Abstract
Purpose
The impact of metabolic syndrome (MetS) on recurrence of atrial fibrillation (AF) after catheter ablation remains uncertain. We conducted a meta-analysis to summarize the relative risks (RR) of AF recurrence after catheter ablation in patients with vs. without MetS and its components.
Methods
Among 839 articles identified from PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials, we included 23 studies with a total of 12,924 patients (7,594 with paroxysmal AF and 5,330 with nonparoxysmal AF) for analysis. Five of these had complete information on MetS components. Variables assessed comprised study design and population characteristics, AF ablation methods, use of anti-arrhythmic drugs, AF recurrence ascertainment methods, adjustment variables, and other quality indicators.
Results
Our meta-analysis found an elevated risk of AF recurrence after ablation in patients with vs. without MetS (pooled RR, 1.63; 95 % confidence interval (CI), 1.25–2.12). Among components of MetS, hypertension was a predictor of AF post-ablation recurrence in studies without adjustment for other MetS components (RR, 1.62; 95 % CI, 1.23–2.13) but not in those adjusting for two or more additional MetS components (RR, 1.03; 95 % CI, 0.88–1.20). There was a borderline association between overweight/obesity and AF recurrence after ablation (RR, 1.27; 95 % CI, 0.99–1.64).
Conclusions
MetS is associated with an increased risk of AF recurrence after catheter ablation. Further study of the MetS and its components as determinants of AF risk could help refine patient selection and improve procedural outcomes.
Keywords: Pulmonary vein isolation, Syndrome X, Glucose intolerance, Hyperlipidemia, Insulin resistance
1 Introduction
Atrial fibrillation (AF) is among the most common cardiac arrhythmias encountered in clinical practice, with more than 2.3 million prevalent cases in the USA [1, 2]. Catheter ablation has emerged as an effective therapy for patients with symptomatic and drug-refractory AF, but success rates are highly variable, ranging from 22.3 to 91.0 % [3]. To explain the heterogeneity of these success rates, several factors have been identified as potential predictors of recurrence after AF catheter ablation, among them left atrial size, nonparoxysmal AF, sleep apnea, and metabolic syndrome (MetS) [4–6].
MetS is defined as a cluster of central obesity, hypertension, dyslipidemia, and glucose intolerance [7]. Higher recurrence rates after AF ablation were first observed in patients with individual components of MetS, such as obesity, diabetes mellitus (DM), and hypertension [5, 8–10]. More recently, MetS has also been associated with higher recurrence after AF ablation [6, 11, 12]. However, data on this association were inconclusive and estimates varied substantially [6, 11, 13]. We therefore conducted a systematic review and meta-analysis to summarize the relative risks (RR) of AF recurrence after catheter ablation in patients with vs. without MetS or its individual components.
2 Methods
2.1 Study selection
We searched PubMed, EMBASE, and Cochrane Central Register of Controlled Trials for studies published up until June 2012 that investigated recurrence of AF after catheter ablation in relation to MetS and its components, including obesity, hypertension, dyslipidemia, and DM or glucose intolerance. Table 1 shows the key words used to search eligible articles. All entries retrieved by this strategy were examined to identify studies that satisfied the following predefined inclusion criteria: (1) assessment of recurrence after AF ablation as either primary or secondary outcomes, (2) reporting of an RR and its standard error or 95 % confidence interval (CI), (3) assessment of MetS and/or its components as major exposure(s) included in the final adjusted model. Although our primary objective is to evaluate the association between MetS and AF ablation outcomes, many prior studies only reported individual components of MetS as independent predictors of post-ablation relapse of AF. To achieve a comprehensive summary of the existing literature, we included a study in the general analysis if it assessed any one of the MetS components in relation to the risk of post-ablation AF recurrence. However, we also contrasted the pooled estimates for each individual component based on studies evaluating MetS overall and all of its components (MetS studies) vs. those evaluating only one or more individual components (non-MetS studies).
Table 1.
Literature search strategy and data extraction
| Search termsa | |
| “Catheter ablation,” “pulmonary vein isolation,” or “atrial AND fibrillation ablation” |
“metabolic syndrome,” “syndrome X,” “insulin resistance,” “hyperglycemia,” “glucose intolerance,” “impaired fasting glucose,” “pre-diabetes,” “dysglycemia,” “obesity,” “body mass index,” “overweight,” “dyslipidemia,” “hyperlipidemia “, “hypertriglyceridemia,” “hypoalphalipoproteinemia,” “hypertension,” or “high blood pressure” |
| Study characteristics | Year of publication, study period, geographic region, sample size, inclusion and exclusion criteria of the study population, characteristics of enrolled subjects, including mean age, gender distribution, the proportion of patients with DM, obesity, hypertension, structural heart disease, and paroxysmal vs. nonparoxysmal AF, mean BMI, mean LAD, LVEF and AF duration, study design, definitions of MetS and its component factors, AF ablation methods, blanking period [46], use of anti-arrhythmic drugs, definition and ascertainment methods of AF recurrence, and variables adjusted in the original analysis |
| Quality indicatorsb | Representativeness of the exposed cohort, selection of the nonexposed cohort, reliability of exposure ascertainment, demonstration that outcome of interest was not present at start of the study, comparability of cohorts, validity of outcome ascertainment, adequacy of follow-up, and loss to follow-up |
The search was restricted to human studies, excluding estimates published solely in the form of letters, commentaries, abstracts, case reports, or reviews AF atrial fibrillation, BMI body mass index, DM diabetes mellitus, LVEF left ventricular ejection faction, LAD left atrial diameter
Search terms were entered as both text words and MeSH terms (if present)
Eight factors based on Newcastle–Ottawa scale [33]
2.2 Data extraction
Three independent investigators (KJL, NT, and MB) extracted the data from eligible articles, and a fourth investigator (SIC) validated the collected data for accuracy. If available, we extracted RRs, including ratios of cumulative incidence, incidence rates, and odds of developing the outcomes of interest, whichever were reported in the original articles. Table 1 lists the variables evaluated in our analysis. Paroxysmal AF was defined as AF self-terminated within 7 days and nonparoxysmal AF as sustained beyond 7 days, including persistent (≤1 year) and long-standing persistent AF (>1 year). Blanking period was defined as a time period since ablation within which the occurrence of AF was not counted as a relapse [14].
