Summary
Third-generation mutant-specific EGFR tyrosine kinase inhibitors are showing robust clinical activity, particularly in lung cancers harboring the EGFRT790M mutation, yet acquired resistance to these agents emerges. Additional mutations in EGFR can confer resistance that, depending on their genomic context, can determine new drug sensitivities of the cancer cells.
In this issue of Clinical Cancer Research, studies by Ercan and colleagues and by Niederst and colleagues identify and characterize three mutations in the EGFR that confer resistance to 3rd generation EGFR tyrosine kinase inhibitors (TKIs) in preclinical models (1, 2). Patients with EGFR mutant lung tumors harboring either an exon 19 deletion or L858R point mutation typically respond to 1st generation, reversible TKIs (i.e., erlotinib or gefitinib), but these tumors are notorious for developing resistance on average 1 year after the beginning of treatment. In half of the resistant cases, a secondary EGFR mutation, EGFRT790M, emerges that confers resistance to these drugs (3). Given its high frequency, strategies to inhibit EGFRT790M have been at the forefront of drug discovery efforts. For instance, the second generation quinazoline irreversible EGFR TKI, afatinib, also approved for first-line treatment of EGFR mutant lung cancer, demonstrates inhibitory capacities against EGFRT790M; however the high concentrations required to inhibit T790M limit its use clinically for this purpose.
Third-generation mutant-specific EGFR inhibitors such as AZD9291, rociletinib (CO-1686) and the tool compound WZ4002, have emerged as new strategies to overcome resistance mediated by EGFRT790M (4-6). In addition to their heightened potency against EGFRT790M, these irreversible covalent pyrimidine-based compounds also inhibit TKI-sensitive mutants (6). Furthermore, they selectively inhibit mutant over wild-type EGFR, and thus have significantly lower toxicities compared to other EGFR TKIs. Two recent studies reported the results of the early phase clinical trials of AZD9291 and rociletinib in patients with TKI-resistant EGFR mutant tumors (7, 8). In tumors harboring the T790M mutation, AZD9291 and rociletinib elicited response rates of 61% and 59%, respectively. In T790M negative tumors more modest response rates of 21% (with AZD9291) and 29% (with rociletinib) were observed.
Despite the impressive results with 3rd-generation EGFR inhibitors, emerging clinical data reveal that acquired resistance to these compounds develops. Very little is known clinically about the mechanisms of resistance to these new agents. Moreover, it is still early to know how closely findings in the clinic will mirror the preclinical studies presented in these papers. To date, in a small series of patients, a tertiary mutation in EGFR, the C797S mutation has been found in ~40% of AZD9291-resistant T790M positive tumors (9). This mutation has not been reported in rociletinib-resistant tumors (10) highlighting potential differences between the two drugs.
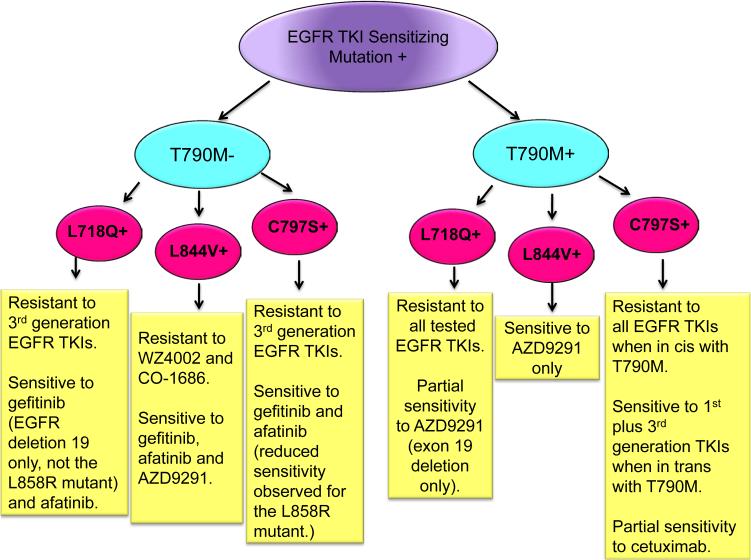
To identify mechanisms of resistance to 3rd generation EGFR inhibitors, Ercan and colleagues performed an N-ethyl-N-nitrosourea (ENU) mutagenesis screen in BAF3 cells expressing EGFR sensitizing mutations with and without the T790M mutation. They identified three tertiary mutations, namely EGFR L718Q, L844V and C797S that conferred resistance to WZ4002. Interestingly, regardless of the genomic context, these mutations conferred resistance to WZ4002 and rociletinib. However, the sensitivity of cells harboring the tertiary mutations to AZD9291 and afatinib, was found to depend on 3 factors: the nature of the original sensitizing EGFR mutation, the presence or absence of EGFRT790M and the identity of the tertiary mutation (Fig. 1). For example, AZD9291 retained activity against L844V (irrespective of the presence of EGFRT790M) but only in certain contexts against L718Q and not against C797S raising the possibility that it may be useful if patients are found to develop the L844V mutation (and possibly L718Q) following rociletinib treatment (1). Remarkably, in most cases, cells with an EGFR TKI sensitizing mutation, without EGFRT790M and with one of these tertiary mutations retain sensitivity to 1st/2nd-generation inhibitors suggesting that these may be useful for treatment of tumors with these genotypes. Cells containing the T790M mutation and the tertiary C797S mutation were the most resistant to known EGFR TKIs. To explore alternative approaches for targeting EGFR, the authors tested the sensitivity of cells with triple mutations to the EGFR antibody, cetuximab and found that L858R/T790M/C797S positive cells exhibited partial sensitivity to this drug.
Figure 1.
