Abstract
Both estradiol and testosterone have been implicated as the steroid critical for modulating women’s sexual desire. By contrast, in all other female mammals only estradiol has been shown to be critical for female sexual motivation and behavior. Pharmaceutical companies have invested heavily in the development of androgen therapies for female sexual desire disorders, but today there are still no FDA approved androgen therapies for women. Nonetheless, testosterone is currently, and frequently, prescribed off-label for the treatment of low sexual desire in women, and the idea of testosterone as a cure-all for female sexual dysfunction remains popular. This paper places the ongoing debate concerning the hormonal modulation of women’s sexual desire within a historical context, and reviews controlled trials of estrogen and/or androgen therapies for low sexual desire in postmenopausal women. These studies demonstrate that estrogen-only therapies that produce periovulatory levels of circulating estradiol increase sexual desire in postmenopausal women. Testosterone at supraphysiological, but not at physiological, levels enhances the effectiveness of low-dose estrogen therapies at increasing women’s sexual desire; however, the mechanism by which supraphysiological testosterone increases women’s sexual desire in combination with an estrogen remains unknown. Because effective therapies require supraphysiological amounts of testosterone, it remains unclear whether endogenous testosterone contributes to the modulation of women’s sexual desire. The likelihood that an androgen-only clinical treatment will meaningfully increase women’s sexual desire is minimal, and the focus of pharmaceutical companies on the development of androgen therapies for the treatment of female sexual desire disorders is likely misplaced.
Keywords: menopause, sexual desire, estradiol, testosterone, hormone therapy
1. Introduction
Ovarian steroids (estradiol, testosterone, and progesterone) modulate sexual desire, or libido, in women. The gradual and age-related cessation of ovarian function associated with natural menopause decreases levels of ovarian steroids, accompanied by diminished sexual desire in a significant portion of postmenopausal women (Dennerstein et al., 2006; Jiroutek et al., 1998; Leiblum et al. 2006). Similarly, women who undergo bilateral oophorectomy (surgical menopause) routinely report a post-operative decline in sexual desire after experiencing an abrupt and pronounced drop in circulating levels of ovarian steroids (Dennerstein et al., 2006; Korse et al., 2009; Leiblum et al. 2006; Sherwin et al., 1985). This menopause-related decrease in sexual desire can be extreme and even debilitating; the Women’s International Study of Health and Sexuality (WISheS) found that roughly 9-percent of naturally and up to 26-percent of surgically postmenopausal women suffer from a persistent and distressing lack of sexual desire (Dennerstein et al., 2006; Leiblum et al. 2006). Steroid hormones do not create sexual desire, but women’s sexual desire is clearly subject to hormonal influence. The identity of the ovarian steroid(s) critical for the modulation of women’s sexual desire, however, remains a topic of debate.
Both estradiol and testosterone have been implicated as the steroid that critically modulates sexual desire in women; although, estradiol seems at first glance to be the more likely candidate for this role. In all other mammalian species that have been studied, estradiol is critical for the expression of species-typical female sexual behavior–female rodents, ungulates, and carnivores all cease mating following ovariectomy, and female mating behavior can be reinstated by exogenous estradiol, without an accompanying androgen (for review see Beach, 1947; Wallen, 1990; 2013). The hormonal modulation of female sexual motivation has been particularly well studied in rhesus monkeys, which share many aspects of reproductive biology in common with women, including an approximately 28 day menstrual cycle with nearly identical patterns of hormonal fluctuation (Wallen et al., 1984; Wilson et al., 1982). Female rhesus monkey sexual motivation varies across the menstrual cycle (Ball & Hartman, 1935; Carpenter, 1942; Cochran, 1979; Gordon, 1981; Keverne, 1976; Michael & Bonsall, 1976; Pomerantz & Goy, 1983; Wallen et al., 1984), and female sexual behavior correlates with estradiol but not testosterone (Wallen et al., 1984; Wilson et al., 1982). Female rhesus monkey sexual motivation decreases following ovariectomy (Keverne, 1976), and treatment with exogenous estradiol increases sexual motivation in ovariectomized females (Keverne, 1976; Wallen & Goy, 1977; Zehr et al., 1998). If women’s sexual desire was under androgenic rather than estrogenic modulation, it would discriminate humans as unique amongst mammals (Wallen, 2013). Nonetheless, testosterone is currently, and frequently, prescribed off-label for the treatment of low libido in postmenopausal women (Bolour & Braunstein, 2005), and the idea of testosterone as a possible cure-all for female sexual dysfunction remains common and popular.
This paper places the ongoing debate concerning the hormonal modulation of women’s sexual desire within a historical context, and reviews controlled trials examining the effectiveness of estrogen and/or androgen therapies for the treatment of low libido in postmenopausal women. We conclude that estrogen-only therapies that produce periovulatory levels of circulating estradiol increase sexual desire in postmenopausal women – likely via a combination of central and peripheral mechanisms. Testosterone at supraphysiological levels, but not at physiological levels, enhances the effectiveness of a low dose estrogen therapy at increasing sexual desire in postmenopausal women; however, the mechanism by which supraphysiological testosterone increases women’s sexual desire in the presence of an estrogen remains unknown. It is possible that testosterone increases women’s sexual desire via its own aromatization to estradiol, and/or via the dynamic relationship between estradiol, testosterone, and sex hormone binding globulin (SHBG). Because effective therapies require supraphysiological amounts of testosterone, it remains unclear whether endogenous testosterone contributes to the modulation of women’s sexual desire.
2. Testosterone and women’s sexual desire: A history
a. The discovery of testosterone and its early uses in women
On June 1, 1889, Charles Brown-Séquard stood before the Sociète de Biologie in Paris and presented the seemingly spectacular results of his now famous auto-experimentation with testicular fluids (Freeman et al., 2001; Hoberman & Yesalis, 1995). Brown-Séquard, then 72, had injected himself with a concoction of his own design–water mixed with equal parts blood from the testicular vein, semen, and testicular fluid, all extracted from the testicles of dogs and guinea pigs–and reported experiencing a number of astonishing health improvements (Brown-Séquard, 1889). According to Brown-Séquard, the day after his very first injection he noticed a pronounced increase in his physical and mental stamina, an alleviation of his troublesome constipation, and even an apparently favorable shortening in the arc of his urine while relieving his bladder (Brown-Séquard, 1889). Although his spectacular claims were never substantiated, Brown-Séquard’s experimentation sparked a wave of international interest in the power of testicular fluids. By the time 1889 drew to a close, over 12,000 physicians worldwide were already prescribing Brown-Séquard’s “Elixir of Life” for conditions ranging from epilepsy to hysteria (Freeman et al., 2001).
In the early 1930’s, three pharmaceutical companies independently charged research teams with the task of identifying and isolating the powerful androgenic factor contained in the testes (Freeman et al., 2001, Hoberman & Yesalis, 1995). The team lead by Karoly Gyula David and backed by the Organon Company of the Netherlands triumphed in this endeavor – in 1935, David’s team published the seminal paper “On crystalline male hormone from testicles” and coined the term “testosterone” (David et al., 1935). Later that same year, and within one week of each other, two different research teams lead by Adolf Butenandt and Leopold Ruzicka developed and published a method for the laboratory preparation of synthetic testosterone – an accomplishment for which both Butenandt and Ruzicka were offered the 1939 Nobel Prize for chemistry. Butenandt, then a member of the Nazi party, was forced to decline the honor by the German government, although he eventually accepted the award in 1949 after World War II had ended (Freeman et al., 2001; Hoberman & Yesalis, 1995).
Following the isolation and synthesis of testosterone in 1935, physicians began prescribing testosterone therapy for a wide range of medical conditions in both men and women (Hoberman & Yesalis, 1995). In women, testosterone therapy was frequently prescribed for the treatment of menstrual complaints and as a tumor suppressant in cases of advanced breast cancer. Several early researchers anecdotally reported that some women treated with high dose testosterone therapies mentioned an unexpected, but not unappreciated, increase in sexual desire (Adair & Hermann, 1946; Geist et al., 1940; Shorr et al., 1938). These women, however, were receiving shockingly supraphysiological amounts of testosterone (75–350 mg/week) that produced somatic virilization, such as lowering of the voice, clitoral enlargement, and growth of unwanted facial hair (Adair & Hermann, 1946; Geist et al., 1940; Shorr, 1938). Nonetheless, in response to these anecdotal reports, Salmon and Geist (1943) directly investigated the ability of supraphysiological amounts of testosterone (20–75mg/week) to increase sexual desire in pre- and postmenopausal women. They found that testosterone therapy successfully increased sexual desire in all of their female participants, but that testosterone in combination with estradiol was more effective at increasing sexual desire in postmenopausal women than was testosterone alone. Despite the supraphysiological doses of testosterone they administered and their lack of placebo controls, Salmon and Geist (1943) inspired an early interest in testosterone as a treatment for low libido in women that continues to this day. Five years later, William H. Perloff, a medical doctor with an interest in the mechanism of human sexual behavior, provided early evidence that estradiol on its own was also capable of increasing women’s sexual desire (Perloff, 1949). Perloff (1949) administered varying dosages of estradiol to his naturally and surgically postmenopausal patients, who consistently reported increased sexual desire in response to estradiol treatment. Despite Perloff’s anecdotal findings, the effects of estradiol therapy on sexual desire in postmenopausal women would not be systematically investigated until the early 1980’s.
b. Women’s sexual desire following adrenalectomy
In 1959, Sheldon E. Waxenberg and his research associates published a highly influential yet problematic study on the effects of adrenalectomy on women’s sexual desire and behavior (Waxenberg et al., 1959). These investigators measured sexual desire and intercourse frequency in 29 women who had undergone bilateral oophorectomy and bilateral adrenalectomy in response to metastatic breast cancer. Seven of the women included in the study had undergone oophorectomy 1–5 years prior to adrenalectomy, and the other 22 women had undergone oophorectomy at the same time as adrenalectomy. The authors reported that, of the 17 women who had reported experiencing some level of sexual desire prior to adrenalectomy, 14 reported a noticeable decrease in desire following surgery. Of the 17 women who had been sexually active prior to adrenalectomy, all decreased their level of sexual activity following surgery, and seven stopped engaging in sexual activity entirely. The authors also reported that five of the seven women who had undergone oophorectomy prior to adrenalectomy reported a decrease in sexual desire following oophorectomy. Six of these seven women reported a decrease in sexual desire following subsequent adrenalectomy, while the seventh reported a complete lack of sexual desire both prior to and after adrenalectomy. Waxenberg et al. (1959) took these results as demonstrating that ovarian steroids had little impact on female sexual functioning, and that adrenal androgens critically modulated women’s sexuality. This study was never replicated, and remains the only study to investigate changes in women’s sexual desire following adrenalectomy.