2.3 Statistical analysis
All RRs extracted were the most finely adjusted estimates in the original studies. If one study presented estimates for multiple component factors of MetS, all of them were considered. If RRs for the same population were reported more than once but in different articles, only the RRs from the most recent or largest one were included. Random effects models were applied to calculate pooled RRs and their 95 % CIs. Heterogeneity of effect estimates was assessed by the Cochran Q test for heterogeneity and the I2 statistic, which quantifies the proportion of total variability attributable to between-study variation [15]. Radom effects meta-regressions were performed to explore sources of heterogeneity. All the extracted variables listed in Table 1 were examined in meta-regression models and we estimated stratified pooled RRs by the variables with p <0.10 in meta-regression. We explored potential publication bias using both Begg’s and Egger’s test [16]. Stata 10.1 (Stata Corp., College Station, TX) was used for all analyses. All reported p values are two sided with a significance level of 0.05.
3 Results
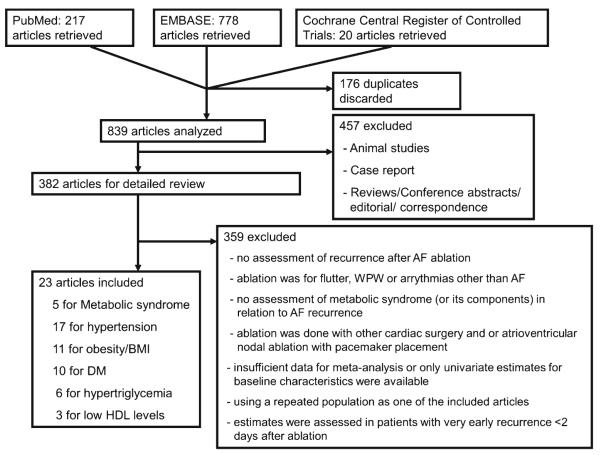
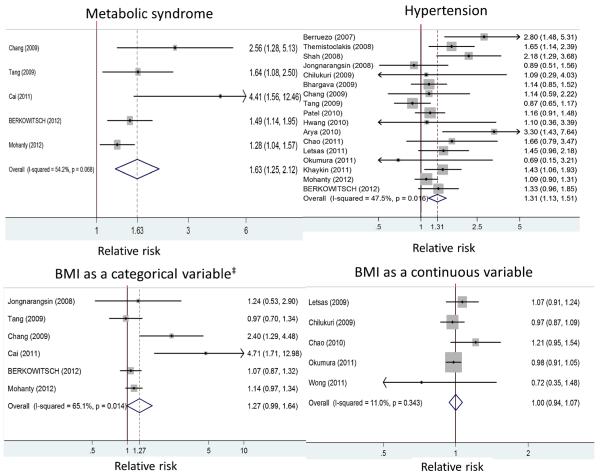
Among 839 articles identified from PubMed, EMBASE, and Cochrane Central Register of Controlled Trials, we included 23 studies [4–6, 8–13, 17–30] with a total of 12,924 patients (7,594 with paroxysmal AF and 5,330 with nonparoxysmal AF) for analysis. Figure 1 shows the literature search process. Table 2 summarizes the characteristics of the included studies. Among the selected studies, 5 studies conducted a comprehensive evaluation of MetS overall and its component factors, 17 assessed hypertension, 11 obesity/overweight (six used BMI as a categorical variable and five as a continuous variable), 10 DM, and 9 dyslipidemia in relation to AF recurrence after ablation (Tables 2 and 3). Meta-analysis results are presented below by exposure categories (Fig. 2).
Fig. 1.
Flow chart of literature search process
Table 2.
Summary of included studies
| First author | Publish year | Sample size | AF recurrence rate (%) | Follow-up (months) | Paroxysmal AF (%) | Mean age | Female (%) | Structural heart disease (%) | HTN (%) | DM (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chang [8] | 2009 | 282 | 29.8 | N/A | 76.6 | 51.6 | 24.5 | 7.3 | 30.6 | 8.1 |
| Cai [11] | 2011 | 186 | 25.3 | N/A | 86.6 | 55.1 | 33.9 | 16.7 (CAD: 9.7) | 34.4 | 10.2 |
| Wokhlu [17] | 2010 | 774 | 35.8 | 36.0 | 55.3 | 54.0 | 19.0 | 11.0b | 36.0 | 6.0 |
| Tang [13] | 2009 | 654 | 37.0 | 15.7 | 79.8 | 57.0 | 28.9 | 11.9 | 55.0 | 11.9 |
| Arya [5] | 2010 | 674 | 24.4 | N/A | 84.6 | 57.3 | 31.0 | 6.0b | 49.7 | N/A |
| Berruezo [18] | 2007 | 148 | 26.4 | 13.1 | 60.8 | 52.0 | 17.6 | 19.6 | 33.8 | N/A |
| Bhargava [19] | 2009 | 1404 | 27.4 | 56.1 | 51.9 | 55.8 | 24.0 | 56.3 | N/A | N/A |
| Chao [20] | 2010 | 132 | 26.5 | 23.0 | 100.0 | 50.7 | 30.3 | 10.5b | 32.6 | 9.1 |
| Chao [9] | 2010 | 228 | 11.0 | 18.8 | 100.0 | 52.3 | 26.8 | 20.4b | 29.7 | 28.5 |
| Chao [21] | 2011 | 232 | 15.9 | 25.4 | 100.0 | 53.4 | 27.6 | 15.5b | 35.4 | 6.5 |
| Chilukuri | 2009 | 210 | 21.4 | 25.0 | 56.7 | 58.0 | 20.0 | N/A | 50.0 | 9.0 |
| Hwang [23] | 2010 | 81 | 37.0 | 9.0 | 71.6 | 51.7 | 14.8 | N/A | 34.6 | 7.4 |
| Jongnarangsin [24] | 2008 | 324 | 39.2 | 7.0 | 72.2 | 57.0 | 24.1 | 11.0b | 46.0 | N/A |
| Khaykin [25] | 2011 | 385 | 42.0 | 33.6 | 63.1 | 58.0 | 23.0 | 24.0 | 45.0 | 28.0 |
| Letsas [26] | 2009 | 72 | 38.9 | 12.5 | 63.9 | 54.9 | 19.0 | 14.0 (CAD: 3) | 19.0 | 22.0 |
| Letsas [27] | 2011 | 226 | 42.0 | 14.4 | 59.3 | 55.9 | 18.6 | 25.7 | 45.6 | N/A |
| Okumura [28] | 2011 | 50 | 42.0 | 14.0 | 56.0 | 61.3 | 24.0 | 8.0 | 42.0 | 10.0 |
| Patel [4] | 2010 | 3000 | 23.1 | 32.0 | 53.4 | 55.7 | 22.8 | 11.1b | 42.9 | 13.3 |
| Shah [10] | 2008 | 264 | 8.7 | 35.0 | 87.1 | 56.0 | 35.0 | 15.0b | 41.0 | N/A |
| Themistoclakis [29] | 2008 | 1298 | 22.5 | 41.0 | 53.9 | 56.1 | 22.0 | 32.0 (CAD, 11) | 33.0 | N/A |
