Abstract
Introduction:
Retinopathy of Prematurity (ROP) represent disease of the eye in premature born children which affects immature blood vessels of the retina during their development. The emergence of retinopathy of prematurity depends on the interaction of multiple factors, such as: gestational age, low birth weight, hypoxia, duration of oxygen supplementation, respiratory distress syndrome, twin pregnancy, anemia, blood transfusions, sepsis, intraventricular hemorrhage, hypotension, hypothermia, etc. If remain unrecognized and untreated it can cause severe visual impairment and blindness in children, but can also be prevented with timely screening.
Goals:
To establish the number of patients with development of retinopathy of prematurity active forms in the observed time period and examine which risk factors have most significant impact on its origin.
Material and methods:
In a clinical, retrospective study we observed a total of 80 premature born children in the period from January to May 2015 with regard to listed risk factors identified for eye examination.
Results:
From a total of 80 premature newborns sample included 48.8% male and 51.2% female children. The active form of ROP developed in 6.2% of cases, while in 93.8% of cases there was a spontaneous resolution. Patients who developed active form of ROP have significantly younger gestational age (26.4±1.5 weeks) and lower birth weight (874±181 grams), lower Apgar score in the first and fifth minute and were longer on oxygen therapy (20±3.4 days).
Conclusion:
Of the potential risk factors that could affect the development of ROP active form following factors have a statistically significant influence: early gestational age, low birth weight, lower Apgar score and prolonged oxygen therapy (p <0.05).
Keywords: Retinopathy of prematurity, premature children, risk factors
1. INTRODUCTION
Retinopathy of prematurity (retinopathia praematurorum, ROP) is a disease of the eye, or specifically blood vessels of the retina and occurs only in premature children. For most of the children retinopathy of prematurity occurs in a mild form so it has spontaneous regression of the disease, unfortunately there may be more severe forms of ROP that cause blindness in one or both eyes. Thanks to the development of the neonatal intensive care unit there is an increase in number of surviving preterm infants with very low birth weight, but at the same time, there is an increase in the incidence of retinopathy of prematurity, why more studies now deals with risk factors that influence ROP occurrence and its prevention (1). The first changes in the eyes of the survived premature babies in terms fibrovascular bundles was described by Terry in 1942 as retrolental fibroplasia, while the term retinopathy of prematurity was introduced by Heath in 1951 (2). Retinopathy of prematurity occurrence is usually associated with premature birth, but the risk for its occurrence represents a consequence of various factors interactive effects (3). Without any doubt it is proved that early gestational age (GA)≤30 weeks and low birth weight (BW)≤1500g are the most important risk factors in the development of ROP, but besides these significant impact also have other factors, such as poor weight gain, reduced IGF increase, the percentage of oxygen in the inhaled air (at first regarded not only as a risk but also directly causative factor for the ROP development), hypoxia, respiratory distress syndrome, twin pregnancy, anemia, blood transfusions, fungal infections, sepsis, intraventricular hemorrhage, etc. (4, 5, 6, 7). International Classification of Retinopathy of Prematurity (ICROP), implies the ROP monitoring according to 3 zones and 5 stages, while additional risk factors of ROP of progression are labeled as “plus disease”, and the development of so-called aggressive ROP or AP-ROP (8). Due to severe visual impairment and possible blindness, it is very important to start ROP screening on time in premature infants at risk, and the world’s revised guidelines, the first initial review indicates a neonatologist at all children born before 31 weeks of GA or BW weight under 1500 grams during 3-4 weeks after birth. Premature infants at GA>31-32 weeks and BW>1500 grams, should be initially reviewed no later than 34 weeks. Also, a neonatologist indicates ophthalmological examination in premature infants of older gestational age if there are clinical risk factors for the ROP development. Further monitoring can be indicated by ophthalmologist according to maturity of retinal blood vessels (9).
Retinopathy of prematurity, according to CRYO-ROP and Etrop studies, can be treated by cryotherapy, laser photocoagulation, cerclage, pars plana vitrectomy (VPP) and application of medications that block the vascular endothelial growth factor (VEGF) (9,10). Even after completed ROP screening long-term monitoring of premature children is necessary due to the increased incidence of strabismus, amblyopia, reactive anomalies, particularly myopia, glaucoma, retinal detachment, etc. (8, 9, 10).
