a 10-bp tandemly repeated sequence displays a unique in vivo pattern of repeat copy number polymorphs. Upon passage in Escherichia coli, a recombinant plasmid containing this domain also displays a unique polymorphic pattern that is different from that seen in the animal. To test the hypothesis that these polymorphisms were slippage induced and that the different polymorphic patterns reflected differences in modes of replication, we performed a series of in vitro primer extension reactions. By utilizing either single- or double-stranded templates containing the repeat domain we were able to correlate in vitro generated repeat polymorphism patterns with those seen in the mitochondria or the bacteria, respectively, thus providing experimental evidence that slippage replication is responsible for a major class of mammalian mutations.
Full text
PDF

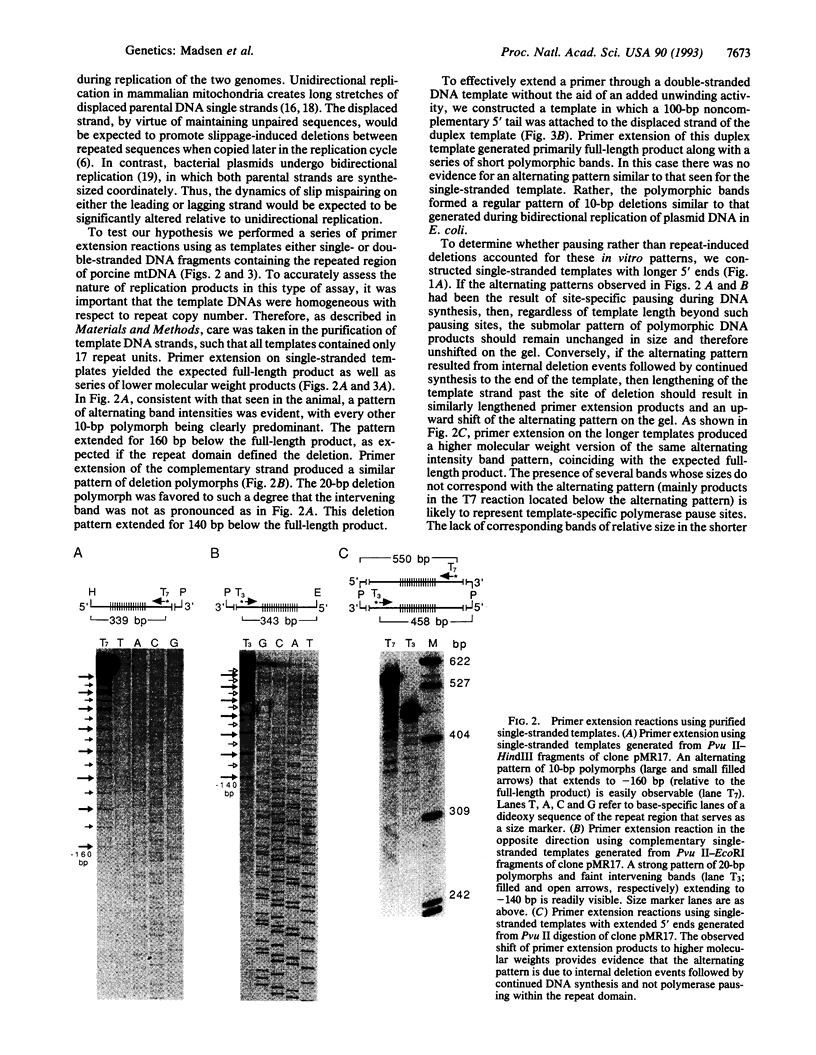
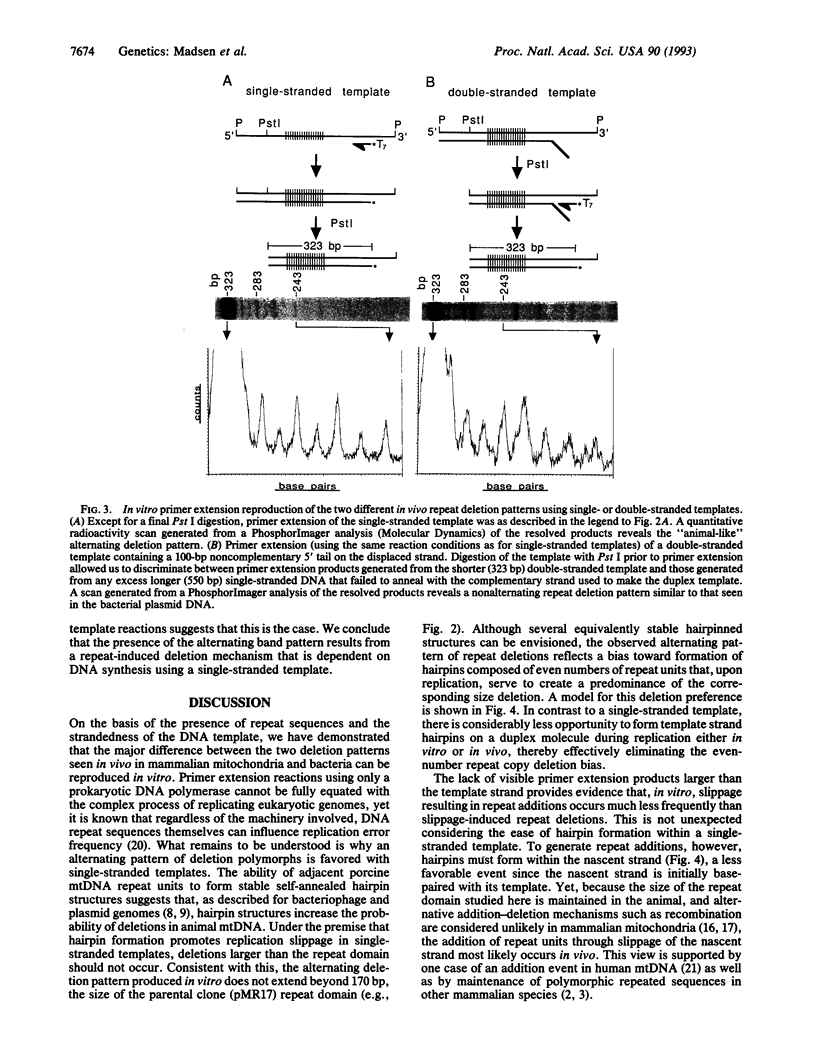
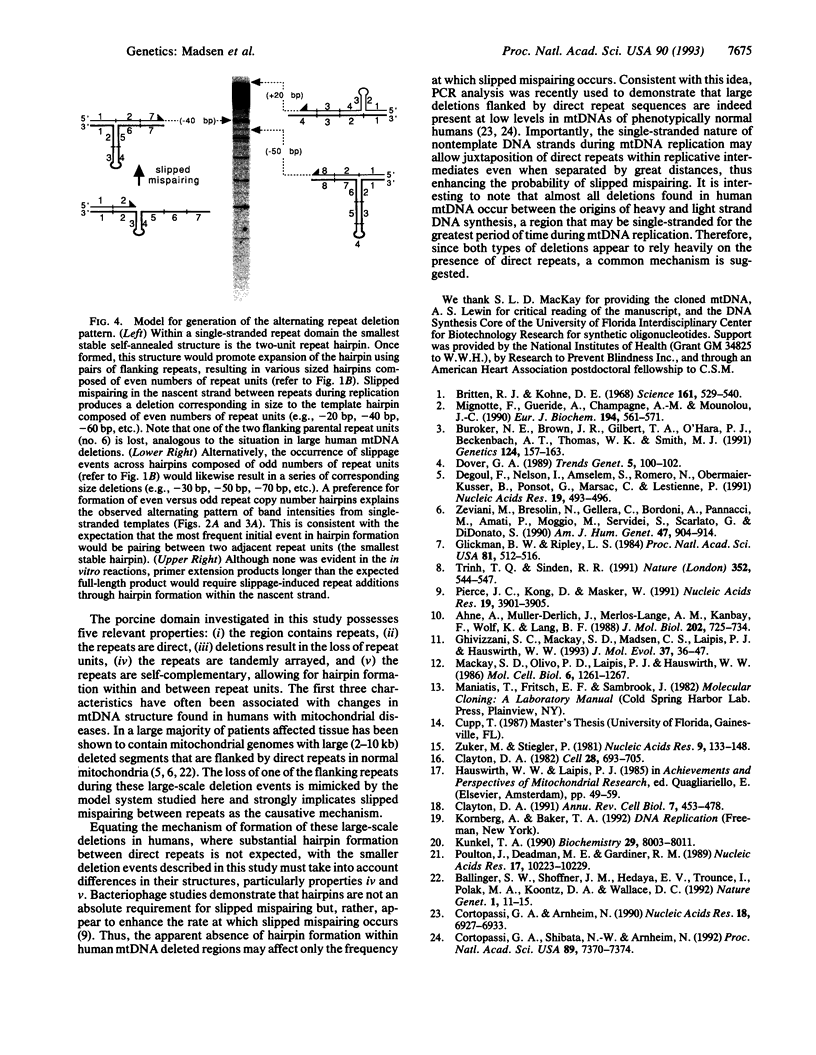
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahne A., Müller-Derlich J., Merlos-Lange A. M., Kanbay F., Wolf K., Lang B. F. Two distinct mechanisms for deletion in mitochondrial DNA of Schizosaccharomyces pombe mutator strains. Slipped mispairing mediated by direct repeats and erroneous intron splicing. J Mol Biol. 1988 Aug 20;202(4):725–734. doi: 10.1016/0022-2836(88)90553-0. [DOI] [PubMed] [Google Scholar]
- Ballinger S. W., Shoffner J. M., Hedaya E. V., Trounce I., Polak M. A., Koontz D. A., Wallace D. C. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet. 1992 Apr;1(1):11–15. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Buroker N. E., Brown J. R., Gilbert T. A., O'Hara P. J., Beckenbach A. T., Thomas W. K., Smith M. J. Length heteroplasmy of sturgeon mitochondrial DNA: an illegitimate elongation model. Genetics. 