Abstract
Apoptosis or programmed cell death is natural way of removing aged cells from the body. Most of the anti-cancer therapies trigger apoptosis induction and related cell death networks to eliminate malignant cells. However, in cancer, de-regulated apoptotic signaling, particularly the activation of an anti-apoptotic systems, allows cancer cells to escape this program leading to uncontrolled proliferation resulting in tumor survival, therapeutic resistance and recurrence of cancer. This resistance is a complicated phenomenon that emanates from the interactions of various molecules and signaling pathways. In this comprehensive review we discuss the various factors contributing to apoptosis resistance in cancers. The key resistance targets that are discussed include (1) Bcl-2 and Mcl-1 proteins; (2) autophagy processes; (3) necrosis and necroptosis; (4) heat shock protein signaling; (5) the proteasome pathway; (6) epigenetic mechanisms; and (7) aberrant nuclear export signaling. The shortcomings of current therapeutic modalities are highlighted and a broad spectrum strategy using approaches including (a) gossypol; (b) epigallocatechin-3-gallate; (c) UMI-77 (d) triptolide and (e) selinexor that can be used to overcome cell death resistance is presented. This review provides a roadmap for the design of successful anti-cancer strategies that overcome resistance to apoptosis for better therapeutic outcome in patients with cancer.
Keywords: Apoptosis, Necrosis, Autophagy, Apoptosis evasion, Nuclear transporters, natural, chemopreventive agents
1. Introduction
According to the GLOBOCAN factsheet (http://globocan.iarc.fr/), there were approximately 14.1 million new cancer cases, 8.2 million cancer deaths and 32.6 million people living with cancer (within 5 years of diagnosis) in the year 2012 worldwide. Among these, 57% (8 million) of new cancer cases, 65% (5.3 million) of the cancer deaths and 48% (15.6 million) of the 5-year prevalent cancer cases occurred in the less/under-developed regions of the world. Cancer treatment requires a careful selection of one or more interventions, such as surgery, radiotherapy, and chemotherapy. However, despite major advances in new targeted drug development and tailored clinical trial designs, therapy resistance is commonly observed in most cancers.
Most cancers harbor significant genetic heterogeneity [1], and patterns of relapse following many therapies are due to evolved resistance to treatment. While efforts have been made to combine targeted therapies, a lack of success, rising drug costs, and significant levels of toxicity have stymied efforts to effectively treat cancer with multi-drug combinations using currently approved therapeutics [2]. Therefore, overcoming therapy resistance mechanisms is one of the most sought-after goals in cancer research.
To accomplish this goal, a non-profit organization entitled Getting to Know Cancer launched an initiative called “The Halifax Project in 2011 with the aim of producing a series of overarching reviews in each of the areas that are widely considered to be cancer hallmarks [3]. This novel approach is premised on many of the insights of genomic sequencing in cancers and it aims to target many disease-specific pathways simultaneously. This is proposed to be done by using low-cost chemistry with little to no toxicity – to address this heterogeneity (in contrast to the limited number of actionable targets that have become the norm in combination chemotherapy).
Our task in the project was to assess the many target choices that exist for resistance to cell death, and to identify up to ten important targets as well as prospective non-toxic approaches that could potentially be combined to produce a low-toxicity therapeutic approach for this area of concern. So our intent here is to discuss the inter-relationship between major mechanisms driving resistance to apoptosis in cancer and then to define a broad-spectrum therapeutic approach aimed at reaching these important targets [3]. In theory, this approach would then be combined with similar approaches being recommended for the other hallmark areas under review in this special issue.
Apoptosis or programmed cell death is evolutionarily conserved process that plays an essential role in organism development and tissue homeostasis [4]. However, in pathological conditions, particularly cancer, cells lose their ability to undergo apoptosis induced death leading to uncontrolled proliferation. Cancer cells are often found to over express many of the proteins that play important roles in resisting the activation of apoptotic cascade. Several mechanisms allow cells to escape programmed cell death and among them is the over expression of the anti-apoptotic molecules. Originally most of the research on apoptosis signaling focused on BH3 pathway proteins leading to acceptance of the Korsmeyers [5] rheostat model. This model predicted a balance between pro-survival and pro-death BH3 members. When the balance shifts toward pro-death signaling, apoptosis occurs, and in instances when pro-survival molecules outnumber pro-death proteins, survival signaling is activated leading to pathological conditions such as cancer and other diseases. With this simplistic model, the drug discovery arena moved at a rapid pace developing small molecule inhibitors (SMI) that interfere with the anti-apoptotic pathways proteins such a B-Cell Lymphoma 2 (Bcl-2), B-Cell Lymphoma extra large (Bcl-xL, Induced myeloid leukemia cell differentiation protein (Mcl-1), Bcl-2-like-protein-2 (BCL2L2/Bcl-w) and Bcl-2 related protein A1 (A1/Bfl1). Nevertheless, most of these approaches have shown little success, and in almost all instances tumor cells become resistant to such apoptosis inducing drugs [6].
Emerging evidence shows that resistance to apoptosis is multi-factorial and involves many secondary players that run either parallel to Bcl-2 signaling or function in complete independence [7]. Here we review the known and emerging pathways that modulate the apoptosis signaling and discuss strategies to overcome apoptosis resistance. We anticipate that a comprehensive understanding of the resistance molecules (and the strategies to target them) will help in the design of clinically successful strategies for cancer in general and specifically in patients who show disease relapse.
2. Role of Bcl-2 family proteins in resistance to apoptosis
One of the major hallmarks of human cancers is the intrinsic or acquired resistance to apoptosis [8,9]. Evasion of apoptosis may contribute to tumor development, progression, and also to treatment resistance, since most of the anticancer therapies that are currently available include chemotherapy, and radio- and immunotherapy (which primarily act by activating cell death pathways i.e., apoptosis in cancer cells). Hence, a better understanding of the molecular mechanisms underlying tumor resistance to apoptotic cell death is expected to provide the basis for a rational approach for the development of molecular targeted therapies.
There are two types of apoptosis programs i.e., intrinsic and extrinsic. The Bcl-2 protein functions through hetero-dimerization with pro-apoptotic members of the BH3 family to prevent mitochondrial pore formation and prevent cytochrome c release and initiation of apoptosis [10] (Fig. 1). However, there is evidence suggesting that Bcl-2 may play an oncogenic role through survival pathways other than its function at the mitochondrial membrane [11]. It has been reported that Bcl-2 activates nuclear factor-κB (NF-κB) by a signaling mechanism that involves Raf-1/MEKK-1-mediated activation of inhibitor of NF-κB kinase subunit beta (IKKβ) [12]. Mortenson and colleagues [13] have shown that overexpression of Bcl-2 increases the activity of AKT and IKK as well as NF-κB transcriptional activity in cancer. While Kumar and colleagues [14] found that Bcl-2-induced tumor cell proliferation and tumor cell invasion were significantly mediated by interleukin-8. Recently, Tucker and colleagues [15] reported that Bcl-2 overexpression leads to the maintenance of cyclin D1a expression, an activity that may occur through p38 mitogen-activated protein kinase (MAPK)-mediated signaling pathways in human lymphoma cell lines. Moreover, down-regulation of Bcl-2 could also modulate the expression of carbonic anhydrase IX (CAIX), vascular endothelial growth factor (VEGF), and pAKT in prostate cancer cell lines [16]. These studies provide evidence in support for a multi-functional role of Bcl-2 in cancer biology that extends beyond its classical role in cell survival. However, even though these early studies encouraged the application of Bcl-2 targeted drugs in a clinical setting, most of the ensuing trials have been rather disappointing [17]. Probably, the drugs are unable to target the entire set of anti-apoptotic proteins or the inhibition efficiency is not robust. Thus, new molecular targets and novel concepts of combination therapies need to gain access into clinical trials – either in neoadjuvant/adjuvant or in palliative treatments.
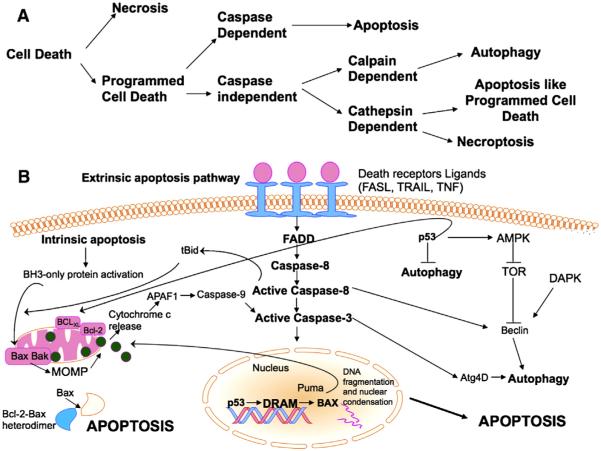
Fig. 1.
The apoptosis pathway: (A) The different paths that a cell can take during the activation of cell death. (B) Apoptosis can be triggered either by external receptor-dependent stimulus (extrinsic) or through internal (intrinsic) mitochondria-mediated signaling. The extrinsic pathway is initiated by the attachment of death receptors with their death initiating ligands, such as FASL, TRAIL or TNF. Consequently, an adaptor molecule, FADD also known as FAS-associated death domain protein, couples the death receptors and this subsequently leads to the activation of caspase-8. Activated caspase-8 can either directly cleave and activate caspase-7 or caspase-3, thereby promoting apoptosis. On the other hand the intrinsic pathway is modulated by the activation of BH3-only proteins sensing different types of cell stress, such as DNA damage or ER stress, and then activating BAX/BAK at mitochondrial outer membrane (MOM). MOM permeabilization (MOMP) leads to release of different apoptosis-mediating molecules, such as cytochrome c, which activates caspase-9. In turn, caspase-9 cleaves and activates caspase-3 and caspase-7, thus triggering apoptotic cell death. Both pathways interface at the point of caspase-3 activation. The formation of autophagosome formation requires activation of Beclin 1 which acts as a component of a multiprotein (PI3K) complex. The crosstalk between autophagy and apoptosis is mediated at least in part by the functional and structural interaction between Beclin 1 and the anti-apoptotic proteins BCL-2 and BCL-XL. Diverse apoptotic stimuli either intrinsic or extrinsic can lead to caspase-mediated cleavage of Beclin 1 rendering it ineffective as an autophagy inducer. The master tumor suppressor p53 has essential roles in both apoptosis and autophagy. At the transcriptional level, p53 upregulates BAX, PUMA and BID or reduces the expression of BCL-2, which antagonizes BAX. In addition to apoptosis, p53 can also induce autophagy through TOR inhibition and also through transcriptional activation of DRAM.
Apoptosis is also deliberated as a stress induced process of cellular communication [18]. In addition to intrinsic and extrinsic processes, there is another pathway that involves T-cell mediated cytotoxicity and perforin-granzyme-dependent killing of the cell where granzyme B and granzyme A proteases are responsible for inducing cell death in this pathway [19]. These intrinsic, extrinsic, and granzyme B have different modes of initiation but have the same outcome: they lead to the activation of a cascade of proteolytic enzymes, members of caspase family [20]. Granzyme A, a serine protease, causes cell death by DNA damage by single-stranded nicks, independent of caspases [21]. The mitochondrial (intrinsic) pathway is regulated by the Bcl-2 family and activated by mitochondrial disruption with subsequent cytochrome c release. Initiators of this pathway include UV irradiation and cytotoxic drugs. An apoptosome is formed by the interaction of cytochrome c, Apaf-1, d-ATP/ATP and procaspase-9 with subsequent initiation of the caspase cascade [22]. Over-expression of Bcl-2 and associated anti-apoptotic proteins Bcl-xL, Mcl-1, A1/Bf1 and Bcl-w occurs in substantial subsets of common cancer types that include pancreatic, ovarian, lymphoma, multiple myeloma, lung adenocarcinoma, prostate adenocarcinoma and others. These Bcl-2 proteins can essentially make cancer cells resistant to a variety of chemotherapeutic agents and therefore these proteins are currently important targets for the development of new anti-cancer agents [23,24].
Myeloid cell leukemia-1 (Mcl-1) is a potent anti-apoptotic protein, a member of the pro-survival Bcl-2 family, its role is emerging as a critical survival factor in a broad range of human cancers [25]. Functional studies have confirmed that Mcl-1 is capable of blocking apoptosis induced by various apoptotic stimuli, including chemotherapy and radiation [26]. Mcl-1 is highly expressed at the protein level in cancer cells and is associated with resistance to chemotherapeutic agents [27]. It has been demonstrated that down-regulation of Mcl-1 enhances the induction of apoptosis and sensitivity of cancer to chemotherapy and radiation [28]. Thus, Mcl-1 represents attractive molecular target for development of a new class of cancer therapy for treatment of cancer.
The most potent small molecule inhibitors of the Bcl-2 subfamily described to date are the Bad-like BH3 mimetics [29]. ABT-737, one of these mimetics, binds with high affinity (Ki 1 nM) to Bcl-2, Bcl-xL and Bcl-w but fails to bind to Mcl-1. It has been demonstrated that resistance to ABT-737 is linked to high expression levels of Mcl-1 and in many instances this resistance can be overcome by treatment with agents that down-regulate, destabilize, or inactivate Mcl-1 [30]. It was also shown that knockdown of Mcl-1 sensitizes human pancreatic cancer cells to ABT-737-induced apoptosis, indicating that Mcl-1 is a relevant therapeutic target in these cancer cells [31]. Recently, a small molecule agent that is a specific inhibitor of Mcl-1 has been developed [32]. The agents showed activity against a panel of pancreatic and other cancer cell lines and works through disruption of interaction between Mcl-1-Bax and Mcl-1-Bak with remarkable activity against sub-cutaneous xenograft models of pancreatic cancer.
Highlighting the importance of apoptotic proteins, recently Stuart Schreiber’s group [33] performed genomic and lineage cancer cell line (CCL) profiling to identify cancer dependencies that are targetable with small molecules and suggested combinations of compounds that mitigate apoptosis resistance. Their Cancer Therapeutic Response Portal (CTRP) suggests candidate dependencies associated with common oncogenes. The first version of the CTRP resulted from the profiling of an Informer Set of Small Molecules (ISSM), many of which target non-altered proteins that work in partnership with oncogenes. Exploiting oncogene-induced dependencies contrasts to an approach based on cancer “hallmarks” [3,34] without first linking these “nononcogene addictions” to specific genomic alterations. For example, navitoclax has been tested in phase I/II clinical trials for small-cell lung cancer [35]; however, the data suggested that navitoclax might best be targeted to patients harboring catenin cadherin-associated protein β 1 (CTNNB1) mutations, which are present in colorectal, hepatocellular, gastric, and endometrial cancers. Indeed it was demonstrated that CTNNB1 mutant CCLs are sensitive to navitoclax in several lineages, though more strongly in some (e.g., gastric) than others. The same selectivity was not observed for ABT-199, a Bcl-2-specific inhibitor [36], suggesting that inhibition of other Bcl-2 family members underlies the differential response. Consistently, Rosenbluh et al. [37] recently showed that knockdown of Bcl-xL in β-catenin-active CCLs impairs proliferation, implicating Bcl-xL as a relevant target for navitoclax in CTNNB1 mutant cancers. So drug specificity and selectivity is significant, however specificity should be broad to cover secondary targets whose presence can lead to resistance to the initial compound. Such is the argument for a “pan-Bcl-2” SMI – a compound which may not bind to all of its targets in the low nanomolar range, but binds to at least Bcl-2 and Mcl-1 to disarm the pro-survival capacities of these key targets. As such, “dirty drugs” may prove useful to knock out a range of Bcl-2-family members. This is the reason why natural compound gossypol from cotton seed has attracted attention for it Bcl-2 inhibitory capacity and has been developed as an anti-cancer agent for clinical application.
3. Autophagy and resistance of cancer
Autophagy serves to maintain intracellular homeostasis through a process that involves lysosomal degradation and recycling of unnecessary or damaged cellular components, and in turn promotes cell survival. Autophagy can prevent cellular damage caused by chemotherapeutics as it attempts to maintain intracellular balance through removal of dysfunctional organelles and eliminating cellular stress. It has been suggested that this temporal survival mechanism may facilitate chemoresistance as a byproduct of its function in keeping the cancer cells alive [38]. Indeed, autophagy has been observed to guard cancer cells against apoptosis upon encountering certain anticancer drugs [39–41]. The majority of relevant preclinical studies using numerous chemotherapeutics including vorinostat, cyclophosphamide, and imatinib have demonstrated that autophagy significantly inhibits the efficacy of several classes of anticancer agents and helps to drive the acquired resistance [42–44]. Furthermore, accumulating evidence has indicated that autophagy is involved in adaptation of cancer cells to chemotherapy [45,46]. These observations suggest that under chemotherapeutic treatments, autophagy is often activated as a cytoprotective mechanism for tumor cell to survive the effects of anticancer drugs which, in turn, may drive chemoresistance. Therefore, the effects of chemotherapy might be improved by inhibiting cytoprotective autophagy to enhance the apoptosis of cancer cells in response to anticancer drugs.
In contrast to its cytoprotective role, autophagy can also lead to cell death (termed “type II programmed cell death”) when induced by excessive cellular stress [47]. More importantly, autophagy-mediated cell death can participate in the upregulation of apoptosis [48,49], and the inhibition of autophagy has been observed to reduce apoptosis in some cancer cells [50]. In this regard, apoptosis and autophagic death may engage each other and share common mechanisms to polarize cellular death response. Furthermore, inhibition of caspase 8 can cause the subsequent activation of autophagy related gene (Atg) Atg6-Atg7-dependent cell death pathway [51], suggesting that autophagy-mediated cell death may serve as a backup mechanism for cell demise in the absence of apoptotic signaling. Due to its paradoxical role in both cell survival and cell death, the outcome of autophagy in cancer cells treated with chemotherapeutic drugs may therefore depend on the type of cancer and stimuli, the progress of tumorigenesis, and the apoptotic status of the cancer cells [52]. As the key mediators in the autophagic process are either products of tumor suppressor genes or oncogenes that often cross the regulatory pathways of apoptosis, clarifying their function during anticancer drug treatment may also help better understand the process of autophagy-driven chemoresistance.
Cell fate dictated by autophagy is regulated by several factors including beclin-1, PI3K, mTOR, Bcl-2, and p53, which are associated with many human disorders [53]. Beclin-1, also called Atg6, is a Bcl-2-homology domain 3 (BH3) protein, that interacts with Vps34 (a class III PI3K), Vps15 (a myristoylated kinase), and UV irradiation resistance-associated tumor suppressor gene (UVRAG) to form a multi-protein interactome that controls the initiation of autophagy, the autophagosome nucleation [54]. Negative regulators to this process include the anti-apoptotic proteins of the Bcl-2 family, which can interact with beclin-1 and inhibit the function of the Vps-UVRAG-beclin-1 multi-protein core complex [55].
It has been suggested that increased apoptosis resistance via anti-apoptotic Bcl-2 family members such as Bcl-2, Bcl-xL, and Mcl-1 can inhibit autophagy induced by chemotherapy, most likely in an attempt to protect cells from the autophagic cell death, and by forming inhibitory complexes with beclin-1 [56]. For example, sorafenib-activated autophagic death in hepatocellular carcinoma (HCC) cells is mediated by the reduced expression of Mcl-1. On the contrary, apogossypolone, a potent anticancer agent that inhibits Bcl-2 and Bcl-xL, has been shown to abrogate the interaction between beclin-1 and Bcl-2/xL and induces protective autophagy in HCC cells [57]. The role of beclin-1 interactome in the crosstalk between apoptosis and autophagy thus emphasizes that disturbances in beclin-1-dependent autophagy can have crucial impact on the apoptotic resistance in chemotherapy.
The ADP ribosylation factor (ARF) tumor suppressor is expressed and accumulated in response to mitogenic stimuli conveyed by oncogenic signals. The majority of ARF localizes to the nucleolus and nucleoplasm, where it antagonizes the E3 ubiquitin ligase muring double minute 2 (MDM2) to provoke MDM2 degradation, thereby stabilizing p53 protein [58,59]. A minor fraction of ARF (smARF) with a smaller molecular weight variant that lacks the nucleolar localization sequence and preferentially localizes to mitochondria, has been shown to induce autophagy [60,61]. Once localized to the mitochondria, smARF can bind to Bcl-xL and disrupt the complex formation of beclin-1 with Bcl-xL, leading to an increased ability of beclin-1 to perform its autophagic functions [62,63]. Heat shock protein 70 (hsp70), a molecular chaperone that is highly expressed in tumor cells, interacts with smARF and regulates the mitochondrial trafficking of smARF, thereby acting as a critical regulator of ARF-mediated autophagy [64]. Recently, ARF has also been identified as a marker for advanced tumors and a lack of ARF function is associated with the inhibition of p53 tumor suppressor function in response to DNA damaging agents as well as ionizing radiation [65,66]. Given that smARF is tightly regulated by proteasomal degradation and preferentially stabilized by metabolic stress, it is speculated that it plays both a pro-survival role and a cytoprotective role in autophagy induced by stress.
Mounting evidence also indicates that ROS are implicated in autophagy induction in cancer therapy. Importantly, the deregulation of ROS formation during autophagic response is associated with cancer initiation, progression, and drug resistance [67,68]. ROS, especially mitochondrial ROS, serve as signaling molecules in inducing autophagy [69]. Atg4, a cysteine protease which cleaves Atg8/LC3 from the outer membrane of autophagosome, is targeted and inactivated by ROS, leading to the lipidation of autophagic process [70]. At the same time, autophagy contributes to the regulation of cellular ROS production by eliminating damaged organelles that may produce high levels of ROS which, in turn, limits chromosomal instability [71].
The selective clearance of damaged mitochondria or mitochondrial autophagy (“mitophagy”) has therefore been suggested to represent an adaptive response to oxidative stress-mediated cell death in several cancer cell lines, mitigating accumulated ROS and protecting cell integrity through the removal of ROS-leaking mitochondria [72]. For instance, anticancer drugs such as 5-fluorouracil (5-FU) elicit protective autophagy against cell death, and the inhibition of autophagy could enhance apoptosis via ROS formation.
Suppressed expression of essential autophagic genes such as beclin-1 and ATG5 has also been observed to enhance the oxaliplatin-induced ROS production and cell death in Caco-2 cells, indicating the role of cytoprotective autophagy in eliminating ROS-induced cell death [73]. Furthermore, ROS-induced DNA damage activates the enzymatic activity of poly(ADP-ribose) polymerase-1(PARP-1), which has been demonstrated to induce protective autophagy through the AMP-activated protein kinase (AMPK) pathway and it instigates the resistance of cancer cells to ROS production by chemotherapeutic drugs [74–76]. Thus, modulating the sensitivity and regulatory mechanism of cells to ROS, such as through the use of antioxidants, could potentially serve as a strategy in chemosensitizing and reducing resistance of cancer cells by blocking ROS-mediated protective autophagy. Evidence of autophagy responding to treatments in cancer cells supports the view that this physiological process can help tumors escape drug-induced cytotoxicity (as a survival mechanism) or potentiate chemotherapeutic efficacy (as a cell death pathway). Selective inhibition or induction of autophagy may therefore help sensitize resistant tumor cells to chemotherapeutic treatments.
4. In vitro and in vivo necrotic cell death and necroptosis
Necrotic cell death is a cell death process that is morphologically characterized by a gain in cell volume, swelling of organelles, plasma membrane rupture and subsequent loss of intracellular contents. This is in contrast to programmed cell death (apoptosis), although it was long thought that necrosis is an uncontrolled cell death that is characterized by progressive loss of cytoplasmic membrane integrity, rapid influx of Na+, Ca2+, and water, resulting in cytoplasmic swelling and nuclear pyknosis [77]. However, there is now an increasing realization that necrotic cell death represents a more programmed form of necrosis, termed necroptosis [78]. The former leads to cellular fragmentation and the release of lysosomal and granular contents into the surrounding extracellular space, with subsequent inflammation. A number of toxic chemicals or physical stresses such as toxins, radiation, heat, trauma, lack of oxygen due to the blockage of blood flow, and other events can cause necrosis. When necrotic cells begin to die, cells swell and holes appear in the plasma membrane and intracellular materials spill out into the surrounding environment [79].
Necrotic cell death is believed to be a consequence of physicochemical stress, such as freeze–thawing or severe hyperthermia, and thus as accidental and uncontrolled [80]. Interestingly, necrotic cell death has emerged as an important and physiologically relevant signaling process that seems to contribute to ovulation [81,82], immune defense [83], death of chondrocytes controlling the longitudinal growth of bones [84], and cellular turnover in the intestine [85]. In vivo studies indicated that removal of inter-digital cells in the paws of Apaf 1−/− mice during embryogenesis occurs by a caspase-independent necrotic-like process [86]. Noteworthy, the occurrence of necrosis in these in vivo models is mostly defined morphologically. Several reports also illustrate the occurrence of necrotic cell death during viral and bacterial infections. For example, HIV-1 shows to kill CD4+T lymphocytes by necrosis [87] and Shigella and Salmonella can induce necrotic cell death of infected neutrophils and macrophages [88]. Although necrosis has been thought to be an unregulated process, recent research suggests that necrosis may occur in two different pathways in a living organism. The first pathway of necrosis initiation involves oncosis, where swellings of the cells occur. The cell then proceeds to blebbing, and it is followed by pyknosis, in which nuclear shrinkage transpires. In the final step of the primary necrosis, the nucleus is dissolved into the cytoplasm known as karyolysis. The second pathway of necrosis involves a secondary form of necrosis that is shown to occur after apoptosis and budding. In this case, cellular changes of necrosis occur in this secondary form of apoptosis, where the nucleus breaks into fragments, which is known as karyorrhexis.
Unlike necrosis, necroptosis is a more programmed form of necrosis that has been shown to be a defense mechanism in organisms against internal pathogens and intracellular infections [89]. Necroptosis was first recognized as a caspase-independent form of cell death that can be triggered by treatment with tumor necrosis factor (TNF) only in the presence of a pan-caspase inhibitor, such as zVAD fluoromethyl ketone. Unlike apoptosis, necroptosis requires that the function of caspase 8 be inhibited or disrupted. Several of the upstream signaling elements of apoptosis and necroptosis are shared, and sensitivity to each death pathway is regulated (sometimes in opposing ways) by an overlapping cluster of regulatory molecules, such as FLICE-like inhibitory protein (FLIP), the deubiquitinases A and cylindromatosis and the cellular inhibitors of apoptosis proteins such as the Baculoviral IAP repeat-containing protein (cIAP1 and cIAP2). At the molecular level, intracellular assembly of a highly regulated complex, the necrosome, can be triggered by death receptors (e.g., tumor necrosis factor receptor 1/TNFR1), by cell-surface toll-like receptors, by DAI (which may act as a cytoplasmic viral RNA sensor). The continuing elucidation of the molecular aspects of various forms of regulated necrosis, including necroptosis, and the efficient design of combination therapies hold promise for our ability to control regulated necrosis in clinical settings.