| Wong [30] | 2011 | 102 | 57.8 | 16.7 | 37.3 | 58.0 | 28.0 | 17.6b | 56.9 | 5.9 |
| Berkowitsch [12] | 2012 | 702 | 47.3 | 15.6 | 59.3 | 58.0 | 29.1 | 8.8b | 69.3 | 6.6 |
| Mohanty [6] | 2012 | 1496 | 34.0 | 21.0 | 29.3 | 62.6 | 26.4 | 12.3b | 52.4 | 4.9 |
| First author | Mean BMI (kg/m2) |
Mean LAD (mm) |
Mean LVEF (%) |
Mean AF duration (years) |
Blanking perioda |
AF ablation strategy | MetS or its component assessed |
Variables adjusted |
|---|---|---|---|---|---|---|---|---|
| Chang [8] | 24.6 | 38.6 | 59.2 | 4.4 | 2 | PVI, linear, and then CFAE sequentially if AF persists for patients with PAF (NPAF gets PVI and linear ablation regardless) |
MetS, BMI≥25, HTN, DM, TG, and HDL levels |
type of AF, CAD, LAD, LVEF, BMI, HTN, DM, TG, and HDL levels |
| Cai [11] | N/A | 35.0 | 68.7 | 5.4 | 3 | PVI, linear, and then CFAE | MetS, BMI≥25, and DM | Obesity, DM, MetS, AF duration, NPAF, duration of AF, LAD, ablation strategy(segmental pulmonary vein isolation vs. circumferential pulmonary vein ablation), ERAF, and procedural failure |
| Wokhlu [17] | 30.3 | N/A | 57.7 | 6.4 | 2 | PVI±linear ablation | DM | Age, DM, HTN, NPAF, family history of AF, LAD, and circumferential PVI |
| Tang [13] | 25.4 | 38.4 | 63.0 | 6.5 | 3 | PVI±linear ablation and CFAE until AF is terminated or all identified CFAE were eliminated |
MetS, BMI≥25, HTN, DM, TG, and HDL levels |
HTN, BMI, fasting blood glucose, TG and HDL levels, MetS, type/duration of AF, LAD, LVEDD, and ablation strategies |
| Arya [5] | N/A | N/A | N/A | 4.0 | 3 | PVI+linear ablation if AF persists |
HTN | LAD, age, gender, HTN, termination of AF during ablation, LV dysfunction, and type of AF, ablation time, and early recurrence(<7 days) |
| Berruezo [18] | N/A | 41.4 | 60.0 | 6.2 | 1 | PVI+linear ablation | HTN | Age, long-standing persistent AF, HTN, LAD, and LVESD |
| Bhargava [19] | N/A | 43.4 | 54.5 | 6.4 | 2 | PVI+SVC isolation+ablation of non-PV triggers+linear ablation (for NPAF) |
HTN | age, gender, LVEF, LAD, duration of AF, hypertension, structural heart disease, and type of AF |
| Chao [20] | 24.5 | 37.3 | N/A | 2 | PVI+SVC isolation or ablation of non-PV ectopy if any |
BMId | BMI, LAD, PA-PDI interval, Left atrial activation time, and left atrial voltage |
|
| Chao [9] | 24.9 | 38.5 | 58.9 | 4.2 | 2 | PVI+SVC isolation or ablation of non-PV ectopy if any |
DM | Age, LAD, LVEF, and the use of AAD |
| Chao [21] | 24,9 | 38.2 | 59.9 | 4.2 | 2 | PVI+SVC isolation or ablation of non-PV ectopy if any |
HTN | Age, HTN, LAD, LVEF, LA total activation time and LA voltage, and renal function |
| Chilukuri | 29 | N/A | 58.0 | 6.4 | 3 | PVI | HTN, BMId, DM | Age, gender, AF duration, BMI, LAD, LVEF, persistent AF, HTN, DM, and OSA |
| Hwang [23] | 24.9 | N/A | 62.6 | 5.1 | 2 | PVI+linear ablation | HTN | age, HTN, persistent AF, PVI method, early recurrence of AF, LVEF, and LA volume index |
| Jongnarangsin [24] | 29.6 | 44.0 | 56.0 | 2 | PVI+CFAE | HTN, BMI≥30 | age, sex, BMI, type/duration of AF, OSA, LAD, LVEF, and HTN |
|
| Khaykin [25] | 29 | 42.4 | N/A | 7.5 | 3 | PVI+CFAE if AF persists | HTN | Early recurrence of AF, NPAF, HTN, LAD, ACEI/ARB use, number of failed AAD, CFAE ablation, and BMI, termination of AF during ablation |
| Letsas [26] | 26.1 | 40.6 | 54.8 | 5.5 | 1 | PVI | HTN, BMId, DM, dyslipidemia |
Age, gender, LVEF, LAD, LVEDD, type/duration of AF, HTN, DM, dyslipidemia, BMI, structural heart disease, AAD use after ablation, use of statins, ACEI, WBC, CRP, and fibrinogen level |
| Letsas [27] | 26.6 | 40.9 | 57.4 | 3 | PVI | HTN | Fibrinogen level, uric acid level, HTN, and CRP |
|
| Okumura [28] | 25.1 | N/A | N/A | 5.9 | 2 | PVI+CFAE if AF persists | HTN and BMId | NPAF, BMI, HTN, MMP-2, carboxyl-terminal telopeptide of collagen type I, and BNP level |
| Patel [4] | 27.1 | 42.6 | 52.9 | N/A | N/A | PVI+linear ablation+SVC isolation |
HTN and DM | Age, LVEF, LAD, HTN, DM, CAD, non-PV triggers, and type of AF |
