Abstract
Background:
Bone lytic lesion in Multiple myeloma are the most commonly presented symptoms which require treatment with bisphosphonates (BPs). BPs are providing supportive care, reducing the rate of skeletal morbidity but evidently not abolishing it, the criteria for stopping their administration have to be different from those used for classic antineoplastic drugs, and they should not be stopped when metastatic bone disease is progressing. Osteonecrosis of the jaw (ONJ) has been associated recently with the use of BPs.
Aim:
The aim of these study is to evaluate the incidence of ONJ in patients with MM treated with mixed biphosphonates.
Patients and methods:
We analyzed total 296 myeloma patients (150 male and 146 female). Mostly effected age group with 58,1% is age more than 60 years up to 88 years, diagnosed in our institution in the period 2005-2015. We used intravenous or oral forms of biphosphonates such as pamidronate, ibandronate, clodronate and zolendronic acid. The patients were evaluated for ONJ.
Results:
The incidence of ONJ in our group of patients treated with Bps was 4,6% from our group of 260 patients 87,8% received BPs therapy and patients which haven’t received BPs 12,2%. From this group, 95,4% (248) didn’t show ONJ, and 4,6% (12) showed ONJ. The period of this treatment with BPs is an important risk factor for development of ONJ, average duration of BPs therapy in patients which show adverse effects is 26.8±13.7 months, from the total number of 12 patients that developed ONJ adverse effects, we have 8 patients which received treatment with Zolendronic acid and the remaining 4 patients which were treated with other BPs combinations without Zolendronic acid.
Conclusions:
All patients treated for MM must continue with the therapy with Zolendronic acid and Pamidronate, each patient must be individually treated according to his response of the treatment (dose, frequency and duration of therapy).
Keywords: Multiple myeloma, osteonecrosis of jaw, biphosphonates
1. INTRODUCTION
Myeloma multiplex (MM) is the second most frequent hematological malignancy, for approximately 10% of all hematological malignancies. It is characterized by malignant plasma cell infiltration of the bone marrow and is associated with an increased level of monoclonal protein in the blood and/or urine. The uncontrolled growth of myeloma cells has many consequences like: skeletal destructions, anemia, and renal and immune impairments. Bisphosphonates (BPs) are potent inhibitors of osteoclast-mediated bone resorption, and it is well accepted that tumor cells in bone, especially breast cancer and myeloma cells, can stimulate osteoclast formation and activity leading to the release of growth factors or cytokines, which will further stimulate cancer cells’ growth and their secretion of osteolytic factors (1). BPs are providing supportive care, reducing the rate of skeletal morbidity but evidently not abolishing it, the criteria for stopping their administration have to be different from those used for classic antineoplastic drugs, and they should not be stopped when metastatic bone disease is progressing. However, criteria to determine whether and for how long an individual patient benefits from their administration are lacking (2-7). New biochemical markers of bone resorption might help identify those patients continuing to benefit from therapy. Even better results have been achieved in patients with multiple myeloma, and the general consensus is that BPs should be started as soon as the diagnosis of lytic disease is made in myeloma patients (8). For multiple myeloma patients who have, on plain radiographs or imaging studies, lytic destruction of bone or spine compression fracture from osteopenia, intravenous pamidronate 90mg delivered over at least 2 hours, or zolendronic acid 4mg delivered over at least 15 minutes every 3 to 4 weeks is recommended (9). Unfortunately long term use of biphosphonates shows adverse effects like osteonecrosis of the jaw ONJ, who is uncommon but potentially serious complication of intravenous application of biphosphonates, because of it, The Update Committee agrees with a recommendations described in the revised US Food and Drug Administration label. Biphosphonate–related osteonecrosis of the jaw sometimes called BRONJ has shown increasing interest by dentists and oral maxillofacial surgeons, it is defined as an area of exposed bone in the maxillofacial region that does not heal within 8 weeks in a patient who is currently receiving biphosphonate medication and has not had radiation to the head-neck region (1). The diagnosis is usually made clinically. More than 300 cases of ONJ associated with intravenous biphosphonate use in patients with cancer have been reported in the medical and dental literature. From our Clinic we have the similar publication in 2010. The number of published cases with biphosphonate-related osteonecrosis of the jaws (BRONJ) is steadily increased, but the definite prevalence is still unknown. The incidence rate is higher in patients with high dose intravenous administration of biphosphonates compared to the oral route of administration, but sometimes the condition occurs also in patients with low dose osteoporotic treatment (10). The estimates of BRONJ for i.v. application range from around 1 to 21% (1, 8, 11, 12) because they have various underlying diseases, also very important is drug potency like zolendronat which is the most potent bisphosphonate and patients received i.v. zolendronat for cancer indication (11, 12). For example, a web-based survey of ONJ in 2004 found that ONJ developed in 10% of patients receiving zoledronate compared to 4% of patients receiving pamidronate (8). As we mentioned one of the risk factor is the type of administration as well as individual local and systemic conditions, asymptomatic form for a long time, do not relate their oral symptoms to the biphosphonate therapy. ONJ is associated with i.v. bisphosphonate a less with oral bisphosphonate (14). The cumulative dose of bisphosphonate is also considered to be an important factor in the development of ONJ and it is determined by the dose, frequency of administration, duration of therapy and half-life of the drug (1, 8, 11, 12, 14).