There are several limitations of the Waxenberg et al. (1959) study that make interpretation difficult. Firstly, the female participants in this study were battling terminal breast cancer; all 29 had recently undergone two major surgeries, oophorectomy and adrenalectomy, in the course of their cancer treatment, and 22 had undergone additional mastectomy. Given the psychological strain associated with battling a terminal illness, and the physical demands of recovering from multiple major surgeries, sexual desire and activity were not likely a primary focus of these women. Secondly, Waxenberg et al. (1959) did not include an adrenalectomy-only treatment group, which would be necessary to demonstrate the importance of adrenal function for women’s sexuality. Therefore, the most that can be confidently concluded from Waxenberg et al. (1959) is that oophorectomy plus adrenalectomy – i.e., a complete elimination of both estrogens and androgens – was associated with the disappearance of women’s sexual behavior and desire in terminally ill cancer patients. Despite these limitations, Waxenberg et al. (1959) had a powerful influence on the field of behavioral endocrinology, and popularized the notion that adrenal androgens were the key modulators of women’s sexual desire – a concept that subsequently guided post-menopausal hormone therapy for more than 25 years.
c. Hormonal fluctuations across the human menstrual cycle
By the early 1970’s, researchers had begun piecing together a composite picture of the hormonal fluctuations across the human menstrual cycle (Abraham, 1974; Korenman & Sherman, 1973; Moghissi et al., 1972). It was widely accepted that the ovaries were the primary source of estrogens in women, and in agreement with this view it was found that levels of circulating estradiol increased steadily throughout the follicular phase of the menstrual cycle, peaked at midcycle roughly 24-hours prior to ovulation, and subsequently decreased and remained at midfollicular levels throughout the luteal phase of the menstrual cycle (Korenman & Sherman, 1973; Moghissi et al., 1972). Waxenberg et al. (1959) had posited that the adrenals were the primary source of androgens in women, which would indicate that circulating testosterone levels (unlike estradiol levels) should not appreciably fluctuate across the menstrual cycle. To the contrary, Abraham (1974) found that women’s levels of circulating testosterone fluctuated across the menstrual cycle, exhibiting a gradual midcycle peak coinciding with the pre-ovulatory peak in estradiol. Abraham (1974) further found that when women were treated with the synthetic glucocorticoid dexamethasone to suppress adrenal function, leaving the ovaries as the only source of testosterone, testosterone’s pattern of fluctuation did not change, but circulating testosterone levels decreased by 20–50% across the menstrual cycle. Abraham (1974) concluded that no more than 50% of women’s circulating testosterone was of adrenal origin, with the ovaries providing the rest. Furthermore, unlike the consistent amount of testosterone secreted by the adrenals, the amount of testosterone secreted by the ovaries fluctuated across the menstrual cycle, such that the ovaries actually released twice as much testosterone as did the adrenals at ovulation. The differences in secretion patterns of adrenal androgens versus ovarian steroids suggested that if adrenal androgens modulated women’s sexual desire, then women’s sexual desire should remain consistent across the menstrual cycle. If, on the other hand, ovarian steroids (estradiol and/or testosterone) modulated women’s sexual desire, then women’s sexual desire should fluctuate across the menstrual cycle and decrease following oophorectomy.
d. Women’s sexual desire across the menstrual cycle and following oophorectomy
The earliest researchers to investigate women’s sexual behavior across the menstrual cycle relied on intercourse frequency as a proxy for women’s sexual desire, and reported that intercourse frequency did not meaningfully fluctuate across the menstrual cycle (James, 1971; Udry & Morris, 1977). However, unlike most female nonprimate mammals, women are physically capable of engaging in sexual intercourse under any hormonal condition, and irrespective of their levels of sexual desire (Wallen, 2001). In consequence, intercourse frequency does not necessarily reflect women’s sexual desire (Wallen, 2001) – women can (and do) engage in sexual intercourse for reasons other than their own sexual desire, and frequency of heterosexual intercourse may be heavily influenced by male sexual motivation (Meston & Buss, 2007; Wallen, 2001). Furthermore, humans are capable of modifying their sexual habits to accommodate a wide range of cultural conventions (Wallen, 2001). For example, sexual intercourse is often intentionally avoided during menstruation in response to cultural taboos (Stanislaw & Rice, 1988), and at midcycle in an effort to avoid pregnancy (Tsui, 1991). In Western nations, intercourse frequency has been shown to vary consistently with external factors such as the days of the week, and to increase around celebrations/holidays (Palmer et al., 1982; Roney & Simmons, 2013; Wilcox et al, 2004). Given the many influences on intercourse frequency, it is perhaps not surprising that the relationship between sexual intercourse and the menstrual cycle is not consistent, but varies with social and cultural context (James, 1971; Matteo & Rissman, 1984; Udry & Morris, 1977; Wilcox et al., 2004).
In contrast to studies of intercourse frequency, studies that directly assessed women’s sexual desire revealed predictable variation in relation to the hormonal fluctuations of the menstrual cycle (Dennerstein et al., 1994; Harvey, 1987; Stanislaw & Rice, 1988; Van Goozen et al., 1997). Stanislaw and Rice (1988) asked 1,066 women to note each day of the month on which they experienced noticeable sexual desire (irrespective of whether they actually engaged in sexual activity on that day). The authors collected sexual desire data for a remarkable 22,365 menstrual cycles, and found a striking midcycle peak in the occurrence of women’s self-reported sexual desire. Harvey (1987) asked 69 women to fill out a daily questionnaire concerning all heterosexual and autosexual behavior, and found that participants reported a significant midcycle peak in rates of masturbation. Interestingly, heterosexual behavior initiated by the participants themselves actually decreased at midcycle; however, none of these women were using a reliable form of contraception, and 87-percent reported being aware of an increased risk of pregnancy at midcycle. Harvey concluded that the midcycle increase in autosexual but not heterosexual behavior reflected a midcycle increase in women’s sexual desire combined with a concerted effort to avoid pregnancy. In support of Harvey’s notion, Matteo and Rissman (1984) studying lesbians and Wilcox et al. (2004) studying women using completely reliable contraceptives both found that partnered sexual activity peaked at midcycle – supporting the idea that women’s patterns of sexual behavior reflect both their hormonal state and their perceived risk of pregnancy.
As laboratory techniques advanced, researchers investigating women’s sexual desire across the menstrual cycle employed increasingly accurate hormone sampling methods to pinpoint the occurrence of ovulation (Dennerstein et al., 1994; Van Goozen et al., 1997; Wilcox et al., 2004). Dennerstein et al. (1994) asked 168 female participants to fill out a daily questionnaire concerning all sexual behavior and to provide a daily urine sample throughout the study. The authors estimated ovulation as occurring 24-hours prior to the midcycle peak in levels of urinary estrogen metabolites, and found that participants’ self-reported levels of sexual interest significantly increased just prior ovulation. Van Goozen et al. (1997) collected daily sexual activity data and 12–14 blood samples from 20 female participants across 1–2 menstrual cycles. The authors defined the ovulatory period of the menstrual cycle as the 1–2 days encompassing the midcycle peak in plasma estradiol, and found that participants reported a significant increase in self-initiated sexual activity (both autosexual and heterosexual) during the ovulatory period of the menstrual cycle. These studies demonstrated that women’s sexual desire consistently exhibited a well defined midcycle peak, irrespective of the measure used to estimate ovulation.
In 1985, Sherwin et al. provided a crucial piece of evidence to indicate that women’s sexual desire was under ovarian rather than adrenal modulation. These investigators assessed sexual desire in 53 healthy, premenopausal women both before and after bilateral oophorectomy for benign health conditions, and found that both sexual desire and frequency of sexual fantasies significantly decreased following oophorectomy. These results contradicted the conclusions of Waxenberg et al. (1959), and provided the first evidence that adrenal androgens alone could not maintain women’s sexual desire in the absence of ovarian steroids. The results of Sherwin et al. (1985) were further substantiated by research with nonhuman primates. Lovejoy and Wallen (1990) used dexamethasone to suppress adrenal function in naturally cycling female rhesus monkeys living in large species-typical social groups, and reported that suppression of adrenal function did not significantly alter rates of female-initiated sexual behavior across the menstrual cycle. The authors concluded that adrenal androgens were not critical for female sexual motivation in nonhuman primates. The results of Lovejoy and Wallen (1990) agreed with those of Sherwin et al. (1985), and indicated that ovarian steroids, not adrenal androgens, were the critical regulators of female sexual motivation in both human and nonhuman primates. Thus by the early 1990’s, researchers had established that women’s sexual desire fluctuated across the menstrual cycle (Dennerstein et al., 1994; Harvey, 1987; Stanislaw & Rice, 1988; Van Goozen et al., 1997) and decreased following oophorectomy (Sherwin et al., 1985). This combined body of work finally ended the idea that adrenal androgens were the key regulators of sexual desire in women and – more than 25 years after Waxenberg et al. (1959) – it became generally accepted that women’s sexual desire was modulated by ovarian steroids (Wallen, 1995).
e. Hormonal correlates of women’s sexual desire
Ovarian steroids modulate sexual desire in women; however, disagreement remains as to whether ovarian estradiol or testosterone is more directly associated with increases in women’s sexual desire. Because both estradiol and testosterone peak at midcycle (Abraham, 1974), either or both steroids could theoretically be responsible for the midcycle peak in women’s sexual desire. Estradiol, however, exhibits a much more pronounced, and briefer, midcycle peak than does testosterone; circulating estradiol levels increase by more than 800-percent over a 3–4 day period at midcycle, whereas circulating testosterone levels increase by roughly 150-percent over a 6–8 day period (Abraham, 1974; Korenman & Sherman, 1973).
Researchers have investigated the relationship between women’s endogenous steroid levels and their sexual desire (Dennerstein et al., 2002; Roney & Simmons, 2013). Roney and Simmons (2013) examined the relationship between estradiol, progesterone, testosterone, and women’s sexual desire across the menstrual cycle. The authors asked 43 naturally cycling female participants (not using hormonal contraceptives) to fill out a daily questionnaire concerning sexual desire and activity across 1–2 menstrual cycles, and to provide a daily saliva sample for hormone analysis throughout the study. The authors examined the relationship between self-reported levels of sexual desire on a given day and steroid hormone levels on that same day, and on one and two days prior to that day (1–2 day lag). The authors reported that salivary estradiol was a significant positive predictor of sexual desire measured two days later, while progesterone was a significant negative predictor of sexual desire at the time of sampling, and at a one or two day lag. By contrast, testosterone did not reliably predict women’s sexual desire at any time point. Thus, Roney and Simmons (2013) provided correlational evidence indicating that circulating estradiol, but not testosterone, was associated with the midcycle peak in women’s sexual desire.
Dennerstein et al. (2002) examined the relationship between waning levels of ovarian estradiol and testosterone and the postmenopausal decline in women’s sexual desire. The authors followed a cohort of 226 perimenopausal women for eight years, as they transitioned from early to late menopause, and charted both hormonal condition and sexual functioning. Estradiol levels were significantly correlated with self-reported levels of both sexual responsiveness and sexual desire across the menopausal transition, but testosterone levels did not significantly correlate with any measure of sexual functioning. The results of Dennerstein et al. (2002) suggest that the menopause-related decline in women’s sexual desire is more closely associated with a loss of circulating estradiol rather than a loss of testosterone; nevertheless, pharmaceutical companies continue to focus on the development of androgen therapies for the treatment of low libido in postmenopausal women.
f. “Female Androgen Insufficiency”
The pharmaceutical industry’s interest in androgen therapies for the treatment of low libido in women is fueled in large part by the concept of “female androgen insufficiency”. In June of 2001, experts in the field of women’s sexual health convened in Princeton, New Jersey, to consider the role androgens play in women’s health and well-being, with a particular focus on women’s sexual functioning (Bachmann et al., 2002). The Princeton Conference released a consensus statement introducing the diagnosis of “female androgen insufficiency”, defined as a pattern of clinical symptoms (including decreased libido) associated with low levels of bioavailable testosterone (Bachmann et al., 2002). Androgen replacement therapy was logically recommended as a treatment for “female androgen insufficiency”, although there were no Food and Drug Administration (FDA) approved androgen therapies for women available at the time (Bachmann et al., 2002). It was noted, however, that the symptoms of “female androgen insufficiency” were based on clinical observations and expert opinion, and that there were no epidemiological data establishing that the symptoms of “female androgen insufficiency” was actually associated with low levels of circulating testosterone (Bachmann et al., 2002).