| Shah [10] | 31 | 54.0 | 56.0 | 6.5 | 12c | PVI | HTN and dyslipidemia | Duration of AF, dyslipidemia, HTN |
| Themistoclakis [29] | N/A | 44.0 | 55.0 | 6.6 | 3 | PVI+SVC isolation | Dyslipidemia | Age, type/duration of AF, LAD, HTN, and SVC isolation |
| Wong [30] | 28 | N/A | N/A | N/A | 3 | PVI±linear ablation | BMId | LA volume and OSA, LVEF, sex, valvulopathy, BMI, and AF chronicity |
| Berkowitsch [12] | 27.0 | N/A | 60.0 | 5.0 | 3 | PVI+CFAE/linear ablation if AF persists |
MetS, BMI≥30, HTN, DM, TG, and HDL levels |
MetS factors, type of AF, LA area/body surface area, eGFR, LVEF, creatinine, age, ablation technique, intraprocedural cardioversion, DCM/HOCM, and uric acid level |
| Mohanty [6] | 28.3 | 43.2 | 56.0 | 4.9 | 3 | PVI+linear ablation+SVC isolation if PV-like potentials were recorded+CFAE if identified |
MetS, obesityd,e, HTN, DM, and dyslipidemia |
Age, sex, comorbidities, LVEF, and LAD |
AAD antiarrhythmic drugs, ACEI angiotensin converting enzyme inhibitor, AF atrial fibrillation, ARB angiotensin receptor blocker, BMI body mass index, BNP brain natriuretic peptide level, CAD coronary artery disease, CFAE (ablation of) sites with complex fractionated atrial electrograms, DM diabetes mellitus, GFR glomerular filtration rate, HDL low high-density lipoprotein levels, HTN hypertension, LAD left atrial diameter, LVEF left ventricular ejection faction, LVEDD Left ventricular end-diastolic dimension, LVESD Left ventricular end-systolic dimension, MetS metabolic syndrome, MMP metalloproteinase, N/A not available, NPAF nonparoxysmal atrial fibrillation, OSA obstructive sleep apnea, PA-PDI interval the time interval from the initiation of the P-wave deflection to the peak of the mitral inflow A-wave on pulse-wave Doppler imaging, PVI pulmonary vein isolation, SVC superior vena cava, TG hypertriglyceridemia
Blanking period: time to recurrence assessment, before which AF recurrence was not counted as a relapse [46]
Proportion of coronary artery disease
Very late recurrence
As a continuous variable
Defined as BMI≥25 or waist circumference 102 cm in men and 88 cm in women
Table 3.
Meta-analysis of the risk of atrial fibrillation recurrence after catheter ablation associated with metabolic syndrome and its component factors
| Exposures | No. of studies | Pooled estimates | p values for association | I-squareda (% (95%CI)) | p values for heterogeneityb |
|---|---|---|---|---|---|
| Metabolic syndrome | 5 | 1.63 (1.25–2.12) | 0.000 | 54 (0–83) | 0.068 |
| Hypertension | 17 | 1.31 (1.13–1.51) | 0.000 | 47 (8–70) | 0.016 |
| Obesity/overweightc | 6 | 1.27 (0.99–1.64) | 0.051 | 65 (16–85) | 0.014 |
| BMId | 5 | 1.00 (0.94–1.07) | 0.961 | 11 (0–81) | 0.343 |
| DM | 10 | 1.11 (0.97–1.26) | 0.129 | 44 (0–73) | 0.064 |
| Hypertriglyceridemia | 6 | 1.07 (0.89–1.29) | 0.484 | 69 (27–87) | 0.007 |
| Low HDL level | 3 | 0.98 (0.96–1.01) | 0.120 | (0–90) | 0.985 |
BMI body mass index, DM diabetes mellitus, HDL low high-density lipoprotein, MetS metabolic syndrome
I2 statistic described by Higgins et al. to assess heterogeneity
Cochrane Q test for heterogeneity
As a continuous variable
Fig. 2.
Forest plots: risk of AF recurrence after catheter ablation associated with metabolic syndrome and its component factors. ‡, as defined in the original articles: BMI≥25 [6, 8, 11, 13] and BMI≥30 [12, 24]
3.1 Metabolic syndrome
Our meta-analysis showed that patients with MetS were at an increased risk of recurrence after AF ablation (RR, 1.63; 95 % CI, 1.25–2.12; Table 3), as compared with those without MetS. Meta-regression revealed that studies with a younger mean age had a significantly higher pooled risk of recurrence (RR, 3.03; 95 % CI, 1.70–5.40) than remaining studies (RR, 1.39; 95 % CI, 1.19–1.62; Table 4). In an exploratory analysis, the studies with a younger mean age were found to have a lower prevalence of hypertension (32.1 vs. 57.1 %; p <0.001) and lower baseline risk of post-ablation AF recurrence among non-MetS controls (21.2 vs. 32.8 %; p <0.001).
Table 4.