5. The role of hsp90 on apoptosis resistance in cancer cells
Heat shock protein 90 (hsp90) is one of the most abundant proteins in eukaryotic cells. It is an enzymatic chaperone molecule with ATPase activity that is highly active in malignant cells and tissues when compared to non-neoplastic cells. In line with its activity pattern and expression profile it has been shown to drive tumor progression by enhancing proliferation, migration and metastasis. In addition, it also confers resistance to programmed cell death by several mechanisms. By its chaperoning activity, hsp90 stabilizes a number of mutated and non-mutated kinases and several anti-apoptotic factors within the cytosol that overall favor resistance to apoptosis and mostly drive proliferation and resistance of tumor cells to various treatment regimens. These factors include, but are not limited to Akt, mutated BRAF, EGFR, JAK2 and the inhibitor of apoptosis protein survivin. Targeting cytosolic hsp90 elicits inhibition of proliferation and depending on the compound and tumor cells involved also apoptosis. The kinase, JAK2, was recently shown to be an interacting partner of hsp90 in a model of polycytemia vera [90], a myeloproliferative disorder that almost always harbors a JAK2 mutation. In line with the interaction of hsp90 and JAK2, a novel hsp90 inhibitor was shown to disrupt this interaction and depleted JAK2 protein levels in vitro and in vivo. Hsp90 signaling also appears instrumental in mediating resistance to tyrosine kinase inhibition (TKI) treatment, specifically with EGFR inhibitory molecules, such as erlotinib and gefitinib. These molecules particularly target mutated EGFR within the intracellular ATP binding domain, especially point mutations in exon 21 (L858R) and deletion located in exon 19. With respect to hsp90, Shimamura et al. [91] have elegantly shown that in several non-small cell lung cancer (NSCLC) cell lines geldanamycin (a prototype hsp90 inhibitor) preferentially elicited depletion of mutated EGFR with subsequent suppression of p-Akt, causing cell death. Moreover, 17-AAG, a derivative of geldanamycin, more efficiently suppressed mutated EGFR compared to the wild-type form. Another important aspect in lung adenocarcinomas is the fact that tumors that are initially responsive to EGFR inhibition due to selective EGFR mutation in exon 19 or exon 21 recur eventually because in the presence of EGFR sensitizing mutations, they generate secondary mutations, such as the T790M EGFR mutation, which in turn confers resistance to erlotinib/gefitinib. One option to bypass a T790M mediated resistance is the administration of so called irreversible tyrosine kinase inhibitors. However, another option appears to be the enzymatic, cytosolic inhibition of hsp90 by 17-AAG [92]. Shimamura et al. [93] have demonstrated in a murine model of lung cancer, harboring both an exon 21 (EGFR inhibitor sensitizing mutation) and the resistance mutation (T790M), that 17-DMAG (a derivative of 17-AAG with optimized in vivo properties) also reduced tumor growth in the presence of the T790M mutation, indicating that hsp90 inhibition may be a worthwhile strategy to combat EGFR tyrosine kinase inhibitor resistance. This was further supported by the depletion of several hsp90 chaperones/downstream molecules in vivo. Given the unfavorable properties of 17-AAG derived molecules, alternative molecules are being tested. One of which is Ganetespib that was recently explored in several lung cancer models, including ones harboring EGFR inhibitor resistance mediating mutations. Ganetespib behaved superiorly compared to 17-AAG and accumulated within neoplastic tissue. Due to the favorable preclinical data it was also recently assessed in a phase-II-clinical trial [94].
Most of the hsp90 functions are ascribed to its presence and function in the cytosol, but recent evidence also suggests that hsp90 is over-expressed in tumor mitochondria and organizes a mitochondrial chaperoning network that antagonizes tumor cell death. In terms of therapeutic applicability, inhibition of hsp90 elicits anti-cancer activity in tumor cells of various origins and therefore has emerged as a viable treatment target for cancer therapy. In 2007, Kang et al. [95] have unraveled a novel tumor chaperone network that is situated in tumor mitochondria. This network consisted of three major players, hsp90 (as referred now as mitochondrial hsp90; mHsp90), TNF receptor-associated protein 1 (TRAP1) and the cell death promoting protein Cyclophilin-D (Cyp-D). TRAP1 is a molecule that shares significant similarities to hsp90 by exhibiting both structural and enzymatic overlap with hsp90. Akin to hsp90, TRAP1 reveals ATPase activity and its ATPase activity is amenable to inhibition by geldanamycin derivatives, such as 17-AAG. Favoring TRAP1 as a suitable “druggable” target, its expression levels were increased in malignant neoplasms from lung, colon, pancreas and breast, prostate and glioblastoma, whereas in non-neoplastic counterparts expression levels were significantly lower [95]. For example, Matsuda et al. [96] have demonstrated that β-hydroxyisovalerylshikonin, a plant derivative with anti-cancer activity, depleted TRAP1 levels in mitochondria in DMS114 (lung cancer) and HL60 (leukemia) cells, respectively. In the matrix of tumor mitochondria, hsp90 and TRAP1 inhibit mitochondrial permeability transition pore (MTP)-dependent cell death initiated by Cyclophilin-D in tumor cells almost independent of their entity. Kang and colleagues also elaborated this concept further by developing two different classes of molecules to specifically inhibit the discovered chaperone network in tumor mitochondria. The first of these molecules was a modified 17-AAG (Ant-GA) molecule that harbored an Antennapedia linker sequence (Ant), which allowed the molecule to accumulate in the matrix of mitochondria and inhibit both mhsp90 and TRAP1. Despite its modification Ant-GA retained its hsp90 inhibitory properties. When incubated, Ant-GA induces disruption of the mitochondrial membrane potential of tumor cells, culminating in cell death independent of their TP53 status, suggesting that a mitochondrial chaperone-targeted treatment approach does not require presence of wild-type p53 which is amongst the most commonly mutated proteins in cancers. To further corroborate the importance of Cyclophilin-D, Kang et al., [97] also made the case that Cyclophilin-D is implicated and instrumental for the cell death elicited by Ant-GA, since cyclosporine-A, an inhibitory molecule of MPT mediated cell death mitigated Ant-GA mediated apoptosis. The second molecule that revealed inhibitory properties of the mitochondrial hsp90 chaperone network was a peptide, called shepherdin. Shepherdin was initially described as a compound that disrupted the interaction of hsp90 and survivin. This peptide induced cell death in various cancer cells with a remarkable efficacy without causing apoptosis in non-neoplastic cells, such as fibroblasts, epithelial cells or astrocytes. The initial form of shepherdin had anti-cancer activity in several skin xenograft models, including prostate cancer. Consistent with its inhibitory cytosolic properties of hsp90, shepherdin treated xenograft tumors exhibited depletion of the hsp90 chaperone client proteins, Akt, and survivin. Most notably, shepherdin was not associated with organ toxicity as provided by a necropsy analysis of shepherdin treated animals. Consistent with this notion is the finding that animals did not exhibit significant weight loss upon administration of the compound. Based on this determination, it was suggested that shepherdin appears to be a fairly safe reagent. However, one pitfall may be the fact that precise pharmacokinetics were not provided. An engineered shorter version of shepherdin showed activity against leukemia cells both in vitro and in vivo [98]. Later on, it was demonstrated that shepherdin also inhibited the mitochondrial hsp90 chaperone network by interacting directly with TRAP1 and mhsp90. Consistent with its pharmacodynamics, shepherdin induces a MPT-dependent cell death specifically in tumor cells, which akin to Ant-GA requires the functional presence of Cyclophilin-D. Along those lines, pretreatment with cyclosporine-A, an inhibitor of the MPT, attenuated shepherdin-mediated cell death. In contrast, adenoviral-mediated over-expression of Bcl-2, that at physiological levels inhibits the translocation of intermembranous cytochrome c into the cytosol, did not significantly suppress cell death mediated by shepherdin, suggesting that this reagent acts independent of the Bcl-2 family of proteins and mainly relies on the MPT pore in the presence of Cyclophilin-D. All in all, shepherdin represents a prototype of a “double hit” hsp90 inhibitor with tumor specific pharmacological effects both in the cytosol and within mitochondria. Newer class of sophisticated molecules Gamitrinibs “GA-mitochondrial matrix inhibitors” have also been tested for their hsp90 inhibitory activity [99]. Gaminitribs are derived from 17-AAG and structurally contain a mitochondrial linker sequence, which enables them to efficiently accumulate in mitochondria. Two molecules have received most attention: Gamitrinib-TPP and Gamitrinib-G4. They are associated with anti-tumor activity in a number of different disease model systems, including breast cancer, lung cancer, colon cancer, prostate cancer lymphoma and glioblastoma (also reviewed in [100]). Collectively, a comprehensive understanding of how hsp90 functions promises not only to provide new avenues for therapeutic intervention, but to shed light on fundamental biological questions of apoptosis evasion.
6. The proteasome pathway and apoptosis resistance
Eukaryotic 26S proteasome is a 2.5 MDa large complex consisting of two 19S regulatory particles and one 20S catalytic core [101]. Each 19S regulatory particle is composed of the lid, which is responsible for recognition and docking of polyubiquitinylated proteins into the 20S catalytic core, and the base, which is in charge of the unfolding and linearization of large proteins. The 20S catalytic core harbors seven distinct α-subunits and β-subunits arranged in a α7β7β7α7 stacked barrel, among which mainly three sets of β-subunits, β1 (caspase-like or peptidyl-glutamyl peptide-hydrolyzing (PGPH)-like), β2 (trypsin-like), and β5 (chymotrypsin-like) are proteolytically active. Unlike common proteolytic enzymes that contain a catalytic triad, the proteasome catalytic subunits belong to a special group termed N-terminal nucleophile hydrolases, which utilizes the side chain of the N-terminal residue as the catalytic nucleophile [102–104]. All three catalytic β-subunits react with peptide bonds of substrates through their OH group of the N-terminal threonine (Thr1), resulting in protein being degraded into small fragments of less than 10 amino acids. It has been well documented that the proteasome is required for cell cycle progression by regulating the turnover of cyclins and cyclin-dependent kinase inhibitors, and therefore inhibition of proteasome function could result in cell cycle arrest. In addition, the ubiquitin-proteasomal system can affect cell survival pathways by regulating the turnover of transcriptional factors responsible for cell survival or apoptosis such as NF-κB, p53, as well as apoptotic proteins such as the Bcl-2 family members and others [105]. Therefore, inhibition of the proteasome is linked with the induction of apoptosis.
In tumor tissues, the proteasome activity is up-regulated by intracellular oncogenic factors, which render the cancer cells more dependent on the proteasome than the normal cells. Enhanced tumor cellular proteasome activity in turn promotes the degradation of tumor suppressor proteins, resulting in cancer cell survival and proliferation as well as the development of resistance to apoptosis [106]. On the other hand, proteasome activity could be suppressed by several endogenous inhibitors as well as various exogenous inhibitors, including some synthetic compounds such as bortezomib and many natural products such as plant polyphenol epigallocatechin-3-gallate (EGCG) [107]. Although the mechanism that is involved is not clear, proteasome inhibition in cancer cells is prone to accumulate pro-apoptotic target proteins and induce cell death. The clinical efficacy of bortezomib in multiple myeloma and other hematologic malignancies lends credence to the concept that targeting the proteasome, making it a promising strategy for cancer treatment.
The proteasome also degrades IκBα, an important inhibitor of the tumor survival factor NF-κB. Many physical (i.e., radiation), chemical (cancer chemotherapeutic agents), viral and biological (cytokines, growth factors) agents induce phosphorylation, ubiquitination and subsequent degradation of IκBα by the proteasome, freeing up NF-κB to translocate to the nucleus and modulate genes involved in proliferation, invasion and tumor survival [108,109]. For example, NFKB upregulates the anti-apoptotic protein Bcl-2 and downregulates the pro-apoptotic protease caspase 8. Therefore, by inhibiting the proteasome, IκBα will accumulate which will inhibit NF-κB from promoting tumor survival. The proteasome is also responsible for degrading the tumor suppressor p53. Many tumor cells inactivate p53 by over expressing p53 master regulator MDM2 [110]. In human tumors that over express MDM2, the inhibition of proteasome pathway is predicted to induce tumor cell apoptosis by accumulating p53. It has been observed that CEP1612, a dipeptidyl proteasome inhibitor, was able to rapidly induce apoptosis in different human cancer cell lines, including breast, prostate, leukemia, lung, bone, brain, and head and neck, but not in human normal fibroblasts and normal breast cells [111].
Proteasome inhibition was also sufficient to overcome apoptotic protection by Bcl-2 or Bcr-Abl oncoprotein. Proteasome inhibition accumulates Bax (but not Bcl-2) protein in mitochondria, resulting in increased ratio of Bax/Bcl-2, associated with cytochrome c release and apoptosis induction [112]. It has also been shown that during TNF-α-induced apoptosis, Bcl-2, but not Bax, protein is degraded through ubiquitin/proteasome-dependent pathway [113] which also increased the Bax/Bcl-2 ratio. Therefore, selectively degrading one or more Bcl-2 family proteins by the proteasome should change the ratio of pro- to anti-apoptotic proteins, which might contribute to the apoptotic commitment and result in overcoming resistance.
7. Epigenetics as a mechanism underlying drug resistance
There is uniform recognition of the importance of epigenetics (heritable non-structural changes in gene expression), as a major mechanism that can drive acquired resistance to chemotherapy [114]. For example, epigenetic mechanisms such as non-coding RNA (microRNAs) and DNA methylation are important drivers that influence the chemo-responsiveness of tumors and acquired drug resistance. Although drug resistance can be overcome using epigenetic therapies in experimental models, clinical studies of epigenetic therapies have highlighted challenges for different cancers, and there is a need to identify more targeted approaches. Here we highlight a few epigenetic mediated drug resistance mechanisms and identify strategies to overcome this challenge.
The breast and ovarian cancer cell line models have provided some of the earlier insights into the different epigenetic mechanisms underlying drug resistance. Studies have shown that taxane resistance in breast cancer may be associated with profound changes in the expression of apoptotic factors such as caspases. The gradual development of taxane resistance over a relatively small number of cell culture passages was shown to correlate with marked downregulation of caspase 9, 7 and Bcl-2 [115]. Such a shutdown of the intrinsic pathway was shown to be associated with a concomitant up regulation of autophagy. Further, low Bcl-2 expression was seen when cells develop a high level of background autophagy and this can be associated with collateral sensitivity to platinum agents, as seen for taxane resistant breast cancer. Moreover, a high level of background autophagy has also been demonstrated in breast cancer cell lines with acquired resistance to anti-endocrine agents such as tamoxifen and also faslodex (fulvestrant). Resistance to agents such as tamoxifen is a major concern when one considers their widespread use in the management of breast cancer, both in the prophylactic and adjuvant settings.
Drug resistance in cancer in terms of changes in the entire epigenome has also been studied. Using the cDNA microarrays of several cancer cell line models made relatively resistant to cytotoxic drugs in vitro, candidate genes have been identified. By incorporating the use of agents that can reverse epigenetic methylation a number of drug resistant cancer cell line models have been screened and compared with their wild-type drug sensitive counterparts. In this way the major genes that we believe are subjected to silencing – generally via methylation of the CpG islands in the promoter region of the gene – and these changes may often be associated with the evolution of drug resistance. For example a series of epithelial ovarian cancer (EOC) cell lines with acquired resistance to paclitaxel and carboplatin and showed that resistance to paclitaxel, often with cross-resistance to carboplatin, occurred with loss of the G2 checkpoint and apoptosis [116]. Among the featured genes included the Polo Like Kinase-2 (PLK2) that was down-regulated due to acquired methylation in the CpG-island at the 5′ end of the gene. These studies demonstrated that by increasing levels of resistance to paclitaxel there was correlation with incrementally decreased expression of PLK2 and increasing CpG island methylation. Based on these studies it was proposed that exposure to chemotherapeutic stress induces methylation in the CpG island of specific “resistance” genes and this seeding effect leads to increasing methylation as the level of resistance increases, a phenomenon previously described in cells exposed to 6-mercaptopurine [117]. A study by Matthew et al. [118] looked at the influence of hypoxia on chemosensitivity in PLK2-deficient tumors. Here, complete resistance to camptothecin pointed to interplay between the tumor microenvironment and PLK2 expression, whereas in the same experiments normoxic cells showed increased drug sensitivity. The same study also showed that PLK2 can inhibit mTOR signaling under the influence of wild-type p53 control. Furthermore, it was found that the cyclin-dependent kinase inhibitor p57kip2 appears to be down-regulated in drug resistant ovarian cancers [119]. It has also been shown that carboplatin-resistant ovarian cancer cells show promoter methylation in the CpG island of the promoter. By silencing otherwise carboplatin-sensitive cells using siRNA directed against p57kip2, carboplatin resistance was recapitulated which was shown by a reduced apoptotic response in those cells when challenged with a sub-lethal dose of platinum agents [119]. However, these cell lines studies need validation from ovarian cancer patient tissue based expression studies to strengthen the role of low p57kip2 and promoter methylation and its association with a poor prognosis and evidence of platinum-refractory disease.
An increasing number of genes involved in cell signaling, migration and adhesion are known to be alternatively spliced and this process appears to be altered during tumor development and progression. Different spliced forms of genes arise as a consequence of alternative pre-mRNA processing and are seen in a number of human malignancies [120,121]. Splicing processes must recognize intron and exon boundaries with accuracy – otherwise there will be changes in nucleotide sequence at the site of exon joining which shifts the reading frame with adverse consequences to the protein coding potential. Splicing is carried out by the “spliceosome” which comprises 5 small nuclear RNAs complexed with several additional proteins. Alternative splicing is one of the many cell processes that are commonly changed in the presence of cancer. These disturbances can result in the production of splice variants with neoplastic potential and could cause transformation of tumors. Alternative splicing contributes to the large number of proteins that can be produced from a much smaller number of genes in the human genome. Aberrations in alternative splicing may also affect cell proliferation, motility and susceptibility to apoptosis, which may be the result of variant mRNA that is tumor-suppressive or oncogenic and can contribute to carcinogenesis [122]. In particular, the family of splicing factors – including SC-35 (a member of the serine rich splicing factors: srsf2) – is recognized to be crucial in controlling the extent of mRNA splicing into active forms of various pro-apoptotic genes such as Bax. Head and neck cancer and ovarian cancer cell lines with acquired resistance to cisplatin have been shown to under express SC-35. Demethylation analyses have revealed that the splicing factor, arginine/serine-rich 2 (SRSF2) gene is silenced via methylation of CpG islands in the promoter region. Other work looking at epigenetic silencing has highlighted a number of genes that when silenced can reduce the apoptotic response of cancer cells to various anticancer agents. These studies can be used to look for biomarkers of chemo-response by detection of methylated DNA in cancer patients.
More recently, it has emerged that splicing efficiency can be affected by splicing enhancers which, in turn, interact with regulators that increase exon inclusion such as the SR family of proteins. SR proteins contain an RNA-binding domain and a characteristic SRSF2 – otherwise referred to as SC-35 – is located on chromosome 17 and contains a large CpG island. SR proteins control alternative splicing events in proto-oncogenes and tumor suppressor genes which leads to profound changes in their cellular activity. Factors such as SF2/ASF and SC35 are associated with transformation may be upregulated in some tumors [123]. In a report by Merdzhanova et al. [124] SC-35 working in conjunction with E2F1 was shown to be upregulated and required for apoptosis using a panel of lung cancer cell lines. E2F1, which is an established transcription factor, is recognized to be stabilized following treatment of cells with DNA-damaging agents such as cyclophosphamide. The upregulation accompanies SC-35 induction and there is evidence that SC-35 is a direct target of E2F1. Further, SC-35 inhibition was then shown to repress apoptosis induced by DNA damaging agents. SC-35 in its phosphorylated form is necessary for apoptosis following chemotherapy treatment with cisplatin and is up-regulated following this treatment [125,126]. These reported observations have led to the consideration that down-regulation of SC-35 – and other splicing factors – as a putative mediator of anticancer drug resistance.
More recently it has been shown that miRNA can have a significant effect on the expression of splicing factors such as SC-35. In drug resistant cell line models that carry significant upregulation of miR-221 and miR222 demonstrate marked downregulation of SC-35, a target gene for both these miRs. Further work should be focused on the influence of miR, splicing events and epigenetics on the modification of the apoptotic response in drug resistant cancers.
8. Nuclear transport in apoptosis resistance
Compartmentalization of proteins inside the eukaryotic cell is an evolutionarily conserved mechanism [127]. Each protein (especially apoptosis inducers) requires proper sub-cellular localization to mediate its specified function [128]. This is especially true for tumor suppressor proteins (TSPs) that usually reside in cell nucleus where they exert their function through sequence specific binding to DNA, modulation of gene expression, and assessment of the integrity of the genome [129]. Mislocalization of proteins has been long recognized to disrupt their function resulting in pathological conditions including cancer [130]. In eukaryotic cells, the cytosol and the nucleus intercommunicate via nuclear pore complexes (NPCs) present in the nuclear membrane [131]. NPCs consist of more than two dozen different proteins [132]. These nucleoporins form a channel and regulate the nucleocytoplasmic transport of various types of RNAs [133], and proteins [134]. The nuclear import and export of most proteins >40 KDa in size, including membrane proteins, occurs through the participation of an evolutionarily conserved family of transport proteins belonging to the karyopherin-β family [135]. Karyopherins-β accomplish either nuclear import and are called importins [136] or nuclear export and are called exportins [137]. Generally, karyopherin-mediated transport occurs through the NPC, which acts as a gateway into and out of the nucleus [138]. Most of karyopherins-β interact directly with their cargoes, but may also be aided by adapter proteins [139]. Karyopherin-α, known also as importin-α, is the most-studied adapter protein [140]. All proteins are synthesized on ribosomes found in the cytoplasm. Nuclear proteins must traverse the NPC following their cytoplasmic synthesis. While nucleocytoplasmic transport is normally a highly regulated process, the aberrant expression of karyopherins has been consistently observed in different cancers and has been linked to apoptosis resistance [141]. Chromosome maintenance region 1 (CRM1) is a karyopherin that exports different protein targets from the nucleus of the cell [142]. CRM1 is the major exporter of tumor suppressor proteins (TSPs) and functions by recognizing a leucine rich nuclear exclusion signal sequences (NESs) in cargo proteins and transports them to the cytoplasm in an energy consuming process that involves RanGTP binding and hydrolysis to RanGDP [143].
It is well recognized that nuclear localization of TSPs and their DNA binding is essential for their regulatory function [144]. Mere activation of apoptosis promoting TSPs (such as FOXO3a, p27, Ikβ and prostate apoptosis response 4/Par-4) is not sufficient for their proper apoptosis induction as over-expression of CRM1 in cancer cells results in TSPs efflux and their functional inactivation. Supporting this, over-expression of CRM1 has been associated with therapy resistance, particularly resistance to apoptosis and poor survival in solid tumors [145]. The prognostic significance of CRM1 in multiple cancers has been established [146]. Its expression is increased in pancreatic ductal adenocarcinoma, renal carcinoma, non-Hodgkin’s lymphomas, mantle cell lymphomas, prostate, breast, colon and other cancers. Unlike K-ras that is found mutated in >65% of cancers, these TSPs to a large extent remain wild type [147]. However, till date, there are no clinically approved drugs that broadly target the activation of TSPs. These multiple lines of evidences support the need for the development of CRM1 targeted drug for cancer treatment.
Inhibition of XPO1 is one approach to restore nuclear localization and activation of multiple TSPs, allowing them to function properly [148]. Therefore, targeted inhibition of CRM1 using specific small molecule inhibitors has been suggested to be a feasible strategy [149]. Leptomycin B (LMB) was the first natural agent identified to irreversibly inhibit XPO1 [150]. LMB is a secondary metabolite produced by Streptomyces spp and was originally discovered as a potent anti-fungal antibiotic [151]. LMB has been shown to be a potent and specific nuclear export inhibits XPO1 and works by alkylating and inhibiting XPO1 through glycosylation of a cysteine residue (cysteine 529) [152]. Nevertheless, LMB demonstrated marked toxicity in both preclinical studies and in a single clinical trial and its clinical development discontinued [153]. Another agent, Leptomycin A (LMA) was discovered together with LMB and showed potency twice as that of LMB [150]. However, its clinical utility has not been evaluated. A novel, small molecule, reversible inhibitor, CBS9106, with XPO1 degrading activity, was shown to have antitumor effects against multiple myeloma cells, both in vitro and in vivo [154]. The development status of this agent is not known. Recently, novel, orally bioavailable, small molecule, drug-like, selective inhibitors of XPO1 mediated nuclear (SINE) have been described [155]. SINE compounds bind irreversibly to the Cys528 NES recognizing residue in XPO1 and block its ability to bind to cargo proteins [156]. SINE have been shown to potently inhibit the growth of multiple cancer cell lines and animal tumor models such as acute myeloid leukemia [157], mantle cell lymphoma [158], and other hematological malignancies [159]. The anti-proliferative activity of SINE against pancreatic ductal adenocarcinoma and Non-Hodgkin’s Lymphoma has been demonstrated [160]. Based on these multiple lines of pre-clinical evidence, the orally bioavailable SINE KPT-330 have rapidly accelerated toward clinical evaluation in solid tumors and hematological malignancies.
CRM1 carries essential roles for the normal function of non-cancerous cells as well. Therefore, the clinical feasibility of any XPO1 targeted strategy has a number of hurdles. While molecular mechanism(s) of action of the first generation XPO1 inhibitor LMB were well defined, the drug proved highly toxic in preclinical models [161] and was discontinued in the clinic, the primary reason being the incomplete understanding and validation of entire sets of pathways modulated by this master exporter. This is coupled with a lack of complete evaluation of the effects of XPO1 inhibition. As recently demonstrated by ours and independent groups, XPO1 interferes with important and complex processes such as miRNA processing [162], epithelial-to-mesenchymal transition [163]. These findings indicate that more advanced pre-clinical work in the right models is required to optimize novel XPO1 inhibitors for applications in cancer and other diseases. Systems biology, particularly mathematical modeling and network analysis, are new approaches that can be used to optimize results in areas where traditional reductionist molecular biology has failed. Mathematical modeling approaches have been utilized to individually assess the consequence of XPO1 inhibition on single pathways. Such approaches have been able to shed light on MAPK/ERK and NF-κB signaling when their nuclear export is blocked, highlighting that the technology, if used correctly, can be applied successfully in XPO1 related research. However, there are no studies to date that have evaluated the differences in the effect of XPO1 inhibition on realignment of proteins in cancer versus normal cell nuclei in any kind of global context. Major unanswered questions remain as to whether there are differences in cellular responses between cell types (cancer with aberrant genome versus normal cells with normal genome versus precancerous lesions). Performing such studies at the systems level will undoubtedly lead to the optimization of XPO1 inhibitor therapies in the clinic, as well as in the design of novel strategies targeting nuclear transport.
9. Apoptosis resistance in different cancers
9.1. Apoptosis resistance in glioblastoma
Among all brain tumors, glioblastoma multiforme (GBM) is the most prevalent brain tumor in humans [164]. It is classified as grade IV astrocytoma by the World Health Organization (WHO) [165]. Various molecular alterations are responsible for development of GBM. Apoptosis resistance plays one of the key roles in tumorigenesis and sustains malignant progression in GBM. GBM patients have a poor prognosis with a median survival of 14.6 months. Surgery, radiotherapy, and the alkylating chemotherapy with TMZ are the standard first line treatment for GBM patients [166,167]. Combination therapeutic strategy with radiotherapy and TMZ significantly improves the median survival, 2 to 5 years, compared to radiotherapy alone in patients with newly diagnosed GBM [168,169]. Therapeutic effect of TMZ on GBM cells depends on the epigenetic silencing of the O6-methylguanine-DNA-methyltransferase (MGMT) gene by promoter methylation [170]. MGMT counteracts chemotherapy-induced DNA damage by restoring the structural integrity of O6-alkylated bases. Unmethylated MGMT promoter is frequently observed in glioblastoma patients and these seem to respond poorly to TMZ treatment [171]. Until now there has been no alternative drug treatment for GBM. Thus, understanding the molecular mechanisms that mediate cellular survival and apoptosis resistance will enable us to exploit the key players to design better drug combinations for targeted therapies for GBM patients.
Erlotinib and gefitinib, which are two epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors, have been evaluated for GBM treatment. The low molecular weights of these inhibitors enable them to cross the blood–brain barrier (BBB). Recent studies show that GBM patients with amplified or overexpressed EGFR responded better to erlotinib than patients with normal EGFR levels [172] and the response depended on low levels of Akt activation. So, Akt phosphorylation may be a direct result of increased EGFR activity. Thus, treatment with EGFR inhibitors can show better clinical responses. Clinical studies indicated that treatment with single tyrosine kinase inhibitor like erlotinib could not effectively inhibit survival signaling because many other RTK were co-activated in GBM cells [173,174]. Two other receptors, platelet-derived growth factor receptor (PDGFR) and c-Met or hepatocyte growth factor receptor (HGFR), are also involved in EGFR function and in maintaining downstream pathway activation [175]. This suggests that carefully designed combination of inhibitors with limited toxicity profiles and maximal additive or synergistic effects may provide more beneficial therapeutic effects [176]. TMZ causes cell cycle arrest at G2/M phase and EGFR inhibitors prevent cells from progressing beyond G1 and may therefore compromise the activity of other cell cycle-specific agents [177]. As the EGFR tyrosine kinase inhibitors show low toxicity, higher dose can be applied, but it may be difficult to predict the functionally active drug in GBM patients. However, the lack of availability of post-treatment tumor tissue for validation of target inhibition results in uncertainties regarding the sufficient inhibition of the EGFR signaling.
