Abstract
Background
On-going malaria transmission is increasingly mediated by outdoor-biting vectors, especially where indoor insecticidal interventions such as long-lasting insecticide treated nets (LLINs) are widespread. Often, the vectors are also physiologically resistant to insecticides, presenting major obstacles for elimination. We tested a combination of electrocuting grids with synthetic odours as an alternative killing mechanism against outdoor-biting mosquitoes.
Methods
An odour-baited device, the Mosquito Landing Box (MLB), was improved by fitting it with low-cost electrocuting grids to instantly kill mosquitoes attracted to the odour lure, and automated photo switch to activate attractant-dispensing and mosquito-killing systems between dusk and dawn. MLBs fitted with one, two or three electrocuting grids were compared outdoors in a malaria endemic village in Tanzania, where vectors had lost susceptibility to pyrethroids. MLBs with three grids were also tested in a large semi-field cage (9.6×9.6×4.5m), to assess effects on biting-densities of laboratory-reared Anopheles arabiensis on volunteers sitting near MLBs.
Results
Significantly more mosquitoes were killed when MLBs had two or three grids, than one grid in wet and dry seasons (P<0.05). The MLBs were highly efficient against Mansonia species and malaria vector, An. arabiensis. Of all mosquitoes, 99% were non-blood fed, suggesting host-seeking status. In the semi-field, the MLBs reduced mean number of malaria mosquitoes attempting to bite humans fourfold.
Conclusion
The improved odour-baited MLBs effectively kill outdoor-biting malaria vector mosquitoes that are behaviourally and physiologically resistant to insecticidal interventions e.g. LLINs. The MLBs reduce human-biting vector densities even when used close to humans, and are insecticide-free, hence potentially antiresistance. The devices could either be used as surveillance tools or complementary mosquito control interventions to accelerate malaria elimination where outdoor transmission is significant.
Background
Current interventions against malaria vectors, notably long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), have been highly successful against the disease [1]. There has also been a decrease in malaria mortality rates between 2000 and 2013 by 47% worldwide and by 54% in Africa, where about 9 in 10 of the malaria deaths occur, mainly in children below five years old [2]. However, malaria remains endemic in sub-Saharan Africa, with an estimate of 198 million malaria cases and 584,000 deaths annually around the world, according to the latest World Malaria Report [2] by the World Health Organization (WHO). Despite the obvious successes and optimism expressed in current strategies, evidence suggests that in order to achieve the target of malaria elimination as stated in the newly launched Global Technical Strategy for Malaria (GTSM), 2016–2030, there are still numerous challenges that must be addressed [3].
One of these challenges is the fact that, while LLINS and IRS have effectively controlled mosquitoes that bite humans indoors and rest indoors [4], a significant proportion of malaria transmission is thought to still happen outdoors [5,6]. Nevertheless, there are other vector species that rest and bites humans or other vertebrates, outdoors [7,8] and are for that reason it becomes hard to control using only the indoor interventions [9]. This outdoor transmission is likely mediated mostly by mosquitoes that naturally bite outdoors and those that have developed behavioural resistance (e.g., avoidance of contact with lethal insecticidal surfaces and/or change of their biting time), adaptive behaviour such as biting people when outdoors [10] or physiological resistance (i.e., failure to be killed after adequate contact with otherwise toxic doses of insecticides) [5].
Various technologies have been proposed for outdoor-mosquito control, one of which is the use of devices baited with synthetic human odours to lure, trap and kill malaria vectors [11,12].
Lure and kill technology has great potential for use in Dengue control mediated by daytime biting Aedes aegypti mosquitoes that cannot be controlled by LLINs. Good efficacy has been demonstrated using oviposition traps baited with volatile grass mixtures as a synthetic oviposition lures for gravid female Ae. aegypti [13,14] and mass trapping of host seeking female Ae. aegypti using odour-baited Biogent-Sentinel (BG-S) traps [15,16]. In addition, lure and kill technology has been extremely successful against arthropods with low reproductive output such tsetse fly vectors of African trypanosomiasis [17] and have theoretical potential against malaria transmission [18]. There is however, only one large scale study currently underway to demonstrate their effectiveness against malarial mosquitoes [19]. Thus, there is a need to further evaluate and refine the technology before it can be recommended for large-scale use.
Recently, an outdoor mosquito control device, named the odour-baited mosquito landing box (MLB), was developed to attract and kill outdoor host-seeking mosquitoes, including major malaria vectors An. arabiensis and An. funestus s.s [12]. During the initial tests, the mosquitoes attracted to the MLB spent only brief periods of time around the device [12], thus limiting lethal contact even when the MLBs were covered with paint-based mixtures of highly effective organophosphates such as pirimiphos-methyl emulsified concentrate. We hypothesised that the reasons for this included the avoidance responses of An. arabiensis, and other mosquito species on conventionally treated insecticidal surfaces that have contact irritancy or non-contact excito-repellent effects [20].
Another possibility was that naturally, the mosquitoes were attracted to the lure but gave up and left the MLB after they found no short-range host cues. Similar behaviours have been observed in experimental hut trials with un-holed LLINs where mosquitoes enter houses to feed but when they are unable to feed on a host escape promptly before adequate contact with sub-lethal doses of either pyrethroids or organochloride insecticides [21]. Mosquitoes also leave experimental huts earlier in the presence of irritant insecticides such as pyrethroids [20,22,23]. However, this behaviour was less pronounced when organophosphates were used in impregnated nets but only after washing [24]. In situations where vector species such as An. arabiensis naturally have alternative blood hosts, e.g., cattle [25,26], and where such vectors contribute significantly to ongoing malaria transmission outdoors, it is important to identify means of killing the vectors instantly when they first make contact with the MLB. In addition, physiological resistance in malaria vector populations is rapidly spreading across Africa and is likely to be a major obstacle to ongoing vector control efforts [27] thus the need to explore non-insecticidal means of killing mosquitoes.
Electrocuting grids (EGs) have been used widely in studying vector behaviours such as flight, oviposition and host seeking responses of both tsetse fly and mosquitoes [28–31]. The technique also has been deployed at the household level and in commercial areas such as restaurants and bars for trapping and killing houseflies and other nuisance flying insects that are visually attracted to light [32,33]. However, such commercially available EGs are not regularly used outdoors in local rural settings [34] because they are expensive and require electrical power. Another limitation of these EG devices is that most of the existing versions are not sufficiently robust under natural settings and not simple to construct locally, especially in rural communities in malaria-prone areas [34].
Here we report improvements to the MLB to address these challenges for improved efficiency, to conserve energy, and to minimize the need for human handling. In summary, these modifications (Fig 1) included: 1) fitting low-cost electrocuting grids (EGs) onto the sides of the MLB to instantly kill transient host-seeking mosquitoes; and 2) the addition of an all-weather light-sensor which automatically switched both the electrocuting system and the odour-dispensing system on at dusk and off at dawn, thus improving safety, saving energy and reducing human handling. The MLB [12] fitted with low-cost, commercially available grids would instantly kill host-seeking mosquito populations effectively where mortality from insecticide treated surfaces is lower due to behavioural avoidance characteristics [7,10,20] or physiological resistance [5,6]. The simple type of electrocuting grid that we used here is widely used domestically for killing flying insects including mosquitoes just by swatting the flies mid-air. The grids fitted in the improved MLB are powered by the solar panel that powers the odour-dispensing unit.
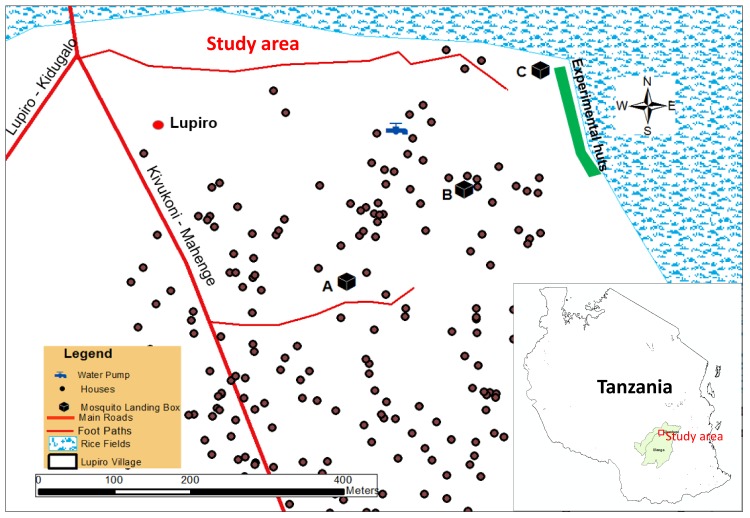
Fig 1. Map showing geographical positioning of the MLBs at A, B and C in Lupiro village, south-eastern Tanzania (Courtesy of Alex J. Limwagu).
This work was motivated by the need for an effective odour-baited mosquito control device that is low-cost, environmentally friendly, non-hazardous to people, easy to maintain, robust and efficient against target vectors.
Methods
Study area
The field study was carried out in Lupiro village (8.385° S, 36.670° E), in the Ulanga district of south-eastern Tanzania, an area of approximately 300 meters above sea level, at the edge of the Kilombero river flood plain (Fig 1). The MLBs with one, two or three grids were positioned at three different locations mainly position A, B and C (Fig 1). The study area experiences two main climatic seasons, a wet season, which peaks between March and June, and the dry season, which peaks between August and October. Annual rainfall is 1200–1800 mm, and the mean daily temperatures are 20–32°C. Most houses in the area are constructed with clay brick walls with either iron or grass-thatched roofs, but a significant proportion have openings on windows, doors and eave spaces. The predominant malaria vectors are the An. arabiensis and An. funestus groups. Interestingly, Anopheles gambiae s.s., which historically dominated the area, is now rarely found, mainly as a result of extensive use of bed nets [4].
Recently, a report of standard WHO insecticide susceptibility tests [35] on field collected adult mosquitoes from this village, showed that An. arabiensis were 100% susceptible to dichlorodiphenyltrichloroethane (DDT) but highly resistant to all pyrethroids [36]. Over the past decade, the Kilombero valley, which was hyper-endemic in the early 2000s, has experienced more than 50% reduction in malaria prevalence and is now classified as meso-endemic [37]. Outdoor transmission is rising and has been claimed to contribute significant proportions of the residual malaria transmissions [5,38].
The semi-field study was conducted at the Ifakara Health Institute’s experimental station located in Kining’ina village (8.11417° S, 36.67864° E) in Kilombero district, south-eastern Tanzania, approximately 6km north of Ifakara town. These experiments were conducted inside two chambers of a large semi-field system (SFS), i.e. screen house chambers measuring (9.6m × 9.6m × 4.5m), as described in [39–41]. During the experiments, the average daily temperature and relative humidity inside the SFS varied between 20.5°C—29.0°C and 50.5% - 80.0%, respectively (The experimental design is described below).