Subgroup analyses and meta-regression for the risk of atrial fibrillation recurrence after catheter ablation associated with metabolic syndrome and its component factors
| Exposures | Variables | Subgroup | No. of studies | Pooled estimates |
p values for association |
p values for heterogeneityb |
p values from meta-regression |
|---|---|---|---|---|---|---|---|
| Metabolic syndrome | Mean age | Low (<median) | 2 | 3.03 (1.70–5.40) | <0.001 | 0.394 | 0.083 |
| High (≥median) | 3 | 1.39 (1.19–1.62) | <0.001 | 0.482 | |||
| HTN | Adjustment for other MetS components | No adjustment | 7 | 1.62 (1.23–2.13) | 0.001 | 0.085 | Ref |
| Adjusted for 1 additional component | 5 | 1.30 (1.00–1.70) | 0.05 | 0.118 | 0.286 | ||
| Adjusted for ≥2 additional components | 5 | 1.08 (0.94–1.24) | 0.295 | 0.454 | 0.042 | ||
| CFAE | No | 10 | 1.54 (1.24–1.92) | <0.001 | 0.053 | 0.043 | |
| Yes | 7 | 1.12 (0.96–1.30) | 0.15 | 0.272 | |||
| Linear ablation | No | 6 | 1.40 (1.09–1.78) | 0.007 | 0.282 | 0.511 | |
| Yes | 11 | 1.29 (1.08–1.54) | 0.005 | 0.015 | |||
| Obesity/overweighta | Proportion of HTN | Low (< median) | 3 | 2.32 (1.20–4.50) | 0.013 | 0.138 | 0.048 |
| High (≥median) | 3 | 1.09 (0.97–1.23) | 0.15 | 0.666 | |||
| Adjustment for OSA | No | 5 | 1.29 (0.98–1.70) | 0.071 | 0.006 | 0.852 | |
| Yes | 1 | 1.24 (0.53–2.90) | 0.62 | – | |||
| Report of follow-up length | No | 2 | 2.97 (1.61–5.50) | 0.001 | 0.267 | 0.025 | |
| Yes | 4 | 1.10 (0.97–1.23) | 0.135 | 0.826 | |||
| DM | Adjustment for other MetS components | No adjustment | 1 | 3.25 (1.21–8.72) | 0.019 | – | Ref |
| Adjusted for 1 additional component | 3 | 1.20 (0.87–1.64) | 0.263 | 0.011 | 0.102 | ||
| Adjusted for ≥2 additional components | 6 | 1.07 (0.95–1.20) | 0.29 | 0.897 | 0.074 | ||
| Dyslipidemia | Timing of recurrence assessment | Investigated very late recurrence (>12 months) | 1 | 4.01 (1.84–8.73) | <0.001 | – | 0.026 |
| Investigated recurrence after 2–3 months | 5 | 1.00 (1.00–1.00) | 0.994 | 0.429 |
Estimates of MetS can potentially vary depending on the diagnostic criteria used. Among the five included studies for MetS, four used National Cholesterol Education Program Adult Treatment Panel III criteria with prevalence of MetS ranging between 18.8 and 49.4 % [8, 11–13] and one used the World Health Organization criteria with a prevalence of 32.4 % [6]. There are no gold standard diagnostic criteria for MetS and its prevalence based on either set of criteria varies without a consistent pattern [31, 32]. In our meta-regression, however, the definition of MetS was not a significant effect modifier (p =0.345).
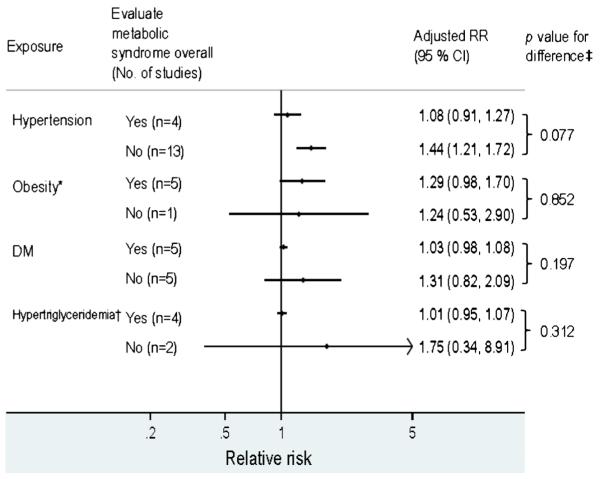
3.2 Hypertension
The risk of post-ablation AF recurrence was elevated in patients with vs. without hypertension (RR, 1.31; 95 % CI, 1.13–1.51) with significant heterogeneity (p =0.016; Table 3). Contrasting results from MetS studies vs. non-MetS studies, we found pooled estimates were only significant in the latter (p =0.077 for the difference; Fig. 3). In a subgroup analysis, hypertension was not independently associated with higher recurrence after AF ablation in studies adjusting for two or more additional MetS components (RR, 1.08; 95 % CI, 0.94–1.24; p =0.042 for the difference from those without such adjustment). The corresponding RR was 1.30 (95 % CI, 1.00–1.70) in studies adjusting for one additional MetS component and 1.62 (95 % CI, 1.23–2.13) in those without such adjustment. Of note, all the estimates for hypertension from MetS studies (n=4) were adjusted for 2 or more MetS components.
Fig. 3.
Comparison between studies investigating metabolic syndrome overall vs. only individual component factors. DM diabetes mellitus, HDL high-density lipoprotein, MetS metabolic syndrome. *, all MetS studies assessed BMI as a categorical variable and estimates for BMI as a continuous variable are not shown here. †, only estimates for hypertriglyceridemia are listed here for comparison as only MetS studies reported estimates for low HDL level (no counterpart for comparison). ‡, p values from meta-regression comparing estimates from MetS studies vs. those evaluating only individual component factors
3.3 Obesity
There was a borderline association between recurrence after AF ablation and BMI as a categorical variable (RR, 1.27; 95 % CI, 0.99 to 1.64; four studies [6, 8, 11, 13] used ≥25 kg/m2 and two studies [12, 24] used ≥30 kg/m2 for categorization) but not as a continuous variable (RR per point increase in BMI, 1.00; 95 % CI, 0.94 to 1.07; Table 3). All the MetS studies evaluated BMI as a categorical variable and their results were not significantly different from non-MetS studies (Fig. 3). Significant heterogeneity was found in studies where BMI was assessed as a categorical variable (p =0.014; Table 3). In these articles, study populations with lower prevalence of hypertension were associated with a higher pooled estimate for the effect of obesity on AF relapse after ablation (p =0.048). The heterogeneity in the estimates for obesity and AF recurrence was not attributable to the cut-off of BMI used (p =0.52). Restriction of the analysis to the two studies using BMI≥30 kg/m2 yielded an RR of 1.08 (95 % CI, 0.88–1.32) [12, 24].
3.4 DM and hyperlipidemia
The risk of AF post-ablation recurrence was not elevated in patients with DM (RR, 1.11; 95 % CI, 0.97–1.26), hypertriglyceridemia (RR, 1.07; 95 % CI, 0.89–1.29), or low high-density lipoprotein (HDL; RR, 0.98, 95 % CI, 0.96–1.01; Table 3). Contrasting results from MetS vs. non-MetS studies, there was no significant difference in estimates for either DM or hypertriglyceridemia (Fig. 3; all estimates for HDL were from MetS studies.). While RRs from studies investigating hypertriglyceridemia appeared to be heterogeneous, there was only one study [10] reporting a positive association (RR, 4.01; 95 % CI, 1.84–8.73) for very late recurrence (recurrence >1 year after ablation). The remaining studies reported consistently null findings for any recurrence after a blanking period of 2–3 months (Table 4).