Many inhibitors of the anti-apoptotic members of the Bcl-2 family have been developed and several of them are under preclinical or clinical trials [178]. Although some of the inhibitors have been tested, only one compound has reached the clinical trial in GBM patients. Brain tumors that overexpress anti-apoptotic Bcl-2 and Bcl-xL proteins can be treated with their inhibitors. ABT-737, a recently developed Bcl-2 inhibitor, is known to induce apoptosis in glioblastoma cells both in vitro and in vivo by releasing the pro-apoptotic Bax protein from its binding partner Bcl-2 [179]. ABT-373 can sensitize tumor cells to the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) as well as to other anti-cancer drugs. But, cells with higher expression of Mcl-1, which is an anti-apoptotic protein of the Bcl-2 family, are found to be less responsive to ABT-737 treatment. So, combination therapy with inhibitors of Mcl-1 and Bcl-2 can be a novel strategy to treat GBM. Recent investigations indicated that the BH3-binding compound HA14-1 can enhance the sensitivity of human glioblastoma cells to both radiotherapy and chemotherapy [180]. Gossypol (AT-101), which is a multi-targeting polyphenol derived from cotton plant, is so far the only Bcl-2 targeting compound tested in clinical trials for treatment of GBMs [181]. Gossypol binds to the BH3 pocket of anti-apoptotic Bcl-2 proteins [182] as well as to other target proteins [183]. Studies suggest that administration of gossypol (20 mg/day) is well tolerated in patients and it has a low but measurable response rate. A Phase II trial of gossypol in recurrent GBM is underway to detect its efficacy against tumor progression and also its toxicity.
The inhibitors of apoptosis (IAP) family proteins like XIAP, cIAP1, cIAP2, ILP2, ML-IAP, and survivin are well known for inhibition of apoptosis in GMB [184,185]. The IAP family proteins are currently known as the baculoviral inhibitors of apoptosis repeat containing (BIRC) proteins. These BIRC proteins can inhibit apoptosis by modulating the activity of different caspases like caspase-9, caspase-7, and caspase-3, which are actively involved in intrinsic pathway of apoptosis in human glioblastoma [186]. In most GBM patients, high levels of BIRC proteins have been detected. Therefore, targeting BIRC proteins to make active caspases available for induction of apoptosis is now an established approach in developing strategy for controlling growth of human glioblastoma cells [187]. Many preclinical trials have been carried out with several small molecule inhibitors directed to BIRC proteins to identify a potential agent capable of inducing apoptosis in different cancers [188]. However, very little research has been performed with BIRC inhibitors in GBM. A recent report showed that BIRC-4 (XIAP) inhibitors synergize with radiation to increase glioblastoma cell apoptosis [189] and targeting BIRC proteins can sensitize cells to TRAIL for induction of apoptosis [190]. Recent investigations also indicated that the endogenous BIRC inhibitor Smac can significantly increase the anti-cancer efficacy of TRAIL in an intracranial glioblastoma xenograft model [191]. Also the use of inhibitors of histone deacetylase and of the proteasome in clinical trials (targeting specific, but poorly characterized aspects of apoptosis) are ongoing at the moment. The versatility and applicability of these preclinical studies will eventually contribute to elucidation of the molecular mechanisms of chemoresistance, which should ultimately result in the identification of more effective therapeutic strategies for avoiding apoptosis resistance in GBM.
9.2. Resistance to apoptosis in melanoma
Melanoma is considered the most aggressive form of skin cancer, derived from activated or genetically altered epidermal melanocytes. Human malignant melanoma is a highly metastatic cancer that is markedly resistant to conventional therapy such as dacarbazine or TMZ. The RAF/MAP kinase pathway has attracted attention because activating mutations of the BRAF serine/threonine kinase has been detected in more than 50% of melanomas. Other mutations occur in NRAS, MEK1, MEK2 as well as c-Kit. Activation of c-Kit results in the stimulation of MAPK and PI3K/AKT pathways resulting in both proliferative and survival advantage. Several other signal transduction pathways have been found to be constitutively active or mutated in other subsets of melanoma tumors including NF-κB. Raf inhibitors in general and specific BRAF inhibitors, including vemurafenib, frequently elicit therapeutic response. However, durable effects are often limited by ERK1/2 pathway reactivation via poorly defined mechanism. Resistance to apoptosis using BRAF specific inhibitors is mediated, in part, by the presence of NRAS mutation and required switch in activation of RAF isoform, CRAF. Furthermore, rebound melanoma growth after initial treatment with BRAF specific inhibitors is associated with elevated activation of PI3K/Akt pathway.
It has been shown that the Bcl-2 positive regulator NF-κB is a key player in human melanoma tumorigenesis. Canonical NF-κB activation in melanoma cells is associated with increased survival and proliferation. In human melanoma, a number of NF-κB-dependent chemokines are constitutively expressed at high levels including CXC ligand 8 (CXCL8 or IL8), interleukin-8 [192], CXCL1, melanoma growth stimulatory activity or MGSA [193], CCL5 (regulated on activation), normal T expressed and secreted, or RANTES [194] and CCL2 (monocyte chemotactic protein-1), or MCP1 [195]. In late stages of metastatic melanoma, hyperactivated NF-κB inhibits pro-apoptotic pathways through the upregulation of (i) tumor necrosis factor receptor-associated factor-1 (TRAF-1) and TRAF-2 [196] to inhibit the TNF-R1/caspase-8-mediated pro-apoptotic pathway TRAIL decoy receptor, inhibiting the TRAIL-mediated cell-death pathway [197,198], and (iii) Fas-associated phosphatase-1 (FAP-1) [199], which down-regulates FAS-R trafficking from cytoplasm to membrane [200]. Furthermore, in late stage metastatic melanoma, activation of NF-κB also enhances several anti-apoptotic molecules such as inhibitor of apoptosis (IAP) [201], caspase-8 (FLICE) and inhibitory protein (FLIP) [202]. NF-κB also promotes Myc activity [203] and the cell cycle regulatory proteins, cyclin D1 and cyclin dependent kinase 2 (CDK2) [204], which further contribute to melanoma tumor growth.
More than 50% of melanoma cells harbor mutations in BRAF signaling protein [205]. BRAF is part of the Raf family of serine/threonine kinases, which are effectors of the small GTPase RAS in the ERK/MAPK pathway. This pathway is activated by several membrane-bound receptors such as receptor tyrosine kinases and G-protein-coupled receptors. BRAF transduces signals through mitogen activated protein kinase (MAPK) [206,207]. MAPK promotes regulation of cell growth, survival and differentiation [208]. Canonical activation of the MAPK pathway occurs when growth factor-growth factor receptors bind which leads to the activation of a RAS family member, any of the three isoforms H, N, and K-RAS. Activated RAS then binds and activates multiple effector proteins, including the three Raf members (ARAF, BRAF and CRAF), leading to subsequent activation of a cascade of kinases including MEK1/2 and ERK1/2. Activated ERK in turn specifically phosphorylates a number of nuclear and cytoplasmic substrates including the ETS transcription factor, which has the net effect of providing a pro-growth and pro-survival signal [209]. Concurring with these assumptions, Erk1/2 signaling has been shown to protect melanoma cells against TRAIL-induced apoptosis by inhibiting the relocation of Bax from the cytosol to mitochondria and that this may reduce TRAIL-mediated release of Smac/DIABLO and induction of apoptosis.
9.3. Apoptosis resistance in pancreatic ductal adenocarcinoma
With mortality rates almost mirroring incidence rates, pancreatic ductal adenocarcinoma (PDAC) is among the most lethal of all cancers. It causes more than 170,000 deaths worldwide and is the fourth leading cause of cancer related deaths in the United States making it a deadly disease in urgent need for newer treatment approaches [210]. PDAC tumors are very heterogeneous and carry alterations in many critical pathways (harbor a robust biological network) rendering the design of therapy against a single pathway unrealistic [211]. The major reasons for this dismal outcome include, lack of early detection markers [212], invasive behavior [213] and intrinsic resistance to therapeutic treatments [214]. Recently, several studies have identified a highly resistant subset of PDAC cancer stem cells (CSCs) with self-renewal capacity and propensity to undergo epithelial to mesenchymal transition [215]. Additionally, the low clinical efficacy of different regimens in PDAC has been attributed to the lack of drug penetrance due to the presence of high desmoplastic stroma that supports a low tumor vasculature [216]. Thus, holistic studies are needed that identify effective regimen against PDAC CSCs, and key molecules that promote desmoplastic stroma in PDAC will provide biomarkers and potential targets to overcome chemo resistance and disease recurrence.
De-regulated apoptosis signaling mechanisms have been attributed as one of the major causes for the drug resistance. Pan-creatic cancer has been shown to over-express Bcl-2 and its family members. Therefore, blockade of Bcl-2 activity should become a novel therapeutic strategy for pancreatic cancer. Many groups have been working to develop anticancer drugs that block the function of Bcl-2 members. Earlier Wang and colleagues have successfully demonstrated that targeted inhibition of Bcl-2 can suppress PDAC growth in vitro and in vivo [217]. Very recently, Abulwerdi et al., [32] exploited Mcl-1 as a therapeutic target using small molecule drugs in PDAC. Both these studies collectively prove that targeted inhibition of BH3 family proteins could be a viable therapeutic strategy against PDAC.
9.4. Apoptosis resistance in colon cancer
Colorectal cancer is among the common forms of cancer worldwide and ranks third among the cancer-related deaths in the US and other Western countries. It is common to both men and women, constituting 10% of new cancer cases in men and 11% in women. Despite recent advancement in therapeutics, the survival rates from metastatic are less than 5%. Growing evidence supports the contention that epithelial cancers including colorectal cancer, the incidence of which increases with aging, are diseases driven by the pluripotent, self-renewing cancer stem cells (CSCs). Dysregulation of Wnt, Notch, Hedgehog and/or TGF-β signaling pathways that are involved in proliferation and maintenance of CSCs leads to the development therapy resistance.
The loss of heterozygosity (LOH) at the bcl-2 gene locus and the expression of the bcl-2 gene has been examined in colorectal carcinoma cell lines and carcinoma tissues. LOH at the bcl-2 locus was detected in 60% (6/10) of colonic carcinomas, all of which were well differentiated adenocarcinomas, whereas LOH was not seen in poorly differentiated ones. Further, tree colorectal carcinoma cell lines, all of which were derived from poorly differentiated adenocarcinomas, expressed considerable levels of bcl-2 mRNA and protein. These results suggest that LOH at the bcl-2 locus is frequently associated with well differentiated adenocarcinomas of colon, and bcl-2 overexpression has implications for the development of poorly differentiated adenocarcinomas of the gastrointestinal tract.
Colon tumors have been shown to exploit the lymphocyte death program by expressing FasL [218]. This may enable colon tumors to mount a “Fas counterattack” against antitumor lymphocytes, impairing antitumor immune responses. FasL-expressing colon tumor-derived cell lines can trigger Fas-mediated apoptosis of co-cultured T cells in vitro. FasL expressed in esophageal cancer has been significantly associated with apoptosis and depletion of tumor-infiltrating lymphocytes (TIL) in vivo. FasL may also facilitate metastatic colonization of Fas-sensitive organs such as the liver, by inducing apoptosis of target organ cells. Normal colonic epithelial cells express Fas and are relatively sensitive to Fas-mediated apoptosis. By contrast, colon tumor-derived cell lines are usually resistant to induction of Fas-mediated apoptosis, and colon cancer cells frequently coexpress Fas and FasL. The mechanisms allowing resistance to Fas-mediated apoptosis are complex, and defects have been identified at several levels of Fas signal transduction. The “Bcl-2 rheostat” may be pitched against apoptosis in colon cancer, in as much as over-expression of Bcl-2, downregulation of Bak, and mutation of Bax are common defects in colon tumors. Caspase-1 is also downregulated in colon cancer. The high frequency of p53 mutations in late-stage cancers may also inhibit Fas signaling. Fundamental defects in apoptosis signaling may contribute to both immuno- and chemoresistance in colon cancer and allow expression of FasL to counterattack antitumor lymphocytes.
9.5. Apoptosis resistance in prostate cancer
With an estimated 233,000 new cases diagnosed and 29,480 deaths, prostate cancer is considered as the top major cause for cancer related deaths in the United States. Hormone ablation therapy is used to manage early stage disease, however, in majority of cases, prostate cancer becomes castrate resistant [219]. The disturbance in apoptosis pathway activation in prostate cancer therapy resistance has been clearly defined [220]. More specifically, Bcl-2 over-expression has been shown to promote androgen ablation resistance [221]. Aside from disturbed apoptotic machinery, a number of studies have demonstrated that lack of autophagy activation may also contribute to chemo- and hormone therapy resistance in prostate tumors [222,223]. Nevertheless, there are reports showing that autophagy pathway activation may even promote apoptosis resistance and this has been linked to therapy resistance of prostate cancer [224,225]. Therefore, it is needless to say that autophagy modulators have been shown to resensitize prostate cancer cells to radiation or chemotherapy [226,227]. Similarly, a number of Bcl-2 inhibitors have shown to reverse prostate cancer chemoresistance [228,229]. Mcl-1 inhibition has also been shown to re-sensitize prostate cancer cells to different chemotherapeutics and targeted therapies [230–232]. Besides Bcl-2 and Mcl-1 overexpression, TRAIL induced apoptosis resistance in prostate cancer has also been reported [233]. The development of TRAIL resistance is both genetic and epigenetic [234] that manifests in apoptosis resistance. Collectively, these studies clearly prove that sensitivity to therapeutics (chemo-, hormone, radio- or targeted) is directly correlated to the presence of functional apoptotic machinery.
9.6. Apoptosis resistance in breast cancer
Breast cancer is the major cause for female cancer mortality in the western world (GLOBACON). Despite the advances in early detection and the greater understanding of the molecular pathways underlying breast cancer biology, a major fraction of patients have recurrent disease that becomes refractory to most of the available therapies [235]. Systemic therapies include the use of cytotoxic, hormonal, and immunotherapy is usually used in the adjuvant, neoadjuvant, and metastatic settings. While these systemic agents are active in the majority of primary breast cancers and also to a great extent in metastatic cases, nevertheless, after a variable period of time, progression occurs. Resistance to therapeutics in breast cancer is multi-factorial. It involves disturbances in the apoptotic machinery, p-glycoprotein and the multidrug resistance protein family, HER-2/neu gene amplification and protein expression, along with the expression of additional members of the epithelial growth factor receptor family, DNA ploidy, p53 gene mutations, cyclin E and p27 dysregulation that cumulatively drive the development of cancer stem cells [236]. A number of studies have linked breast cancer drug resistance to disturbed or over-expression of pro-survival factors including Bcl-1, Mcl-1 and other BH3 family members [237]. This is confirmed from the observation that BH3 mimetic ABT-737 can sensitize BH3 protein over-expressing breast cancer cells [238]. Studies have also linked disturbed proteasome signaling to breast cancer chemo and radio resistance [239]. These findings become particularly relevant as proteasome inhibitor bortezomib treatment causes suppression of MDRs leading to induction of apoptosis in breast tumor models [240]. In summary these studies prove that the inherent resistant traits of breast cancer are associated with malfunctioning of the apoptosis signaling. Targeting the different pathways that either induce apoptosis or suppress the pro-survival signaling is expected to resulting in overcoming drug resistance to various therapies.
9.7. Apoptosis resistance in leukemia’s (focus on chronic lymphocytic leukemia and acute promyelocytic leukemia)
Chronic lymphocytic leukemia (CLL) is the most common B-cell malignancy in the Western world and is characterized by the accumulation of mature CD5 positive B lymphocytes in peripheral blood, bone marrow, and lymphoid organs [241]. CLL disease is generally associated to apoptosis resistance. A combination of alterations in the apoptotic machinery as well as micro-environmental survival signals within the CLL cells cause a cell death resistance. Recent studies suggest that most CLL clones include activated cells that proliferate at appreciable rates [242,243]. Thus, the balance between proliferation and cell death is extremely within patients and refiects the different disease progression [244].
In CLL, defects in the intrinsic and extrinsic apoptotic pathways have been described. B-cells can be resistant to both CD95/Fas [245] and TRAIL death receptors induction [246]. The intrinsic, or mitochondrial, pathway is regulated by the Bcl-2 family proteins, classified into anti-apoptotic (Bcl-2, Bcl-XL, Bcl-w, Mcl-1, A1) and pro-apoptotic (Bax, Bak, Bim, Bad, Bid, Hrk, Noxa, Puma) members [247]. In CLL the overexpression of anti-apoptotic proteins has implicated in resistance to apoptosis [248]. Aberrant expression of Bcl-2 is common in CLL patients and is associated with poor response to chemotherapy and decreased overall survival [249]. Moreover, Mcl-1 protein expression was correlated with adverse prognostic factors, such as disease stage, IgVH mutation status, ZAP-70 positivity and CD38 expression [250] and, was found to be predictive of the clinical outcomes of CLL patients [251,252]. In B-cells, several signals are involved in regulation of Bcl-2 family proteins, including BCR, cytokine signaling and microRNA. CD40 signaling has been suggested to trigger up-regulation of the anti-apoptotic proteins Mcl-1, A1 and Bcl-xL [253,254]. BCR signaling has been reported to be able to regulate Mcl-1, through hyper-activation of Akt [255] which also contributes to maintain cell survival by phosphorylating and inactivating the pro-apoptotic factor proteins Bad and Bim [256,257]. Given the key role that anti-apoptotic proteins play in CLL, they became an attractive target for the creation of novel therapeutic agents, such as anti-sense methodology [258], small molecules mimicking the action of BH3 domain [259], and microRNAs (mainly miRNA-15a and miRNA-16-1) [260]. For several decades, front-line therapy for CLL has been represented by treatment with alkylating agents and purine analogs [261]. Recently, significant clinical outcomes have been achieved using chemo-immunotherapy, such as rituximab, a chimeric monoclonal antibody against CD20, administrated in combination with fludarabine or fludarabine plus cyclophosphamide [262,263]. Nevertheless, the relapse remains problematic, particularly in older patients, thus the identification of innovative and specific agents against CLL remains of high interest [264].
Very interesting is the use of a cottonseed extract derivative, gossypol, which acts as a BH3-mimetic, interfering with the functions of Mcl-1, Bcl-2 and Bcl-xL proteins (from highest to lowest affinity) and displacing pro-death partners to induce apoptosis [265–267]. However, despite this encouraging data and the abundant literature describing the molecular mechanisms triggered by phytochemicals to inhibit cell growth and induce apoptosis in cancer cells, only few of them entered clinical trials [268].
Acute promyelocytic leukemia (APML or APL for short) is a subtype of acute myelogenous leukemia (AML). APL is characterized by an abnormal accumulation of immature granulocytes. The disease harbors chromosomal translocation involving the retinoic acid receptor alpha (RARα or RARA) gene and is distinguished from other forms of AML by its responsiveness to all-trans retinoic acid therapy. The role of Bcl-2 proteins in apoptosis resistance to retinoic acid therapy has been intensively investigated. It has been shown that the ability of retinoid-induced cells to undergo apoptosis depends on the level of expression and the functional interaction between Bcl-2 and Bax [269]. Highlighting its significance, autophagy and Beclin 1, an autophagic protein, were shown to be upregulated during the course of all trans retinoic acid (ATRA)-induced neutrophil/granulocyte differentiation of an APL-derived cell line. This induction of autophagy is associated with downregulation of Bcl-2 and inhibition of mTOR activity. Further, other studies have shown that a BH3 domain mimetic, JY-1-106, which antagonizes the anti-apoptotic BCL-2 family members B-cell lymphoma-extra large (BCL-xL) and myeloid cell leukemia-1 (MCL-1) alone and in combination with retinoids reduced cell viability in HL-60 APL cells alone and in combination with retinoids. The combination had the greatest impact on cell viability by stimulating apoptosis. These studies indicate that dual BCL-xL/MCL-1 inhibitors and retinoids could work cooperatively in APL.
10. Role of different natural compounds (phytochemicals) against apoptosis resistance
Since the early eighties, when apoptotic cell death appeared on the stage of molecular cancer field, scientists understood that bypassing apoptotic resistance resulted in novel therapeutic solutions against cancer. These efforts successfully involved novel and canonical anticancer drugs, but also stimulated the interest to explore the possibility that natural compounds may re-sensitize tumor cells to pro-apoptotic drugs. However, many of the studies that followed have been limited to preclinical laboratory experiments (largely confined to cell lines). More importantly, it is difficult to figure out how molecules that are structurally and functionally different (i.e., stability, bioavailability, biotransformation etc.) can behave similarly, and remove the resistance occurring in cancer cells to apoptotic induction.
It must be imagined that a unique mechanism, common to many phytochemicals, is responsible for their ability to re-establish the lost sensitivity to apoptosis. The well-known antioxidant capacity shared by the large majority of these compounds has been evoked to explain the experimental evidence. However, the “antioxidant hypothesis” is weakened by paradoxes and contradictions. In fact, the scavenging activity of the phytochemicals against ROS chemically converts them into oxidative products which display a high reactivity toward thiols and can lead to the loss of protein function. Therefore, paradoxically, the net result between protection offered by many phytochemicals and damage caused by its toxic products may weigh in favor of the latter. As an example, in lung cells, quercetin efficiently protects against H2O2-induced DNA damage, but this positive effect is counteracted by the reduction in GSH level, an increase in LDH leakage and cytosolic-free calcium concentration [270]. Quercetin may also form quercetin–quinone (QQ) species when the molecule is employed as an antioxidant. QQ, like other semiquinone radicals and quinones, is toxic because of its ability to arylate protein thiols. Protection against QQ may arise from GSH which, when present at the right concentration, quickly traps QQ [271]. Accordingly with this example, a pivotal review from Ursini’s group explains how the major mechanism of action for nutritional antioxidants is the paradoxical oxidative activation of the Nrf2-Keap1 (nuclear factor erythroid 2-related factor 2-Kelch-like ECH associated protein 1) signaling pathway, since kinetic constraints indicate that in vivo scavenging of radicals by these compounds is ineffective in antioxidant defense [272].
Nonetheless, although this preamble illustrate a critical challenge in this area of research, recent studies have demonstrated that treatment with phytochemicals may represent an applicable therapeutic strategy to bypass apoptotic resistance in specific types of cancers [273,274].
Pleiotropy”’ and “synergism” are two terms often associated to the effects of natural compounds to explain their multiple biological activities. The former refers to their ability to bind and interfere with the activity of several effectors that insist on one or more pathways, converging on the same cellular process, for example apoptosis, cell cycle arrest, autophagy. The latter indicates the property to enhance the efficacy of chemotherapeutic drugs in co-treatment protocols with the advantage to limit toxicity for patients. Both features, pleiotropy and synergism, find rationale applications in the physiopathology of cancer and is highly relevant to some specific diseases such as CLL, a form leukemia which remains indolent for decades. In fact, patients with CLL may survive for many years without any treatment because of the relatively slow progression rate of the disease. This feature makes CLL patients ideal models to test the pleiotropic properties of natural chemo-preventers possessing limited toxicity. In fact, in the temporal window during which patient’s remains asymptomatic, chronic administration of a specific phytochemical may retard disease onset. Moreover, when the disease progresses to clinically relevant forms and requires pharmacological interventions, the same compound can be introduced in the therapeutic protocol as adjuvant to synergistically improve the efficacy of the therapy. Supplementation with biologically active phytochemicals or phytochemicals-enriched food may represent an important approach to modify the clinical progression of cancers. However, to move from speculation to clinical application, further fundamental steps are required, such as well designed, randomized, control clinical trials.
10.1. EGCG
Mechanistic studies suggest that polyphenols have multiple intracellular targets, one of which is the proteasome complex [275,276]. Several botanically derived agents such as green tea catechins, isoflavones, anthocyanins, anthocyanidins, quercetin (3,3′,4 5,7-pentahydroxyflavone), apigenin and curcumin have been identified as potent proteasome inhibitors, with a more favorable toxicity profile compared to velcade and bortezomib and may be ideal candidates for therapeutic applicability in cancer chemoprevention and treatment. The goal of this section is to focus on providing a model of a botanically derived agent green tea polyphenol that has been demonstrated to induce apoptosis through targeting of the ubiquitin/proteasome pathway.
Among the constituents of green tea extracts (GTE), laboratory studies have identified EGCG as the most potent chemopreventive agent which appears to affect a number of molecular processes including induction of apoptosis and inhibition of tumor growth and angiogenesis [277–280]. More recently EGCG has been found to affect several cancer-related proteins including p27, Bcl-2 or Bcr-Abl oncoproteins, Bax, matrix metalloproteinases (MMP-2 and MMP-9) [281], the androgen receptor, EGF receptor, activator proteins 1(AP1), and some cell cycle regulators [282–284]. Based on their studies of GTE in cell culture systems, Adhami et al. [285] were able to demonstrate that EGCG (in GTE) induces apoptosis, cell growth inhibition and cyclin kinase inhibitor WAF-1/p21-mediated cell cycle-dysregulation. Using cDNA microarrays, they also observed the EGCG treatment of prostate cancer cells results in induction of genes that functionally exhibit growth-inhibitory effects and repression of genes that belong to the G-protein signaling network. These data confirm that GTEs exert potent and selective in vitro and in vivo pro-apoptotic activity on prostate cancer cells. Although there are several mechanisms by which EGCG may operate in prostate carcinogenesis, EGCG has been demonstrated to potently and selectively inhibit the proteasome activity in intact human cells leading to the accumulation of IkB-α and p27 proteins, and growth arrest [286]. This inhibition of proteasome activity by EGCG occurred at or near physiological concentrations similar to that found in the body fluids of green tea drinkers. Other studies have shown that a mixture of green tea polyphenols (polyphenon E) is equally potent inhibiting the proteasome activity as purified EGCG [287]. Polyphenon E preferentially inhibits the proteasomal chymotrypsin-like activities over other activities; b. Polyphenon E inhibits proteasome activity in intact cells in a concentration-dependent manner. Treatment of polyphenon E, at all used concentrations in both cell lines, increased accumulation of the proteasome target protein p27Kip1 in human multiple myeloma and prostate cancer cells. Similar to purified EGCG, using a cell-free proteasome assay it has been shown that Polyphenon E significantly inhibits the chymotrypsin-like activity of the purified rabbit 20S proteasome with an IC50 value of 0.88 µM. To investigate whether polyphenon E specifically inhibits the proteasomal chymotrypsin-like activity, its effects on the PGPH-like and trypsin-like activities of the purified 20S proteasome were determined. Polyphenon E inhibited PGPH-like activity of the purified rabbit 20S proteasome with an IC50 value of 7 µM. The IC50 value for trypsin-like activity was above 100 µM, thus demonstrating that polyphenon E preferentially inhibits the proteasomal chymotrypsin-like activities over other activities. The most significant study analyzed the effect of 2 mg/diet of Polyphenon E in a phase I/II clinical trial on asymptomatic CLL patients (Rai stage 0 to II). The results of the study indicated that in 69% of patients a substantial decline in absolute lymphocyte count (>20%) and/or reduction of lymphadenopathy (>30%) during the 6 months of active treatment [288,289]. Also flavopiridol, a semisynthetic flavonoidal alkaloid, originated from an Indian plant and known for its ability to inhibit cyclin-dependent kinases, achieved significant clinical activity in patients with relapsed CLL, including those with high-risk genomic features and bulky lymphadenopathy [290,291].
These data strongly suggest that the proteasome, an important large multi subunit protease complex in the cell, is a cancer-related molecular target of green tea catechins and that inhibition of the proteasome activity by EGCG may be the primary pathway by which tea catechins, specifically EGCG, induce prostate epithelial cell apoptosis. This appears to be accomplished via the proteasome inhibition pathway i.e., IκB-α protein expression, accumulation of p27 proteins and decreasing NFKB DNA-binding activity, and resulting in the inhibition of prostate cell survival and the induction of apoptosis, thereby decreasing progression from HGPIN to prostate [292,293] – similar to the effects of bortezomib and velcade (PS-341) [294–296].
The proteasome has been proven to be an excellent target for developing anticancer drugs, but several side effects (including nausea, fatigue, diarrhea, peripheral neuropathy, rapidly reversible reduction in platelets, and reversible thrombocytopenia) have been observed with bortezomib. Therefore, it is necessary to identify less toxic proteasome inhibitors with similar potency to bortezomib or different novel proteasome inhibitors, as we have proposed in this study. Additionally, although it is clear that the ester carbon of EGCG is important for mediating the proteasome-inhibitory activity, EGCG is very unstable under physiological conditions. Therefore, a series of EGCG analogs are evolving that are aimed at improving stability and bioavailability of EGCG. Among them, peracetate-protected or the pro-drug of EGCG was found to have increased bioavailability, stability, and proteasome-inhibitory activities against various human cancer cells and tumors compared to EGCG, suggesting its potential use for cancer prevention and treatment [297]. The early laboratory and Phase I trials have demonstrated GTPs, specifically EGCG, have a completely different chemical structure from bortezomib. Most importantly, (−)-EGCG and other catechin mixtures at varying doses has been found to be relatively better tolerated in clinical trials, in contrast to the observed AEs seen in the bortezomib trials, and thus appear to have significant potential to be tested in clinical trials.
