Abstract
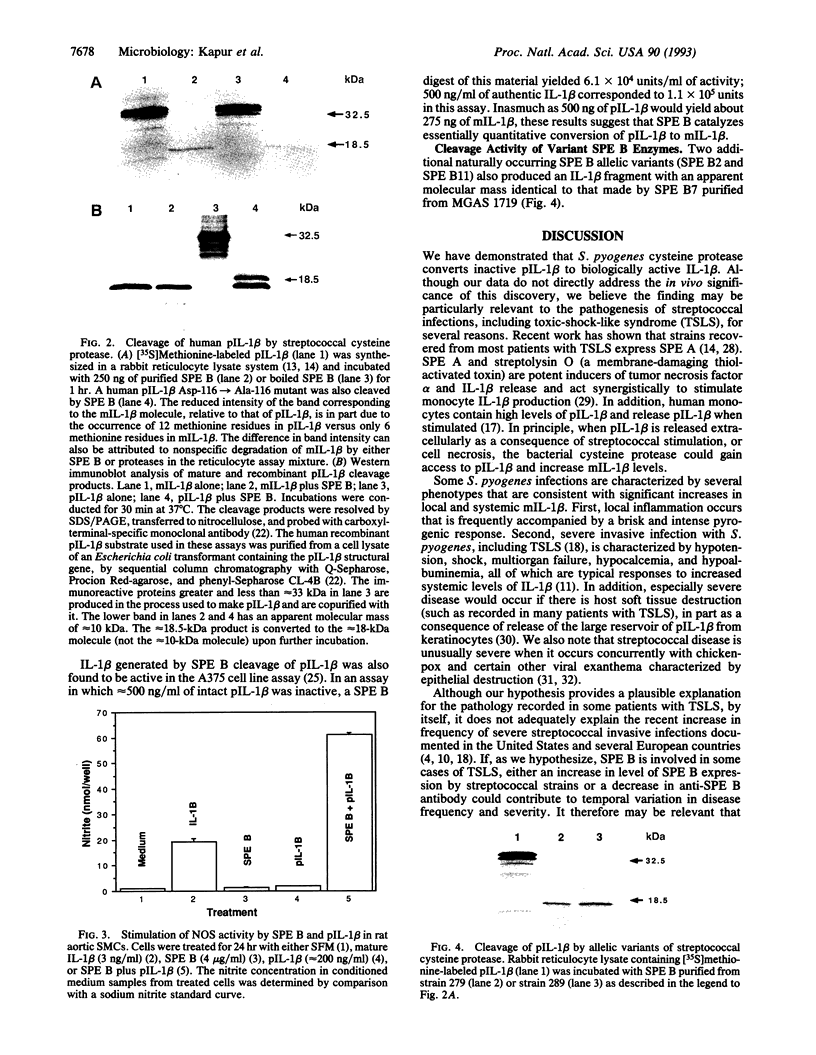
Streptococcal pyrogenic exotoxin B (SPE B), a conserved extracellular cysteine protease expressed by the human pathogenic bacterium Streptococcus pyogenes, was purified and shown to cleave inactive human interleukin 1 beta precursor (pIL-1 beta) to produce biologically active IL-1 beta. SPE B cleaves pIL-1 beta one residue amino-terminal to the site where a recently characterized endogenous human cysteine protease acts. IL-1 beta resulting from cleavage of pIL-1 beta by SPE B induced nitric oxide synthase activity in vascular smooth muscle cells and killed of the human melanoma A375 line. Two additional naturally occurring SPE B variants cleaved pIL-1 beta in a similar fashion. By demonstrating that SPE B catalyzes the formation of biologically active IL-1 beta from inactive pIL-1 beta, our data add a further dimension to an emerging theme in microbial pathogenesis that bacterial and viral virulence factors act directly on host cytokine pathways. The data also contribute to an enlarging literature demonstrating that microbial extracellular cysteine proteases are important in host-parasite interactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcamí A., Smith G. L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992 Oct 2;71(1):153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Björck L., Akesson P., Bohus M., Trojnar J., Abrahamson M., Olafsson I., Grubb A. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature. 1989 Jan 26;337(6205):385–386. doi: 10.1038/337385a0. [DOI] [PubMed] [Google Scholar]
- Black R. A., Kronheim S. R., Cantrell M., Deeley M. C., March C. J., Prickett K. S., Wignall J., Conlon P. J., Cosman D., Hopp T. P. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J Biol Chem. 1988 Jul 5;263(19):9437–9442. [PubMed] [Google Scholar]
- Black R. A., Kronheim S. R., Merriam J. E., March C. J., Hopp T. P. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J Biol Chem. 1989 Apr 5;264(10):5323–5326. [PubMed] [Google Scholar]
- Black R. A., Kronheim S. R., Sleath P. R. Activation of interleukin-1 beta by a co-induced protease. FEBS Lett. 1989 Apr 24;247(2):386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- Campetella O., Martínez J., Cazzulo J. J. A major cysteine proteinase is developmentally regulated in Trypanosoma cruzi. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):145–149. doi: 10.1016/0378-1097(90)90184-r. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Kozlosky C. J., Mosley B., Nelson N., Van Ness K., Greenstreet T. A., March C. J., Kronheim S. R., Druck T., Cannizzaro L. A. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992 Apr 3;256(5053):97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- ELLIOTT S. D. The crystallization and serological differentiation of a streptococcal proteinase and its precursor. J Exp Med. 1950 Sep;92(3):201–218. doi: 10.1084/jem.92.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin A. E., Bouvier J., Sakanari J. A., Craik C. S., McKerrow J. H. Amplification and sequencing of genomic DNA fragments encoding cysteine proteases from protozoan parasites. Mol Biochem Parasitol. 1990 Feb;39(1):1–8. doi: 10.1016/0166-6851(90)90002-4. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Leme V. M., Cancado J. R., Gazzinelli G., Scharfstein J. Identification and partial characterization of Trypanosoma cruzi antigens recognized by T cells and immune sera from patients with Chagas' disease. Infect Immun. 1990 May;58(5):1437–1444. doi: 10.1128/iai.58.5.1437-1444.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach D., Knöll H., Köhler W., Ozegowski J. H., Hríbalova V. Isolation and characterization of erythrogenic toxins. V. Communication: identity of erythrogenic toxin type B and streptococcal proteinase precursor. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Sep;255(2-3):221–233. [PubMed] [Google Scholar]
- Gooding L. R. Virus proteins that counteract host immune defenses. Cell. 1992 Oct 2;71(1):5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hackett S. P., Stevens D. L. Streptococcal toxic shock syndrome: synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J Infect Dis. 1992 May;165(5):879–885. doi: 10.1093/infdis/165.5.879. [DOI] [PubMed] [Google Scholar]
- Hauser A. R., Schlievert P. M. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J Bacteriol. 1990 Aug;172(8):4536–4542. doi: 10.1128/jb.172.8.4536-4542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazuda D. J., Lee J. C., Young P. R. The kinetics of interleukin 1 secretion from activated monocytes. Differences between interleukin 1 alpha and interleukin 1 beta. J Biol Chem. 1988 Jun 15;263(17):8473–8479. [PubMed] [Google Scholar]
- Hellman U., Wernstedt C., Cazzulo J. J. Self-proteolysis of the cysteine proteinase, cruzipain, from Trypanosoma cruzi gives a major fragment corresponding to its carboxy-terminal domain. Mol Biochem Parasitol. 1991 Jan;44(1):15–21. doi: 10.1016/0166-6851(91)90216-s. [DOI] [PubMed] [Google Scholar]
- Hogquist K. A., Nett M. A., Unanue E. R., Chaplin D. D. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. E., Norrby A., Bergholm A. M., Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988-1989. J Infect Dis. 1992 Jul;166(1):31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- KELLNER A., ROBERTSON T. Myocardial necrosis produced in animals by means of crystalline streptococcal proteinase. J Exp Med. 1954 May 1;99(5):495–503. doi: 10.1084/jem.99.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene W. E., Petitt M. G., Allen S., McKerrow J. H. The major neutral proteinase of Entamoeba histolytica. J Exp Med. 1986 Mar 1;163(3):536–549. doi: 10.1084/jem.163.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostura M. J., Tocci M. J., Limjuco G., Chin J., Cameron P., Hillman A. G., Chartrain N. A., Schmidt J. A. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Campetella O., Frasch A. C., Cazzulo J. J. The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is antigenic in human infections. Infect Immun. 1991 Nov;59(11):4275–4277. doi: 10.1128/iai.59.11.4275-4277.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerrow J. H. Parasite proteases. Exp Parasitol. 1989 Jan;68(1):111–115. doi: 10.1016/0014-4894(89)90016-7. [DOI] [PubMed] [Google Scholar]