3.5 Quality assessment
For all the exposure categories, out of the eight indicators of Newcastle–Ottawa scale [33], we identified a few associations where the quality of the study could appreciably affect the reported estimates. First, adjustment for additional MetS components could substantially attenuate the positive associations between hypertension and AF recurrence (Table 4). Second, the heterogeneity in estimates for DM was eliminated after excluding the only study without adjustment for additional MetS components (Table 4). Besides, we could not assess the follow-up adequacy for two studies investigating obesity and AF recurrence as they did not report follow-up duration. These two studies yielded a significantly higher pooled RR than that from the remaining studies (p =0.025; Table 4). While nondifferential loss to follow-up would have biased the results towards the null, more nonobese patients lost to follow-up remains a plausible explanation of these findings. Variation in AF ablation methods was not shown to be a significant effect modifier in most of the exposure categories. There was a borderline effect modification in one instance, whereby those who did not undergo ablation of sites with complex fractionated atrial electrograms [34] appeared to have somewhat higher hypertension-associated risk of AF recurrence (Table 4).
In the included studies, recurrence of AF was defined as a relapse of “AF alone” or “AF, atrial flutter, or atrial tachycardia” for a duration of at least 30 or 60 s, or without a specified duration. In our meta-regression, these variations in definition did not influence reported risks of AF recurrence in relation to MetS and its components appreciably, nor did the frequency or length of follow-up, methods of detecting AF recurrence, or length of “blanking period” materially affect our results (data not shown).
3.6 Publication bias
While all the prior studies reported positive associations between MetS and the risk of post-ablation AF recurrence, the tests for small-study effect were significant (p for Begg’s test=0.027; p for Egger’s test=0.002), which indicates small studies tended to find more extreme estimates. Adjustment for possible influence of unpublished articles based on “Trim and Fill” method [35] yielded a conservative pooled RR of 1.43 (95 % CI, 1.05–1.93) for AF recurrence associated with MetS. Moreover, we calculated the fail-safe number for meta-analysis, which estimated the number of unpublished null studies needed to abolish the currently observed association [36]. It was 42 for the association between MetS and post-ablation recurrence of AF, which is greater than the accepted threshold for robustness (>10 plus 5 times the original number of studies) [36]. Alternatively, the small study effect can be an unbiased effect with clinical implications [37]. In our analysis, comparing studies with sample sizes less vs. more than the median, small studies had populations with a younger mean age (53.0 vs. 60.2; p <0.001) and lower prevalence of patients with hypertension (32.1 vs. 57.1 %; p <0.001), both of which variables were associated with higher RRs of AF recurrence associated with MetS. For all the other exposure categories, Begg’s and Egger’s tests revealed no evidence of publication bias (data not shown).
4 Discussion
Our meta-analysis found that MetS was associated with a 60 % increase in AF recurrence rates after catheter ablation. Among the MetS components, overweight or obesity was a borderline predictor of AF relapse after ablation. Hypertension was associated with AF post-ablation recurrence only in studies without adjustment for other MetS components.
The mechanism underlying the association between MetS and post-ablation recurrence of AF remains unclear. The pathophysiology of AF is believed to involve a trigger (a rapidly firing focus often located inside one or more pulmonary veins) and its interaction with an arrhythmogenic atrial substrate to form a reentrant circuit or circuits [38]. This process is perpetuated by electrical and structural remodeling of the atria. Prior studies have demonstrated that MetS may play an important role in these remodeling processes. For electrical remodeling, MetS has been associated with atrial conduction disturbance and dispersion of refractoriness between the right and left atria [8, 39]; and obesity with a shortened effective refractory period in the pulmonary veins [40]. These electrophysiological abnormalities are thought to stabilize multiple wavelet reentry circuits and maintain AF. MetS is also linked to several features contributing to structural remodeling of the atria. First, MetS, hypertension, obesity, and insulin resistance are associated with left atrial dilation [8, 11, 41], which predisposes to the development of AF substrate characterized by increased nonuniform anisotropy and macroscopic slowing of conduction and promotes reentrant circuits in the atria [42]. Second, myocardial fibrosis, possibly caused by silent ischemia in DM or by oxidative stress and a proinflammatory state in MetS [43], may serve as an anchor for reentrant circuits, alter the wave-front propagation, and thus sustain AF [39, 44].
In our analysis, the MetS studies reporting higher RRs of AF post-ablation relapse were found to have a younger mean age and lower prevalence of patients with hypertension, which are also associated with a lower baseline recurrence risk in the non-MetS controls. While a possible interpretation of these findings could be that MetS is more predictive of AF recurrence in a younger population with fewer risk factors for AF than in an older population, these observations come from meta-regression with only aggregate (rather than individual-level) data, and should only be viewed as hypothesis generating.
Many prior studies reported individual components of MetS as independent predictors of post-ablation relapse of AF without evaluating all the MetS components [5, 8–11, 17, 18, 25, 29]. We contrasted estimates for each of the MetS components based on studies with comprehensive evaluation of MetS and those without (i.e., MetS and non-MetS studies). We found no significant difference between the two sources except that pooled estimates for hypertension were significant in the non-MetS studies but not in the MetS studies. This difference can be explained by adjustment for other MetS components: hypertension predicted post-ablation recurrence of AF only in studies without adjustment for other MetS components. Among other MetS components, only overweight or obesity had a borderline association after adjusting for other MetS components. The two studies [8, 11] reporting significant associations adjusted their estimates for MetS and thus this association cannot be explained solely by surrogate effect of MetS. The extent to which the MetS as a composite measure exceeds the predictive value for post-ablation AF recurrence contained in its individual components requires further study, but our findings imply MetS may be a more reliable predictor of AF recurrence than any of its components alone.
A prior meta-analysis has reported that obstructive sleep apnea (OSA) diagnosed by polysomnography, but not by Berlin questionnaire, was associated with lower long-term success rates of AF ablation [45]. Among our included studies, there was only one study that adjusted for OSA when estimating the association between obesity and post-ablation AF recurrence and it reported null findings [24]. It is important to note that none of the estimates from the prior meta-analysis for OSA in relation to AF recurrence was adjusted for MetS, nor were the estimates for MetS in the current analysis adjusted for OSA. As both MetS and OSA are associated with obesity, further studies taking into account the correlation between the three factors are needed to confirm these associations.