A history of dental disease, including invasive dental procedures, dental trauma and periodontal disease, may also be an important risk factor for the development of ONJ in association with bisphosphonates (8, 11). The most representative study, however with a retrospective review of 830 patients, showed an ONJ incidence of 8,2%.
2. AIM
The aim of these study was to evaluate the incidence of ONJ in patients with MM treated with mixed biphosphonates, even with zolendronic acid in a period of 10 years, in our institution, with is rapid increased because in our last study in 2010 we had a incidence of 1% from observed patients (14).
3. PATIENTS AND METHODS
We retrospectively analyzed total 296 myeloma patients (150 male and 146 female) with average age of 62 years ±10,3 years. Mostly effected age group with 58,1% is age more than 60 years up to 88 years, diagnosed in our institution in the period 2005-2015. Bone disease affects more than 70% of patients with MM and is associated with pain, pathological fractures and hypercalcaemia. Bone disease is important morbidity factor in patients with MM, and because of that measures for reducing morbidity from skeletal involvement are important in optimizing and improving a patient’s quality of life. Monthly infusion of biphosphonates reduces skeletal events and modifies the natural history of bone disease in MM.
We used intravenous or oral forms of biphosphonates such as pamidronate, ibandronate, clodronate and zolendronic acid. Mostly common we used oamidronate 90mg i.v. or ibandronate 6mg i.v. per month or zolendronic acid 4mg per month. Oral biphosphonates we used like standard dose of clodronate or ibandronate every day all month long. Details of the disease such as lasting of the type of MM treatment, biphosphonates therapy and lasting of the same was analyzed for all patients. The patients which developed adverse effects of biphosphonates treatment like osteonecrosis of the jaw (mostly mandible) and maxilla, were treated with surgical debridement and received broad spectrum of antibiotics before and after the surgery and afterwards the bisphosponate therapy was stopped after the X-Ray signs of ONJ.
4. RESULTS
The incidence of ONJ in our group of patients treated with biphosphonates was 4,6% from our group of 260 patients 87,8% received BPs therapy and patients which haven’t received BPs 12,2%. From this group of 260 patients, 95,4% (248) didn’t show ONJ, and 4,6% (12) showed ONJ. According to Bamias et al., the incidence of ONJ in patients with MM treated with BPs was 11 patients, 9,9%. In our study from 2010 this percentage was 1% (14). The incidence has grown because we started using zolendronic acid which is the most commonly referred biphosphonate is causing ONJ. Our results co-relate with the world experiences, in the literature. The period of this treatment with BPs is an important risk factor for development of ONJ.
Biphosphonate therapy was received by 260 (87,8%) patients, and 36 (12,2%) didn’t receive biphosphonate therapy (Table 1).
Table 1.
Distribution of patients according to adverse effect from biphosphonate therapy
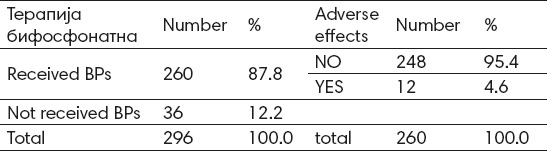
Adverse effect from biphosphonate therapy is not registered with 94,4 patients which received biphosphonate therapy, and adverse effects are registered (such as osteonecrosis of the jaw) with 4,6% of patients which received biphosphonate therapy (Table 1).
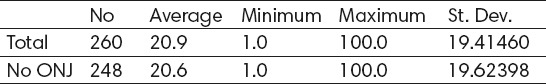
Average duration of biphosphonate therapy in patients is 20.9±19.4 months, minimum 1 month and maximum 100 months (Table 2, Figure 2). Average duration of biphosphonate therapy in patients which don’t show adverse effects is 20.6±19.6 months, minimum 1 month, and maximum 100 months (Table 2 and Figure 2). Average duration of biphosphonate therapy in patients which show adverse effects is 26.8±13.7 months, minimum 6 months and maximum 63 months (Table 2 and Figure 2). According to t-test the difference is statistically insignificant for p<0.05 (t=-1.07329, p=0.284145).
Table 2.
Average lasting of biphosphonate therapy with patients which have registered adverse effects
![]()
Figure 1.
