Abstract
This study tested the hypothesis that ABT-888 (velparib), a poly (ADP-ribose) polymerase (PARP) inhibitor, can modulate temozolomide (TMZ) resistance in recurrent TMZ refractory glioblastoma patients. The combination regimen (TMZ/ABT-888) was tested using 2 randomized schedules (5 versus 21 days), with 6-month progression free survival (PFS6) as the primary endpoint. The maximum tolerated dose (MTD) for TMZ using the 21 day of 28 TMZ schedule, in concert with 40 mg BID ABT-888 was determined in a phase I portion of this study, and previously reported to be 75 mg/m2 (arm1). The MTD for ABT-888 (40 mg BID) and the 5 of 28 day TMZ (150-200 mg/m2) schedule was known from prior trials (arm 2). Two cohorts were studied: bevacizumab (BEV) naïve (n=151), and BEV refractory (n=74). Overall ten patients were ineligible. The incidence rate of grade 3/4 myelosuppression over all was 20.0%. For the BEV refractory cohort, the PFS 6 was 4.4%; for the BEV naïve cohort, PFS6 was 17%. Overall survival was similar for both arms in both the BEV naïve (median survival time (MST) 10.3M; 95% CI, 8.4-12) and BEV refractory cohort (MST 4.7 M; 95%CI, 3.5-5.6). The median PFS was essentially the same for both arms and both cohorts at ~2.0M (95% CI, 1.9-2.1).
Keywords: Glioblastoma, ABT-888, Temozolomide, Velparib
Introduction
A large multinational study demonstrated the effectiveness of temozolomide (TMZ) with and after radiation in the management of newly diagnosed glioblastoma (GBM) after maximal safe resection [1]. This approach provides a median survival time (MST) of 12-16 months with only 10% of patients surviving 5 years or more [2]. More recently a phase III study has provided evidence for improved survival with an alternating electrical field technology used in conjunction with TMZ in newly diagnosed patients, but data are not yet available in a peer-reviewed format [3].
To date, no therapy, with the exception of carmustine wafers [4] in a select subset of patients, (based on 6 month survival data) has provided level 1 evidence for survival benefit in patients with recurrent GBM. Although bevacizumab (BEV) was approved by the US FDA for recurrent GBM, its benefit is reflected in improved edema control and progression free survival (PFS) only. More significantly, no drug therapy to date has had any demonstrated activity after progression on BEV [5].
Relative to the above discussion, it was recognized that there might be a further opportunity to exploit the usefulness of TMZ: At least two major resistance mechanisms to TMZ exist. High levels of O6 alkylguanine DNA alkyltransferase (AGAT) have been shown to be a significant resistance mechanism for TMZ and additionally has prognostic significance [6]. The gene locus for the production of AGAT is methyl-guanine-methyl-transferase (MGMT). If MGMT is methylated, levels of AGAT production are low, which confers TMZ sensitivity; if MGMT is unmethylated, AGAT levels are high, and this in turn confers resistance by reversing temozolomide-induced alkylation. Multiple strategies have been pursued to address MGMT-related resistance. These strategies include patient selection and/or multiday dosing (e.g., 21 days of TMZ) to diminish AGAT levels [7], (as AGAT is a suicide enzyme, and prolonged exposure to TMZ reduces its levels), as well as the use of specific AGAT-inhibitors.
Interestingly, only 7% of the alkylation of temozolomide involves the O6 position of guanine. Far more common (i.e., approximately 70%) is the alkylation of the N-7 position of guanine and N-3 position of adenine. These sites of methylation relate to by base excision pathways, for which poly (ADP-ribose) polymerase (PARP) activity is required [8]. Consistent with these observations, PARP has been directly shown to be related to TMZ resistance [9, 10].
With the aforementioned background, the RTOG conducted a phase I/II trial of TMZ and the PARP inhibitor ABT-888 [11] in recurrent (TMZ resistant) GBM patients. The doses of ABT-888 for this study were derived from a phase 0 study [12] an ongoing phase I trial with ABT-888 in subjects with nonhematologic malignancies and metastatic melanoma (Abbive Investigator's Brochure, 2010) [11]. Additionally, the ABT-888 pharmacokinetic results following the 20 and 40 mg BID doses achieved the steady-state exposures (AUC) that were effective in murine efficacy models and were thus selected. The maximum tolerated dose (MTD) for ABT-888 (40 mg BID) with a 5 of 28 days TMZ dose (150-200 mg/m2) was known. The 21 day TMZ dose was determined in a phase I portion of this study, and previously reported to be 75 mg/m2 [13]. Two cohorts were studied: BEV naïve, and BEV refractory.