We need to acknowledge several limitations. First, although our primary objective was to evaluate recurrence after AF ablation in relation to MetS, there were only five studies directly assessing this association. We included studies investigating only individual components of MetS as they have been reported as independent predictors of AF post-ablation recurrence. However, these estimates were subject to confounding by other MetS components and the pooled estimates for the component factors of MetS derived from the 5 MetS studies may not be comparable with those derived by the remaining studies. Second, for meta-analyses to combine and integrate results of independent studies together, the studies should be similar and comparable. However, we found substantial heterogeneity among estimates for MetS, hypertension, obesity, DM, and hypertriglyceridemia. While stratification of the analysis according to some of the study characteristics provided more homogeneous subgroups, some subgroup analyses were based on relatively few studies and thus the results should be interpreted with caution. In addition, because the studies in question were not randomized controlled trials, but observational studies, the possibility of residual confounding cannot be excluded.
5 Conclusions
Our meta-analysis found that MetS is associated with a 60 % increase in AF recurrence rates after catheter ablation, but did not show hypertension to be independently related to, or overweight/obesity consistently associated with, this outcome. In light of the wide variation in reported studies regarding definition of exposure(s), assessment of covariates, and ascertainment of outcomes, the observed summary association points up the need to better understand MetS and its components as determinants of procedural success. The present findings suggest that presence of the MetS may help clinicians identify patients at higher risk of recurrence after AF ablation who might benefit from close postprocedural follow-up and early management for recurrent AF, which, if not well rate controlled, can be associated with secondary cardiomyopathy and eventually heart failure. While many of the MetS components are modifiable, the impact of treating MetS on AF ablation outcome remains to be investigated.
Acknowledgments
Financial support Dr. Kizer is supported by R01 HL094555 from the National Heart, Lung and Blood Institute.
Footnotes
Conflict of interest None declared.
Contributor Information
Kueiyu Joshua Lin, Department of Epidemiology, Harvard School of Public Health, 677 Huntington Avenue, Boston, MA 02115, USA; Department of Medicine, Jacobi Medical Center and Albert Einstein College of Medicine, Bronx, NY, USA.
Soung Ick Cho, Department of Medicine, Jacobi Medical Center and Albert Einstein College of Medicine, Bronx, NY, USA.
Nidhish Tiwari, Department of Medicine, Jacobi Medical Center and Albert Einstein College of Medicine, Bronx, NY, USA.
Michael Bergman, Department of Medicine, Jacobi Medical Center and Albert Einstein College of Medicine, Bronx, NY, USA.
Jorge R. Kizer, Department of Medicine, Montefiore Medical Center and Albert Einstein College of Medicine, Bronx, NY, USA; Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA
Eugen C. Palma, Department of Medicine, Montefiore Medical Center and Albert Einstein College of Medicine, Bronx, NY, USA
Cynthia C. Taub, Department of Medicine, Montefiore Medical Center and Albert Einstein College of Medicine, Bronx, NY, USA
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA : the Journal of the American Medical Association. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 3.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 4.Patel D, Mohanty P, Di Biase L, Shaheen M, Lewis WR, Quan K, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circulation Arrhythmia and Electrophysiology. 2010;3:445–451. doi: 10.1161/CIRCEP.109.858381. [DOI] [PubMed] [Google Scholar]
- 5.Arya A, Hindricks G, Sommer P, Huo Y, Bollmann A, Gaspar T, et al. Long-term results and the predictors of outcome of catheter ablation of atrial fibrillation using steerable sheath catheter navigation after single procedure in 674 patients. Europace. 2010;12:173–180. doi: 10.1093/europace/eup331. [DOI] [PubMed] [Google Scholar]
- 6.Mohanty S, Mohanty P, Di Biase L, Bai R, Pump A, Santangeli P, et al. Impact of metabolic syndrome on procedural outcomes in patients with atrial fibrillation undergoing catheter ablation. Journal of the American College of Cardiology. 2012;59:1295–1301. doi: 10.1016/j.jacc.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 7.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 8.Chang SL, Tuan TC, Tai CT, Lin YJ, Lo LW, Hu YF, et al. Comparison of outcome in catheter ablation of atrial fibrillation in patients with versus without the metabolic syndrome. American Journal of Cardiology. 2009;103:67–72. doi: 10.1016/j.amjcard.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Chao TF, Suenari K, Chang SL, Lin YJ, Lo LW, Hu YF, et al. Atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation associated with diabetes mellitus or impaired fasting glucose. American Journal of Cardiology. 2010;106:1615–1620. doi: 10.1016/j.amjcard.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 10.Shah AN, Mittal S, Sichrovsky TC, Cotiga D, Arshad A, Maleki K, et al. Long-term outcome following successful pulmonary vein isolation: pattern and prediction of very late recurrence. Journal of Cardiovascular Electrophysiology. 2008;19:661–667. doi: 10.1111/j.1540-8167.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 11.Cai L, Yin Y, Ling Z, Su L, Liu Z, Wu J, Du H, Lan X, Fan J, Chen W, Xu Y, Zhou P, Zhu J, Zrenner B. Predictors of late recurrence of atrial fibrillation after catheter ablation. International Journal of Cardiology. 2013;164:82–87. doi: 10.1016/j.ijcard.2011.06.094. [DOI] [PubMed] [Google Scholar]
- 12.Berkowitsch A, Kuniss M, Greiss H, Wojcik M, Zaltsberg S, Lehinant S, et al. Impact of impaired renal function and metabolic syndrome on the recurrence of atrial fibrillation after catheter ablation: a long term follow-up. Pacing and Clinical Electrophysiology: PACE. 2012;35:532–543. doi: 10.1111/j.1540-8159.2012.03350.x. [DOI] [PubMed] [Google Scholar]
- 13.Tang RB, Dong JZ, Liu XP, Long DY, Yu RH, Kalifa J, et al. Metabolic syndrome and risk of recurrence of atrial fibrillation after catheter ablation. Circulation Journal. 2009;3:438–443. doi: 10.1253/circj.cj-08-0832. [DOI] [PubMed] [Google Scholar]