In green tea, the catechin EGCG has been extensively studied for the biological activities and cellular targets, among which the proteasome attracts more attention [277]. Both naturally-occurring (−)-EGCG and synthetic enantiomer (+)-EGCG are able to potently and specifically inhibit the chymotrypsin-like activity of proteasome β5 subunit in an irreversible way with IC50 range 86–194 nM in vitro and 1–10 µM in vivo [280]. Of note, EGCG is able to interact with not only the β5 subunit in constitutive proteasome but also the β5i subunit in interferon-γ inducible immunoproteasome (referring to as BrAAP activity) with even higher affinity [298]. Pre-clinical studies have demonstrated the chemosensitizing effect of EGCG and other catechins in vitro and in vivo. It has also been shown that EGCG reversed drug resistance by inhibiting the drug efflux transporter P-gp. For example, Kitagawa et al. [299] reported that catechins increased the cellular accumulation of P-gp substrates in human cervical epidermal carcinoma cells. The chemosensitization by EGCG through the inhibition of P-gp expression/activity has also been validated in Caco-2 human intestinal adenocarcinoma cells (which are often used as a model for intestinal transport studies mediated by P-gp) [300] and tamoxifen-resistant MCF-7 human breast cancer cells [301], as well as in the xenograft mouse models using doxorubicin-resistant KB-A1 cells [302] or doxorubicin-resistant BEL-7404/DOX hepatocarcinoma cells [303]. The study with tamoxifen-resistant MCF-7 cells also found that EGCG was able to inhibit the activity of the BCRP transporter [304]. EGCG was also reported to enhance the sensitivity of glioblastoma to temozolomide, and of prostate carcinoma to doxorubicin [305], and of breast carcinoma to paclitaxel [306] in corresponding ectopic or orthotopic xenograft mouse models. Furthermore, the peracetate-protected prodrug of EGCG also exhibited a chemosensitizing effect in the treatment of leukemia cells by augmenting the efficacy of conventional chemotherapy daunorubicin and cytosine arabinoside [307]. A very recent study reported that polymer-based nanoparticle of polyphenols EGCG and theaflavin retained biological effectiveness with over 20-fold dose advantage than EGCG/theaflavin in exerting anti-cancer effects and also enhanced the potential of cisplatin in several different tumor cell types [308].
Encouraged by the preclinical results, a few studies were conducted using green tea extract (GTE) as monotherapy or a complementary/alternative medicine (CAM) in cancer patients. Although its anticancer activity was unsatisfactory, the oral consumption of GTE was well tolerated in these studies [309–311]. After a temporary suspension of EGCG in clinical trials by the US Food and Drug Administration (FDA) for additional review of toxicity, a tea extract named polyphenon E further proved the safety of tea polyphenols. In a polyphenon E phase I clinical trial in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia, most patients showed declines in absolute lymphocyte count and/or lymphadenopathy after daily oral consumption of Polyphenon E. A phase II trial using 2 g polyphenon E twice a day to evaluate its efficacy showed that it was well tolerated by patients with CLL and durable declines in the absolute lymphocyte count and/or lymphadenopathy were observed in the majority of patients [288]. Furthermore, two clinical studies using polyphenon E in combination with erlotinib are currently ongoing in patients with advanced non-small cell lung cancer and premalignant lesions of the head and neck, respectively. The chemosensitizing effect of polyphenon E in clinical settings remains to be determined. The in vitro and in vivo studies suggest that tea polyphenols (especially EGCG) may serve as powerful agents for reversing tumor drug resistance and enhancing the efficacy of chemotherapy.
10.2. Resveratrol
Isolated from the skin of red grapes, resveratrol received much attention due to its association with the French Paradox (i.e., the observation of low coronary disease incidence in high fat diet consuming French population who consume Red Wine-a supposedly major source of resveratrol). Later on a number of laboratories have demonstrated the anti-cancer activity of resveratrol in multiple tumor models. Studies have since shown that resveratrol can reverse apoptosis resistance and also sensitize cancer cells to different apoptosis inducing agents (chemotherapeutics). For example, Sprouse and Herbert [312] very recently demonstrated that resveratrol can augment paclitaxel treatment in paclitaxel resistant breast cancer cell line models. In another study Huq and colleagues [313] showed that resveratrol can overcome chemoresistance in ovarian cancer. In nasophyrangeal carcinoma, resveratrol has been shown to cause expansion of ER, and ER caspase mediated apoptosis [314]. Similarly, it was demonstrated that resveratrol sensitizes tamoxifen in estrogen receptor resistance breast cancer models that show epithelial-to-mesenchymal transition [315]. In a glioma model, resveratrol was shown to suppress X linked inhibitor of apoptotic proteins [316]. While Díaz-Chávez [317] utilized a proteomic approach to investigate the mechanism of resveratrol mediated chemosensitization and highlighted the role of hsp27 (at least in the model they tested).
Other studies support the apoptosis and necrosis inducing effects of resveratrol in different cancer models. Aside from resveratrol, studies have also focused on its biological analog piceatannol as well. For example, Farrand and colleagues [318] demonstrated that piceatannol enhances cisplatin sensitivity in ovarian cancer via modulation of p53, X-linked inhibitor of apoptosis protein (XIAP), and mitochondrial fission. Several natural compounds have been employed to assess their anti-apoptotic effects in in vitro models of CLL. For example, resveratrol (3,4′,5-trihydroxystilbene), a phytoalexin well known for its healthy properties, induced apoptosis in WSU-CLL cells (derived from a CLL patient resistant to fludarabine) and in B cells isolated from CLL patients; instead, normal peripheral blood mononuclear cells were slightly affected. The effect of this compound, used at 10–50 µM concentration, was correlated to a down-regulation of two anti-apoptotic proteins, inducible nitric oxide synthase and Bcl-2 [319,320]. Recently, it has been reported that oral administration of resveratrol (5 g/day) for 4 weeks, to three CLL patients, decreased circulating white blood cell counts and directly lowered O-linked β-N-acetylglucosamine (O-GlcNAc) levels in leukemic cells through proteasomal activation; this is an interesting result considering that CLL cells are characterized by high levels of proteins that are post-translationally modified by O-GlcNAc moieties [321]. Resveratrol, in combination with quercetin, induced apoptosis and cell cycle arrest in G0/G1 in human 232B4 cell line derived from CLL [322]. Collectively, these studies very clearly demonstrate that grape derivative resveratrol and its analogs can either induce apoptosis by themselves or can sensitize resistant cells to apoptosis inducers.
10.3. Curcumin
Curcumin, which is the major component of turmeric has been used in folklore medicine for centuries. The first reports on anti-cancer activity of curcumin were reported in 1985 [323]. Since then more than 3000 studies have been reported on the different anti-cancer mechanisms of curcumin and related analogs (numbers from PubMed search). Numerous studies have shown the apoptotis inducing effects of curcumin and analogs in cancer cell lines (the readers are referred to several outstanding reviews in the literature [324]).
In primary B-CLL cells curcumin induced apoptosis with a mean EC50 of 5.5 µM, fourfold lower than the EC50 observed in normal mononuclear cells (21.8 µM). The molecule blocks NF-κB signaling, probably through inhibition of IκB [325]. Some authors observed an induction of apoptosis in primary B-cells using curcumin in association with other natural compounds, such as rapamycin and EGCG. In the former case, the effect was associated with a decreased expression of Bcl-2 and an increased level of the pro-apoptotic factor Bax [326]. Sequential administration of curcumin and EGCG led to a substantial increase in B-cells death, overcoming stromal protection [327,328]. These are some examples showing the potency of curcumin in overcoming resistance to apoptosis. The synergism shown by curcumin with other natural compounds need to be examined in greater details in order to move these combinations in clinical studies.
10.4. Quercetin
Quercetin a phytochemical found in a broad range of fruits, vegetables and beverage, is widely known for its antioxidant, anti-inflammatory, anti-proliferative and apoptotic effects both in cell culture and animal models [329,330]. Quercetin at relatively low concentrations (10–20 µM) is able to down-regulate Mcl-1 and thus sensitize to apoptosis the B-cells isolated from CLL patients [331,332], with the maximal effect observed in combined treatments with apoptotic inducers (see below). Moreover, recent studies have reported that quercetin affects Hh, Wnt, as well as Notch signaling, emphasizing the potential efficacy of the molecule against CLL [333].
10.5. Isothiocyanates
Several studies have documented the cancer-preventive activity of many isothiocyanates (ITCs), occurring in cruciferous vegetables. They produce their anticancer effects through distinct but interconnected signaling pathways [334]. Sulforaphane, a well-studied ITC, restored chemosensitivity to DOX in mouse fibroblasts with p53 mutated at codon 220 [335]. The clinical pattern observed for the above reported mutation sustains a strong oncogenic potential and a lack of chemotherapy response. Sulforaphane was able to increase the efficacy of DOX, allowing its administration at levels within the concentration range clinically achievable [336]. The mechanism by which Sulforaphane reversed DOX resistance involves a rapid depletion of glutathione that renders cells more susceptible to DOX-induced oxidative stress, stress-induced damage, and apoptosis induction. The same study has shown that, pre-treatment with N-acetyl-cysteine, a glutathione precursor, prevented Sulforaphane plus DOX-induced apoptosis. Since the clinical doses of DOX currently being used during therapy are associated with severe cardiotoxicity [337], Gupta et al. [338] analyzed the ability of phenylethyl ITC (PEITC) to enhance the cytotoxic effects of low (sub-toxic) concentrations of DOX in human breast carcinoma cells stably transfected with the oncogene HER2. PEITC increased the cytotoxicity of DOX. Moreover, DOX treatment suppressed HER2 expression modestly, whereas the combination of PEITC with DOX markedly decreased the expression of HER2 and the phosphorylation of STAT3, known to play a critical role in the expression of cell proliferative pathways [339]. Consistently, combination treatment showed increased cleavage of caspase 3 and PARP as compared to individual treatment indicating apoptosis. In colorectal cancer cells, co-administration of Sulforaphane potentiated the growth inhibition activity of oxaliplatin through a reduction of intracellular ATP levels, activation of caspase-3, DNA fragmentation, and PARP cleavage [340]. Cisplatin was also tested in combination with PEITC in endometrial cancer cells [341] or with BITC in leukemia cells [342]. The combination therapy induced an increase in caspase-3 activity and increased levels of cleaved PARP. Such effects were not cell-specific since these results could also be observed in breast cancer cells. The sensitization induced by ITCs was partly mediated by ERK and Noxa activation. Interestingly, no effect was found in normal breast cells or lymphocytes.
ITCs also enhanced the efficacy of cisplatin when used against human non-small cell lung cancer cells [343]. Neither cellular platinum accumulation nor DNA platination accounts for this effect. Protein binding is important for the induction of apoptosis by ITCs [344]. ITCs covalently modify cysteine residues in tubulin, resulting in tubulin conformational changes, disruption of microtubule polymerization, and ultimately apoptosis. Tubulin-binding agents such as paclitaxel are commonly used in combination with platinum drugs to treat non-small cell lung cancer [345]. Thus, β-tubulin depletion may correlate and be important for sensitization to platinum compounds by ITCs.
Taken together, the evidence reported above indicates that EGCG and ITCs have an enormous potential for enhancing tumor cell sensitivity to therapy. However, some recent results sound a cautionary note. EGCG and ITCs are able to increase Nrf2 expression [346], a key transcription factor inducing a cytoprotective gene array. Since many standard chemotherapeutic agents are cytotoxic, their cytotoxic effects can be abrogated by tumor cells by upregulation of Nrf2 signaling, thereby yielding a more aggressive tumor [347]. Even if pharmacologic induction of Keap1–Nrf2-signaling axis allows for pulsed rather than permanent induction [348], further studies are necessary before suggesting the use of EGCG or ITCs in patients with established cancers who are undergoing chemotherapy treatment.
10.6. Marine derived agents with apoptosis inducing properties
Aside from different plant derived natural products, nature has bestowed many other types of anti-cancer compounds from marine sources as well. A key word search on ‘Marine anticancer agents’ reveals more than 3000 hits in PubMed. Many of these studies directly point to the apoptotic inducing potential of marine derived compounds in cancer cell lines and animal xenograft models [349–358]. For those seeking additional detail in this area, we refer the reader to an excellent literature review on the topic [359].
In summary, there is ample laboratory and early phase clinical evidence demonstrating that natural agents (both plant and marine derived) can impact important signaling pathways associated with apoptosis. However, a lot remains to be learned on their exact mechanism of action especially in relation to overcoming resistance to apoptosis. Further studies on these agents are anticipated to enhance our knowledge that in turn will drive their rapid clinical use in combination with standard chemotherapeutics or targeted therapies against therapy resistant cancers.
11. Broad spectrum approach to obtain synergies between various different approaches
Given that the heterogeneity that is present in most cancers, t is our assumption that the complete arrest of the various sub-populations of immortalized cells in any given cancer will require simultaneous actions on mechanisms that are important for several aspects of cancer’s biology. We therefore believe that it is important to be able to anticipate synergies that might be achieved by acting on specific targets and with specific approaches (i.e., when contemplating an approach aimed at a broad-spectrum of targets). Accordingly, in this review, we also explored cross-hallmark relationships that have been found between the prioritized target sites and the approaches that we identified in this review.
Specifically, when evidence in the literature showed that the targets and approaches that we identified as relevant for apoptosis resistance were also therapeutically relevant for other aspects of cancer’s biology (i.e., anti-carcinogenic) we noted them as having “complementary” cross-hallmark effects. While those that were found to have pro-carcinogenic actions were noted as having “contrary” effects. In instances where reports on relevant actions in other aspects of cancer biology were mixed (i.e., reports showing both pro-carcinogenic potential and anti-carcinogenic potential), the term “controversial” was used. Finally, in instances where no literature support was found to document the relevance of a target site or approach in a particular aspect of cancer’s biology, we documented this as “no known relationship”. These validation results are shown below in tabular form in Tables 1 and 2.
Table 1.
Relationships between resistance to apoptosis targets and cancer hallmarks.
| Targets related to resistance to apoptosis |
Inhibit Bcl-2 | Inhibit Mcl-1 | Activate tumor autophagy | Activate tumor necrosis | Inhibit hsp90 | Inhibit proteasome | Inhibit nuclear exporter CRM1 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Other cancer hallmarks | |||||||||||||
| Genomic instability | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Sustained proliferative signaling | + | [360,361] | + | [32,362] | +/− | [363,364] | +/− | [365,366] | + | [367,368] | + | [369,370] | + |
| [141] | |||||||||||||
| Tumor promoting inflammation | + | [371,372] | + | [373,374] | − | [375,376] | +/− | [377,378] | + | [379,380] | + | [381–383] | 0 |
| Evasion of Anti–growth Signaling | + | [384–386] | + | [237,387] | + | [388] | + | [389] | + | [390,391] | + | [392,393] | + |
| Replicative immortality | +/− | [395,396] | + | [362] | +/− | [397–399] | +/− | [400] | + | [401,402] | + | [403,404] | [394] |
| Dysregulated metabolism | + | [405–407] | + | [408–410] | + | [411–413] | + | [414–417] | + | [418–420] | + | [421] | 0 |
| Immune system evasion | + | [422] | + | [423] | 0 | + | [424] | 0 | + | [425–427] | 0 | ||
| Angiogenesis | + | [428] | + | [429] | +/− | [430] | − | [431] | + | [432] | + | [433] | + |
| [434] | |||||||||||||
| Tissue invasion and metastasis | + | [435] | + | [436] | + | [437,438] | + | [439] | - | [440,441] | + | [442] | + |
| [443] | |||||||||||||
| Tumor microenvironment | + | [444,445] | + | [444] | +/− | [446,447] | − | [448] | + | [449] | + | [450] | + |
| [451] | |||||||||||||
Prioritized targets were evaluated for known effects in other cancer hallmark areas. Targets that were found to have complementary, anti-carcinogenic actions reported in another hallmark area were indicated with “+”, while targets that were found to have pro-carcinogenic actions in another hallmark area were indicated with “-”. In instances where reports on relevant actions in other hallmark areas were mixed (i.e., reports showing both anti-carcinogenic potential and pro-carcinogenic potential), the symbol “+/−” was used. Finally, in instances where no literature support was found to document the relevance of a target in a particular aspect of cancer‘s biology, we documented this as “0”. These cross-hallmark relationships are reported in the upper rows of the table.
Table 2.
Relationships of the approaches to different cancer hallmarks.
| Approaches | Gossypol | EGCG | UMI-77 | Triptolide | Selinexor | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Other cancer hallmarks | ||||||||||
| Genomic instability | ||||||||||
| Sustained proliferative signaling | 0 | + | [452,453] | 0 | 0 | 0 | ||||
| Tumor promoting inflammation | + | [454,455] | + | [456,457] | + | [32] | + | [458,459] | 0 | |
| Evasion of anti-growth signaling | + | [460,461] | + | [462,463] | 0 | + | [464,465] | 0 | ||
| Replicative immortality | + | [466,467] | + | [468–470] | + | [32] | + | [471] | 0 | |
| Dysregulated metabolism | + | [472,473] | + | [474,475] | 0 | + | [476] | 0 | ||
| Immune system evasion | + | [477] | + | [478] | 0 | + | [479–481] | 0 | ||
| Angiogenesis | 0 | + | [482,483] | 0 | − | [484] | 0 | |||
| Tissue invasion and metastasis | + | [454] | + | [485] | 0 | + | [486] | 0 | ||
| Tumor microenvironment | + | [487] | + | [488] | + | [32] | + | [489,490] | + | [491,492] |
| + | [493] | + | [483,494] | + | [32] | + | [495,496] | + | [497] | |
Selected approaches were evaluated for reported actions in other cancer hallmark areas. Approaches that were found to have complementary, anti-carcinogenic actions in a particular hallmark area were were indicated with “+”, while approaches that were found to have pro-carcinogenic actions in a particular hallmark area were indicated with “-”. In instances where reports on relevant actions in other hallmarks were mixed (i.e., reports showing both anti-carcinogenic and pro-carcinogenic potential), the symbol “+/−” was used. Finally, in instances where no literature support was found to document the relevance of an approach in a particular aspect of cancer’s biology, we documented this as “0”. These cross-hallmark relationships are reported in the upper rows of the table.
It is predicted that future cancer therapeutics will be based on network pharmacology strategies that will likely involve empirical testing of mixtures of constituents. Therefore, we wanted to create a starting point for other researchers who might be considering translational projects. We anticipated interest in approaches reported to exhibit a large number of anti-carcinogenic actions across the hallmarks and we anticipated that a lack of pro-carcinogenic potential might be important to identify in advance (since targets or approaches that have been shown to exert pro-carcinogenic actions would potentially represent a confounding and unwanted influence/factor in empirical research).
Our cross validation exercise showed that in some instances, the underlying evidence used to support the indication of a cross-hallmark relationship was robust, consisting of multiple studies involving detailed in vitro and in vivo findings. In other instances, however, the underlying evidence that was used to report the existence of a cross-hallmark relationship was quite weak (e.g., consisting of only a single in vitro study involving a single cell-type). Additionally, there are examples of approaches that are known to exert different effects at different dose levels and in different tissues but dose-levels and cell/tissue types were not used to discriminate when gathering together these reported actions. Nonetheless, given that the overarching goal in this project was to create a foundation that would allow researchers to look systematically across the literature in each of these areas, the tables should serve as a useful starting point as long as they are approached with caveats in mind and a degree of caution. Essentially, we believe that this heuristic should be useful to consider synergies that might be anticipated in testing that involves certain targets and/or mixtures of chemical constituents that are being considered for therapeutic effects.
12. Conclusions and future directions
Resistance to apoptosis is multi-factorial involving the interaction of various signaling pathways at multiple levels. Therefore, the use of single pathway targeted agents to commit cancer cells to undergo apoptosis is not a feasible strategy (except in isolated cases). Hence, this requires a careful selection of treatment strategies that are based on a comprehensive understanding of the biological networks involved in resistance. This article presents some of the major genetic and epigenetic drivers of apoptosis resistance and provides a list of prioritized targets and the approaches that can be utilized against them to overcome de novo or acquired resistance. In this review, first we discussed how Bcl-2 family proteins play critical role in the biology of apoptosis activation/resistance. We presented knowledge on the development of agents such as gossypol and synthetic compounds particularly ABT-737 that have entered clinical trials. Additionally, we also highlighted problems associated with Bcl-2 inhibition and the development of resistance by one of its family member Mcl-1 making it an attractive target. In this direction, specific drugs against Mcl-1 are being investigated in the pre-clinical setting and some of these agents are expected to enter clinical evaluation in the near future. Additional cell death mechanisms such as autophagy and necrosis were also discussed and strategies, particularly the use of natural agents such EGCG were highlighted. The role of chaperone protein hsp70 in apoptosis resistance was evaluated and suggestions to overcome this critical protein marker using natural products were presented. The article also discusses the molecular mechanisms that support the resistance to apoptosis in different cancers such as glioblastoma, multiple myeloma, CLL, prostate cancer, breast cancer, colon cancer and pancreatic cancer. The role of epigenetic players, particularly miRNAs, in the development of apoptosis resistance was also highlighted. The cross-validation tables (Tables 1 and 2) are offered here as a simple heuristic framework that is intended to help researchers approach the topic of anticipated synergies. Although, these attempts do not represent a homogenous set of underlying data, it is hoped that it can serve as a starting point for the translational research that will be needed for the design of effective natural product based therapies. Rigorous experimentation will obviously be needed to determine whether or not actual synergies between the identified approaches emerge that can be predicted and clinically applied. A much broader range of targets overall may be the only chance we will have to address cancer associate heterogeneity. It is a promising approach, but a considerable amount of encompassing research needs to follow to determine methodological validity. Collectively, these unique targets and specific strategies may bring forward a broad form of therapy to overcome resistance to apoptosis resulting in better treatment outcome in patients suffering from cancer.
Acknowledgements
NIH Grants R21 1R21CA16984801 and 1R21CA17597401 to RMM is acknowledged. This work was partially supported by the Scientific Research Foundation for the Returned Oversea Scholars and State Education Ministry and Scientific and Technological Innovation Project, Harbin (2012RFLXS011 to HJ Yang), and NIH/NCI (1R01CA20009, 5R01CA127258-05 and R21CA184788 to Q.P. Dou, and NIH P30 CA22453 to Karmanos Cancer Institute). HY Hsu was funded by grants from the Ministry of Science and Technology of Taiwan (NSC93-2314-B-320-006 and NSC94-2314-B-320-002) and LT Lin was supported by a grant from Taipei Medical University (TMU101-AE3-Y19) NIH Grant K08 NS083732 to MDS is acknowledged. Dr. Gian Luigi Russo acknowledges the Fulbright Research Scholar program 2013-14. Work in CF’s lab described here was supported by Ministero dell’Istruzione dell’Università e della Ricerca (grant number: 200974K3JC). Dr. Clement G. Yedjou acknowledges NIH grant 5G12RR013459-13 through the RCMI-Center for Environmental Health at Jackson State University. Dr. Georgakilas was supported by an EU Marie Curie Reintegration Grant MC-CIG-303514, Greek National funds through the Operational Program ‘Educational and Lifelong Learning of the National Strategic Reference Framework (NSRF)-Research Funding Program: THALES (Grant number MIS 379346) and COST Action CM1201 ‘Biomimetic Radical Chemistry’. The work reported by Nagi B. Kumar was funded by the National Institute of Health – National Cancer Institute R01 CA12060-01A1. Work in Carmela Famignori’s lab described here was supported by Ministero dell’Istruzione dell’Università e della Ricerca (grant number: 200974K3JC). Dr. Swapan K Ray and Mrinmay Chakrabarti acknowledge the support by United Soybean Board (USB Chesterfield, M, United States). The USB had no involvement in the writing of the manuscript or the decision to submit the manuscript for publication. Amr Amin was supported partially by Al-Jalila Foundation 2015, Zayed Center for Health Sciences 2015, and Terry Fox Foundation 2014. NIH grant 1 R21 CA188818 01A1 to Asfar Azmi is acknowledged.