Improvement of the Mosquito Landing Boxes by fitting low-cost electrocuting grids and light sensors that trigger automatic on/off operation
To improve efficiency and ease of use, the MLBs were fitted with solar-driven low-voltage electrocuting grids (EGs) to kill attracted mosquitoes instantly without destroying essential morphological and molecular features for mosquito identification and pathogen monitoring (Fig 2A and 2B).
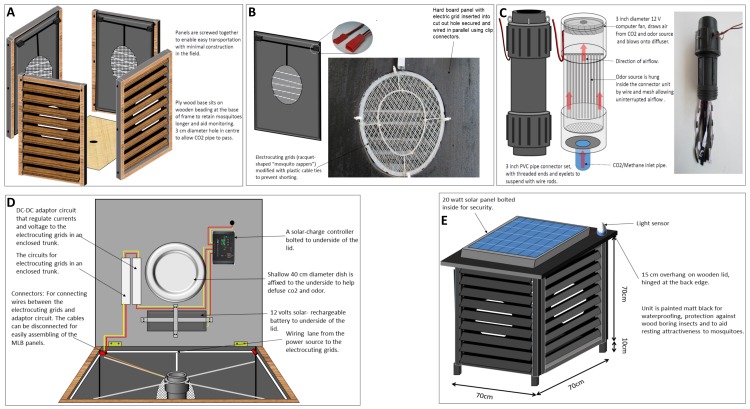
Fig 2.
(A) The panels of the Odour-baited Mosquito Landing Box (MLB), fitted with low-voltage electrocuting grids (EGs). (B) A close look of the EG inserted and secured into a hard wooded board of the panel of the MLB. (C) The odour-dispensing unit of the MLB. (D) Both the electrical system and the odour-dispensing unit are powered by a 12-volts solar-rechargeable battery regulated by solar-charger controller and adapter circuit system, allowing for a passive but effective mosquito control and surveillance system. (E) An automated on/off light sensitive switch that activates the device at dusk and stops it at dawn.
Commercially available racquet-shaped “mosquito zappers” (manufactured by LiTian Electronic Limited Company, Yiwu City, China), were purchased at the equivalent of 4 USD/unit from local retail stores and modified (by inserting plastic cable between the grid wires to prevent short-circuiting) for use as the grids on the sides of the MLB (Fig 2B). The grids were supported by rectangular pieces of hard board on each side of the box so that only a small circular portion of the soft boards, equal to the size of the grids, were visibly open whenever the landing surfaces were tilted (Fig 2B). The EGs were inserted on the inside sections behind the louvers and powered by the solar system which ran the odour-dispensing system. The louvers were adjusted to an angle to allow attracted mosquitoes to pass through, but also provide protection of the grids against rainfall. The energy that drives the 12 volts fan of the odour-dispensing system of the MLB (Fig 2C) from the solar-rechargeable battery was only regulated by the solar charger-controller (Fig 2D). To be able to minimize excess currents that could burn and destroy the morphological features of the mosquitoes, the electricity coming from the solar battery through the charger controller was regulated by DC-DC adapter circuit with an output range of 1.5–3 volts and a maximum 1.2 amperes and supplied to the EGs (Fig 2D). Due to some voltage drop, the voltages and currents measured in the EGs were between 0.28 and 0.75 volts and 0.35 and 1.39 amperes, respectively. We also fitted an all-weather-light sensitive sensor to switch on the odour-dispensing unit and the grids of the MLB at dusk and switch them off at dawn (Fig 2E). This automated system ensured that: a) the solar battery power is saved and unused during the day, b) the devices are not in any way hazardous to children who might touch them during the day, and c) no daily human servicing is necessary for these devices, other than the experimenter collecting data.
The estimations of potential features including community acceptance, sustainability, communal benefits, limitations and target-product profile of the effective odour-baited mosquito traps has been described in detail in the previous article [18]. The original cost of fabrication, purchasing of electrical solar parts, assembling, and annual maintenance of one experimental MLB prototype was 190 USD. However, the cost will be lower following mass production of the MLBs using cheaper and long-lasting materials, preferably plastic which is estimated to be 25USD per MLB (personal communication with Sarah J. Moore), and or adopting a community-based delivery mechanism.
Comparative evaluation of the killing efficacy of MLBs fitted with electrocuting grids against wild populations of free-flying mosquitoes
Three MLBs (the first one fitted with an EG on only one side, the second fitted with EGs on two sides, and the third fitted with EGs on three sides) were positioned 50–150 meters apart, and at least 30 meters from the nearest house, in the study village (Fig 1). White linoleum was placed at the bottom of each of the devices so that any dead mosquitoes dropping down could be recovered and counted. The boxes were raised on four pedestals in water moats to prevent any ants from entering and scavenging the dead mosquitoes.
The MLBs were baited with the Ifakara blend of mosquito attractants [42], a highly attractive synthetic human odour that was demonstrated in experimental hut studies to be more attractive than humans at long-range. The blend comprises hydrous solutions of ammonia (2.5%), L-lactic acid (85%), and other aliphatic carboxylic acids, namely propionic acid (C3) at 0.1%, butanoic acid (C4) at 1%, pentanoic acid (C5) at 0.01%, 3-methylbutanoic acid (3mC4) at 0.001%, heptanoic acid (C7) at 0.01%, octanoic acid (C8) at 0.01% and tetradecanoic acid (C14) at 0.01% dispensed via nylon strips, supplemented with CO2 gas flowing at 500 ml/min [12,42,43]. The odour-dispensing system was similar to the one described in [12], and was powered by an automated solar-battery driven mechanism (Fig 2C).
The MLBs with one, two or three grids were compared using a 3 × 3 fully randomised Latin square experiment replicated over 21 nights, in three different locations in the study village (Fig 1):
Location A was inside the village, approximately 150 m from the edge of the village, and the device was located 30 m from the nearest household.
Location B was in the middle of the village, approximately 100 m from the edge of the village, in an area with one human dwelling.
Location C was at the edge of the village, close to an area regularly cultivated for vegetables and rice using traditional irrigation systems.
To minimize positional bias on mosquito catches, the three MLBs were rotated nightly among the three locations to ensure that at the end of the 21 nights, each MLB had been at each of the three separate locations once every three nights. The order of the rotations of the three MLBs was randomised after every three nights to counter potential effects of any cyclical variations in the natural diurnal vector densities.
These experiments had no blank controls with no grids or without any bait, thus it was limited to comparing MLBs with varying surfaces area of grids. This was because our initial tests had demonstrated that un-baited MLBs did not attract host-seeking mosquitoes [12]. Furthermore, use of MLBs without grids as a control was not feasible since the MLB is designed as a lure-and-kill station without any trapping mechanism [12]. We therefore relied on previous published observations that mosquitoes visited baited-MLBs, but not un-baited ones and that these mosquitoes could be sampled intermittently during the night using drop nets [12]. Hence, no additional controls were necessary for the current study.
The experiments were done during the wet season (May-June 2013) and repeated in the dry season (August-September 2014), to capture the vector abundances at both times of the year.
Semi-field evaluation to determine effects of odour-baited MLBs fitted with electrocuting grids on human-biting densities of female An. arabiensis mosquitoes
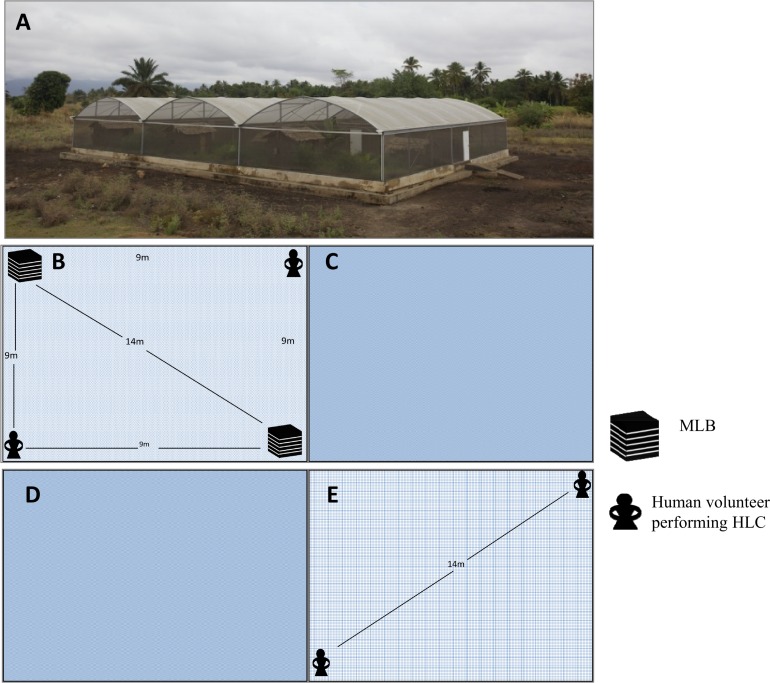
A comparative crossover study design was used in evaluating the effect of odour-baited MLBs fitted with electrocuting grids on human-biting densities against An. arabiensis for 12 nights (3 days/week) inside two equal sized chambers of a semi-field system (SFS) [40] (Fig 3A). Two SFS chambers were used whereby the treatment chamber contained two odour-baited MLBs fitted with three electrocuting grids each, and two human volunteers performing human landing catch (HLC) (Fig 3B). Each night, 500 nulliparous laboratory-reared unfed female An. arabiensis mosquitoes, aged 6–9 day old, were released at the centre of each SFS chamber two hours before the volunteers went in to begin HLC, so that the mosquitoes could acclimatise to the SFS environmental conditions. In the treatment chamber, the MLBs were placed at two diagonally opposite corners, 14 meters apart, on flat surfaces covered with clear plastic linoleum for easy visualization of any dead mosquitoes around the device (Fig 3B). The MLBs were baited with dirty nylon socks worn for 12 hours and carbon dioxide gas generated from yeast-molasses fermentation [44]. The MLBs were interchanged randomly inside the treated chamber nightly to minimize position bias. Pair of male human volunteers aged 18–30 years performing HLC were positioned at adjacent corners, so that they were also on diagonally opposite ends, 14 meters apart, and 9 meters from the adjacent MLB (Fig 3B). The HLC was carried out for 30 minutes at every two hours intervals from 2000Hrs through 0600Hrs, to collect mosquitoes landing and attempting to bite the volunteers. The remaining mosquitoes flying around in the chamber were collected using backpack aspirators from 0700Hrs to 0800Hrs. The two chambers of the SFS (Fig 3C and 3D) were left vacant acting as a buffer zone to minimize possible contamination between the treatment and control chambers. In the control chamber, a pair of male human volunteers aged 18–30 years, sat at two opposite corners performed HLC for 30 minutes at every two hours intervals, to collect mosquitoes landing and attempting to bite them (Fig 3E) in the absence of the MLB. Pairs of volunteers in each chamber were randomly interchanged nightly between treatment and control chambers to minimize bias due to differential attractiveness of individuals to mosquitoes [45,46].