Soon after the introduction of “female androgen insufficiency”, the pharmaceutical industry began investing heavily in the development of androgen therapies for the treatment of low libido in women. Fueled by the success of Viagra, pharmaceutical companies were determined to capitalize on the potential multi-billion dollar market for female sexual-disorder treatments (Tsao, 2004). In 2004, Proctor & Gamble and Watson Pharmaceuticals tried to fast-track FDA approval of Intrinsa, a 300 ug/day transdermal testosterone patch for the treatment of low libido in women (Spark, 2005). Much to the surprise of an enthusiastic media, who were already heralding Intrinsa as the “female Viagra”, the FDA advisory panel rejected Intrinsa – citing concerns about unanticipated adverse side-effects with long-term use (Spark, 2005; Tsao, 2004).
It was not until 2005, three years after the introduction of the notion of “female androgen insufficiency”, that anyone investigated whether androgen levels differ between women with low libido and women without sexual dysfunction – in other words, whether androgen levels predict “female androgen insufficiency”. Davis et al. (2005) used the Profile of Female Sexual Functioning (PFSF), a validated measurement tool developed specifically for the diagnosis of low sexual desire, to assess sexual functioning in 1423 randomly selected women between the ages of 18–75. The authors also collected a fasting blood sample from each participant, to be assayed for testosterone. Women were excluded who suffered from any major illness (including psychiatric illness), or who were currently taking medications known to influence sexual functioning, such as antidepressants and oral contraceptives. Davis et al. (2005) found that low serum testosterone levels were not associated with low scores on any of the sexual domains assessed by the PFSF, including sexual desire, and concluded that testosterone levels were not predictive of “female androgen insufficiency”.
Thus “female androgen insufficiency” was never empirically established, and the concept has largely fallen out of favor; nonetheless, the concept drove an early and ongoing interest in testosterone that likely influenced the pharmaceutical industry to pursue androgen therapies for women. Pharmaceutical companies have now invested millions of dollars towards the development of an androgen therapy for female sexual desire disorders, but today there are still no FDA approved androgen therapies for women. Testosterone continues to be prescribed off-label for the treatment of low libido in women (Bolour & Braunstein, 2005), although the role that androgens play in the modulation of women’s sexual desire remains controversial.
3. Hormone therapies for low libido in postmenopausal women
Researchers have invested a great deal of effort over the past three decades in assessing whether estrogen or androgen therapies are more effective at increasing sexual desire in postmenopausal women. In 2004, Alexander et al. reviewed all double-blind randomized controlled trials to date examining the effects of estrogen and/or androgen therapies on sexual desire and/or functioning in postmenopausal women (Dennerstein et al., 1980; Flöter et al., 2002; Hays et al., 2003; Lobo et al., 2003; Myers et al., 1990; Nathorst-Boos et al., 1993; Sherwin et al., 1985; Sherwin, 1991; Shifren et al., 2000; Wiklund et al., 1993). The authors concluded that certain estrogen therapies were associated with an improvement in female sexual functioning, but that androgen therapies were only effective at improving female sexual functioning when administered in combination with an estrogen (Alexander et al., 2004). We analyzed the ten studies included in the Alexander et al. (2004) review, focusing specifically on circulating hormone levels produced by treatment and their relationship to sexual desire. In addition, we analyzed five studies excluded from Alexander et al. (2004) because they were not double-blind randomized controlled trials (Burger et al., 1987; Davis et al., 1995; Dow et al., 1983; Sarrel et al., 1998; Sherwin & Gelfand, 1987), and eight double-blind randomized controlled trials published after 2004 that investigated the effects of estrogen and/or androgen therapies on sexual desire in postmenopausal women (Braunstein et al., 2005; Buster et al., 2005; Davis et al. 2006a, 2006b; 2008; Panay et al., 2010; Shifren et al., 2006; Simon et al., 2005). Table 1 provides details of all studies included in the following review.
Table 1.
Controlled trials of hormone therapies for low libido in postmenopausal women.
| DOUBLE-BLIND/RANDOMIZED TRIALS | |||||||
|---|---|---|---|---|---|---|---|
| An estrogen, testosterone, and/or an estrogen + testosterone versus placebo | |||||||
| Treatment | Participants | Measure of sexual desire | E2 (pg/m L) | T (ng/dL) | Comments | Effects on sexual desire | |
| Dennerstein et al. (1980) | 12 months (3-month crossover design) E: 50 ug/d ethinyl estradiol (oral) P: 250 ug/d levonorge strel (oral) E+P: 50 ug/d ethinyl estradiol (oral) plus 250 ug/d levonorge strel (oral) Pl: placebo (oral) |
N=36 Menopause: Surgical |
Self-rating analogue scale (0–100) | Not reported | Not reported | No HRT in last 2 weeks | E increased sexual desire as compared to placebo |
| Sherwin et al. (1985) | 6 months (3-month crossover design) E: 6.3 mg estradiol valerate/28d (IM) T: 96.0 mg testosterone enanthate/28d (IM) E+T: 150 mg testosterone enanthate benzilic acid hydrozone plus 7.5 mg estradiol dienanthate plus 1.0mg estradiol benzoate/28d (IM) Pl: 0.5 mL sesame oil (IM) |
N=53 Menopause: Surgical |
Daily menopausal rating scale (DMRS) |
Preoperative baseline* E: 49.4 (12.3) T: 42.1 (11.1) E+T: 67.2 (10.4) Pl: 69.4 (8.9) 72hrs post-injection 1* E: 301.9 (13.1) T: 107.8 (8.4) E+T: 243.8 (12.4) Pl: 11.2 (3.9) 72hrs post-injection 2* E: 289.1 (15.4) T: 98.3 (9.6) E+T: 236.2 (14.8) Pl: 6.2 (2.3) *Reflects estradiol plus estrone [mean (SEM)] |
Preoperative baseline E: 99.6 (4.7) T: 71.9 (10.3) E+T: 81.1 (5.3) Pl: 114.1 (9.6) 72hrs post-injection 1 E: 89.4 (3.6) T:111.1 (6.6) E+T: 133.1 (12.4) Pl: 87.8 (1.6) 72hrs post-injection 2 E: 48.4 (2.6) T: 115.3 (6.2) E+T: 129.2 (11.6) Pl: 44.7 (3.2) [mean (SEM)] |
No previous use of HRT; treatment began immediately following oophorectomy Sexual desire assessed monthly across study |
T and E+T increased sexual desire more than did E and placebo at all time points Sexual desire never differed between T and E+T groups E never increased sexual desire as compared to placebo |
| Myers et al. (1990) | 8 weeks E: 0.625 mg/d CEE (oral) E+P: 0.625 mg/d CEE plus 5 mg/d MPA (oral) E+MT: 0.625 mg/d CEE plus 5 mg/d MT (oral) Pl: placebo (oral) |
N=40 Menopause: Natural |
Daily log |
Baseline All groups: ~5 Range across 8 weeks All groups: <40 [mean] |
Baseline All groups: 10–25 Range across 8 weeks* All groups: 10–25 *Reflects endogenous T only, no measure of MT [mean] |
No HRT in last 3 months | E and E+MT did not increase sexual desire as compared to placebo |
| Sherwin (1991) | 12 months (each month: 3 weeks treatment, 1 week no treatment) Low E: 0.625 mg/d CEE (oral) High E: 1.25 mg/d CEE (oral) |
N=48 Menopause: Natural |
Daily menopausal rating scale (DMRS) |
Baseline Low E: 40.0 (3.0) High E: 77.0 (10.0) Range across 12 months Low E: 98.0 (2.9) – 130.2 (14.0) High E: 98.0 (2.9) – 119.0 (2.9) [mean (SD)] |
Baseline Low E: 48.7 (0.9) High E: 39.2 (1.4) Range across 12 months Low E: 46.7 (1.4) – 50.1 (1.2) High E: 38.9 (0.3) – 43.8 (1.2) [mean (SD)] |
No HRT in last 6 months No placebo pill given during hormone free week |
Low E and High E increased sexual desire as compared to no treatment |
| Davis et al. (2008) | 24 weeks 150T: 150 ug/d TTP 300T: 300 ug/d TTP Pl: placebo (patch) |
N= ~578 Menopause: Natural or Surgical |
Profile of Female Sexual Function (PFSF) |
Baseline 150T: 5.0 (3.0, 12.0) 300T: 6.0 (3.0, 14.0) Pl: 6.0 (3.0, 13.0) At 24 weeks 150T: 9.0 (5.0–23.0) 300T: 10.0 (5.0, 24.5) Pl: 8.0 (4.0, 33.0) [median (10th, 90th percentile ranges)] |
Baseline 150T: 16.5 (9.0, 27.0) 300T: 15.0 (8.0, 26.0) Pl: 15.0 (8.0, 27.0) At 24 weeks 150T: 42.0 (23.0, 72.0) 300T: 67.0 (30.0, 111.0) Pl: 16.0 (8, 26) [median (10th, 90th percentile ranges)] |
No HRT in last 3 months (last 7 months for implantable T) Sexual desire assessed at 12 and 24 weeks of treatment 4 participant s receiving T were diagnosed with breast cancer during the study (as compared with none in the placebo group) |
150T increased sexual desire as compared to placebo at 24 weeks, but not at 12 weeks, but mean PFSF sexual desire score achieved was not reported 300T increased sexual desire as compared to placebo at 12 and 24 weeks, but mean PFSF sexual desire score achieved was <35 |
| An estrogen + testosterone versus an estrogen + placebo | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Participants | Measure of sexual desire | E2 (pg/mL) | T (ng/dL) | Comments | Effects on sexual desire | |
| Sarrel et al. (1998) | 12 weeks (2 weeks previous HRT, 2 weeks single-blind placebo, 8 weeks double-blind treatment) E: 1.25 mg/d esterified estrogen (oral) E+MT: 1.25 mg/d esterified estrogen (oral) plus 2.5 mg/d MT (oral) |
N=20 Menopause: Natural or Surgical |
Sexual Activity and Libido Scale |
Baseline E: 5.6 (2.7) E+MT: 29.0 (71.6) At 4 weeks E: 66.5 (30.8) E+MT: 40.8 (17.7) At 8 weeks E: 70.2 (31.2) E+MT: 47.0 (23.3) [mean (SD)] |
Not reported | Participants were taking an estrogen therapy for ≥4 months prior to study onset | E and E+MT did not increase sexual desire as compared to baseline No comparison of E and E+MT groups |
| Shifren et al. (2000) | 9 months (3-month crossover design) E+Pl: 0.625–2.5 mg/d CEE (oral) plus placebo patch E+150T: 0.625–2.5 mg/d CEE (oral) plus 150 ug/d TTP E+300T: 0.625–2.5 mg/d CEE (oral) plus 300 ug/d TTP |
N=75 Menopause: Surgical |
Brief index of sexual functioning for women (BISF-W) |
Baseline All groups: 36 (22) At 4, 8, and 12 weeks (average) E+Pl: 40 (57) E+150T: 42 (27) E+300T: 45 (43) [mean (SD)] |
Baseline All groups: 21 (10) At 4, 8, and 12 weeks (average) E+Pl: 22 (12) E +150T: 64 (25) E+300T: 102 (39) [mean (SD)] |
Participants were taking an estrogen therapy for ≥2 months prior to study onset and experiencing low libido, T or placebo added to their existing estrogen regimen | E+150T and E+300T did not increase sexual desire as compared to E+Pl |
| Flöter et al. (2002) | 12 months (6-month crossover design) E+Pl: 2 mg/d estradiol valerate (oral) E+T: 2 mg/d estradiol valerate (oral) plus 40 mg/d testosterone undecanoate (oral) |
N=44 Menopause: Surgical |
McCoy’s Female Sexuality Questionnaire |
Baseline Both groups: <19.9 At 24 weeks E+Pl: 66.8 (41.2) E+T: 47.2 (27.3) [mean (SD)] |
Baseline Both groups: 23.1 (13.0) At 24 weeks E+Pl: 25.9 (37.5) E+T: 141.2 (118.2) [mean (SD] |
No HRT in last 2 months | Both E+Pl and E+T increased sexual desire as compared to baseline E+T increased sexual desire more than did E+Pl |
| Lobo et al. (2003) | 16 weeks E+Pl: 0.625 mg/d esterified estrogen (oral) E+MT: 0.625 mg/d esterified estrogen (oral) plus 1.25 mg/d MT (oral) |