2. MATERIAL AND METHODS
The survey was conducted as clinical, descriptive and retrospective study, including a total of 80 premature children of both genders, who were followed in the period from January to May 2015. Data for the study were obtained from the Protocol for ROP screening among premature children, and which was selected for the first ophthalmic examination by neonatologists according to previously set criteria for screening. The following criteria for the screening were taken into account: gestational age≤32 weeks or birth weight ≤1500 grams, as well as children at older gestational age and higher birth weight, which had some of the risk factors such as: low Apgar score, duration of oxygen supplementation, sepsis, blood transfusion, respiratory distress syndrome, infection, microcephaly, hydrocephaly, twin pregnancy, etc. The first ophthalmology examination was conducted in intensive neonatal care unit of the Pediatric Clinic, University Clinical Center Sarajevo, and controls at the Pediatric’s Department of the Eye Clinic and scheduled by ophthalmologist indication according to the development of the ocular retinal blood vessels, and was usually performed at 1, 2, 3 or 4 weeks, until the moment when the development of retinal blood network was completed and when the screening program was ended. The development of blood vessels network is followed by zones, while the observed changes on fundus were: dilatation and tortuosity of blood vessels, ischemic zones, bleeding, changes in the optic nerve and signs of “plus disease” and AP-ROP. Review of children was carried out in complete mydriasis achieved by drops of Sol. Phenilneprin 2.5% and 0.5% Sol. Cycopnetolat using indirect ophthalmoscope and 20 dioptres lens. Children with observed changes in the blood vessels and suspicious ischemic zones, had an active form of ROP which required treatment weather by application of anti-VEGF factors, laser photo coagulation or by vitreoretinal surgery, according to choice of ophthalmologists and surgeon. Results of the analysis are presented in tables and charts by number of cases, percentage, mean, median, standard deviation, standard error with the maximum and minimum values. To test the difference and impact of certain parameters following tests were used: chi-square test, Student’s t test, analysis of variance ANOVA and Pearson’s linear correlation coefficient. Results of these tests were considered statistically significant with p<0.05 or at the 95% confidence level. The analysis was conducted using the statistical package IBM SPSS Statistics 21.0.
3. RESULTS
Sample included a total of 80 premature children, 41 females (51.3%) and 39 (48.8%) male. The analysis of gestational age (GA) indicated that the mean age is 29.9±2.9 weeks, the youngest patient is in the age of 24 and the oldest at the age of 37 weeks, with majority of patients were at GA between 25 and 30 weeks (Figure 1).
Figure 1.
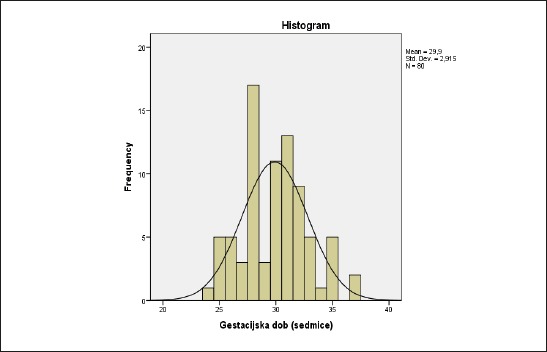
Distribution of gestational age
The average birth weight (BW) in the total sample is 1422.2±515 grams, with lowest of 640 and highest of 3500 grams. The largest number of treated patients had a BW between 900 and 1300 grams (Figure 2).
Figure 2.
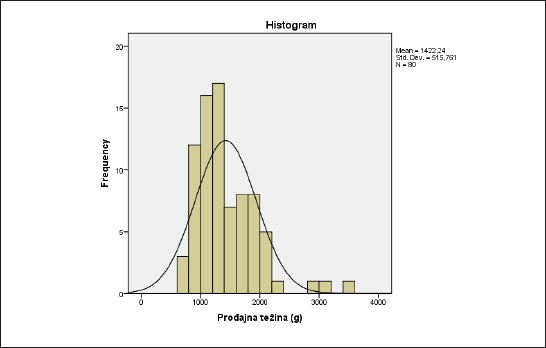
The distribution of birth weight
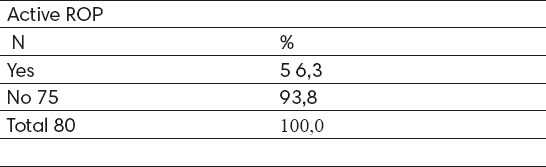
The active form of ROP developed in 5 (6.2%) cases and in 75 (93.8%) cases there was spontaneous regression of the disease (Table 1).