Fig 3.
(A) A semi-field system (SFS) in the Kilombero district of south-eastern Tanzania, where semi-field study was carried out [40]. (B) Treatment chamber of the SFS contained odor- baited Mosquito Landing Box (MLB) and human volunteers performing human landing catching (HLC). (C and D) Empty chambers of the SFS, which were used as buffer to reduce inter-chamber bias during the orientation of host-seeking mosquitoes. (E) Control chamber of the SFS contained only volunteers performing HLC.
Mosquito collections, identification and assessment for Plasmodium falciparum infection
In the field experiments, mosquitoes were collected in the mornings. Electrocuted mosquitoes were gently removed individually from the EG surfaces using forceps (Fig 4), from the inside surfaces of the MLB and from the raised linoleum floors. A water moat was always placed at the base of the surfaces to prevent ants from scavenging on the mosquitoes. The mosquitoes were then identified morphologically and sorted by taxa and sex. Taxonomy was done using dichotomous keys for the Anopheles of Africa, south of the Sahara [47]. The mosquitoes were also sorted as either blood-fed or non-blood fed, gravid or semi-gravid.
Fig 4.
(A) Photograph of electrocuted wild mosquitoes stuck in the grid underneath the louvers of the Mosquito Landing Box (MLB). (B) A pile of mosquitoes after they fell from the electrocuting grid. (C) A close-up look of the collection of electrocuted Anopheles gambiae s.l (circled with spotted wings) and (D) A microscopic image of the head parts of the Anopheles gambiae s.l, and (E) all the morphologically features of the Culex species.
Members of the An. gambiae complex and An. funestus group were stored separately for further individual species identification. A subsample of 690 An. gambiae s.l and 271 An. funestus group were randomly selected for species identification in the molecular laboratory at the Ifakara Health Institute using DNA-polymerase chain reaction (PCR) [48]. For the An. gambiae complex, multiplex PCR was done following the procedures of Scott et al. [49], while for the An. funestus group, DNA was extracted [50], and PCR was performed according to the procedures of Koekemoer et al. [51].
Female Anopheles mosquitoes identified as belonging to the An. gambiae complex and the An. funestus group were also separately pooled in groups of two (minimum) to ten (maximum) and stored for further examination to assess if they were infected with P. falciparum sporozoites, using circumsporozoite protein enzyme-linked immunosorbent assay (CSP-ELISA) techniques [52]. To remove false positives, all the sporozoite positive ELISA lysates were boiled at 100°C for 10 minutes to verify presence of Plasmodium protozoan antigen, which are heat stable [53,54].
For the semi-field experiments, each volunteer performing HLC recaptured only mosquitoes landing on him during scheduled collection periods of 30 minutes at every two hour intervals from 2000Hrs through 0600Hrs. Each morning, the leftover mosquitoes flying around in both chambers were collected using backpack aspirators. The number of mosquitoes killed by the MLB inside the treated chamber was recorded for the different MLBs after every two hours throughout the experimental night. All live collected mosquitoes were killed using petroleum vapours. By assessing the number of mosquitoes landing on the human volunteers inside the chambers with MLBs (treatment chamber) or without the MLBs (control chamber), we were able to assess whether presence of MLBs fitted with electrocuting grids in an enclosed environment affected the human-biting densities of laboratory-reared An. arabiensis.
Data Analysis
Data were entered into excel spreadsheets and saved as comma-separated value (CSV) files. Data analysis was done using R statistical software version 3.1.1 [55]. Data were fitted using Generalized Linear Mixed Effects statistical Models (GLMMs) to describe effects of the different variables on mosquito catches. Since the data were evidently over-dispersed, we used the packages lme4 [56], MASS and glmmADMB [57] to fit either Poisson distribution models or negative binomial distribution models with log-link functions to transform the data distributions. Numbers of individual mosquito species were assessed as a function of two fixed factors, the number of grids on the MLB and positions of the MLB, each time comparing additive versus multiplicative models, using Akaike Information Criteria (AIC) values [58]. Experimental days were treated as a random factor to account for the natural nightly heterogeneity in mosquito counts. The results of the parameter estimates were exponentiated to obtain the estimated mean number of mosquitoes killed each night by each MLB at different locations, and the associated relative rates (RR) of collecting a mosquito in any of the MLBs, when compared to the reference MLB with one grid, when located at the first location. The final data were summarised in tables and box plots to display the variability. Similarly, data collected from the semi-field studies on human biting densities between the control and treatment chambers where compared by using GLMMs, whereby number of mosquitoes recaptured was treated as a of function treatment, round and collection time. Number of mosquitoes recaptured in each chamber were treated as numeric variable, round and treatment as a factor variable.
Results
Molecular identification of the mosquito species
In all the experimental field tests in both dry and wet seasons, 9,685 mosquitoes in total were sampled from the three odour-baited mosquito landing boxes fitted with electrocuting grids. Of these, 3533 (36%) were members of the Anopheles gambiae complex, 107 (1%) were of An. funestus group, 826 (9%) were other Anopheles species, 1,230 (13%) were Culex species and 3989 (41%) were Mansonia species mosquitoes. Of the mosquitoes that were submitted for sibling species identification using PCR as described above, only 260 An. gambiae s.l and 57 from the An. funestus group were successfully identified. Of the An. gambiae s.l. samples, 98% (256/260) were An. arabiensis, while 2% (4/260) were An. quadriannulatus. For the An. funestus group, 54% (31/57) were identified as An. rivulorum, 25% (14/57) were An. funestus s.s and 21% (12/57) were An. leesoni. An. arabiensis and An. funestus s.s are the two predominant malaria-carrying vectors in the study area. We experienced high proportions of non-amplified mosquitoes during our molecular analysis for species identification, especially for the An. funestus group, (A summary of PCR results is presented in details in Table 1). Since An. arabiensis constituted 98% of the An. gambiae complex mosquitoes, the term An. arabiensis is used in subsequent sections to refer to all members of the complex.
Table 1. A summary of a sub-sample of the malaria vectors caught, that were submitted for sibling species identification using DNA-polymerase chain reaction (PCR) technique.
| Species complex or group | Total number assayed by PCR | Total number and percentage successfully amplified | Percentage not amplified |
|---|---|---|---|
| Anopheles gambiae s.l | 690 | Subtotal = 260: Anopheles arabiensis: 98% (256/260); Anopheles quandianilatus: 2% (4/260) | 62% (430/690) |
| Anopheles funestus group | 271 | Subtotal = 57: Anopheles rivolurum: 54% (31/57); Anopheles funestus s.s: 25% (14/57); Anopheles lessoni: 21% (12/57) | 79% (214/271) |
| Grand Total | 961 | 317 | 644 |
Field tests conducted during the wet season
In total, 4,986 mosquitoes were collected from all three odour-baited MLBs equipped with EGs during the first round of the tests over the 21 nights in the wet season. Of these, there were 1,541 An. arabiensis (31%), 38 An. funestus (1%), 356 mosquitoes belonging to any other Anopheles species (7%), 554 Culex species (11%), and 2,497 Mansonia species (50%). Ninety nine percentage of all the mosquitoes caught were non-blood fed, suggesting that they were host-seeking mosquitoes.
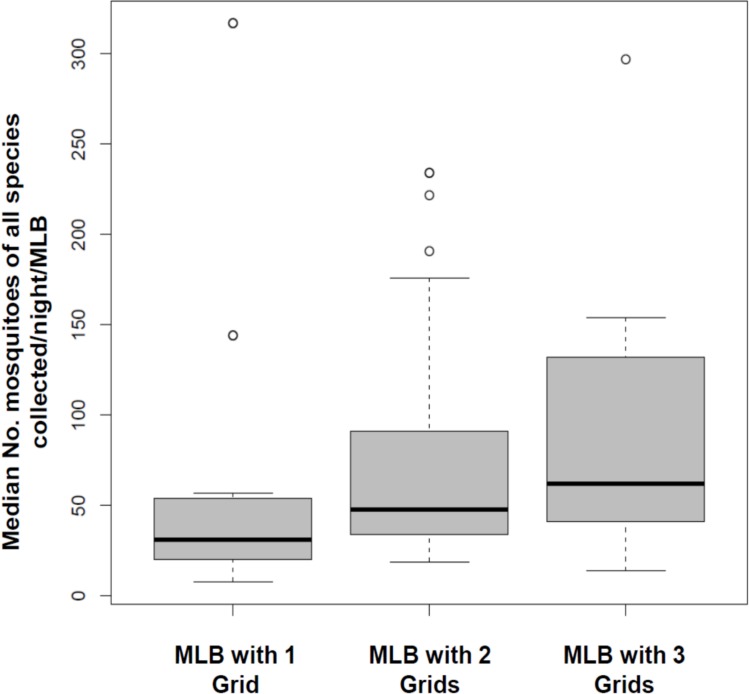
The estimated mean numbers of mosquitoes killed by each of the MLBs are shown in Tables 2 and 3. The number of mosquitoes collected increased with increasing grids in the MLBs. The MLB fitted with three grids killed significantly more An. arabiensis (RR = 1.59 [1.37–1.84], P < 0.001) than the MLB with just one grid, but significantly fewer of the other Anopheles species (RR = 0.41 [0.30–058], P < 0.001), and fewer Mansonia mosquitoes (0.73 [0.65–0.82], P < 0.001). The MLB with 3 grids also killed more An. funestus (RR = 1.56 [0.64–3.81], P = 0.335) and more Culex mosquitoes 1.90 [0.98–2.59], P = 0.329) although not by a statistically significant margin for either taxon. Similarly, the MLB fitted with two grids killed significantly more An. arabiensis (RR = 1.59 [1.37–1.84], P < 0.001) and more Culex mosquitoes (RR = 1.89 [1.03–2.64], P < 0.005) than the MLB fitted with one grid. However, the number of An. funestus killed by this MLB with two grids was similar to the MLB with one grid (RR = 0.95 [0.36–2.52], P = 0.913).