- 14.Andrade JG, Khairy P, Verma A, Guerra PG, Dubuc M, Rivard L, et al. Early recurrence of atrial tachyarrhythmias following radiofrequency catheter ablation of atrial fibrillation. Pacing and Clinical Electrophysiology: PACE. 2012;35:106–116. doi: 10.1111/j.1540-8159.2011.03256.x. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 17.Wokhlu A, Hodge DO, Monahan KH, Asirvatham SJ, Friedman PA, Munger TM, et al. Long-term outcome of atrial fibrillation ablation: impact and predictors of very late recurrence. Journal of Cardiovascular Electrophysiology. 2010;21:1071–1078. doi: 10.1111/j.1540-8167.2010.01786.x. [DOI] [PubMed] [Google Scholar]
- 18.Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. European Heart Journal. 2007;28:836–841. doi: 10.1093/eurheartj/ehm027. [DOI] [PubMed] [Google Scholar]
- 19.Bhargava M, Di Biase L, Mohanty P, Prasad S, Martin DO, Williams-Andrews M, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm: the Official Journal of the Heart Rhythm Society. 2009;6:1403–1412. doi: 10.1016/j.hrthm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Chao TF, Sung SH, Wang KL, Lin YJ, Chang SL, Lo LW, et al. Associations between the atrial electromechanical interval, atrial remodelling and outcome of catheter ablation in paroxysmal atrial fibrillation. Heart. 2011;97:225–230. doi: 10.1136/hrt.2010.212373. [DOI] [PubMed] [Google Scholar]
- 21.Chao TF, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, et al. Associations between renal function, atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Circulation Journal: Official Journal of the Japanese Circulation Society. 2011;75:2326–2332. doi: 10.1253/circj.cj-11-0178. [DOI] [PubMed] [Google Scholar]
- 22.Chilukuri K, Dalal D, Marine JE, Scherr D, Henrikson CA, Cheng A, et al. Predictive value of obstructive sleep apnoea assessed by the Berlin Questionnaire for outcomes after the catheter ablation of atrial fibrillation. Europace. 2009;11:896–901. doi: 10.1093/europace/eup064. [DOI] [PubMed] [Google Scholar]
- 23.Hwang HJ, Lee JM, Joung B, Lee BH, Kim JB, Lee MH, et al. Atrial Electroanatomical remodeling as a determinant of different outcomes between two current ablation strategies: circumferential pulmonary vein isolation vs pulmonary vein isolation. Clinical Cardiology. 2010;33:E69–E74. doi: 10.1002/clc.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jongnarangsin K, Chugh A, Good E, Mukerji S, Dey S, Crawford T, et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. Journal of Cardiovascular Electrophysiology. 2008;19:668–672. doi: 10.1111/j.1540-8167.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 25.Khaykin Y, Oosthuizen R, Zarnett L, Essebag V, Parkash R, Seabrook C, et al. Clinical predictors of arrhythmia recurrences following pulmonary vein antrum isolation for atrial fibrillation: predicting arrhythmia recurrence post-PVAI. Journal of Cardiovascular Electrophysiology. 2011;22:1206–1214. doi: 10.1111/j.1540-8167.2011.02108.x. [DOI] [PubMed] [Google Scholar]
- 26.Letsas KP, Weber R, Burkle G, Mihas CC, Minners J, Kalusche D, et al. Pre-ablative predictors of atrial fibrillation recurrence following pulmonary vein isolation: the potential role of inflammation. Europace. 2009;11:158–163. doi: 10.1093/europace/eun309. [DOI] [PubMed] [Google Scholar]
- 27.Letsas KP, Siklody CH, Korantzopoulos P, Weber R, Burkle G, Mihas CC, Kalusche D, Arentz T. The impact of body mass index on the efficacy and safety of catheter ablation of atrial fibrillation. International Journal of Cardiology. 2013;164:94–98. doi: 10.1016/j.ijcard.2011.06.092. [DOI] [PubMed] [Google Scholar]
- 28.Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune T, Kofune M, et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. Journal of Cardiovascular Electrophysiology. 2011;22:987–993. doi: 10.1111/j.1540-8167.2011.02059.x. [DOI] [PubMed] [Google Scholar]
- 29.Themistoclakis S, Schweikert RA, Saliba WI, Bonso A, Rossillo A, Bader G, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm : the official journal of the Heart Rhythm Society. 2008;5:679–685. doi: 10.1016/j.hrthm.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 30.Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. Journal of the American College of Cardiology. 2011;57:1745–1751. doi: 10.1016/j.jacc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Deepa M, Farooq S, Datta M, Deepa R, Mohan V. Prevalence of metabolic syndrome using WHO, ATPIII and IDF definitions in Asian Indians: the Chennai Urban Rural Epidemiology Study (CURES-34) Diabetes/Metabolism Research and Reviews. 2007;23:127–134. doi: 10.1002/dmrr.658. [DOI] [PubMed] [Google Scholar]
- 32.Sundstrom J, Riserus U, Byberg L, Zethelius B, Lithell H, Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ. 2006;332:878–882. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 34.Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC, Allessie MA. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 35.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg MS. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution: International Journal of Organic Evolution. 2005;59:464–468. [PubMed] [Google Scholar]
- 37.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology. 2000;53:1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 38.Wyse DG, Gersh BJ. Atrial fibrillation: a perspective: thinking inside and outside the box. Circulation. 2004;109:3089–3095. doi: 10.1161/01.CIR.0000132611.01101.DC. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circulation Research. 2007;101:839–847. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 40.Munger TM, Dong YX, Masaki M, Oh JK, Mankad SV, Borlaug BA, et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. Journal of the American College of Cardiology. 2012;60:851–860. doi: 10.1016/j.jacc.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 41.Wang TJ, Parise H, Levy D, D’Agostino RB, Sr., Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA : the Journal of the American Medical Association. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 42.Schotten U, Neuberger HR, Allessie MA. The role of atrial dilatation in the domestication of atrial fibrillation. Progress in Biophysics and Molecular Biology. 2003;82:151–162. doi: 10.1016/s0079-6107(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 43.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 44.Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. International Journal of Cardiology. 2007;115:135–143. doi: 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 45.Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. The American Journal of Cardiology. 2011;108:47–51. doi: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 46.Dewire J, Calkins H. State-of-the-art and emerging technologies for atrial fibrillation ablation. Nature Reviews Cardiology. 2010;7:129–138. doi: 10.1038/nrcardio.2009.232. [DOI] [PubMed] [Google Scholar]