The overall goal of this study was to provide a foundation for future studies in newly diagnosed and recurrent GBM. Inherent in this long-term goal is the recognition that ABT-888 as a PARP inhibitor is a known radiation sensitizer and might be useful in GBM patients whose disease is not MGMT methylated for which many believe the efficacy of TMZ in negligible. It was the intent of the current study to treat TMZ-resistant patients as a proof of principal study, (i.e., to confirm that PARP modulation can result in restoration of TMZ sensitivity. If confirmed this strategy may be useful in newly diagnosed patients in whom PARP inhibition may be unusually beneficial (e.g., those with non-methylated MGMT GBM). Further it was hoped that this approach might provide a salvage therapy for patient who had progressed on BEV. In this regard, as the prognosis at recurrence for BEV naïve, versus BEV treated patients, is dramatically different [5], patients were placed in different treatment cohorts on this basis. The report to follow summarizes the results of this study
Patients and methods
Patient Population
Histologically proven intracranial glioblastoma or gliosarcoma (phase II) or any high-grade glioma (phase I), with imaging confirmation of tumor progression or regrowth. Patients were required to have completed a course of radiation therapy (phases I and II) and at least 2 adjuvant cycles of temozolomide (phase II). Phase II patients could not have had more than 2 prior regimens for recurrent disease for glioblastoma/gliosarcoma; phase I patients were required not to have more than 3 prior treatments. Other Criteria included: Karnofsky performance status ≥ 70; Age ≥ 18; CBC/differential obtained within 14 days prior to registration, with adequate bone marrow function defined as follows: Absolute neutrophil count (ANC) ≥ 1.5 × 109/L, Platelets ≥ 100 × 109/L, Hemoglobin ≥ 10.0 g/dl, White blood cell count (WBC) ≥ 3 × 109/L; Adequate liver function within 14 days prior to registration, defined as follows: SGOT [AST] < 3.0 × the upper limit of normal (ULN), SGPT [ALT] < 3.0 X ULN Bilirubin ≤ 1.25 X ULN; Adequate renal function, defined as follows, within 14 days prior to registration: Creatinine < 1.7 mg/dL and normal urinary protein; 28 days from the administration of any investigational agent; 28 days from administration of prior cytotoxic therapy with the following exceptions: 42 days from administration of nitrosoureas, 21 days from administration of procarbazine,14 days from administration of non-cytotoxic agents.
Summary of Patient Enrollment
The phase II components of this study were conducted through two separate randomized phase II studies: one for the BEV-naïve group and the other for the BEV-failure group. The phase II component opened to accrual on March 6, 2012, with a target sample size of 212, 142 for the BEV-naïve group and 70 for the BEV-failure group. The study on the BEV-naïve group was temporarily closed to accrual on November 21, 2012, and reopened on April 17, 2013 following a protocol-specified efficacy analysis. The BEV-naïve and BEV-failure groups were closed to accrual on July 23, 2013 and January 28, 2013, respectively, with a total of 225 patients enrolled, 151 for the BEV-naïve group and 74 for the BEV-failure group. Overall 10 patients (4.4%) were found to be ineligible secondary to: less than 2 cycles of adjuvant TMZ; no prior TMZ with radiation; no MRI evidence of progression; TMZ given <28days prior to registration, and therefore excluded from analysis. Table 1 lists the distributions of pretreatment characteristics for all eligible patients. The distributions of the stratification factors appeared balanced between the two randomized treatment arms for each group.
Table 1.