References
- [1].Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ringash J, Au HJ, Siu LL, Shapiro JD, Jonker DJ, Zalcberg JR, et al. Quality of life in patients with K-RAS wild-type colorectal cancer: the CO.20 phase 3 randomized trial. Cancer. 2014;120:181–9. doi: 10.1002/cncr.28410. [DOI] [PubMed] [Google Scholar]
- [3].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [4].Du TA. Cell death: balance through a bivalent regulator. Nat Rev Mol Cell Biol. 2013;14:546. doi: 10.1038/nrm3637. [DOI] [PubMed] [Google Scholar]
- [5].Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–32. [PubMed] [Google Scholar]
- [6].Morin PJ. Drug resistance and the microenvironment: nature and nurture. Drug Resist Updat. 2003;6:169–72. doi: 10.1016/s1368-7646(03)00059-1. [DOI] [PubMed] [Google Scholar]
- [7].Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46:308–16. doi: 10.1007/s12033-010-9321-2. [DOI] [PubMed] [Google Scholar]
- [8].Ferguson LR, Baguley BC. Multidrug resistance and mutagenesis. Mutat Res. 1993;285:79–90. doi: 10.1016/0027-5107(93)90054-j. [DOI] [PubMed] [Google Scholar]
- [9].Baguley BC. Multidrug resistance in cancer. Methods Mol Biol. 2010;596:1–14. doi: 10.1007/978-1-60761-416-6_1. [DOI] [PubMed] [Google Scholar]
- [10].Masood A, Azmi AS, Mohammad RM. Small molecule inhibitors of bcl-2 family proteins for pancreatic cancer therapy. Cancers (Basel) 2011;3:1527–49. doi: 10.3390/cancers3021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2013;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- [12].Vitagliano O, Addeo R, D’Angelo V, Indolfi C, Indolfi P, Casale F. The Bcl-2/Bax and Ras/Raf/MEK/ERK signaling pathways: implications in pediatric leukemia pathogenesis and new prospects for therapeutic approaches. Expert Rev Hematol. 2013;6:587–97. doi: 10.1586/17474086.2013.827415. [DOI] [PubMed] [Google Scholar]
- [13].Mortenson MM, Galante JG, Gilad O, Schlieman MG, Virudachalam S, Kung HJ, et al. BCL-2 functions as an activator of the AKT signaling pathway in pancreatic cancer. J Cell Biochem. 2007;102:1171–9. doi: 10.1002/jcb.21343. [DOI] [PubMed] [Google Scholar]
- [14].Kumar P, Ning Y, Polverini PJ. Endothelial cells expressing Bcl-2 promotes tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor invasion. Lab Investig: J Techn Methods Pathol. 2008;88:740–9. doi: 10.1038/labinvest.2008.46. [DOI] [PubMed] [Google Scholar]
- [15].Tucker CA, Kapanen AI, Chikh G, Hoffman BG, Kyle AH, Wilson IM, et al. Silencing Bcl-2 in models of mantle cell lymphoma is associated with decreases in cyclin D1, nuclear factor-kappaB, p53, bax, and p27 levels. Mol Cancer Ther. 2008;7:749–58. doi: 10.1158/1535-7163.MCT-07-0302. [DOI] [PubMed] [Google Scholar]
- [16].Anai S, Shiverick K, Medrano T, Nakamura K, Goodison S, Brown BD, et al. Downregulation of BCL-2 induces downregulation of carbonic anhydrase IX, vascular endothelial growth factor, and pAkt and induces radiation sensitization. Urology. 2007;70:832–7. doi: 10.1016/j.urology.2007.06.1118. [DOI] [PubMed] [Google Scholar]
- [17].Patel MP, Masood A, Patel PS, Chanan-Khan AA. Targeting the Bcl-2. Curr Opin Oncol. 2009;21:516–23. doi: 10.1097/CCO.0b013e328331a7a4. [DOI] [PubMed] [Google Scholar]
- [18].Wang JY. Nucleo-cytoplasmic communication in apoptotic response to genotoxic and inflammatory stress. Cell Res. 2005;15:43–8. doi: 10.1038/sj.cr.7290263. [DOI] [PubMed] [Google Scholar]
- [19].Ewen CL, Kane KP, Bleackley RC. A quarter century of granzymes. Cell Death Differ. 2012;19:28–35. doi: 10.1038/cdd.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lord SJ, Rajotte RV, Korbutt GS, Bleackley RC. Granzyme B: a natural born killer. Immunol Rev. 2003;193:31–8. doi: 10.1034/j.1600-065x.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- [21].Chowdhury D, Beresford PJ, Zhu P, Zhang D, Sung JS, Demple B, et al. The exonuclease TREX1 is in the SET complex and acts in concert with NM23-H1 to degrade DNA during granzyme A-mediated cell death. Mol Cell. 2006;23:133–42. doi: 10.1016/j.molcel.2006.06.005. [DOI] [PubMed] [Google Scholar]
- [22].Yuan S, Akey CW. Apoptosome structure, assembly, and procaspase activation. Structure. 2013;21:501–15. doi: 10.1016/j.str.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Azmi AS, Mohammad RM. Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J Cell Physiol. 2009;218:13–21. doi: 10.1002/jcp.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Azmi AS, Wang Z, Philip PA, Mohammad RM, Sarkar FH. Emerging Bcl-2 inhibitors for the treatment of cancer. Expert Opin Emerg Drugs. 2011;16:59–70. doi: 10.1517/14728214.2010.515210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009;66:1326–36. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Warr MR, Shore GC. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med. 2008;8:138–47. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- [27].Quinn BA, Dash R, Azab B, Sarkar S, Das SK, Kumar S, et al. Targeting Mcl-1 for the therapy of cancer. Expert Opin Investig Drugs. 2011;20:1397–411. doi: 10.1517/13543784.2011.609167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Karami H, Baradaran B, Esfehani A, Sakhinia M, Sakhinia E. Down-regulation of Mcl-1 by small interference RNA induces apoptosis and sensitizes HL-60 leukemia cells to etoposide. Asian Pac J Cancer Prev. 2014;15:629–35. doi: 10.7314/apjcp.2014.15.2.629. [DOI] [PubMed] [Google Scholar]
- [29].Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- [30].Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–13. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lestini BJ, Goldsmith KC, Fluchel MN, Liu X, Chen NL, Goyal B, et al. Mcl1 down-regulation sensitizes neuroblastoma to cytotoxic chemotherapy and small molecule Bcl2-family antagonists. Cancer Biol Ther. 2009;8:1587–95. doi: 10.4161/cbt.8.16.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Abulwerdi F, Liao C, Liu M, Azmi AS, Aboukameel A, Mady AS, et al. A novel small-molecule inhibitor of mcl-1 blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther. 2014;13:565–75. doi: 10.1158/1535-7163.MCT-12-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Basu A, Bodycombe NE, Cheah JH, Price EV, Liu K, Schaefer GI, et al. An interactive resource to identify cancer genetic and lineage dependencies targeted by small molecules. Cell. 2013;154:1151–61. doi: 10.1016/j.cell.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [35].Gandhi L, Camidge DR, Ribeiro de OM, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–16. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- [37].Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–73. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–7. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu D, Yang Y, Liu Q, Wang J. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med Oncol. 2011;28:105–11. doi: 10.1007/s12032-009-9397-3. [DOI] [PubMed] [Google Scholar]
- [40].Xi G, Hu X, Wu B, Jiang H, Young CY, Pang Y, et al. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett. 2011;307:141–8. doi: 10.1016/j.canlet.2011.03.026. [DOI] [PubMed] [Google Scholar]
- [41].Sharma N, Thomas S, Golden EB, Hofman FM, Chen TC, Petasis NA, et al. Inhibition of autophagy and induction of breast cancer cell death by mefloquine, an antimalarial agent. Cancer Lett. 2012;326:143–54. doi: 10.1016/j.canlet.2012.07.029. [DOI] [PubMed] [Google Scholar]
- [42].Carew JS, Nawrocki ST, Giles FJ, Cleveland JL. Targeting autophagy: a novel anticancer strategy with therapeutic implications for imatinib resistance. Biologics. 2008;2:201–4. doi: 10.2147/btt.s1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Carew JS, Medina EC, Esquivel JA, Mahalingam D, Swords R, Kelly K, et al. Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation. J Cell Mol Med. 2010;14:2448–59. doi: 10.1111/j.1582-4934.2009.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Carew JS, Kelly KR, Nawrocki ST. Autophagy as a target for cancer therapy: new developments. Cancer Manag Res. 2012;4:357–65. doi: 10.2147/CMAR.S26133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dupere-Richer D, Kinal M, Menasche V, Nielsen TH, Del RS, Pettersson F, et al. Vorinostat-induced autophagy switches from a death-promoting to a cytoprotective signal to drive acquired resistance. Cell Death Dis. 2013;4:e486. doi: 10.1038/cddis.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F, et al. Cisplatin-induced downregulation of miR-199a-5p increases drug resistance by activating autophagy in HCC cell. Biochem Biophys Res Commun. 2012;423:826–31. doi: 10.1016/j.bbrc.2012.06.048. [DOI] [PubMed] [Google Scholar]
- [47].Wang SY, Yu QJ, Zhang RD, Liu B. Core signaling pathways of survival/death in autophagy-related cancer networks. Int J Biochem Cell Biol. 2011;43:1263–6. doi: 10.1016/j.biocel.2011.05.010. [DOI] [PubMed] [Google Scholar]
- [48].Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- [49].Li X, Wu WK, Sun B, Cui M, Liu S, Gao J, et al. Dihydroptychantol A, a macrocyclic bisbibenzyl derivative, induces autophagy and following apoptosis associated with p53 pathway in human osteosarcoma U2OS cells. Toxicol Appl Pharmacol. 2011;251:146–54. doi: 10.1016/j.taap.2010.12.007. [DOI] [PubMed] [Google Scholar]
- [50].Wang W, Fan H, Zhou Y, Duan P, Zhao G, Wu G. Knockdown of autophagy-related gene BECLIN1 promotes cell growth and inhibits apoptosis in the A549 human lung cancer cell line. Mol Med Rep. 2013;7:1501–5. doi: 10.3892/mmr.2013.1379. [DOI] [PubMed] [Google Scholar]
- [51].Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–2. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- [52].Pan X, Zhang X, Sun H, Zhang J, Yan M, Zhang H. Autophagy inhibition promotes 5-fluorouraci-induced apoptosis by stimulating ROS formation in human non-small cell lung cancer A549 cells. PLoS ONE. 2013;8:e56679. doi: 10.1371/journal.pone.0056679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Salminen A, Kaarniranta K, Kauppinen A. Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: impact on the aging process. Ageing Res Rev. 2013;12:520–34. doi: 10.1016/j.arr.2012.11.004. [DOI] [PubMed] [Google Scholar]
- [54].He C, Wei Y, Sun K, Li B, Dong X, Zou Z, et al. Beclin 2 functions in autophagy, degradation of G protein-coupled receptors, and metabolism. Cell. 2013;154:1085–99. doi: 10.1016/j.cell.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Marquez RT, Xu L. Bcl-2:Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res. 2012;2:214–21. [PMC free article] [PubMed] [Google Scholar]
- [56].Tai WT, Shiau CW, Chen HL, Liu CY, Lin CS, Cheng AL, et al. Mcl-1-dependent activation of Beclin 1 mediates autophagic cell death induced by sorafenib and SC-59 in hepatocellular carcinoma cells. Cell Death Dis. 2013;4:e485. doi: 10.1038/cddis.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cheng P, Ni Z, Dai X, Wang B, Ding W, Rae SA, et al. The novel BH-3 mimetic apogossypolone induces Beclin-1- and ROS-mediated autophagy in human hepatocellular carcinoma [corrected] cells. Cell Death Dis. 2013;4:e489. doi: 10.1038/cddis.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pomerantz J, Schreiber-Agus N, Liegeois NJ, Silverman A, Alland L, Chin L, et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2’s inhibition of p53. Cell. 1998;92:713–23. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- [59].Sherr CJ, Weber JD. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–9. doi: 10.1016/s0959-437x(99)00038-6. [DOI] [PubMed] [Google Scholar]
- [60].Reef S, Zalckvar E, Shifman O, Bialik S, Sabanay H, Oren M, et al. A short mitochondrial form of p19ARF induces autophagy and caspase-independent cell death. Mol Cell. 2006;22:463–75. doi: 10.1016/j.molcel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- [61].Balaburski GM, Hontz RD, Murphy ME. p53 and ARF: unexpected players in autophagy. Trends Cell Biol. 2010;20:363–9. doi: 10.1016/j.tcb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gozuacik D. Identification of human cancer-related genes by naturally occurring Hepatitis B Virus DNA tagging. Oncogene. 2001;20:6233–40. doi: 10.1038/sj.onc.1204835. [DOI] [PubMed] [Google Scholar]
- [63].Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–34. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- [64].Pimkina J, Murphy ME. Interaction of the ARF tumor suppressor with cytosolic HSP70 contributes to its autophagy function. Cancer Biol Ther. 2011;12:503–9. doi: 10.4161/cbt.12.6.15976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–7. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- [66].Junttila MR, Karnezis AN, Garcia D, Madriles F, Kortlever RM, Rostker F, et al. Selective activation of p53-mediated tumour suppression in high-grade tumours. Nature. 2010;468:567–71. doi: 10.1038/nature09526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dewaele M, Maes H, Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy. 2010;6:838–54. doi: 10.4161/auto.6.7.12113. [DOI] [PubMed] [Google Scholar]
- [68].Nicolau-Galmes F, Asumendi A, onso-Tejerina E, Perez-Yarza G, Jangi SM, Gardeazabal J, et al. Terfenadine induces apoptosis and autophagy in melanoma cells through ROS-dependent and -independent mechanisms. Apoptosis. 2011;16:1253–67. doi: 10.1007/s10495-011-0640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–52. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- [70].Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–60. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–81. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Azad MB, Chen Y, Henson ES, Cizeau J, Millan-Ward E, Israels SJ, et al. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shi Y, Tang B, Yu PW, Tang B, Hao YX, Lei X, et al. Autophagy protects against oxaliplatin-induced cell death via ER stress and ROS in Caco-2 cells. PLoS ONE. 2012;7:e51076. doi: 10.1371/journal.pone.0051076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Boldogh I, Roy G, Lee MS, Bacsi A, Hazra TK, Bhakat KK, et al. Reduced DNA double strand breaks in chlorambucil resistant cells are related to high DNA-PKcs activity and low oxidative stress. Toxicology. 2003;193:137–52. doi: 10.1016/j.tox.2003.08.013. [DOI] [PubMed] [Google Scholar]
- [75].Huang Q, Wu YT, Tan HL, Ong CN, Shen HM. A novel function of poly(ADP-ribose) polymerase-1 in modulation of autophagy and necrosis under oxidative stress. Cell Death Differ. 2009;16:264–77. doi: 10.1038/cdd.2008.151. [DOI] [PubMed] [Google Scholar]
- [76].Munoz-Gamez JA, Rodriguez-Vargas JM, Quiles-Perez R, guilar-Quesada R, Martin-Oliva D, de MG, et al. PARP-1 is involved in autophagy induced by DNA damage. Autophagy. 2009;5:61–74. doi: 10.4161/auto.5.1.7272. [DOI] [PubMed] [Google Scholar]
- [77].Wyllie AH. Death from inside out: an overview. Philos Trans R Soc Lond B: Biol Sci. 1994;345:237–41. doi: 10.1098/rstb.1994.0099. [DOI] [PubMed] [Google Scholar]
- [78].Giampietri C, Starace D, Petrungaro S, Filippini A, Ziparo E. Necroptosis: molecular signalling and translational implications. Int J Cell Biol. 2014;2014:490275. doi: 10.1155/2014/490275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Van CS, Van den BW. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214–23. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- [80].Walker NI, Harmon BV, Gobe GC, Kerr JF. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- [81].Murdoch WJ, Wilken C, Young DA. Sequence of apoptosis and inflammatory necrosis within the formative ovulatory site of sheep follicles. J Reprod Fertil. 1999;117:325–9. doi: 10.1530/jrf.0.1170325. [DOI] [PubMed] [Google Scholar]
- [82].Murdoch WJ. Plasmin-tumour necrosis factor interaction in the ovulatory process. J Reprod Fertil Suppl. 1999;54:353–8. [PubMed] [Google Scholar]
- [83].Jaattela M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2003;4:416–23. doi: 10.1038/ni0503-416. [DOI] [PubMed] [Google Scholar]
- [84].Roach HI, Clarke NM. Physiological cell death of chondrocytes in vivo is not confined to apoptosis. New observations on the mammalian growth plate. J Bone Joint Surg Br. 2000;82:601–13. doi: 10.1302/0301-620x.82b4.9846. [DOI] [PubMed] [Google Scholar]
- [85].Barkla DH, Gibson PR. The fate of epithelial cells in the human large intestine. Pathology. 1999;31:230–8. doi: 10.1080/003130299105043. [DOI] [PubMed] [Google Scholar]
- [86].Chautan M, Chazal G, Cecconi F, Gruss P, Golstein P. Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr Biol. 1999;9:967–70. doi: 10.1016/s0960-9822(99)80425-4. [DOI] [PubMed] [Google Scholar]
- [87].Lenardo MJ, Angleman SB, Bounkeua V, Dimas J, Duvall MG, Graubard MB, et al. Cytopathic killing of peripheral blood CD4(+) T lymphocytes by human immunodeficiency virus type 1 appears necrotic rather than apoptotic and does not require env. J Virol. 2002;76:5082–93. doi: 10.1128/JVI.76.10.5082-5093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- [89].Cho Y, McQuade T, Zhang H, Zhang J, Chan FK. RIP1-dependent and independent effects of necrostatin-1 in necrosis and T cell activation. PLoS ONE. 2011;6:e23209. doi: 10.1371/journal.pone.0023209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Marubayashi S, Koppikar P, Taldone T, bdel-Wahab O, West N, Bhagwat N, et al. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest. 2010;120:3578–93. doi: 10.1172/JCI42442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Shimamura T, Lowell AM, Engelman JA, Shapiro GI. Epidermal growth factor receptors harboring kinase domain mutations associate with the heat shock protein 90 chaperone and are destabilized following exposure to gel-danamycins. Cancer Res. 2005;65:6401–8. doi: 10.1158/0008-5472.CAN-05-0933. [DOI] [PubMed] [Google Scholar]
- [92].Shimamura T, Li D, Ji H, Haringsma HJ, Liniker E, Borgman CL, et al. Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinase inhibitor resistance. Cancer Res. 2008;68:5827–38. doi: 10.1158/0008-5472.CAN-07-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shimamura T, Perera SA, Foley KP, Sang J, Rodig SJ, Inoue T, et al. Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin Cancer Res. 2012;18:4973–85. doi: 10.1158/1078-0432.CCR-11-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Socinski MA, Goldman J, El-Hariry I, Koczywas M, Vukovic V, Horn L, et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin Cancer Res. 2013;19:3068–77. doi: 10.1158/1078-0432.CCR-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–70. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- [96].Masuda Y, Shima G, Aiuchi T, Horie M, Hori K, Nakajo S, et al. Involvement of tumor necrosis factor receptor-associated protein 1 (TRAP1) in apoptosis induced by beta-hydroxyisovalerylshikonin. J Biol Chem. 2004;279:42503–15. doi: 10.1074/jbc.M404256200. [DOI] [PubMed] [Google Scholar]
- [97].Fortugno P, Beltrami E, Plescia J, Fontana J, Pradhan D, Marchisio PC, et al. Regulation of survivin function by Hsp90. Proc Natl Acad Sci USA. 2003;100:13791–6. doi: 10.1073/pnas.2434345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gyurkocza B, Plescia J, Raskett CM, Garlick DS, Lowry PA, Carter BZ, et al. Antileukemic activity of shepherdin and molecular diversity of hsp90 inhibitors. J Natl Cancer Inst. 2006;98:1068–77. doi: 10.1093/jnci/djj300. [DOI] [PubMed] [Google Scholar]
- [99].Kang BH, Plescia J, Song HY, Meli M, Colombo G, Beebe K, et al. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J Clin Invest. 2009;119:454–64. doi: 10.1172/JCI37613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Siegelin MD. Inhibition of the mitochondrial Hsp90 chaperone network: a novel, efficient treatment strategy for cancer. Cancer Lett. 2013;333:133–46. doi: 10.1016/j.canlet.2013.01.045. [DOI] [PubMed] [Google Scholar]
- [101].Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- [102].Kisselev AF, Songyang Z, Goldberg AL. Why does threonine, and not serine, function as the active site nucleophile in proteasomes. J Biol Chem. 2000;275:14831–7. doi: 10.1074/jbc.275.20.14831. [DOI] [PubMed] [Google Scholar]
- [103].Moore BS, Eustaquio AS, McGlinchey RP. Advances in and applications of proteasome inhibitors. Curr Opin Chem Biol. 2008;12:434–40. doi: 10.1016/j.cbpa.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Borissenko L, Groll M. 20S proteasome and its inhibitors: crystallographic knowledge for drug development. Chem Rev. 2007;107:687–717. doi: 10.1021/cr0502504. [DOI] [PubMed] [Google Scholar]
- [105].Jesenberger V, Jentsch S. Deadly encounter: ubiquitin meets apoptosis. Nat Rev Mol Cell Biol. 2002;3:112–21. doi: 10.1038/nrm731. [DOI] [PubMed] [Google Scholar]
- [106].Voorhees PM, Orlowski RZ. The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol. 2006;46:189–213. doi: 10.1146/annurev.pharmtox.46.120604.141300. [DOI] [PubMed] [Google Scholar]
- [107].Richardson PG, Hideshima T, Anderson KC. Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control. 2003;10:361–9. doi: 10.1177/107327480301000502. [DOI] [PubMed] [Google Scholar]
- [108].Chen S, Fribley A, Wang CY. Potentiation of tumor necrosis factor-mediated apoptosis of oral squamous cell carcinoma cells by adenovirus-mediated gene transfer of NF-kappaB inhibitor. J Dent Res. 2002;81:98–102. [PubMed] [Google Scholar]
- [109].Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- [110].Jentsch S. Ubiquitin-dependent protein degradation: a cellular perspective. Trends Cell Biol. 1992;2:98–103. doi: 10.1016/0962-8924(92)90013-d. [DOI] [PubMed] [Google Scholar]
- [111].An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–75. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- [112].Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci USA. 2000;97:3850–5. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Dimmeler S, Breitschopf K, Haendeler J, Zeiher AM. Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: a link between the apoptosome and the proteasome pathway. J Exp Med. 1999;189:1815–22. doi: 10.1084/jem.189.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Glasspool RM, Teodoridis JM, Brown R. Epigenetics as a mechanism driving polygenic clinical drug resistance. Br J Cancer. 2006;94:1087–92. doi: 10.1038/sj.bjc.6603024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ajabnoor GM, Crook T, Coley HM. Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis. 2012;3:e260. doi: 10.1038/cddis.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Syed N, Coley HM, Sehouli J, Koensgen D, Mustea A, Szlosarek P, et al. Polo-like kinase Plk2 is an epigenetic determinant of chemosensitivity and clinical outcomes in ovarian cancer. Cancer Res. 2011;71:3317–27. doi: 10.1158/0008-5472.CAN-10-2048. [DOI] [PubMed] [Google Scholar]
- [117].Bredberg A, Bodmer W. Cytostatic drug treatment causes seeding of gene promoter methylation. Eur J Cancer. 2007;43:947–54. doi: 10.1016/j.ejca.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Matthew EM, Hart LS, Astrinidis A, Navaraj A, Dolloff NG, Dicker DT, et al. The p53 target Plk2 interacts with TSC proteins impacting mTOR signaling, tumor growth and chemosensitivity under hypoxic conditions. Cell Cycle (Georgetown, Tex) 2009;8:4168–75. doi: 10.4161/cc.8.24.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Coley HM, Safuwan NA, Chivers P, Papacharalbous E, Giannopoulos T, Butler-Manuel S, et al. The cyclin-dependent kinase inhibitor p57(Kip2) is epigenetically regulated in carboplatin resistance and results in collateral sensitivity to the CDK inhibitor seliciclib in ovarian cancer. Br J Cancer. 2012;106:482–9. doi: 10.1038/bjc.2011.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lee MP, Feinberg AP. Aberrant splicing but not mutations of TSG101 in human breast cancer. Cancer Res. 1997;57:3131–4. [PubMed] [Google Scholar]
- [121].Fischer DC, Noack K, Runnebaum IB, Watermann DO, Kieback DG, Stamm S, et al. Expression of splicing factors in human ovarian cancer. Oncol Rep. 2004;11:1085–90. [PubMed] [Google Scholar]
- [122].Piekielko-Witkowska A, Wiszomirska H, Wojcicka A, Poplawski P, Boguslawska J, Tanski Z, et al. Disturbed expression of splicing factors in renal cancer affects alternative splicing of apoptosis regulators, oncogenes, and tumor suppressors. PLoS ONE. 2010;5:e13690. doi: 10.1371/journal.pone.0013690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Karni R, de SE, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–93. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Merdzhanova G, Edmond V, De SS, Van den BA, Corcos L, Brambilla C, et al. E2F1 controls alternative splicing pattern of genes involved in apoptosis through upregulation of the splicing factor SC35. Cell Death Differ. 2008;15:1815–23. doi: 10.1038/cdd.2008.135. [DOI] [PubMed] [Google Scholar]
- [125].Edmond V, Brambilla C, Brambilla E, Gazzeri S, Eymin B. SRSF2 is required for sodium butyrate-mediated p21(WAF1) induction and premature senescence in human lung carcinoma cell lines. Cell Cycle (Georgetown, Tex) 2011;10:1968–77. doi: 10.4161/cc.10.12.15825. [DOI] [PubMed] [Google Scholar]
- [126].Edmond V, Moysan E, Khochbin S, Matthias P, Brambilla C, Brambilla E, et al. Acetylation and phosphorylation of SRSF2 control cell fate decision in response to cisplatin. EMBO J. 2011;30:510–23. doi: 10.1038/emboj.2010.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Diekmann Y, Pereira-Leal JB. Evolution of intracellular compartmentalization. Biochem J. 2013;449:319–31. doi: 10.1042/BJ20120957. [DOI] [PubMed] [Google Scholar]
- [128].Van BK, Corces VG. Nuclear organization and genome function. Annu Rev Cell Dev Biol. 2012;28:163–87. doi: 10.1146/annurev-cellbio-101011-155824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Schneider R, Grosschedl R. Dynamics and interplay of nuclear architecture, genome organization, and gene expression. Genes Dev. 2007;21:3027–43. doi: 10.1101/gad.1604607. [DOI] [PubMed] [Google Scholar]
- [130].Nagano A, Arahata K. Nuclear envelope proteins and associated diseases. Curr Opin Neurol. 2000;13:533–9. doi: 10.1097/00019052-200010000-00005. [DOI] [PubMed] [Google Scholar]
- [131].Raices M, D’Angelo MA. Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat Rev Mol Cell Biol. 2012;13:687–99. doi: 10.1038/nrm3461. [DOI] [PubMed] [Google Scholar]
- [132].Grossman E, Medalia O, Zwerger M. Functional architecture of the nuclear pore complex. Annu Rev Biophys. 2012;41:557–84. doi: 10.1146/annurev-biophys-050511-102328. [DOI] [PubMed] [Google Scholar]
- [133].Oeffinger M, Zenklusen D. To the pore and through the pore: a story of mRNA export kinetics. Biochim Biophys Acta. 2012;1819:494–506. doi: 10.1016/j.bbagrm.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Walde S, Kehlenbach RH. The part and the whole: functions of nucleoporins in nucleocytoplasmic transport. Trends Cell Biol. 2010;20:461–9. doi: 10.1016/j.tcb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- [135].Xu D, Farmer A, Chook YM. Recognition of nuclear targeting signals by Karyopherin-beta proteins. Curr Opin Struct Biol. 2010;20:782–90. doi: 10.1016/j.sbi.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Chook YM, Suel KE. Nuclear import by karyopherin-betas: recognition and inhibition. Biochim Biophys Acta. 2011;1813:1593–606. doi: 10.1016/j.bbamcr.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Fornerod M, Ohno M. Exportin-mediated nuclear export of proteins and ribonucleoproteins. Results Probl Cell Differ. 2002;35:67–91. doi: 10.1007/978-3-540-44603-3_4. [DOI] [PubMed] [Google Scholar]
- [138].Marelli M, Dilworth DJ, Wozniak RW, Aitchison JD. The dynamics of karyopherin-mediated nuclear transport. Biochem Cell Biol. 2001;79:603–12. [PubMed] [Google Scholar]
- [139].Yoneda Y. Nuclear pore-targeting complex and its role on nuclear protein transport. Arch Histol Cytol. 1996;59:97–107. doi: 10.1679/aohc.59.97. [DOI] [PubMed] [Google Scholar]
- [140].Marfori M, Lonhienne TG, Forwood JK, Kobe B. Structural basis of high-affinity nuclear localization signal interactions with importin-alpha. Traffic. 2012;13:532–48. doi: 10.1111/j.1600-0854.2012.01329.x. [DOI] [PubMed] [Google Scholar]