Table 2. Estimated mean number of Anopheles mosquitoes collected per night from the odour-baited mosquito landing boxes (MLBs) fitted with one, two or three electrocuting grids during the wet season or dry season testsϕ.
| Anopheles arabiensis | Anopheles funestus | Other Anopheles | ||||||||
| Round 1 (Wet season) | No. Grids on MLB | Mean [95% CI] | RR [95% CI] | P value | Mean [95% CI] | RR [95% CI] | P value | Mean [95% CI] | RR [95% CI] | P value |
| One Grid | 13.82 [9.90–19.28] | REF | REF | 0.15 [0.05–0.45] | REF | REF | 1.51 [0.73–3.13] | REF | REF | |
| Two Grids | 21.97 [18.95–25.47] | 1.59 [1.37–1.84] | <0.001 | 0.14 [0.05–0.36] | 0.95 [0.36–2.52] | 0.913 | 1.14 [0.12–10.71] | 0.75 [0.08–7.09] | 0.049 | |
| Three Grids | 36.04 [9.16–141.76] | 2.61 [0.66–10.26] | <0.001 | 0.23 [0.09–0.55] | 1.56 [0.64–3.81] | 0.335 | 0.63 [0.45–0.88] | 0.41 [0.30–0.58] | <0.001 | |
| Anopheles arabiensis | Anopheles funestus | Other Anopheles | ||||||||
| Round 2 (Dry season) | No. Grids on MLB | Mean [95% CI] | RR [95% CI] | P value | Mean [95% CI] | RR [95% CI] | P value | Mean [95% CI] | RR [95% CI] | P value |
| One Grid | 17.67 [15.64–19.96] | REF | REF | 0.03 [0.01–0.14] | REF | REF | 0.21 [0.09–0.47] | REF | REF | |
| Two Grids | 32.65 [29.07–36.67] | 1.85 [1.65–2.08] | <0.001 | 0.13 [0.06–0.31] | 4.79 [2.08–11.02] | <0.001 | 0.49 [0.32–0.73] | 2.35 [1.56–3.53] | <0.001 | |
| Three Grids | 28.79 [25.54–32.45] | 1.63 [1.45–1.84] | <0.001 | 0.02 [0.01–0.05] | 0.73 [0.29–1.85] | 0.515 | 0.40 [0.26–0.63] | 1.95 [1.25–3.04] | 0.003 | |
ϕ The RR stands for relative rate while REF stands for a reference category.
All the estimations were generated using Generalized Linear Mixed Effects Models in R [55].
Table 3. Estimated mean number of non-malaria mosquitoes and overall number of mosquitoes collected per night from the odour-baited mosquito landing boxes (MLBs) fitted with one, two or three electrocuting grids during the wet season or dry season testsϕ.
| Culex species | Mansonia species | All mosquito species combined | ||||||||
| Round 1 (Wet season) | No. Grids on MLB | Mean [95% CI] | RR [95% CI] | P value | Mean [95% CI] | RR [95% CI] | P value | Mean [95% CI] | RR [95% CI] | P value |
| One Grid | 2.61 [1.79–3.81] | REF | REF | 14.66 [10.87–19.75] | REF | REF | 20.24 [14.72–27.84] | REF | REF | |
| Two Grids | 4.39 [2.68–4.88] | 1.89 [1.03–2.64] | 0.029 | 12.08 [10.85–13.45] | 0.82 [0.74–0.92 | <0.001 | 99.36 [82.35–119.89] | 4.91 [4.07–5.92] | <0.001 | |
| Three Grids | 3.39 [2.56–4.15] | 1.90 [0.98–2.59] | 0.329 | 10.68 [9.50–12.01] | 0.73 [0.65–0.82] | <0.001 | 41.53 [34.37–50.19] | 2.05 [1.69–2.48] | <0.001 | |
| Culex species | Mansonia species | All mosquito species combined | ||||||||
| Round 2 (Dry season) | No. Grids on MLB | Mean [95% CI] | RR [95% CI] | P value | Mean [95% CI] | RR [95% CI] | P value | Mean [95% CI] | RR [95% CI] | P value |
| One Grid | 1.93 [1.24–2.99] | REF | REF | 1.59 [1.01–2.51] | REF | REF | 19.07 [13.77–26.41 | REF | REF | |
| Two Grids | 2.78 [2.24–3.44] | 1.44 [1.16–1.79] | <0.001 | 2.05 [1.65–2.54 | 1.29 [1.04–1.59] | 0.02 | 30.02 [27.77–32.45] | 1.57 [1.46–1.70 | <0.001 | |
| Three Grids | 3.70 [2.97–4.62] | 1.92 [1.54–2.39] | <0.001 | 4.16 [3.32–5.21] | 2.61 [2.08–3.27] | <0.001 | 33.05 [30.49–35.82] | 1.73 [1.59–1.88] | <0.001 | |
ϕ The RR stands for relative rate while REF stands for a reference category.
All the estimations were generated using Generalized Linear Mixed Effects Models in R [55].
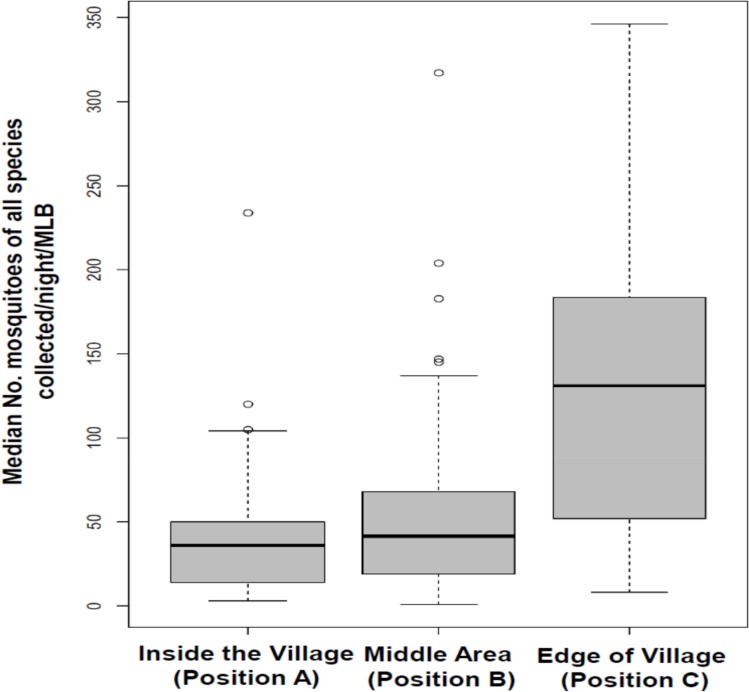
We also observed statistically significant effects of location on number of mosquitoes of different species collected. When any of the MLBs, regardless of number of grids, was at position C at the edge of the village close to an area regularly cultivated for vegetables and rice using traditional irrigation systems, we collected at least 4 times more mosquitoes of all species combined than at position A inside the village, approximately 150 m from the edge of the village. Specifically, at position C, we collected 5.4 times more An. funestus (RR = 5.37 [2.04–14.15], P < 0.001), 5.3 times more of the other Anopheles mosquitoes (RR = 5.26 [3.83–7.23], P < 0.001), 4.2 times more Culex (RR = 4.23 [3.28–5.46], P < 0.001) and 5.4 times more Mansonia mosquitoes (RR = 5.43 [4.79–6.14], P < 0.001) than at position A. However, there were significantly fewer An. arabiensis mosquitoes sampled when the MLB was at position C relative to position A (RR = 0.70 [0.62–0.80], P < 0.001). Similarly, we collected more of the non-malaria mosquitoes, Culex (RR = 1.68 [1.26–2.24], P < 0.001), other Anopheles species (RR = 1.18 [0.81–1.73], P = 0.39) and Mansonia (RR = 1.63 [1.41–1.88), P < 0.001)) at position B in the middle of the village (approximately 100 m from the edge of the village, in an area with one human dwelling near it), but fewer An. arabiensis (RR = 0.83 [0.72–0.94, P = 0.01) and the same number of An. funestus (RR = 0.99 [0.29–3.46], P = 0.99) compared to position A.
Field tests conducted during the dry season
Results were generally similar to those of the wet season and are given in Tables 2 and 3. Overall, 4,699 mosquitoes were collected from all the three odour-baited MLBs fitted with electric grids during the dry season tests. Of these, 1,992 (42%) were An. arabiensis, 69 (2%) were An. funestus, 470 (10%) were other Anopheles species, 676 (14%) were Culex species and 1,492 (32%) were Mansonia species. Relative to the MLB with one grid, significantly more mosquitoes (all species combined) were collected at the MLB with three grids (RR = 1.73 [1.59–1.88], P < 0.001) or the one with two grids (RR = 1.57 [1.46–1.70], P < 0.001). Regarding effect of position, we also observed that relative to position A, 3.8 times more mosquitoes of all species were collected when MLBs were at position C (RR = 3.84 [3.53–4.17], P < 0.001), and 1.8 times more when MLBs were at position B (RR = 1.83 [1.68–2.00], P < 0.001).
Upon morphological identification, we determined that the MLB with three grids killed significantly more An. arabiensis, (RR = 1.63 [1.45–1.84], P < 0.001), more Culex mosquitoes (RR = 1.92 [1.54–2.39], P < 0.001) and more Mansonia mosquitoes (RR = 4.16 [3.32–5.21], P < 0.001) than the MLB with one grid. There was no significant difference in numbers of An. funestus caught from the MLB with three grids compared to the MLB with one grid. The MLB with two grids also killed more An. arabiensis (RR = 1.85 [1.65–2.08], P < 0.001), more An. funestus (RR = 4.79 [2.08–11.02], P < 0.001) more Culex (RR = 1.44 [1.16–1.79], P < 0.001) and more Mansonia mosquitoes (RR = 1.29 [1.04–1.59], P < 0.02), compared to the MLB with one grid.
Interestingly, there was no difference in the number of mosquitoes collected between the wet season (first round) and the dry season (second round) except for Mansonia species, for which there were higher numbers in the wet season relative to the dry season (RR = 1.80 [1.17–2.79], P < 0.007). Figs 5 and 6 show the variations in mosquito collections by number of grids and positions respectively.
Fig 5. Comparison of mosquitoes (all species combined) killed by odour-baited mosquito landing boxes fitted with one, two or three electrocuting grids on the sides.
Fig 6. Comparison of mosquitoes (all species combined) that were collected when the odour-baited mosquito landing boxes were located at the three different positions.