Pretreatment Characteristics
| ARM1/BEV-NAIVE (n=73) | ARM2/BEV-NAIVE (n=73) | ARM1/BEV-FAILURE (n=32) | ARM2/BEV-FAILURE (n=37) | |
|---|---|---|---|---|
| Age (years), Median (Min – Max) | 56(21 – 77) | 56(20 - 81) | 56.5(20 - 70) | 57 (38 - 77) |
| Gender | ||||
| Male | 53 ( 72.6%) | 47 ( 64.4%) | 20 ( 62.5%) | 23 ( 62.2%) |
| Female | 20 ( 27.4%) | 26 ( 35.6%) | 12 ( 37.5%) | 14 ( 37.8%) |
| Karnofsky Performance Status* | ||||
| 70-80 | 29 ( 39.7%) | 28 ( 38.4%) | 22 ( 68.8%) | 26 ( 70.3%) |
| 90-100 | 44 ( 60.3%) | 45 ( 61.6%) | 10 ( 31.3%) | 11 ( 29.7%) |
| Neurologic Function | ||||
| No symptoms | 27 ( 37.0%) | 33 ( 45.2%) | 9 ( 28.1%) | 7 ( 18.9%) |
| Minor symptoms | 30 ( 41.1%) | 27 ( 37.0%) | 9 ( 28.1%) | 13 ( 35.1%) |
| Moderate symptoms (fully active) | 9 ( 12.3%) | 10 ( 13.7%) | 9 ( 28.1%) | 10 ( 27.0%) |
| Moderate symptoms (required assistance) | 7 ( 9.6%) | 3 ( 4.1%) | 5 ( 15.6%) | 7 ( 18.9%) |
| Surgery (Initial Brain Tumor) | ||||
| None | 0 ( 0.0%) | 0 ( 0.0%) | 1 ( 3.1%) | 0 ( 0.0%) |
| Biopsy only | 5 ( 6.8%) | 6 ( 8.2%) | 2 ( 6.3%) | 4 ( 10.8%) |
| Subtotal resection | 27 ( 37.0%) | 25 ( 34.2%) | 10 ( 31.3%) | 14 ( 37.8%) |
| Gross total resection | 39 ( 53.4%) | 41 ( 56.2%) | 19 ( 59.4%) | 18 ( 48.6%) |
| Other | 2 ( 2.7%) | 1 ( 1.4%) | 0 ( 0.0%) | 1 ( 2.7%) |
| Recent resection* | ||||
| No - biopsy only | 42 ( 57.5%) | 42 ( 57.5%) | 23 ( 71.9%) | 29 ( 78.4%) |
| Yes | 31 ( 42.5%) | 31 ( 42.5%) | 9 ( 28.1%) | 8 ( 21.6%) |
| Measurable disease (mm2) | (n=62) | (n=65) | (n=26) | (n=33) |
| Median | 42.885 | 119 | 283 | 506 |
| Min - Max | 0.49 - 2044.25 | 0.3599 - 8160 | 7.8736 - 7800 | 1.4 - 4464 |
Stratification factor.
Treatment
The combination regimen (TMZ/ABT-888) was tested using 2 randomized schedules (21 versus 5 days; arms 1 & 2 respectively), with 6-month PFS (PFS6) as the primary endpoint. The MTD for ABT-888 (40 mg BID) with a 5 of 28 days TMZ schedule (150-200 mg/m2) was known, the 21 day TMZ dose in concert with 40 mg BID of ABT-888 was determined in a phase 1 portion of this study [12]. That dose of TMZ, 75 mg/m2, was then utilized in the phase II portion of the study. Two different cohorts of patients were studied: BEV naïve, and BEV refractory, on the same treatment options mentioned above.
Statistical Methods
Study Design for Primary Endpoints
The primary objective of the phase II component of this study is to determine whether the combination of TMZ and ABT-888 (using two different schedules, 5-day and 21-day) improved the PFS6 rate in patients with recurrent GBM who had been previously treated with TMZ. The phase II component was conducted through two separate randomized phase II studies: one for the BEV-naïve group and the other for the BEV-failure group.