- [141].van der Watt PJ, Maske CP, Hendricks DT, Parker MI, Denny L, Govender D, et al. The Karyopherin proteins, Crm1 and Karyopherin beta1, are overexpressed in cervical cancer and are critical for cancer cell survival and proliferation. Int J Cancer. 2009;124:1829–40. doi: 10.1002/ijc.24146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 2007;17:193–201. doi: 10.1016/j.tcb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- [143].Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–11. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- [144].Shaulsky G, Goldfinger N, Tosky MS, Levine AJ, Rotter V. Nuclear localization is essential for the activity of p53 protein. Oncogene. 1991;6:2055–65. [PubMed] [Google Scholar]
- [145].Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr Med Chem. 2008;15:2648–55. doi: 10.2174/092986708786242859. [DOI] [PubMed] [Google Scholar]
- [146].Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clin Invest Med. 2009;32:E315. [PubMed] [Google Scholar]
- [147].Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- [148].Mao L, Yang Y. Targeting the nuclear transport machinery by rational drug design. Curr Pharm Des. 2013;19:2318–25. doi: 10.2174/1381612811319120018. [DOI] [PubMed] [Google Scholar]
- [149].Yashiroda Y, Yoshida M. Nucleo-cytoplasmic transport of proteins as a target for therapeutic drugs. Curr Med Chem. 2003;10:741–8. doi: 10.2174/0929867033457791. [DOI] [PubMed] [Google Scholar]
- [150].Hamamoto T, Seto H, Beppu T. Leptomycins A and B, new antifungal antibiotics. II. Structure elucidation. J Antibiot (Tokyo) 1983;36:646–50. doi: 10.7164/antibiotics.36.646. [DOI] [PubMed] [Google Scholar]
- [151].Hamamoto T, Gunji S, Tsuji H, Beppu T. Leptomycins A and B, new antifungal antibiotics. I. Taxonomy of the producing strain and their fermentation, purification and characterization. J Antibiot (Tokyo) 1983;36:639–45. doi: 10.7164/antibiotics.36.639. [DOI] [PubMed] [Google Scholar]
- [152].Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–7. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Newlands ES, Rustin GJ, Phase Brampton MH I trial of elactocin. Br J Cancer. 1996;74:648–9. doi: 10.1038/bjc.1996.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Sakakibara K, Saito N, Sato T, Suzuki A, Hasegawa Y, Friedman JM, et al. CBS9106 is a novel reversible oral CRM1 inhibitor with CRM1 degrading activity. Blood. 2011;118:3922–31. doi: 10.1182/blood-2011-01-333138. [DOI] [PubMed] [Google Scholar]
- [155].Kalid O, Toledo WD, Shechter S, Sherman W, Shacham S. Consensus Induced Fit Docking (cIFD): methodology, validation, and application to the discovery of novel Crm1 inhibitors. J Comput Aided Mol Des. 2012;26:1217–28. doi: 10.1007/s10822-012-9611-9. [DOI] [PubMed] [Google Scholar]
- [156].Inoue H, Kauffman M, Shacham S, Landesman Y, Yang J, Evans CP, et al. CRM1 blockade by selective inhibitors of nuclear export (SINE) attenuates kidney cancer growth. J Urol. 2013;189:2317–26. doi: 10.1016/j.juro.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Etchin J, Sun Q, Kentsis A, Farmer A, Zhang ZC, Sanda T, et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia. 2013;27:66–74. doi: 10.1038/leu.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhang K, Wang M, Tamayo AT, Shacham S, Kauffman M, Lee J, et al. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Exp Hematol. 2013;41:67–78. doi: 10.1016/j.exphem.2012.09.002. [DOI] [PubMed] [Google Scholar]
- [159].Azmi AS, Al-Katib A, Aboukameel A, McCauley D, Kauffman M, Shacham S, et al. Selective inhibitors of nuclear export for the treatment of non-Hodgkin’s Lymphomas. Haematologica. 2013;98:1098–106. doi: 10.3324/haematol.2012.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Azmi AS, Aboukameel A, Bao B, Sarkar FH, Philip PA, Kauffman M, et al. Selective inhibitors of nuclear export block pancreatic cancer cell proliferation and reduce tumor growth in mice. Gastroenterology. 2012;144:447–56. doi: 10.1053/j.gastro.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Mutka SC, Yang WQ, Dong SD, Ward SL, Craig DA, Timmermans PB, et al. Identification of nuclear export inhibitors with potent anticancer activity in vivo. Cancer Res. 2009;69:510–7. doi: 10.1158/0008-5472.CAN-08-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Muqbil I, Bao B, bou-Samra AB, Mohammad RM, Azmi AS. Nuclear export mediated regulation of microRNAs: potential target for drug intervention. Curr Drug Targets. 2013;14:1094–100. doi: 10.2174/1389450111314100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Azmi AS. Unveiling the role of nuclear transport in epithelial-to-mesenchymal transition. Curr Cancer Drug Targets. 2013;13:906–14. doi: 10.2174/15680096113136660096. [DOI] [PubMed] [Google Scholar]
- [164].Robert M, Wastie M. Glioblastoma multiforme: a rare manifestation of extensive liver and bone metastases. Biomed Imaging Interv J. 2008;4:e3. doi: 10.2349/biij.4.1.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Dreyfuss JM, Johnson MD, Park PJ. Meta-analysis of glioblastoma multiforme versus anaplastic astrocytoma identifies robust gene markers. Mol Cancer. 2009;8:71. doi: 10.1186/1476-4598-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115:3512–8. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- [167].Das A, Banik NL, Ray SK. Modulatory effects of acetazolomide and dexamethasone on temozolomide-mediated apoptosis in human glioblastoma T98G and U87MG cells. Cancer Invest. 2008;26:352–8. doi: 10.1080/07357900701788080. [DOI] [PubMed] [Google Scholar]
- [168].Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29:142–8. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443–9. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Spiegl-Kreinecker S, Pirker C, Filipits M, Lotsch D, Buchroithner J, Pichler J, et al. O6-Methylguanine DNA methyltransferase protein expression in tumor cells predicts outcome of temozolomide therapy in glioblastoma patients. Neuro Oncol. 2010;12:28–36. doi: 10.1093/neuonc/nop003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Krakstad C, Chekenya M. Survival signalling and apoptosis resistance in glioblastomas: opportunities for targeted therapeutics. Mol Cancer. 2010;9:135. doi: 10.1186/1476-4598-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Haas-Kogan DA, Prados MD, Tihan T, Eberhard DA, Jelluma N, Arvold ND, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–7. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- [173].Brem S, Tsanaclis AM, Gately S, Gross JL, Herblin WF. Immunolocalization of basic fibroblast growth factor to the microvasculature of human brain tumors. Cancer. 1992;70:2673–80. doi: 10.1002/1097-0142(19921201)70:11<2673::aid-cncr2820701118>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [174].Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62:200–7. [PubMed] [Google Scholar]
- [175].Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–90. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- [176].Huang TT, Sarkaria SM, Cloughesy TF, Mischel PS. Targeted therapy for malignant glioma patients: lessons learned and the road ahead. Neurotherapeutics. 2009;6:500–12. doi: 10.1016/j.nurt.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Hirose Y, Berger MS, Pieper RO. p53 effects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res. 2001;61:1957–63. [PubMed] [Google Scholar]
- [178].Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–7. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- [179].Tagscherer KE, Fassl A, Campos B, Farhadi M, Kraemer A, Bock BC, et al. Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene. 2008;27:6646–56. doi: 10.1038/onc.2008.259. [DOI] [PubMed] [Google Scholar]
- [180].Manero F, Gautier F, Gallenne T, Cauquil N, Gree D, Cartron PF, et al. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006;66:2757–64. doi: 10.1158/0008-5472.CAN-05-2097. [DOI] [PubMed] [Google Scholar]
- [181].Bushunow P, Reidenberg MM, Wasenko J, Winfield J, Lorenzo B, Lemke S, et al. Gossypol treatment of recurrent adult malignant gliomas. J Neurooncol. 1999;43:79–86. doi: 10.1023/a:1006267902186. [DOI] [PubMed] [Google Scholar]
- [182].Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, Guo J, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–42. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- [183].Dodou K, Anderson RJ, Small DA, Groundwater PW. Investigations on gossypol: past and present developments. Expert Opin Investig Drugs. 2005;14:1419–34. doi: 10.1517/13543784.14.11.1419. [DOI] [PubMed] [Google Scholar]
- [184].Srinivasula SM, Ashwell JD. IAPs: what’s in a name. Mol Cell. 2008;30:123–35. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].George J, Banik NL, Ray SK. Survivin knockdown and concurrent 4-HPR treatment controlled human glioblastoma in vitro and in vivo. Neuro Oncol. 2010;12:1088–101. doi: 10.1093/neuonc/noq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Karmakar S, Banik NL, Ray SK. Combination of all-trans retinoic acid and paclitaxel-induced differentiation and apoptosis in human glioblastoma U87MG xenografts in nude mice. Cancer. 2008;112:596–607. doi: 10.1002/cncr.23223. [DOI] [PubMed] [Google Scholar]
- [187].Das A, Banik NL, Ray SK. Retinoids induced astrocytic differentiation with down regulation of telomerase activity and enhanced sensitivity to taxol for apoptosis in human glioblastoma T98G and U87MG cells. J Neurooncol. 2008;87:9–22. doi: 10.1007/s11060-007-9485-1. [DOI] [PubMed] [Google Scholar]
- [188].Lacasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–75. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- [189].Joo KM, Kim SY, Jin X, Song SY, Kong DS, Lee JI, et al. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Investig: J Techn Methods Pathol. 2008;88:808–15. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- [190].Vellanki SH, Grabrucker A, Liebau S, Proepper C, Eramo A, Braun V, et al. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia. 2009;11:743–52. doi: 10.1593/neo.09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/T. Nat Med. 2002;8:808–15. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- [192].Singh SK, Morbach H, Nanki T, Girschick HJ. Differential expression of chemokines in synovial cells exposed to different Borrelia burgdorferi isolates. Clin Exp Rheumatol. 2005;23:311–22. [PubMed] [Google Scholar]
- [193].Richmond A, Lawson DH, Nixon DW, Chawla RK. Characterization of autostimulatory and transforming growth factors from human melanoma cells. Cancer Res. 1985;45:6390–4. [PubMed] [Google Scholar]
- [194].Mrowietz U, Schwenk U, Maune S, Bartels J, Kupper M, Fichtner I, et al. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br J Cancer. 1999;79:1025–31. doi: 10.1038/sj.bjc.6690164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Bottazzi B, Walter S, Govoni D, Colotta F, Mantovani A. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J Immunol. 1992;148:1280–5. [PubMed] [Google Scholar]
- [196].Lowman HB, Slagle PH, DeForge LE, Wirth CM, Gillece-Castro BL, Bourell JH, et al. Exchanging interleukin-8 and melanoma growth-stimulating activity receptor binding specificities. J Biol Chem. 1996;271:14344–52. doi: 10.1074/jbc.271.24.14344. [DOI] [PubMed] [Google Scholar]
- [197].Bernard D, Quatannens B, Vandenbunder B, Abbadie C. Rel/NF-kappaB transcription factors protect against tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by up-regulating the TRAIL decoy receptor DcR1. J Biol Chem. 2001;276:27322–8. doi: 10.1074/jbc.M011183200. [DOI] [PubMed] [Google Scholar]
- [198].Oya M, Ohtsubo M, Takayanagi A, Tachibana M, Shimizu N, Murai M. Constitutive activation of nuclear factor-kappaB prevents TRAIL-induced apoptosis in renal cancer cells. Oncogene. 2001;20:3888–96. doi: 10.1038/sj.onc.1204525. [DOI] [PubMed] [Google Scholar]
- [199].Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995;268:411–5. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- [200].Ivanov VN, Lopez BP, Maulit G, Sato TA, Sassoon D, Ronai Z. FAP-1 association with Fas (Apo-1) inhibits Fas expression on the cell surface. Mol Cell Biol. 2003;23:3623–35. doi: 10.1128/MCB.23.10.3623-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Deveraux QL, Roy N, Stennicke HR, Van AT, Zhou Q, Srinivasula SM, et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–23. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–4. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- [203].Kraehn GM, Utikal J, Udart M, Greulich KM, Bezold G, Kaskel P, et al. Extra c-myc oncogene copies in high risk cutaneous malignant melanoma and melanoma metastases. Br J Cancer. 2001;84:72–9. doi: 10.1054/bjoc.2000.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Hinz M, Gottschling D, Eritja R, Seliger H. Synthesis and properties of 2′ -deoxycytidine triphosphate carrying c-myc tag sequence. Nucleosides Nucleotides Nucleic Acids. 2000;19:1543–52. doi: 10.1080/15257770008045445. [DOI] [PubMed] [Google Scholar]
- [205].Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- [206].Beeram M, Patnaik A, Rowinsky EK. Regulation of c-Raf-1: therapeutic implications. Clin Adv Hematol Oncol. 2003;1:476–81. [PubMed] [Google Scholar]
- [207].Beeram M, Patnaik A, Rowinsky EK. Raf: a strategic target for therapeutic development against cancer. J Clin Oncol. 2005;23:6771–90. doi: 10.1200/JCO.2005.08.036. [DOI] [PubMed] [Google Scholar]
- [208].Gray-Schopfer VC, da Rocha DS, Marais R. The role of B-RAF in melanoma. Cancer Metastasis Rev. 2005;24:165–83. doi: 10.1007/s10555-005-5865-1. [DOI] [PubMed] [Google Scholar]
- [209].Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- [210].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- [211].Kitano H. Cancer robustness: tumour tactics. Nature. 2003;426:125. doi: 10.1038/426125a. [DOI] [PubMed] [Google Scholar]
- [212].Haugk B. Pancreatic intraepithelial neoplasia – can we detect early pancreatic cancer. Histopathology. 2010;57:503–14. doi: 10.1111/j.1365-2559.2010.03610.x. [DOI] [PubMed] [Google Scholar]
- [213].Mihaljevic AL, Michalski CW, Friess H, Kleeff J. Molecular mechanism of pancreatic cancer – understanding proliferation, invasion, and metastasis. Langenbecks Arch Surg. 2010;395:295–308. doi: 10.1007/s00423-010-0622-5. [DOI] [PubMed] [Google Scholar]
- [214].Sheikh R, Walsh N, Clynes M, O’Connor R, McDermott R. Challenges of drug resistance in the management of pancreatic cancer. Expert Rev Anticancer Ther. 2010;10:1647–61. doi: 10.1586/era.10.148. [DOI] [PubMed] [Google Scholar]
- [215].Dangi-Garimella S, Krantz SB, Shields MA, Grippo PJ, Munshi HG. Epithelialmesenchymal transition and pancreatic cancer progression. 2012. [PubMed]
- [216].Rasheed ZA, Matsui W, Maitra A. Pathology of pancreatic stroma in PDAC. 2012. [PubMed]
- [217].Wang Z, Azmi AS, Ahmad A, Banerjee S, Wang S, Sarkar FH, et al. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and induces apoptosis in pancreatic cancer: involvement of Notch-1 signaling pathway. Cancer Res. 2009;69:2757–65. doi: 10.1158/0008-5472.CAN-08-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].O’Connell J, Bennett MW, Nally K, Houston A, O’Sullivan GC, Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and counterattack in the tumor-immune conflict. Ann N Y Acad Sci. 2000;910:178–92. doi: 10.1111/j.1749-6632.2000.tb06708.x. [DOI] [PubMed] [Google Scholar]
- [219].Karantanos T, Evans CP, Tombal B, Thompson TC, Montironi R, Isaacs WB. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol. 2015;67:470–9. doi: 10.1016/j.eururo.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Berchem GJ, Bosseler M, Sugars LY, Voeller HJ, Zeitlin S, Gelmann EP. Androgens induce resistance to bcl-2-mediated apoptosis in LNCaP prostate cancer cells. Cancer Res. 1995;55:735–8. [PubMed] [Google Scholar]
- [221].Bonkhoff H, Fixemer T, Remberger K. Relation between Bcl-2, cell proliferation, and the androgen receptor status in prostate tissue and precursors of prostate cancer. Prostate. 1998;34:251–8. doi: 10.1002/(sici)1097-0045(19980301)34:4<251::aid-pros2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- [222].Balakumaran BS, Herbert JT, Febbo PG. MYC activity mitigates response to rapamycin in prostate cancer through 4EBP1-mediated inhibition of autophagy. Autophagy. 2010;6:281–2. doi: 10.4161/auto.6.2.10921. [DOI] [PubMed] [Google Scholar]
- [223].Balakumaran BS, Porrello A, Hsu DS, Glover W, Foye A, Leung JY, et al. MYC activity mitigates response to rapamycin in prostate cancer through eukaryotic initiation factor 4E-binding protein 1-mediated inhibition of autophagy. Cancer Res. 2009;69:7803–10. doi: 10.1158/0008-5472.CAN-09-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Chang PC, Wang TY, Chang YT, Chu CY, Lee CL, Hsu HW, et al. Autophagy pathway is required for IL-6 induced neuroendocrine differentiation and chemoresistance of prostate cancer LNCaP cells. PLoS ONE. 2014;9:e88556. doi: 10.1371/journal.pone.0088556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Bennett HL, Stockley J, Fleming JT, Mandal R, O’Prey J, Ryan KM, et al. Does androgen-ablation therapy (AAT) associated autophagy have a pro-survival effect in LNCaP human prostate cancer cells. BJU Int. 2013;111:672–82. doi: 10.1111/j.1464-410X.2012.11409.x. [DOI] [PubMed] [Google Scholar]
- [226].Ziparo E, Petrungaro S, Marini ES, Starace D, Conti S, Facchiano A, et al. Autophagy in prostate cancer and androgen suppression therapy. Int J Mol Sci. 2013;14:12090–106. doi: 10.3390/ijms140612090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Giampietri C, Petrungaro S, Padula F, D’Alessio A, Marini ES, Facchiano A, et al. Autophagy modulators sensitize prostate epithelial cancer cell lines to TNF-alpha-dependent apoptosis. Apoptosis. 2012;17:1210–22. doi: 10.1007/s10495-012-0752-z. [DOI] [PubMed] [Google Scholar]
- [228].Parrondo R, de Las PA, Reiner T, Perez-Stable C. ABT-737, a small molecule Bcl-2/Bcl-xL antagonist, increases antimitotic-mediated apoptosis in human prostate cancer cells. Peer J. 2013;1:e144. doi: 10.7717/peerj.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Zhang XQ, Huang XF, Hu XB, Zhan YH, An QX, Yang SM, et al. Apogossypolone, a novel inhibitor of antiapoptotic Bcl-2 family proteins, induces autophagy of PC-3 and LNCaP prostate cancer cells in vitro. Asian J Androl. 2010;12:697–708. doi: 10.1038/aja.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Jackson RS, Placzek W, Fernandez A, Ziaee S, Chu CY, Wei J, et al. Sabutoclax, a Mcl-1 antagonist, inhibits tumorigenesis in transgenic mouse and human xenograft models of prostate cancer. Neoplasia. 2012;14:656–65. doi: 10.1593/neo.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Zheng L, Yang W, Zhang C, Ding WJ, Zhu H, Lin NM, et al. GDC-0941 sensitizes breast cancer to ABT-737 in vitro and in vivo through promoting the degradation of Mcl-1. Cancer Lett. 2011;309:27–36. doi: 10.1016/j.canlet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- [232].Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H, Dong XW, et al. Synergistic anti-tumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther. 2011;10:1264–75. doi: 10.1158/1535-7163.MCT-10-1091. [DOI] [PubMed] [Google Scholar]
- [233].Voelkel-Johnson C. TRAIL-mediated signaling in prostate, bladder and renal cancer. Nat Rev Urol. 2011;8:417–27. doi: 10.1038/nrurol.2011.81. [DOI] [PubMed] [Google Scholar]
- [234].Shin EA, Sohn EJ, Won G, Choi JU, Jeong M, Kim B, et al. Upregulation of microRNA135a-3p and death receptor 5 plays a critical role in Tanshinone I sensitized prostate cancer cells to TRAIL induced apoptosis. Oncotarget. 2014;5:5624–36. doi: 10.18632/oncotarget.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Videira M, Reis RL, Brito MA. Deconstructing breast cancer cell biology and the mechanisms of multidrug resistance. Biochim Biophys Acta. 2014;1846:312–25. doi: 10.1016/j.bbcan.2014.07.011. [DOI] [PubMed] [Google Scholar]
- [236].Filipova A, Seifrtova M, Mokry J, Dvorak J, Rezacova M, Filip S, et al. Breast cancer and cancer stem cells: a mini-review. Tumori. 2014;100:363–9. doi: 10.1700/1636.17886. [DOI] [PubMed] [Google Scholar]
- [237].Booy EP, Henson ES, Gibson SB. Epidermal growth factor regulates Mcl-1 expression through the MAPK-Elk-1 signalling pathway contributing to cell survival in breast cancer. Oncogene. 2011;30:2367–78. doi: 10.1038/onc.2010.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F, et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci USA. 2012;109:2766–71. doi: 10.1073/pnas.1104778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Elfadl D, Hodgkinson VC, Long ED, Scaife L, Drew PJ, Lind MJ, et al. A pilot study to investigate the role of the 26S proteasome in radiotherapy resistance and loco-regional recurrence following breast conserving therapy for early breast cancer. Breast. 2011;20:334–7. doi: 10.1016/j.breast.2011.02.017. [DOI] [PubMed] [Google Scholar]
- [240].Panischeva LA, Kakpakova ES, Rybalkina EY, Stavrovskaya AA. Influence of proteasome inhibitor bortezomib on the expression of multidrug resistance genes and Akt kinase activity. Biochemistry (Mosc) 2011;76:1009–16. doi: 10.1134/S0006297911090045. [DOI] [PubMed] [Google Scholar]
- [241].Caligaris-Cappio F. B-chronic lymphocytic leukemia: a malignancy of anti-self B cells. Blood. 1996;87:2615–20. [PubMed] [Google Scholar]
- [242].Damle RN, Calissano C, Chiorazzi N. Chronic lymphocytic leukaemia: a disease of activated monoclonal B cells. Best Pract Res Clin Haematol. 2010;23:33–45. doi: 10.1016/j.beha.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115:755–64. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Tinhofer I, Marschitz I, Kos M, Henn T, Egle A, Villunger A, et al. Differential sensitivity of CD4+ and CD8+ T lymphocytes to the killing efficacy of Fas (Apo-1/CD95) ligand+ tumor cells in B chronic lymphocytic leukemia. Blood. 1998;91:4273–81. [PubMed] [Google Scholar]
- [245].Macfarlane M, Inoue S, Kohlhaas SL, Majid A, Harper N, Kennedy DB, et al. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ. 2005;12:773–82. doi: 10.1038/sj.cdd.4401649. [DOI] [PubMed] [Google Scholar]
- [246].Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- [247].Buggins AG, Pepper CJ. The role of Bcl-2 family proteins in chronic lymphocytic leukaemia. Leuk Res. 2010;34:837–42. doi: 10.1016/j.leukres.2010.03.011. [DOI] [PubMed] [Google Scholar]
- [248].Pepper C, Hoy T, Bentley DP. Bcl-2/Bax ratios in chronic lymphocytic leukaemia and their correlation with in vitro apoptosis and clinical resistance. Br J Cancer. 1997;76:935–8. doi: 10.1038/bjc.1997.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. 1996;10:456–9. [PubMed] [Google Scholar]
- [250].Pepper C, Lin TT, Pratt G, Hewamana S, Brennan P, Hiller L, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112:3807–17. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- [251].Awan FT, Kay NE, Davis ME, Wu W, Geyer SM, Leung N, et al. Mcl-1 expression predicts progression-free survival in chronic lymphocytic leukemia patients treated with pentostatin, cyclophosphamide, and rituximab. Blood. 2009;113:535–7. doi: 10.1182/blood-2008-08-173450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Veronese L, Tournilhac O, Verrelle P, Davi F, Dighiero G, Chautard E, et al. Low MCL-1 mRNA expression correlates with prolonged survival in B-cell chronic lymphocytic leukemia. Leukemia. 2008;22:1291–3. doi: 10.1038/sj.leu.2405052. [DOI] [PubMed] [Google Scholar]
- [253].Hallaert DY, Jaspers A, van Noesel CJ, van Oers MH, Kater AP, Eldering E. c-Abl kinase inhibitors overcome CD40-mediated drug resistance in CLL: implications for therapeutic targeting of chemoresistant niches. Blood. 2008;112:5141–9. doi: 10.1182/blood-2008-03-146704. [DOI] [PubMed] [Google Scholar]
- [254].Smit LA, Hallaert DY, Spijker R, de GB, Jaspers A, Kater AP, et al. Differential Noxa/Mcl-1 balance in peripheral versus lymph node chronic lymphocytic leukemia cells correlates with survival capacity. Blood. 2007;109:1660–8. doi: 10.1182/blood-2006-05-021683. [DOI] [PubMed] [Google Scholar]
- [255].Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–55. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- [256].Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- [257].Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–23. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- [258].Durig J, Duhrsen U, Klein-Hitpass L, Worm J, Hansen JB, Orum H, et al. The novel antisense Bcl-2 inhibitor SPC2996 causes rapid leukemic cell clearance and immune activation in chronic lymphocytic leukemia. Leukemia. 2011;25:638–47. doi: 10.1038/leu.2010.322. [DOI] [PubMed] [Google Scholar]
- [259].Billard C. Design of novel BH3 mimetics for the treatment of chronic lymphocytic leukemia. Leukemia. 2012;26:2032–8. doi: 10.1038/leu.2012.88. [DOI] [PubMed] [Google Scholar]
- [260].Nana-Sinkam SP, Croce CM. MicroRNA in chronic lymphocytic leukemia: transitioning from laboratory-based investigation to clinical application. Cancer Genet Cytogenet. 2010;203:127–33. doi: 10.1016/j.cancergencyto.2010.09.007. [DOI] [PubMed] [Google Scholar]
- [261].Hallek M. Therapy of chronic lymphocytic leukaemia. Best Pract Res Clin Haematol. 2010;23:85–96. doi: 10.1016/j.beha.2009.12.002. [DOI] [PubMed] [Google Scholar]
- [262].Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371:1017–29. doi: 10.1016/S0140-6736(08)60456-0. [DOI] [PubMed] [Google Scholar]
- [263].Elter T, Gercheva-Kyuchukova L, Pylylpenko H, Robak T, Jaksic B, Rekhtman G, et al. Fludarabine plus alemtuzumab versus fludarabine alone in patients with previously treated chronic lymphocytic leukaemia: a randomised phase 3 trial. Lancet Oncol. 2011;12:1204–13. doi: 10.1016/S1470-2045(11)70242-X. [DOI] [PubMed] [Google Scholar]
- [264].bou-Nassar K, Brown JR. Novel agents for the treatment of chronic lymphocytic leukemia. Clin Adv Hematol Oncol. 2010;8:886–95. [PubMed] [Google Scholar]
- [265].Balakrishnan K, Wierda WG, Keating MJ, Gandhi V. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2008;112:1971–80. doi: 10.1182/blood-2007-12-126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113:149–53. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Etxebarria A, Landeta O, Antonsson B, Basanez G. Regulation of antiapoptotic MCL-1 function by gossypol: mechanistic insights from in vitro reconstituted systems. Biochem Pharmacol. 2008;76:1563–76. doi: 10.1016/j.bcp.2008.08.003. [DOI] [PubMed] [Google Scholar]
- [268].Russo M, Spagnuolo C, Tedesco I, Russo GL. Phytochemicals in cancer prevention and therapy: truth or dare? Toxins (Basel) 2010;2:517–51. doi: 10.3390/toxins2040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Bruel A, Karsenty E, Schmid M, McDonnell TJ, Lanotte M. Altered sensitivity to retinoid-induced apoptosis associated with changes in the subcellular distribution of Bcl-2. Exp Cell Res. 1997;233:281–7. doi: 10.1006/excr.1997.3594. [DOI] [PubMed] [Google Scholar]
- [270].Russo GL, Russo M, Spagnuolo C, Tedesco I, Bilotto S, Iannitti R, et al. Quercetin: a pleiotropic kinase inhibitor against cancer. Cancer Treat Res. 2014;159:185–205. doi: 10.1007/978-3-642-38007-5_11. [DOI] [PubMed] [Google Scholar]