Table 1.
Active ROP identified during screening
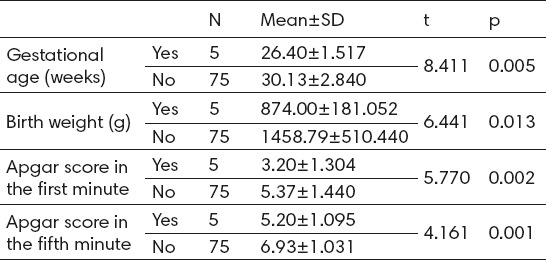
According to gender, 2 (5.1%) male patients and 3 (7.3%) female patients developed active ROP, but without statistically significant difference (p>0.05). Analysis of gestational age indicates that patients who developed active ROP were statistically significantly born earlier or with mean GA of 26.4±1.5 weeks compared to those who did not develop active ROP–30.1±2.8 week. Also, birth weight was statistically significantly lower in patients who developed active ROP–874±181 grams compared to patients who did not develop active ROP–1458.8±510.4 grams. Patients who developed active ROP had statistically lower first minute Apgar score (3.2±1.3 : 5.4±1.4) and five-minute Apgar score (5.20±1.1 : 6.9±1.03) compared to those patients who did not develop active ROP (Table 2).
Table 2.
The analysis of GA, BW and Apgar score according to active ROP
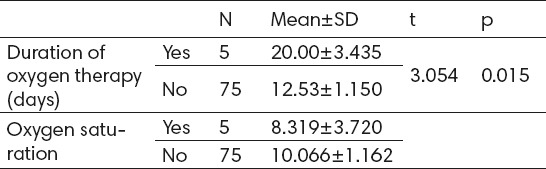
Patients with active ROP had a longer duration of oxygen therapy–20±3.4 days compared to those who did not develop active ROP–12.5±1.1 days with statistically significant difference and correlation (p<0.05). Oxygen saturation rate is lower in patients with active ROP – 83.8±8,3%, compared to patients without active ROP–88.5±10.1%, but without statistically significant difference (Table 3).
Table 3.
Oxygen therapy duration and oxygen saturation according to active ROP
Of the total number of patients with infection 3 (10.7%) cases developed active ROP and 2 (3.8%) cases without infection indicating that there is no statistically significant influence on the development of active ROP but that there is still a certain influence (p>0.05). Given the small number of patients with microcephaly (N=4) could not be verified its influence on the formation of active ROP, but of 4 patients with microcephaly 1 (25%) developed the active form of ROP and 3 (75.0%) did not. Neither one of 3 patients with hydrocephaly did not developed active ROP indicating that hydrocephalus has no influence on the formation of active ROP. Of the 15 patients with intraventricular hemorrhage 2 (13.3%) cases developed active ROP as well as 3 (4.6%) patients without intraventricular hemorrhage, indicating that intraventricular hemorrhage has a certain influence on the development of active ROP, but not statistically significant (p>0.05) (Table 4).
Table 4.
Analysis of risk factors impact on active ROP development
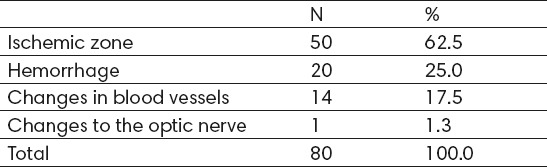
The most common changes observed in the retina were: ischemic zone in 50 (62.5%) cases, bleeding in 20 cases (25%), changes in the blood vessels in 14 (17.5%) cases, and only 1 (1.3%) case the changes in the optic nerve (Table 5)
Table 5.
Changes in the retina observed at baseline examination
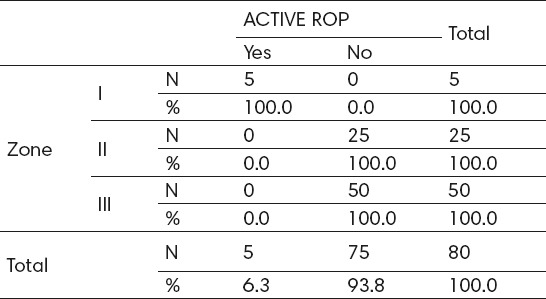
Analysis of patients according to zones revealed the following: From the total number of patients (N = 80), 5 (6.3%) cases were zone I, 25 (31.3%) zone II, and the largest number of 50 (62.5 %) zone III. All patients with active ROP were zone I, while patients in the zone II or III, have not developed active ROP, indicating significant impact of zones on the development of active ROP (p<0.05) (Table 6).