For the null hypothesis, assuming no improvement in efficacy for the BEV-naïve group, the PFS6 rate was set to be 15%, according to Wong et al. [14]. For the alternative hypothesis, the PFS6 rate was set at 30%. The study was designed to provide 90% power for the detection of a 15% absolute increase in the PFS6 rate. To control for type I error, the significance level was 0.10 (one-sided). Simon's min-max two-stage principle was used to calculate the sample size [15]. The first stage required 34 analyzable patients with measurable disease after surgery for each arm. If 5 or fewer experienced 6-month PFS, then the null hypothesis that the PFS6 rate for the experimental arm is less than 15% could not be rejected. Otherwise, accrual would continue to a total of 53 analyzable patients with measurable disease after surgery for each arm. If 11 or fewer patients experienced 6-month PFS among the 53 patients, we would not reject the null hypothesis that the PFS6 rate for the experimental arm is less than 15%; if 12 or more patients experienced 6-month PFS, we would reject the null hypothesis that the PFS6 rate of the experimental arm is less than 15% and conclude that PFS6 is at least 30%. The total registration sample size was 71 patients for each arm to ensure that there would be enough eligible patients with measurable disease after surgery.
For the BEV-failure group, the PFS6 rate was reported as 0% based on a literature review [16]. For the null hypothesis, assuming no improvement in efficacy for the BEV-failure group, the PFS6 rate was set to be 2%. For the alternative hypothesis, the PFS6 rate was set at 15%, with a 13% absolute increase. The study was designed to provide 90% power for the detection of this 13% absolute increase in the PFS6 rate, requiring 26 analyzable patients with measurable disease after surgery for each arm, to detect the projected absolute increase in PFS6 at a significance level of 0.10 (one-sided). If at least 2 patients were progression free at 6 months, we would record this regimen to be promising in this patient group. To ensure that there would be enough analyzable patients with measurable disease after surgery, the study for the BEV-failure group required a total sample size of 70 patients, 35 per arm.
The crude incidence rate of treatment response (complete response (CR) and partial response (PR)) by central review was evaluated as a secondary endpoint. For the BEV-naïve group, the analysis was to be performed on the first 53 analyzable patients with measurable disease after surgery for each arm. For the BEV-failure group, the analysis would be performed on the first 26 analyzable patients with measurable disease after surgery for each arm. The crude incidence rate of treatment response were also planned to be calculated on patients with no measurable disease after surgery by treatment arm and group. Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method for each treatment arm in both groups, based on all the eligible patients. OS was defined as the interval from randomization to death due to any cause, and PFS as the interval from randomization to progression or death, whichever occurred first.
Results
Treatment Adverse Events
Adverse events (AEs) were scored by CTCAE version 4.0; Table 2 summarizes all the observed toxicities. For all the eligible patients, the incidence rate of grade 3/4 myelosuppression was 20.0%. For Bev refractory patients: in arm 1 (n=32) the grade 3/4 incidence of neutropenia and thrombocytopenia was 12.6% and 25.0% respectively; in arm 2 (n=37) it was 8.1% and 5.4% respectively. For BEV naïve patients: in arm 1 (n=73) the grade 3/4 incidence of neutropenia and thrombocytopenia was 19.1% and 16.4 respectively; in arm 2 (n=73) it was 4.1% and 8.2% respectively. There were only two patients with grade 3 or higher CD4 lymphopenia, and both were grade 3 cases, one in arm 2 of the BEV naïve group and the other in arm 1 of the BEV refractory group.
Table 2.