- [271].Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–37. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- [272].Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Gundala SR, Yang C, Mukkavilli R, Paranjpe R, Brahmbhatt M, Pannu V, et al. Hydroxychavicol, a betel leaf component, inhibits prostate cancer through ROS-driven DNA damage and apoptosis. Toxicol Appl Pharmacol. 2014;280:86–96. doi: 10.1016/j.taap.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Singh T, Sharma SD, Katiyar SK. Grape proanthocyanidins induce apoptosis by loss of mitochondrial membrane potential of human non-small cell lung cancer cells in vitro and in vivo. PLoS ONE. 2011;6:e27444. doi: 10.1371/journal.pone.0027444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Shen M, Schmitt S, Buac D, Dou QP. Targeting the ubiquitin-proteasome system for cancer therapy. Expert Opin Ther Targets. 2013;17:1091–108. doi: 10.1517/14728222.2013.815728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Kumar N, Crocker T, Smith T, Connors S, Pow-Sang J, Spiess PE, et al. Prostate Cancer Chemoprevention Targeting Men with High-Grade Prostatic Intraepithelial Neoplasia (HGPIN) and Atypical Small Acinar Proliferation (ASAP): Model for Trial Design and Outcome Measures. J Clin Trials. 2012:2. doi: 10.4172/jctr.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–30. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- [278].Le Y, Cui Y, Iribarren P, Ying G, Wang JM. Manipulating chemoattractant and receptor genes. In Vivo (Athens, Greece) 2002;16:1–23. [PubMed] [Google Scholar]
- [279].Kazi A, Wang Z, Kumar N, Falsetti SC, Chan TH, Dou QP. Structure-activity relationships of synthetic analogs of (−)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Res. 2004;24:943–54. [PubMed] [Google Scholar]
- [280].Smith DM, Wang Z, Kazi A, Li LH, Chan TH, Dou QP. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol Med. 2002;8:382–92. [PMC free article] [PubMed] [Google Scholar]
- [281].Adhami VM, Ahmad N, Mukhtar H. Molecular targets for green tea in prostate cancer prevention. J Nutr. 2003;133:2417S–24S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- [282].Liang YC, Lin-Shiau SY, Chen CF, Lin JK. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (−)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J Cell Biochem. 1997;67:55–65. doi: 10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- [283].Chung JY, Huang C, Meng X, Dong Z, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure-activity relationship and mechanisms involved. Cancer Res. 1999;59:4610–7. [PubMed] [Google Scholar]
- [284].Liang YC, Lin-Shiau SY, Chen CF, Lin JK. Inhibition of cyclin-dependent kinases 2 and 4 activities as well as induction of Cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (−)-epigallocatechin-3-gallate. J Cell Biochem. 1999;75:1–12. [PubMed] [Google Scholar]
- [285].Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res. 2007;13:1611–9. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- [286].Kazi A, Daniel KG, Smith DM, Kumar NB, Dou QP. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem Pharmacol. 2003;66:965–76. doi: 10.1016/s0006-2952(03)00414-3. [DOI] [PubMed] [Google Scholar]
- [287].Kumar N, Chornokur G. Molecular targeted therapies using botanicals for prostate cancer chemoprevention. Transl Med (Sunnyvale) 2012;(Suppl. 2):005. doi: 10.4172/2161-1025.S2-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Shanafelt TD, Call TG, Zent CS, Leis JF, LaPlant B, Bowen DA, et al. Phase 2 trial of daily, oral Polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer. 2013;119:363–70. doi: 10.1002/cncr.27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Shanafelt TD, Call TG, Zent CS, LaPlant B, Bowen DA, Roos M, et al. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J Clin Oncol. 2009;27:3808–14. doi: 10.1200/JCO.2008.21.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–8. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Phelps MA, Lin TS, Johnson AJ, Hurh E, Rozewski DM, Farley KL, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–45. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Molinari M, Watt KD, Kruszyna T, Nelson R, Walsh M, Huang WY, et al. Acute liver failure induced by green tea extracts: case report and review of the literature. Liver Transpl. 2006;12:1892–5. doi: 10.1002/lt.21021. [DOI] [PubMed] [Google Scholar]
- [293].Pedros C, Cereza G, Garcia N, Laporte JR. Liver toxicity of Camellia sinensis dried etanolic extract. Med Clin (Barc) 2003;121:598–9. doi: 10.1016/s0025-7753(03)74026-3. [DOI] [PubMed] [Google Scholar]
- [294].Tahmatzopoulos A, Gudegast C, Stockle M, Wullich B, Unteregger G, Zwergel U, et al. Proteasome inhibitors: induction of apoptosis as new therapeutic option in prostate cancer. Aktuelle Urol. 2004;35:491–6. doi: 10.1055/s-2004-830048. [DOI] [PubMed] [Google Scholar]
- [295].Papandreou CN, Logothetis CJ. Bortezomib as a potential treatment for prostate cancer. Cancer Res. 2004;64:5036–43. doi: 10.1158/0008-5472.CAN-03-2707. [DOI] [PubMed] [Google Scholar]
- [296].Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib) Cancer Invest. 2004;22:304–11. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- [297].Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- [298].Mozzicafreddo M, Cuccioloni M, Cecarini V, Eleuteri AM, Angeletti M. Homology modeling and docking analysis of the interaction between polyphenols and mammalian 20S proteasomes. J Chem Inf Model. 2009;49:401–9. doi: 10.1021/ci800235m. [DOI] [PubMed] [Google Scholar]
- [299].Kitagawa S, Nabekura T, Kamiyama S. Inhibition of P-glycoprotein function by tea catechins in KB-C2 cells. J Pharm Pharmacol. 2004;56:1001–5. doi: 10.1211/0022357044003. [DOI] [PubMed] [Google Scholar]
- [300].Jodoin J, Demeule M, Beliveau R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim Biophys Acta. 2002;1542:149–59. doi: 10.1016/s0167-4889(01)00175-6. [DOI] [PubMed] [Google Scholar]
- [301].Farabegoli F, Papi A, Bartolini G, Ostan R, Orlandi M. (−)-Epigallocatechin-3-gallate downregulates Pg-P and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine. 2010;17:356–62. doi: 10.1016/j.phymed.2010.01.001. [DOI] [PubMed] [Google Scholar]
- [302].Mei Y, Qian F, Wei D, Liu J. Reversal of cancer multidrug resistance by green tea polyphenols. J Pharm Pharmacol. 2004;56:1307–14. doi: 10.1211/0022357044364. [DOI] [PubMed] [Google Scholar]
- [303].Liang G, Tang A, Lin X, Li L, Zhang S, Huang Z, et al. Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. Int J Oncol. 2010;37:111–23. [PubMed] [Google Scholar]
- [304].Chen TC, Wang W, Golden EB, Thomas S, Sivakumar W, Hofman FM, et al. Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett. 2011;302:100–8. doi: 10.1016/j.canlet.2010.11.008. [DOI] [PubMed] [Google Scholar]
- [305].Stearns ME, Amatangelo MD, Varma D, Sell C, Goodyear SM. Combination therapy with epigallocatechin-3-gallate and doxorubicin in human prostate tumor modeling studies: inhibition of metastatic tumor growth in severe combined immunodeficiency mice. Am J Pathol. 2010;177:3169–79. doi: 10.2353/ajpath.2010.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Luo T, Wang J, Yin Y, Hua H, Jing J, Sun X, et al. (−)-Epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res. 2010;12:R8. doi: 10.1186/bcr2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Davenport A, Frezza M, Shen M, Ge Y, Huo C, Chan TH, et al. Celastrol and an EGCG pro-drug exhibit potent chemosensitizing activity in human leukemia cells. Int J Mol Med. 2010;25:465–70. doi: 10.3892/ijmm_00000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Singh M, Bhatnagar P, Srivastava AK, Kumar P, Shukla Y, Gupta KC. Enhancement of cancer chemosensitization potential of cisplatin by tea polyphenols poly(lactide-co-glycolide) nanoparticles. J Biomed Nanotechnol. 2011;7:202. doi: 10.1166/jbn.2011.1268. [DOI] [PubMed] [Google Scholar]
- [309].Laurie SA, Miller VA, Grant SC, Kris MG, Ng KK. Phase I study of green tea extract in patients with advanced lung cancer. Cancer Chemother Pharmacol. 2005;55:33–8. doi: 10.1007/s00280-004-0859-1. [DOI] [PubMed] [Google Scholar]
- [310].Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–6. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- [311].Choan E, Segal R, Jonker D, Malone S, Reaume N, Eapen L, et al. A prospective clinical trial of green tea for hormone refractory prostate cancer: an evaluation of the complementary/alternative therapy approach. Urol Oncol. 2005;23:108–13. doi: 10.1016/j.urolonc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- [312].Sprouse AA, Herbert BS. Resveratrol augments paclitaxel treatment in MDA-MB-231 and paclitaxel-resistant MDA-MB-231 breast cancer cells. Anticancer Res. 2014;34:5363–74. [PubMed] [Google Scholar]
- [313].Huq F, Yu JQ, Beale P, Chan C, Arzuman L, Nessa MU, et al. Combinations of platinums and selected phytochemicals as a means of overcoming resistance in ovarian cancer. Anticancer Res. 2014;34:541–5. [PubMed] [Google Scholar]
- [314].Chow SE, Kao CH, Liu YT, Cheng ML, Yang YW, Huang YK, et al. Resveratrol induced ER expansion and ER caspase-mediated apoptosis in human nasopharyngeal carcinoma cells. Apoptosis. 2014;19:527–41. doi: 10.1007/s10495-013-0945-0. [DOI] [PubMed] [Google Scholar]
- [315].Shi XP, Miao S, Wu Y, Zhang W, Zhang XF, Ma HZ, et al. Resveratrol sensitizes tamoxifen in antiestrogen-resistant breast cancer cells with epithelial-mesenchymal transition features. Int J Mol Sci. 2013;14:15655–68. doi: 10.3390/ijms140815655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Lopez PL, Filippi-Chiela EC, Silva AO, Cordero EA, Garcia-Santos D, Pelegrini AL, et al. Sensitization of glioma cells by X-linked inhibitor of apoptosis protein knockdown. Oncology. 2012;83:75–82. doi: 10.1159/000337978. [DOI] [PubMed] [Google Scholar]
- [317].Diaz-Chavez J, Fonseca-Sanchez MA, Arechaga-Ocampo E, Flores-Perez A, Palacios-Rodriguez Y, Dominguez-Gomez G, et al. Proteomic profiling reveals that resveratrol inhibits HSP27 expression and sensitizes breast cancer cells to doxorubicin therapy. PLoS ONE. 2013;8:e64378. doi: 10.1371/journal.pone.0064378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Farrand L, Byun S, Kim JY, Im-aram A, Lee J, Lim S, et al. Piceatannol enhances cisplatin sensitivity in ovarian cancer via modulation of p53, X-linked inhibitor of apoptosis protein (XIAP), and mitochondrial fission. J Biol Chem. 2013;288:23740–50. doi: 10.1074/jbc.M113.487686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Roman V, Billard C, Kern C, Ferry-Dumazet H, Izard JC, Mohammad R, et al. Analysis of resveratrol-induced apoptosis in human B-cell chronic leukaemia. Br J Haematol. 2002;117:842–51. doi: 10.1046/j.1365-2141.2002.03520.x. [DOI] [PubMed] [Google Scholar]
- [320].Billard C, Izard JC, Roman V, Kern C, Mathiot C, Mentz F, et al. Comparative antiproliferative and apoptotic effects of resveratrol, epsilon-viniferin and vine-shots derived polyphenols (vineatrols) on chronic B lymphocytic leukemia cells and normal human lymphocytes. Leuk Lymphoma. 2002;43:1991–2002. doi: 10.1080/1042819021000015952. [DOI] [PubMed] [Google Scholar]
- [321].Tomic J, McCaw L, Li Y, Hough MR, Ben-David Y, Moffat J, et al. Resveratrol has anti-leukemic activity associated with decreased O-GlcNAcylated proteins. Exp Hematol. 2013;41:675–86. doi: 10.1016/j.exphem.2013.04.004. [DOI] [PubMed] [Google Scholar]
- [322].Gokbulut AA, Apohan E, Baran Y. Resveratrol and quercetin-induced apoptosis of human 232B4 chronic lymphocytic leukemia cells by activation of caspase-3 and cell cycle arrest. Hematology. 2013;18:144–50. doi: 10.1179/1607845412Y.0000000042. [DOI] [PubMed] [Google Scholar]
- [323].Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- [324].Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39:2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- [325].Everett PC, Meyers JA, Makkinje A, Rabbi M, Lerner A. Preclinical assessment of curcumin as a potential therapy for B-CLL. Am J Hematol. 2007;82:23–30. doi: 10.1002/ajh.20757. [DOI] [PubMed] [Google Scholar]
- [326].Hayun R, Okun E, Berrebi A, Shvidel L, Bassous L, Sredni B, et al. Rapamycin and curcumin induce apoptosis in primary resting B chronic lymphocytic leukemia cells. Leuk Lymphoma. 2009;50:625–32. doi: 10.1080/10428190902789181. [DOI] [PubMed] [Google Scholar]
- [327].Ghosh AK, Kay NE, Secreto CR, Shanafelt TD. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin Cancer Res. 2009;15:1250–8. doi: 10.1158/1078-0432.CCR-08-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Angelo LS, Kurzrock R. Turmeric and green tea: a recipe for the treatment of B-chronic lymphocytic leukemia. Clin Cancer Res. 2009;15:1123–5. doi: 10.1158/1078-0432.CCR-08-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem Pharmacol. 2012;83:6–15. doi: 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- [330].Spagnuolo C, Russo M, Bilotto S, Tedesco I, Laratta B, Russo GL. Dietary polyphenols in cancer prevention: the example of the flavonoid quercetin in leukemia. Ann NY Acad Sci. 2012;1259:95–103. doi: 10.1111/j.1749-6632.2012.06599.x. [DOI] [PubMed] [Google Scholar]
- [331].Russo M, Spagnuolo C, Volpe S, Mupo A, Tedesco I, Russo GL. Quercetin induced apoptosis in association with death receptors and fludarabine in cells isolated from chronic lymphocytic leukaemia patients. Br J Cancer. 2010;103:642–8. doi: 10.1038/sj.bjc.6605794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Russo M, Spagnuolo C, Volpe S, Tedesco I, Bilotto S, Russo GL. ABT-737 resistance in B-cells isolated from chronic lymphocytic leukemia patients and leukemia cell lines is overcome by the pleiotropic kinase inhibitor quercetin through Mcl-1 down-regulation. Biochem Pharmacol. 2013;85:927–36. doi: 10.1016/j.bcp.2013.01.011. [DOI] [PubMed] [Google Scholar]
- [333].Okuhashi Y, Itoh M, Nara N, Tohda S. Effects of combination of notch inhibitor plus hedgehog inhibitor or Wnt inhibitor on growth of leukemia cells. Anti-cancer Res. 2011;31:893–6. [PubMed] [Google Scholar]
- [334].Fimognari C, Turrini E, Ferruzzi L, Lenzi M, Hrelia P. Natural isothiocyanates: genotoxic potential versus chemoprevention. Mutat Res. 2012;750:107–31. doi: 10.1016/j.mrrev.2011.12.001. [DOI] [PubMed] [Google Scholar]
- [335].Fimognari C, Nusse M, Lenzi M, Sciuscio D, Cantelli-Forti G, Hrelia P. Sulforaphane increases the efficacy of doxorubicin in mouse fibroblasts characterized by p53 mutations. Mutat Res. 2006;601:92–101. doi: 10.1016/j.mrfmmm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- [336].Geisler S, Lonning PE, Aas T, Johnsen H, Fluge O, Haugen DF, et al. Influence of TP53 gene alterations and c-erbB-2 expression on the response to treatment with doxorubicin in locally advanced breast cancer. Cancer Res. 2001;61:2505–12. [PubMed] [Google Scholar]
- [337].Volkova M, Russell R., III Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7:214–20. doi: 10.2174/157340311799960645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Gupta P, Srivastava SK. Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Med. 2012;10:80. doi: 10.1186/1741-7015-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T, et al. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 1997;8:1267–76. [PubMed] [Google Scholar]
- [340].Kaminski BM, Weigert A, Brune B, Schumacher M, Wenzel U, Steinhilber D, et al. Sulforaphane potentiates oxaliplatin-induced cell growth inhibition in colorectal cancer cells via induction of different modes of cell death. Cancer Chemother Pharmacol. 2011;67:1167–78. doi: 10.1007/s00280-010-1413-y. [DOI] [PubMed] [Google Scholar]
- [341].Wang X, Govind S, Sajankila SP, Mi L, Roy R, Chung FL. Phenethyl isothiocyanate sensitizes human cervical cancer cells to apoptosis induced by cisplatin. Mol Nutr Food Res. 2011;55:1572–81. doi: 10.1002/mnfr.201000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Lee Y, Kim YJ, Choi YJ, Lee JW, Lee S, Chung HW. Enhancement of cisplatin cytotoxicity by benzyl isothiocyanate in HL-60 cells. Food Chem Toxicol. 2012;50:2397–406. doi: 10.1016/j.fct.2012.04.014. [DOI] [PubMed] [Google Scholar]
- [343].Mi L, Wang X, Govind S, Hood BL, Veenstra TD, Conrads TP, et al. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 2007;67:6409–16. doi: 10.1158/0008-5472.CAN-07-0340. [DOI] [PubMed] [Google Scholar]
- [344].Mi L, Gan N, Cheema A, Dakshanamurthy S, Wang X, Yang DC, et al. Cancer preventive isothiocyanates induce selective degradation of cellular alpha- and beta-tubulins by proteasomes. J Biol Chem. 2009;284:17039–51. doi: 10.1074/jbc.M901789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Ramalingam S, Belani CP. Paclitaxel for non-small cell lung cancer. Expert Opin Pharmacother. 2004;5:1771–80. doi: 10.1517/14656566.5.8.1771. [DOI] [PubMed] [Google Scholar]
- [346].Hu L, Miao W, Loignon M, Kandouz M, Batist G. Putative chemopreventive molecules can increase Nrf2-regulated cell defense in some human cancer cell lines, resulting in resistance to common cytotoxic therapies. Cancer Chemother Pharmacol. 2010;66:467–74. doi: 10.1007/s00280-009-1182-7. [DOI] [PubMed] [Google Scholar]
- [347].Nair S, Barve A, Khor TO, Shen GX, Lin W, Chan JY, et al. Regulation of Nrf2- and AP-1-mediated gene expression by epigallocatechin-3-gallate and sulforaphane in prostate of Nrf2-knockout or C57BL/6J mice and PC-3 AP-1 human prostate cancer cells. Acta Pharmacol Sin. 2010;31:1223–40. doi: 10.1038/aps.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Slocum SL, Kensler TW. Nrf2: control of sensitivity to carcinogens. Arch Toxicol. 2011;85:273–84. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- [349].Sagar S, Esau L, Holtermann K, Hikmawan T, Zhang G, Stingl U, et al. Induction of apoptosis in cancer cell lines by the Red Sea brine pool bacterial extracts. BMC Complement Altern Med. 2013;13:344. doi: 10.1186/1472-6882-13-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Rengarajan T, Rajendran P, Nandakumar N, Balasubramanian MP, Nishigaki I. Cancer preventive efficacy of marine carotenoid fucoxanthin: cell cycle arrest and apoptosis. Nutrients. 2013;5:4978–89. doi: 10.3390/nu5124978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Farnaes L, Coufal NG, Kauffman CA, Rheingold AL, DiPasquale AG, Jensen PR, et al. Napyradiomycin derivatives, produced by a marine-derived actinomycete, illustrate cytotoxicity by induction of apoptosis. J Nat Prod. 2014;77:15–21. doi: 10.1021/np400466j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Farnaes L, La Clair JJ, Fenical W. Napyradiomycins CNQ525.510B and A80915C target the Hsp90 paralogue Grp94. Org Biomol Chem. 2014;12:418–23. doi: 10.1039/c3ob41355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Haste NM, Farnaes L, Perera VR, Fenical W, Nizet V, Hensler ME. Bactericidal kinetics of marine-derived napyradiomycins against contemporary methicillin-resistant Staphylococcus aureus. Mar Drugs. 2011;9:680–9. doi: 10.3390/md9040680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Kumar SR, Hosokawa M, Miyashita K. Fucoxanthin: a marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar Drugs. 2013;11:5130–47. doi: 10.3390/md11125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Nguyen VT, Lee JS, Qian ZJ, Li YX, Kim KN, Heo SJ, et al. Gliotoxin isolated from marine fungus Aspergillus sp. induces apoptosis of human cervical cancer and chondrosarcoma cells. Mar Drugs. 2014;12:69–87. doi: 10.3390/md12010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [356].Garcia-Caballero M, Canedo L, Fernandez-Medarde A, Medina MA, Quesada AR. The marine fungal metabolite, AD0157, inhibits angiogenesis by targeting the Akt signaling pathway. Mar Drugs. 2014;12:279–99. doi: 10.3390/md12010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Garcia-Caballero M, Mari-Beffa M, Canedo L, Medina MA, Quesada AR. Toluquinol, a marine fungus metabolite, is a new angiosuppresor that interferes with the Akt pathway. Biochem Pharmacol. 2013;85:1727–40. doi: 10.1016/j.bcp.2013.04.007. [DOI] [PubMed] [Google Scholar]
- [358].Goncalves AM, de Lima AB, da Silva Barbosa MC, de Camargos LF, de Oliveira JT, de Souza BC, et al. Synthesis and biological evaluation of novel 3-alkylpyridine marine alkaloid analogs with promising anticancer activity. Mar Drugs. 2014;12:4361–78. doi: 10.3390/md12084361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [359].Beesoo R, Neergheen-Bhujun V, Bhagooli R, Bahorun T. Apoptosis inducing lead compounds isolated from marine organisms of potential relevance in cancer treatment. Mutat Res Fundam Mol Mech Mutagen. 2014 doi: 10.1016/j.mrfmmm.2014.03.005. http://dx.doi.org/10.1016/j.mrfmmm.2014.03.005. [DOI] [PubMed]
- [360].Yin Y, Chen X, Zhang CD, Liu PF, Duan YR, Fan YR, et al. Asymmetric siRNA targeting the bcl2 gene inhibits the proliferation of cancer cells in vitro and in vivo. Int J Oncol. 2013;42:253–60. doi: 10.3892/ijo.2012.1691. [DOI] [PubMed] [Google Scholar]
- [361].Zhen Y, Liu Z, Yang H, Yu X, Wu Q, Hua S, et al. Tumor suppressor PDCD4 modulates miR-184-mediated direct suppression of C-MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell Death Dis. 2013;4:e872. doi: 10.1038/cddis.2013.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Bolesta E, Pfannenstiel LW, Demelash A, Lesniewski ML, Tobin M, Schlanger SE, et al. Inhibition of Mcl-1 promotes senescence in cancer cells: implications for preventing tumor growth and chemotherapy resistance. Mol Cell Biol. 2012;32:1879–92. doi: 10.1128/MCB.06214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Chen N, Eritja N, Lock R, Debnath J. Autophagy restricts proliferation driven by oncogenic phosphatidylinositol 3-kinase in three-dimensional culture. Oncogene. 2013;32:2543–54. doi: 10.1038/onc.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Kaminskyy VO, Piskunova T, Zborovskaya IB, Tchevkina EM, Zhivotovsky B. Suppression of basal autophagy reduces lung cancer cell proliferation and enhances caspase-dependent and -independent apoptosis by stimulating ROS formation. Autophagy. 2012;8:1032–44. doi: 10.4161/auto.20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Sha W, Olesch C, Hanaka H, Radmark O, Weigert A, Brune B. Necrosis in DU145 prostate cancer spheroids induces COX-2/mPGES-1-derived PGE2 to promote tumor growth and to inhibit T cell activation. Int J Cancer. 2013;133:1578–88. doi: 10.1002/ijc.28181. [DOI] [PubMed] [Google Scholar]
- [366].Dutta S, Going JJ, Crumley AB, Mohammed Z, Orange C, Edwards J, et al. The relationship between tumour necrosis, tumour proliferation, local and systemic inflammation, microvessel density and survival in patients undergoing potentially curative resection of oesophageal adenocarcinoma. Br J Cancer. 2012;106:702–10. doi: 10.1038/bjc.2011.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Chang DJ, An H, Kim KS, Kim HH, Jung J, Lee JM, et al. Design, synthesis, and biological evaluation of novel deguelin-based heat shock protein 90 (HSP90) inhibitors targeting proliferation and angiogenesis. J Med Chem. 2012;55:10863–84. doi: 10.1021/jm301488q. [DOI] [PubMed] [Google Scholar]
- [368].Zhao M, Ma J, Zhu HY, Zhang XH, Du ZY, Xu YJ, et al. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the trinity of CK2, Cdc37 and Hsp90. Mol Cancer. 2011;10:104. doi: 10.1186/1476-4598-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].Hui B, Shi YH, Ding ZB, Zhou J, Gu CY, Peng YF, et al. Proteasome inhibitor interacts synergistically with autophagy inhibitor to suppress proliferation and induce apoptosis in hepatocellular carcinoma. Cancer. 2012;118:5560–71. doi: 10.1002/cncr.27586. [DOI] [PubMed] [Google Scholar]
- [370].Bono C, Karlin L, Harel S, Mouly E, Labaume S, Galicier L, et al. The human immunodeficiency virus-1 protease inhibitor nelfinavir impairs proteasome activity and inhibits the proliferation of multiple myeloma cells in vitro and in vivo. Haematologica. 2012;97:1101–9. doi: 10.3324/haematol.2011.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Vogler M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 2012;19:67–74. doi: 10.1038/cdd.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Hsu A, Bruno RS, Lohr CV, Taylor AW, Dashwood RH, Bray TM, et al. Dietary soy and tea mitigate chronic inflammation and prostate cancer via NFkappaB pathway in the Noble rat model. J Nutr Biochem. 2011;22:502–10. doi: 10.1016/j.jnutbio.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Zhang HY, Zhang Q, Zhang X, Yu C, Huo X, Cheng E, et al. Cancer-related inflammation and Barrett’s carcinogenesis: interleukin-6 and STAT3 mediate apoptotic resistance in transformed Barrett’s cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G454, 60. doi: 10.1152/ajpgi.00458.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Chen LS, Balakrishnan K, Gandhi V. Inflammation and survival pathways: chronic lymphocytic leukemia as a model system. Biochem Pharmacol. 2010;80:1936–45. doi: 10.1016/j.bcp.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Chang CP, Su YC, Lee PH, Lei HY. Targeting NFKB by autophagy to polarize hepatoma-associated macrophage differentiation. Autophagy. 2013;9:619–21. doi: 10.4161/auto.23546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Liu EY, Ryan KM. Autophagy and cancer – issues we need to digest. J Cell Sci. 2012;125:2349–58. doi: 10.1242/jcs.093708. [DOI] [PubMed] [Google Scholar]
- [377].Chopra M, Lang I, Salzmann S, Pachel C, Kraus S, Bauerlein CA, et al. Tumor necrosis factor induces tumor promoting and anti-tumoral effects on pancreatic cancer via TNFR1. PLoS ONE. 2013;8:e75737. doi: 10.1371/journal.pone.0075737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Snow BJ, Peppard RF, Guttman M, Okada J, Martin WR, Steele J, et al. Positron emission tomographic scanning demonstrates a presynaptic dopaminergic lesion in Lytico-Bodig. The amyotrophic lateral sclerosis-parkinsonism-dementia complex of Guam. Arch Neurol. 1990;47:870–4. doi: 10.1001/archneur.1990.00530080052010. [DOI] [PubMed] [Google Scholar]
- [379].Du Y, Moulick K, Rodina A, Aguirre J, Felts S, Dingledine R, et al. High-throughput screening fluorescence polarization assay for tumor-specific Hsp90. J Biomol Screen. 2007;12:915–24. doi: 10.1177/1087057107306067. [DOI] [PubMed] [Google Scholar]
- [380].Arthur JC, Lich JD, Aziz RK, Kotb M, Ting JP. Heat shock protein 90 associates with monarch-1 and regulates its ability to promote degradation of NF-kappaB-inducing kinase. J Immunol. 2007;179:6291–6. doi: 10.4049/jimmunol.179.9.6291. [DOI] [PubMed] [Google Scholar]
- [381].Cortes Sempere M, Rodriguez Fanjul V, Sanchez Perez I, Perona R. The role of the NFkappaB signalling pathway in cancer. Clin Transl Oncol: Off Publ Fed Spanish Oncol Soc Natl Cancer Inst Mexico. 2008;10:143–7. doi: 10.1007/s12094-008-0171-3. [DOI] [PubMed] [Google Scholar]
- [382].Dalla Via L, Nardon C, Fregona D. Targeting the ubiquitin-proteasome pathway with inorganic compounds to fight cancer: a challenge for the future. Fut Med Chem. 2012;4:525–43. doi: 10.4155/fmc.11.187. [DOI] [PubMed] [Google Scholar]
- [383].Op den Kamp CM, Langen RC, Minnaard R, Kelders MC, Snepvangers FJ, Hesselink MK, et al. Pre-cachexia in patients with stages I–III non-small cell lung cancer: systemic inflammation and functional impairment without activation of skeletal muscle ubiquitin proteasome system. Lung Cancer. 2012;76:112–7. doi: 10.1016/j.lungcan.2011.09.012. [DOI] [PubMed] [Google Scholar]
- [384].Deng J, Shimamura T, Perera S, Carlson NE, Cai D, Shapiro GI, et al. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007;67:11867–75. doi: 10.1158/0008-5472.CAN-07-1961. [DOI] [PubMed] [Google Scholar]
- [385].Cai J, Ahmad S, Jiang WG, Huang J, Kontos CD, Boulton M, et al. Activation of vascular endothelial growth factor receptor-1 sustains angiogenesis and Bcl-2 expression via the phosphatidylinositol 3-kinase pathway in endothelial cells. Diabetes. 2003;52:2959–68. doi: 10.2337/diabetes.52.12.2959. [DOI] [PubMed] [Google Scholar]