Table 6.
Detected active ROP by screening according to zones. X2=80.0; p=0.000; rho=0.662; p=0.000
Gestational age was statistically significantly higher (p<0.05) in patients with ischemic zone (30.48±2.525) compared to patients without ischemic zone (28.93±3.29), while the birth weight was slightly higher (p>0.05) in patients with ischemic zone (1433.9±352.68 g) as compared to patients without ischemic zone (1402.8±476.84 g). Twin pregnancy was present in 12 (24%) patients and 3 (10%) patients without ischemic zones, or without significant difference (p>0.05). Apgar scores in the first and fifth minute statistically significantly distinguished the presence of ischemic zone. So Apgar during 1 minute in patients with ischemic zone had mean of 4.7±1.55 and in patients without 5.56±1.41, while in 5 minute in patients with ischemic zone Apgar mean was 6.4±1.248 compared to patients without ischemic zone 7.08±0.94. Duration of oxygen therapy in days was significantly different and was longer in patients with 18.37±11.38 compared to patients without ischemic zone–9.78±10.14. The oxygen saturation was not significantly different, although it was slightly lower in patients with–85.15±10.43 compared to patients without ischemic zone–89.83±10.79. Significant difference was observed in the case of hydrocephalus, which was observed in patients with ischemic zone, but was not recorded in 3 (10%) of patients without ischemic zone (p<0.05).
The analysis of the risk factors impact on the incidence of bleeding in the retina was observed statistically significant difference or the impact in the event of hydrocephalus was observed in 3 (15%) patients and none of the patients without bleeding. The analysis of risk factors on changes in the retinal blood vessels indicate a significant effect only in the case of Apgar score at 1 and 5 minutes. Apgar score in the first minute was significantly lower in patients with–4.50±1.51 compared to patients without changes in retinal blood vessels–5.39±1.49. The same trend also follows Apgar score at 5 minutes which on average was 6.21±1.48 in patients with, compared to 0.98±6.95 in the group of patients with no changes in the retinal blood vessels.
Analysis of correlation between risk factors and the development of retinal blood vessels according to zones, the results obtained show significant influence of Apgar score at 1 and 5 minutes, as well as the duration of oxygen therapy. Apgar score in the first minute was the lowest in the patient with zone I–3.20±1.30, the highest for patients in case of zone III–5.54±1.36 with the significant difference (p<0.05). Also Apgar score at 5 minutes, was the lowest in the patients with zone I–5.20±1.09 and the highest for patients with zone III–7.14±0.81. The duration of oxygen therapy was longest for changes in zone II–22.20±12,258, and the lowest for Zone III–7.70±6.53 with the statistically significant difference (p <0.05).
4. DISCUSSION
In this retrospective study we observe a total of 80 preterm children who, according to the relevant criteria, were selected for retinopathy of prematurity screening. Gender structure of respondents indicated the higher representation of female children (51.2%) compared to men (48.8%). Average GA was 29.9±2.9 weeks, of which the youngest child was at GA of 24 weeks and the oldest at 37 weeks of gestation. Average BW was 1422.2±515 grams with lowest weight of 640 and maximum of 3500 grams. The active form of ROP is developed in 5 (6.2%) children which needed interventions in terms of anti-VEGF factors (intravitreal injections of Avastin in 3 cases) application, in one case argon laser photocoagulation and in one case pars plana vitrectomy. In the remaining 75 (93.8%) cases there was a spontaneous disease regression. Patients in the zone I were born the earliest 26.40±1,52 GA and patients in zone III the latest–31.02±2.34 week, with significant statistical difference (p<0.05). Birth weight was also lowest in patients in zone I–874.00±181.05 grams and the highest for patients in zone III–1601.9±433.57 grams with significant statistical difference (p<0.05). Apgar score in the first minute was the lowest for the patient in zone I–3.20±1.30 and the highest for patients in zone III–5.54±1.358 with statistically significant difference (p<0.05). Apgar score at 5 minutes, was the lowest for the patient zone I–5.20±1.095 and the highest for patients in zone III–7.1±0.81 with statistically significant difference (p<0.05). The duration of oxygen therapy was longest for patients in zone II–22.20±12,26 and the lowest for patients in zone III–7.70±6.53 with statistically significant difference (p<0.05).