Number of Patients with an Adverse Event by System Organ Class and Grade. Any Relationship to Protocol Treatment
| ARM1/BEV-NAIVE (n=73) Grade | ARM2/BEV-NAIVE (n=73) Grade | ARM1/BEV-FAILURE (n=32) Grade | ARM2/BEV-FAILURE (n=37) Grade | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| System Organ Class | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| Investigations | 9 | 11 | 26 | 7 | 0 | 20 | 22 | 16 | 2 | 0 | 3 | 6 | 7 | 7 | 0 | 13 | 5 | 6 | 2 | 0 |
| CD4 lymphocytes decreased | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphocyte count decreased | 2 | 5 | 23 | 1 | 0 | 4 | 15 | 11 | 2 | 0 | 1 | 6 | 3 | 3 | 0 | 2 | 5 | 5 | 1 | 0 |
| Neutrophil count decreased | 4 | 11 | 9 | 5 | 0 | 6 | 6 | 3 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 1 | 0 |
| Platelet count decreased | 23 | 6 | 9 | 3 | 0 | 32 | 10 | 6 | 0 | 0 | 10 | 3 | 4 | 4 | 0 | 18 | 2 | 1 | 1 | 0 |
| Blood and lymphatic system disorders | 20 | 3 | 1 | 0 | 0 | 23 | 4 | 0 | 0 | 0 | 7 | 1 | 1 | 1 | 0 | 7 | 3 | 1 | 0 | 0 |
| Cardiac disorders | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Ear and labyrinth disorders | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Endocrine disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Eye disorders | 5 | 2 | 0 | 0 | 0 | 4 | 3 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 0 | 0 |
| Gastrointestinal disorders | 20 | 17 | 1 | 0 | 0 | 15 | 18 | 3 | 1 | 0 | 8 | 7 | 3 | 0 | 0 | 10 | 4 | 1 | 0 | 0 |
| General disorders/administration site conditions | 19 | 16 | 2 | 0 | 2 | 23 | 14 | 4 | 0 | 1 | 7 | 9 | 2 | 0 | 0 | 9 | 10 | 3 | 0 | 0 |
| Immune system disorders | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infections and infestations | 1 | 3 | 5 | 0 | 0 | 0 | 9 | 3 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 4 | 1 | 0 | 0 |
| Injury, poisoning and procedural complications | 3 | 4 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 |
| Metabolism and nutrition disorders | 17 | 7 | 3 | 0 | 0 | 20 | 8 | 5 | 0 | 0 | 8 | 5 | 3 | 1 | 0 | 9 | 3 | 3 | 2 | 0 |
| Musculoskeletal and connective tissue disorders | 9 | 8 | 4 | 0 | 0 | 8 | 8 | 4 | 0 | 0 | 4 | 7 | 6 | 0 | 0 | 3 | 7 | 4 | 0 | 0 |
| Neoplasms benign, malignant and unspecified | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 2 | 0 | 3 |
| Nervous system disorders | 23 | 24 | 5 | 4 | 1 | 19 | 19 | 13 | 2 | 0 | 8 | 10 | 4 | 0 | 0 | 8 | 6 | 7 | 1 | 1 |
| Psychiatric disorders | 10 | 8 | 2 | 0 | 0 | 7 | 10 | 4 | 0 | 0 | 8 | 3 | 2 | 0 | 0 | 4 | 8 | 0 | 0 | 0 |
| Renal and urinary disorders | 5 | 2 | 0 | 0 | 0 | 5 | 2 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| Reproductive system and breast disorders | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | 4 | 2 | 1 | 0 | 1 | 9 | 4 | 0 | 0 | 0 | 4 | 1 | 0 | 1 | 0 | 1 | 4 | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | 10 | 3 | 0 | 0 | 0 | 6 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 |
| Social circumstances | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Vascular disorders | 2 | 7 | 0 | 1 | 0 | 1 | 5 | 6 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
Includes adverse events where relationship to protocol treatment is missing.
Adverse events were graded with CTCAE version 4. (Specific toxicities types in italics).
Tests for Primary Endpoints
6-month PFS status
Overall, only162 (75.3%) patients were evaluable for 6-month PFS, as 3 patients received no therapy, 21 (9.8%) patients did not complete cycle 1 of therapy, and 29 patients had no measurable disease after surgery. For the BEV-failure group, among the first 26 evaluable in arm 2, only 1 (3.8%) experienced 6-month PFS, less than the two patients needed to claim this regimen as efficacious. Therefore, we were unable to reject the null hypothesis that the PFS6 rate is less than 2%. For arm 1, since the targeted accrual for the primary endpoint of this study was 26 analyzable patients with measurable disease after surgery and only 19 had been identified from the 36 accrued, the parameters for the rejection region of the primary endpoint hypothesis test needed to be adjusted. With a sample size of 19 evaluable patients, the design provided an adjusted power of 86% at a significance level of 0.10. The rejection region continued to define that if at least 2 patients were progression free at 6 months, we would be able to claim this regimen as efficacious. Of these 19 patients, only 1 (5.3%) experienced 6 month PFS, and therefore, we are unable to reject the null hypothesis that the PFS6 rate for the combination of TMZ and ABT-888 is less than 2% in the BEV-failure cohort.