- Mizutani H., Black R., Kupper T. S. Human keratinocytes produce but do not process pro-interleukin-1 (IL-1) beta. Different strategies of IL-1 production and processing in monocytes and keratinocytes. J Clin Invest. 1991 Mar;87(3):1066–1071. doi: 10.1172/JCI115067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murta A. C., Persechini P. M., Padron T. de S., de Souza W., Guimarães J. A., Scharfstein J. Structural and functional identification of GP57/51 antigen of Trypanosoma cruzi as a cysteine proteinase. Mol Biochem Parasitol. 1990 Nov;43(1):27–38. doi: 10.1016/0166-6851(90)90127-8. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Hauser A. R., Kim M. H., Schlievert P. M., Nelson K., Selander R. K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Kapur V., Kanjilal S., Shah U., Musher D. M., Barg N. L., Johnston K. H., Schlievert P. M., Henrichsen J., Gerlach D. Geographic and temporal distribution and molecular characterization of two highly pathogenic clones of Streptococcus pyogenes expressing allelic variants of pyrogenic exotoxin A (Scarlet fever toxin). J Infect Dis. 1993 Feb;167(2):337–346. doi: 10.1093/infdis/167.2.337. [DOI] [PubMed] [Google Scholar]
- Nakai S., Mizuno K., Kaneta M., Hirai Y. A simple, sensitive bioassay for the detection of interleukin-1 using human melanoma A375 cell line. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1189–1196. doi: 10.1016/0006-291x(88)90266-5. [DOI] [PubMed] [Google Scholar]
- Parmely M., Gale A., Clabaugh M., Horvat R., Zhou W. W. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect Immun. 1990 Sep;58(9):3009–3014. doi: 10.1128/iai.58.9.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C. A., Black R. A., Kronheim S. R., Greenstreet T. A., Sleath P. R., Salvesen G. S., Pickup D. J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992 May 15;69(4):597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Reed S. L., Keene W. E., McKerrow J. H. Thiol proteinase expression and pathogenicity of Entamoeba histolytica. J Clin Microbiol. 1989 Dec;27(12):2772–2777. doi: 10.1128/jcm.27.12.2772-2777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Burden T., Schini V. B., Elizondo E., Junquero D. C., Vanhoutte P. M. Platelet-derived growth factor suppresses and fibroblast growth factor enhances cytokine-induced production of nitric oxide by cultured smooth muscle cells. Effects on cell proliferation. Circ Res. 1992 Nov;71(5):1088–1100. doi: 10.1161/01.res.71.5.1088. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Hruby D. E., Maliszewski C. R., Pickup D. J., Sims J. E., Buller R. M., VanSlyke J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992 Oct 2;71(1):145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- Stevens D. L. Invasive group A streptococcus infections. Clin Infect Dis. 1992 Jan;14(1):2–11. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992 Apr 30;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Upton C., Mossman K., McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992 Nov 20;258(5086):1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- Yu C. E., Ferretti J. J. Frequency of the erythrogenic toxin B and C genes (speB and speC) among clinical isolates of group A streptococci. Infect Immun. 1991 Jan;59(1):211–215. doi: 10.1128/iai.59.1.211-215.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. E., Ferretti J. J. Molecular epidemiologic analysis of the type A streptococcal exotoxin (erythrogenic toxin) gene (speA) in clinical Streptococcus pyogenes strains. Infect Immun. 1989 Dec;57(12):3715–3719. doi: 10.1128/iai.57.12.3715-3719.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]