Plasmodium infection rates in the field-sampled mosquitoes
In total, 1,230 individual mosquitoes from the An. gambiae complex and the An. funestus group were tested for P. falciparum circumsporozoite protein (CSP). We used a modified enzyme-linked immunosorbent assay (ELISA) technique that involved boiling the ELISA lysate for 10 minutes at 100°C, to exclude any heat stable antigens that may occasionally express as false positives in CSP-ELISAs [53], which is highly likely in zoophilic Anopheles species [54,59]. The initial sporozoite rate for An. arabiensis was 0.84% (i.e. 8 out of 958) but after boiling, the rate dropped to 0.2% (2 of 958). None of the An. funestus (in which half were An. rivulorum) tested positive for P. falciparum circumsporozoite protein.
Comparisons of number of An. arabiensis mosquitoes landing on volunteers inside semi-field chambers with or without MLBs fitted with electrocuting grids
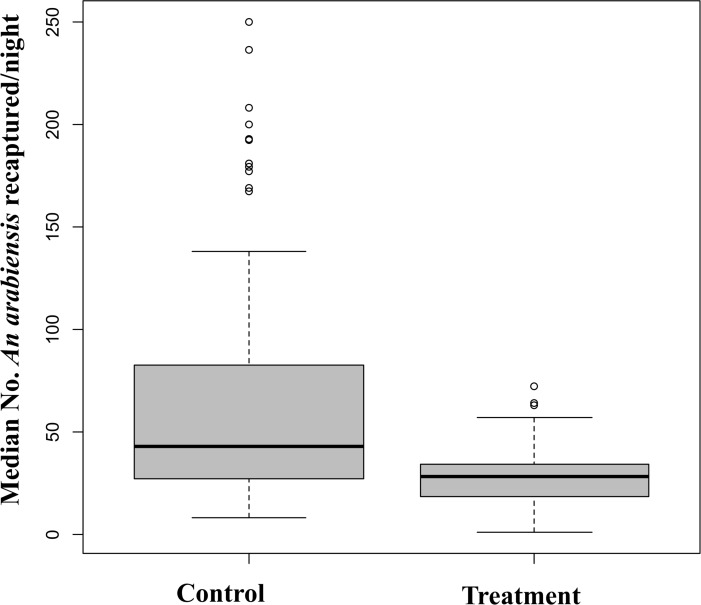
Overall, there were statistically more An. arabiensis mosquitoes collected by HLC inside the control chambers without MLBs than in the treatment chambers with MLBs (P < 0.001). Despite the daily replenishment of the adult mosquito populations, there were still 57.2% fewer mosquitoes in the treatment chamber than in the controls, presumably because they were killed by the MLBs. Of the total of 6,000 female An. arabiensis mosquitoes released in control chamber at a rate of 500/night over the 12 nights of testing, the two volunteers recaptured a total of 2,791 (46.6%) and 2,866 (47.8%), respectively. On the other hand, of the 6,000 female An. arabiensis mosquitoes released over the 12 nights of testing in treatment chambers, where 2 MLBs fitted with electrocuting grids had been located, the two volunteers recaptured only 1,194 (19.9%) and 1,229 (20.5%), respectively. When the mean numbers of live mosquitoes recaptured in each chamber at each interval of collection were compared, the result showed significantly higher numbers of live mosquitoes recaptured in the control than in the treated chamber (Fig 7). Each night, four times fewer mosquitoes (RR = 4.39 [3.92–4.93], P <0.001), were recaptured alive from the treatment chamber with MLB, which indicated the killing effect of the device fitted with electrocuting grids inside the treated chamber against the control setup. The mean nightly number of mosquitoes caught in the chamber with MLB was 14.27 [10.77–18.89], while the mean in the chamber without MLB was 62.69 [53.06–74.07].
Fig 7. Mean number of live mosquitoes recaptured for 30 minutes collection sessions at each 2 hours intervals by human landing catch (HLC) in the control and treatment chambers.
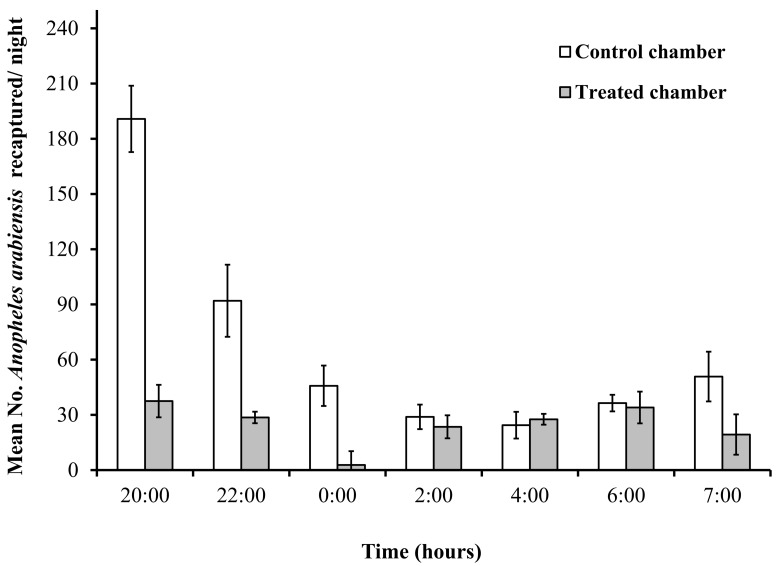
Since the collections were conducted after every two hours intervals, we could assess the times of night when the host-seeking activity was strongest, and therefore when most of the released mosquitoes were recaptured. Most of the recaptured mosquitoes had been collected at the beginning of night from 2000Hrs to 2200Hrs, and the number gradually decreased with time in both the control and treatment chambers, though there was a minor increase in the morning collection at 0700Hrs (Fig 8).
Fig 8. Mean number of live adult An. arabiensis mosquitoes recaptured by human landing catch (HLC) during each of the half-hour collection sessions at the end of each 2-hour interval in the treatment chamber with the MLBs and in the control chamber without MLBs.
Discussion
In Africa, malaria transmission still occurs primarily indoors [60,61], but the relative proportion that occurs outdoors is increasing, particularly in communities where indoor interventions are used extensively [6,62]. Odour-baited technologies for attracting and killing disease vectors by using lethal insecticide targets have been considered as potential options against outdoor-biting malaria vectors [11]. Such technologies must be designed to avoid known vector control challenges such as development of insecticide resistance after prolonged use as well as behavioural resilience or avoidance by some vector species or populations [20,63].
The Mosquito Landing Box (MLB) [12], fitted with electrocuting grids (EGs) may offer a quick-acting, non-chemical control mechanism against outdoor-biting malaria vectors, even if these vectors spend only brief periods on the device, and even if the mosquitoes are physiologically resistant to insecticides commonly used for vector control. Furthermore, grids may reduce associated labour costs and eliminate the need for insecticides, thus improving environmental safety and create opportunities to address the management of insecticide resistance [64] in mosquito populations. The addition of a light-based sensor ensured that the device is automatically switched on at dusk and off at dawn also conserves energy, increases longevity of the odour lure as it was being emanated only at night, and eliminates unwanted effects on daytime flying non-target insects. This also improves the safety associated with the device, especially in places where children might play and touch the devices during the day.
The number of mosquitoes collected increased with increased number of grids in the MLB. Significantly more mosquitoes of all species were killed by the three-grid MLB relative to one-grid MLB, and slightly more by the two-grid MLB than the one-grid MLB, a trend attributable to the increase in mosquito contact surface areas when the number of grids is increased. An. arabiensis was the most abundant member of the An. gambiae complex identified representing 98% of the total sample size. Only one other member of the complex, An. quadriannulatus, was found. We also have extensive evidence from previous indoor and outdoor studies that An. gambiae s.s., which was formerly the dominant sibling species in this complex, is now nearly extinct [4]. The MLB prototype that we used is designed specifically to target outdoor-biting vector populations, regardless of species. Moreover, its effects on non-malaria mosquitoes such as Culex and Mansonia species, as we observed in this study could improve its level of acceptability, as it would also be effective against other mosquito-borne illnesses and against nuisance biting mosquitoes.
The majority of the other Anopheles mosquitoes caught during the experiments were morphologically identified as An. coustani (considered as secondary malaria vectors). Since vector species such as An. arabiensis seek blood hosts both outdoors and indoors, depending on availability of the hosts [7,8], efficacy of the MLB against this species suggests that the device can be effective as a complementary and non-chemical option targeting them. Interestingly, some of the An. funestus s.s., that are known to feed and rest mainly indoors, were caught outdoors. However, it is unknown what proportion of the population the outdoor collections represent, as indoor collections were not conducted as part of this study. Since An. funestus is known to be a major contributor to the on-going residual malaria transmission in east Africa [65,66] as well as a recent study in west Africa by Moiroux et al. [62,67]. This observation further justifies the need for supplementary interventions to target these species that are increasingly biting outdoors.
The ELISA assays determined that the sprozoite rate was 0.84% for An. arabiensis before boiling, but only 0.2% was confirmed positive after boiling. False positive results are known to occur with samples that feed on animals, such as An. parensis and the An. marshallii group [53,54,59]. None of the An. funestus, of which half were An. rivulorum, were found positive with malaria parasites. However, further collections and analyses should be done since this species previously has been found infectious with malaria sporozoites [68–70], particularly in areas with widespread indoor interventions [70]. No ELISA was done on other Anopheles species, the majority of which were An. coustani. However, the earlier work done by Gillies & De Meillon [71] in Tanzania, following the dissection of salivary gland, 1 out of the 567 dissected An. coustani was found positive with malaria parasites, and yet there was no evidence that this species had a minor potential malaria transmission. Recently, there has been a suggestion that this species may be a secondary vectors that might play a role in malaria transmission in the Taveta area of Kenya [72], however the ELISA tests done in that study were not confirmed by either boiling the lysate or doing PCR and the results must therefore be questioned. This species has also been reported to be a vector of the Rift Valley fever virus during the disease epidemics [73]. Although the main target of this study was malaria mosquitoes (for which the estimated mean catches are shown in (Table 2), it also was important to assess overall impact of the devices on all mosquito species combined, including the culicines. This is particularly important as the biting densities of non-malaria mosquitoes could influence people’s perception on whether a malaria control intervention is effective [74].
The differences due to location are important when determining optimal positions necessary to achieve maximum impact on vector densities. For example, at least four times more mosquitoes of all species were collected when the MLB (regardless of number of grids) was placed at position C, which was at the edge of the village, nearest to vector breeding sites, relative to the device at position A, which was 150 meters from the nearest mosquito breeding site and inside the village (Fig 6). The present results match the findings of many previous reports on strong associations between high numbers of adult mosquito densities and distance from nearest aquatic breeding sites [75,76]. Similar observations have also been made of relationships between increases in malaria cases in houses near mosquito breeding sites, suggesting that general mosquito control interventions should be either more focused on the households close to the breeding sites or located to intercept mosquitoes flying between these aquatic sites and human dwellings [77,78].