Sensitivity of cells containing different combinations of primary, secondary and tertiary mutations in EGFR. A schematic diagram of primary EGFR TKI-sensitizing mutations [EGFR exon 19 DEL or L858R (purple bubble)] in the presence or absence of the secondary EGFRT790M mutation (green bubbles) and with different tertiary mutations (red bubbles) is shown. Possible combinations of EGFR mutations and the sensitivities of these mutants to different generations of TKIs based on preclinical studies are shown.
Niederst and colleagues used a different approach and cultured patient-derived erlotinib-resistant T790M positive tumor cells with increasing concentrations of WZ4002 until resistance emerged. Comparison of sequencing data from resistant clones to the parental TKI-sensitive counterparts revealed the presence of the C797S mutation. These cells were resistant to all generations of EGFR TKIs and were found to harbor T790M and C797S in cis. Interestingly, transfection experiments determined that when these mutations are in trans, cells are sensitive to a combination of a first and third generation TKI (Fig. 1). Finally, consistent with the findings by Ercan et al., when C797S occurs in the absence of T790M, resistance to 3rd-generation inhibitors is observed but sensitivity to 1st-generation inhibitors remains (2). In fact, it has been shown that erlotinib can suppress EGFR bearing the C797 mutation (11).
Prior experience with resistance to 1st-generation EGFR TKIs has taught us that understanding resistance mechanisms can be critical to determining ways in which to overcome it, and we are now starting to see that the same may be true with resistance to the 3rd-generation inhibitors. The studies presented in this edition of CCR reveal that whether the EGFR tertiary mutations are found in the presence or absence of the T790M resistance mutation, and even more specifically on the same or different allele, may impact which (if any) EGFR inhibitors are likely to be effective. These data also suggest that in certain cases, combination therapies including a 1st-generation plus a 3rd-generation EGFR inhibitor, or an EGFR TKI plus the EGFR antibody cetuximab may be more effective depending on the molecular findings at resistance. This is remarkably powerful information that may impact clinical decision-making if these mutations are identified and the drug sensitivities of the mutants are confirmed in patients.
Many studies have now clearly demonstrated the importance of repeat biopsies at the time of resistance to EGFR inhibitors. Over the last few years, the detection of the T790M mutation after the development of resistance to 1st-generation TKIs has led many patients to clinical trials of 3rd-generation inhibitors, which have shown significant efficacy. Whether the same is true after resistance to 3rd-generation inhibitors remains to be seen, however it is an area that is worth pursuing.
The findings in these papers raise questions regarding appropriate treatments and mechanisms of resistance in EGFR-mutant NSCLC. First, how will we sequence these agents in the clinic? We now have several EGFR TKIs approved for use in this patient population, and there may be more in the future. Trials are currently ongoing to compare 1st-generation with 3rd-generation EGFR inhibitors in TKI-naïve patients, and it will be critical to not only determine the clinical efficacy but also the mechanisms of resistance to these drugs when used in this setting. Second, will combination strategies surpass single-agent treatments in this patient population? The studies by Ercan and Niederst lend weight to combining multiple generations of EGFR TKIs as well as EGFR TKIs plus EGFR antibodies in certain situations, yet it is still not evident clinically when this may be useful. We have seen in many illnesses including cancers and infectious diseases that multi-drug combinations can lead to cures more than sequential use of single agents. Clinical trials to test the value of such approaches in this disease and that incorporate pre- and post-treatment biopsies will be required to further improve the treatment of EGFR-mutant lung cancer.
Acknowledgments
Grant Support
D. Ayeni is supported by a predoctoral fellowship from the National Science Foundation (DGE-1122492). K. Politi is supported by the NCI of the NIH under award numbers R01CA120247, R01 CA121210, and R01CA195720; the U.S. Department of Defense; and Yale University. S.B. Goldberg is supported by the U.S. Department of Defense and the Hope Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
K. Politi reports receiving a commercial research grant from AstraZeneca and Kolltan; is listed as an inventor on a patent application for EGFRT790M mutation testing, which is licensed to MolecularMD by Memorial Sloan Kettering Cancer Center; and is a consultant/advisory board member for the National Comprehensive Cancer Network (NCCN) and Takeda. S.B. Goldberg reports receiving a commercial research grant from AstraZeneca and is a consultant/advisory board member for Clovis Oncology. No potential conflicts of interest were disclosed by the other author.
References
- 1.Ercan D, Choi HG, Yun C- H, Capelletti M, Xie T, Eck MJ, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res. 2015 May 6; doi: 10.1158/1078-0432.CCR-14-2789. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niederst MJ, Hu H, Mulvey HE, Lockerman EL, Garcia AR, Piotrowska Z, et al. The allelic context of the C797S mutation acquired upon treatment with third generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res. 2015 May 11; doi: 10.1158/1078-0432.CCR-15-0560. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–61. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter AO, Sjin RT, Haringsma HJ, Ohashi K, Sun J, Lee K, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov. 2013;3:1404–15. doi: 10.1158/2159-8290.CD-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009;462:1070–4. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–99. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 8.Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372:1700–9. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 9.Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nature Med. 2015;21:560–2. doi: 10.1038/nm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piotrowska Z, Niederst MJ, Karlovich CA, Wakelee HA, Neal JW, Mino-Kenudson M, et al. Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of T790M-positive cancers with a third-generation EGFR inhibitor. Cancer Discov. 2015;5:1–10. doi: 10.1158/2159-8290.CD-15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godin-Heymann N, Ulkus L, Brannigan BW, McDermott U, Lamb J, Maheswaran S, et al. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol Cancer Ther. 2008;7:874–9. doi: 10.1158/1535-7163.MCT-07-2387. [DOI] [PubMed] [Google Scholar]