N=218 Menopause: Natural or Surgical |
Brief Index of Sexual Functioning for Women (BISF-W) Sexual Interest Questionnaire (SIQ) |
Baseline E+MT: 36.2 (35.3) E+Pl: 33.6 (25.8) Change from baseline at 16 weeks E+MT: +3.1 (32.6) E+Pl: −9.6 (47.0) [mean (SD)] |
Baseline E+MT: 18.9 (8.4 E+Pl: 20.8 (1.1 Change from baseline at 16 weeks* E+MT: −3.7 (6.8) E+Pl: −0.1 (7.4) *Reflects endogenous T only, no measure of MT [mean (SD)] |
Participants were taking an estrogen therapy for ≥3 months prior to study onset and experiencing low libido, MT or placebo added to their existing estrogen regimen | E+MT increased sexual desire as compared to E+Pl |
| Braunstein et al. (2005) | 24 weeks E+Pl: ≥0.625 mg/d CEE (or similar) plus placebo (patch) E+150T: ≥0.625 mg/d CEE (or similar) plus 150 ug/d TTP E+300T: ≥0.625 mg/d CEE (or similar oral estrogen) plus 300 ug/d TTP E+450T: ≥0.625 mg/d CEE (or similar) plus 450 ug/d TTP |
N=318 Menopause: Surgical |
Profile of Female Sexual Function (PFSF) |
At baseline E+Pl: 31.5 (17–57) E+150T: 27.0 (16–51) E+300T: 26 (14–43) E+450T: 25 (17–50) At 24 weeks E+Pl: 33 (19–73) E+150T: 29.5 (17–45.5) E+300T: 37 (19–53) E+450T: 31 (17.5–55) [median (10th, 90th percentile ranges)] |
At baseline E+Pl: 16 (13–22) E+150T:15 (11–19) E+300T:17 (12–25) E+450T: 15.5 (11–21) At 24 weeks E+Pl: 18 (14–22) E+150T: 44.5 (31.5–66) E+300T: 91 (67.5–130) E+450T: 122.5 (78–182) [median (10th, 90th percentile ranges)] |
Participants were taking an oral estrogen therapy for ≥3 months prior to study onset and experiencing low libido, T or placebo was added to their existing estrogen regimen | E+150T and E+450T did not increase sexual desire as compared to E+Pl E+300T increased sexual desire as compared to E+Pl, but mean PFSF sexual desire score achieved was <35 (mean increase in sexual desire did not differ b/w E+300T and E+450T, even though E+300T increased sexual desire significantly more than did E+Pl and E+450T did not) |
| Buster et al. (2005) | 24 weeks E+Pl: an estrogen therapy (oral or transdermal) plus placebo (patch) E+300T: an estrogen therapy (oral or transdermal) plus 300 ug/d TTP |
N=417 Menopause: Surgical |
Profile of Female Sexual Function (PFSF) |
At baseline E+Pl: 30.0 (9, 89) E+300T: 30.0 (10, 95) At 24 weeks E+Pl: 29.0 (9, 82) E+300T: 26.5 (8, 106.5) [median (10th, 90th percentile ranges)] |
At baseline E+Pl: 16.0 (8, 28) E+300T: 15.0 (8, 30) At 24 weeks E+Pl: 15.0 (7, 26) E+300T: 65.5 (23, 146) [median (10th, 90th percentile ranges)] |
Participants were taking an estrogen therapy (oral or transdermal) for ≥3 months prior to study onset and experiencing low libido, T or placebo was added to their existing estrogen regimen | E+300T increased sexual desire as compared to E+Pl, but mean PFSF sexual desire score achieved was not reported |
| Simon et al. (2005) | 24 weeks E+Pl: an estrogen therapy (oral or transdermal) plus placebo (patch) E+300T: an estrogen therapy (oral or transdermal) plus 300 ug/d TTP |
N= 451 Menopause: Surgical |
Profile of Female Sexual Function (PFSF) |
Baseline E+Pl: 29.5 (10, 110) E+300T: 29 (10, 84) At 24 weeks E+Pl: 32 (10, 114) E+300T: 31 (12, 99) [median (10th, 90th percentile ranges)] |
Baseline E+Pl: 17 (8, 28) E+300T: 16 (8, 29) At 24 weeks E+Pl: 16 (7, 28) E+300T: 70 (33, 139) [median (10th, 90th percentile ranges)] |
Participants were taking an estrogen therapy (oral or transdermal) for ≥3 months prior to study onset and experiencing low libido, T or placebo was added to their existing estrogen regimen | E+300T increased sexual desire as compared to E+Pl, but mean PFSF sexual desire score achieved was <35 |
| Davis et al. (2006b) | 24 weeks E+Pl: ≥50 ug/d E2 (transdermal) plus placebo (patch) E+300T: ≥50 ug/d E2 (transdermal) plus 300 ug/d TTP |
N=61 Menopause: Surgical |
Profile of Female Sexual Function (PFSF) |
Baseline E+Pl: 56 (27, 75) E+300T: 57 (31, 102.5) At 24 weeks E+Pl: 47 (22, 71) E+300T: 69 (46, 81) [median (10th, 90th percentile ranges)] |
Baseline E+Pl: 17 (13,24) E+300T: 15 (13,18) At 24 weeks E+Pl: 17 (11,22) E+300T: 63 (38, 84.5) [median (10th, 90th percentile ranges)] |
Participants were taking an estrogen therapy (transdermal) for ≥ 3 months prior to study onset and experiencing low libido, T or placebo was added to their existing estrogen regimen | E+300T increased sexual desire as compared to E+Pl, but mean PFSF sexual desire score achieved was ~40 |
| Shifren et al. (2006) | 24 weeks E+Pl: an estrogen therapy (oral) plus placebo (patch) E+300T: an estrogen therapy (oral) plus 300 ug/d TTP |
N=433 Menopause: Natural |
Profile of Female Sexual Function (PFSF) |
Baseline E+Pl: 21 (7, 61) E+300T: 20 (5, 53) At 24 weeks E+Pl: 18 (5, 42) E+300T: 21 (6, 59) [median (10th, 90th percentile ranges)] |
Baseline E+Pl: 16 (9, 30) E+300T: 17 (9, 31) At 24 weeks E+Pl: 15 (8, 28) E+300T: 54 (20, 119) [median (10th, 90th percentile ranges)] |
Participants were taking an oral estrogen therapy for ≥3 months prior to study onset and experiencing low libido, T or placebo was added to their existing estrogen regimen Participants with intact uteri were also taking a progestin throughout the study |
E+300T increased sexual desire as compared to E+Pl, but mean PFSF sexual desire score achieved was <35 |
| Panay et al. (2010) | 24 weeks Pl: previous HRT plus placebo (patch) 300T: previous HRT plus 300 ug/d TTP |
N= 207 Menopause: Natural |
Profile of Female Sexual Function (PFSF) |
Baseline E+Pl: 19.6 E+300T: 18.9 At 24 weeks E+Pl: 16.0 E+300T: 16.8 [mean] |
Baseline E+Pl: 23.2 E+300T: 20.7 At 24 weeks E+Pl: 22.9 E+300T: 67.8 [mean] |
Participants were taking an estrogen therapy (oral or transdermal), an estrogen plus a progestin (oral), or no HRT prior to study onset, T or placebo was added to their existing hormone regimen | 300T increased sexual desire as compared to placebo, but mean PFSF sexual desire score achieved was <35 |
| An estrogen + testosterone versus an estrogen + testosterone + letrozole | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Participants | Measure of sexual desire | E2 (pg/mL) | T (ng/dL) | Comments | Effects on sexual desire | |
| Davis et al. (2006a) | 16 weeks E+T+Pl: E2 (≥50 ug/d transdermal patch or ≥1 mg/day transdermal gel) plus 2 mg T (transdermal gel) plus placebo (oral) E+T+L: E2 (≥50 ug/d transdermal patch or ≥1 mg/day transdermal gel) plus 2 mg T (transdermal gel) plus 2.5 mg/d letrozole (oral) |
N=60 Menopause: Natural or Surgical |
Sabbatsberg Sexual Self-rating Scale (SSS) |
Baseline E+T: 64.0 E+T+L: 58.6 At 16 weeks E+T: 53.1 E+T+L: 54.2 [median] |
Baseline E+T: 19.3 (15.9) E+T+L: 17.9 (13.0) At 16 weeks E+T: 59.4 (61.4) E+T+L: 62.5 (77.8) [mean (SD)] |
Participants were taking an estrogen therapy (transdermal) for ≥8 weeks prior to study onset and experiencing low libido, T or T plus letrozole (an aromatase inhibitor) was added to their existing estrogen regimen No treatment group received E2 without concurrent T |
Sexual desire did not differ between E+T+Pl and E+T+L groups |
| NOT DOUBLE-BLIND AND/OR NOT RANDOMIZED TRIALS | |||||||
| Dow et al. (1983) |
Single-blind, randomized 6 months E: 50 mg E2 (implant) E+T: 50 mg E2 plus 100 mg T (implant) |
N=40 Menopause: Natural or surgical |
Self-rating analogue scale | Not reported | Not reported | Participants were not using HRT prior to study onset and were experiencing low libido Sexual desire assessed at 2 and 6 months of treatment Six participants with intact uteri also received a progestin for 7 days of each month |
Both E and E+T increased sexual desire as compared to baseline at both time points Sexual desire did not differ b/w E and E+T groups at either time point |
| Burger (1987) |
Single-blind, randomized 6 weeks E: 40 mg E2 (implant) E+T: 40 mg E2 plus 50 mg T (implant) |
N=20 Menopause: Natural or Surgical |
Self-rating analogue scale (0–100) | Not reported |
At 6 weeks E: not reported E+T: 100.9 [mean] |
Participants were taking an estrogen therapy (oral) prior to study onset and experiencing low libido Two week wash-out period prior to treatment onset |
Only E+T increased sexual desire as compared to baseline E+T increased sexual desire more than did E |
| Sherwin & Gelfand (1987) |
Open label, not randomized 4 weeks E: 10.0 mg estradiol valerate (IM) E+T: 150.0 mg testosterone enanthate benzilic acid hydrozone plus 7.5 mg estradiol dienanthate plus 1.0 mg estradiol benzoate (IM) CON: no hormone |
N=44 Menopause: Surgical |
Daily Menopausal Rating Scale (DMRS) |
Baseline E: 33.0 (1.6) E+T: 67.9 (1.3) CON: 4.9 (1.2) Range across 4 treatment weeks E: 66.9 (6.1) – 363.8 (8.1) E+T: 90.1 (1.6) – 262.2 (4.8) CON: 5.3 (1.4) [mean (SEM)] |
Baseline E: 25 (1) E+T: 194 (3) CON: 40 (1) Range across 4 treatment weeks E: 18 (1) – 26 (1) E+T: 210 (3) – 284 (4) CON: 22 (1) [mean (SEM)] |
Participants in E and E+T groups had been taking HRT for ≥2 years prior to study onset, and continued previous HRT throughout the study following an initial 8-week washout period Participants in CON group had never taken HRT and received no hormone treatment during study Sexual desire assessed weekly across study |
E+T increased sexual desire as compared to baseline at weeks 1–3, but not at week 4 E+T increased sexual desire as compared to E and CON at all 4 time points Sexual desire never differed b/w E and CON groups E+T group had supraphysiological T levels, and higher T levels than E and CON groups, at baseline, but sexual desire did not differ b/w groups at baseline |
| Davis et al. (1995) |
Single-blind, randomized 2 years E: 50 mg E2 (3-month implant) E+T: 50 mg E2 plus 50 mg T (3-month implant) |
N=33 Menopause: Natural or Surgical |
Sabbatsberg Sexual Self-Rating Scale (SSS) |
Baseline E: 61.3 (55.0) E+T: 27.5 (200.8) Range across study E: 94.8 (40.0) – 232.4 (86.9) E+T: 95.6 (61.8) – 204.6 (102.4) [mean (SD)] |
Baseline E: 31.7 (11.5) E+T: 34.6 (15.9) Range across study E: 28.8 (17.0) –49.0 (15.3) E+T: 63.4 (28.8) – 77.8 (40.1) [mean (SD)] |
Some, but not all, participants were taking an estrogen therapy up until study onset Sexual desire assessed every 6 months across study 10 participants in each group with intact uteruses also received 5–10 mg MPA (oral) or 2.5 mg norethisterone 12d/month (oral) |
Both E and E+T increased sexual desire as compared to baseline at all time points Grand means for sexual desire across the study did not differ b/w E and E+T groups |
Normal ranges for testosterone and estradiol in premenopausal women:
Testosterone: 15-50 ng/dl (Abraham, 1974; Braunstein et al., 2011)
Estradiol: 50-400 pg/ml (Abraham, 1974; Korenman & Sherman, 1973)
E2 = estradiol
T = testosterone
CEE = conjugated equine estrogens
HRT = hormone replacement therapy
MPA = medroxyprogesterone acetate
MT = methyltestosterone
TTP = transdermal testosterone patch
a. An estrogen and/or testosterone versus placebo