1990 Jan;124(1):157–163. doi: 10.1093/genetics/124.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol. 1991;7:453–478. doi: 10.1146/annurev.cb.07.110191.002321. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Cortopassi G. A., Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990 Dec 11;18(23):6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortopassi G. A., Shibata D., Soong N. W., Arnheim N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7370–7374. doi: 10.1073/pnas.89.16.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degoul F., Nelson I., Amselem S., Romero N., Obermaier-Kusser B., Ponsot G., Marsac C., Lestienne P. Different mechanisms inferred from sequences of human mitochondrial DNA deletions in ocular myopathies. Nucleic Acids Res. 1991 Feb 11;19(3):493–496. doi: 10.1093/nar/19.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover G. A. Slips, strings and species. Trends Genet. 1989 Apr;5(4):100–102. doi: 10.1016/0168-9525(89)90038-3. [DOI] [PubMed] [Google Scholar]
- Ghivizzani S. C., Mackay S. L., Madsen C. S., Laipis P. J., Hauswirth W. W. Transcribed heteroplasmic repeated sequences in the porcine mitochondrial DNA D-loop region. J Mol Evol. 1993 Jul;37(1):36–37. doi: 10.1007/BF00170460. [DOI] [PubMed] [Google Scholar]
- Glickman B. W., Ripley L. S. Structural intermediates of deletion mutagenesis: a role for palindromic DNA. Proc Natl Acad Sci U S A. 1984 Jan;81(2):512–516. doi: 10.1073/pnas.81.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Misalignment-mediated DNA synthesis errors. Biochemistry. 1990 Sep 4;29(35):8003–8011. doi: 10.1021/bi00487a001. [DOI] [PubMed] [Google Scholar]