For each arm in the BEV-naïve group, among the first 53 evaluable patients, 9 (17.0%) experienced 6-month PFS, less than the number of patients needed (12) to claim efficacy for this regimen in this patient group. Therefore, we are unable to reject the null hypothesis that PFS6 for the combination of TMZ and ABT-888 is less than 15% in the BEV-naïve cohort.
Results for the Secondary Endpoints
Response Rates
In Bev-failure group, on arm 1, there was 1 CR (5.3%), and no PR; no CR or PR on arm2. In the BEV-naïve group, there was also 1 CR (1.9%), and 1 PR (1.9%), both on arm 2, for an overall response rate of 3.8%]; additionally, on arm 1 of the BEV-naïve group, there was 1 stable disease (1.9%). %). For the 29 eligible patients with no measurable disease (19 from BEV-naïve group and 10 from BEV-failure group), there was no CR or PR.
OS and PFS
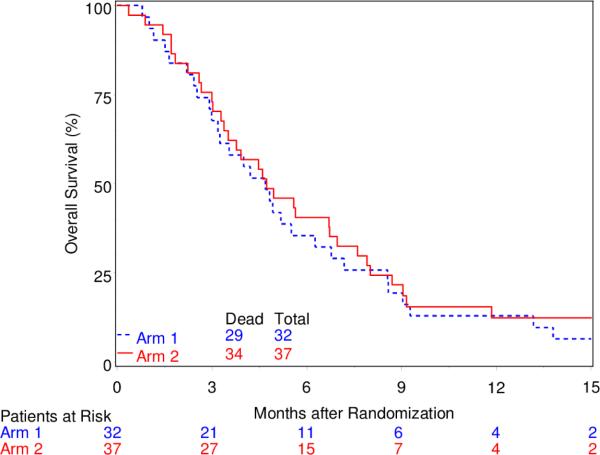
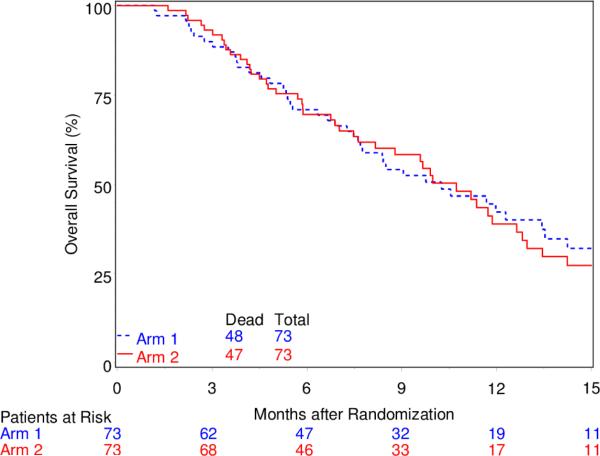
For both the BEV-failure and BEV-naïve group, OS and PFS were similar between the treatment arms. Specifically, for the BEV-failure group, MST was 4.7 months (95% CI, 3.0-6.3) for arm 1 and 4.7 months (95% CI, 3.4-7.0) for arm 2, and the Kaplan-Meier curves for OS did not differ by treatment arm (Figure 1). Median PFS time was 2.2 months (95% CI, 1.1-3.2) and 1.9 months (95% CI, 1.8-2.1), respectively for arm 1 and 2. For the BEV-naïve group, MST was 10.3 (95% CI, 7.6-13.4) months for arm 1 and 10.7 (95% CI, 7.6-12.6) for arm 2, and the Kaplan-Meier curves for OS again show no difference between the two arms (Figure 2). Median PFS time was 2.0 months (95% CI, 1.8-2.5) and 2.1 months (95% CI, 1.9-2.3), respectively for arm 1 and 2.
Fig. 1.
Overall Survival for BEV-Failure Group
Fig. 2.