Over the past 30 years, seven double-blind randomized trials have examined the effectiveness of estrogen-only therapies at improving sexual functioning in postmenopausal women (Dennerstein et al., 1980; Hays et al., 2003; Myers et al., 1990; Nathorst-Boos et al., 1993; Sherwin, 1991; Sherwin et al., 1985; Wiklund et al., 1993). Three of these seven studies, however, did not include a direct measure of sexual desire (Hays et al., 2003; Nathorst-Boos et al., 1993; Wiklund et al., 1993) and thus were not included in the present review. The four remaining studies produced conflicting results; two found that an estrogen-only therapy was effective at increasing sexual desire in postmenopausal women (Dennerstein et al., 1980; Sherwin, 1991), and two found that self-reported levels of sexual desire did not significantly differ between participants receiving an estrogen-only therapy and placebo controls (Myers et al., 1990; Sherwin et al., 1985). These contradictory results may reflect differences in the estrogen treatments administered in these four studies, which produced widely varying levels of circulating estradiol. For example, Myers et al. (1990) administered a conjugated equine estrogen (CEE) therapy alone or in combination with methyltestosterone to 40 naturally menopausal women, and found that neither treatment significantly increased self-reported levels of sexual desire as compared to placebo. Their CEE therapy, however, produced circulating estradiol levels averaging less than 40 pg/ml throughout the study. In naturally cycling pre-menopausal women, estradiol levels normally range between 100 and 400 pg/mL during the periovulatory phase of menstrual cycle (Korenman & Sherman, 1973; Moghissi et al., 1972) – the portion of the menstrual cycle associated with the midcycle peak in women’s sexual desire. Thus, the estrogen therapy administered by Myers et al. (1990) would not have been expected to increase women’s sexual desire, given the very low levels of estradiol it produced. Conversely, Sherwin (1991) administered a CEE therapy that produced periovulatory levels of circulating estradiol (>95 pg/mL) to 48 naturally menopausal women, and found that participants’ self-reported levels of sexual desire were higher during weeks of the month when they were receiving CEE therapy as compared to hormone free weeks. However, these results must be interpreted with caution; Sherwin (1991) did not administer a placebo during the hormone free weeks, and thus it is not possible to confirm that their CEE therapy increased participants’ sexual desire as compared to placebo.
Sherwin et al. (1985) conducted one of the most comprehensive investigations to date of the effectiveness of estrogen and testosterone therapies at improving sexual functioning in postmenopausal women. The authors administered an estrogen-only treatment (estradiol valerate), a testosterone-only treatment (testosterone enanthate), an estrogen in combination with testosterone (estradiol dienanthate, estradiol benzoate, testosterone enanthate benzilic acid hydrozone), or placebo to 53 surgically menopausal women immediately following oophorectomy. The authors found that self-reported levels of sexual desire did not differ between the testosterone-only and estrogen+testosterone treatment groups, and that both treatments increased sexual desire more than did the estrogen-only treatment and placebo. Conversely, self-reported levels of sexual desire did not differ between the estrogen-only and placebo treatment groups. However, the dosage of testosterone administered produced markedly supraphysiological levels of circulating testosterone in both the testosterone-only and estrogen+testosterone treatment groups (>100 ng/dL as compared to normal levels of 15–50 ng/dL). Unfortunately, the authors reported circulating estrogen levels produced by their four treatments as a combination of estradiol plus estrone (a relatively weak estrogen, typically present in high concentrations), making it difficult to determine whether their estrogen-only treatment produced periovulatory levels of circulating estradiol.
Apart from Sherwin et al. (1985), only one other double-blind randomized controlled trial has administered testosterone to postmenopausal women in the absence of a concurrent estrogen therapy (Davis et al., 2008). Davis et al. (2008) treated 814 postmenopausal women who were not taking an estrogen therapy with one of two different dosages of testosterone (150 ug/d and 300 ug/d) via transdermal patch or a placebo. The authors reported that the 300 ug/d transdermal testosterone patch (TTP) significantly increased sexual desire at both 12- and 24-weeks of treatment as compared to placebo; however, the authors also reported that the 300 ug/d TTP produced supraphysiological levels of circulating testosterone (>60 ng/dl). Furthermore, the increase in sexual desire reported for the women in the 300 ug/d TTP treatment group was of questionable clinical relevance. The authors used the PFSF to assess sexual desire, and reported that the mean sexual desire score for participants in the 300 ug/d TTP treatment group never exceeded 40, the clinical cutoff for low sexual desire (Dennerstein et al., 2006; McHorney et al., 2004). Thus, the women in the 300 ug/d TTP treatment group were still experiencing clinically low sexual desire, while simultaneously experiencing supraphysiological levels of circulating testosterone. Additionally, the authors reported that the 150 ug/d TTP, which produced physiological levels of circulating testosterone (>50 ng/dL), did not increase sexual desire at 12 weeks of treatment, but did increase sexual desire at 24 weeks of treatment, as compared to placebo. Changes in sexual desire across the human menstrual cycle occur within a 14-day period, and it is unclear why 24 weeks of treatment would be required to find an effect of testosterone on women’s sexual desire in this particular case. Unfortunately, the authors only reported the effect of the 150 ug/d TTP, but did not provide any data, so it is not possible to know how large an effect of testosterone was found. Today, Davis et al. (2008) remains the only study to administer physiological testosterone to postmenopausal women in the absence of a concurrent estrogen therapy, and the effects of physiological testosterone on women’s sexual desire remain unclear. The results of Sherwin et al. (1985) and Davis et al. (2008) agree with the much earlier work of Salmon and Geist (1942), and demonstrate that supraphysiological levels of testosterone are capable of increasing sexual desire in postmenopausal women in the absence of a concurrent estrogen therapy.
b. An estrogen versus an estrogen plus testosterone
Ten double-blind randomized controlled trials have compared the effectiveness of an estrogen therapy alone and in combination with testosterone at increasing sexual desire in postmenopausal women (Braunstein et al., 2005; Buster et al., 2005; Davis et al., 2006b; Floter et al., 2002; Lobo et al., 2003; Panay et al., 2010; Sarrel et al., 1998; Shifren et al., 2000; 2006; Simon et al., 2005). Sarrel et al. (1998) administered an esterified estrogen (EE) therapy by itself or in combination with methyltestosterone to postmenopausal women, and reported that neither treatment increased sexual desire as compared to baseline. Their EE treatment, however, produced low levels of circulating estradiol (40–70 pg/ml), which may explain why neither treatment increased participants’ sexual desire. Lobo et al. (2003) administered methyltestosterone or placebo to 218 postmenopausal women currently taking, but dissatisfied with, an estrogen therapy, and reported that methyltestosterone increased sexual desire as compared to placebo; however, estradiol levels were again very low in these women (<40 pg/ml), which may explain why they were dissatisfied with their estrogen therapies to begin with. There is currently no assay for methyltestosterone, and thus the authors of these two studies were unable to determine if their methyltestosterone treatments were physiological. Flöter et al. (2002) administered estradiol valerate alone or in combination with testosterone propionate to 50 surgically postmenopausal women. The authors reported that both treatments significantly increased sexual desire as compared to baseline, but that the combined treatment increased sexual desire more than did the estradiol-only treatment. The authors also reported, however, that their combined estradiol+testosterone treatment produced supraphysiological levels of circulating testosterone (>140 ng/dl). The results of Floter et al. (2002) and Lobo et al. (2003) suggest that testosterone may enhance the effectiveness of a low dose estrogen therapy at increasing sexual desire in postmenopausal women.
In the seven remaining estrogen versus estrogen+testosterone studies, researchers administered either testosterone (via TTP) or placebo to postmenopausal women currently taking, but dissatisfied with, an estrogen therapy (Braunstein et al., 2005; Buster et al., 2005; Davis et al., 2006b; Panay et al., 2010; Shifren et al., 2000; 2006; Simon et al., 2005). Six of these seven studies found that adding the 300 ug/d TTP to an existing estrogen regimen significantly increased women’s sexual desire as compared to placebo (Braunstein et al., 2005; Buster et al., 2005; Davis et al., 2006b; Panay et al., 2010; Shifren et al., 2006; Simon et al., 2005). However, the 300 ug/d TTP consistently produces supraphysiological levels of circulating testosterone (>50ng/dl; Braunstein et al., 2005; Buster et al., 2005; Davis et al., 2006b; Panay et al., 2010; Shifren et al., 2000; Shifren et al., 2006; Simon et al., 2005). By contrast, the 150 ug/d TTP, that produces physiological levels of circulating testosterone (<50 ng/dl), has proven ineffective at increasing sexual desire in postmenopausal women currently taking an estrogen therapy (Braunstein et al., 2005; Shifren et al., 2000). For example, Braunstein et al. (2005) administered the 150 ug/d, 300 ug/d, 450 ug/d TTP, or placebo to 318 surgically menopausal women currently taking an estrogen therapy. The authors reported that, consistent with findings of other TTP studies, the 300 ug/d TTP significantly increased participants’ sexual desire as compared to placebo, but neither the 150 ug/d TTP nor the 450 ug/d TTP increased sexual desire. Why the 450 ug/d TTP, which produced supraphysiological levels of testosterone (>100 ng/dl) that were significantly higher than the levels produced by the 300 ug/d TTP, did not similarly increase sexual desire remains unresolved. However, and as with Davis et al. (2008), the improvements in sexual desire reported in these six TTP studies, while statistically significant, left participants with levels of sexual desire that would still clinically be considered dysfunctional. The results of these six TTP studies again suggest that supraphysiological levels of testosterone–with the exception of the 450 ug/d TTP dose–enhance the effectiveness of an estrogen therapy at increasing sexual desire in postmenopausal women, but not to levels that would reverse the clinical condition of low sexual desire.