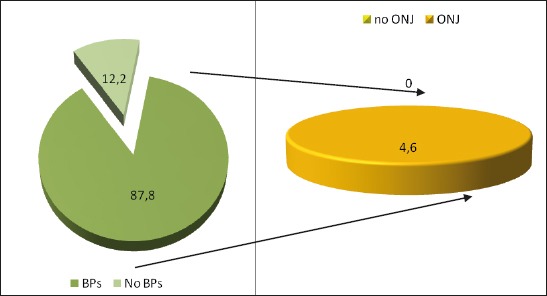
Graphic presentation of the distribution of patients according to adverse eff ect from bisphosphonate therapy
Figure 2.
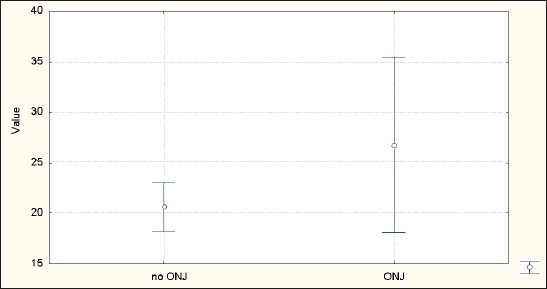
Average lasting of biphosphonate therapy duration with patients which have registered adverse eff ects(ONJ)
Single-type-biphosphonate therapy is used by 54,6% of patients and combination of biphopshonate therapy is used by 45,4% of patients (Table 3 and Figure 3).
Table 3.
Use of biphosphonates type

Figure 3.
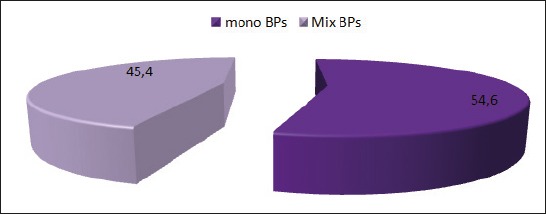
Graphic showing of the usage of bisphosphonate type
From the total number of 12 patients that developed ONJ adverse effects, we have 8 patients which received treatment with Zolendronic acid and the remaining 4 patients which were treated with other BPs combinations without Zolendronic acid. Patients received i.v. zolendronic and pamidronate and only one received per os. Therefore the use of Zolendronic acid is a major risk factor in the incidence of ONJ.
5. DISCUSSION
The use of biphosphonates seems to be associated with the development of ONJ. Our results correspond with those from the literature: the first one is time of exposure, their cumulative effect, type of biphosphonate mostly zolendronic acid, form of application like parenteral versus oral form, and previous dental procedures, like tooth extraction. Also potential risk factors for ONJ other than BPs include corticosteroids, coagulopathy, alcohol abuse, tobacco use, infections and inflammation. But steel prevention is superior to treatment, the establishment of oral hygiene and pre-emptive surgical treatment prior to application of BPs therapy is recommended. Conservative management with antibiotic therapy, improved local oral hygiene measures, 0,12% chlorhexidine mouth rinses and 2% potassium iodine solution maybe will relief significant symptoms. Most important is surgical debridement, which probably becomes the treatment of choice for the management of ONJ. The time of exposure to BPs was strongly associated with the development of ONJ, the duration of BPs treatment in the study of Pavkovic M. et al. was 24.7±17.7 months, with median time 24 months and range of 2-84 months, compared to the Bamias A. et al. study where the median time of exposure to BPs and occurrence of ONJ was 39,3 months. This is explained because of the lesser use of zolendronic acid, compared to the other studies. In all patients that showed a higher percentage, a treatment with Zolendronic acid was implemented and that’s why we have an increased percentage compared to the 2010 study. When surgical treatment becomes unavoidable referral to a specialist oral and maxillofacial surgeon is recommended, extraction and all types of surgery to the jaws should be avoided. According to Cheng et al. there is a novel approach of slow extraction using orthodontic bands (15). One of the option is laser therapy at low intensity, segmental osteotomies and hyperbaric oxygen, but all these therapies are based on individual approach to the patient (16-19). Patients that take BPs can sometimes show ON of the jaws but most commonly it is associated with trauma, predominantly dental extraction. We can not predict who has a higher risk for BRONJ while using BPs. One of the persistent conclusions is the correlation between duration of therapy and occurrence of BRONJ. We have also used antibiotics while treating BRONJ but with small effects. Debridement’s from surgery and wound closure worsens the symptoms and even excessive osseous surgery has resulted in enlargement of areas of necrotic bone. The conclusion is that the best way is to use conservative debridement. However, the choice between surgery and conservative therapy is a difficult issue and must be made on an individual basis (20). All those patients, treated with antibiotics and surgically treated, which developed ONJ had a small benefit from the same, the most important is the prophylaxis and the preventive measures: education of the patient about potential adverse effects associated with the therapy, early detection and non-surgical of oral conditions, maintenance of oral hygiene program.