Thomas K. at all. in their study on 9187 children, found that 1163 (12.7%) infants developed severe ROP. Lower gestational age, male sex, small for gestational age, patent ductus arteriosus, late onset sepsis, more than two blood transfusions, use of inotropes and outborn statues were associated with an increased risk of severe ROP. Younger, smaller and sicker small infants had higher adjusted risks of severe ROP and rates varied significantly among sites (11). Enomoto H at all. found in clinical records of 143 newborn infants with a gestational age of 32 weeks or less were reviewed. Severe ROP was diagnosed when photocoagulation due to progression to stage 3 was identified or when ‘plus disease’ developed. The factors were evaluated with univariate and multivariate logistic regression analyses between the groups with severe (n=24) and non-severe (n=119) ROP. Gestational age, birth weight, duration of oxygen supplementation, duration of directional positive air pressure and maximum fraction of inspiratory oxygen (FiO2) were significantly associated with severe ROP in the univariate analyses. In the multivariate analysis, a longer duration of oxygen supplementation and a higher maximum FiO2 were revealed as significant risk factors associated with severe ROP (12). Park SH at all. determined the incidence and clinical features and risk factors for retinopathy of prematurity in Korean infants with birth weight (BW)> 1500 g. A total of 201 consecutive infants with BW> 1500 g from January 2009 to December 2013 were included. The overall incidence of ROP was 11.94% and that of treatment-requiring ROP was 3.98%. Two patients with gestational age (GA)> 32 weeks and BW>1500 g had treatment-requiring ROP. 15 eyes from eight infants with type I ROP required laser photocoagulation. The mean BWs and gas in the treatment-requiring ROP group were significantly lower than those in the no or mild ROP group. Total duration of oxygen supplementation, surfactant usage, respiratory distress syndrome, bronchopulmonary dysplasia, antibiotic use for more than 14 days and the number of ROPs associated risk factors significantly increased the likelihood of treatment-requiring ROP (p = 0.002, p = 0.008, p = 0.008, p = 0.000, p = 0.015, and p = 0.004, respectively) (13). Yau GS at all. determined the incidence and risk factors of retinopathy of prematurity (ROP) among new-born Chinese infants of multiple Gestations. A retrospective review of medical records was performed for all neonates of multiple Gestations screened for ROP between January 2007 and December 2012 in two neonatal intensive care units in Hong Kong. Screening was offered to very low birth weight (VLBW; ≤1500 g) and / or preterm (≤32 weeks gestation) neonates. The mean gestational age (GA) was 30.8 ± 2.4 weeks and the mean birth weight (BW) was 1284.8 ± 267.4 g. The incidence of ROP and Type 1 ROP was 11.8% and 3.9%, respectively. On univariate analysis, GA younger, lighter birth weight, postnatal hypotension, inotropes use, bronchopulmonary disease, intraventricular hemorrhage and were common independent risk factors for the development of ROP and Type 1 ROP (all P ≤ 0.04),. On multivariate analysis, younger GA, surfactant use, invasive mechanical ventilation, mean higher oxygen concentration, thrombocytopenia, intraventricular hemorrhage, total parental nutrition, and hypoglycemia, were significant risk factors for ROP. For Type 1 ROP, there were no significant risk factors (14). Khalesi N at all.in their study detects possible risk factors for retinopathy of prematurity (ROP), and a leading cause of childhood blindness treatable, among premature neonates. In this retrospective study, 60 premature neonates with ROP and 60 premature infants without ROP were entered and compared. Variables such as gestational age, birth weight, oxygen therapy, phototherapy, and so on were gathered and compared between the two groups. Gestational age (lower in the ROP group), first-minute Apgar scores (lower mean scores in the ROP group), birth weight, phototherapy, and oxygen therapy were discovered factors that affect the occurrence of ROP among premature infants. Higher birth weight and more advanced gestational age were protective factors for ROP. Oxygen therapy and multiple birth are risk factors of ROP and these can be used for prediction of ROP occurrence (16). Kavurt S at all.to evaluate the incidence, risk factors and severity of retinopathy of prematurity and neonatal intensive care unit and to evaluate its relationship with gestational age. Of the 495 neonates screened, 43 (8.7%) infants were small for gestational age; the frequency of severe retinopathy of prematurity was 5.8%. Sepsis and being small for gestational age were independent risk factors for severe retinopathy of prematurity (17).