Four additional studies, all published prior to 2004, also investigated the effects of estradiol and/or testosterone on sexual desire and functioning in postmenopausal women, but were excluded from the Alexander et al. (2004) review because they were not double-blind randomized trials (Burger et al., 1987; Davis et al., 1995; Dow et al., 1983; Sherwin & Gelfand,1987). Dow et al. (1983), Burger et al. (1987), and Davis et al. (1995) were single-blind experiments, and Sherwin and Gelfand (1987) was an open-label study. Despite these considerable limitations, these four studies influenced the academic conversation concerning the hormonal regulation of female sexual desire, and therefore warrant discussion. Dow et al. (1983), Burger et al. (1987), and Davis et al. (1995) all investigated the effects of estradiol alone or in combination with testosterone on sexual desire and functioning in postmenopausal women. Burger et al. (1987) reported that only estradiol in combination with testosterone increased sexual desire as compared to baseline, but also reported that their estradiol+testosterone treatment produced supraphysiological levels of circulating testosterone (>100 ng/dl). Furthermore, the authors did not report levels of circulating estradiol produced by either treatment, leaving unresolved whether their estradiol-only treatment produced periovulatory levels of circulating estradiol. Dow et al. (1983) and Davis et al. (1995) both reported that estradiol alone and in combination with testosterone increased participants’ sexual desire as compared to baseline, and that self-reported levels of sexual desire did not differ between estradiol-only and estradiol+testosterone treatment groups. Importantly, these two studies administered the same estradiol-only treatment, and Davis et al. 1995 reported that this treatment produced periovulatory levels of circulating estradiol (90–250 pg/ml), which may explain why these two studies found that estradiol alone was as effective at increasing sexual desire in postmenopausal women as was estradiol in combination with supraphysiological amounts of testosterone.
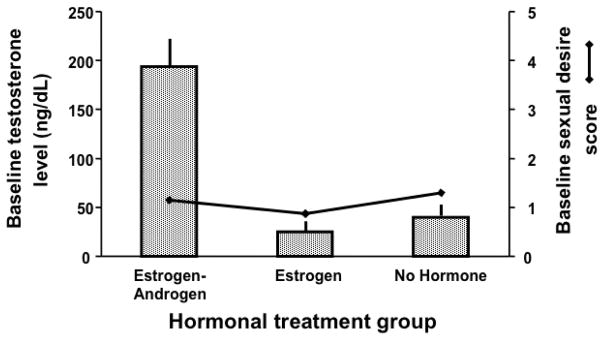
Sherwin and Gelfand (1987) investigated sexual desire and functioning in 44 surgically menopausal women, 33 of whom had already been using an estradiol-only therapy or a combined estradiol+testosterone therapy for at least two years, and 11 of whom had never used a hormone therapy. This study was not randomized and not blind; after an eight-week washout period, participants were instructed to resume taking the hormone therapies they had been using prior to the onset of the study. Participants who had never used a hormone therapy were assigned to the control group, and remained untreated throughout the study. Sherwin and Gelfand (1987) found that women in the estradiol+testosterone treatment group reported significantly higher levels of sexual desire than did women in the estradiol-only and control treatment groups. Conversely, self-reported levels of sexual desire never differed between the estradiol-only and control treatment groups. The authors interpreted these results as indicating that testosterone, not estradiol, was critical for the occurrence of women’s sexual desire. To the contrary, however, Sherwin and Gelfand (1987) actually provides striking evidence that elevated testosterone levels are not sufficient to increase sexual desire in postmenopausal women in the absence of estradiol. Sexual desire and circulating levels of estradiol and testosterone were measured at the end of the eight-week washout period, before participants resumed taking their previous hormone therapies. The women who had previously been taking the combined estradiol+testosterone therapy still had strikingly supraphysiological levels of testosterone (>200 ng/dL) at the end of the eight-week washout period; however, their self-reported levels of sexual desire at that time were as low as those of the women in the other two treatment groups, who both had very low levels of testosterone (<30 ng/dL; Figure 1). Unlike testosterone, estradiol levels were low and the same for women in all three treatment groups at the end of the eight-week washout period, matching their low levels of sexual desire. Women in the estradiol+testosterone treatment group only reported increased sexual desire when they resumed taking their estradiol+testosterone treatment, which elevated their circulating levels of both estradiol and testosterone. Furthermore, although testosterone levels remained supraphysiological throughout the study for women in the estradiol+testosterone treatment group, their sexual desire only increased in the first treatment week, and decreased thereafter – following the pattern of their circulating estradiol levels, which likewise increased in the first treatment week, and decreased thereafter. Interestingly, women in the estradiol-only treatment group did not report increased sexual desire after resuming treatment, even though the estradiol-only treatment produced periovulaotry levels of circulating estradiol. Today, Sherwin and Gelfand (1987) remains the only study to find that periovulatory levels of estradiol did not increase sexual desire in postmenopausal women (Davis et al., 1995; Dennerstein et al., 1980; Floter et al., 2002; Sherwin, 1991). Why the results of this study differ from those of other studies using treatments that produced similar levels of circulating estradiol remains unexplained.
Figure 1. Baseline testosterone levels do not predict sexual desire (Sherwin & Gelfand, 1987).
Mean baseline serum testosterone levels (±SEM) and mean baseline sexual desire scores for women in the three hormonal treatment groups after an eight-week washout period, but prior to participants’ resumption of their previous hormone therapies. All three treatment groups showed low levels of sexual desire that did not vary with testosterone levels, as the estrogen-only and no hormone groups had low levels of testosterone, while the estrogen+androgen treatment group had supraphysiological levels of testosterone (>50 ng/dL). Thus a striking difference in testosterone was not reflected in differences in sexual desire, indicating that variation in testosterone does not predict sexual desire. (Figure based on data from Sherwin and Gelfand, 1987.)
c. Summary
In summary, four out of five studies found that estrogen-only therapies that produced periovulatory levels of circulating estradiol increased sexual desire in postmenopausal women (Dow et al., 1983; Davis et al., 1995; Dennerstein et al., 1980; Sherwin, 1991; Sherwin & Gelfand, 1987). Estradiol presumably impacts female sexual functioning by acting on the central nervous system to increase sexual desire; however, these central effects are likely moderated by peripheral effects of estradiol acting directly on the genitals. Estradiol acts on the walls of the vagina to increase lubrication (Dennerstein et al., 1980; Nathorst-Böös et al., 1993). Dyspareunia (painful intercourse) related to genitourinary atrophy is commonly associated with sexual dysfunction in postmenopausal women (Avis et al., 2005; Gracia et al. 2002), and decreasing dyspareunia by increasing vaginal lubrication could conceivably indirectly increase women’s sexual desire by making sexual intercourse more pleasurable.
Ten out of twelve studies found that supraphysiological testosterone enhanced the effectiveness of an estrogen therapy at increasing sexual desire in postmenopausal women (Burger et al., 1987; Flöter et al., 2002; Sherwin et al., 1985; Sherwin & Gelfand, 1987; Braunstein et al., 2005; Buster et al., 2005; Davis et al., 2006b; Panay et al., 2010; Shifren et al., 2006; Simon et al., 2005). The reason for this improved effectiveness of an estrogen in combination with supraphysiological testosterone remains unknown, but may reflect testosterone’s aromatization to estradiol, and/or the dynamic relationship between estradiol, testosterone, and sex hormone binding globulin (SHBG; Burke and Anderson, 1972).
d. Potential roles of aromatase and sex hormone binding globulin (SHBG) in the modulation of women’s sexual desire
Evidence indicates that low-dose estrogen therapies are generally more effective at increasing sexual desire in hypogonadal woman when administered in combination with supraphysiological testosterone; however, it remains unclear how and why testosterone has this effect. One possibility is that testosterone influences woman’s sexual desire via its aromatization to estradiol. While the gonads are the primary source of estrogens in both men and women, testosterone can be directly metabolized to estradiol in breast, bone, adipose, and brain tissue (amongst others) via the enzyme aromatase (for review see Simpson, 2000). The aromatization of testosterone to estradiol in many different parts of the brain could account for testosterone’s ability to improve the effectiveness of an estrogen therapy at increasing women’s sexual desire. In this view, the addition of testosterone to an estrogen therapy would result in an increase in the amount of intracellular estradiol in any of the neural target tissues that aromatize testosterone, which would increase sexual desire.
Only one study has directly investigated whether aromatization contributes to testosterone’s ability to enhance the effectiveness of an estrogen therapy at increasing women’s sexual desire. Davis et al. (2006a) administered testosterone (as a topical gel) both alone or in combination with an orally-administered aromatase inhibitor (letrozole) to 76 postmenopausal women currently taking, but dissatisfied with, an estrogen therapy. The authors found no difference in self-reported levels of sexual desire between the testosterone-only and testosterone+letrozole treatment groups, and concluded that testosterone was capable of influencing women’s sexual desire without aromatization to estradiol. However, no data were presented showing that the dosage of letrozole administered in this study suppressed the aromatization of testosterone. As evidence to the contrary, levels of circulating estradiol did not differ between the testosterone-only and testosterone+letrozole treatment groups, even though estradiol levels should have been lower in the group receiving the aromatase inhibitor – as was found in a similar study of men treated with testosterone or testosterone plus an aromatase inhibitor (Bagatell et al., 1994). As the authors did not include a placebo control group that did not receive testosterone, it is not possible to confirm that their testosterone treatment, with or without letrozole, improved participants’ sexual desire as compared to placebo. Today, it remains unclear whether testosterone is capable of influencing women’s sexual desire without aromatization to estradiol.
A second possibility is that testosterone enhances the effectiveness of a low-dose estrogen therapy at increasing women’s sexual desire via its preferential binding to sex hormone binding globulin (SHBG). SHBG is a steroid-binding protein that circulates in the blood and reversibly binds both estradiol and testosterone (as well as other estrogens and androgens), although it binds testosterone with twice the affinity that it binds estradiol (Burke & Anderson, 1972). The majority of estradiol and testosterone in the blood circulates bound to SHBG at any given time, and only the relatively small unbound (free) fractions of either steroid (1–3%) are considered biologically active (Burke & Anderson, 1972; Rosner, 1990; Selby, 1990). The equilibrium between bound and free concentrations of both steroids is modulated by their differential binding affinities for SHBG. Because SHBG preferentially binds testosterone, an increase in circulating testosterone increases the amount of SHBG bound to testosterone, which liberates previously SHBG-bound estradiol (Burke & Anderson, 1972). In this dynamic system, the addition of testosterone to an estrogen therapy would theoretically increase circulating levels of unbound and biologically active estradiol, which would increase sexual desire (Wallen, 2001). There is currently little evidence to support either the aromatization or SHBG explanation for the enhanced effectiveness of an estrogen therapy in combination with supraphysiological testosterone, and the two possibilities are not mutually exclusive – testosterone may enhance the effectiveness of an estrogen therapy at increasing women’s sexual desire via its aromatization to estradiol, or via its preferential binding to SHBG, or both. Future work on the roles of estrogens and androgens as modulators of women’s sexual desire should investigate these potential mechanisms by which testosterone could influence the effectiveness of an estrogen therapy at increasing women’s sexual desire.