6. CONCLUSION
The conclusion of this study comes to similar conclusions as other authors. All patients treated for MM must continue with the therapy with Zolendronic acid and Pamidronate, each patient must be individually treated according to his response of the treatment (dose, frequency and duration of therapy). The medications must be administrated in a monthly frequency the first year and every three months in the forthcoming years. Involvement of surgery should be avoided and put to a lesser level, only in cases where it is mandatory.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with biphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 2.Stewart AF. Clinical practice: Hypercalcemia associated with cancer. N Engl J Med. 2005;352:272–279. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 3.Hillner BE, Ingle JN, Berenson JR, et al. American Society of Clinical Oncology guidelines on the role of biphosphonates in breast cancer. J Clin Oncol. 2000;18:1378–1391. doi: 10.1200/JCO.2000.18.6.1378. [DOI] [PubMed] [Google Scholar]
- 4.Saad F. Treatment of bone complications in advanced prostate cancer: Rationale for biphosphonate use and results of a phase III trial with zolendronic acid. Semin Oncol. 2002;29:S19–S27. doi: 10.1053/sonc.2002.37418. [DOI] [PubMed] [Google Scholar]
- 5.Rosen LS. Efficacy and safety of zolendronic acid in the treatment of bone metastases associated with lung cancer and other solid tumors. Semin Oncol. 2002;29:S28–S32. doi: 10.1053/sonc.2002.37416. [DOI] [PubMed] [Google Scholar]
- 6.Rosen LS, Gordon D, Tchekmedyian S, et al. Zolendronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: A phase III, double-blind, randomized trial-the Zolendronica Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 7.Ruggiero SL, Mehtotra B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of biphosphonates: A review of 63 cases. J Oral Maxxilofax Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Durie BGM, Katz M, Crowley J. Osteonecrosis of the jaws and biphosphonates. N Engl J Med. 2005;353:99. doi: 10.1056/NEJM200507073530120. [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, et al. American Society of clinical oncology. Clinical practice guidelines update on the role of biphoshonates in mulltiple myeloma. J Clin Oncol. 2007;25:2464–2472. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 10.Carter GD, Goss AN. Bisphosphonate and avascular necrosis of the jaws. Aust Dent J. 2003;48:268. [PubMed] [Google Scholar]
- 11.Marx RE. Pamindronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J Oral Maxxilofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 12.Badros A, Weikel D, Salama A. et al. OSteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol. 2006;23:8580–8587. doi: 10.1200/JCO.2005.04.2465. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, Kastritis E, Bamia C, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zolendronic acid. Ann Oncol. 2009;20:117–120. doi: 10.1093/annonc/mdn554. [DOI] [PubMed] [Google Scholar]
- 14.Assael LA. New foundations in understanding osteonecrosis of the jaw. J Oral Maxiloofax Surg. 2004;62:125–126. doi: 10.1016/j.joms.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Pavkovic M, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with biphosphonates, Contributions. Soc Biol Med Sci, MASA, XXXI. 2010;2:39–49. [PubMed] [Google Scholar]
- 16.Cheng A, Mavrokokki A, Carter G, et al. The dental implications of biphosphonates and bone disease. Aust Dent J. 2005;50:S4–13. doi: 10.1111/j.1834-7819.2005.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 17.Lemound J, Eckardt A, Kokemuller H, et al. Bisphophnate azsociated osteonecrosis of the myofascial flap. Clinical Oral Investigations. 2012;16(4):1143–1152. doi: 10.1007/s00784-011-0596-x. [DOI] [PubMed] [Google Scholar]
- 18.Vescovi P, Merigo E, Manfredi M, et al. Nd: YAG laser biostimulation in the treatment of bisphophonates-associated osteonecrosis of the jaw: clinical experience in 28 cases. Photomedicine and Laser Surgery. 2008;26(1):37–46. doi: 10.1089/pho.2007.2181. [DOI] [PubMed] [Google Scholar]
- 19.Freiberger JJ. Utility of hyperbaric oxygen in treatment of bisphosphonate-related osteonecrosis of the jaws. Journal of Oral and Maxillofacial Surgery. 2009;67(5):96–106. doi: 10.1016/j.joms.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Vescovi P, Merigo E, Meleti M, et al. Conservative surgical management of stage I bisphosphonate-related osteonecrosis of the jaw. International Journal of Dentistry. 2014:8. doi: 10.1155/2014/107690. ID 107690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holzinger D, Seemann R, Klug C, et al. Long-term success of surgery in biphosphonate-related osteonecrosis of the jaws (BRONJs) Oral Oncology. 2013;49(1):66–70. doi: 10.1016/j.oraloncology.2012.07.008. [DOI] [PubMed] [Google Scholar]