Overall Survival for BEV-Naive Group
Discussion
This study was designed as a proof of principle relating to the potential ability of the PARP inhibitor ABT-888 (veliparib) to overcome TMZ resistance. Protocol eligibility required prior TMZ exposure. At the time of protocol conception the availability MGMT-methylation status [17] was not mandated, primarily for practical considerations, as well as because the hypothesis was deemed to be “AGAT-independent”. Indeed, the overriding concept for the study was to capitalize on methylation at the N3 and N7 positions, presumed to be independent of the effect of MGMT and related more to base excision repair with PARP as a necessary co-factor. Hence there was no prospective to plan to collect such data. Relative to this assumption, it is of interest that at least one group of investigators has recently evolved preclinical data suggesting a relationship between MGMT methylation status and the ability of ABT-888 to overcome TMZ resistance. This has resulted in the activation of an Alliance Oncology study (A071102): “A Phase II/III Randomized Trial of Veliparib or Placebo in Combination with Adjuvant Temozolomide in Newly Diagnosed Glioblastoma with MGMT Promoter Hypermethylation.” The A071102 trial tests the veliparib-TMZ synergy concept using arm 1, i.e., 5/28 day treatment schedule, which was far better tolerated than the arm 2, i.e., 21/28 day schedule in our study. Parenthetically, in our study MGMT methylation data was obtained retrospectively for the patients demonstrating response: the 2 CR's were methylated; the 1 PR was not methylated; data were not available for the one patient with stable disease. In any case, our trial categorically demonstrates very low clinical activity of the combination of TMZ and veliparib in both the Bev-naïve and refractory recurrent GBM cohorts, with absolutely no clinical suggestion of synergy, in patients unselected for MGMT-methylation; this observation should be considered in light of the known ~ 40% MGMT-methylation rate anticipated in a newly diagnosed GBM cohort.
Our data relating to the differing prognosis of BEV-naïve and BEV refractory patients as illustrated in Figures 1 and 2 is completely consistent with a review of the literature (i.e.,16 phase II studies) encompassing 995 BEV progressed patients [5]. Magnus et al noted that the median time to progression (+/−SD) on BEV was 4.2 M (+/− 2.1), and median survival after progression on BEV was 3.8M (+/−1) [5]. The data reported here adds to this literature and again illustrates the dismal prognosis of GBM patients after progression on BEV. The OS data in the BEV-naïve group is similarly consistent overall survival BEV-naïve patients [18, 19]. Presumably these patients received further therapy (which was not recorded), which likely included BEV.
In summary the combination of TMZ and ABT-888 did not significantly improve PFS6 for either the BEV-naïve or BEV-failure patients with recurrent GBM who have been previously treated with TMZ. For both groups of patients, the objective response rate was low, and did not differ by treatment arm, and the OS and PFS were similar for both treatment arms.
Acknowledgement
The authors wish to thank our senior data manager, Sandrine Geinoz PhD, CCRP for her significant contributions.
Funding
This project was supported by grants U10CA21661, U10CA180868, U10CA180822, U10CA37422, and UG1CA189867 from the National Cancer Institute (NCI) and AbbVie.
Footnotes
Conflicts of Interest
H. Ian Robins reports grants and personal fees from Novocure, personal fees from Abbvie, outside the submitted work. Mark R Gilbert reports personal fees and non-financial support from Merck, personal fees from Genentech Roche, personal fees from Abbvie, personal fees from Wellcome Trust, and personal fees from Foundation Medicine, outside the submitted work. Frank S Lieberman reports grants and personal fees from Roche (Genentech), grants from Novacure, grants from Celldex, and grants from Stemline, outside the submitted work. Nimish Mohile reports personal fees from Novocure, personal fees from Abbvie, outside the submitted work. Howard Colman reports personal fees from Hoffman La Roche, personal fees from Genentech, personal fees from Sigma Tau, personal fees from Proximagen/Upsher Smith, personal fees from Foundation Medicine, personal fees from Merck, outside the submitted work. Santosh Kesari reports personal fees from Novocure, personal fees from Sigma Tau, grants from Celldex, grants from Tocagen, grants from NWBT, grants from Novartis, outside the