4. Discussion
It has been known since the early 1940’s that supraphysiological amounts of testosterone increase sexual desire in women; however, the relationship between endogenous/physiological levels of testosterone and women’s sexual desire remains controversial. Estradiol on its own (at periovulatory levels) increases sexual desire in naturally and surgically postmenopausal women (Dow et al., 1983, Davis et al., 1995; Dennerstein et al., 1980; Sherwin, 1991). Supraphysiological testosterone, but not physiological testosterone, enhances the effectiveness of a low dose estrogen therapy at increasing sexual desire in postmenopausal women (Burger et al., 1987; Braunstein et al., 2005; Buster et al., 2005; Davis et al., 2006b; Panay et al., 2010; Shifren et al., 2006; Simon et al., 2005; Flöter et al., 2002; Lobo et al., 2003; Sherwin et al., 1985); however, few studies have added testosterone to an estrogen therapy that produces periovulatory levels of circulating estradiol (Dow et al., 1983; Davis et al., 1995, Sherwin & Gelfand, 1987). It is unclear whether testosterone, even at supraphysiological levels, is capable of further increasing sexual desire in women experiencing periovulatory levels of estradiol. Furthermore, the mechanism by which testosterone increases women’s sexual desire in the presence of an estrogen remains unknown. Testosterone may work peripherally to modulate levels of free estradiol via its preferential binding to SHBG, or work centrally to increase estradiol levels in the brain via its aromatization to estradiol, or both. The data suggest that it is not possible to rule-out a role for testosterone in the modulation of women’s sexual desire; however, the exact nature of that role remains unknown.
5. Conclusions
There is little support for the notion that testosterone is the critical libidinal hormone for women. The likelihood that an androgen-only clinical treatment will meaningfully increase women’s sexual desire is minimal, and the focus of pharmaceutical companies on the development of androgen therapies for the treatment of female sexual desire disorders is likely misplaced. Given that elevated estradiol levels within physiological range increase women’s sexual desire without concurrent androgen therapy, the use of supraphysiological testosterone to treat low sexual desire in women may be inappropriate. Future studies should focus on establishing the threshold level of estradiol required to reliably produce a meaningful increase in sexual desire in post-menopausal women, and examining the comparative effectiveness of different estradiol treatment regimens (e.g. chronic, cyclical, as-needed). Whether safety concerns about exposure to elevated estradiol can be addressed via novel steroid formulations or treatment regimens should also needs to be investigated.
HIGHLIGHTS.
Hormone therapies for low sexual desire in postmenopausal women are reviewed
Estradiol, at periovulatory levels, increases sexual desire in postmenopausal women
Supraphysiological testosterone increases sexual desire in postmenopausal women
Physiological testosterone does not increase sexual desire in postmenopausal women
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development 1R21HD078077-01, and by the National Science Foundation Graduate Training Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CITATIONS
- Abraham GE. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. Journal of Clinical Endocrinology & Metabolism. 1974;39(2):340–346. doi: 10.1210/jcem-39-2-340. [DOI] [PubMed] [Google Scholar]
- Adair FE, Herrmann JB. The use of testosterone propionate in the treatment of advanced carcinoma of the breast. Annals of Surgery. 1946;123(6):1023. [PMC free article] [PubMed] [Google Scholar]
- Alexander JL, Kotz K, Dennerstein L, Kutner SJ, Wallen K, Notelovitz M. The effects of postmenopausal hormone therapies on female sexual functioning: a review of double-blind, randomized controlled trials. Menopause. 2004;11(6 Part 2 of 2):749–765. doi: 10.1097/01.gme.0000142887.31811.97. [DOI] [PubMed] [Google Scholar]
- Avis NE, Zhao X, Johannes CB, Ory M, Brockwell S, Greendale GA. Correlates of sexual function among multi-ethnic middle-aged women: results from the Study of Women’s Health Across the Nation (SWAN) Menopause. 2005;12(4):385–398. doi: 10.1097/01.GME.0000151656.92317.A9. [DOI] [PubMed] [Google Scholar]
- Bachmann G, Bancroft J, Braunstein G, Burger H, Davis S, Dennerstein L, … Traish A. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertility and Sterility. 2002;77(4):660–665. doi: 10.1016/s0015-0282(02)02969-2. [DOI] [PubMed] [Google Scholar]
- Bagatell CJ, Heiman JR, Rivier JE, Bremner WJ. Effects of endogenous testosterone and estradiol on sexual behavior in normal young men. The Journal of Clinical Endocrinology & Metabolism. 1994;78(3):711–716. doi: 10.1210/jcem.78.3.8126146. [DOI] [PubMed] [Google Scholar]
- Ball J, Hartman CG. Sexual excitability as related to the menstrual cycle in the monkey. American Journal of Obstetrics and Gynecology. 1935;29:117–119. [Google Scholar]
- Beach FA. Evolutionary changes in the physiological control of mating behavior in mammals. Psychological Review. 1947;54(6):297. doi: 10.1037/h0056549. [DOI] [PubMed] [Google Scholar]
- Bolour S, Braunstein G. Testosterone therapy in women: a review. International Journal of Impotence Research. 2005;17(5):399–408. doi: 10.1038/sj.ijir.3901334. [DOI] [PubMed] [Google Scholar]
- Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, … Watts NB. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Archives of Internal Medicine. 2005;165(14):1582. doi: 10.1001/archinte.165.14.1582. [DOI] [PubMed] [Google Scholar]
- Braunstein GD, Reitz RE, Buch A, Schnell D, Caulfield MP. Testosterone reference ranges in normally cycling healthy premenopausal women. Journal of Sexual Medicine. 2011;8(10):2924–2934. doi: 10.1111/j.1743-6109.2011.02380.x. [DOI] [PubMed] [Google Scholar]
- Brown-Sequard CE. The effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. Lancet. 1889;2(3438):105. [Google Scholar]
- Burger H, Hailes J, Nelson J, Menelaus M. Effect of combined implants of oestradiol and testosterone on libido in postmenopausal women. British Medical Journal. 1987;294(6577):936. doi: 10.1136/bmj.294.6577.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CW, Anderson DC. Sex-hormone-binding globulin is an oestrogen amplifier. Nature. 1972;240:38–40. doi: 10.1038/240038a0. [DOI] [PubMed] [Google Scholar]
- Buster JE, Kingsberg SA, Aguirre O, Brown C, Breaux JG, Buch A, … Casson P. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstetrics & Gynecology. 2005;105(5 Part 1):944–952. doi: 10.1097/01.AOG.0000158103.27672.0d. [DOI] [PubMed] [Google Scholar]
- Carpenter CR. Sexual behavior is free ranging rhesus monkeys (Macaca mulatta): I. Specimens, procedures and behavioral characteristics of estrus. Journal of Comparative Psychology. 1942;33(1):113–142. [Google Scholar]
- Cochran CG. Proceptive patterns of behavior throughout the menstrual cycle in female rhesus monkeys. Behavioral and Neural Biology. 1979;27:342–353. doi: 10.1016/s0163-1047(79)92412-9. [DOI] [PubMed] [Google Scholar]
- David KG, Dingemanse E, Freud J, Laqueur E. On crystalline male hormone from testicles (testosterone) Hoppe Seylers Z Physiol Chem. 1935;233:281. [Google Scholar]
- Davis SR, McCloud P, Strauss BJ, Burger H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas. 1995;21(3):227–236. doi: 10.1016/0378-5122(94)00898-h. [DOI] [PubMed] [Google Scholar]
- Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self-reported sexual function in women. Journal of the American Medical Association. 2005;294(1):91–96. doi: 10.1001/jama.294.1.91. [DOI] [PubMed] [Google Scholar]
- Davis SR, Goldstat R, Papalia MA, Shah S, Kulkarni J, Donath S, Bell RJ. Effects of aromatase inhibition on sexual function and well-being in postmenopausal women treated with testosterone: a randomized, placebo-controlled trial. Menopause. 2006a;13(1):37–45. doi: 10.1097/01.gme.0000168061.32917.83. [DOI] [PubMed] [Google Scholar]
- Davis SR, Van Der Mooren MJ, van Lunsen RH, Lopes P, Ribot J, Rees M, … Purdie DW. Efficacy and safety of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Menopause. 2006b;13(3):387–396. doi: 10.1097/01.gme.0000179049.08371.c7. [DOI] [PubMed] [Google Scholar]
- Davis SR, Moreau M, Kroll R, Bouchard C, Panay N, Gass M, … Studd J. Testosterone for low libido in postmenopausal women not taking estrogen. New England Journal of Medicine. 2008;359(19):2005–2017. doi: 10.1056/NEJMoa0707302. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Burrows GD, Wood C, Hyman G. Hormones and sexuality: effect of estrogen and progestogen. Obstetrics & Gynecology. 1980;56(3):316–317. [PubMed] [Google Scholar]
- Dennerstein L, Gotts G, Brown JB, Morse CA, Farley TM, Pinol A. The relationship between the menstrual cycle and female sexual interest in women with PMS complaints and volunteers. Psychoneuroendocrinology. 1994;19(3):293–304. doi: 10.1016/0306-4530(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertility and Sterility. 2002;77:42–48. doi: 10.1016/s0015-0282(02)03001-7. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Koochaki P, Barton I, Graziottin A. Hypoactive sexual desire disorder in menopausal women: a survey of Western European women. The Journal of Sexual Medicine. 2006;3(2):212–222. doi: 10.1111/j.1743-6109.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- Dow MGT, Hart DM, Forrest CA. Hormonal treatments of sexual unresponsiveness in postmenopausal women: a comparative study. British Journal of Obstetrics & Gynaecology. 1983;90(4):361–366. doi: 10.1111/j.1471-0528.1983.tb08924.x. [DOI] [PubMed] [Google Scholar]
- Flöter A, Nathorst-Böös J, Carlström K, Von Schoultz B. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric. 2002;5(4):357–365. [PubMed] [Google Scholar]
- Freeman ER, Bloom DA, McGuire EJ. A brief history of testosterone. The Journal of Urology. 2001;165(2):371–373. doi: 10.1097/00005392-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Geist SH, Salmon UJ, Gaines JA, Walter RI. The biologic effects of androgen (Testosterone Propionate) in women. Journal of the American Medical Association. 1940;114(16):1539–1544. [Google Scholar]
- Gordon TP. Reproductive behavior in the rhesus monkey: social and endocrine variables. American Zoologist. 1981;21(1):185–195. [Google Scholar]
- Gracia CR, Freeman EW, Sammel MD, Lin H, Mogul M. Hormones and sexuality during transition to menopause. Obstetrics & Gynecology. 2007;109(4):831–840. doi: 10.1097/01.AOG.0000258781.15142.0d. [DOI] [PubMed] [Google Scholar]
- Harvey SM. Female sexual behavior: fluctuations during the menstrual cycle. Journal of Psychosomatic Research. 1987;31(1):101–110. doi: 10.1016/0022-3999(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, … Valanis BG. Effects of estrogen plus progestin on health-related quality of life. New England Journal of Medicine. 2003;348(19):1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- Hoberman JM, Yesalis CE. The history of synthetic testosterone. Scientific American. 1995;272(2):60–65. doi: 10.1038/scientificamerican0295-76. [DOI] [PubMed] [Google Scholar]