Unexpectedly, there was no difference in number of mosquito catches at the end of the wet season relative to the dry season, except for Mansonia species that increased significantly at the end of the wet season, perhaps because the study area is a rice growing area with breeding sites available throughout the year [79,80]. Moreover, some species, such as An. arabiensis, tend to maintain their population size throughout most of the year even when the breeding sites are dry, since their eggs can remain dormant to resist desiccation [81]. Also, the persistent number of An. funestus during the dry season may be due to their preference for breeding in permanent water bodies and as well as in hidden, small, man-made habitats [82].
The mosquito lure used here has been demonstrated previously to attract be more proportions of mosquito species than humans at long-range [42]. During these experiments, the synthetic lure used [42], was prepared by hand and dispensed using locally available nylon strips [43]. However, the blend have been lately improved and packed into simple long-lasting pellets that can be widely used hence reducing cost and time for labour. Similar techniques are already being implemented by commercial manufacturers such as Biogents (BG) Ltd (Germany) for large-scale use against the dengue vector Ae. aegypti [83]. No other insects (non-target organisms) were captured suggesting that the MLB baited with the synthetic lures that we used here selectively attract mosquitoes and have minimal environmental impact. Moreover, in this study, nearly all female mosquitoes collected at any of the positions were not blood fed, suggesting that they were in a host-seeking state or looking for a resting site after emerging. Additional studies should be done to determine whether a non-odour-baited MLB could also acts as an attractive outdoor resting box. As an effective control tool against such vector populations, the MLB would be effective against outdoor-biting vectors, which otherwise perpetuate residual malaria, even where indoor interventions already are common.
In the semi-field assays that we conducted to assess potential effects of the electrocuting grid-fitted MLBs on human-biting densities of the vectors, the results clearly demonstrate that at medium range of host seeking (10 metres) MLBs were significantly more attractive to mosquitoes than the human volunteers and reduced human biting densities. The reduction in mosquitoes recaptured by HLC from the treatment chamber compared to the control chamber showed the impact of MLB fitted with the electrocuting grids in removing host seeking vectors from the environment. In this experiment, we also observed that more mosquitoes were recaptured at the beginning of experimental nights in both control and treatment chambers than later in the night, although there was a small peak also in the morning (Fig 8). This pattern matches the naturally observed nightly biting patterns of An. arabiensis in many parts of Africa [5] and such high numbers of mosquitoes recaptured during the early hours of the night have been reported previously [25,84]. While this experiment did not allow us to assess the performance of the device at varying vector abundances or in the presence of LLIN use, the apparent mass killing effect demonstrated in the study suggests we can achieve desirable levels of community protection, if the device is used consistently including during dry seasons when densities are low. When the device is scaled up, both temporal and spatial targeting will be essential to ensure maximum benefit.
We have demonstrated that the improved Mosquito Landing Boxes with electrocuting grids are effective weapons against malaria vectors and non-malaria vectors outdoors. The MLB might also be used as a surveillance tool to measure vector population densities and disease transmission outdoors. Since the MLB is baited with synthetic human lures, it is an alternative to the ethically questionable practice of human landing catches to potentially infectious mosquitoes. The HLC method subjects volunteers to an increased risk of being bitten by infectious mosquitoes [85] and is extremely arduous and labour intensive. Also, due to variability between individuals’ attractiveness to mosquitoes and ability to catch landing mosquitoes, having a standardised system that measures outdoor man-vector contact in a similar way across and between sites, as is done with the CDC light trap for indoor biting mosquitoes [86], is highly desirable.
In addition to attracting and killing potentially infectious and nuisance mosquitoes, the MLB may be able to supply lighting to households in rural communities from the power from its solar panel. This concept was originally aimed at reducing potential barriers to acceptance of the MLB by community members and may significantly increase uptake because users have an obvious personal benefit to use the device. The “home lighting” concept has been demonstrated on a small scale in rural south-eastern Tanzanian communities (Okumu et al., unpublished data), where the main electricity coverage remains below 10% [87–89]. In those demonstrations, nine specially designed experimental huts and two village houses, were supplied with solar-powered lighting systems, using the same solar panel that powers the odour-dispensing unit and electrocuting grids in the MLBs. The MLBs could improve livelihoods by controlling disease vectors and providing basic lighting, thus reducing risks associated with common rural light sources such as kerosene lamps. We expect that since the MLBs can provide energy for basic lighting, pupils’ home-study, and mobile phone charging, they stand to improve opportunities for acceptability and sustainability of this technology in rural and remote communities. However, future social-anthropological studies are recommended to assess the community acceptability on the cost, maintenance and sustainability of the MLB.
One limitation of this device is the need for CO2 gas, a major component of the mosquito lures that we used. In the field studies we used industrial CO2 gas, delivered in pressurized cylinders and dispensed through calibrated flow metres (Glass Precision Engineering Ltd., United Kingdom). The CO2 gas is an important component of mosquito lures. It is necessary for activating mosquitoes and synergizing other lure components [90,91]. While industrial CO2 is effective for experimental and demonstration purposes, it is unsustainable on a large-scale in rural sub-Saharan Africa, but there are several alternatives including CO2 gas generated from yeast-sugar fermentation [92] or yeast-molasses fermentation [44], as we used in our semi-field tests. For field applications, we can consider either alternative or host odours suctioned directly from human dwellings [12,93,94].
Another limitation of our study was the high proportion of non-amplified field mosquitoes during our molecular analysis for species identification. We could not determine causes of the poor amplification rates in this study, which was also observed in other studies ongoing at the same time. However, we hypothesised problems arising from suboptimal specimen handling, preservation and storage conditions, and long waiting times for analysis that that degrade the DNA. Also in our laboratory, we have been using single legs or wing only during DNA extraction processes that might produce less DNA than extraction from the abdomen or legs and wings. Also, it might be a technical problem in the laboratory as similar challenge has been experienced by other scientists who have been using the same laboratory during the same period. Some of the non-amplified samples were re-assayed in a separate laboratory and confirmed successfully amplified. We therefore have also a similar plan to repeat these assays on the non-amplified samples in a separate laboratory.
Conclusion and Recommendations
This study has shown that it is possible to target outdoor-biting mosquitoes, including major malaria vectors, using the odour-baited mosquito landing box (MLB) fitted with affordable electrocuting grids and automated sensors. It also demonstrated that using MLBs close to humans reduced their risk of mosquitoes attempting to feed on them sowing that the odour blend is effective at medium range and is not likely to lure in mosquitoes that then go and bite people located close to the traps, for instance if used in the peri-domestic space. This non-chemical technology instantly kills host-seeking mosquitoes that would naturally bite outdoors or those that might have changed behaviours from indoor-biting and now increasingly bite outdoors due to selection pressure from the indoor insecticide-based interventions such as LLINS and IRS. In addition, this method may be effective in managing vector populations that are physiologically resistant to insecticide-based interventions, as was observed in the field studies reported here, conducted in an area where malaria vectors are resistant to pyrethroids. Electric grids eliminate the need for applying insecticides to the MLB, and ensure high killing efficacy even if the mosquitoes are behaviourally or physiologically resistant. The MLB may allow us to preserve the effectiveness of the existing interventions such as LLINs and IRS by removing resistance alleles from the population using an entirely new insecticide-free killing strategy. Moreover, MLBs with electrocuting grids might support an integrated approach for controlling and for effectively monitoring of densities and infectiousness of disease-transmitting mosquitoes without the need for human landing catches, the current gold standard method for measuring outdoor mosquito densities. Other than the malaria vectors, the MLB could target simultaneously non-malaria mosquitoes such as Culex and Mansonia species that are potential vectors of lymphatic filariasis and arboviruses, and are also major sources of biting nuisance to humans. Reducing nuisance biting will increase consumer satisfaction with this method. Further studies are recommended to assess and compare the monitoring efficacy of the MLB equipped with grids with other existing tools, as well as the potential effects of the tool on malaria transmission and incidence rates in communities. We also suggest comparative evaluation studies using MLBs with grids to determine species variations on outdoor collections relative to indoor mosquito populations.
Ethical Consideration
Prior to the commencement of the study, a detailed explanation of the aims, study procedures, risks and benefits were provided to the owners of the land (Position A and B) where the MLBs were located. Position C is a hired area by Ifakara Health Institute (IHI) where experimental huts are located near the irrigation rice fields for field trials. Written consents were sought from the head of the households upon agreement to be involved in the study. All the households that were located nearby the study areas were provided with bed nets and topical repellents. Participation was voluntary and household members were free to withdraw from the study in case of any inconveniences. However, none of the study participants withdrew from the study.
For semi-field experiments, both verbal and written consents forms were sought from the volunteers prior to the start of the experiment. To reduce the risks of being bitten by mosquitoes, the volunteers were provided with long sleeved jackets with ventilated hoods and gloves during mosquito collections. The volunteers received free access to malaria tests using Rapid Diagnostic Test (RDT) and they had access to malaria treatment using the first line drug, artemether lumefantrine (Coartem), in case of any malaria cases (which in this particular experiment there was none). We did not provide chemoprophylaxis to the volunteers because mosquitoes used were laboratory-reared females with no prior blood-meals.
Ethical review and approval was granted by the institutional review board of Ifakara Health Institute (Ref: IHI/IRB/NO.030) and The Medical Research Coordinating Committee at the National Institute of Medical Research in Tanzania (Ref: NIMR/HQ/R.8a/Vol.IX/1222). An ethics waiver was provided from the Human Research Ethics Committee of the University of the Witwatersrand, Johannesburg. The permission to publish this study was granted by the director general of the National Institute of Medical Research in Tanzania (Ref: NIMR/HQ/P.12 VOL XVII/19).
Acknowledgments
We wish to express our sincere gratitude to the community of Lupiro for allowing us to conduct this study in their village. We offer special thanks to the members of the households for the consent to use some pieces of land to place our traps for evaluation. We greatly appreciate Jason Moore for assisting with the new schema diagrams of the MLB. Thanks to Prof. George Corliss for critical reviewing both the research protocol and draft manuscript and Dr. Givemore Munhenga, Dr. Riann Christian for reviewing the research protocol and Paul Kaefer for the edits and grammar corrections. We thank you Deogratius R. Kavishe for assisting us on PCR and ELISA diagnostics. We are grateful to the project team Irene R. Moshi, Mercy Opiyo, Alex J. Limwagu, Edith P. Madumla, Emanuel W. Kaindoa, Stephen P. Mwangungulu, Johnson K. Swai, Lina Finda, Halfan Ngowo, Haika G. Minja, Dickson W. Lweotoijera, and Robert D. Sumaye for their helpful comments and recommendations on our experimental study protocols. We also thank Dr. Katharina Kreppel and Dr. Samson Kiware for their advice during R statistical analysis. We sincerely thank you the editor and anonymous reviewers for their invaluable comments and recommendations on this manuscript.