5. CONCLUSION
The analysis of risk factors that influenced the development of active ROP revealed that statistically significant effect was observed in case of gestational age, birth weight, Apgar score in the first and fifth minute, as well as longer duration of oxygen therapy. Specifically, patients whose blood vessels were in zone I and who later developed the active form of ROP, were born at the earliest gestational age, with the lowest birth weight and lowest Apgar score in the first and fifth minute, and with a maximum duration of oxygen therapy. Timely screening and adequate treatment are essential in the prevention of blindness in children.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Ober RR, Palmer EA, Drack AV, Wright KW. Retinopathy of prematurity. Handbook of pediatric retinal disease. 2006;10:284–338. [Google Scholar]
- 2.Terry TL. Extreme Prematurity and Fibroblastic Overgrowth. Preliminary Report. Am J Ophthamlol. 1942;25:203–204. [Google Scholar]
- 3.Smith LEH. Pathogenesis of retinopathy of prematurity. Growth Hormone & IGF Research 2004. 14:140–144. doi: 10.1016/j.ghir.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Saugstad OD. Oxygen and retinopathy of prematurity. J Perinatol. 2006;26:46–50. doi: 10.1038/sj.jp.7211475. [DOI] [PubMed] [Google Scholar]
- 5.Kinsey VE, Patz A, Stern L. Oxygen and retrolental fibroplasias: in defense of data. Pediatrics. 1978;62:439–440. [PubMed] [Google Scholar]
- 6.Tin W. Oxygen therapy:50 years of uncertainty. Pediatrics. 2002;110:615–616. doi: 10.1542/peds.110.3.615. [DOI] [PubMed] [Google Scholar]
- 7.Darlow BA, Hutchinson JL, Henderson-Smart DJ, Donoghue DA, Simpson JM, Evans NJ. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics. 2005;115:990–996. doi: 10.1542/peds.2004-1309. [DOI] [PubMed] [Google Scholar]
- 8.The Committee for the Classification of Retinopathy of Prematurity: an international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 9.Vatavuk Z, Benčić G, AndrijevićDerk B, Mandić Z. Suvremeni pristup liječenju retinopatije nedonoščadi. Suvremeni dijagnostičko-terapijski postupci u oftalmologiji. Medix, listopad. 2008;78:113–116. [Google Scholar]
- 10.Mutlu FM, Sadici SU. Treatment of retinopathy of prematurity: a review of conventional and promising new therapeutic option. Int J Ophthalmol. 2013;6:228–226. doi: 10.3980/j.issn.2222-3959.2013.02.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas K, Shah PS, Canning R, Harrison A, Lee SK, Dow KE. Retinopathy of prematurity: Risk factors and variability in Canadian neonatal intensive care units. J Neonatal Perinatal Med. 2015 Oct 24;8(3):207–214. doi: 10.3233/NPM-15814128. doi:10.3233/NPM-15814128. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto H, Miki A, Matsumiya W, Honda S. Evaluation of Oxygen Supplementation Status as a Risk Factor Associated with the Development of Severe Retinopathy of Prematurity. Ophthalmologica. 2015;234(3):135–138. doi: 10.1159/000433565. doi:10.1159/000433565. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Yum HR, Kim S, Lee YC. Retinopathy of prematurity in Korean infants with birthweight greater than 1500 g. Br J Ophthalmol. 2015 Oct 19; doi: 10.1136/bjophthalmol-2015-306960. pii: bjophthalmol-2015-306960. doi:10.1136/bjophthalmol-2015-306960. [DOI] [PubMed] [Google Scholar]
- 14.Yau GS, Lee JW, Tam VT, Yip S, Cheng E, Liu CC, Chu BC, Wong IY. Incidence and risk factors for retinopathy of prematurity in multiple gestations: a Chinese population study. Medicine (Baltimore) 2015 May;94(18):e867. doi: 10.1097/MD.0000000000000867. doi:10.1097/MD.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalesi N, Shariat M, Fallahi M, Rostamian G. Evaluation of risk factors for retinopathy in preterm infant: a case-control study in a referral hospital in Iran. Minerva Pediatr. 2015 Jun;67(3):231–237. [PubMed] [Google Scholar]
- 16.Kavurt S, Özcan B, Aydemir O, Bas AY, Demirel N. Risk of retinopathy of prematurity in small for gestational age premature infants. Indian Pediatr. 2014 Oct;51(10):804–806. doi: 10.1007/s13312-014-0506-9. [DOI] [PubMed] [Google Scholar]