- James WH. The distribution of coitus within the human intermenstruum. Journal of Biosocial Science. 1971;3:159–171. doi: 10.1017/s0021932000007902. [DOI] [PubMed] [Google Scholar]
- Jiroutek MR, Chen MH, Johnston CC, Longcope C. Changes in reproductive hormones and sex hormone-binding globulin in a group of postmenopausal women measured over 10 years. Menopause. 1998;5(2):90–94. [PubMed] [Google Scholar]
- Keverne EB. Sexual receptivity and attractiveness in the female rhesus monkey. Advances in the Study of Behavior. 1976;7:155–200. [Google Scholar]
- Korenman SG, Sherman BM. Further studies of gonadotropin and estradiol secretion during the preovulatory phase of the human menstrual cycle. Journal of Clinical Endocrinology and Metabolism. 1973;36(6):1205–1209. doi: 10.1210/jcem-36-6-1205. [DOI] [PubMed] [Google Scholar]
- Korse CM, Bonfrer JM, van Beurden M, Verheijen RH, Rookus MA. Estradiol and testosterone levels are lower after oophorectomy than after natural menopause. Tumor Biology. 2009;30(1):37–42. doi: 10.1159/000199449. [DOI] [PubMed] [Google Scholar]
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS) Menopause. 2006;13(1):46–56. doi: 10.1097/01.gme.0000172596.76272.06. [DOI] [PubMed] [Google Scholar]
- Lobo RA, Rosen RC, Yang HM, Block B, Van Der Hoop RG. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertility and Sterility. 2003;79(6):1341–1352. doi: 10.1016/s0015-0282(03)00358-3. [DOI] [PubMed] [Google Scholar]
- Lovejoy J, Wallen K. Adrenal suppression and sexual initiation in group-living female rhesus monkeys. Hormones and Behavior. 1990;24(2):256–269. doi: 10.1016/0018-506x(90)90008-l. [DOI] [PubMed] [Google Scholar]
- Matteo S, Rissman EF. Increased sexual activity during the midcycle portion of the human menstrual cycle. Hormones and Behavior. 1984;18:249–255. doi: 10.1016/0018-506x(84)90014-x. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Rust J, Golombok S, Davis S, Bouchard C, Brown C, … Derogatis L. Profile of Female Sexual Function: a patient-based, international, psychometric instrument for the assessment of hypoactive sexual desire in oophorectomized women. Menopause. 2004;11(4):474–483. doi: 10.1097/01.gme.0000109316.11228.77. [DOI] [PubMed] [Google Scholar]
- Meston CM, Buss DM. Why humans have sex. Archives of Sexual Behavior. 2007;36(4):477–507. doi: 10.1007/s10508-007-9175-2. [DOI] [PubMed] [Google Scholar]
- Michael RR, Bonsall RW. Peri-ovulatory synchronization of behavior in male and female rhesus monkeys. Nature. 1977;265:463–465. doi: 10.1038/265463a0. [DOI] [PubMed] [Google Scholar]
- Moghissi KS, Syner FN, Evans TN. A composite picture of the menstrual cycle. American Journal of Obstetrics and Gynecology. 1972;114(3):405. doi: 10.1016/0002-9378(72)90617-5. [DOI] [PubMed] [Google Scholar]
- Myers LS, Dixen J, Morrissette D, Carmichael M, Davidson JM. Effects of estrogen, androgen, and progestin on sexual psychophysiology and behavior in postmenopausal women. Journal of Clinical Endocrinology & Metabolism. 1990;70(4):1124–1131. doi: 10.1210/jcem-70-4-1124. [DOI] [PubMed] [Google Scholar]
- Nathorst-Böös J, Wiklund I, Mattsson LÅ, Sandin K, Schoultz BV. Is sexual life influenced by transdermal estrogen therapy?: A double blind placebo controlled study in postmenopausal women. Acta Obstetricia et Gynecologica Scandinavica. 1993;72(8):656–660. doi: 10.3109/00016349309021160. [DOI] [PubMed] [Google Scholar]
- Palmer JD, Udry JR, Morris NM. Diurnal and weekly, but no lunar rhythms in humans copulation. Human Biology. 1982;54:111–121. [PubMed] [Google Scholar]
- Panay N, Al-Azzawi F, Bouchard C, Davis SR, Eden J, Lodhi I, … Sturdee DW. Testosterone treatment of HSDD in naturally menopausal women: the ADORE study. Climacteric. 2010;13(2):121–131. doi: 10.3109/13697131003675922. [DOI] [PubMed] [Google Scholar]
- Perloff WH. Role of the hormones in human sexuality. Psychosomatic Medicine. 1949;11(3):133–139. doi: 10.1097/00006842-194905000-00001. [DOI] [PubMed] [Google Scholar]
- Pomerantz SM, Goy RW. Proceptive behavior of the female rhesus monkeys during tests with tethered males. Hormones and Behavior. 1983;17:237–248. doi: 10.1016/0018-506x(83)90023-5. [DOI] [PubMed] [Google Scholar]
- Roney JR, Simmons ZL. Hormonal predictors of sexual motivation in natural menstrual cycles. Hormones and Behavior. 2013;63(4):636–645. doi: 10.1016/j.yhbeh.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Rosner W. The Functions of Corticosteroid-Binding Globulin and Sex Hormone-Binding Globulin: Recent Advances. Endocrine Reviews. 1990;11(1):80–91. doi: 10.1210/edrv-11-1-80. [DOI] [PubMed] [Google Scholar]
- Salmon UJ, Geist SH. Effect of androgens upon libido in women. The Journal of Clinical Endocrinology. 1943;3(4):235–238. [Google Scholar]
- Sarrel P, Dobay B, Wiita B. Estrogen and estrogen-androgen replacement in postmenopausal women dissatisfied with estrogen-only therapy: sexual behavior and neuroendocrine responses. Journal of Reproductive Medicine. 1998;43(10):847–856. [PubMed] [Google Scholar]
- Selby C. Sex hormone binding globulin: origin, function and clinical significance. Annals of Clinical Biochemistry. 1990;27(6):532–541. doi: 10.1177/000456329002700603. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosomatic Medicine. 1985;47(4):339–351. doi: 10.1097/00006842-198507000-00004. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM. The role of androgen in the maintenance of sexual functioning in oophorectomized women. Psychosomatic Medicine. 1987;49(4):397–409. doi: 10.1097/00006842-198707000-00009. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. Journal of Clinical Endocrinology & Metabolism. 1991;72(2):336–343. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, … Mazer NA. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. New England Journal of Medicine. 2000;343(10):682–688. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Davis SR, Moreau M, Waldbaum A, Bouchard C, DeRogatis L, … Kroll R. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 Study. Menopause. 2006;13(5):770–779. doi: 10.1097/01.gme.0000243567.32828.99. [DOI] [PubMed] [Google Scholar]
- Shorr E, Papanicolaou GN, Stimmel BF. Neutralization of Ovarian Follicular Hormone in Women by Simultaneous Administration of Male Sex Hormone. Experimental Biology and Medicine. 1938;38(5):759–762. [Google Scholar]
- Simon J, Braunstein G, Nachtigall L, Utian W, Katz M, Miller S, … Davis S. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. Journal of Clinical Endocrinology & Metabolism. 2005;90(9):5226–5233. doi: 10.1210/jc.2004-1747. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Aromatization of androgens in women: current concepts and findings. Fertility and Sterility. 2002;77:6–10. doi: 10.1016/s0015-0282(02)02984-9. [DOI] [PubMed] [Google Scholar]
- Spark RF. Intrinsa fails to impress FDA advisory panel. International journal of impotence research. 2005;17(3):283–284. doi: 10.1038/sj.ijir.3901308. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Rice FJ. Correlations between sexual desire and menstrual cycle characteristics. Archives of Sexual Behavior. 1988;17(6):499–508. doi: 10.1007/BF01542338. [DOI] [PubMed] [Google Scholar]
- Tsao A. Can Intrinsa be a Viagra for women? Bloomberg Business. 2004 Oct 27; Retrieved from http://www.bloomberg.com/bw/stories/2004-10-27/can-intrinsa-be-a-viagra-for-women.
- Tsui AO, de Silva SV, Marinshaw R. Pregnancy avoidance and coital behavior. Demography. 1991;28(1):101–117. [PubMed] [Google Scholar]
- Udry JR, Morris NM. The distribution of events in the human menstrual cycle. Journal of Reproductive Fertility. 1977;51:419–425. doi: 10.1530/jrf.0.0510419. [DOI] [PubMed] [Google Scholar]
- Van Goozen SHM, Wiegant VM, Endert E, Helmond FA, Van de Poll NE. Psychoendocrinological assessment of the menstrual cycle: the relationship between hormones, sexuality and mood. Archives of Sexual Behavior. 1997;26(4):359–382. doi: 10.1023/a:1024587217927. [DOI] [PubMed] [Google Scholar]
- Wallen K, Goy RW. Effects of estradiol benzoate, estrone, and propionates of testosterone or dihydrotestosterone on sexual and related behaviors of OVX rhesus monkeys. Hormones and Behavior. 1977;9(3):228–248. doi: 10.1016/0018-506x(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Wallen K, Winston LA, Gaventa S, Davis-DaSilva M, Collins DC. Periovulatory changes in female sexual behavior and patters of ovarian steroid secretion in group-living rhesus macaques. Hormones and Behavior. 1984;18:431–450. doi: 10.1016/0018-506x(84)90028-x. [DOI] [PubMed] [Google Scholar]
- Wallen K. Desire and ability: hormones and the regulation of female sexual behavior. Neuroscience and Biobehavioral Reviews. 1990;14:233–241. doi: 10.1016/s0149-7634(05)80223-4. [DOI] [PubMed] [Google Scholar]
- Wallen K. The evolution of female sexual desire. Sexual Nature/Sexual Culture. 1995:57–79. [Google Scholar]
- Wallen K. Sex and context: hormones and primate sexual motivation. Hormones and Behavior. 2001;40:339–357. doi: 10.1006/hbeh.2001.1696. [DOI] [PubMed] [Google Scholar]
- Wallen K. Women are not as unique as thought by some: comment on “Hormonal predictors of sexual motivation in natural menstrual cycles”, by Roney and Simmons. Hormones and Behavior. 2013;63(4):634–635. doi: 10.1016/j.yhbeh.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Waxenberg SE, Drellich MG, Sutherland AM. The role of hormones in human behavior. I. Changes in female sexuality after adrenalectomy. Journal of Clinical Endocrinology & Metabolism. 1959;19(2):193–202. doi: 10.1210/jcem-19-2-193. [DOI] [PubMed] [Google Scholar]
- Wiklund I, Karlberg J, Mattsson LA. Quality of life of postmenopausal women on a regimen of transdermal estradiol therapy: a double-blind placebo-controlled study. American Journal of Obstetrics and Gynecology. 1993;168(3 Pt 1):824. doi: 10.1016/s0002-9378(12)90828-5. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Dunson DB, McConnaughey DR, Kesner JS, Weinberg CR. On the frequency of intercourse around ovulation: evidence for biological influences. Human Reproduction. 2004;19(7):1539–1543. doi: 10.1093/humrep/deh305. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Gordon TP, Collins DC. Variation in ovarian steroids associated with the annual mating period in female rhesus monkeys (Macaca mulatta) Biology of Reproduction. 1982;27:530–539. doi: 10.1095/biolreprod27.3.530. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Maestripieri D, Wallen K. Estradiol increases female sexual initiation independent of male responsiveness in rhesus monkeys. Hormones and Behavior. 1998;33:95–103. doi: 10.1006/hbeh.1998.1440. [DOI] [PubMed] [Google Scholar]