- MacKay S. L., Olivo P. D., Laipis P. J., Hauswirth W. W. Template-directed arrest of mammalian mitochondrial DNA synthesis. Mol Cell Biol. 1986 Apr;6(4):1261–1267. doi: 10.1128/mcb.6.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte F., Gueride M., Champagne A. M., Mounolou J. C. Direct repeats in the non-coding region of rabbit mitochondrial DNA. Involvement in the generation of intra- and inter-individual heterogeneity. Eur J Biochem. 1990 Dec 12;194(2):561–571. doi: 10.1111/j.1432-1033.1990.tb15653.x. [DOI] [PubMed] [Google Scholar]
- Pierce J. C., Kong D., Masker W. The effect of the length of direct repeats and the presence of palindromes on deletion between directly repeated DNA sequences in bacteriophage T7. Nucleic Acids Res. 1991 Jul 25;19(14):3901–3905. doi: 10.1093/nar/19.14.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton J., Deadman M. E., Gardiner R. M. Tandem direct duplications of mitochondrial DNA in mitochondrial myopathy: analysis of nucleotide sequence and tissue distribution. Nucleic Acids Res. 1989 Dec 25;17(24):10223–10229. doi: 10.1093/nar/17.24.10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh T. Q., Sinden R. R. Preferential DNA secondary structure mutagenesis in the lagging strand of replication in E. coli. Nature. 1991 Aug 8;352(6335):544–547. doi: 10.1038/352544a0. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Bresolin N., Gellera C., Bordoni A., Pannacci M., Amati P., Moggio M., Servidei S., Scarlato G., DiDonato S. Nucleus-driven multiple large-scale deletions of the human mitochondrial genome: a new autosomal dominant disease. Am J Hum Genet. 1990 Dec;47(6):904–914. [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]