Data Availability
All minimum data required to replicate the study are available within the manuscript.
Funding Statement
The Global Health Discovery program of the Bill and Melinda Gates Foundation through Grand Challenges Explorations Award (Grant Number: 53214 and OPP103574), the Low and Middle Income Countries Stars in Global Health Program at the Grand Challenges Canada (Grant Number: 0012-01-01-01-01) awarded to FOO. Websites links available at http://gcgh.grandchallenges.org/explorations/pages/introduction.aspx and http://www.grandchallenges.ca/. Wellcome Trust Masters Fellowship (Grant number: WT104029/Z/14/Z) awarded to the lead author, NSM. http://www.wellcome.ac.uk/. FOO is also supported by a Wellcome Trust Intermediate Research Fellowship (Grant number: WT102350/Z/13/Z). http://www.wellcome.ac.uk/. MC is supported by a DST/NRF South African Research Chairs Initiative grant. SJM is supported by Bill & Melinda Gates Foundation OPP1081737 administered by Notre Dame University, http://gcgh.grandchallenges.org/explorations/pages/introduction.aspx. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. (2015) The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO (2014) World Malaria Report Geneva, World Health Organization. [Google Scholar]
- 3.WHO (2015) Global Techinical Strategy for Malaria 2016–2030. Geneva, World Health Organization. [Google Scholar]
- 4.Russell TL, Lwetoijera DW, Maliti D, Chipwaza B, Kihonda J, Charlwood JD, et al. (2010) Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar J 9:187 10.1186/1475-2875-9-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF (2011) Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J 10:80 10.1186/1475-2875-10-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. (2011) Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J 10:184 10.1186/1475-2875-10-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pates H, Curtis C (2005) Mosquito behavior and vector control. Ann Rev Entomol 50: 53–70. [DOI] [PubMed] [Google Scholar]
- 8.Takken W, Verhulst NO (2013) Host preferences of blood-feeding mosquitoes. Ann Rev Entomol 58: 433–453. [DOI] [PubMed] [Google Scholar]
- 9.The malERA Consultative Group on Vector Control (2011) A research agenda for malaria eradication: vector control. PLoS Med 8: e1000401 10.1371/journal.pmed.1000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durnez L, Coosemans M (2013) Residual transmission of malaria: an old issue for new approaches. New insights into malaria vectors: 671–704. [Google Scholar]
- 11.Okumu FO, Madumla EP, John AN, Lwetoijera DW, Sumaye RD (2010) Attracting, trapping and killing disease-transmitting mosquitoes using odor-baited stations-The Ifakara Odor-Baited Stations. Parasit Vectors 3: 12 10.1186/1756-3305-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matowo NS, Moore J, Mapua S, Madumla EP, Moshi IR, Kaindoa EW, et al. (2013) Using a new odour-baited device to explore options for luring and killing outdoor-biting malaria vectors: a report on design and field evaluation of the Mosquito Landing Box. Parasit Vectors 6: 137 10.1186/1756-3305-6-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eiras AE, Buhagiar TS, Ritchie SA (2014) Development of the Gravid Aedes Trap for the capture of adult female container–exploiting mosquitoes (Diptera: Culicidae). J Med Entomol 51: 200–209. [DOI] [PubMed] [Google Scholar]
- 14.Eiras AE, Resende MC (2009) Preliminary evaluation of the" Dengue-MI" technology for Aedes aegypti monitoring and control. Cadernos de Saúde Pública 25: S45–S58. [DOI] [PubMed] [Google Scholar]
- 15.Degener CM, Eiras AE, Azara TM, Roque R, Rösner S, Codeço CT, et al. (2014) Evaluation of the effectiveness of mass trapping with BG-sentinel traps for dengue vector control: a cluster randomized controlled trial in Manaus, Brazil. J Med Entomol 51: 408–420. [DOI] [PubMed] [Google Scholar]
- 16.Rapley LP, Johnson PH, Williams CR, Silcock RM, Larkman M, Long SA, et al. (2009) A lethal ovitrap -based mass trapping scheme for dengue control in Australia: II. Impact on populations of the mosquito Aedes aegypti. Med Vet Entomol 23: 303–316. 10.1111/j.1365-2915.2009.00834.x [DOI] [PubMed] [Google Scholar]
- 17.Vale GA, Lovemore DF, Flint S, Cockbill GF (1988) Odour-baited targets to control tsetse flies, Glossina spp.(Diptera: Glossinidae) in Zimbabwe. Bull Entomol Res 78: 31–49. [Google Scholar]
- 18.Okumu FO, Govella NJ, Moore SJ, Chitnis N, Killeen GF (2010) Potential benefits, limitations and target product-profiles of odor-baited mosquito traps for malaria control in Africa. PLoS One 5: e11573 10.1371/journal.pone.0011573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiscox A, Maire N, Kiche I, Silkey M, Homan T, Oria P, et al. (2012) The SolarMal Project: innovative mosquito trapping technology for malaria control. Malar J 11: 045. [Google Scholar]
- 20.Muirhead-Thomson RC (1960) The significance of irritability, behaviouristic avoidance and allied phenomena in malaria eradication. Bull World Health Organ 22: 721–734. [PMC free article] [PubMed] [Google Scholar]
- 21.Okumu FO, Mbeyela E, Lingamba G, Moore J, Ntamatungiro AJ, Kavishe DR, et al. (2013) Comparative field evaluation of combinations of long-lasting insecticide treated nets and indoor residual spraying, relative to either method alone, for malaria prevention in an area where the main vector is Anopheles arabiensis. Parasit Vectors 6: 46 10.1186/1756-3305-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson G (1953) Experiments on the effect of residual insecticides in houses against Anopheles gambiae and An. funestus. Bull Entomol Res 44: 231–254. [Google Scholar]
- 23.Lines J, Myamba J, Curtis C (1987) Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania. Med Vet Entomol 1: 37–51. [DOI] [PubMed] [Google Scholar]
- 24.Miller JE, Lindsay S, Armstrong J (1991) Experimental hut trials of bednets impregnated with synthetic pyrethroid or organophosphate insecticide for mosquito control in The Gambia. Med Vet Entomol 5: 465–476. [DOI] [PubMed] [Google Scholar]
- 25.Tirados I, Costantini C, Gibson G, Torr SJ (2006) Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol 20: 425–437. [DOI] [PubMed] [Google Scholar]
- 26.Fornadel CM, Norris LC, Glass GE, Norris DE (2010) Analysis of Anopheles arabiensis blood feeding behavior in southern Zambia during the two years after introduction of insecticide-treated bed nets. Am J Trop Med Hyg 83: 848–853. 10.4269/ajtmh.2010.10-0242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V (2011) Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 27: 91–98. 10.1016/j.pt.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 28.Vale GA (1974) New field methods for studying the responses of tsetse flies (Diptera, Glossinidae) to hosts. Bull Entomol Res 64: 199–208. [Google Scholar]
- 29.Knols BGJ, Mboera LEG, Takken W (1998) Electric nets for studying odour- mediated host-seeking behaviour of mosquitoes. Med Vet Entomol 12: 116–120. [DOI] [PubMed] [Google Scholar]
- 30.Torr SJ, Della Torre A, Calzetta M, Costantini C, Vale GA (2008) Towards a fuller understanding of mosquito behaviour: use of electrocuting grids to compare the odour-orientated responses of Anopheles arabiensis and An. quadriannulatus in the field. Med Vet Entomol 22: 93–108. 10.1111/j.1365-2915.2008.00723.x [DOI] [PubMed] [Google Scholar]
- 31.Torr S (1988) The flight and landing of tsetse (Glossina) in response to components of host odour in the field. Physiol Entomol 13: 453–465. [Google Scholar]
- 32.Frick T, Tallamy D (1996) Density and diversity of non-target insects killed by suburban electric insect traps. Entomol News 107: 77–82. [Google Scholar]
- 33.Urban JE, Broce A (2000) Killing of flies in electrocuting insect traps releases bacteria and viruses. Curr Microbiol 41: 267–270. [DOI] [PubMed] [Google Scholar]
- 34.Majambere S, Masue D, Mlacha Y, Govella NJ, Magesa SM, Killeen GF (2013) Advantages and limitations of commercially available electrocuting grids for studying mosquito behaviour. Parasit Vectors 6: 53 10.1186/1756-3305-6-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO (2013) Test procedures for insecticide resistance monitoring in malaria vector mosquitoes Geneva, World Health Organization. [Google Scholar]
- 36.Okumu FO, Chipwaza B, Madumla EP, Mbeyela E, Lingamba G, Moore J, et al. (2012) Implications of bio-efficacy and persistence of insecticides when indoor residual spraying and longlasting insecticide nets are combined for malaria prevention. Malar J 11: 378 10.1186/1475-2875-11-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Malaria Control Programme (Tanzania), WHO (Tanzania), Ifakara Health Institute (Tanzania), KEMRI-Wellcome Trust (Kenya) (2013) An epidemiological profile of malaria and its control in mainland Tanzania. Report funded by Roll Back Malaria and Department for International Development-UK July 2013.
- 38.Govella NJ, Okumu FO, Killeen GF (2010) Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am J Trop Med Hyg 82: 415–419. 10.4269/ajtmh.2010.09-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lwetoijera D, Harris C, Kiware S, Dongus S, Devine GJ, McCall PJ, et al. (2014) Effective autodissemination of pyriproxyfen to breeding sites by the exophilic malaria vector Anopheles arabiensis in semi-field settings in Tanzania. Malar J 13: 161 10.1186/1475-2875-13-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson HM, Ng'habi KR, Walder T, Kadungula D, Moore SJ, Lyimo I, et al. (2008) Establishment of a large semi-field system for experimental study of African malaria vector ecology and control in Tanzania. Malar J 7: 158 10.1186/1475-2875-7-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng'habi KR, Mwasheshi D, Knols BG, Ferguson HM (2010) Establishment of a self-propagating population of the African malaria vector Anopheles arabiensis under semi-field conditions. Malar J 9: 356 10.1186/1475-2875-9-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okumu FO, Killeen GF, Ogoma S, Biswaro L, Smallegange RC, Mbeyela E, et al. (2010) Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS One 5: e8951 10.1371/journal.pone.0008951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okumu F, Biswaro L, Mbeleyela E, Killeen GF, Mukabana R, Moore SJ (2010) Using nylon strips to dispense mosquito attractants for sampling the malaria vector Anopheles gambiae ss. J Med Entomol 47: 274–282. [DOI] [PubMed] [Google Scholar]
- 44.Mweresa CK, Omusula P, Otieno B, Van Loon JJ, Takken W, Mukabana WR (2014) Molasses as a source of carbon dioxide for attracting the malaria mosquitoes Anopheles gambiae and Anopheles funestus. Malar J 13: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verhulst NO, Andriessen R, Groenhagen U, BukovinszkinéK G, Schulz S, Takken W, et al. (2010) Differential attraction of malaria mosquitoes to volatile blends produced by human skin bacteria. PLoS One 5: e15829 10.1371/journal.pone.0015829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukabana WR (2002) Differential attractiveness of humans to the African malaria vector Anopheles gambiae Giles: effects of host characteristics and parasite infection. Wageningen: PhD thesis, Wageningen University.