- [386].Trisciuoglio D, Iervolino A, Zupi G, Del Bufalo D. Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol Biol Cell. 2005;16:4153–62. doi: 10.1091/mbc.E04-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Chandra A, Lan S, Zhu J, Siclari VA, Qin L. Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression. J Biol Chem. 2013;288:20488–98. doi: 10.1074/jbc.M112.447250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Chen S, Han Q, Wang X, Yang M, Zhang Z, Li P, et al. IBP-mediated suppression of autophagy promotes growth and metastasis of breast cancer cells via activating mTORC2/Akt/FOXO3a signaling pathway. Cell Death Dis. 2013;4:e842. doi: 10.1038/cddis.2013.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Nakagami H, Cui TX, Iwai M, Shiuchi T, Takeda-Matsubara Y, Wu L, et al. Tumor necrosis factor-alpha inhibits growth factor-mediated cell proliferation through SHP-1 activation in endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22:238–42. doi: 10.1161/hq0202.104001. [DOI] [PubMed] [Google Scholar]
- [390].Chandarlapaty S, Scaltriti M, Angelini P, Ye Q, Guzman M, Hudis CA, et al. Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010;29:325–34. doi: 10.1038/onc.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [391].Duval M, Le Boeuf F, Huot J, Gratton JP. Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase. Mol Biol Cell. 2007;18:4659–68. doi: 10.1091/mbc.E07-05-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [392].Yang Y, Ikezoe T, Saito T, Kobayashi M, Koeffier HP, Taguchi H. Proteasome inhibitor PS-341 induces growth arrest and apoptosis of non-small cell lung cancer cells via the JNK/c-Jun/AP-1 signaling. Cancer Sci. 2004;95:176–80. doi: 10.1111/j.1349-7006.2004.tb03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [393].Cascone T, Morelli MP, Morgillo F, Kim WY, Rodolico G, Pepe S, et al. Synergistic anti-proliferative and pro-apoptotic activity of combined therapy with bortezomib, a proteasome inhibitor, with anti-epidermal growth factor receptor (EGFR) drugs in human cancer cells. J Cell Physiol. 2008;216:698–707. doi: 10.1002/jcp.21444. [DOI] [PubMed] [Google Scholar]
- [394].Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–14. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [395].Johnson VL, Cooper IR, Jenkins JR, Chow SC. Effects of differential overexpression of Bcl-2 on apoptosis, proliferation, and telomerase activity in Jurkat T cells. Exp Cell Res. 1999;251:175–84. doi: 10.1006/excr.1999.4557. [DOI] [PubMed] [Google Scholar]
- [396].Mandal M, Kumar R. Bcl-2 modulates telomerase activity. J Biol Chem. 1997;272:14183–7. doi: 10.1074/jbc.272.22.14183. [DOI] [PubMed] [Google Scholar]
- [397].Aoki H, Iwado E, Eller MS, Kondo Y, Fujiwara K, Li GZ, et al. Telomere 3′ overhang-specific DNA oligonucleotides induce autophagy in malignant glioma cells. FASEB J. 2007;21:2918–30. doi: 10.1096/fj.06-6941com. [DOI] [PubMed] [Google Scholar]
- [398].Iwado E, Daido S, Kondo Y, Kondo S. Combined effect of 2-5A-linked antisense against telomerase RNA and conventional therapies on human malignant glioma cells in vitro and in vivo. Int J Oncol. 2007;31:1087–95. [PubMed] [Google Scholar]
- [399].Capparelli C, Guido C, Whitaker-Menezes D, Bonuccelli G, Balliet R, Pestell TG, et al. Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Cell Cycle (Georgetown, Tex) 2012;11:2285–302. doi: 10.4161/cc.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Jain MV, Paczulla AM, Klonisch T, Dimgba FN, Rao SB, Roberg K, et al. Interconnections between apoptotic, autophagic and necrotic pathways: implications for cancer therapy development. J Cell Mol Med. 2013;17:12–29. doi: 10.1111/jcmm.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Restall IJ, Lorimer IA. Induction of premature senescence by hsp90 inhibition in small cell lung cancer. PLoS ONE. 2010;5:e11076. doi: 10.1371/journal.pone.0011076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [402].Chan KC, Ting CM, Chan PS, Lo MC, Lo KW, Curry JE, et al. A novel Hsp90 inhibitor AT13387 induces senescence in EBV-positive nasopharyngeal carcinoma cells and suppresses tumor formation. Mol Cancer. 2013;12:128. doi: 10.1186/1476-4598-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Torres CA, Perez VI. Proteasome modulates mitochondrial function during cellular senescence. Free Radic Biol Med. 2008;44:403–14. doi: 10.1016/j.freeradbiomed.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [404].Chondrogianni N, Stratford FL, Trougakos IP, Friguet B, Rivett AJ, Gonos ES. Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J Biol Chem. 2003;278:28026–37. doi: 10.1074/jbc.M301048200. [DOI] [PubMed] [Google Scholar]
- [405].Hu T, Zhang C, Tang Q, Su Y, Li B, Chen L, et al. Variant G6PD levels promote tumor cell proliferation or apoptosis via the STAT3/5 pathway in the human melanoma xenograft mouse model. BMC Cancer. 2013;13:251. doi: 10.1186/1471-2407-13-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [406].Chio CC, Lin JW, Cheng HA, Chiu WT, Wang YH, Wang JJ, et al. MicroRNA-210 targets antiapoptotic Bcl-2 expression and mediates hypoxia-induced apoptosis of neuroblastoma cells. Arch Toxicol. 2013;87:459–68. doi: 10.1007/s00204-012-0965-5. [DOI] [PubMed] [Google Scholar]
- [407].MacFarlane M, Robinson GL, Cain K. Glucose – a sweet way to die: metabolic switching modulates tumor cell death. Cell Cycle (Georgetown, Tex) 2012;11:3919–25. doi: 10.4161/cc.21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [408].Kim SM, Yun MR, Hong YK, Solca F, Kim JH, Kim HJ, et al. Glycolysis inhibition sensitizes non-small cell lung cancer with T790M mutation to irreversible EGFR inhibitors via translational suppression of Mcl-1 by AMPK activation. Mol Cancer Ther. 2013;12:2145–56. doi: 10.1158/1535-7163.MCT-12-1188. [DOI] [PubMed] [Google Scholar]
- [409].Choi HN, Jin HO, Kim JH, Hong SE, Kim HA, Kim EK, et al. Inhibition of S6K1 enhances glucose deprivation-induced cell death via downregulation of anti-apoptotic proteins in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2013;432:123–8. doi: 10.1016/j.bbrc.2013.01.074. [DOI] [PubMed] [Google Scholar]
- [410].McBrayer SK, Cheng JC, Singhal S, Krett NL, Rosen ST, Shanmugam M. Multiple myeloma exhibits novel dependence on GLUT4, GLUT8, and GLUT11: implications for glucose transporter-directed therapy. Blood. 2012;119:4686–97. doi: 10.1182/blood-2011-09-377846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Pal K, Cao Y, Gaisina IN, Bhattacharya S, Dutta SK, Wang E, et al. Inhibition of GSK-3 induces differentiation and impaired glucose metabolism in renal cancer. Mol Cancer Ther. 2014;13:285–96. doi: 10.1158/1535-7163.MCT-13-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [412].Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- [413].Klarer AC, O’Neal J, Imbert-Fernandez Y, Clem A, Ellis SR, Clark J, et al. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) induces autophagy as a survival mechanism. Cancer Metab. 2014;2:2. doi: 10.1186/2049-3002-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [414].Wanka C, Brucker DP, Bahr O, Ronellenfitsch M, Weller M, Steinbach JP, et al. Synthesis of cytochrome C oxidase 2: a p53-dependent metabolic regulator that promotes respiratory function and protects glioma and colon cancer cells from hypoxia-induced cell death. Oncogene. 2012;31:3764–76. doi: 10.1038/onc.2011.530. [DOI] [PubMed] [Google Scholar]
- [415].Qin JZ, Xin H, Nickoloff BJ. 2-deoxyglucose sensitizes melanoma cells to TRAIL-induced apoptosis which is reduced by mannose. Biochem Biophys Res Commun. 2010;401:293–9. doi: 10.1016/j.bbrc.2010.09.054. [DOI] [PubMed] [Google Scholar]
- [416].Pradelli LA, Beneteau M, Chauvin C, Jacquin MA, Marchetti S, Munoz-Pinedo C, et al. Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene. 2010;29:1641–52. doi: 10.1038/onc.2009.448. [DOI] [PubMed] [Google Scholar]
- [417].Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, et al. A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol Cancer Ther. 2008;7:3546–55. doi: 10.1158/1535-7163.MCT-08-0569. [DOI] [PubMed] [Google Scholar]
- [418].Mayer P, Harjung A, Breinig M, Fischer L, Ehemann V, Malz M, et al. Expression and therapeutic relevance of heat-shock protein 90 in pancreatic endocrine tumors. Endocr Relat Cancer. 2012;19:217–32. doi: 10.1530/ERC-11-0227. [DOI] [PubMed] [Google Scholar]
- [419].Beck R, Verrax J, Gonze T, Zappone M, Pedrosa RC, Taper H, et al. Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem Pharmacol. 2009;77:375–83. doi: 10.1016/j.bcp.2008.10.019. [DOI] [PubMed] [Google Scholar]
- [420].Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94:15–21. doi: 10.1111/j.1349-7006.2003.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [421].Busacca S, Chacko AD, Klabatsa A, Arthur K, Sheaff M, Barbone D, et al. BAK and NOXA are critical determinants of mitochondrial apoptosis induced by bortezomib in mesothelioma. PLoS ONE. 2013;8:e65489. doi: 10.1371/journal.pone.0065489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [422].Zheng JM, Wang DK, Jiang HM. bcl-2 expression in small cell lung cancer: a mechanism for apoptosis antagonism and immune evasion. Di 1 Jun Yi Da Xue Xue Bao = Acad J First Med Coll PLA. 2005;25:1537–9. [PubMed] [Google Scholar]
- [423].Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8:409–22. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- [424].Kareva I, Hahnfeldt P. The emerging hallmarks of metabolic reprogramming and immune evasion: distinct or linked. Cancer Res. 2013;73:2737–42. doi: 10.1158/0008-5472.CAN-12-3696. [DOI] [PubMed] [Google Scholar]
- [425].Niebler M, Qian X, Hofler D, Kogosov V, Kaewprag J, Kaufmann AM, et al. Post-translational control of IL-1beta via the human papillomavirus type 16 E6 oncoprotein: a novel mechanism of innate immune escape mediated by the E3-ubiquitin ligase E6-AP and p53. PLoS Pathogens. 2013;9:e1003536. doi: 10.1371/journal.ppat.1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [426].Zhang X, Dawson CW, He Z, Huang P. Immune evasion strategies of the human gamma-herpesviruses: implications for viral tumorigenesis. J Med Virol. 2012;84:272–81. doi: 10.1002/jmv.22267. [DOI] [PubMed] [Google Scholar]
- [427].Ayshamgul H, Ma H, Ilyar S, Zhang LW, Abulizi A. Association of defective HLA-I expression with antigen processing machinery and their association with clinicopathological characteristics in Kazak patients with esophageal cancer. Chin Med J (Engl) 2011;124:341–6. [PubMed] [Google Scholar]
- [428].Nor JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM, et al. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61:2183–8. [PubMed] [Google Scholar]
- [429].Pignochino Y, Grignani G, Cavalloni G, Motta M, Tapparo M, Bruno S, et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol Cancer. 2009;8:118. doi: 10.1186/1476-4598-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [430].Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both. Cancer Res. 2006;66:9349–51. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- [431].Edwards JG, Swinson DE, Jones JL, Muller S, Waller DA, O’Byrne KJ. Tumor necrosis correlates with angiogenesis and is a predictor of poor prognosis in malignant mesothelioma. Chest. 2003;124:1916–23. doi: 10.1378/chest.124.5.1916. [DOI] [PubMed] [Google Scholar]
- [432].Sun J, Liao JK. Induction of angiogenesis by heat shock protein 90 mediated by protein kinase Akt and endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2004;24:2238–44. doi: 10.1161/01.ATV.0000147894.22300.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [433].Oikawa T, Sasaki T, Nakamura M, Shimamura M, Tanahashi N, Omura S, et al. The proteasome is involved in angiogenesis. Biochem Biophys Res Commun. 1998;246:243–8. doi: 10.1006/bbrc.1998.8604. [DOI] [PubMed] [Google Scholar]
- [434].Knauer SK, Kramer OH, Knosel T, Engels K, Rodel F, Kovacs AF, et al. Nuclear export is essential for the tumor-promoting activity of survivin. FASEB J. 2007;21:207–16. doi: 10.1096/fj.06-5741com. [DOI] [PubMed] [Google Scholar]
- [435].Zuo J, Ishikawa T, Boutros S, Xiao Z, Humtsoe JO, Kramer RH. Bcl-2 overexpression induces a partial epithelial to mesenchymal transition and promotes squamous carcinoma cell invasion and metastasis. Mol Cancer Res: MCR. 2010;8:170–82. doi: 10.1158/1541-7786.MCR-09-0354. [DOI] [PubMed] [Google Scholar]
- [436].Likui W, Qun L, Wanqing Z, Haifeng S, Fangqiu L, Xiaojun L. Prognostic role of myeloid cell leukemia-1 protein (Mcl-1) expression in human gastric cancer. J Surg Oncol. 2009;100:396–400. doi: 10.1002/jso.21344. [DOI] [PubMed] [Google Scholar]
- [437].Lv Q, Wang W, Xue J, Hua F, Mu R, Lin H, et al. DEDD interacts with PI3KC3 to activate autophagy and attenuate epithelial-mesenchymal transition in human breast cancer. Cancer Res. 2012;72:3238–50. doi: 10.1158/0008-5472.CAN-11-3832. [DOI] [PubMed] [Google Scholar]
- [438].Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [439].Seki N, Hayakawa Y, Brooks AD, Wine J, Wiltrout RH, Yagita H, et al. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res. 2003;63:207–13. [PubMed] [Google Scholar]
- [440].Koga F, Kihara K, Neckers L. Inhibition of cancer invasion and metastasis by targeting the molecular chaperone heat-shock protein 90. Anticancer Res. 2009;29:797–807. [PubMed] [Google Scholar]
- [441].Yan W, Xiao J, Liu T, Huang W, Yang X, Wu Z, et al. The effects of Hsp90 expression alteration on spinal metastases of breast carcinoma. Tumour Biol. 2013;34:1391–7. doi: 10.1007/s13277-012-0584-z. [DOI] [PubMed] [Google Scholar]
- [442].Zhu S, Yao F, Li WH, Wan JN, Zhang YM, Tang Z, et al. PKC?-dependent activation of the ubiquitin proteasome system is responsible for high glucose-induced human breast cancer MCF-7 cell proliferation, migration and invasion. Asian Pac J Cancer Prevent: APJCP. 2013;14:5687–92. doi: 10.7314/apjcp.2013.14.10.5687. [DOI] [PubMed] [Google Scholar]
- [443].Inoue H, Kauffman M, Shacham S, Landesman Y, Yang J, Evans CP, et al. CRM1 blockade by selective inhibitors of nuclear export attenuates kidney cancer growth. J Urol. 2013;189:2317–26. doi: 10.1016/j.juro.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [444].Buggins AG, Pepper C, Patten PE, Hewamana S, Gohil S, Moorhead J, et al. Interaction with vascular endothelium enhances survival in primary chronic lymphocytic leukemia cells via NF-kappaB activation and de novo gene transcription. Cancer Res. 2010;70:7523–33. doi: 10.1158/0008-5472.CAN-10-1634. [DOI] [PubMed] [Google Scholar]
- [445].Anai S, Sakamoto N, Sakai Y, Tanaka M, Porvasnik S, Urbanek C, et al. Dual targeting of Bcl-2 and VEGF: a potential strategy to improve therapy for prostate cancer. Urol Oncol. 2011;29:421–9. doi: 10.1016/j.urolonc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- [446].Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–75. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [447].Zhu H, Wang D, Liu Y, Su Z, Zhang L, Chen F, et al. Role of the Hypoxia-inducible factor-1 alpha induced autophagy in the conversion of non-stem pancreatic cancer cells into CD133+ pancreatic cancer stem-like cells. Cancer Cell Int. 2013;13:119. doi: 10.1186/1475-2867-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [448].Mo J, Sun B, Zhao X, Gu Q, Dong X, Liu Z, et al. Hypoxia-induced senescence contributes to the regulation of microenvironment in melanomas. Pathol Res Pract. 2013;209:640–7. doi: 10.1016/j.prp.2013.07.004. [DOI] [PubMed] [Google Scholar]
- [449].Walsby E, Pearce L, Burnett AK, Fegan C, Pepper C. The Hsp90 inhibitor NVP-AUY922-AG inhibits NF-kappaB signaling, overcomes microenvironmental cytoprotection and is highly synergistic with fludarabine in primary CLL cells. Oncotarget. 2012;3:525–34. doi: 10.18632/oncotarget.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [450].Skandalis SS, Aletras AJ, Gialeli C, Theocharis AD, Afratis N, Tzanakakis GN, et al. Targeting the tumor proteasome as a mechanism to control the synthesis and bioactivity of matrix macromolecules. Curr Mol Med. 2012;12:1068–82. doi: 10.2174/156652412802480943. [DOI] [PubMed] [Google Scholar]
- [451].Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28:155–65. doi: 10.1038/leu.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [452].Valenti D, de Bari L, Manente GA, Rossi L, Mutti L, Moro L, et al. Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells. Biochim Biophys Acta. 2013;1832:2085–96. doi: 10.1016/j.bbadis.2013.07.014. [DOI] [PubMed] [Google Scholar]
- [453].Zhang Q, Fu H, Pan J, He J, Ryota S, Hara Y, et al. Effect of dietary Polyphenon E and EGCG on lung tumorigenesis in A/J Mice. Pharm Res. 2010;27:1066–71. doi: 10.1007/s11095-010-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [454].Pang X, Wu Y, Wu Y, Lu B, Chen J, Wang J, et al. (−)-Gossypol suppresses the growth of human prostate cancer xenografts via modulating VEGF signaling-mediated angiogenesis. Mol Cancer Ther. 2011;10:795–805. doi: 10.1158/1535-7163.MCT-10-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [455].Zhang XQ, Huang XF, Mu SJ, An QX, Xia AJ, Chen R, et al. Inhibition of proliferation of prostate cancer cell line, PC-3, in vitro and in vivo using (−)-gossypol. Asian J Androl. 2010;12:390–9. doi: 10.1038/aja.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [456].Deng YT, Lin JK. EGCG inhibits the invasion of highly invasive CL1-5 lung cancer cells through suppressing MMP-2 expression via JNK signaling and induces G2/M arrest. J Agric Food Chem. 2011;59:13318–27. doi: 10.1021/jf204149c. [DOI] [PubMed] [Google Scholar]
- [457].Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1alpha. Carcinogenesis. 2011;32:1881–9. doi: 10.1093/carcin/bgr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [458].Liu J, Shen M, Yue Z, Yang Z, Wang M, Li C, et al. Triptolide inhibits colon-rectal cancer cells proliferation by induction of G1 phase arrest through upregulation of p21. Phytomedicine. 2012;19:756–62. doi: 10.1016/j.phymed.2012.02.014. [DOI] [PubMed] [Google Scholar]
- [459].Zhao F, Huang W, Ousman T, Zhang B, Han Y, Clotaire DZ, et al. Triptolide induces growth inhibition and apoptosis of human laryngocarcinoma cells by enhancing p53 activities and suppressing E6-mediated p53 degradation. PLoS ONE. 2013;8:e80784. doi: 10.1371/journal.pone.0080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [460].Wang J, Jin L, Li X, Deng H, Chen Y, Lian Q, et al. Gossypol induces apoptosis in ovarian cancer cells through oxidative stress. Mol BioSyst. 2013;9:1489–97. doi: 10.1039/c3mb25461e. [DOI] [PubMed] [Google Scholar]
- [461].Soderquist R, Bates DJ, Danilov AV, Eastman A. Gossypol overcomes stroma-mediated resistance to the BCL2 inhibitor ABT-737 in chronic lymphocytic leukemia cells ex vivo. Leukemia. 2013;27:2262–4. doi: 10.1038/leu.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [462].Hardtner C, Multhoff G, Falk W, Radons J. (−)-Epigallocatechin-3-gallate, a green tea-derived catechin, synergizes with celecoxib to inhibit IL-1-induced tumorigenic mediators by human pancreatic adenocarcinoma cells Colo357. Eur J Pharmacol. 2012;684:36–43. doi: 10.1016/j.ejphar.2012.03.039. [DOI] [PubMed] [Google Scholar]
- [463].Hoffmann J, Junker H, Schmieder A, Venz S, Brandt R, Multhoff G, et al. EGCG downregulates IL-1RI expression and suppresses IL-1-induced tumorigenic factors in human pancreatic adenocarcinoma cells. Biochem Pharmacol. 2011;82:1153–62. doi: 10.1016/j.bcp.2011.07.063. [DOI] [PubMed] [Google Scholar]
- [464].Park B, Sung B, Yadav VR, Chaturvedi MM, Aggarwal BB. Triptolide, histone acetyltransferase inhibitor, suppresses growth and chemosensitizes leukemic cells through inhibition of gene expression regulated by TNF-TNFR1-TRADD-TRAF2-NIK-TAK1-IKK pathway. Biochem Pharmacol. 2011;82:1134–44. doi: 10.1016/j.bcp.2011.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [465].Pan J. RNA polymerase – an important molecular target of triptolide in cancer cells. Cancer Lett. 2010;292:149–52. doi: 10.1016/j.canlet.2009.11.018. [DOI] [PubMed] [Google Scholar]
- [466].Jiang J, Ye W, Lin YC. Gossypol inhibits the growth of MAT-LyLu prostate cancer cells by modulation of TGFbeta/Akt signaling. Int J Mol Med. 2009;24:69–75. [PubMed] [Google Scholar]
- [467].Jiang J, Sugimoto Y, Liu S, Chang HL, Park KY, Kulp SK, et al. The inhibitory effects of gossypol on human prostate cancer cells-PC3 are associated with transforming growth factor beta1 (TGFbeta1) signal transduction pathway. Anticancer Res. 2004;24:91–100. [PubMed] [Google Scholar]
- [468].Chang CM, Chang PY, Tu MG, Lu CC, Kuo SC, Amagaya S, et al. Epigallocatechin gallate sensitizes CAL-27 human oral squamous cell carcinoma cells to the anti-metastatic effects of gefitinib (Iressa) via synergistic suppression of epidermal growth factor receptor and matrix metalloproteinase-2. Oncol Rep. 2012;28:1799–807. doi: 10.3892/or.2012.1991. [DOI] [PubMed] [Google Scholar]
- [469].Hsu YC, Liou YM. The anti-cancer effects of (−)-epigallocatechin-3-gallate on the signaling pathways associated with membrane receptors in MCF-7 cells. J Cell Physiol. 2011;226:2721–30. doi: 10.1002/jcp.22623. [DOI] [PubMed] [Google Scholar]
- [470].Hwang KC, Lee KH, Jang Y, Yun YP, Chung KH. Epigallocatechin-3-gallate inhibits basic fibroblast growth factor-induced intracellular signaling transduction pathway in rat aortic smooth muscle cells. J Cardiovasc Pharmacol. 2002;39:271–7. doi: 10.1097/00005344-200202000-00014. [DOI] [PubMed] [Google Scholar]
- [471].Huang W, He T, Chai C, Yang Y, Zheng Y, Zhou P, et al. Triptolide inhibits the proliferation of prostate cancer cells and down-regulates SUMO-specific protease 1 expression. PLoS ONE. 2012;7:e37693. doi: 10.1371/journal.pone.0037693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [472].Sahin F, Avci CB, Gunduz C, Sezgin C, Simsir IY, Saydam G. Gossypol exerts its cytotoxic effect on HL-60 leukemic cell line via decreasing activity of protein phosphatase 2A and interacting with human telomerase reverse transcriptase activity. Hematology. 2010;15:144–50. doi: 10.1179/102453309X12583347113771. [DOI] [PubMed] [Google Scholar]
- [473].Moon DO, Kim MO, Choi YH, Lee HG, Kim ND, Kim GY. Gossypol suppresses telomerase activity in human leukemia cells via regulating hTERT. FEBS Lett. 2008;582:3367–73. doi: 10.1016/j.febslet.2008.08.029. [DOI] [PubMed] [Google Scholar]
- [474].Wang X, Hao MW, Dong K, Lin F, Ren JH, Zhang HZ. Apoptosis induction effects of EGCG in laryngeal squamous cell carcinoma cells through telomerase repression. Arch Pharmacal Res. 2009;32:1263–9. doi: 10.1007/s12272-009-1912-8. [DOI] [PubMed] [Google Scholar]
- [475].Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008;103:509–19. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [476].Kiviharju TM, Lecane PS, Sellers RG, Peehl DM. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin Cancer Res. 2002;8:2666–74. [PubMed] [Google Scholar]
- [477].Schwartz PS, Manion MK, Emerson CB, Fry JS, Schulz CM, Sweet IR, et al. 2-Methoxy antimycin reveals a unique mechanism for Bcl-x(L) inhibition. Mol Cancer Ther. 2007;6:2073–80. doi: 10.1158/1535-7163.MCT-06-0767. [DOI] [PubMed] [Google Scholar]
- [478].Huang KH, Kuo KL, Chen SC, Weng TI, Chuang YT, Tsai YC, et al. Down-regulation of glucose-regulated protein (GRP) 78 potentiates cytotoxic effect of celecoxib in human urothelial carcinoma cells. PLoS ONE. 2012;7:e33615. doi: 10.1371/journal.pone.0033615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [479].Tan BJ, Chiu GN. Role of oxidative stress, endoplasmic reticulum stress and ERK activation in triptolide-induced apoptosis. Int J Oncol. 2013;42:1605–12. doi: 10.3892/ijo.2013.1843. [DOI] [PubMed] [Google Scholar]
- [480].Chen L, Liu Q, Huang Z, Wu F, Li Z, Chen X, et al. Tripchlorolide induces cell death in lung cancer cells by autophagy. Int J Oncol. 2012;40:1066–70. doi: 10.3892/ijo.2011.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [481].Wu PP, Liu KC, Huang WW, Ma CY, Lin H, Yang JS, et al. Triptolide induces apoptosis in human adrenal cancer NCI-H295 cells through a mitochondrial-dependent pathway. Oncol Rep. 2011;25:551–7. doi: 10.3892/or.2010.1080. [DOI] [PubMed] [Google Scholar]
- [482].Tarnow J. Art and pitfalls of preoperative anesthesia information. Anasthesiol Intens Notfallmed Schmerzther: AINS. 1999;34:723–4. doi: 10.1055/s-1999-224. [DOI] [PubMed] [Google Scholar]
- [483].Jang JY, Lee JK, Jeon YK, Kim CW. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [484].Liang M, Fu J. Triptolide inhibits interferon-gamma-induced programmed death-1-ligand 1 surface expression in breast cancer cells. Cancer Lett. 2008;270:337–41. doi: 10.1016/j.canlet.2008.05.025. [DOI] [PubMed] [Google Scholar]
- [485].Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440–52. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- [486].Ma JX, Sun YL, Wang YQ, Wu HY, Jin J, Yu XF. Triptolide induces apoptosis and inhibits the growth and angiogenesis of human pancreatic cancer cells by downregulating COX-2 and VEGF. Oncol Res. 2013;20:359–68. doi: 10.3727/096504013X13657689382932. [DOI] [PubMed] [Google Scholar]
- [487].Wolter KG, Wang SJ, Henson BS, Wang S, Griffith KA, Kumar B, et al. (−)-gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia. 2006;8:163–72. doi: 10.1593/neo.05691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [488].Brohem CA, Massaro RR, Tiago M, Marinho CE, Jasiulionis MG, de Almeida RL, et al. Proteasome inhibition and ROS generation by 4-nerolidylcatechol induces melanoma cell death. Pigment Cell Melanoma Res. 2012;25:354–69. doi: 10.1111/j.1755-148X.2012.00992.x. [DOI] [PubMed] [Google Scholar]
- [489].Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, et al. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65–72. [PubMed] [Google Scholar]
- [490].Pacak K, Sirova M, Giubellino A, Lencesova L, Csaderova L, Laukova M, et al. NF-kappaB inhibition significantly upregulates the norepinephrine transporter system, causes apoptosis in pheochromocytoma cell lines and prevents metastasis in an animal model. Int J Cancer. 2012;131:2445–55. doi: 10.1002/ijc.27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [491].Cheng Y, Holloway MP, Nguyen K, McCauley D, Landesman Y, Kauffman MG, et al. XPO1 (CRM1) inhibition represses STAT3 activation to drive a survivin-dependent oncogenic switch in triple-negative breast cancer. Mol Cancer Ther. 2014;13:675–86. doi: 10.1158/1535-7163.MCT-13-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [492].Etchin J, Sanda T, Mansour MR, Kentsis A, Montero J, Le BT, et al. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. Br J Haematol. 2013;161:117–27. doi: 10.1111/bjh.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [493].Voss V, Senft C, Lang V, Ronellenfitsch MW, Steinbach JP, Seifert V, et al. The pan-Bcl-2 inhibitor (−)-gossypol triggers autophagic cell death in malignant glioma. Mol Cancer Res: MCR. 2010;8:1002–16. doi: 10.1158/1541-7786.MCR-09-0562. [DOI] [PubMed] [Google Scholar]
- [494].Zgheib A, Lamy S, Annabi B. Epigallocatechin gallate targeting of membrane type 1 matrix metalloproteinase-mediated Src and Janus kinase/signal transducers and activators of transcription 3 signaling inhibits transcription of colony-stimulating factors 2 and 3 in mesenchymal stromal cells. J Biol Chem. 2013;288:13378–86. doi: 10.1074/jbc.M113.456533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [495].Hung FM, Chen YL, Huang AC, Hsiao YP, Yang JS, Chung MT, et al. Triptolide induces S phase arrest via the inhibition of cyclin E and CDC25A and triggers apoptosis via caspase- and mitochondrial-dependent signaling pathways in A375.S2 human melanoma cells. Oncol Rep. 2013;29:1053–60. doi: 10.3892/or.2013.2230. [DOI] [PubMed] [Google Scholar]
- [496].Carter BZ, Mak DH, Shi Y, Fidler JM, Chen R, Ling X, et al. MRx102, a triptolide derivative, has potent antileukemic activity in vitro and in a murine model of AML. Leukemia. 2012;26:443–50. doi: 10.1038/leu.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [497].Zhong Y, El-Gamal D, Dubovsky JA, Beckwith KA, Harrington BK, Williams KE, et al. Selinexor suppresses downstream effectors of B-cell activation, proliferation and migration in chronic lymphocytic leukemia cells. Leukemia. 2014;28:1158–63. doi: 10.1038/leu.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]