submitted work. Minesh Mehta reports personal fees from Abbvie, personal fees from BMS, personal fees from Celldex, grants and personal fees from Novelos, personal fees from Phillips, personal fees from Roche, personal fees from Elekta, personal fees from Novartis, personal fees from Cavion, grants and personal fees from Novocure, personal fees from Pharmacyclics, outside the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. doi:352/10/987 [pii] 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. doi:S1470-2045(09)70025-7 [pii] 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Wong E, Scott C, Taillibert S, Kanner A, Kesari S, Ram Z. Interim Analysis of the EF-14 Trial: A Prospective, Multi-center Trial of NovoTTF-100A Together With Temozolomide Compared to Temozolomide Alone in Patients with Newly Diagnosed GBM. Neuro-Oncology. 2014;16:v167–v167. abstr NT-140 doi:10.1093/neuonc/nou265.40. [Google Scholar]
- 4.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 5.Magnuson W, Ian Robins H, Mohindra P, Howard S. Large volume reirradiation as salvage therapy for glioblastoma after progression on bevacizumab. J Neurooncol. 2014;117:133–139. doi: 10.1007/s11060-014-1363-z. doi:10.1007/s11060-014-1363-z. [DOI] [PubMed] [Google Scholar]
- 6.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. doi:352/10/997 [pii] 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 7.Tolcher AW, Gerson SL, Denis L, Geyer C, Hammond LA, Patnaik A, Goetz AD, Schwartz G, Edwards T, Reyderman L, Statkevich P, Cutler DL, Rowinsky EK. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88:1004–1011. doi: 10.1038/sj.bjc.6600827. doi:10.1038/sj.bjc.6600827 6600827 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tentori L, Graziani G. Chemopotentiation by PARP inhibitors in cancer therapy. Pharmacol Res. 2005;52:25–33. doi: 10.1016/j.phrs.2005.02.010. doi:S1043-6618(05)00042-3 [pii] 10.1016/j.phrs.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Tentori L, Orlando L, Lacal PM, Benincasa E, Faraoni I, Bonmassar E, D'Atri S, Graziani G. Inhibition of O6-alkylguanine DNA-alkyltransferase or poly(ADP-ribose) polymerase increases susceptibility of leukemic cells to apoptosis induced by temozolomide. Mol Pharmacol. 1997;52:249–258. doi: 10.1124/mol.52.2.249. [DOI] [PubMed] [Google Scholar]
- 10.Wedge SR, Porteous JK, Newlands ES. 3-aminobenzamide and/or O6-benzylguanine evaluated as an adjuvant to temozolomide or BCNU treatment in cell lines of variable mismatch repair status and O6-alkylguanine-DNA alkyltransferase activity. Br J Cancer. 1996;74:1030–1036. doi: 10.1038/bjc.1996.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepeak LM, Leal T, Robins HI. Preclinical and clinical development of Velaparib (ABT-888): a poly (ADP-ribose) polymerase inhibitor. Drugs of the Future. 2010;35:815–822. [Google Scholar]
- 12.Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, Ji J, Monks A, Low JA, Chen A, Murgo AJ, Collins J, Steinberg SM, Eliopoulos H, Giranda VL, Gordon G, Helman L, Wiltrout R, Tomaszewski JE, Doroshow JH. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol. 2009;27:2705–2711. doi: 10.1200/JCO.2008.19.7681. doi:10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robins HI, Wang M, Gilbert MR, Chakravarti A, Grimm S, Penas-Prado M, Chaudhary R, Anderson PJ, Elinzano H, Gilbert RA, Mehta M. Phase I results from RTOG 0929, a randomized phase I/II study of ABT-888 (veliparib) in combination with temozolomide (TMZ) in recurrent, TMZ-resistant glioblastoma. Neuro Oncol. 2012;14 abstr NO-14. [Google Scholar]
- 14.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 15.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled clinical trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 16.Lassman AB, Iwamoto FM, Gutin PH. Patterns of relapse and prognosis *after* bevacizumab (BEV) failure in recurrent glioblastoma (GBM). J Clin Oncol. 2008;26:96S. doi: 10.1212/WNL.0b013e3181bc0184. abstr 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wick W, Weller M, van den Bent M, Sanson M, Weiler M, von Deimling A, Plass C, Hegi M, Platten M, Reifenberger G. MGMT testing--the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10:372–385. doi: 10.1038/nrneurol.2014.100. doi:10.1038/nrneurol.2014.100. [DOI] [PubMed] [Google Scholar]
- 18.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:JCO.2008.19.8721 [pii] 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 19.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. doi:JCO.2008.16.3055 [pii] 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]