- 47.Gillies M, Coetzee M (1987) A Supplement to the Anophelinae of Africa South of the Sahara. Publications of the South African Institute for Medical Research 55: 1–143. [Google Scholar]
- 48.Paskewitz SM, Collins F (1990) Use of the polymerase chain reaction to identify mosquito species of the Anopheles gambiae complex. Med Vet Entomol 4: 367–373. [DOI] [PubMed] [Google Scholar]
- 49.Scott JA, Brogdon WG, Collins FH (1993) Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg 49: 520–529. [DOI] [PubMed] [Google Scholar]
- 50.Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V (1987) A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg 37: 37–41. [DOI] [PubMed] [Google Scholar]
- 51.Koekemoer LL, Kamau L, Hunt RH, Coetzee M (2002) A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg 66: 804–811. [DOI] [PubMed] [Google Scholar]
- 52.Beier JC, Perkins PV, Koros JK, Onyango FK, Gargan TP, Wirtz RA, et al. (1990) Malaria sporozoite detection by dissection and ELISA to assess infectivity of afrotropical Anopheles (Diptera: Culicidae). J Med Entomol 27: 377–384. [DOI] [PubMed] [Google Scholar]
- 53.Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, et al. (2011) False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar J 10: 195 10.1186/1475-2875-10-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bashar K, Tuno N, Ahmed TU, Howlader AJ (2013) False positivity of circumsporozoite protein (CSP)-ELISA in zoophilic anophelines in Bangladesh. Acta Trop 125: 220–225. 10.1016/j.actatropica.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 55.R Core Team (2012) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2012. ISBN 3-900051-07-0. [Google Scholar]
- 56.Bates D (2005) Fitting linear mixed models in R. R news 5: 27–30. [Google Scholar]
- 57.Bolker B, Skaug H, Magnusson A, Nielsen A (2012) Getting started with the glmmADMB package. Available at glmmadmb r-forge r-project org/glmmADMB pdf.
- 58.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, et al. (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24: 127–135. 10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 59.Koekemoer LL, Rankoe EM, la Grange JP, Govere J, Coetzee M (2001) False detection of Plasmodium falciparum sporozoites in Anopheles marshallii group mosquitoes. J Am Mosq Control Assoc 17: 160–165. [PubMed] [Google Scholar]
- 60.Huho B, Briet O, Seyoum A, Sikaala C, Bayoh N, Gimnig J, et al. (2012) Consistently high baseline estimates for the proportion of human exposure to rural African malaria vector populations that occurred indoors. Malar J 11: P50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seyoum A, Sikaala CH, Chanda J, Chinula D, Ntamatungiro AJ, Hawela M, et al. (2012) Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, South-east Zambia. Parasit Vectors 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moiroux N, Gomez MB, Pennetier C, Elanga E, Djenontin A, Chandre F, et al. (2012) Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis 206:1622–1629. 10.1093/infdis/jis565 [DOI] [PubMed] [Google Scholar]
- 63.Sokhna C, Ndiath MO, Rogier C (2013) The changes of mosquito vectors behavior and the emerging resistance to insecticide will challenge the decline of malaria. Clin Microbiol Infect 19: 902–907. 10.1111/1469-0691.12314 [DOI] [PubMed] [Google Scholar]
- 64.WHO (2012) Global Plan for Insecticide Resistance Management in Malaria Vectors. Geneva, World Health Organization. [Google Scholar]
- 65.Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, McCall PJ, et al. (2014) Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar J 13: 331 10.1186/1475-2875-13-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCann RS, Ochomo E, Bayoh MN, Vulule JM, Hamel MJ, Gimnig JE, et al. (2014) Re-emergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. Am J Trop Med Hyg 90: 597–604. 10.4269/ajtmh.13-0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moiroux N, Damien GB, Egrot M, Djenontin A, Chandre F, Corbel V, et al. (2014) Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS One 9: e104967 10.1371/journal.pone.0104967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilkes TJ, Matola YG, Charlwood JD (2008) Anopheles rivulorum, a vector of human malaria in Africa. Med Vet Entomol 10: 108–110. [DOI] [PubMed] [Google Scholar]
- 69.Kawada H, Dida GO, Sonye G, Njenga SM, Mwandawiro C, Minakawa N (2012) Reconsideration of Anopheles rivulorum as a vector of Plasmodium falciparum in Western Kenya: some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasit Vectors 5: 230 10.1186/1756-3305-5-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gillies MT, Smith A (1960) The effect of residual house- spraying campaign in East Africa on species balance in the Anopheles funestus group. The repalcement of An. funestus giles by a An. rivulorum leeson. Bull Entomol Res 51: 243–252. [Google Scholar]
- 71.Gillies MT, De Meillon B (1968) The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). Publications of the South African Institute for Medical Research, Johannesburg,54:1–344. [Google Scholar]
- 72.Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C (2013) The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors 6: 114 10.1186/1756-3305-6-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ratovonjato J, Olive M-M, Tantely LM, Andrianaivolambo L, Tata E, Razainirina J, et al. (2011) Detection, isolation, and genetic characterization of Rift Valley fever virus from Anopheles coustani, Anopheles squamosus, and Culex antennatus of the Haute Matsiatra region, Madagascar. Vector Borne Zoonotic Dis 11: 753–759. 10.1089/vbz.2010.0031 [DOI] [PubMed] [Google Scholar]
- 74.Stephens С, Masamu E, Kiama M, Keto A, Kinenekejo M (1995) Knowledge of mosquitos in relation to public and domestic control activities in the cities of Dar es Salaam and Tanga. Bull World Health Organ, 73: 97–104. [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou G, Munga S, Minakawa N, Githeko AK, Yan G (2007) Spatial relationship between adult malaria vector abundance and environmental factors in western Kenya highlands. Am J Trop Med Hyg 77: 29–35. [PubMed] [Google Scholar]
- 76.Minakawa N, Seda P, Yan G (2002) Influence of host and larval habitat distribution on the abundance of African malaria vectors in western Kenya. Am J Trop Med Hyg 67: 32–38. [DOI] [PubMed] [Google Scholar]
- 77.Oesterholt M, Bousema J, Mwerinde O, Harris C, Lushino P, Masokoto A, et al. (2006) Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malar J 5:98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ribeiro J, Seulu F, Abose T, Kidane G, Teklehaimanot A (1996) Temporal and spatial distribution of anopheline mosquitos in an Ethiopian village: implications for malaria control strategies. Bull World Health Organ 74: 299–305. [PMC free article] [PubMed] [Google Scholar]
- 79.Briët OJ, Dossou Yovo J, Akodo E, Van De Giesen N, Teuscher TM (2003) The relationship between Anopheles gambiae density and rice cultivation in the savannah zone and forest zone of Cote d'Ivoire. Trop Med Int Health 8: 439–448. [DOI] [PubMed] [Google Scholar]
- 80.Smith T, Charlwood J, Kihonda J, Mwankusye S, Billingsley P, Meuwissen J, et al. (1993) Absence of seasonal variation in malaria parasitaemia in an area of intense seasonal transmission. Acta Trop 54: 55–72. [DOI] [PubMed] [Google Scholar]
- 81.Minakawa N, Githure JI, Beier JC, Yan G (2001) Anopheline mosquito survival strategies during the dry period in western Kenya. J Med Entomol 38: 388–392. [DOI] [PubMed] [Google Scholar]
- 82.Charlwood J, Vij R, Billingsley P (2000) Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. Am J Trop Med Hyg 62: 726–732. [DOI] [PubMed] [Google Scholar]
- 83.Biogents (2014) Biogents mimics human scent.
- 84.Elliott R (1968) Studies on man-vector contact in some malarious areas in Colombia. Bull Entomol Res 38: 239–253. [PMC free article] [PubMed] [Google Scholar]
- 85.Service M (1977) Critical-review of procedures for sampling populations of adult mosquitos. Bull Entomol Res 67: 343–382. [Google Scholar]
- 86.Lines J, Curtis C, Wilkes T, Njunwa K (1991) Monitoring human-biting mosquitoes (Diptera: Culicidae) in Tanzania with light-traps hung beside mosquito nets. Bull Entomol Res 81: 77–84. [Google Scholar]
- 87.NBS (2013) Tanzania National Bureau of Statistics. National Bureau of Statistics, Ministry of Finance, Dar es Salaam. [Google Scholar]
- 88.NBS (2010) Tanzania Demographic and Health Survey. National Bureau of Statistics, Ministry of Finance, Dar es Salaam. [Google Scholar]
- 89.ESRF U (2014) Tanzania Human Development Report. Economic Transformation for Human Development. [Google Scholar]
- 90.Gillies MT (1980) The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bull Entomol Res 70: 525–532. [Google Scholar]
- 91.Dekker T, Geier M, Cardé RT (2005) Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol 208: 2963–2972. [DOI] [PubMed] [Google Scholar]
- 92.Smallegange RC, Schmied WH, van Roey KJ, Verhulst NO, Spitzen J, Mukabana WR, et al. (2010) Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae. Malar J 9: 292 10.1186/1475-2875-9-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Costantini C, Gibson G, Brady J, Merzagora L, Coluzzi M (1993) A new odour-baited trap to collect host-seeking mosquitoes. Parassitologia 35: 5. [PubMed] [Google Scholar]
- 94.Mboera LEG, Knols BGJ, Takken W, Della Torre A (1997) The response of Anopheles gambiae s.l. and Anopheles funestus (Diptera: Culicidae) to tents baited with human odour or carbon dioxide in Tanzania. Bull of Entomolol Res 87: 173–178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All minimum data required to replicate the study are available within the manuscript.